Abstract
Background
Examine the impact of a multi-faceted, clinical decision support (CDS)-enabled intervention on magnetic resonance imaging (MRI) use in adult primary care patients with low back pain.
Methods
After a baseline observation period, we implemented a CDS targeting lumbar-spine MRI use in primary care patients with low back pain through our computerized physician order entry (CPOE) as well as two accountability tools: 1) mandatory peer-to-peer consultation when test utility was uncertain and 2) quarterly practice pattern variation reports to providers. Our primary outcome measure was rate of lumbar-spine MRI use. Secondary measures included utilization of MRI of any body part, comparing to that of a concurrent national comparison, as well as proportion of lumbar-spine MRI performed in the study cohort that was adherent to evidence-based guideline. Chi-square, t-tests, and logistic regression were used to assess pre- and post-intervention differences.
Results
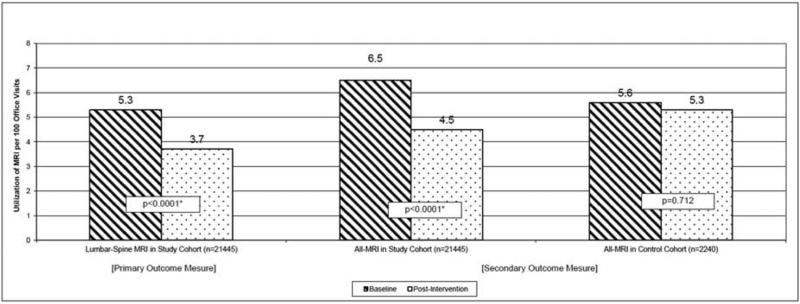
In the study cohort, pre-intervention, 5.3% of low back pain-related primary care visits resulted in lumbar-spine MRI compared to 3.7% of visits post-intervention (p<0.0001, Adjusted Odds Ratio 0.68). There was a 30.8% relative decrease (6.5% vs. 4.5%, p<0.0001, Adjusted Odds Ratio 0.67) in the use of MRI of any body part by the primary care providers in the study cohort. This difference was not detected in the control cohort (5.6% vs. 5.3%, p=0.712). In the study cohort, adherence to evidence-based guideline in the use of lumbar-spine MRI increased from 78% to 96% (p=0.0002).
Conclusions
CDS and associated accountability tools may reduce potentially inappropriate imaging in patients with low back pain.
Keywords: imaging use, health information technology, clinical decision support
Introduction
With the substantial financial investment associated with the Health Information Technology for Economic and Clinical Health (HITECH) provisions of the American Recovery and Reinvestment Act of 2009 comes great expectations that health information technology (HIT) will not only enhance patient safety and improve quality of care, but also reduce waste such as unnecessary high-cost medical imaging. Yet, the impact of HIT on healthcare delivery remains largely unclear. Kellermann noted that we have yet to fully capitalize on the $81 billion in annual cost savings that was originally projected1. In fact, McCormick et al reported that HIT may even be associated with an unintended consequence of increasing cost2.
Low back pain (LBP) is very common3 affecting approximately 70-85% of Americans over their lifetimes4 and one quarter of U.S. adults report LBP within the previous 3 months5. The estimated direct healthcare costs associated with spine problems exceeded $85 billion, representing 9% of national health expenditures6. While lumbar spine magnetic resonance imaging (LS-MRI) is the preferred diagnostic examination for most spinal diseases (e.g., cauda equina syndrome, infection, or neoplasm), its value in the investigation of simple back pain may be limited7, as imaging abnormalities and clinical symptoms are poorly correlated8 and routine imaging is not associated with better pain relief, higher functioning, or improved quality of life9–12. Based on an extensive systematic review, the joint guidelines of the American College of Physicians and the American Pain Society (ACP/APS) recommend against routine imaging in patients with nonspecific LBP (i.e., no severe or progressive neurologic deficits or evidence of serious underlying conditions)13. Qaseem et al. identified imaging in patients with nonspecific LBP to be one clinical situation that does not reflect high-value care14.
Despite evidence that routine imaging does not improve patient outcomes, clinical practice is often inconsistent with the ACP/APS guidelines and the use of LS-MRI has continued to increase, and with wide practice variation15,16. Mafi et al17 recently found that the management of back pain has relied increasingly on guideline discordant care, with more frequent use of narcotics and high-cost imaging since 1999. The purpose of this study was to examine the impact of a multi-faceted, clinical decision support (CDS)-enabled intervention based on the published ACP/APS guidelines,18 on the use of MRI in adult primary care patients with low back pain.
