Abstract
Routine pulmonary ultrasound for diagnosis of disease or injury relies on interpretation of image features, such as comet-tail artifacts, which can also be indicative of the poorly understood phenomenon of ultrasound-induced pulmonary capillary hemorrhage (PCH). Evans blue extraction and bronchoalveolar lavage (BAL) were evaluated for assessment of PCH induced by ultrasound scanning. Rats anesthetized with ketamine with or without xylazine received sham or scanning for 5 min with a 7.6 MHz linear array. Evans blue extraction and BAL albumin measurements failed to demonstrate significant increases for scanning, even though the induction of comet-tail artifacts was significant. BAL cell counts had an insignificant increase relative to shams at a near-threshold Mechanical Index (MI) of 0.52 (P=0.07), but a highly significant increase at MI=0.9 (P=0.001). The possibility of xylazine-induced elevated albumin was tested, but no significant decrease was found for sham or scanned rats with ketamine-only anesthesia. Interestingly, without xylazine, the widths of comet-tail artifacts in the ultrasound images were significantly smaller (P=0.001) and cell counts in BAL fluid also were reduced (P=0.014). The BAL cell-count method provides a valuable additional means of PCH quantification.
Keywords: Pulmonary ultrasound, Mechanical index, Comet-tail artifact, Bronchoalveolar lavage, Rat anesthesia
Introduction
Diagnostic ultrasound imaging of lung can induce pulmonary capillary hemorrhage (PCH). The induction of PCH by pulsed ultrasound was discovered more than 20 yr ago by Child et al. (1990). Research has indicated that the phenomenon occurs in mice, rats and pigs and may be characterized by a threshold for a specific situation (American Institute of Ultrasound in Medicine, 2000; Church et al. 2008). Initially, the lung was expected to receive mostly incidental exposure, such as during echocardiography, and therefore the risk of patient injury was thought to be low. However, the use of trans-thoracic diagnostic ultrasound for direct lung examination has grown rapidly in recent years (Volpicelli, 2013). Various features in the images, such as comet-tail artifacts (CTAs), are interpreted to diagnose interstitial syndrome, pulmonary edema and pulmonary effusion, among others. More research is needed to define the possible risks of PCH for patients and to provide suitable safety guidance.
In a previous study, a diagnostic ultrasound machine (HDI 5000, Philips Healthcare, Andover MA USA) with 7.6 MHz (CL15-7) linear array was used to image the right lung of anesthetized rats in a warmed water bath (Miller 2012). The image immediately displayed growing comet-tail artifacts (CTAs) for a Mechanical Index (MI) of 0.9. Upon examination of the lung, a hemorrhage region corresponded to the region of CTAs in the scan plane. PCH was observed for several groups of rats scanned at a range of MI settings, and a threshold was indicated for an MI of about 0.44. This result indicates a greater sensitivity to direct pulmonary ultrasound than was expected from earlier findings.
Previously, the PCH was observed by ultrasound imaging and by stereomicroscopy of the excised lungs (Miller 2012). A quantitative measure of PCH volume would also be a valuable parameter. Histological assessment of PCH volumes is difficult and somewhat uncertain due to distortion during fixation and processing. In this study, two alternative methods were tried. First, Evans blue IV injection before scanning, with subsequent extraction of the dye from lung tissue samples, was tested as a PCH characterization method. This method has been used to characterize various types of edema (Green et al. 1988), including pulmonary edema as a measure of assessing permeability injury (Kelher et al. 2009). Second, bronchoalveolar lavage (BAL), which is useful in research on lung contusion (Raghavendran et. al. 2005; 2008), was used to characterize the ultrasonic PCH by measurement of albumin and cell counts in BAL fluid.
