Abstract
Background & Aims
Loss of parietal cells causes the development of spasmolytic polypeptide-expressing metaplasia (SPEM), through transdifferentiation of chief cells. In the presence of inflammation, SPEM can advance into a more proliferative metaplasia with increased expression of intestine-specific transcripts. We used L635 to induce acute SPEM with inflammation in mice and investigated the roles of inflammatory cells in the development of SPEM.
Methods
To study the adaptive immune system, Rag1 knockout (Rag1KO), interferon g-deficient (IFNgKO), and wild type (control) mice received L635 for 3 days. To study the innate immune system, macrophages were depleted by intraperitoneal injection of clodronate liposomes 2 days before and throughout L635 administration. Neutrophils were depleted by intraperitoneal injection of an antibody against Ly6G 2 days before and throughout L635 administration. Pathology and immunohistochemical analyses were used to determine depletion efficiency, metaplasia, and proliferation. To characterize SPEM in each model, gastric tissues were collected and levels of Cftr, Dmbt1, and Gpx2 mRNAs were measured. Markers of macrophage polarization were used to identify subpopulations of macrophages recruited to the gastric mucosa.
Results
Administration of L635 to Rag1KO, IFNgKO, and neutrophil-depleted mice led to development of proliferative SPEM and upregulation of intestine-specific transcripts in SPEM cells, similar to controls. However, macrophage-depleted mice given L635 showed significant reductions in numbers of SPEM cells, SPEM cell proliferation, and expression of intestine-specific transcripts, compared with control mice given L635. In mice given L635, as well as patients with intestinal metaplasia, M2 macrophages were the primary inflammatory component.
Conclusion
Results from studies of mouse models and human metaplastic tissues indicate that M2 macrophages promote the advancement of SPEM in the presence of inflammation.
Keywords: Immune depletion, acute injury, gastric cancer, CD68
Introduction
Gastric adenocarcinoma is the second highest cause of cancer-related death in the world.1 Due to a lack of early clinical manifestations, gastric cancer frequently presents as late stage disease. Helicobacter pylori (H. pylori) infection is the major predisposing factor for gastric cancer, causing chronic inflammation and oxyntic atrophy in the gastric mucosa.2 The parietal cell loss disrupts the homeostatic glandular environment and chief cells transdifferentiate into spasmolytic polypeptide expressing metaplasia (SPEM).3, 4 Increasing data suggest that intestinal metaplasia (IM) arises from SPEM in humans, supporting the hypothesis that SPEM is the critical initial pre-neoplastic metaplasia predisposing to gastric adenocarcinoma.5–7
Helicobacter felis (H. felis) infection in mice recapitulates the inflammatory and pre-neoplastic cascade of human H. pylori infection.3 In the murine Helicobacter infection model, SPEM develops after 6 to 12 months of infection. As in human infection with H. pylori, there appear to be two phases in the development of metaplasia. First, the infection induces parietal cell loss or oxyntic atrophy. Oxyntic atrophy is required for the induction of metaplasia in humans.8, 9 In the presence of on-going inflammation, metaplasia then evolves and expands. Given the long latency for SPEM development in H. felis-infected mice, our group developed two acute SPEM models. The administration of either drugs DMP-777 or L635 induces SPEM by selectively ablating parietal cells.10, 11 Mice treated with DMP-777 for 14 days develop SPEM without the presence of significant inflammation, likely due to the ability of DMP-777 to also inhibit elastase activity.10 In contrast, L635-treated mice develop an advanced proliferative SPEM with intestinal characteristics (previously designated as SPEM-IC) in just 3 days of treatment,3, 4 which is associated with both loss of parietal cells and a prominent inflammatory infiltrate. The phenotype of mice treated L635 for 3 days is similar to that for mice infected with H. felis for 6 months or more.4 Thus, the L635 model appears to bypass the initial phases of H. felis infection that leads to oxyntic atrophy by directly inducing parietal cell loss acutely. While mice do not develop typical goblet cell intestinal metaplasia in either the L635-treatment or Helicobacter infection models, they do develop advanced proliferative SPEM that is characterized by the expression of specific upregulated intestinal transcripts (Cftr, Dmbt1 and Gpx2) and an increase in proliferative SPEM cells.4, 12, 13 Similarly, Varon et al. observed an increase in intestinal characteristics in murine metaplasia associated with long-term Helicobacter infection.14 Studies with DMP-777 treatment demonstrate that loss of parietal cells even without inflammation leads to the development of SPEM from transdifferentiation of chief cells; however, the presence of inflammation in L635-treated mice leads to more rapid SPEM induction as well as promotion of both increased proliferation and a more intestinalized phenotype.4 Thus, inflammation is a key factor in the advancement of SPEM to a more aggressive metaplastic phenotype. Nevertheless, the precise immune cell populations responsible for the progression of metaplasia are not known.
Four distinct inflammatory cell populations are most frequently associated with Helicobacter infection in the stomach: B-cells, interferon-γ (IFNγ) secreting T-cells, neutrophils, and macrophages.15 Through the manipulation of specific immune cells, previous studies have shown that T-cells contribute to parietal cell loss and the development of metaplasia in Helicobacter infection.16 However, chronic inflammation associated with Helicobacter infection is predominately made up of neutrophils and macrophages. These phagocytic cells migrate into the mucosa to engulf debris and propagate the inflammatory response.17 Similarly, during acute induction of SPEM with L635, there is a significant influx of T-cells, B-cells, neutrophils and macrophages that migrate into the mucosa.3 Still, little is known about which immune cells promote the advancement of SPEM.
In the present studies, we have sought to assess the influence of specific immune cell populations on the advancement of SPEM following the induction of parietal cell loss. To address the specific immune components, we evaluated the presence and characteristics of L635-induced SPEM in various mouse models of depleted immune cells. Rag1 knockout mice (Rag1KO) deficient in T- and B-cells, IFNγ knockout mice (IFNγKO), neutrophil-depleted mice (Ly6G antibody-treated), and macrophage-depleted mice (clodronate-treated) were each administered L635 to induce acute parietal cell loss and SPEM. Our findings indicated that M2 macrophages are the critical immune cell driver of the induction of metaplasia following loss of parietal cells.