Materials and Methods
Study setting and cohort
Our study site consists of an integrated health system, centered around an urban academic quaternary care hospital, with an outpatient network that spans 183 practices and 1,200 physicians. The requirement to obtain informed consent was waived by the system's Institutional Review Board for this HIPAA-compliant study. The study cohort included all adult patients who presented with LBP to a primary care physician (PCP) affiliated with our institution between 2007 and 2010. To identify primary care visits for LBP-related conditions, we queried our institutional billing database to identify all primary care encounters of patients aged 18 or older with an associated primary or top 2 secondary diagnosis of LBP using International Classification of Diseases 9th Revision (ICD-9) codes (Appendix Table 1)17,19.
Control cohort
To account for secular differences in MRI utilization, we selected a control cohort consisting of primary care visits of patients with LBP captured from the publicly available National Ambulatory Medical Care Survey (NAMCS) during the same time period. The NAMCS survey was designed to be representative of outpatient care in the United States, with data collected using a standardized form completed during each patient visit. NAMCS included data on patient's demographics, medications listed, laboratory and imaging studies ordered during the visit, as well as up to 3 diagnoses derived from ICD-9 codes. NAMCS does not provide details of the specific body part imaged with MRI, hence the need to compare MRI of any body part utilization. Using surveys conducted between 2007 and 2010, we included only primary care visits in adult patients aged 18 or older. We used ICD-9 diagnosis (primary or secondary) to identify back pain-related visits based on the same codes as for the study cohort.
Intervention
After a baseline data-gathering observational period of 7 quarters, we implemented a multi-faceted intervention to promote guideline adherence in the use of LS-MRI in patients with LBP-related primary care visits in the study cohort. Our institution's computerized physician order entry (CPOE) system for imaging (Percipio, Medicalis Corp, San Francisco, CA) is integrated into our health information technology infrastructure20. Based on the clinical history input via the CPOE system, real-time CDS launches, advising the orderer regarding the best diagnostic strategy if evidence is available. The CDS content for LS-MRI is derived from the ACP/APS guidelines13, which are based on systematic review and supported by moderate quality evidence. In the absence of any clinical “red flags” (for which LS-MRI would be considered appropriate), CDS suggests the LS-MRI is not indicated (Figure 1). The clinician may cancel the request, or ignore the CDS and proceed with the order. Pre-intervention, LS-MRI orders were placed via the CPOE system but did not trigger CDS. Only PCPs received the intervention, triggered based on their primary practice affiliation; medical and surgical subspecialists and emergency physicians placed orders for LS-MRI without receiving CDS.
In addition to CDS, our intervention included two components we termed accountability tools. The first was a mandatory near real-time peer-to-peer telephonic consultation with a radiologist or internist familiar with the evidence before order completion when the orderer ignored a ‘not indicated’ CDS alert. The orderer could alternatively avoid the peer-to-peer consultation workflow by cancelling the order. As a second accountability tool, quarterly practice pattern variation reports were sent to individual PCPs, depicting their LS-MRI utilization (number of LS-MRIs ordered per number of LBP-related visits) in comparison to peers.
Data collection and sources
Patient demographics and imaging use in the study cohort were collected from electronic medical records. Any MRI ordered on the day of primary care visit from a primary care site, or an LS-MRI order from a specialist or PCP within 30 days after the date of primary care visit, was attributed to the visit. Similar data of patient demographics and MRI of any body part ordering patterns in the control cohort was collected directly from the NAMCS database. Due to the design of the NAMCS survey, the specific body part of MRI and subsequent imaging orders from specialists were not available.
To evaluate whether LS-MRI orders were guideline-adherent in the study cohort, two board-certified attending physicians reviewed the medical records. Based on power calculation with alpha of 0.05, power of 0.8, and confidence interval of 15%, charts of two hundred randomly-selected patients with visits in the pre- and post-intervention periods (100 in each group) were reviewed to determine whether each study ordered was in adherence with the ACP/APS guidelines. Records were also reviewed to verify concordance between physician note documentation and CPOE system input. For example, a case would be considered not concordant if review of the physician note showed that an order was guideline-adherent while the LS-MRI order requisition (entered into the CPOE system) illustrated otherwise.