Materials and Methods
All in vivo animal procedures were conducted with the approval and guidance of the University Committee on Use and Care of Animals (UCUCA). 41 female rats (CD IGS strain, Charles River, Wilmington, MA, USA) 8–10 weeks of age and weighing an average of 223 gm (st. dev. 13 g) were used for this study, with three lost from the study due to technical problems. The general methods were described previously (Miller 2012). Anesthesia with ketamine (91 mg kg−1) and xylazine (9 mg kg−1) was used for 33 rats (2 lost), and ketamine alone (100 mg kg−1) was used for 8 rats (1 lost). The purpose of the omission of the xylazine for anesthesia in some rats was to evaluate the possibility of xylazine-induced elevation of permeability. Xylazine has been reported to induce pulmonary edema, although with higher doses of 21–42 mg/kg (Amouzadeh et al. 1991, 1993). The right thorax of all rats was shaved and depilated for ultrasound transmission. The rats were mounted in a 38 °C degassed water bath for ultrasound exposures of the right lung. This exposure method provides reproducible ultrasound coupling and exposure, and maintains the body temperature of the rats. Ten minutes after scanning or sham scanning, the rats were sacrificed by exsanguination under anesthesia for evaluation of the lungs.
A Phillips HDI 5000 (Philips Healthcare, Andover MA USA) diagnostic ultrasound machine with CL15-7 linear array was used, as described previously (Miller 2012). This probe was set up in the water bath to scan the right cranial or middle lobe in B mode for 5 min with 2 cm image depth, 1 cm focal depth, and 39 frames per second. The probe was partially in contact with the skin, and the pleural surface was at a depth of about 5–6 mm. The center frequency was 7.6 MHz with a pulse repetition frequency of 10 kHz. The MI level was set by the on-screen readout to 0.52, which was just above the PCH threshold, or to the maximum 0.9. These settings were previously estimated to yield in situ peak rarefactional pressure amplitudes of about 1.2 or 1.9 MPa, respectively. The size of the region with PCH was estimated from the width of the CTAs in the lung image, and by measurement of the hemorrhage area on excised lungs. The PCH also was characterized by two new methods: Evans blue extraction and bronchoalveolar lavage (BAL). Tissue samples from the scanned region of lung after BAL also were fixed in neutral buffered formalin and processed for histology by the University of Michigan Comprehensive Cancer Center, Research Histology and Immunoperoxidase Laboratory, to detect the retention of cells in the lung after BAL.
The Evans blue evaluation was modeled after published methods (Green et al. 1988; Kelher et al. 2009). Evans blue at 20 mg/ml in saline was injected at 30 mg/kg via tail vein at anesthesia. Evans blue has a high affinity for albumin, and therefore is an indicator for capillary permeability. At sacrifice, blood was obtained for a plasma sample, and the lung circulation was cleared by 20 ml phosphate buffered saline perfusion into the right ventricle. The trachea was occluded and the lung was removed intact for photography. The length and width of the hemorrhage region was measured on the lungs. Right lung lobes (cranial or middle lobe with PCH for scans, or both cranial and middle lobe for shams) were removed and placed in 9 times the volume of the lung sample (by weight) of formamide (F-5786, Sigma-Aldrich, St. Louis MO, USA) for extraction. The lung samples were placed in formamide, minced, subjected to a brief vacuum to reduce the amount of gas remaining in the lungs, and then incubated at 60 °C overnight. After centrifugation at 3200×g for 30 min the supernatant fluid was measured using a spectrophotometer for Evans blue absorbance at 620 nm. For measuring the optical densities, the extracted samples were measured undiluted after centrifugation, while the plasma samples were diluted to 4 μl/ml with formamide.
The BAL evaluation followed the methods of Raghavendran et al. (2005, 2008). The chest was opened and the lung circulation was cleared as described above. The right lung was isolated and instilled with 5 ml of phosphate buffered saline (PBS pH 7.4, Gibco, Life Technologies Corp., Grand Island NY, USA) twice with collection of the BAL fluid each time. The BAL procedure precluded size measurement on the lungs. For the higher MI exposures, the fluid was typically pink due to the presence of erythrocytes. The second lavage sample gave reduced results, judging by the pink color of the samples, and only the first sample was analyzed. The samples were centrifuged to separate the cells, which were counted using a cell counter (Multisizer 3, Beckman Coulter, Fullerton, CA). The supernatant was assayed for albumin concentration using an enzyme-linked immunoassay kit (GenWay Biotech Inc., San Diego CA).