Methods
Treatment of Animals
L635 treatment
Each experimental group consisted of three male mice. L635 (synthesized by the Chemical Synthesis Core of the Vanderbilt Institute of Chemical Biology), dissolved in deionized DNA and RNA-free water, was administered by oral gavage (350 mg/kg) once a day for three consecutive days. Neutrophils were depleted through intraperitoneal injection of anti-Ly6G antibody (Leaf, BioLegend, San Diego, CA) (100 µg) two days prior to and throughout the three day L635 administration. Control mice received intraperitoneal injections of a non-specific isotype-matched IgG antibody. Macrophages were depleted by intraperitoneal injection of clodronate-containing liposomes (Encapsula NanoSciences, Brentwood, TN) (10 mg/kg) two days prior to and throughout the three days of L635 administration. Control mice received liposomes alone (10 mg/kg). Mice were sacrificed on the third day of L635 administration.
DMP-777 treatment
Three male mice were used for each experimental group. DMP-777 (a gift from DuPont- Merck Co.) dissolved in 1% methylcellulose was administered by oral gavage (350mg/kg) once a day for 8 consecutive days. Macrophages were depleted using four intraperitoneal injections of clodronate-containing liposomes (10 mg/kg) every other day of DMP-777 treatment. Control mice received liposomes (10 mg/kg) with or without DMP-777-treatment. Mice were sacrificed the ninth day.
For detailed methods, see Supplemental Material.
Results
Rag1 and IFNγ knockout mice develop acute proliferative SPEM
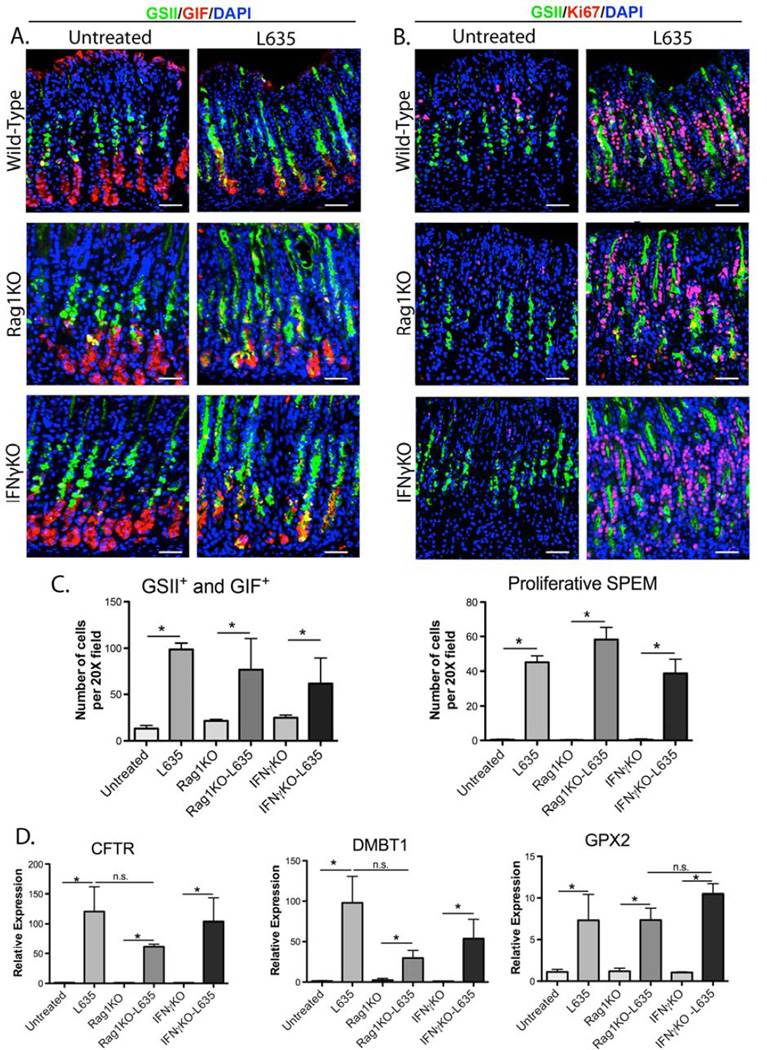
To determine the role of the adaptive immune system in the development of proliferative SPEM, wild type, Rag1KO and IFNγKO mice were administered L635 for three days and stomach cell lineages were analyzed. Histologic examination revealed parietal cell loss and significant inflammatory infiltration in the fundus of the stomach in all L635-treated mice (Supplemental Figure 1). Upon L635 treatment, neutrophils increased by 6-fold in wild type mice, with no significant difference observed in L635-treated Rag1KO or IFNγKO mice (Supplemental Figure 2A and C). F4/80 positive cells increased three to five-fold in wild type, Rag1KO, and IFNγKO L635-treated mice compared to untreated mice (Supplemental Figure 2B and C). L635-treated Rag1KO and IFNγKO mice did not have a significant change in F4/80 positive cells compared to wild-type L635-treated mice (Supplemental Figure 2C). L635-treated Rag1KO and IFNγKO mice developed SPEM (as defined by intrinsic factor and GSII-lectin co-positive cells),18 similar to L635- treated wild-type mice (Figure 1A and C). Furthermore, L635-treated Rag1KO and IFNγKO mice showed a 40 to 50-fold increase in SPEM cell proliferation similar to wildtype L635-treated mice (as defined by Ki67, gastric intrinsic factor, and GSII-lectin triplepositive cells) (Figure 1B and 1C). To determine whether Rag1KO or IFNγKO mice develop advanced proliferative SPEM, the expression of SPEM-associated intestinal transcripts was analyzed by qRT-PCR (Figure 1D). Cftr, Dmbt1, and Gpx2 expression was significantly upregulated (55-fold, 30-fold, and 7-fold, respectively) in L635-treated Rag1KO mice (Figure 1D). L635-treated IFNγKO mice showed similar changes in Cftr, Dmbt1 and Gpx2 expression comparable to changes observed in L635-treated wild type (Figure 1D). These results all indicate that the presence of T- and B-cells or IFNγ is not necessary for the development of advanced proliferative SPEM after acute parietal cell loss.
Figure 1. Wild type, Rag1KO and IFNγKO mice develop acute proliferative SPEM after L635-treatment.