Statistical analyses
The primary outcome measure in our study cohort was the intensity of LSMRI use, defined as the number of completed LS-MRI examinations that were ordered by PCP per LBP-related visit. As a secondary measure, we also examined the intensity of MRI of any body part use, an element that is captured by the NAMCS survey, thus allowing us to compare utilization in the study cohort to that of a concurrent control. MRI use intensity in the pre-intervention period was compared to that post-intervention. For MRI of any body part, the change in MRI use intensity between the pre- and post-intervention periods was compared to the control cohort to account for secular confounders. We also examined in the study cohort the rates of utilization of LS-MRI by both primary care and specialists, adherence rate to ACP/APS guideline for LS-MRI use, as well as the rate of follow-up LBP-related primary care visits within 30 days of the index visit. The 30-day follow-up timeframe was based on the ACP guideline recommendation of follow-up within 4 weeks13. Analyses were performed using JMP 10 (SAS Institute, Cary, NC). Chi-square and t-tests were used to assess pre- and post-intervention differences. To adjust for demographic differences between the study and control cohorts, a logistic regression was performed. A two-tailed p-value of <0.05 was defined as statistically significant.
Results
Between 2007 and 2010, there were 21,445 LBP-related primary care visits (8,437 pre-intervention and 13,008 post-intervention) by patients aged 18 or older in the study cohort. There were 2,240 (945 pre-intervention and 1,295 post-intervention) LBP-related primary care visits in the control cohort. Overall, 3.7% of primary care encounters in the pooled study and control cohorts were LBP-related (3.6% in the study cohort; 6.5% in the control). In the study cohort, the mean patient age was 53.0 years and 69.7% of patients were female. This represented a slighter older and more female-concentrated cohort than the control (50.5 years mean age, 57.3% female). Details of the patient demographic characteristics of the study and control cohorts are shown in Table 1.
Table 1.
Patient Characteristics of Study and Control Cohorts
| Characteristic | Study Cohort (n=21,445) | Control Cohort (n=2,240) | P-value |
|---|---|---|---|
| Gender | |||
| Female (n; %) | 14,950 (69.7%) | 1,283 (57.3%) | <0.0001* |
| Age (years: average ± standard dev) | 53.0±15.6 | 50.5±15.8 | <0.0001* |
| Race / Ethnicity (n; %) | <0.0001* | ||
| Caucasian | 13,563 (63.2%) | 1,259 (56.2%) | |
| Black / African American | 3,785 (17.7%) | 274 (12.2%) | |
| Hispanic | 2,080 (9.7%) | 190 (8.5%) | |
| Asian | 614 (2.9%) | 27 (1.2%) | |
| Other | 1,403 (6.5%) | 490 (21.9%) |
Denotes statistical significance
Overall, 920 (4.3%) LBP-related primary care visits were associated with a LS-MRI ordered from the primary care practice on the day of visit in the study cohort. During the study period, we observed a decreased intensity in the use of LS-MRI among patients with LBP in the study cohort. In the pre-intervention phase, 5.3% of LBP visits (443/8437) were associated with a LS-MRI order; after our CDS-enabled interventions were implemented, utilization decreased by a relative 30.2% (p<0.0001), to a rate of 3.7% of LBP-related primary care visits (n=477/13,008). The approximately 30% relative decrease in LS-MRI utilization intensity in the study cohort post-intervention persisted even after accounting for baseline demographic differences in age, gender, and race between the study and control cohorts (Adjusted Odds Ratio 0.68, p<0.0001) (Table 2).
Table 2.