For the Evans blue testing, the groups with ketamine and xylazine anesthesia included 4 shams, 3 rats scanned at MI=0.52 and 4 rats scanned at MI=0.9. For the BAL testing with ketamine and xylazine anesthesia, the groups included 7 shams, 4 scanned at MI=0.52 and 6 scanned at MI=0.9. In addition, groups of 4 rats were anesthetized with ketamine alone for sham and MI=0.9 scanning, to test the influence of xylazine on the results. Statistical analyses between groups were performed by the t-test or the Mann-Whitney Rank Sum (MWRS) test using SigmaPlot for Windows V. 11.0 (Systat Software Inc., San Jose CA, USA), with statistical significance assumed at P<0.05. In addition, a two way ANOVA (Holm-Sidek test) was used to gauge the relative importance and interaction of xylazine and scanning in the BAL cell count results.
Results
The results of the Evans blue method are presented in Table 1. One animal had a negative outcome (no visible PCH) for scanning at MI=0.52. The optical densities indicated a low level of albumin in the lung by comparison to the diluted plasma samples (Table 1). The results for the scanned groups were not significantly different from sham results. The lengths of the CTAs in the images and the measured hemorrhage areas on the lungs (Table 1) were both significantly increased relative to the zero values in shams for the MI=0.9 scans, but not for MI=0.52 with only two of the three outcomes positive for PCH. Therefore, the Evans blue extraction method was not sufficiently sensitive to pick up the ultrasound-induced PCH.
Table 1.
Results for means and standard deviations for the Evans blue test method.
| Group MI | Weight gm | Sample OD | Plasma OD | Area mm2 | Length mm |
|---|---|---|---|---|---|
| sham | 0.27 ± 0.07 | 0.26 ± 0.14 | 0.17 ± 0.03 | 0.0 | 0.0 |
| 0.52 | 0.19 ± 0.03 | 0.47 ± 0.10 | 0.18 ± 0.01 | 1.4 ± 1.9 | 1.4 ± 1.5 |
| 0.9 | 0.18 ± 0.01 | 0.49 ± 0.23 | 0.17 ± 0.01 | 18 ± 7* | 10.1 ± 3.1* |
The PCH areas from lung surface measurements and lengths from the ultrasound images were found to be statistically significantly different (*) from the corresponding sham for MI=0.9. Data presented as the means with standard deviations: MI, Mechanical Index of exposure; OD, optical density.
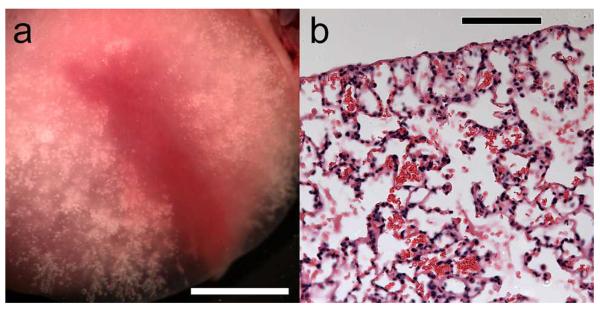
The results of the BAL cell-count method are shown in Fig. 1. For ketamine plus xylazine anesthesia, the MI=0.52 increase was insignificant relative to shams (P=0.07 MWRS-test), but the MI=0.9 increase was highly significant (P=0.001 MWRS-test). Residual erythrocytes were often noted in the lung after lavage, as shown in Fig. 2a. Although this residual feature was initially thought to be due to capillary hemorrhage into the lung interstitium, histological examination of the lavaged lobe revealed that the red cells were primarily in the alveolar space, see Fig. 2b. This suggests that the cell counts did not completely reflect the true amount of PCH, due to limited retrieval of the cells from alveolar spaces. The width of the lung surface image with comets was 5.8±4.7 mm (not significant with 3 positive and one negative result) for MI=0.52, and 10.9±1.7 mm (P<0.01, MWRS-test) for MI=0.9, relative to zero seen for shams. By visual inspection of the lavaged lungs (Fig. 2a), the PCH regions appeared to be substantial at about 13 mm long, 2 mm wide and ~2 mm deep (often equal to the thickness of the lung near the edge of the lobe) for the MI=0.9 scans.
Figure 1.
