Abstract
Purpose
Obesity (as defined by body mass index) hasn’t been consistently associated with higher mortality in older adults. However, total body mass includes fat and muscle which have different metabolic effects. This study was designed to test the hypothesis that greater muscle mass in older adults will be associated with lower all-cause mortality.
Methods
All-cause mortality was analyzed by the year 2004 in 3,659 participants from the National Health and Nutrition Examination Survey III, who were 55 years (65 years if women) or older at the time of the survey (1988–94). I ndividuals who were underweight or died in the first 2 years of follow-up, were excluded so as to remove frail elders from the sample. Skeletal muscle mass was measured using bioelectrical impedance and muscle mass index was defined as muscle mass divided by height squared. Modified Poisson regression and proportional hazards regression were used to examine the relationship of muscle mass index with all-cause mortality risk and rate respectively, adjusted for central obesity (waist hip ratio)and other significant covariates.
Results
In adjusted analyses, total mortality was significantly lower in the fourth quartile of muscle mass index compared to the first: adjusted risk ratio 0.81 (95% confidence interval 0.71 – 0.91) and adjusted hazard ratio 0.80 (95% confidence interval 0.66 – 0.97).
Conclusions
This study demonstrates the survival predication ability of relative muscle mass and highlights the need to look beyond total body mass in assessing the health of older adults.
Keywords: muscle, mortality
INTRODUCTION
A high level of adiposity, as implied by a body mass index (BMI) more than 30 kg/m2, is linked to abnormal glucose metabolism(1) and incidence of cardiovascular disease(2), and is strongly associated with increased mortality in multiple prospective cohort studies(3). In older adults, however, all-cause mortality may be lower in obese than in non-obese older adults(4). This seemingly protective role for obesity in older ages is not solely due to confounding by weight loss in severely ill individuals at the end of life. Studies that have controlled for this possibility by excluding those with recent weight loss, those with end-stage diseases (e.g., cancer), and those who died in the first few years after BMI assessment, have also found that obesity is not associated with increased mortality in older adults(5, 6).
However, adiposity and muscle mass have opposite associations with glucose metabolism(7) and health risks(8) and hence possibly opposite associations with mortality risk. Since old age is a time of changing balance between fat and muscle mass, total body mass (and BMI) becomes less useful in assessing the metabolic health of older adults. In contrast, waist hip ratio, which indirectly reflects the relative abundance of central fat over peripheral fat and muscle (specifically, gluteal muscle) does have positive associations with all-cause mortality even in older individuals(9). A few studies have also found that muscle strength is related inversely to mortality risk in older adults (10, 11).
It has been noted that muscle mass index, defined as muscle mass divided by the square of height (analogous to BMI but with muscle mass instead of total body mass), is associated with better insulin sensitivity and lower risk of pre-diabetes or diabetes(12). Hence we hypothesized that muscle mass index would be associated inversely with all-cause mortality in older adults. To test this hypothesis we examined the association of muscle mass index with all-cause mortality over 10–16 years in a nationally representative cohort of older adults.
METHODS
Design and Methods
The Third National Health and Nutrition Examination Survey (NHANES III) was a national survey conducted from 1988 through 1994, using a stratified, multistage, probability cluster design(13).
We restricted our analysis to the 4321 older participants (men 55 years and older, and women, 65 years and older), who were not underweight (BMI more than18.5 kg/m2) or undernourished (waist circumference at least 50cm), and did not die in the first two years after the NHANES examination. A higher minimum age criterion was used for women because of the later onset of cardiovascular disease (14)and longer life expectancy in women (15) relative to men. Of these 4321 eligible participants, 662 were excluded for missing the primary exposure (because of contraindications such as implanted pacemakers and defibrillators and/or congestive heart failure), leaving us with a final study sample of size 3659.
Measurements: Exposures
Total skeletal muscle mass was calculated from measurements of bioelectrical impedance using the Valhalla Scientific Body Composition Analyzer 1990 B, obtained after fasting for at least six hours(in which all fluids other than water were also restricted)(13). The bioelectrical impedance -based approach relies on the relationship between body composition and body water content, and this may be disturbed in pathological states which increase whole body water, such as cardiac failure. Hence participants with diagnosed congestive heart failure were excluded. The bioelectrical impedance measurement was converted to total skeletal muscle mass (in kg) via the calibration equation of Janssen et al (16).
with height measured in cm, bioelectrical impedance measured in ohms, sex coded 1 for men and 0 for women, and age measured in years. Muscle mass index, the ratio of skeletal muscle mass to the square of body height, in kg/m2, was calculated, and sex- specific quartiles of muscle mass index were created.
Measurements: Outcomes
NHANES III participants were assessed for mortality over the years 1986 – 2004 by the National Center for Health Statistics Research Data Center using three sources of information: a) a probabilistic match to a National Death Index record; b) the Social Security Administration; c) the Centers for Medicare and Medicaid Services. Participants whose deaths resulted from accidents, suicide, homicide, firearms, and war were treated as not having died and censored at the recorded time of death.