Results of Logistic Regression on the Use of Magnetic Resonance Imaging Controlling for Patient Characteristics in Study Cohort
| Variable | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| Primary Outcome Measure: LS-MRI Utilization | |||
| Patient Age (by year) | 1.008 per year | 1.004-1.013 | 0.0002* |
| Patient Gender (reference = Female) | 1.23 | 1.07-1.42 | 0.004* |
| Race/Ethnicity (reference = Caucasian) | 0.150 | ||
| Asian | 0.99 | 0.65-1.45 | |
| Black/African American | 0.79 | 0.65-0.95 | |
| Hispanic | 1.05 | 0.83-1.31 | |
| Other | 0.98 | 0.74-1.28 | |
| Intervention | 0.68 | 0.59-0.77 | <0.0001* |
| Secondary Outcome Measure: MRI of any body part Utilization | |||
| Patient Age (by year) | 1.008 per year | 1.005-1.012 | <0.0001* |
| Patient Gender (reference = Female) | 1.26 | 1.11-1.42 | 0.0004* |
| Race/Ethnicity (reference = Caucasian) | 0.178 | ||
| Asian | 1.11 | 0.77-1.55 | |
| Black/African American | 0.83 | 0.69-0.98 | |
| Hispanic | 1.06 | 0.86-1.30 | |
| Other | 1.01 | 0.79-1.28 | |
| Intervention | 0.67 | 0.59-0.75 | <0.0001* |
denotes statistical significance
CI = confidence
LS = Lumbar spine
MRI = magnetic resonance imaging
In the study cohort, 1,251 (5.3%) LBP-related primary care visits were associated with and order for an MRI of any body part. 73.5% of these MRIs were for lumbar-spine (920/1251). In the pre-intervention phase, 6.5% of LBP visits (n=546/8437) were associated with an MRI of any body part order; after intervention, the utilization of MRI of any body part decreased by a relative 30.8% (p<0.0001), to a rate of 4.5% of LBP-related primary care visits (n=584/13,008). In contrast, in the control cohort of NAMCS surveyed visits, the use of MRI of any body part did not change significantly (p=0.712) over the same timeframe (Figure 2). Similar to the primary outcome measure, the approximately 30% relative decrease in MRI of any body part utilization intensity in the study cohort post-intervention persisted even after accounting for baseline demographic differences in age, gender, and race between the study and control cohorts (Adjusted Odds Ratio 0.67, p<0.0001) (Table 2).
Utilization of Magnetic Resonance Imaging in Back-Pain Related Primary Care Office Visits
Table 3 depicts results for the tertiary outcome measures in the study cohort. There was a statistically significant relative increase of 22.7% (2.2% vs. 2.7%) in the rate of LS-MRI ordered by outpatient specialists (e.g., orthopedics, neurosurgery, rheumatology, etc.) within 30 days of a patient's index primary care visit (p=0.0292), which suggests that some of the LS-MRI use may have simply shifted to ordering by specialists. However, the overall percentage of LBP-related visits that resulted in a LS-MRI within 30 days of the index visit, remained significantly different in the pre- and post- intervention periods, even after accounting for exams that were ordered by specialists (8.9% vs. 7.8%, relative 12% decrease, p=0.0023).
Table 3.
Analysis of Tertiary Outcome Measures in the Study CohortA
| Outcome Measure | Pre-Intervention | Post-Intervention | % Change | p-value |
|---|---|---|---|---|
| Lumbar Spine MRI ordered by any outpatient providers within 30 days of index primary care visit | 753 (8.9%) | 1009 (7.8%) | −12.3% | 0.0023* |
| Lumbar Spine MRI ordered by specialty clinics within 30 days | 188 (2.2%) | 352 (2.7%) | +22.7% | 0.0292* |
| Lumbar Spine MRI ordered by primary care outpatient providers within 30 days | 565 (6.7%) | 657 (5.1%) | −23.9% | <0.001* |
| Follow-up PCP visit within 30 days | 855 (10.1%) | 1224 (9.4%) | −6.9% | 0.080 |
| Guideline adherence rate in the use of Lumbar Spine MRI based on manual chart review | 78/100 (78%) | 96/100 (96%) | +23.1% | 0.0002* |
PCP = primary care physician
MRI = magnetic resonance imaging
denotes statistical significance
Due do design of NAMCS survey, tertiary outcome measure was not possible in the control cohort
In the study cohort, pre-intervention, 78% of LS-MRI orders were adherent to the evidence-based guideline, compared to 96% after intervention (p=0.0002). There was 89% (89/100) concordance between users’ input into the CPOE system and the PCP clinic notes. The majority of the non-concordance was due to incomplete documentation (n=7 of 100; 7%) of clinical information in clinic notes compared to LS-MRI order. In 4/100 instances (4%), discordance was noted with conflicting clinical information entered in clinic notes compared to LS-MRI order.