A. Immunofluorescence staining for SPEM using gastric intrinsic factor (GIF) in red co-labeled with GSII-lectin (green) and DAPI (blue). GSII-lectin and GIF co-positive cells at the base of the glands were considered SPEM. B. Immunofluorescence staining for proliferation using Ki67 (red) co-labeled with GSII-lectin (green) and DAPI (blue) to determine the presence of SPEM cell proliferation before and after L635 administration in wild type, Rag1KO and IFNγKO mice. Ki67, GIF, and GSII-lectin triple-positive cells were considered proliferative SPEM. C. Quantitation of GSII+ and GIF+ cells (SPEM) and proliferative SPEM cells in wild type, Rag1KO and IFNγKO mice, (*p=0.05). D. qPCR of advanced proliferative SPEM markers. All L635- treated mice showed significant increases in the expression in intestinal transcript markers Cftr, Dmbt1 and Gpx2 (*p=0.05). Scale bars: 50 µm.
Neutrophil-depleted mice develop acute proliferative SPEM
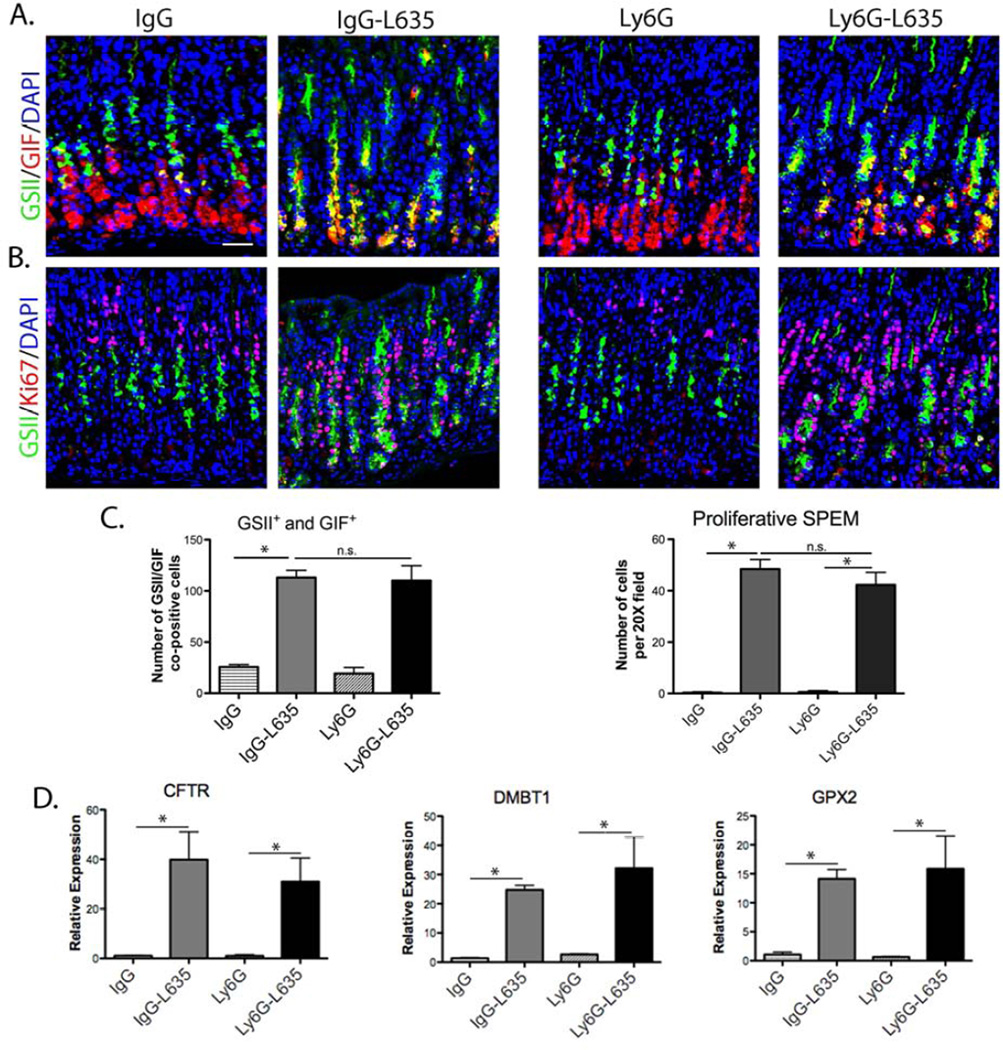
Neutrophils are potent phagocytic cells19 that are recruited to the gastric fundus in L635- treated mice.3 Neutrophils were depleted in mice using an anti-Ly6G antibody20 to determine the role of neutrophils in the development of SPEM. Control mice were dosed with a matched anti-IgG, and L635 was administered to both groups of mice. Prominent parietal cell loss and inflammation were observed by H&E within the fundic mucosa of all mice (Supplemental Figure 3A). Immunostaining for Ly6B.2, a neutrophil and polymorphonuclear cell marker, confirmed effective depletion of neutrophils in L653-Ly6G-treated mice compared to control L635-IgG-treated mice (Supplemental Figure 3B and D). Macrophage and dendritic cells labeled by F4/80 antibody were not significantly altered by neutrophil depletion (Supplemental Figure 3C and D). The number of SPEM cells increased five-fold, while SPEM cell proliferation increased 40 to 50-fold, in both neutrophil-depleted and control L635-treated mice (Figure 2A, B, and C). Quantitative PCR for intestinal transcripts revealed a significant upregulation of Cftr, Dmbt1 and Gpx2 in both neutrophil-depleted and control L635-treated mice (Figure 2D). These results indicate that neutrophils are not necessary for the development of acute advanced proliferative SPEM.
Figure 2. Neutrophil-depletion in mice does not alter the development acute advanced proliferative SPEM after L635 treatment.