Measurements: Covariates
Age, race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American and other) sex, smoking status (current, past, never) and doctor-diagnosed cancer (yes/no) were obtained from self-reports. Five-year age categories were created, from 55–59 years upward; the last was >= 95 years. Waist circumference, body height and body weight were measured, and central obesity was defined as waist circumference > 88 cms in women and > 102 cms in men (17).
Serum insulin and plasma glucose were measured from fasting blood samples (from participants who had fasted 6 hours or more). Plasma glucose level was measured with a hexokinase enzymatic reference method (COBAS MIRA; Roche Diagnostics, Indianapolis, IN) and serum insulin level by means of a radioimmunoassay (Pharmacia Diagnostics, Uppsala, Sweden)(13). Insulin resistance was approximated using the Homeostasis model assessment of insulin resistance (HOMA-IR) using the formula below (18):
HOMA-IR = fasting glucose (in mmol/L) × fasting insulin (in μU/ml) / 22.5 Glycosylated hemoglobin (HbA1c) was measured using the Primus Automated HPLC system (model CLC330 (Primus I), Primus Corp, Kansas City, MO)(13). Diabetes mellitus was defined by the presence of one or more of the following three conditions(19): 1) HbA1C ≥6.5%, 2) fasting glucose ≥ 7 mmol/L (126 mg/dl), or 3) self-report of diabetes (based upon the participant’s response to the question “have you ever been told by a doctor that you have diabetes or sugar diabetes?”) Pre-diabetes was defined by the presence of HbA1C≥6.0% or fasting glucose ≥ 5.5 mmol/L(100mg/dL), in the absence of diabetes.
Total serum cholesterol and high density lipoprotein (HDL) cholesterol were measured using a Hitachi 737 Analyzer (Boehringer-Mannheim Diagnostics, Indianapolis, IN)(13). Total cholesterol was categorized into three groups: less than 200 mg/dl, 200 to 240 mg/dl, and more than or equal to 240 mg/dL. HDL was marked as low if less than or equal to 40mg/dl.
Serum C-reactive protein levels (CRP) were measured using latex-enhanced nephelometry (Behring Nephelometer Analyzer System; Behring Diagnostics, Somerville, NJ)(13). CRP levels were categorized based on clinical thresholds as between <0.3mg/dl, >=0.3 but <1.0mg/dl, and ≥ 1.0mg/dl.
A set of blood pressure measurements was obtained during the 4-hour physical examination at the mobile examination center (13). Hypertension was defined as mean systolic blood pressure ≥140 mm Hg, mean diastolic blood pressure ≥90 mm Hg, or self-report of hypertension(13).
Finally, serum creatinine was measured by the modified kinetic Jaffe reaction using a Hitachi Model 737 multichannel analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN)(13).
Statistical Analyses
We examined the risk of all-cause mortality before December 2004 by sex-specific quartiles of muscle mass index, and used Poisson regression with robust estimation of standard errors to adjust for covariates. We chose to employ modified Poisson regression over ordinary logistic regression because the outcome was not rare; risk of mortality in each of the muscle mass index quartiles was more than 10%. We first adjusted only for demographics (age, sex, race/ethnicity) smoking status, cancer history and central obesity. We then added cardiovascular risk factors: inflammation (CRP); hypertension; HDL cholesterol; total cholesterol and serum creatinine, and finally glucose metabolism measures: (HOMA-IR; HbA1C; diabetes mellitus and pre-diabetes).
We also examined mortality rates (number of deaths per 10,000 person-months) by sex-specific quartiles of muscle mass index, and used Cox proportional hazards regression (with person-time censored at December 31, 2004 for those who were still alive at that date) to adjust for the same covariates.
To address the possibility that longevity associations with muscle mass primarily reflect the cardiovascular and metabolic consequences of fat mass, we also examined mortality risk and rate as a function of total non-muscle mass. In these analyses, we used sex-specific quartiles of non-muscle mass index, defined as BMI minus muscle mass index, as the primary predictor, and adjusted for all the same covariates except for central obesity (a marker of trunkal fat mass). To contrast the mortality associations of muscle mass index against those of BMI, we ran parallel analyses with sex-specific quartiles of BMI.
To make the results representative of the US population we used NHANES mobile examination center weights (with robust standard error estimation), and to account for the NHANES survey design, we modeled clustering at the NHANES geographic (primary) sampling units using generalized estimating equations in the Poisson regressions and the Wei-Lin approach in Cox proportional hazards regressions (20).
We used SAS, version 9.2 (SAS Institute Inc, Cary, NC) for all the analyses.