The results of the BAL method for albumin concentration (left), and cell counts (right). No albumin scan results were significantly different from the shams. The cell counts for MI=0.9 were statistically greater than the sham results with (** P=0.001 MWRS-test), or without xylazine (* P=0.028, t-test), but the MI=0.52 result was not (P=0.07 MWRS-test). Interestingly, the scan result without xylazine was significantly less than the result with xylazine (P=0.014, MWRS-test).
Figure 2.
Photomicrographs of (a) a cranial lung lobe after lavage, which has red color remaining and no residual air retained in the hemorrhage region, and (b) histological image of the residual erythrocytes remaining in the alveolar space after lavage. In (b) the alveolar septa appeared to be intact with no apparent interstitial hemorrhage. Scale bars: (a) 5 mm, (b) 100 μm.
The BAL albumin results for scanning were not significantly different from shams, as shown in Fig. 1. This insensitivity for the albumin test was thought to be due to a relatively high background concentration for the sham group, possibly due to the use of xylazine. However, the sham results for ketamine-only anesthesia were not significantly different from the ketamine plus xylazine sham result, see Fig. 1. This shows that the use of xylazine at the recommended dosages for rat anesthesia did not elevate significantly the BAL albumin level in these brief experiments. Once again, the sham and scan results for the albumin concentrations also were not significantly different for ketamine only.
However, the scan cell counts with ketamine only were statistically greater than the shams (P=0.028, t-test), see Fig. 1. Interestingly, the scan results for ketamine only were significantly less than those for ketamine plus xylazine anesthesia (P=0.014, MWRS-test). A two way ANOVA (with or without scan and with or without xylazine) revealed that both scan (P<0.001) and xylazine (P=0.004) were significant, and that there was a statistically significant interaction (P=0.005) between the scan and xylazine in the variation of results. In addition, a reduction in CTAs for ketamine only was clearly displayed in the ultrasound images. The widths of the lung surface images with comets was 10.9±1.7 mm for ketamine plus xylazine and 1.4±1.1 mm for ketamine only (P=0.001, t-test).
4. Discussion
The previous study of diagnostic ultrasound induced PCH utilized ultrasound imaging and visual inspection of the lungs to characterize the PCH (Miller 2012). This present study was undertaken to characterize ultrasound induced PCH by two new methods, which were indicative of PCH volume: Evans blue dye extraction from lung samples with measurement of optical density, and bronchoalveolar lavage (BAL) with measurement of albumin and cell counts in BAL fluid.
Neither the Evans blue nor the BAL albumin results obtained from scanned samples were significantly greater than those from sham samples. As noted above, higher dosages of xylazine have been reported to induce pulmonary edema. For example, an increase in the ratio of lung weight to body weight was reported for 21 mg/kg xylazine after 24 h (Amouzadeh et al. 1991). The possibility of confounding by a high background of albumin, due to xylazine-induced pulmonary edema, was tested by omitting the xylazine from the anesthesia. However, this hypothesis was not supported for these short duration experiments with a lower dosage (9 mg/kg). The background (sham) values may represent the basic level of permeability induced by the BAL methods, since even the use of moderate pressure in the pulmonary circulation is known to induce edema (Tsukimoto et al. 1994). That is, the step of blood clearance from the pulmonary circulation may be a source of some of the background. In the Evans blue method, for which the blood clearance is essential, the background values also may be elevated by incomplete clearance. The sensitivity of PCH detection was much better using the ultrasound measurements of CTA formation, or the simple inspection of the lungs, owing to the lack of PCH in sham lungs.
The results obtained by counting the cells in the BAL fluid were more sensitive for characterizing the PCH than the measurement of BAL albumin. The cell counts for scanned rats were significantly greater than shams for MI=0.9. The significant reduction in scan-induced PCH for MI=0.9 with ketamine-only anesthesia was an interesting result. There was a synergistic interaction between xylazine and scanning, which suggests that anesthesia and its physiological perturbation of the pulmonary microvascular are important for the induction of PCH. The lavage did not completely capture the PCH: histological examination revealed that some of the erythrocytes remained in the alveolar space after the lavage, see Fig. 2. For comparison, lung samples, which were not subjected to lavage, were presented in Figs. 3 and 4 of Miller (2012). The cell count method therefore underestimates the PCH magnitude to some extent. Nevertheless, this method provides a valuable additional means for characterization of ultrasound-induced PCH.