A. Immunofluorescence staining of SPEM cells with antibodies against GIF (red) co-labeled with GSII-lectin (green) and DAPI (blue) in mice treated with either control anti-IgG or anti-Ly6G. B. Immunofluorescence staining of proliferating cells with antibodies against Ki67 (red) colabeled with GSII-lectin (green) and DAPI (blue), in untreated or L635-treated mice administered either control anti-IgG or anti-Ly6G to deplete neutrophils. C. Quantitation of GSII+ and GIF+ cells (SPEM) and proliferative SPEM cells per 20X field. IgG-L635-treated mice had a significant increase in SPEM cells and proliferative SPEM cells (*p=0.05) compared to anti-IgG untreated mice. In mice treated with anti-Ly6G and L635 we observed significant increases in SPEM cells and proliferative SPEM cells (*p=0.05) compared to anti-Ly6G untreated mice. D. qPCR of intestinal transcript markers. All L635-treated mice showed similar increases in Cftr, Dmbt1 or Gpx2 expression (*p=0.05). Scale bars: 50 µm.
Macrophages are involved in the development of acute L635-induced SPEM
Macrophages make up a significant portion of the inflammatory infiltrate in L635-treated mice.3 Therefore, we evaluated the impact of macrophage depletion using clodronate on the development of SPEM induced by L635. Mice were treated with either clodronate-containing liposomes or control liposomes without clodronate for two days prior to and throughout the three days of L635 administration. All L635-treated mice showed significant parietal cell loss (Supplemental Figure 4A). Mice treated with L635 and either control liposomes or clodronate demonstrated a 10 to 15-fold increase in neutrophils (Supplemental Figure 4B and D). Therefore neutrophil recruitment was not significantly affected by macrophage depletion (Supplemental Figure 4D).
The antibody F4/80 was utilized to identify macrophages and dendritic cells to confirm macrophage depletion by clodronate treatment. In untreated mice an average of 50 tissue-resident macrophages and/or dendritic cells was observed per 20X field (Supplemental Figure 4C). These resident immune cells serve to alert the host to invading pathogens and to clear dead cells.17 After L635 administration, F4/80 positive cells increased three-fold in control mice; however, the number of F4/80 positive cells in the gastric fundic mucosa of clodronate-L635-treated mice remained unchanged and similar to untreated mice (Supplemental Figure 4C and D). These findings suggest that clodronate-containing liposomes depleted systemic macrophages, but not tissueresident macrophages or dendritic cells in the stomach.
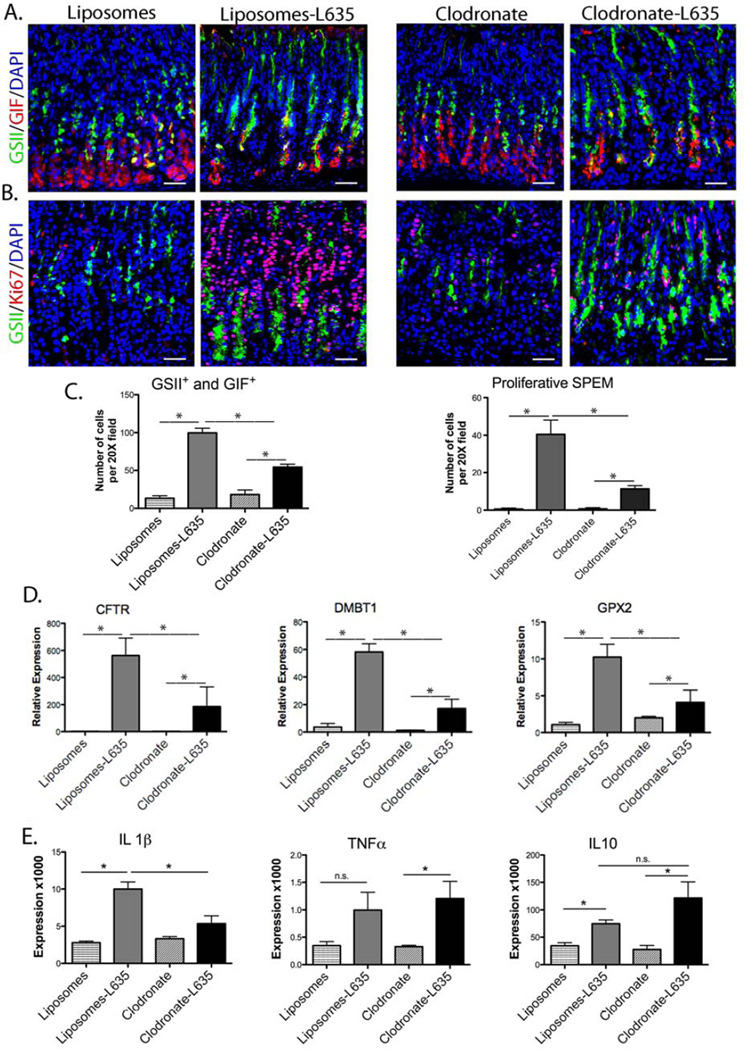
After L635 administration, control mice showed a five-fold increase in SPEM cells, while clodronate-treated mice L635 elicited only a two-fold increase in the number of SPEM cells (Figure 3A and C). Furthermore, SPEM cell proliferation increased 40-fold in control L635-treated mice, but only 10-fold in mice treated with both clodronate and L635 (Figure 3B and C). Therefore, macrophage depletion significantly attenuated the number of SPEM cells per gland and the proliferative response in SPEM following L635 administration (Figure 3A, B, and C). Notably, mice treated with both clodronate and L635 demonstrated significantly lower levels of intestinal transcripts for Cftr, Dmbt1 and Gpx2 compared to the L635-treated control mice (Figure 3D).
Figure 3. Macrophage depletion inhibits the development of proliferative SPEM following L635 treatment.
A. Immunofluorescence staining of SPEM cells with antibodies against GIF (red) co-labeled with GSII-lectin (green) and DAPI (blue) in mice treated with either control liposomes or clodronate-containing liposomes, with or without L635-treatment. B. Immunofluorescence staining of proliferating SPEM cells with antibodies against Ki67 (red) co-labeled with GSII-lectin (green) and DAPI (blue). C. Quantitation of the number of GSII+ and GIF+ cells (SPEM) and proliferative SPEM cells. Mice treated with both clodronate and L635 had significantly reduced SPEM cell numbers and SPEM cell proliferation (*p=0.05) compared to mice treated with liposomes and L635. D. qPCR showing relative expression of intestinal transcript markers. Clodronate treatment significantly reduced the expression of Cftr, Dmbt1 and Gpx2 in L635-treated mice (*p=0.05). E. qPCR for IL1β, TNFα and IL10 cytokines. Clodronate treatment only significantly reduced IL1β expression (*p=0.05). Scale bars: 50 µm.
