Abstract
BACKGROUND & AIMS
The gastric cancer-causing pathogen Helicobacter pylori upregulates spermine oxidase (SMOX) in gastric epithelial cells, causing oxidative stress-induced apoptosis and DNA damage. A subpopulation of SMOXhigh cells are resistant to apoptosis, despite their high levels of DNA damage. Because epidermal growth factor receptor (EGFR) activation can regulate apoptosis, we determined its role in SMOX-mediated effects.
METHODS
SMOX, apoptosis, and DNA damage were measured in gastric epithelial cells from H pylori-infected Egfrwa5 mice (which have attenuated EGFR activity), Egfr wild-type mice, or in infected cells incubated with EGFR inhibitors or deficient in EGFR. Phosphoproteomic analysis was performed. Two independent tissue microarrays containing each stage of disease, from gastritis to carcinoma, and gastric biopsies from Colombian and Honduran cohorts were analyzed by immunohistochemistry.
RESULTS
SMOX expression and DNA damage were decreased, and apoptosis increased in H pylori-infected Egfrwa5 mice. H pylori-infected cells with deletion or inhibition of EGFR had reduced levels of SMOX, DNA damage, and DNA damagehigh apoptosislow cells. Phosphoproteomic analysis revealed increased EGFR and ERBB2 signaling. Immunoblot analysis demonstrated the presence of a phosphorylated (p)EGFR–ERBB2 heterodimer and pERBB2; knockdown of ErbB2 facilitated apoptosis of DNA damagehigh apoptosislow cells. SMOX was increased in all stages of gastric disease, peaking in tissues with intestinal metaplasia, whereas pEGFR, pEGFR–ERBB2, and pERBB2 were increased predominantly in tissues demonstrating gastritis or atrophic gastritis. Principal component analysis separated gastritis tissues from patients with cancer vs those without cancer. pEGFR, pEGFR–ERBB2, pERBB2, and SMOX were increased in gastric samples from patients whose disease progressed to intestinal metaplasia or dysplasia, compared with patients whose disease did not progress.
CONCLUSIONS
In an analysis of gastric tissues from mice and patients, we identified a molecular signature (based on levels of pEGFR, pERBB2, and SMOX) for the initiation of gastric carcinogenesis.
Keywords: marker, prognostic factor, risk, signal transduction pathways
Introduction
Helicobacter pylori is a microaerophilic Gram-negative bacteria that selectively colonizes the human stomach. The infection is acquired in childhood and induces gastritis, and in a subgroup of patients, this progresses to gastric cancer through a cascade of histologic lesions consisting of multifocal atrophic gastritis (MAG), intestinal metaplasia (IM), and dysplasia.1,2 Gastric cancer is the second leading cause of cancer-related deaths worldwide. Because half of the world’s population is infected, H pylori incurs a substantial disease burden.3 Moreover, epidemiologic studies suggest that H pylori prevalence is inversely correlated with a number of diseases including asthma and gastroesophageal reflux disease.4,5 A greater understanding of the specific mediators of H pylori-induced gastric cancer is therefore needed to establish markers of disease risk.
Adherence of H pylori to gastric epithelial cells has been shown to stimulate numerous signaling pathways, including epidermal growth factor receptor (EGFR) activation.6 EGFR tyrosine kinases are key regulators of oncogenic transformation and tumor progression, and are composed of four avian erythroblastic leukemia-associated viral oncogene B (ERBB) homologues: namely EGFR (ERBB1), ERBB2, ERBB3, and ERBB4.7,8 Binding of a ligand to these receptors initiates homodimerization and/or heterodimerization and subsequent tyrosine kinase activation.9 While the ligand for ERBB2 remains unknown, heterodimerization of EGFR with ERBB2 induces phosphorylation of ERBB2 and activation of an intracellular signaling cascade that modulates cellular responses.10,11
H pylori infection induces apoptosis and DNA damage in gastric epithelial cells.12–16 The accumulation of cells with damaged DNA plays a crucial role in the process of carcinogenesis. We have reported that H pylori infection induces the expression of spermine oxidase (SMOX), an enzyme that catabolizes the polyamine spermine to spermidine and produces H2O2 as a byproduct.12,13 The resulting oxidative stress causes apoptosis in epithelial cells, but also increases DNA damage.12,13 Further, H pylori infection results in the generation of a subpopulation of gastric epithelial cells with high levels of DNA damage that are resistant to apoptosis.12 We have also reported that the H pylori-stimulated increase in phosphorylated EGFR (pEGFR) protects epithelial cells from apoptosis.14 In the current report, we demonstrate that increased SMOX expression and DNA damage in H pylori infection is dependent on pEGFR. We used a phosphoproteomics approach to establish the involvement of the ERBB2 signaling pathway, and then directly demonstrate that interference with EGFR activation or ERBB2 eliminates cells with DNA damage that are resistant to apoptosis. Our studies also reveal that in human subjects, SMOX, pEGFR, the pEGFR–ERBB2 heterodimer, and pERBB2 constitute a biologically relevant molecular signature for progression of disease.
Materials and Methods
Reagents
Cell and Culture Conditions, Bacteria, and Mice
Mouse conditionally immortalized stomach epithelial (ImSt) and EGFR−/− ImSt cells were grown and co-cultured with H pylori cagA+ strains PMSS1 or 7.13 at a multiplicity of infection of 200 as described.12,17 C57BL/6 wild-type (WT) and Egfrwa5 mice possessing an antimorphic EGFR allele that attenuates EGFR phosphorylation,18 were infected with PMSS1 for 8 weeks.
Human Subjects
Four human study populations were used (see Supplementary Methods). 1) Surgical specimens from Vanderbilt University Hospital from gastric resections; 209 cores from 84 cases in two tissue microarrays (TMAs). 2) A TMA of gastric tissues purchased from US Biomax, Inc. (Rockville, MD). 3) Biopsies from a longitudinal cohort from the Andean high gastric cancer risk region of Colombia.19 There were 976 original cases randomized to H pylori treatment or placebo in 1991; antral biopsies from the 3-year follow-up were used as the baseline and were selected based on having multifocal atrophic gastritis, H pylori-positive status, and follow-up data being available at the 16-year timepoint. Amongst these patients, 25/68 progressed to metaplasia or dysplasia (36.7%, 95% CI 25.9% – 48.5%) at 16 years, a rate of progression of 3.48 per 100 person years (95% CI 2.4 – 4.9). 4) Antral biopsies from H pylori-positive subjects from a high gastric cancer region of western Honduras.20
Additional Molecular Assays
See Supplementary Methods for description of real-time PCR; immunoblotting and immunoprecipitation; flow cytometry for SMOX, DNA damage and apoptosis; transfection of siRNA against ErbB2 and H pylori serology.
Immunohistochemistry for SMOX, pEGFR and pERBB2; Ligation Assay for pEGFR–ERBB2 Heterodimer
Stable Isotope Labeling by Amino Acids in Cell Culture (SILAC)
Peptide Digestion, Phosphopeptide Enrichment, Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS) and Mass Spectrometry Data Analysis
Pathway Analysis, Motif analysis, and Heat Maps
Phosphoproteins were analyzed as described in Supplementary Methods.