Discussion
Recent healthcare reform efforts aim to improve quality, reduce waste, and enhance value21. Clinical guidelines have been proposed as a way to increase clinical efficiency and minimize inappropriate care22,23. However, wide gaps between evidence and practice exist24,25,26, and significant implementation barriers persist27. In our study, we found that implementing a multi-faceted intervention including education using CDS and accountability tools was associated with a 32-33% decrease in LS-MRI and MRI of any body part use intensity while improving guideline-adherent practice. Given national promotion of adoption and meaningful use of HIT28, these findings support the notion that HIT-enabled interventions using CDS can help improve quality and reduce waste by promoting evidence-based practice for diagnostic imaging.
Comparing to previous studies of imaging CDS, we observed a slightly greater improvement in guideline adherence than others29,30. In a time-series study, making appropriateness guidelines available in a CPOE system in two European emergency departments decreased non-conforming radiology orders from 33.2% to 26.9% (p=0.0001)31. Blackmore et al found that the use of imaging CDS was associated with a 23% decrease in the utilization rate of lumbar MRI for low back pain in a retrospective cohort study29. Although HIT in the form of CDS likely played a critical role in our intervention, we believe our higher guideline adherence rates were due to the combined effect of CDS and complementary accountability tools. These tools highlight to providers the importance of quality and value, and the quarterly practice variation reports and peer-to-peer consultation likely reinforced this message regularly.
Although we found an adjusted 32% reduction in LS-MRI utilization on the same day as the index primary care visit post-intervention, it is important to note that part of this decrease did not necessarily translate into reduction in use of LS-MRI in the 30-day interval post the index primary care visit. Our findings show that some patients still underwent LS-MRI studies, requested either through the PCPs or specialists, within 30 days of the index visit. Some of the studies that were ordered through primary care subsequently may represent care that is guideline-adherent, performed in patients whose symptoms persisted despite conservative medical management. Yet, we also noted that the LS-MRI utilization rate actually increased, from 2.2% to 2.7% (p=0.0292), when examining those ordered by a specialist. This shift of ordering pattern to specialty providers in which the intervention was not implemented may have offset some of the MRI use reductions ordered by PCPs. Further research is needed to examine the impact of our intervention in non-primary care settings.
Our study has several limitations. First, we could not measure the specific impact of individual components of our intervention (i.e., CDS, quarterly reporting, and peer-to-peer consultation) on ordering behavior. However, we chose to implement a multi-faceted intervention strategy as previous research has found that interventions that target multiple behavioral factors are more likely to result in change32–34. Second, it is possible that our observed decline in imaging use may not be solely due to our intervention but also confounders, such as increased public awareness of harm associated with inappropriate imaging, and the publication of the ACP guidelines during the study period. However, small-to-no decline in imaging use was observed in the control cohort, which argues that guideline publication alone may not be an effective intervention for changing clinical practice35. Due to design of the NAMCS survey, body-specific imaging data (i.e., LS-MRI) was not available. The difference in data collection methodology between the study and control cohorts (health records in the study cohort vs. survey in the control cohort) represents another limitation. However, other studies over the same time period have found that MRI use in the Medicare population based on claims data36 is consistent with that revealed in NAMCS surveys. Additionally, our study was performed at a single academic medical center; thus the generalizability of our findings in other settings is unclear. Furthermore, we used billing data in cohort identification, which may not have captured all eligible patients. Only orders placed through our institution were included, potentially underestimating imaging for our patients at outside institutions. However, such occurrences are estimated to be small and are thus unlikely to influence our findings. Finally, we did not assess the impact of our intervention on patient or provider satisfaction which will be an important topic for future enquiry to help define best practices for implementing CDS-enabled interventions.
Conclusion
A multi-faceted intervention of evidence-CDS, supplemented by near real-time technology-enabled consequences for overriding CDS and quarterly practice pattern variation reporting, may be a valuable strategy to reduce potentially inappropriate imaging.
Clinical Significance.
Evidence-based clinical decision support (CDS), with embedded consequences for ignoring evidence, was associated with a statistically significant decrease in lumbar-spine MRI use in patients with low back pain.
A targeted CDS-enabled intervention was associated with an absolute increase in guideline adherence rate in the use of MRI.
Health information technology tools can help improve quality and reduce waste by promoting evidence-based practice for diagnostic imaging.
Acknowledgments
Funding Disclosure: This study was funded in part by Grant 1UC4EB012952-01 from the National Institute of Biomedical Imaging and Bioengineering.
Appendix
Appendix Table 1.