We have previously shown that IL-1β and IL10 expression significantly increases in SPEM associated with inflammation.3 Thus, we sought to evaluate how depletion of macrophages might affect these specific cytokine levels. As previously noted,3 control mice demonstrated a significant increase in IL-1β following L635 administration (Figure 3E). However, IL-1β cytokine expression was significantly reduced in clodronate-L635-treated mice compared to L635-treated mice (Figure 3E). In contrast, clodronate treatment had no significant effect on TNFα or IL10 expression, which can be produced by other cells besides macrophages. IFNγ and IL4 were undetectable in all samples (data not shown).
DMP-777 induced SPEM does not recruit macrophages
DMP-777 drug treatment induces SPEM over an 8-day treatment without appreciable inflammation.10 In contrast with SPEM induced by L635 treatment, DMP-777-induced SPEM demonstrates only a low proliferative rate. Furthermore, DMP-777-treated mice demonstrated only a two-fold increase in F4/80+ cells compared to untreated mice, significantly less than the three-fold increase in L635-treated mice (data not shown). To determine whether DMP-777 induced SPEM is influenced by macrophage depletion, DMP-777-treated mice were treated with either clodronate-containing or control liposomes. Control and macrophage-depleted DMP-777-treated mice both developed SPEM (Supplemental Figure 5A), and had similar low levels of proliferative SPEM cells (Supplemental Figure 5B and F). Additionally, the number of F4/80+ cells was not impacted by clodronate treatment (Supplemental Figure 5D and G). Other groups have noted that clodronate treatment does not influence tissue resident macrophage or dendritic cell populations in some organs.21, 22 Importantly, we observed a lack of immunostaining for the macrophage marker CD68 and only a rare Arginase II positive cell in DMP-777 treated mice, indicating neither M2 macrophages nor macrophages were recruited into the gastric mucosa during DMP-777 treatment (Supplemental Figure 5D and 5E). A previous study showed local expansion of tissue resident macrophages in association with tissue maintenance or inflammation.23 Thus, the increase in F4/80+ cells in DMP-777 treated mice likely reflects the expansion of tissue resident dendritic cells within the gastric mucosa. The lack of an effect of macrophage depletion on SPEM development induced by DMP-777 supports the concept that infiltration by macrophages promotes the progression to an advanced proliferative metaplasia.
Macrophages in L635-treated mice express M2 polarization markers
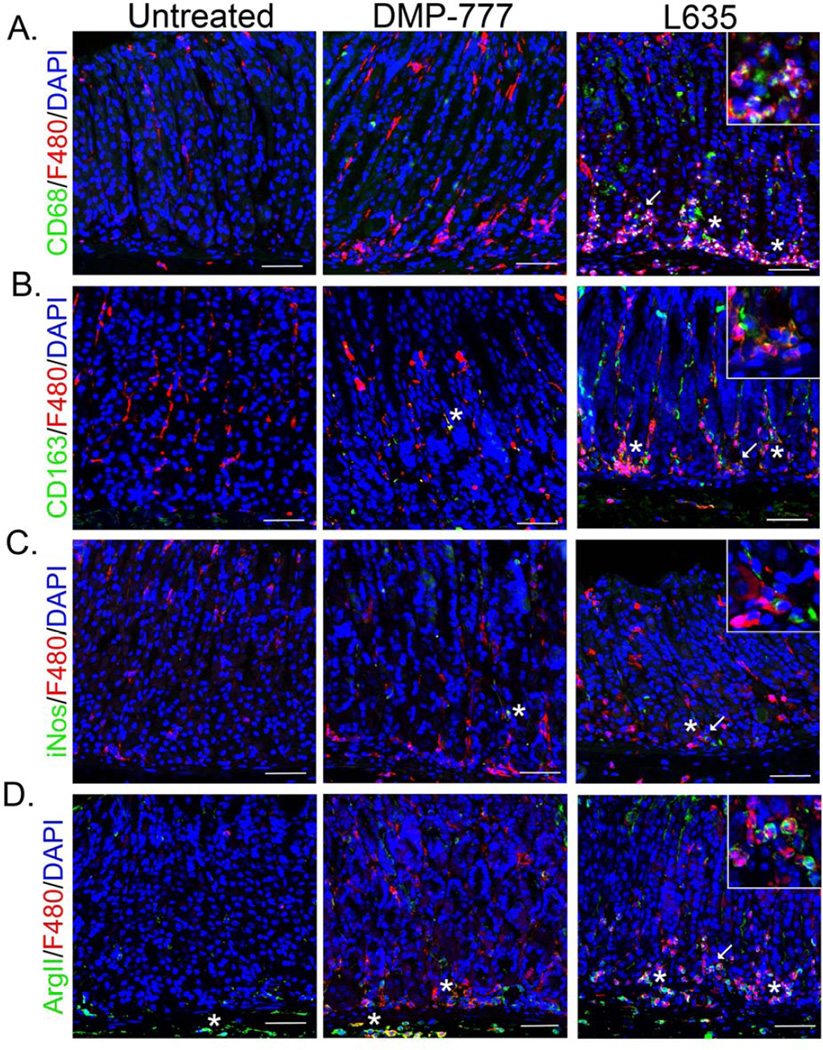
Macrophage activation is typically described as either “classical” (M1) or “alternative” (M2).24 M1 polarized macrophages are considered pro-inflammatory and are associated with iNos expression.25 In contract, M2 macrophages are associated with wound healing and are anti-inflammatory, but are also known to promote neoplasia.26 We assessed the characteristics of the infiltrating macrophages after L635 treatment to determine M1 or M2 polarization. The F4/80+ cells at the base of glands after L635-treatment were identified as macrophages through co-staining with CD68 (Figure 4A). The macrophages present in L635-treated mice were also positive for the M2 marker and hemoglobin-scavenger receptor CD163, but were negative for the M1 marker iNos (Figure 4B and C).27, 28 Furthermore, L635-treated mice displayed a predominance of Arginase II (ArgII) positive macrophages, likely an M2 marker as Arginase II competes for similar substrates as iNos (Figure 4D).29 In contrast, as noted above, F4/80+ cells in DMP-777-treated mice were negative for macrophage markers (Figure 4) with the exception of rare ArgII+ cells in the submucosa (Figure 4D).