Acknowledgments
This study was supported by the Office of the Vice President for Research, University of Michigan, and by the National Institutes of Health via grants nos. HL102013 and HL116434.
Abbreviations
- BAL
bronchoalveolar lavage
- MI
Mechanical Index
- PCH
pulmonary capillary hemorrhage
- CTA
comet tail artifact
- MWRS
Mann-Whitney rank sum
References
- Amouzadeh HR, Sangiah S, Qualls CW, Jr, Cowell RL, Mauromoustakos A. Xylazine-induced pulmonary edema in rats. Toxicol Appl Pharmacol. 1991;108:417–427. doi: 10.1016/0041-008x(91)90088-v. [DOI] [PubMed] [Google Scholar]
- Amouzadeh HR, Qualls CW, Jr, Wyckoff JH, 3rd, Dzata GK, Sangiah S, Mauromoustakos A, Stein LE. Biochemical and morphological alterations in xylazine-induced pulmonary edema. Toxicol Pathol. 1993;21:562–571. doi: 10.1177/019262339302100607. [DOI] [PubMed] [Google Scholar]
- American Institute of Ultrasound in Medicine Section 4--bioeffects in tissues with gas bodies. J Ultrasound Med. 2000;19:97–108. 154–68. doi: 10.7863/jum.2000.19.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child SZ, Hartman CL, Schery LA, Carstensen EL. Lung damage from exposure to pulsed ultrasound. Ultrasound Med Biol. 1990;16:817–825. doi: 10.1016/0301-5629(90)90046-f. [DOI] [PubMed] [Google Scholar]
- Church CC, Carstensen EL, Nyborg WL, Carson PL, Frizzell LA, Bailey MR. The risk of exposure to diagnostic ultrasound in postnatal subjects: nonthermal mechanisms. J Ultrasound Med. 2008;27:565–592. doi: 10.7863/jum.2008.27.4.565. [DOI] [PubMed] [Google Scholar]
- Green TP, Johnson DE, Marchessault RP, Gatto CW. Transvascular flux and tissue accrual of Evans blue: effects of endotoxin and histamine. J Lab Clin Med. 1988;111:173–183. [PubMed] [Google Scholar]
- Kelher MR, Masuno T, Moore EE, Damle S, Meng X, Song Y, Liang X, Niedzinski J, Geier SS, Khan SY, Gamboni-Robertson F, Silliman CC. Plasma from stored packed red blood cells and MHC class I antibodies causes acute lung injury in a 2-event in vivo rat model. Blood. 2009;113:2079–2087. doi: 10.1182/blood-2008-09-177857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DL. Induction of pulmonary hemorrhage in rats during diagnostic ultrasound. Ultrasound Med Biol. 2012;38:1476–1482. doi: 10.1016/j.ultrasmedbio.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Raghavendran K, Davidson BA, Woytash JA, Helinski JD, Marschke CJ. Manderscheid PA, Notter RH, Knight PR. The evolution of isolated bilateral lung contusion from blunt chest trauma in rats: cellular and cytokine responses. Shock. 2005;24:132–138. doi: 10.1097/01.shk.0000169725.80068.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendran K, Davidson BA, Knight PR, Wang Z, Helinski J, Chess PR, Notter RH. Surfactant dysfunction in lung contusion with and without superimposed gastric aspiration in a rat model. Shock. 2008;30:508–517. doi: 10.1097/SHK.0b013e3181673fc5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukimoto K, Yoshimura N, Ichioka M, Tojo N, Miyazato I, Marumo F, Mathieu-Costello O, West JB. Protein, cell, and LTB4 concentrations of lung edema fluid produced by high capillary pressures in rabbit. J Appl Physiol. 1994;76:321–327. doi: 10.1152/jappl.1994.76.1.321. [DOI] [PubMed] [Google Scholar]
- Volpicelli G. Lung sonography. J Ultrasound Med. 2013;32:165–171. doi: 10.7863/jum.2013.32.1.165. [DOI] [PubMed] [Google Scholar]