ICD9 Inclusion Codes for Cohort Identification
| ICD9 Code | Description |
|---|---|
| 307.89 | Psychogenic backache |
| 721.3 | Lumbosacral spondylosis w/o myelopathy |
| 721.5 | Kissing spine (Baastrup Disease) |
| 721.6 | Ankylosing vertebral hyperostosis |
| 721.7 | Traumatic spondylopathy |
| 721.8 | Other allide disorders of spine |
| 721.9 | Spondylosis of unspecified site w/o myelopathy |
| 722.1 | Displacement of thoracic or lumbar disc w/o myelopathy |
| 722.2 | Degeneration of intervertebral disc, site unspecified |
| 722.3 | Schmorl's bides |
| 722.5 | Degeneration of thoracic or lumbar intervertebral disc |
| 722.6 | Degeneration of intervertebral disc, site unspecified |
| 722.9 | Other and unspecified disc disorder of unspecified region |
| 724 | Other and unspecified disorders of back |
| 724.0 | Spinal stenosis, not cervical |
| 724.1 | Pain in thoracic spine |
| 724.2 | Lumbago |
| 724.3 | Sciatica |
| 724.4 | Back pain with radiation, unspec |
| 724.5 | Backache, unspecified |
| 724.6 | Disorders of sacrum (including lumbosacral junction) |
| 733.10 | Pathologic fractures, unspecified site |
| 733.13 | Pathologic fractures: vertebrae |
| 733.93 | Stress fracture of other bone |
| 738.4 | Acquired spondylolisthesis |
| 738.5 | Other acquired deformity of back or spine |
| 739.2 | Nonallopathic lesions-thoracic, not elsewhere classified |
| 739.3 | Nonallopathic lesions-lumbar, not elsewhere classified |
| 739.4 | Nonallopathic lesions-sacral, not elsewhere classified |
| 756.11 | Spondylolysis |
| 756.12 | Spondylolisthesis |
| 846.0 | Lumbosacral sprain |
| 846.1 | Sacroiliac (ligament) sprain |
| 846.2 | Sacrospinatus (ligament) sprain |
| 846.3 | Sacrotuberous (ligament) sprain |
| 846.8 | Other specified sites of sacroiliac region sprain |
| 846.9 | Unspecified site of sacroiliac region sprain |
| 847.2 | Thoracic sprain |
| 847.3 | Sacral sprain |
| 847.9 | Sprain – unspecified site of back |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure: Dr. Khorasani is a consultant to Medicalis Corporation. Dr. Khorasani is named on US Patent 6,029,138 held by Brigham and Women's Hospital on Clinical Decision Support-related software licensed to Medicalis Corporation in the year 2000. As the result of this licensing, Brigham and Women's Hospital and its parent organization, Partners Healthcare Inc., have equity and royalty interests in Medicalis. Dr. Seltzer is President of Brigham Radiology Research and Education Foundation, which has been an equity holder in Medicalis Corporation. Dr. Seltzer has been the recipient of research grants from Siemens, GE, and Toshiba, and is on the Board of Directors of Association of University Radiologists, Society of Chairs of Academic Radiology Departments, and the Academy of Radiology Research. Dr. Gershanik is a stockholder in Amgen, Eli Lilly and Company, General Electric Company, Johnson & Johnson, and Pfizer, Inc.
All authors have access to the data a role in writing the manuscript.