RESULTS
The complete NHANES sample that met eligibility criteria (age>=55 years for men, >=65 years for women; BMI>18.5 kg/m2; waist size >= 50 cm; survived at least 2 years) had 4,321 participants. The study sample, that also had valid bioelectrical impedance measurements (N=3,659), was representative of the larger, complete NHANES sample (Table 1).
Table 1.
Descriptive Statistics: Median (Inter-Quartile Range) or Percentage*
| Analytic sample (N= 3659) | Total NHANES sample (N= 4321)** | |
|---|---|---|
|
| ||
| Age (years) | 69.0 (64.0 to 75.0) | 69.0 (64.0 to 75.0) |
|
| ||
| Gender: Male | 57.2% | 56.9% |
|
| ||
| Race/Ethnicity: | ||
| Non-Hispanic White | 85.2% | 84.6% |
| Non-Hispanic lack | 07.4% | 07.7% |
| Mexican-American | 02.1% | 02.3% |
| Other | 05.3% | 05.4% |
|
| ||
| Body mass index (kg/m2) | 26.4 (23.8 to 29.7) | 26.5 (23.8 to 29.8) |
|
| ||
| Waist circumference (cm) | 97.8 (89.4 to 105.5) | 97.8 (89.4 to 105.9) |
|
| ||
| Smoking Status | ||
|
| ||
| Past | 43.2% | 43.5% |
|
| ||
| Current | 14.8% | 15.1% |
|
| ||
| Serum Creatinine (mg/dl) | 1.1 (1.0 to 1.3) | 1.1 (1.0 to 1.3) |
|
| ||
| Glycosylated hemoglobin (%) | 5.5 (5.2 to 5.9) | 5.6 (5.2 to 5.9) |
|
| ||
| HOMA-IR | 2.2 (1.6 to 3.5) | 2.3 (1.6 to 3.5) |
|
| ||
| Pre-diabetes | 36.1% | 35.8% |
|
| ||
| Diabetes | 17.5% | 18.6% |
|
| ||
| Hypertension | 61.4% | 62.9% |
|
| ||
| Serum C-reactive protein (mg/dL) | 0.21 (0.21 to 0.44) | 0.21 (0.21 to 0.44) |
|
| ||
| HDL cholesterol (mg/dL) | 47 (39 to 58) | 47 (39 to 58) |
|
| ||
| Serum cholesterol (mg/dL) | 219 (193 to 246) | 219 (193 to 246) |
|
| ||
| Cancer | 7.8% | 7.9% |
|
| ||
| Mortality by December 2004 | 48.0% | 50.3% |
Descriptive statistics computed with observations weighted by NHANES MEC weights.
Those in the NHANES III sample who were older, or equal to, than 55 years (male) or 65 years (female), not underweight or undernourished, who survived at least 2 years after the NHANES exam.
For definitions of generalized obesity, central obesity, pre-diabetes, diabetes, see text: Methods section
HDL = High density lipoprotein cholesterol
HOMA IR = Homeostatic Model Assessment of Insulin Resistance.
The sex-specific 25th, 50th, and 75th percentiles of muscle mass index, the primary predictor, were 6.2, 6.9, and 7.6 kg/m2 in women, and 9.2, 10.0, and 10.8 kg/m2 in men. For comparison, the sex-specific 25th, 50th, and 75th percentiles of BMI were 22.8, 25.9 and 29.6kg/m2 in women, and 24.4, 26.8 and 29.7 kg/m2 in men.
Median length of follow up in the study sample was 158 person-months (13.2 person-years), and there were 2012 deaths. Median age at death was 82.7 years; seven individuals were 100 years or older at the time of death. Median age at end of follow up (or censoring) for those not known to have died was 80.6 years; 10 individuals were 100 years or older at end of follow up.
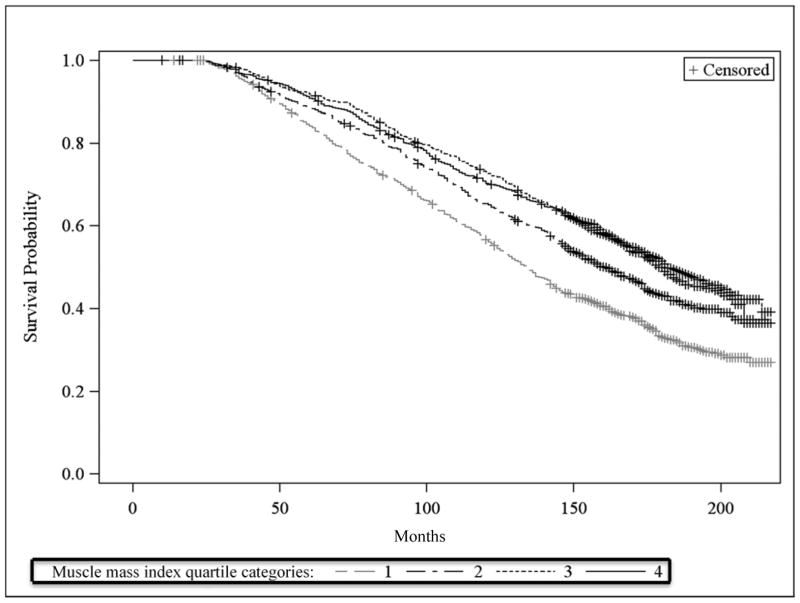
Unadjusted all-cause mortality risk was significantly higher in the lowest muscle mass index quartile compared to the highest muscle mass index quartile (58% compared to 41%; relative reduction of 30%) (Table 2a). Unadjusted mortality rates were also significantly higher in the lowest muscle mass index quartile compared to the highest muscle mass index quartile (42.5 per 10,000 person-months compared to 27.9 per 10,000 person-months; relative reduction of 34%). However, both mortality risk and mortality rate in the third quartile were not significantly different from that in the fourth quartile (Table 2a). Kaplan Meier survival curves by muscle mass index quartiles similarly shows higher survival in the top two quartiles than in the bottom two quartiles, but little difference between the top two quartiles (Figure 1).