Figure 4. Macrophages are M2 polarized in L635-treated mice, but not in DMP-777- treated mice.
Immunofluorescence of fundic mucosa from untreated, DMP-777 and L635-treated mice. A. Identification of macrophages by co-positive CD68 (green) and F4/80 (red) cells. F4/80 positive cells, negative for CD68 staining were considered dendritic cells. F4/80 positive cells at the base of glands in L635-treated mice were also CD68 positive. Inset in L635 panel illustrating dual-positive F4/80 and CD68 cells. B. Immunofluorescence staining of CD163 (green) marked M2 polarized macrophages. Several CD163 positive M2 polarized macrophages co-labeling with F4/80 are present in L635-treated mice. Inset showing CD163 and F4/80 co-positive cells. C. Immunofluorescence staining of the M1 marker iNos (green), F4/80 (red) and DAPI (blue). Inset in L635 showing rare iNos and F4/80 positive cells. D. Immunofluorescence of Arginase II (green), an M2 marker, with F4/80 (red) and DAPI (blue). DMP-777-treated mice had a few Arginase II and F4/80 co-positive cells, mostly localized to the submucosa. L635-treated mice have several Arginase II and F4/80 copositive cells within the mucosa. Scale bars: 50 µm. All insets show enlarged view of area marked by arrow. All co-positive cells marked by asterisk.
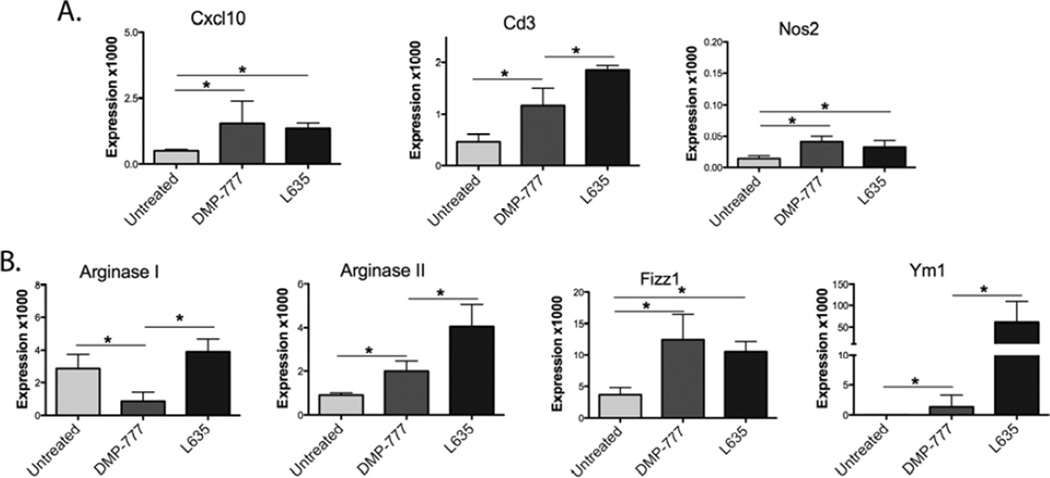
To further characterize macrophage polarization, M1 and M2 transcript expression was analyzed using RNA from fresh whole fundic tissue for qRT-PCR (Figure 5). M1 markers Cxcl10, Cd3 and Nos2 were all significantly upregulated after DMP-777 and L635 administration, but both the absolute expression levels and the magnitude of expression changes were small (Figure 5A). In contrast, the expression of the M2 marker Ym1 increased 75-fold and ArgII expression increased 5-fold (Figure 5B) in L635-treated mice.M2 macrophages produce high levels of Ym1, which is proposed to deposit extracellular matrix for wound healing.30, 31 Fizz1 increased to equal levels with both DMP-777 and L635 treatment, which may be reflective of Fizz1 expression in both macrophages and dendritic cells.31 Therefore, while a few iNos (protein expression of Nos2) positive M1 cells can be detected, the majority of macrophages infiltrating into the mucosa after L635-treatment were M2 polarized.
Figure 5. M2 marker transcripts are markedly increased in L635-treated mice.
Quantitative PCR showing mRNA expression from whole fresh fundus of M1 and M2 markers normalized to GAPDH, shown as expression level multiplied by 1000 for scale. A. M1 markers Cxcl10, Cd3, and Nos2 all significantly increase (*p=0.05) in DMP-777 and L635-treated mice. However, the expression levels overall of the M1 markers are very low. B. M2 markers Arginase I, Arginase II, Fizz1, and Ym1 all significantly increase in L635-treated mice (*p=0.05).
M2 macrophages increase in human SPEM and intestinal metaplasia
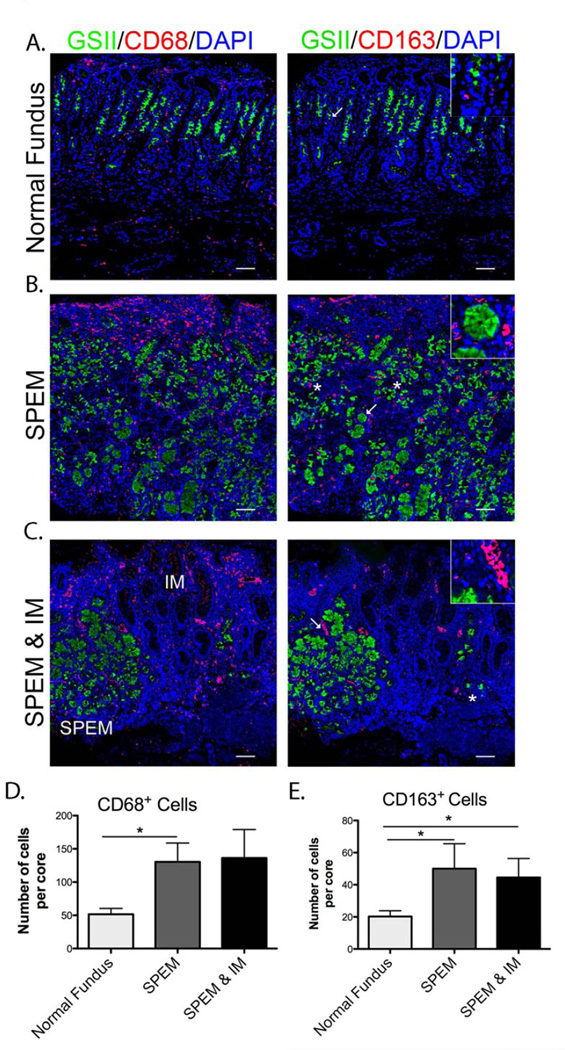
Based on the characteristics of macrophages found in the murine model, we examined macrophages associated with stomach metaplasia in humans. Normal human fundic mucosa contained CD68+ macrophages (Figure 6A and D). However, in mucosa with SPEM and intestinal metaplasia (IM), the number of CD68+ macrophages significantly increased two to three-fold (Figure 6B, C and D). Typically macrophages were dispersed throughout the SPEM area, while in IM the macrophages clustered together in regions around blood vessel-like structures (Figure 6B and C inset). Normal gastric tissue demonstrated only a few M2 polarized macrophages labeled with CD163 (Figure 6A and E), which increased two to three-fold SPEM and IM (Figure 6B, C and E).