Isolation of Epithelial Cells from Gastric Tissue
Gastric epithelial cells were isolated as described.12
Statistical Analysis
Quantitative data are shown as the mean, ± SE as appropriate. Analyses are in the Supplementary Methods.
Results
Genetic Attenuation of EFGR Activation in Mice Leads to Decreased Levels of SMOX and DNA Damage in vivo During H pylori Infection
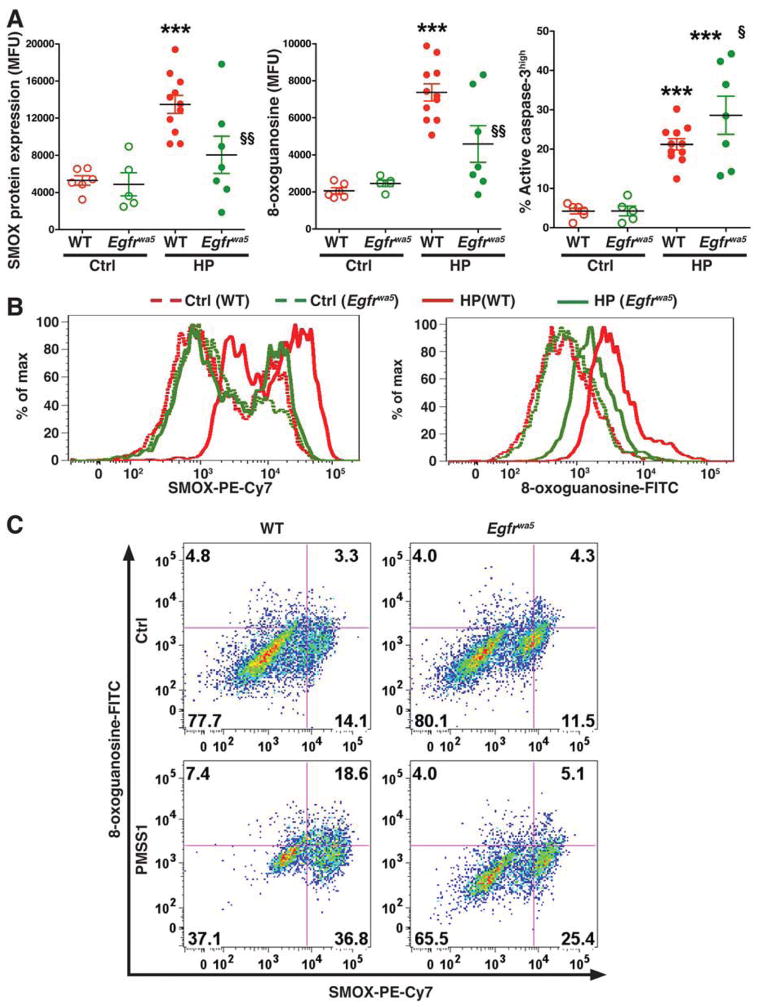
We have reported that SMOX induced by H pylori infection causes oxidative stress that leads to both DNA damage and apoptosis in gastric epithelial cells, and that infection causes phosphorylation of EGFR in vitro and in vivo.12,14,17 We therefore investigated the role of EGFR in the regulation of H pylori-induced SMOX expression and DNA damage in gastric epithelial cells using a mouse model. When assessed by flow cytometry (Figure 1A), gastric epithelial cells isolated from WT mice infected with H pylori for 2 months demonstrated increased levels of SMOX protein, the DNA damage marker 8-oxoguanosine, and the apoptosis marker active caspase-3, compared to cells isolated from uninfected WT mice. Epithelial cells from infected Egfrwa5 mice with defective EGFR activation, when compared to those from WT mice, exhibited decreased levels of SMOX and 8-oxoguanosine (Figure 1A and B). In contrast, apoptosis was further increased in gastric epithelial cells from infected Egfrwa5 mice when compared to WT mice (Figure 1A). When SMOX and 8-oxoguanosine were analyzed simultaneously (Figure 1C), there was a marked increase in the SMOXhigh,8-oxoguanosinehigh cells in infected WT mice, whereas the percentage of these cells in infected Egfrwa5 mice was similar to that in uninfected WT mice. These findings indicate that EGFR transactivation is needed in vivo to generate a population of cells at increased risk for transformation that harbor SMOX-mediated oxidative DNA damage, even though levels of histologic gastritis and H pylori colonization were not different in Egfrwa5 mice when compared to WT mice (Supplementary Figure 1 A and B).
Figure 1.
Levels of SMOX protein, DNA damage, and apoptosis in mice infected with H pylori. C57BL/6 wild-type (WT) and Egfrwa5 mice were infected with PMSS1 for 8 weeks, and gastric epithelial cells were isolated at 2 months post-inoculation. (A) Summary data for levels of SMOX, 8-oxoguanosine, and active caspase-3, measured by flow cytometry. ***P < .001 vs uninfected WT control (Ctrl); §P < .05, §§P < .01 vs infected WT mice. (B) Representative histograms for SMOX and 8-oxoguanosine. (C) Representative dot plots for simultaneous analysis of SMOX and 8-oxoguanosine.
Levels of SMOX Expression and Phosphorylated EGFR Are Increased in Preneoplastic Lesions in Human Gastric Tissues
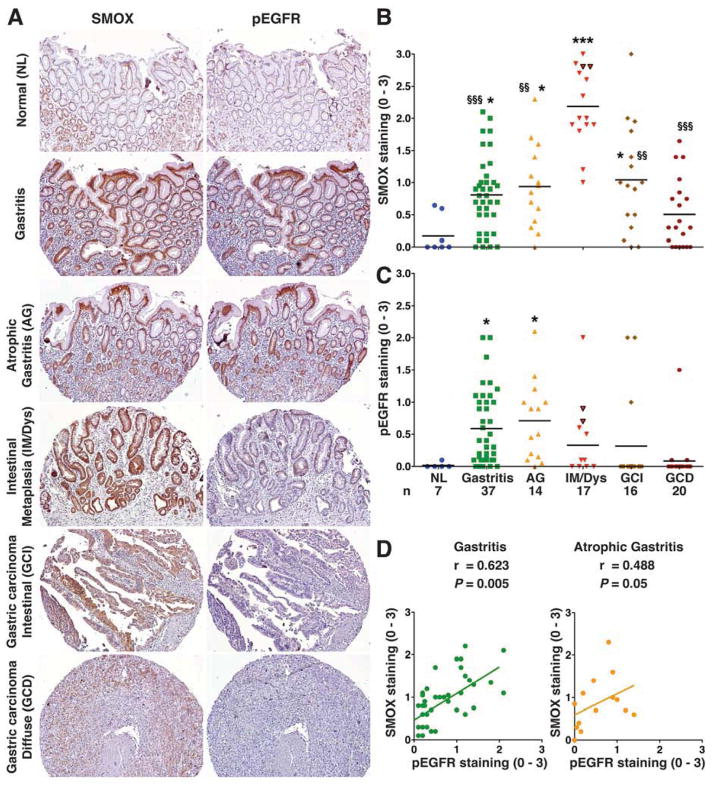
To provide direct clinical relevance for an interaction between EGFR activation and SMOX, we generated and immunostained a tissue microarray (TMA) for SMOX and pEGFR. As shown in Figure 2A, SMOX staining in normal gastric tissues was weak and restricted to the surface epithelium. Staining was strongly increased in surface epithelial cells and within deep glands in tissues with non-atrophic gastritis, remained increased in atrophic gastritis, and was significantly further increased in IM, where it reached maximum levels (Figure 2A and 2B). In intestinal type gastric cancer specimens, SMOX staining was increased compared to normal tissues and there were some tissues with high expression, but overall, staining was less than in IM, and SMOX was further decreased in cancer of the diffuse type (Figure 2A and B). We obtained similar results when SMOX levels were assessed by immunofluorescence (Supplementary Figure 1). Additionally, marked nuclear localization of SMOX was detected on high power images when examined by immunohistochemical or immunofluorescent staining (Supplementary Figure 2). pEGFR staining was significantly increased in gastritis and atrophic gastritis tissues, but was relatively attenuated in IM and gastric cancer (Figure 2A and C). pEGFR and SMOX levels were correlated in gastritis and atrophic gastritis tissues (Figure 2D), but not in more advanced lesions (not shown). Levels of SMOX or pEGFR in gastritis tissue cores did not correlate with acute or chronic inflammation, or with severity of atrophy (Supplementary Table 1). In a separate TMA, staining levels also peaked for SMOX in IM, and for pEGFR in gastritis and atrophic gastritis, respectively (Supplementary Figure 3). These results indicate that the interaction of pEGFR and SMOX expression is associated with initiation of the progression to preneoplastic lesions.1,21
Figure 2.