REFERENCES
- 1.Kellermann AL, Jones SS. What it will take to achieve the as-yet-unfulfilled promises of health information technology. Health Aff (Millwood) 2013;32(1):63–68. doi: 10.1377/hlthaff.2012.0693. doi:10.1377/hlthaff.2012.0693. [DOI] [PubMed] [Google Scholar]
- 2.McCormick D, Bor DH, Woolhandler S, Himmelstein DU. Giving Office-Based Physicians Electronic Access To Patients’ Prior Imaging And Lab Results Did Not Deter Ordering Of Tests. Health Aff. 2012;31(3):488–496. doi: 10.1377/hlthaff.2011.0876. doi:10.1377/hlthaff.2011.0876. [DOI] [PubMed] [Google Scholar]
- 3.Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169(3):251–258. doi: 10.1001/archinternmed.2008.543. doi:10.1001/archinternmed.2008.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354(9178):581–585. doi: 10.1016/S0140-6736(99)01312-4. doi:10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 5.Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine. 2006;31(23):2724–2727. doi: 10.1097/01.brs.0000244618.06877.cd. doi:10.1097/01.brs.0000244618.06877.cd. [DOI] [PubMed] [Google Scholar]
- 6.Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299(6):656–664. doi: 10.1001/jama.299.6.656. doi:10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 7.Chou R, Fu R, Carrino JA, Deyo RA. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373(9662):463–472. doi: 10.1016/S0140-6736(09)60172-0. doi:10.1016/S0140-6736(09)60172-0. [DOI] [PubMed] [Google Scholar]
- 8.Van Tulder MW, Assendelft WJ, Koes BW, Bouter LM. Spinal radiographic findings and nonspecific low back pain. A systematic review of observational studies. Spine. 1997;22(4):427–434. doi: 10.1097/00007632-199702150-00015. [DOI] [PubMed] [Google Scholar]
- 9.Modic MT, Obuchowski NA, Ross JS, et al. Acute low back pain and radiculopathy: MR imaging findings and their prognostic role and effect on outcome. Radiology. 2005;237(2):597–604. doi: 10.1148/radiol.2372041509. doi:10.1148/radiol.2372041509. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert FJ, Grant AM, Gillan MGC, et al. Low back pain: influence of early MR imaging or CT on treatment and outcome--multicenter randomized trial. Radiology. 2004;231(2):343–351. doi: 10.1148/radiol.2312030886. doi:10.1148/radiol.2312030886. [DOI] [PubMed] [Google Scholar]
- 11.Kendrick D, Fielding K, Bentley E, Kerslake R, Miller P, Pringle M. Radiography of the lumbar spine in primary care patients with low back pain: randomised controlled trial. BMJ. 2001;322(7283):400–405. doi: 10.1136/bmj.322.7283.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerry S, Hilton S, Dundas D, Rink E, Oakeshott P. Radiography for low back pain: a randomised controlled trial and observational study in primary care. Br J Gen Pract. 2002;52(479):469–474. [PMC free article] [PubMed] [Google Scholar]
- 13.Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478–491. doi: 10.7326/0003-4819-147-7-200710020-00006. [DOI] [PubMed] [Google Scholar]
- 14.Qaseem A, Alguire P, Dallas P, et al. Appropriate use of screening and diagnostic tests to foster high-value, cost-conscious care. Ann Intern Med. 2012;156(2):147–149. doi: 10.7326/0003-4819-156-2-201201170-00011. doi:10.1059/0003-4819-156-2-201201170-00011. [DOI] [PubMed] [Google Scholar]
- 15.Srinivas SV, Deyo RA, Berger ZD. Application of “less is more” to low back pain. Arch Intern Med. 2012;172(13):1016–1020. doi: 10.1001/archinternmed.2012.1838. doi:10.1001/archinternmed.2012.1838. [DOI] [PubMed] [Google Scholar]
- 16.Lurie JD, Birkmeyer NJ, Weinstein JN. Rates of advanced spinal imaging and spine surgery. Spine. 2003;28(6):616–620. doi: 10.1097/01.BRS.0000049927.37696.DC. doi:10.1097/01.BRS.0000049927.37696.DC. [DOI] [PubMed] [Google Scholar]
- 17.Mafi JN, McCarthy EP, Davis RB, Landon BE. Worsening Trends in the Management and Treatment of Back Pain. JAMA Intern Med. 2013 doi: 10.1001/jamainternmed.2013.8992. doi:10.1001/jamainternmed.2013.8992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American College of Emergency Physicians [October 24, 2012];ACEP Policy Statement on Health Information Technology. Available at: http://www.acep.org/Content.aspx?id=29534.