Table 2.
Unadjusted and Adjusted all-cause mortality risk (% mortality by December 2004) and rate (number of deaths per 10,000 person-months) as a function of sex-specific quartiles of:
- Muscle mass index
- Non muscle mass index
- Body Mass Index (BMI).
| a) Muscle mass index | |||||
|---|---|---|---|---|---|
| Muscle mass index Lowest Quartile |
Muscle mass index 2nd Quartile |
Muscle mass index 3rd Quartile |
Muscle mass index Highest Quartile |
p for trend* | |
| Absolute Risk (%) | 58.0 | 51.9 | 41.3 | 40.8 | <.0001 |
| Adjusted Relative Risk (Risk Ratio)** | ref | 0.97 (0.85–1.10) | 0.81 (0.70–0.94) | 0.81 (0.71–0.91) | 0.0003 |
| Absolute Rate (per 10,000 person-months) | 42.5 | 36.4 | 27.4 | 27.9 | <.0001 |
| Adjusted Relative Rate (Hazard Ratio)** | ref | 0.94 (0.76–1.16) | 0.74 (0.60–0.90) | 0.80 (0.66–0.97) | 0.006 |
| b) Non muscle mass index | |||||
|---|---|---|---|---|---|
| Non muscle mass index Lowest Quartile |
Non muscle mass index 2nd Quartile |
Non muscle mass index 3rd Quartile |
Non muscle mass index Highest Quartile |
p for trend* | |
| Absolute Risk (%) | 49.6 | 44.9 | 48.6 | 49.1 | 0.8 |
| Adjusted Relative Risk (Risk Ratio) ∫ | ref | 0.94 (0.83–1.05) | 0.91 (0.79–1.05) | 0.96 (0.83–1.11) | 0.5 |
| Absolute Rate (per 10,000 person-months) | 35.6 | 30.7 | 33.9 | 33.5 | 0.7 |
| Adjusted Relative Rate (Hazard Ratio) ∫ | ref | 0.87 (0.71–1.10) | 0.84 (0.68–1.00) | 0.84 (0.67–1.10) | 0.1 |
| c) Body mass index(BMI) | |||||
|---|---|---|---|---|---|
| BMI Lowest Quartile |
BMI 2nd Quartile |
BMI 3rd Quartile |
BMI Highest Quartile |
p for trend* | |
| Absolute Risk (%) | 50.5 | 48.0 | 47.6 | 45.9 | 0.2 |
| Adjusted Relative Risk (Risk Ratio)§ | ref | 0.99 (0.89–1.10) | 0.91 (0.80–1.00) | 0.92 (0.80–1.10) | 0.12 |
| Absolute Rate (per 10,000 person-months) | 36.1 | 33.4 | 32.9 | 31.2 | 0.09 |
| Adjusted Relative Rate (Hazard Ratio)§ | ref | 1.0 (0.84–1.20) | 0.86 (0.71–1.30) | 0.85 (0.69–1.40) | 0.25 |
p value for linear trend across the four quartiles
Muscle mass index (skeletal muscle mass divided by height-squared); quartile cut-points: 6.2, 6.9 and 7.6 kg/m2 for women, and 9.2, 10.0, and 10.8 kg/m2 for men.
Adjusted for age (in half-decade categories), sex, race, central obesity, current smoking, past smoking, cancer (yes/no), C reactive protein categories: <0.3, 0.3–1.0, and ≥1.0mg/dl, hypertension, low HDL cholesterol (≤40 mg/dL), total cholesterol (categorized as <200, 200–240, and ≥240 mg/dL), HOMA-IR (log-transformed), HbA1C (log-transformed), diabetes, pre-diabetes and serum creatinine.
HDL = High density lipoprotein cholesterol
HOMA IR = Homeostatic Model Assessment of Insulin Resistance.