Levels of SMOX and pEGFR in human TMA. (A) Immunohistochemistry for SMOX and pEGFR at 100X magnification. (B, and C) Scoring of SMOX and pEGFR staining intensity in epithelial cells from immunohistochemistry analysis. (D) Correlation (Pearson coefficient) between SMOX and pEGFR levels. In B and C, each circle represents a different TMA core; *P < .05, ***P < .001 vs normal tissue; §§P < .01, §§§P < 0.001 vs IM/Dys tissues. Triangles with black borders represent cores with dysplasia. For B and C, the number of patient cores for each diagnosis is indicated. NL, normal; Gastritis, non-atrophic gastritis; AG, atrophic gastritis; IM/Dys, intestinal metaplasia/dysplasia; GCI, gastric carcinoma intestinal type; GCD, gastric carcinoma diffuse type.
Pharmacological Inhibitors of EGFR Phosphorylation Decrease Levels of H pylori-induced Spermine Oxidase Expression and DNA Damage
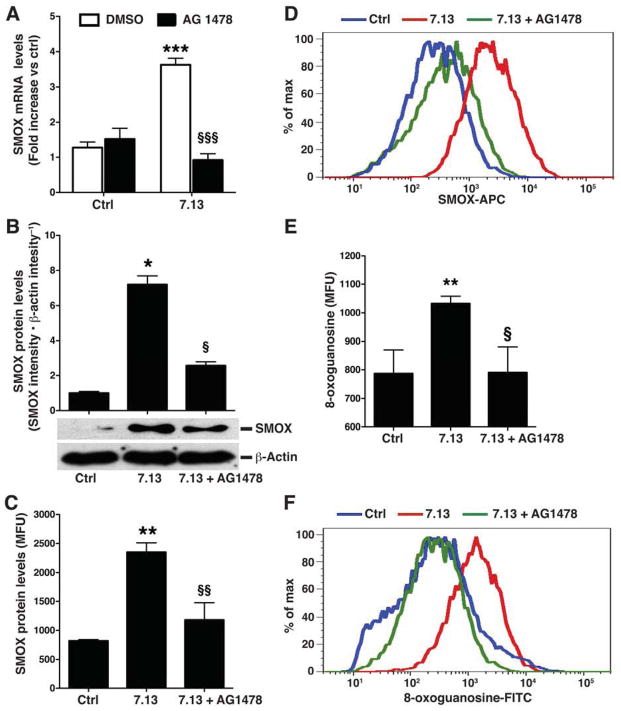
To more definitively establish the role of EGFR activation in SMOX induction, we pretreated conditionally immortalized ImSt cells with EGFR tyrosine kinase inhibitors AG1478 (Figure 3A) or PD53035 (Supplementary Figure 4), and found that the pretreatment attenuated the levels of SMOX mRNA expression stimulated by H pylori strain 7.13. AG1478 decreased levels of H pylori-induced SMOX protein expression by 78% and 76% when assessed by Western blotting, and flow cytometry, respectively (Figure 3B–D). There was a concomitant decrease in H pylori-induced oxidative DNA damage in AG1478-treated cells (Figure 3E and F). AG1478 similarly reduced SMOX expression and DNA damage in ImSt cells infected with H pylori strain PMSS1 (Supplementary Figure 5). Treatment of ImSt cells with the prototype EGFR ligand, EGF, which readily induces phosphorylation of EGFR (Supplementary Figure 6), failed to induce SMOX or DNA damage (Supplementary Figure 7), indicating that phosphorylation of EGFR alone is not sufficient to induce SMOX. Thus, H pylori-mediated induction of SMOX in gastric epithelial cells requires phosphorylation of EGFR, and additional H pylori-specific constituents.12
Figure 3.
Effect of inhibition of EGFR on levels of SMOX and DNA damage in gastric epithelial cells. Imst cells were pretreated with the EGFR inhibitor AG1478 (150 nM) for 45 min and co-cultured with H pylori 7.13. (A) After 6 h, levels of SMOX mRNA were measured by real-time PCR. (B) After 24 h, levels of SMOX protein were measured by Western blotting and quantified by densitometry. (C) Levels of SMOX protein after 24 h by flow cytometry. (D) Representative histogram for SMOX. (E) DNA damage measured after 24 h by flow cytometry for 8-oxoguanosine. (F) Representative histogram for 8-oxoguanosine. For A, ***P < .001 vs DMSO control, §§§P <.001 vs DMSO + 7.13; for B, C, and E, ***P < .001 vs DMSO, §§§P <.001 vs DMSO + 7.13. Data are from 3 experiments in duplicate.