- 19.Jarvik JG, Comstock BA, Bresnahan BW, et al. Study protocol: the Back Pain Outcomes using Longitudinal Data (BOLD) registry. BMC Musculoskelet Disord. 2012;13:64. doi: 10.1186/1471-2474-13-64. doi:10.1186/1471-2474-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ip IK, Schneider LI, Hanson R, et al. Adoption and meaningful use of computerized physician order entry with an integrated clinical decision support system for radiology: ten-year analysis in an urban teaching hospital. J Am Coll Radiol. 2012;9(2):129–136. doi: 10.1016/j.jacr.2011.10.010. doi:10.1016/j.jacr.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Iglehart JK. The New Era of Medical Imaging — Progress and Pitfalls. New England Journal of Medicine. 2006;354(26):2822–2828. doi: 10.1056/NEJMhpr061219. doi:10.1056/NEJMhpr061219. [DOI] [PubMed] [Google Scholar]
- 22.Conroy M, Shannon W. Clinical guidelines: their implementation in general practice. Br J Gen Pract. 1995;45(396):371–375. [PMC free article] [PubMed] [Google Scholar]
- 23.Barosi G. Strategies for dissemination and implementation of guidelines. Neurol Sci. 2006;27(Suppl 3):S231–234. doi: 10.1007/s10072-006-0624-9. doi:10.1007/s10072-006-0624-9. [DOI] [PubMed] [Google Scholar]
- 24.Grol R. Improving the quality of medical care: building bridges among professional pride, payer profit, and patient satisfaction. JAMA. 2001;286(20):2578–2585. doi: 10.1001/jama.286.20.2578. [DOI] [PubMed] [Google Scholar]
- 25.Grol R. Successes and failures in the implementation of evidence-based guidelines for clinical practice. Med Care. 2001;39(8 Suppl 2):II46–54. doi: 10.1097/00005650-200108002-00003. [DOI] [PubMed] [Google Scholar]
- 26.Doumit G, Gattellari M, Grimshaw J, O'Brien MA. Local opinion leaders: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2007;(1):CD000125. doi: 10.1002/14651858.CD000125.pub3. doi:10.1002/14651858.CD000125.pub3. [DOI] [PubMed] [Google Scholar]
- 27.Clarkson JE. Getting research into clinical practice - barriers and solutions. Caries Res. 2004;38(3):321–324. doi: 10.1159/000077772. doi:10.1159/000077772. [DOI] [PubMed] [Google Scholar]
- 28.Buntin MB, Jain SH, Blumenthal D. Health information technology: laying the infrastructure for national health reform. Health Aff (Millwood) 2010;29(6):1214–1219. doi: 10.1377/hlthaff.2010.0503. doi:10.1377/hlthaff.2010.0503. [DOI] [PubMed] [Google Scholar]
- 29.Blackmore CC, Mecklenburg RS, Kaplan GS. Effectiveness of clinical decision support in controlling inappropriate imaging. J Am Coll Radiol. 2011;8(1):19–25. doi: 10.1016/j.jacr.2010.07.009. doi:10.1016/j.jacr.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Sistrom CL, Dang PA, Weilburg JB, Dreyer KJ, Rosenthal DI, Thrall JH. Effect of Computerized Order Entry with Integrated Decision Support on the Growth of Outpatient Procedure Volumes: Seven-year Time Series Analysis1. Radiology. 2009;251(1):147–155. doi: 10.1148/radiol.2511081174. doi:10.1148/radiol.2511081174. [DOI] [PubMed] [Google Scholar]
- 31.Carton M, Auvert B, Guerini H, et al. Assessment of radiological referral practice and effect of computer-based guidelines on radiological requests in two emergency departments. Clin Radiol. 2002;57(2):123–128. doi: 10.1053/crad.2001.0827. doi:10.1053/crad.2001.0827. [DOI] [PubMed] [Google Scholar]
- 32.Solomon DH. Techniques to Improve Physicians’ Use of Diagnostic Tests: A New Conceptual Framework. JAMA: The Journal of the American Medical Association. 1998;280(23):2020–2027. doi: 10.1001/jama.280.23.2020. doi:10.1001/jama.280.23.2020. [DOI] [PubMed] [Google Scholar]
- 33.Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523–530. doi: 10.1197/jamia.M1370. doi:10.1197/jamia.M1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raja AS, Gupta A, Ip IK, Mills A, Khorasani R. The Use of Decision Support to Measure Documented Adherence to a National Imaging Quality Measure. Academic Radiology. doi: 10.1016/j.acra.2013.10.017. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams CM, Maher CG, Hancock MJ, et al. Low back pain and best practice care: A survey of general practice physicians. Arch Intern Med. 2010;170(3):271–277. doi: 10.1001/archinternmed.2009.507. doi:10.1001/archinternmed.2009.507. [DOI] [PubMed] [Google Scholar]
- 36.Sharpe RE, Jr, Levin DC, Parker L, Rao VM. The Recent Reversal of the Growth Trend in MRI: A Harbinger of the Future? J Am Coll Radiol. 2013;10(8):599–602. doi: 10.1016/j.jacr.2013.01.023. doi:10.1016/j.jacr.2013.01.023. [DOI] [PubMed] [Google Scholar]