Figure 6. M2 macrophage infiltration in human SPEM and IM.
A. Left panel: Normal human mucosa labeled with GSII-Lectin (green) marking mucous neck cells and CD68 (red) for macrophages. Right panel shows CD163 (red) antibody labeling for M2 macrophages, GSII-Lectin (green) and DAPI (blue) in a serial section. B. Left panel: SPEM in human tissue immunolabeled with GSII-Lectin (green), CD68 (red) and DAPI (blue), showing an increased number of CD68+ macrophages. Right panel shows an increase in CD163 (red) M2 marker staining cells in a serial section. Inset in right panel is showing SPEM surrounded by CD163+ macrophages C. Left panel: SPEM (GSII-Lectin in green) and IM show increased CD68+ (red) macrophages clustered around vessel-like structures. Right panel: CD163 staining in a serial section demonstrates macrophages were M2 polarized in SPEM and IM. Inset showing M2 macrophages cluster around vessel-like structures. D. Quantitation of CD68+ macrophages per core in normal, SPEM and IM. CD68 positive cells significantly increase (*p=0.05) from normal fundus to SPEM, and SPEM/IM. D. Quantitation of CD163 positive cells per core. The number of M2 polarized macrophages significantly increased (*p=0.05) from normal to SPEM and SPEM/IM tissue. Scale bars: 50 µm. All insets show enlarged view of area marked by arrow.
Discussion
The loss of parietal cells, also known as oxyntic atrophy, is considered an absolute requirement for the development of both metaplasia and intestinal-type gastric cancer.7–9 Nevertheless, the exact cause of parietal cell loss during chronic Helicobacter infection remains unclear. Previous studies have investigated the role of immune factors during Helicobacter infection and their impact on parietal cell loss and the development of metaplasia. Severe combined immune deficient (SCID) mice do not undergo parietal cell loss following H. pylori infection, implicating the adaptive immune system in the development of Helicobacter-associated oxyntic atrophy.32 Roth et al. found that Rag1 knockout mice, deficient in mature T and B lymphocytes, do not lose parietal cells when infected with H. pylori.16, 33 In further studies, they found that T-cells were necessary for parietal cell loss during H. pylori infection.16 Therefore, the T-cell-mediated response to H. pylori infection results in oxyntic atrophy, a prerequisite for induction of metaplasia.16, 34
While the loss of parietal cells during Helicobacter infection requires the influence of T-cells, the development of metaplasia appears to relate directly to parietal cell loss. Our recent investigations have demonstrated that SPEM is derived from transdifferentiation of chief cells in mice.3–5, 7 In the present studies, L635-treated Rag1KO mice developed proliferative SPEM and upregulated intestinal transcripts similar to wild type L635-treated mice. Thus, while T-cells are necessary for parietal cell loss in chronic Helicobacter models to facilitate the development of SPEM,16 T-cells are not necessary for the development of SPEM in the acute L635 treatment model. It is important to note that DMP-777 and L635 treatments directly induce parietal cell loss bypassing the mechanisms that require T-cell influences to induce oxyntic atrophy in association with Helicobacter infection. These models can be used to decouple the immune influences required to induce oxyntic atrophy from the mechanisms of chief cell transdifferentiation into metaplasia. Furthermore, studies using the drugs DMP-777 and L635 have demonstrated that SPEM does not progress to an advanced proliferative metaplasia without the presence of inflammation.4, 10 Thus, the present study addresses the role of different immune factors in the development of advanced SPEM after parietal cell loss using the drug L635.
Previous studies have evaluated the role of specific cytokines secreted by T-cells such as IFNγ in oxyntic atrophy and SPEM.18, 35 IFNγ secreted by T-cells drives several different immune processes, including the activation of macrophages and upregulation of IFNγ target genes in epithelial cells.13, 36 Liu et al. used a knockout of Hip1r, a model of parietal cell loss through apoptosis, crossed to IFNγ knockout mice to show reduced metaplasia initially; but over a longer time course, loss of IFNγ did not impair the development of SPEM.18, 37 In contrast, Helicobacter-infected mice overexpressing IFNγ through the H+/K+-ATPase promoter develop less Helicobacter-associated gastric pathology due to increased epithelial autophagy and decreased parietal cell loss, indicating that IFNγ may also have a cytoprotective role.35 Using a similar model to overexpress IFNγ, Syu et al. reported IFNγ induced metaplasia.38 In the present study, L635-treated IFNγKO mice developed SPEM similar to wild type mice. The difference in our findings may depend on experimental conditions used to induce parietal cell loss. Furthermore, the mouse models manipulating IFNγ likely focus on mechanisms related to promotion of chronic parietal cell loss, whereas the L635 treatment model causes rapid toxic loss of parietal cells. The L635 treatment model therefore focuses on the regulation of the processes influencing the transdifferentiation of chief cells into SPEM and their development to more advanced proliferative metaplasia. Our present data indicate that IFNγ is not a requisite mediator in the development of acute advanced SPEM.