Levels of SMOX Expression, DNA Damage, and Cells With DNA Damage and Resistance to Apoptosis Are Decreased in H pylori-infected Gastric Epithelial Cells That Lack EGFR
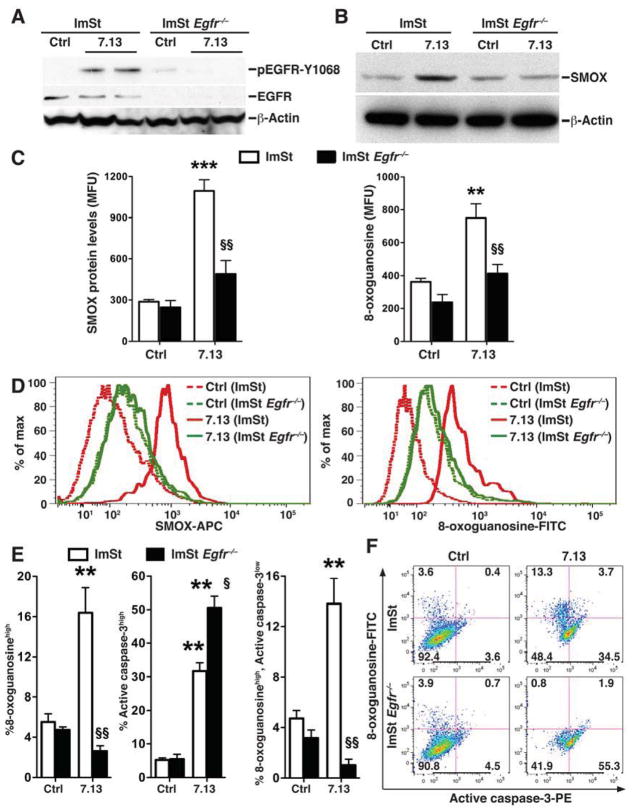
Survival of cells with damaged DNA is an important step in carcinogenesis. We have reported that H pylori infection in vitro and in vivo results in a subpopulation of 8-oxoguanosinehigh, active caspase-3low (DNA damagehigh, apoptosislow) cells. We used ImSt cells from Egfr−/− mice crossed to the immortomouse17 to determine the role of EGFR in the generation of 8-oxoguanosinehigh, active caspase-3low cells during H pylori infection. As expected, in Egfr−/− ImSt cells, treatment with EGF no longer induced phosphorylation of EGFR, and phosphorylation of the downstream MAP kinase, ERK1/2, was not detected, despite intact total ERK protein levels (Supplementary Figure 6). When activated with H pylori, the increase in pEGFR levels in WT cells was eliminated in Egfr−/− cells (Figure 4A). In contrast to WT cells, SMOX levels were reduced to basal levels in infected Egfr−/− cells (Figure 4B). When assessed by flow cytometry, there was a significant attenuation in the levels of increased SMOX and 8-oxoguanosine in infected Egfr−/− cells compared to the marked increases that were observed in WT cells (Figure 4C and D). Similarly, with H pylori infection there was an increase in the percentage of WT ImSt cells exhibiting oxidative DNA damage that was completely eliminated in Egfr−/− cells (Figure 4E and F). In contrast, the increase percentage of apoptosis in WT cells was further increased in Egfr−/− cells (Figure 4E and F). Importantly, generation of the 8-oxoguanosinehigh, active caspase-3low cell subpopulation in WT cells exposed to H pylori was significantly reduced in Egfr−/− cells (Figure 4E and F). Consistent with these data, addition of the EGFR inhibitor AG1478 also prevented the generation of the oxoguanosinehigh, active caspase-3low subpopulation in ImSt cells infected with H pylori (data not shown). Collectively, these data indicate that pEGFR is required for the increased levels of SMOX and DNA damage in H pylori-infected gastric epithelial cells, and also protects cells with DNA damage from undergoing apoptosis.
Figure 4.
Levels of SMOX protein, DNA damage, and apoptosis in ImSt Egfr−/− cells. WT and Egfr−/− Imst cells were co-cultured with H pylori 7.13 for 24 hrs. (A and B) Western blotting for pEGFR, EGFR, SMOX, and β-actin. (C and D) Levels of SMOX and 8-oxoguanosine, and representative histograms, respectively, by flow cytometry. (E) Percentage of cells that are 8-oxoguanosinehigh (left), active caspase-3high (middle), and 8-oxoguanosinehigh, active caspase-3low (right). (F) Representative dot plots. In C and E, **P < .01, ***P < .001 vs uninfected WT control; §P < 0.05, §§P < .01 vs 7.13-infected WT. n=3 experiments in duplicate.
Quantitative Phosphoproteomics Identifies Activation of EGFR and ERBB2 Signaling Pathways in H pylori-infected Gastric Epithelial Cells
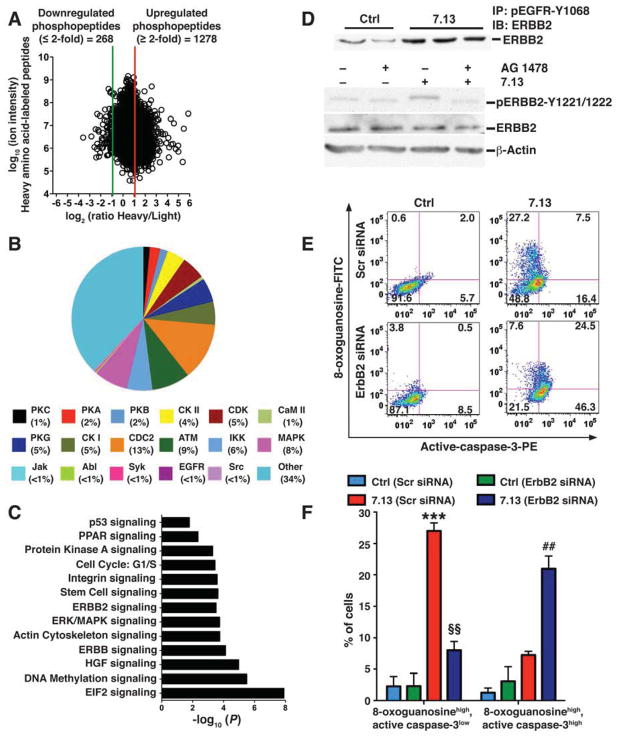
To identify the signaling pathways that are activated via phosphorylation during H pylori infection in gastric epithelial cells we next took an unbiased, quantitative SILAC-based phosphoproteomics approach in which uninfected and H pylori-infected cells were labeled with light and heavy amino acids, respectively. We detected 5218 unique phosphopeptides in ImSt cells. H pylori activation increased the levels of 1278 unique phosphopeptides by ≥2–fold (Figure 5A), which corresponded to 779 unique phosphoproteins. Analysis of upregulated phosphopeptides for kinase substrate distribution by KinasePhos indicated the presence of substrates for 18 kinases, including EGFR and its downstream kinases ERK and AKT (Figure 5B). Examination of upregulated phosphoproteins by Ingenuity Pathway Analysis demonstrated significant enrichment of several signaling pathways, including EGFR and ERBB2, but not ERBB3 or ERBB4 (Figure 5C). Phosphoproteins enriched within ERBB2 signaling pathways showed a significant increase in their respective phosphopeptides with H pylori activation (Supplementary Figure 8A). Representative tandem mass spectra of phosphopeptides enriched in ERBB2 signaling are shown as Supplementary Figure 9. When we analyzed samples from ImSt cells in which amino acid labels were swapped in a second experiment, we observed a mirror image of Figure 5A for phosphopeptides (Supplementary Figure 8B). Additionally, enrichment of pathways (Supplementary Figure 8C) were similar to the findings in Figure 5C. Taken together, the pathway and kinase substrate distribution analyses indicate that H pylori infection can activate numerous signaling pathways as early events in gastric epithelial cells, including EGFR and ERBB2, which are known to regulate diverse functions relevant to our studies, including apoptosis.8
Figure 5.