HbA1C = Blood level of glycosylated hemoglobin
p value for linear trend across the four quartiles
Non muscle mass index quartile cut-points: 16.5, 19.2 and 22.2 kg/m2 for women, and 14.7, 16.8 and 19.1 kg/m2 for men.
Adjusted for age (in half-decade categories), sex, race, current smoking, past smoking, cancer (yes/no), C reactive protein categories: <0.3, 0.3–1.0, and ≥1.0 mg/dl, hypertension, low HDL cholesterol (≤40 mg/dL), total cholesterol (categorized as <200, 200–240, and ≥240 mg/dL), HOMA-IR (log-transformed), HbA1C (log-transformed), diabetes, pre-diabetes and serum creatinine.
HDL = High density lipoprotein cholesterol
HOMA IR = Homeostatic Model Assessment of Insulin Resistance.
HbA1C = Blood level of glycosylated hemoglobin.
p value for linear trend across the four quartiles
BMI = body mass index (weight in kg divided by height-squared); quartile cut-points: 22.8, 25.9 and 29.6 kg/m2 for women, and 24.4, 26.8 and 29.7 kg/m2 for men.
Adjusted for age (in half-decade categories), sex, race, current smoking, past smoking, cancer (yes/no), C reactive protein categories: <0.3, 0.3–1.0, and ≥1.0 mg/dl, hypertension, low HDL cholesterol (≤40 mg/dL), total cholesterol (categorized as <200, 200–240, and ≥240 mg/dL), HOMA-IR (log-transformed), HbA1C (log-transformed), diabetes, pre-diabetes, and serum creatinine.
HDL = High density lipoprotein cholesterol
HOMA IR = Homeostatic Model Assessment of Insulin Resistance.
HbA1C = Blood level of glycosylated hemoglobin.
Figure 1.
Kaplan Meier curves for survival by sex-specific quartiles of muscle mass index
Muscle mass index quartile category cut-points: 6.2, 6.9 and 7.6 kg/m2 for women, and 9.2, 10.0 and 10.8 kg/m2 for men.
In the fully adjusted models, mortality in the 3rd and 4th highest quartiles of muscle mass index were significantly lower than in the bottom quartile, but not different from each other. Also, mortality in the 2nd muscle mass index quartile was not different from that in the bottom (reference) quartile (Table 2a). Compared to the lowest quartile of muscle mass index, the mortality risk ratio for the third quartile was 0.84(95% confidence interval [CI] 0.74 – 0.94) when adjusted only for demographics, smoking, cancer, and central obesity, was 0.80 (95% CI 0.70–0.92) after additional adjustment for cardiovascular risk factors and serum creatinine, and 0.81 (95% CI 0.70–0.94) in the fully adjusted model (which also included glucose metabolism measures) of Table 2a. Similarly, the mortality hazard ratio for the third quartile compared to the bottom quartile of muscle mass index was 0.78 (95% CI 0.66–0.93) adjusted for demographics, smoking, cancer, and central obesity, 0.73 (95% CI 0.60–0.87) with additional adjustment for cardiovascular risk factors and serum creatinine, and 0.74 (95% CI 0.60–0.90) in the fully adjusted model of Table 2a.
In contrast, mortality risk (likewise, mortality rate) remained essentially unchanged from the lowest to the highest sex specific quartile of non-muscle mass index : 49.6% in the highest to 49.1% in the lowest, p for mortality risk trend 0.8 (likewise, 35.6 per 10,000 person-months in the highest to 33.5 per 10,000 person-months in the lowest; p for mortality rate trend 0.7) (Table 2b). In the fully adjusted models (adjusted for all the same covariates as before except central obesity) mortality was not significantly different across quartiles of non-muscle mass index; adjusted mortality risk ratio for the highest compared to the lowest quartile was 0.96 (95% CI 0.83–1.11), p for trend across quartiles 0.5; adjusted mortality hazard ratio 0.84 (95% CI 0.67–1.10), p for trend across quartiles 0.1 (Table 2b).
There was a small and statistically non-significant decline in mortality risk (50.5% to 45.9%, p for trend 0.2) and mortality rate (36.1 per 10,000 person-months to 31.2 per 10,000 person-months, p for trend 0.09) from the lowest to the highest quartile of BMI (Table 2c). BMI associations with mortality risk and rate remained statistically non-significant in the fully adjusted models (after adjusting for all covariates except central obesity): The adjusted mortality risk ratio for the highest compared to the lowest quartile of BMI was 0.92 (95% CI 0. 80–1.10; p for trend across the quartiles 0.12), and the adjusted mortality hazard ratio for the highest compared to the lowest quartile of BMI was 0.85 (95% CI 0.69–1.40; p for trend across the quartiles 0.25) (Table 2c).