Effect of H pylori infection on ImSt cell phosphoproteome assessed by SILAC, and role of ERBB2 in apoptosis. ImSt cells were cultured in light or heavy medium; the cells grown in heavy medium were co-cultured with H pylori 7.13 for 30 min. After lysis and enrichment, phosphopeptides were analyzed by LC-MS/MS. (A) Dot plot of the relative abundance of heavy vs light amino acid-labeled phosphopeptides (A). Red lines: 2–fold increase; green line 2–fold decrease. (B) Distribution of phosphopeptide substrates for catalytic kinases is shown for upregulated phosphoproteins. (C) Graphical representation of significantly enriched biological pathways with H pylori infection in ImSt cells (P; significance level). (D) Upper panel: ImSt cells cultured in normal medium with H pylori for 30 min, pEGFR was immunoprecipitated with anti-pEGFR (Y1068) antibody, and immunoprecipitate was probed with anti-ERBB2 antibody. Lower panel: Imst cells were pretreated with the EGFR inhibitor AG1478 for 45 min and co-cultured with H pylori for 30 min and cell lysates were analyzed for pERBB2 (Y1221/1222) and ERBB2 by Western blotting. (E and F) ImSt cells were transfected with either Scr siRNA or ErbB2 siRNA and co-cultured with 7.13 for 24 hrs. (E and F) Representative dot plots for simultaneous analysis of 8-oxoguanosine and active-caspase-3, and summary data, respectively. ***P < .001 vs control Scr siRNA; §§P < .01 vs 7.13-activated ErbB2 siRNA; ##P < .01 vs 7.13-activated ErbB2 siRNA. Data are from 3 experiments in duplicate.
H pylori Infection Causes pEGFR–ERBB2 Dimerization, and Phosphorylation of ERBB2 That Depends on Phosphorylation of EGFR and Leads to Apoptosis Resistance
Our phosphoproteomics data indicate that H pylori infection can activate EGFR and ERBB2 signaling in gastric epithelial cells. ERBB2 can form a heterodimer with pEGFR, which then leads to phosphorylation of ERBB2 at tyrosine(Y1221/1222) and thus activation of the ERBB2 signaling pathway.22,23 Immunoprecipitation with anti-pEGFR(Y1068) and Western blotting with anti-ERBB2 antibody indicated an increase in the pEGFR–ERBB2 heterodimer in H pylori-infected ImSt cells (Figure 5D). In contrast, there was no dimerization of pEGFR with ERBB3 or ERBB4 (data not shown). Western blot analysis demonstrated that H pylori infection increased pERBB2(Y1221/1222) in ImSt cells; this was attenuated by AG1478 (Figure 5D), indicating that H pylori infection leads to dimerization of pEGFR and ERBB2 and that phosphorylation of ERBB2 within infected cells depends on pEGFR.
To assess the biological significance of pERBB2, we performed knockdown of ErbB2 using siRNA in ImSt cells (Supplementary Figure 9A). Knockdown of ErbB2 did not change the levels of H pylori-induced SMOX expression or overall DNA damage (Supplementary Figure 9B), but instead significantly decreased the 8-oxoguanosinehigh active caspase-3low subpopulation (Figure 5E and F), with a concurrent increase in the 8-oxoguanosinehigh active caspase-3high subpopulation (Figure 5E and F). Together, these data indicate that ERBB2 activation protects 8-oxoguanosinehigh cells from apoptosis.
Levels of pEGFR–ERBB2 Heterodimer and pERBB2 Are Increased in Human Biopsies with Gastric Inflammation
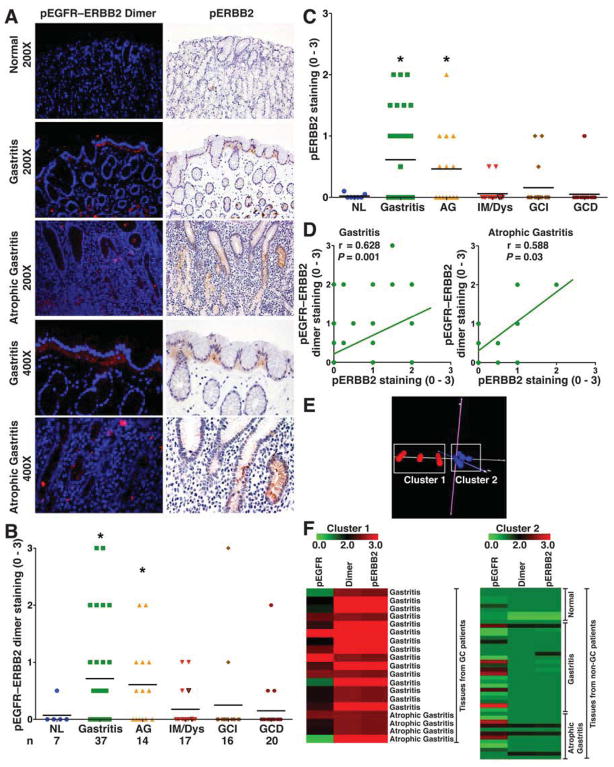
To assess levels of the pEGFR–ERBB2 heterodimer and pERBB2 in clinical samples, we utilized the TMA described in Figure 2. We detected increased levels of both the pEGFR–ERBB2 heterodimer (Figure 6A and B) and pERBB2 (Figure 6A and C) in tissues with either gastritis or atrophic gastritis when compared to normal controls. As shown in the higher power fields in Figure 6A, there was increased basolateral pEGFR–ERBB2 and pERBB2 staining in surface epithelial cells and in deep gastric glands. Staining for pEGFR–ERBB2 and pERBB2 was less prominent in IM and GC of the intestinal or diffuse type (Figure 6B and C). Levels of pEGFR–ERBB2 and pERBB2 staining was significantly correlated in gastritis and atrophic gastritis (Figure 6D), consistent with pEGFR–ERBB2 dimerization leading to activation of ERBB2 in the early stages of H pylori-associated disease. In the second TMA, we confirmed that pERBB2 was less prominent in IM and GC when compared to gastritis or atrophic gastritis (Supplementary Figure 3).
Figure 6.
pEGFR–ERBB2 dimer and pERBB2 in human tissue microarray. (A) Immunofluorescence-based PLA for pEGFR–ERBB2 dimer and immunohistochemistry for pERBB2 (Y1221/1222). (B and C) Scoring of pEGFR–ERBB2 dimer and pERBB2 (Y1221/1222) staining intensity in epithelial cells from PLA and immunohistochemistry analysis, respectively. (D) Correlation (Pearson coefficient) between levels of pEGFR–ERBB2 dimer and pERBB2 (Y1221/1222). (E) Principal component analysis of TMA cores for the levels of pEGFR, pEGFR–ERBB2 dimer, and pERBB2 in gastritis and atrophic gastritis tissues from 32 non-cancer and 19 cancer subjects. (F) Heat maps for levels of pEGFR, pEGFR–ERBB2 dimer, and pERBB2 in Cluster 1 and Cluster 2. In B and C, *P < .05 vs normal tissues.