DISCUSSION
As hypothesized, in older Americans, muscle mass relative to body height was inversely associated with all-cause mortality over 10–16 year follow up. This inverse relationship was not explained by either traditional cardiovascular risk factors (dyslipidemia, hypertension, and inflammation) or glucose dysregulation (pre diabetes, diabetes, insulin resistance, and dysglycemia), suggesting that relative muscle mass is an independent prognostic marker for survival in older adults.
Traditional cardiovascular risk factors and glucose dysregulation are linked to the development of atherosclerotic vascular disease and reduced cardiac output (21, 22) which in turn lead to reduced blood flow to skeletal muscle, and thus potentially, to reduced muscle mass(23). The cardiovascular risk factors and markers of glucose dysregulation and inflammation in this study may not have completely captured the extent of subclinical cardiovascular disease, and the independent prognostic ability of relative muscle mass may be a reflection of the mortality risks conferred by subclinical cardiovascular disease in older adults.
Alternately, factors that lead to better-than-average relative muscle mass, such as genetic predisposition and a consistently active lifestyle over the life-course, are likely to also increase cardio-respiratory fitness, a major predictor of improved survival(24). The survival prediction ability of relative muscle mass may simply reflect the protective role of cardio-respiratory fitness. Finally, a potential causal pathway from muscle mass to longevity is through the role of muscle as a reliable protein reserve, which is vital in protecting an individual following a prolonged illness (25).
Even if there are no causal links between muscle mass and longevity, this study definitively demonstrates that muscle mass relative to body height has independent predictive ability for all-cause mortality in older adults. This is the first study to establish this in a large, nationally representative cohort. Previous studies have uncovered associations between muscle function (strength, power, speed) and mortality, but have failed to find associations between muscle bulk and mortality (10, 11, 26, 27). With a few exceptions, prior studies were either small or in select populations(28–31) In a younger (50–64 years at baseline), Danish cohort, Bigaard et al. noted an inverse association between skeletal muscle mass and mortality(32). Our study extends the findings of Bigaard et al. to an older, nationally-representative cohort from the United States.
It is increasingly being recognized that total body mass is an inadequate marker of prognosis in older adults(4–6) although it is still standard clinical practice to counsel patients regarding their BMI. A recent meta-analysis noted that while older adults (65 years of age or older) with BMI ≥ 35 (grade 2 or 3 obesity) indeed had increased mortality relative to normal weight adults (BMI 18.5 to 25), older adults with grade 1 obesity (30≤BMI<35) were not at increased mortality risk, and overweight older adults (25<=BMI<30) actually had lower mortality (33). This is in sharp contrast to the declines in mortality across the quartiles of relative muscle mass, documented in this study in a national cohort, pointing to the potential usefulness of muscle mass in the assessment of prognosis in older adults. It should be noted that bioelectrical impedance is easily measured in a physician’s office (using a device that looks like, and takes up as much space as, a weighing scale with two grip handles and costs a few hundred dollars) and can be quickly converted to muscle mass index for routine assessment of relative muscle mass in a busy clinical practice.
Our study had some limitations that should be acknowledged. First, as discussed above, a cause-effect relationship between muscle mass and survival cannot be established by a prospective cohort study. However, the value of muscle mass index as an independent predictor can be established, given the national representativeness and size of the cohort.
Second, we used bioelectrical impedance to estimate muscle mass. It has been suggested that age-related hydrostatic disturbances affect the validity of bioelectrical impedance measurements in older age groups(34). However, the bioelectrical impedance to muscle mass conversion equation we used has been validated against a magnetic resonance imaging based gold standard, in a multiethnic sample of adults between 18–86 years(16). Although the bioelectrical impedance measurements in NHANES III were performed after at least a 6 hour fast (in which all fluids other than water were also restricted), large volume water consumption during the fast could have caused errors in muscle mass estimates. Finally, dual x-ray absorptiometry (DXA) based measures of fat free mass are also subject to error when there is alteration of total body water(35). Further, DXA estimates total muscle mass from 2D projected images without regard to muscle composition. Lipid infiltration of muscle, which is common with obesity, with sedentary lifestyles, and with aging (36–38), leads to overestimation of effective muscle mass by DXA. Unlike DXA, bioelectrical impedance-because it relies on conductivity- does not count intra-muscular fat (which does not conduct electricity) as muscle. bioelectrical impedance may therefore provide a more accurate measurement of functioning muscle mass, in addition to being less invasive, less expensive, easier to use, and faster than the other three main techniques commonly used to measure skeletal muscle mass (DXA, computed tomography and magnetic resonance imaging).
Third, individuals with high relative muscle mass may have low relative fat mass, and the latter may be the root cause for their improved survival. This potential confound is of concern when muscle mass is indexed to body weight (since increases in fat mass translate to reductions in the muscle mass fraction of body weight) but is less relevant for muscle mass indexed to body height, the approach adopted here. We also controlled for clinical measures of central obesity in all analyses. In addition, we examined total non-muscle mass as a predictor and found no associations of non-muscle mass with survival. However, bone is heavier than fat and thus non-muscle mass is a poor surrogate for fat mass. Thus more direct measures of fat mass, using DXA or whole body/abdominal CT will be required to confirm our findings regarding the mortality prediction ability of fat mass in older adults.
Despite these limitations, this study establishes the independent survival prediction ability of muscle mass as measured by bioelectrical impedance in older adults, using data from a large, nationally representative cohort. This is in sharp contrast to body mass index, whose association with mortality in older adults is inconsistent, at best. We conclude that measurement of muscle mass relative to body height should be added to the toolbox of clinicians caring for older adults. Future research should determine the type and duration of exercise interventions that improve muscle mass and potentially increase survival in well, older adults.
Clinical significance.
Muscle mass, independent of fat mass and cardiovascular and metabolic risk factors, is inversely associated with mortality risk in older adults
These findings suggest that anabolic processes, that promote muscle bulk, may be associated with longer survival.
Changes in body composition, rather than adiposity alone, should be considered when counseling older adults on preventative health behaviours.
Acknowledgments
Funding source: the study was partially supported by the Analysis and Cost Effectiveness Resource Core of the UCLA Older Adults Independence Center (NIH/NIA Grant, P30 AG028748).
The study was partially supported by the Analysis and Cost Effectiveness Resource Core of the UCLA Older Adults Independence Center (NIH/NIA Grant, P30 AG028748).
Footnotes
Conflict of interest statement: no conflicts of interest to disclose.
Author roles: PS manuscript conception, data analysis, data interpretation and writing final manuscript. ASK assisted in data analysis, interpretation of data and manuscript revisions.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Preethi Srikanthan, Division of Endocrinology, Department of Medicine, David Geffen School of Medicine at UCLA Los Angeles, California, USA
Arun S. Karlamangla, Division of Geriatrics, Department of Medicine, David Geffen School of Medicine at UCLA, Los Angeles, California, USA.
References
- 1.Kohrt WM, Kirwan JP, Staten MA, Bourey RE, King DS, Holloszy JO. Insulin resistance in aging is related to abdominal obesity. Diabetes. 1993;42(2):273–81. [PubMed] [Google Scholar]
- 2.Dey DK, Lissner L. Obesity in 70-year-old subjects as a risk factor for 15-year coronary heart disease incidence. Obes Res. 2003;11(7):817–27. doi: 10.1038/oby.2003.113. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. Jama. 2006;295(13):1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 4.Heiat A, Vaccarino V, Krumholz HM. An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Arch Intern Med. 2001;161(9):1194–203. doi: 10.1001/archinte.161.9.1194. [DOI] [PubMed] [Google Scholar]
- 5.Grabowski DC, Ellis JE. High body mass index does not predict mortality in older people: analysis of the Longitudinal Study of Aging. Journal of the American Geriatrics Society. 2001;49(7):968–79. doi: 10.1046/j.1532-5415.2001.49189.x. [DOI] [PubMed] [Google Scholar]
- 6.Woo J, Ho SC, Sham A. Longitudinal changes in body mass index and body composition over 3 years and relationship to health outcomes in Hong Kong Chinese age 70 and older. Journal of the American Geriatrics Society. 2001;49(6):737–46. doi: 10.1046/j.1532-5415.2001.49150.x. [DOI] [PubMed] [Google Scholar]
- 7.Snijder MB, Dekker JM, Visser M, Yudkin JS, Stehouwer CD, Bouter LM, et al. Larger thigh and hip circumferences are associated with better glucose tolerance: the Hoorn study. Obes Res. 2003;11(1):104–11. doi: 10.1038/oby.2003.18. [DOI] [PubMed] [Google Scholar]
- 8.Seidell JC, Perusse L, Despres JP, Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family Study. Am J Clin Nutr. 2001;74(3):315–21. doi: 10.1093/ajcn/74.3.315. [DOI] [PubMed] [Google Scholar]
- 9.Srikanthan P, Seeman TE, Karlamangla AS. Waist-hip-ratio as a predictor of all-cause mortality in high-functioning older adults. Ann Epidemiol. 2009;19(10):724–31. doi: 10.1016/j.annepidem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. 2002;57(10):B359–65. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- 11.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61(1):72–7. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 12.Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 96(9):2898–903. doi: 10.1210/jc.2011-0435. [DOI] [PubMed] [Google Scholar]
- 13.CDC. The Third National Health and Nutrition Examination Survey (NHANES III 1988–94) Reference Manuals and Reports [CD-ROM] Bethesda, MD: National Center for Health Statistics; 1996. [Google Scholar]
- 14.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 15.United Nations; Department of Economic and Social Affairs, editor. World Population Prospects: The 2006 Revision, Highlights. New York City: 2007. [Google Scholar]
- 16.Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol. 2000;89(2):465–71. doi: 10.1152/jappl.2000.89.2.465. [DOI] [PubMed] [Google Scholar]