Levels of pEGFR, pERBB2 and pEGFR–ERBB2 Distinguish Cancer vs Non-cancer Patients
A subgroup analysis of gastritis and atrophic gastritis tissues from cancer and non-cancer patients failed to identify significant differences between these two groups in staining intensity of pEGFR, pERBB2 or pEGFR–ERBB2 as individual parameters. However, principal component analysis of these variables combined, generated 2 distinct clusters (Figure 6E). Presented as heat map (Figure 6F), tissues in Cluster 1 exhibited higher levels of pEGFR, pERBB2, and pEGFR–ERBB2 staining intensity; all of these gastritis and atrophic gastric tissues were from stomachs of cancer patients. In contrast, the tissues in Cluster 2 exhibited low levels of staining intensity and were all from non-cancer patients (Figure 6F).
Validation of TMA Findings in Biopsies From H pylori-infected Subjects
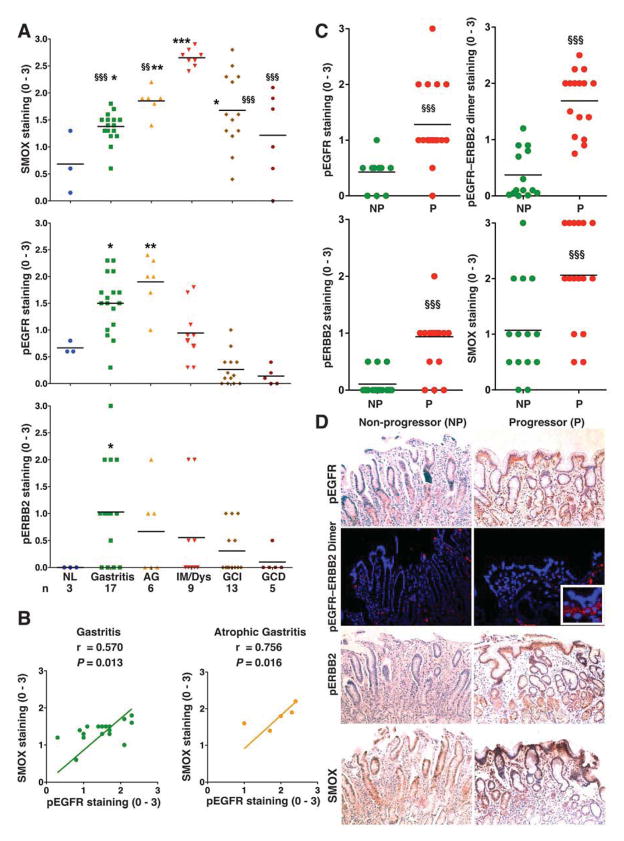
To provide translational significance, we used endoscopic antral biopsies from Honduras, and selected gastritis and preneoplastic lesions (atrophy and complete IM) where H pylori infection was confirmed by Steiner staining, and/or serology in cancer cases. Similar to our TMA datasets, we detected a stepwise increase in SMOX staining from gastritis to IM, and increases in pEGFR in gastritis and atrophic gastritis, and pERBB2 in gastritis; levels of SMOX were again increased in intestinal type GC, while pEGFR and pERBB2 were not (Figure 7A, Supplementary Figure 11). Also, there was a positive correlation between pEGFR and SMOX levels in gastritis and atrophic gastritis (Figure 7B), as in the TMA data. Levels of SMOX or pEGFR in gastritis biopsies did not correlate with acute or chronic inflammation, or with severity of infection (Supplementary Table 2).
Figure 7.
Levels of molecular signature in Honduras cohort and Colombian progressors vs non-progressors. (A) Scoring of SMOX, pEGFR, and pERBB2 staining in epithelial cells of Honduran biopsies. (B) Correlation (Pearson coefficient) between SMOX and pEGFR levels in patients with gastritis and atrophic gastritis. (C) Scoring and (D) representative immunohistochemistry at 100X for SMOX, pEGFR, and pERBB2, and PLA for pEGFR–ERBB2 heterodimer at 200X, with 400X insert, in progressors (n=14) vs non-progressors (n=16). (D) In A, *P < .05, **P < .01 ***P < .001 vs normal; §§P < .01, §§§P < 0.001 vs IM/Dys. In C, §§§P < 0.001 vs non-progressors.
Levels of pEGFR, pERBB2, pEGFR–ERBB2, and SMOX Predict Progression of Disease From Atrophic Gastritis to IM or Dysplasia
Since we observed that gastritis or atrophic gastritis tissues from cancer patients exhibited higher levels of pEGFR, pEGFR–ERBB2, and pERBB2 compared to these same tissues from non-cancer patients, we tested whether this pathway may represent a molecular signature for progression of disease. We analyzed gastric biopsies of patients with multifocal atrophic gastritis from a longitudinal cohort from Colombia, that were all H pylori positive at baseline, and progressed or not to IM/dysplasia after 13 more years of follow-up. Levels of pEGFR, pEGFR–ERBB2 heterodimer, and pERBB2 staining intensity at baseline were higher in patients that progressed to IM or dysplasia compared to non-progressors (Figure 7C and D). Consistent with our findings that EGFR signaling contributes to SMOX expression, there was also an increase in SMOX staining in progressors vs non-progressors. Progressors and non-progressors had similar H pylori density, and there was no difference in staining results in progressors based on H pylori status after 13 years of follow-up (Supplementary Figure 12).
Discussion
This study reveals a new function of activated EGFR during H pylori infection. Although pEGFR is linked to repair of irradiation-induced DNA damage,24 we have demonstrated that pEGFR is an upstream event that leads to H pylori-induced DNA damage in gastric epithelial cells. Our data indicate that activation of EGFR is required for H pylori-induced increases in SMOX that causes DNA damage in gastric epithelial cells. Either pharmacologic or genetic inhibition of EGFR resulted in attenuated SMOX expression and DNA damage with H pylori infection. Overexpression of constitutively-activate EGFR in glioblastoma cells increases reactive oxygen species and DNA damage,25 but herein EGF did not increase SMOX or DNA damage. Thus, pEGFR is not sufficient, but is required to increase SMOX expression and DNA damage in H pylori-infected gastric epithelial cells. Inhibition of EGFR abrogated H pylori-stimulated SMOX mRNA expression, and we reported that H pylori upregulates SMOX promoter activity, suggesting EGFR signaling may act to enhance transcription.13 SILAC data show increased phosphorylation of AP1 complex components, and the minimal promoter of SMOX reveals an AP1 binding site (data not shown).
SMOX mRNA and protein expression is increased in endoscopic biopsies from patients with gastritis.12 We now extended these studies by using two independent TMAs from gastric resections, and a case series of gastric biopsies that spanned the spectrum of disease from gastritis to carcinoma. Strikingly, levels of SMOX were highest in cases with IM. This suggests a potential causal role for SMOX in the development of metaplasia; the finding that intestinal type cells express higher levels of SMOX may reflect that these cells are substantially different from gastric gland cells. Nuclear SMOX levels were also increased in tissues with gastritis, atrophic gastritis, IM, or GC indicating more proximity of SMOX to cellular DNA, and increased likelihood of oxidative DNA damage.
The current study demonstrated that levels of SMOX decreased in gastric cancer when compared to IM tissues, although SMOX levels in cancer were higher than in normal tissues. These data are similar to that reported in a prostate TMA, in which there was maximal staining in prostatic intraepithelial neoplasia (PIN) vs inflammation and cancer.26 The decrease in SMOX in both prostate and gastric cancer relative to earlier stages in neoplastic progression may be due to loss of differentiation or trans-differentiation of epithelial cells, or persistence of cells in tumors that are low expressers of SMOX, since cells with high levels of SMOX are more likely to undergo apoptosis.
In the case of pEGFR staining, in both TMAs and in the Honduran biopsies there was a strong correlation with SMOX expression in gastritis or atrophic gastritis tissues, but not in IM or GC. Similarly, in another study in prostate tissues, EGFR was increased in benign stages, decreased in PIN, and absent in prostate cancer.27 EGFR overexpression has been associated with poor prognosis in gastric cancer, although only 40% of the gastric cancer tissues were positive for EGFR overexpression.28,29 TMA data showing that levels of pEGFR are increased in gastritis and atrophic gastritis, but not in IM, dysplasia or GC, indicate that EGFR signaling and associated SMOX induction could be involved in initiation of gastric carcinogenesis.
H pylori infection generates a subpopulation of epithelial cells with high levels of DNA damage that are resistant to apoptosis12 and pEGFR protects cells from H pylori-induced apoptosis.14 We now show that deletion of EGFR eliminated the SMOXhigh,8-oxoguanosinehigh, active caspase-3low cell population. The EGFR inhibitor AG1478 produced similar results (data not shown). Our studies indicate that the dual effect of increased pEGFR and SMOX causes DNA damage, and pEGFR interaction with ERBB2 prevents clearance of cells with DNA damage. Our SILAC experiments demonstrate that H pylori upregulates EGFR and downstream ERBB2, but not ERBB3 or ERBB4 signaling. Although ERBB2–4 are expressed in gastric tissues or cell lines, their role in H pylori-induced carcinogenesis is not known.30–32
Anti-EGFR(Y1068) antibody immunoprecipitated ERBB2, but not ERBB3 or ERBB4, indicating a specific interaction of pEGFR with ERBB2. While ERBB2 is increased in 15–35% of intestinal type gastric adenocarcinomas, pERBB2 has not been investigated.33 pERBB2 leads to activation of downstream signaling that can regulate apoptosis.34 Herein, ERBB2 knockdown increased H pylori-induced apoptosis, and eliminated DNA damagehighapoptosislow cells. Knockdown of ERBB2 reduced pAKT (data not shown), which has been implicated in survival of H pylori-infected cells. Increased pEGFR signaling may inappropriately protect cells with DNA damage from apoptosis by activation of ERBB2 and AKT, leading to cells with aberrant DNA.
Similar to the pEGFR staining, there was increased staining for pEGFR–ERBB2 in gastritis and atrophic gastritis tissues. The correlation of levels of downstream pERBB2 with EGFR/ERBB2 suggests that the heterodimer is functional. The PCA data indicate that in adjacent gastritis or atrophic gastritis tissues from patients with gastric cancer there were higher levels of pEGFR, pEGFR–ERBB2, and pERBB2, compared to tissues from patients that did not have gastric cancer, highlighting the importance of pEGFR–ERBB2 in activating ERBB2. Increased pERBB2 was confirmed on a second TMA and in the Honduran biopsies.
While it is established that H pylori causes chronic active gastritis and is the strongest risk factor for gastric carcinogenesis, our data indicate that the severity of gastritis or infection does not predict the intensity of the pEGFR/pErB2/SMO molecular signature. However, H pylori infection per se may not be the main determinant, as we have shown that patients harboring strains with functional CagA exhibit higher SMOX expression and associated DNA damage12, and that phylogeographic origin of strains is a likely determinant of histologic disease progression and DNA damage.35. Further, the clinical importance of the proposed molecular signature was validated in H pylori-positive Honduran cases along the cascade from gastritis to carcinoma, and in a longitudinal Colombian cohort. In the latter, pEGFR, pERBB2, and SMOX were upregulated in baseline biopsies from subjects whose histologic lesions later progressed to IM or dysplasia compared to those that did not.
Because antibiotic-based eradication of H pylori is not feasible worldwide, is associated with increased risk for multiple diseases, and may not be beneficial once IM is present, strategies are greatly needed to select patients for eradication or institution of endoscopic surveillance. Our studies suggest that SMOX, pEGFR, pEGFR–ERBB2, and ERBB2 represent key molecular mediators of disease progression.
Supplementary Material
Acknowledgments
Funding: Supported by National Institutes of Health grants R01DK053620 and R01AT004821 (to K.T.W.), P01CA028842 (to K.T.W and P.C.), P01CA116087 (to R.M.P., D.P.B., K.T.W.), R01CA077955 and R01DK058587 (to R.M.P.), R01CA051085 and R01CA098454 (to R.A.C.), K01AT007324 (to R.C.), the Flow Cytometry and Proteomics Cores of the Vanderbilt Digestive Disease Research Center grant (P30DK058404), the Flow Cytometry Core of the Vanderbilt Ingram Cancer Center (P30CA68485), UL1RR024975 (Vanderbilt CTSA, Pilot Project to KTW), and Merit Review Grant 1I01BX001453 from the Office of Medical Research, Department of Veterans Affairs (to K.T.W.)
Abbreviations
- EGFR
epidermal growth factor receptor
- ERBB
erythroblastic leukemia-associated viral oncogene B
- GC
gastric cancer
- IM
intestinal metaplasia
- ImSt
immortalized stomach
- MFU
mean fluorescence units
- MOI
multiplicity of infection
- PCR
polymerase chain reaction
- PMSS1
pre-mouse Sydney strain 1
- SILAC
stable isotope labeling by amino acids in cell culture
- SMOX
spermine oxidase
- Scr
scrambled
- siRNA
small interfering RNA
- TMA
tissue microarray
Footnotes
Conflicts of Interest: None
Writing Assistance: None
Author names in bold designate shared co-first authorship.