- 17.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285(19):2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Carson AP, Reynolds K, Fonseca VA, Muntner P. Comparison of A1C and fasting glucose criteria to diagnose diabetes among U.S. adults. Diabetes care. 2010;33(1):95–7. doi: 10.2337/dc09-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei L, Lin D. The robust inference for the Cox proportional hazards model. J Am Statist Assoc. 1989;84:1074–8. [Google Scholar]
- 21.AlZadjali MA, Godfrey V, Khan F, Choy A, Doney AS, Wong AK, et al. Insulin resistance is highly prevalent and is associated with reduced exercise tolerance in nondiabetic patients with heart failure. Journal of the American College of Cardiology. 2009;53(9):747–53. doi: 10.1016/j.jacc.2008.08.081. [DOI] [PubMed] [Google Scholar]
- 22.Gaddam KK, Ventura HO, Lavie CJ. Metabolic syndrome and heart failure--the risk, paradox, and treatment. Current hypertension reports. 2011;13(2):142–8. doi: 10.1007/s11906-011-0179-x. [DOI] [PubMed] [Google Scholar]
- 23.Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, Tracy R, et al. Associations of subclinical cardiovascular disease with frailty. The journals of gerontology Series A, Biological sciences and medical sciences. 2001;56(3):M158–66. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 24.Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Hooker SP, et al. Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA : the journal of the American Medical Association. 2007;298(21):2507–16. doi: 10.1001/jama.298.21.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stini WA. Body composition and longevity: is there a longevous morphotype? Medical anthropology. 1991;13(3):215–29. doi: 10.1080/01459740.1991.9966049. [DOI] [PubMed] [Google Scholar]
- 26.Cesari M, Pahor M, Lauretani F, Zamboni V, Bandinelli S, Bernabei R, et al. Skeletal muscle and mortality results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2009;64(3):377–84. doi: 10.1093/gerona/gln031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K, et al. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. The journals of gerontology Series A, Biological sciences and medical sciences. 2000;55(3):M168–73. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- 28.Miller MD, Crotty M, Giles LC, Bannerman E, Whitehead C, Cobiac L, et al. Corrected arm muscle area: an independent predictor of long-term mortality in community-dwelling older adults? Journal of the American Geriatrics Society. 2002;50(7):1272–7. doi: 10.1046/j.1532-5415.2002.50316.x. [DOI] [PubMed] [Google Scholar]
- 29.Wannamethee SG, Shaper AG, Lennon L, Whincup PH. Decreased muscle mass and increased central adiposity are independently related to mortality in older men. The American journal of clinical nutrition. 2007;86(5):1339–46. doi: 10.1093/ajcn/86.5.1339. [DOI] [PubMed] [Google Scholar]
- 30.Wijnhoven HA, Snijder MB, van Bokhorst-de van der Schueren MA, Deeg DJ, Visser M. Region-specific fat mass and muscle mass and mortality in community-dwelling older men and women. Gerontology. 2012;58(1):32–40. doi: 10.1159/000324027. [DOI] [PubMed] [Google Scholar]
- 31.Toss F, Wiklund P, Nordstrom P, Nordstrom A. Body composition and mortality risk in later life. Age and ageing. 2012;41(5):677–81. doi: 10.1093/ageing/afs087. [DOI] [PubMed] [Google Scholar]
- 32.Bigaard J, Frederiksen K, Tjonneland A, Thomsen BL, Overvad K, Heitmann BL, et al. Body fat and fat-free mass and all-cause mortality. Obesity research. 2004;12(7):1042–9. doi: 10.1038/oby.2004.131. [DOI] [PubMed] [Google Scholar]
- 33.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA : the journal of the American Medical Association. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritz P, Vol S, Berrut G, Tack I, Arnaud MJ, Tichet J. Influence of gender and body composition on hydration and body water spaces. Clin Nutr. 2008;27(5):740–6. doi: 10.1016/j.clnu.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Proctor DN, O’Brien PC, Atkinson EJ, Nair KS. Comparison of techniques to estimate total body skeletal muscle mass in people of different age groups. Am J Physiol. 1999;277(3 Pt 1):E489–95. doi: 10.1152/ajpendo.1999.277.3.E489. [DOI] [PubMed] [Google Scholar]
- 36.Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49(4):467–72. doi: 10.1016/s0026-0495(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 37.Guo ZK. Intramyocellular lipid kinetics and insulin resistance. Lipids Health Dis. 2007;6:18. doi: 10.1186/1476-511X-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crane JD, Devries MC, Safdar A, Hamadeh MJ, Tarnopolsky MA. The effect of aging on human skeletal muscle mitochondrial and intramyocellular lipid ultrastructure. J Gerontol A Biol Sci Med Sci. 65(2):119–28. doi: 10.1093/gerona/glp179. [DOI] [PubMed] [Google Scholar]