Author contributions:
Rupesh Chaturvedi: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis
Mohammad Asim: acquisition of data; technical support
M. Blanca Piazuelo: acquisition of data, analysis and interpretation of data, drafting of the manuscript
Fang Yan: material support, acquisition of data
Daniel P. Barry: technical support; critical revision of manuscript for intellectual content
Johanna Carolina Sierra: technical support
Alberto G. Delgado: technical support, acquisition of data
Shalisha Hill: technical support, acquisition of data
Robert A. Casero, Jr.: material support, and critical revision of manuscript for intellectual content
Luis E Bravo: material support, acquisition of data
Ricardo L. Dominguez: material support, acquisition of data
Pelayo Correa: material support, obtained funding
D. Brent Polk: material support, critical revision of manuscript for intellectual content, obtained funding
M. Kay Washington: material support, acquisition of data
Kristie L. Rose: acquisition of data, analysis and interpretation of data, drafting of the manuscript
Kevin L. Schey: analysis and interpretation of data, critical revision of manuscript for intellectual content
Douglas R. Morgan: material support, acquisition of data, analysis and interpretation of data
Richard M. Peek, Jr.: material support, critical revision of manuscript for intellectual content, obtained funding
Keith T. Wilson: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of manuscript for important intellectual content, statistical analysis, obtained funding, study supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wroblewski LE, Peek RM, Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 3.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37 (Suppl 8):S4–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis. 2008;198:553–560. doi: 10.1086/590158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koike T, Ohara S, Sekine H, et al. Helicobacter pylori infection inhibits reflux esophagitis by inducing atrophic gastritis. Am J Gastroenterol. 1999;94:3468–3472. doi: 10.1111/j.1572-0241.1999.01593.x. [DOI] [PubMed] [Google Scholar]
- 6.Peek RM, Jr, Fiske C, Wilson KT. Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol Rev. 2010;90:831–858. doi: 10.1152/physrev.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 8.Yarden Y, Sliwkowski MX. Untangling the ERBB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 9.Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–672. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- 10.Graus-Porta D, Beerli RR, Daly JM, et al. ERBB-2, the preferred heterodimerization partner of all ERBB receptors, is a mediator of lateral signaling. EMBO J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrett TP, McKern NM, Lou M, et al. Crystal structure of a truncated epidermal growth factor receptor extracellular domain bound to transforming growth factor alpha. Cell. 2002;110:763–773. doi: 10.1016/s0092-8674(02)00940-6. [DOI] [PubMed] [Google Scholar]
- 12.Chaturvedi R, Asim M, Romero-Gallo J, et al. Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology. 2011;141:1696–1708. e1691–1692. doi: 10.1053/j.gastro.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu H, Chaturvedi R, Cheng Y, et al. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res. 2004;64:8521–8525. doi: 10.1158/0008-5472.CAN-04-3511. [DOI] [PubMed] [Google Scholar]
- 14.Yan F, Cao H, Chaturvedi R, et al. Epidermal growth factor receptor activation protects gastric epithelial cells from Helicobacter pylori-induced apoptosis. Gastroenterology. 2009;136:1297–1307. e1291–1293. doi: 10.1053/j.gastro.2008.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farinati F, Cardin R, Degan P, et al. Oxidative DNA damage accumulation in gastric carcinogenesis. Gut. 1998;42:351–356. doi: 10.1136/gut.42.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moss SF, Calam J, Agarwal B, et al. Induction of gastric epithelial apoptosis by Helicobacter pylori. Gut. 1996;38:498–501. doi: 10.1136/gut.38.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sierra JC, Hobbs S, Chaturvedi R, et al. Induction of COX-2 expression by Helicobacter pylori is mediated by activation of epidermal growth factor receptor in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2013;305:G196–203. doi: 10.1152/ajpgi.00495.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee D, Cross SH, Strunk KE, et al. Wa5 is a novel ENU-induced antimorphic allele of the epidermal growth factor receptor. Mamm Genome. 2004;15:525–536. doi: 10.1007/s00335-004-2384-2. [DOI] [PubMed] [Google Scholar]
- 19.Mera R, Fontham ET, Bravo LE, et al. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005;54:1536–1540. doi: 10.1136/gut.2005.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan DR, Dominguez RL, Keku TO, et al. Gastric cancer and the high combination prevalence of host cytokine genotypes and Helicobacter pylori in Honduras. Clin Gastroenterol Hepatol. 2006;4:1103–1111. doi: 10.1016/j.cgh.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 21.Polk DB, Peek RM., Jr Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macdonald-Obermann JL, Piwnica-Worms D, Pike LJ. Mechanics of EGF receptor/ERBB2 kinase activation revealed by luciferase fragment complementation imaging. Proc Natl Acad Sci U S A. 2012;109:137–142. doi: 10.1073/pnas.1111316109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Macdonald-Obermann J, Westfall C, et al. Quantitation of the effect of ERBB2 on epidermal growth factor receptor binding and dimerization. J Biol Chem. 2012;287:31116–31125. doi: 10.1074/jbc.M112.373647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodemann HP, Dittmann K, Toulany M. Radiation-induced EGFR-signaling and control of DNA-damage repair. Int J Radiat Biol. 2007;83:781–791. doi: 10.1080/09553000701769970. [DOI] [PubMed] [Google Scholar]
- 25.Nitta M, Kozono D, Kennedy R, et al. Targeting EGFR induced oxidative stress by PARP1 inhibition in glioblastoma therapy. PLoS One. 2010;5:e10767. doi: 10.1371/journal.pone.0010767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodwin AC, Jadallah S, Toubaji A, et al. Increased spermine oxidase expression in human prostate cancer and prostatic intraepithelial neoplasia tissues. Prostate. 2008;68:766–772. doi: 10.1002/pros.20735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibrahim GK, Kerns BJ, MacDonald JA, et al. Differential immunoreactivity of epidermal growth factor receptor in benign, dysplastic and malignant prostatic tissues. J Urol. 1993;149:170–173. doi: 10.1016/s0022-5347(17)36032-9. [DOI] [PubMed] [Google Scholar]
- 28.Chen C, Yang JM, Hu TT, et al. Prognostic Role of Human Epidermal Growth Factor Receptor in Gastric Cancer: A Systematic Review and Meta-analysis. Arch Med Res. 2013;44:380–389. doi: 10.1016/j.arcmed.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Moon WS, Tarnawski AS, Chai J, et al. Reduced expression of epidermal growth factor receptor related protein in gastric cancer. Gut. 2005;54:201–206. doi: 10.1136/gut.2003.027078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noguchi H, Sakamoto C, Wada K, et al. Expression of heregulin alpha, ERBB2, and ERBB3 and their influences on proliferation of gastric epithelial cells. Gastroenterology. 1999;117:1119–1127. doi: 10.1016/s0016-5085(99)70397-5. [DOI] [PubMed] [Google Scholar]
- 31.Wu WK, Tse TT, Sung JJ, et al. Expression of ERBB receptors and their cognate ligands in gastric and colon cancer cell lines. Anticancer Res. 2009;29:229–234. [PubMed] [Google Scholar]
- 32.Lee J, Kim S, Kim P, et al. A novel proteomics-based clinical diagnostics technology identifies heterogeneity in activated signaling pathways in gastric cancers. PLoS One. 2013;8:e54644. doi: 10.1371/journal.pone.0054644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho EY, Park K, Do I, et al. Heterogeneity of ERBB2 in gastric carcinomas: a study of tissue microarray and matched primary and metastatic carcinomas. Mod Pathol. 2013;26:677–684. doi: 10.1038/modpathol.2012.205. [DOI] [PubMed] [Google Scholar]
- 34.Yamaoka T, Yan F, Cao H, et al. Transactivation of EGF receptor and ERBB2 protects intestinal epithelial cells from TNF-induced apoptosis. Proc Natl Acad Sci U S A. 2008;105:11772–11777. doi: 10.1073/pnas.0801463105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Sablet T, Piazuelo MB, Shaffer CL, et al. Phylogeographic origin of Helicobacter pylori is a determinant of gastric cancer risk. Gut. 2011;60:1189–1195. doi: 10.1136/gut.2010.234468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.