While shorter lived than macrophages, neutrophils are a highly effective phagocytic cells that are tightly controlled due to their high cytotoxicity.19 Neutrophil-associated tissue damage caused by extravasation, increased vascular permeability, neutrophil-derived destructive enzymes and reactive oxygen species is well documented.19, 39 Nevertheless, neutrophils play an essential role in clearing Helicobacter infection.40 Typically, phagocytic neutrophils are scarce in the normal stomach mucosa and enter during infection or injury,15, 16 driven in part by chemokines released by epithelial cells39 and tissue-resident macrophages and dendritic cells.41–44 After L635 administration, there is increased neutrophil infiltration, but neutrophil-depleted L635-treated mice developed similar levels of proliferative SPEM and intestinalizing transcript expression as in L635-treated control mice. Therefore, neutrophils are not necessary for the development of advanced SPEM in this model.
Acute injury or infection immediately stimulates antigen-presenting cells, tissue-resident macrophages and dendritic cells, to engulf tissue debris and foreign antigens.19 These cells secrete cytokines that not only influence the adaptive immune system through T-cell differentiation, but also promote the homeostasis of surrounding epithelial cells, while increasing the inflammatory response.17 The short time course of L635 treatment to ablate parietal cells and induce SPEM in just three days facilitated examination of the influence of macrophages on SPEM induction using clodronate treatment, an experimental paradigm that is not practical in conjunction with chronic Helicobacter infection models. Clodronate-treated mice demonstrated the same number of F4/80-positive cells with or without L635 treatment. The remaining F4/80-positive cells were likely tissue resident macrophages and dendritic cells that do not label for CD68, and are unaffected by clodronate treatment.21 Recently, Hashimoto et al. suggested that tissue-resident macrophages and circulating monocytes are independently maintained at a steady state, and therefore tissue resident macrophages and invading monocytes have varied responses and may not behave similarly.45 Indeed, we have observed that DMP-777 treatment increased the number of CD68-negative, F4/80-positive cells, but clodronate had no effect on the induction of SPEM by DMP-777. L635-treated macrophage-depleted mice demonstrated a significant reduction in SPEM cell numbers, SPEM cell proliferation and expression of intestinal transcripts. Therefore, while the discrete role of dendritic cells in SPEM induction remains unclear, the results here indicate that infiltrating macrophages contribute to the advanced metaplastic phenotype in L635-induced SPEM.
Macrophage secretion of several different pro-inflammatory cytokines is well documented.42, 46 Cytokine transcript expression for IL-1β, which is secreted by activated macrophages, is increased significantly in L635-treated and H. felis-infected mice.3, 46 Notably, macrophage-depleted L635-treated mice have significantly reduced IL-1β transcript expression compared to L635-treated control mice. IL-1β cytokine acts to propagate the inflammatory response and inhibits gastric acid secretion.47 Transgenic mice that overexpress human IL-1β in the stomach develop spontaneous gastritis and eventually dysplasia after a year.48 However the specific role of IL-1β in the advancement of SPEM is still unknown. Tumor necrosis factor alpha (TNFα) and IL-10, which are secreted by activated macrophages and other inflammatory cell types as well as mucosal cells, increase significantly in H. felis mice.3, 47 Nevertheless, since depletion of macrophages did not reduce TNFα or IL-10 levels in L635-treated mice, these cytokines may not contribute to the progression of metaplasia in the acute SPEM model. However, it is likely that specific macrophage cytokines contribute to the advancement of SPEM.
M2 polarized macrophages are attributed to the progression of several reported disease states.49, 50 Inhibiting macrophage infiltration using a monocyte chemoattractant protein-1 (MCP-1, CCL2) antagonist led to tumor regression in Gan mice, a murine model of gastric cancer.50 In GAN mice, infiltrating macrophages were M2 polarized, supporting our findings that M2 macrophages are important in the advancement of metaplasia.51 While we observed a small increase in M1 marker expression, it was minor compared to the dramatic increase observed in M2 marker expression. This finding, coupled with positive staining of CD163 and Arginase II, indicates that macrophages present in L635-treated mice are M2 polarized, but are not present in DMP-777-treated mice. Therefore, while neither CD68+ macrophages nor M2 macrophages are required for the induction of SPEM, M2 macrophages are important for advancing the metaplastic phenotype. This result in mice was supported in human metaplasia, where half of the observed macrophages where M2 polarized. Fehlings et al. reported similar findings of increased CD163+ cells in H. pylori-infected patients,52 supporting the concept that M2 macrophages promote SPEM progression and intestinal metaplasia.
In summary, our data suggest that circulating monocytes invade the gastric mucosa during L635 administration and promote the advancement of SPEM. The macrophages associated with advanced proliferative metaplasia were polarized towards M2, a phenotype associated with promotion of tumorogenesis in mice and humans.28, 50, 51 While T-cells and neutrophils may have critical influences on the induction of parietal cell loss in the gastric mucosa during Helicobacter infection, these immune cell populations do not appear to have significant direct influences on the emergence of SPEM from transdifferentiation of chief cells or the advancement of SPEM to a more proliferative metaplasia with intestinal characteristics. Further investigations will be required to define the discrete mediators released by M2 macrophages that can promote the process of metaplastic transition.
Supplementary Material
ACKNOWLEDGEMENTS
These studies were supported by grants from a Department of Veterans Affairs Merit Review Award and NIH RO1 DK071590, as well as a grant from the Martell Foundation (to J.R.G). This work was supported by core resources of the Vanderbilt Digestive Disease Center, (P30 DK058404) the Vanderbilt-Ingram Cancer Center (P30 CA68485, Chemical Synthesis Core), and imaging supported by both the Vanderbilt Combined Imaging Shared Resource and the Vanderbilt Digital Histology Shared Resource. We thank Drs. Rupesh Chaturvedi and Keith Wilson for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The Authors have no conflicts of interest.
Author roles:
Petersen: Designed experiments, performed studies, analyzed data, prepared figures and drafted manuscript.
Weis: Designed experiments, analyzed data, drafted manuscript.
Nam: Designed experiments, analyzed data, drafted manuscript.
Sousa: Designed experiments, analyzed data, drafted manuscript.
Fingleton: Designed experiments, analyzed data, drafted manuscript.
Goldenring: Designed experiments, drafted manuscript.
References
- 1.de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 2.Blaser MJ, Parsonnet J. Parasitism by the "slow" bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J Clin Invest. 1994;94:4–8. doi: 10.1172/JCI117336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nam KT, Lee HJ, Sousa JF, et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037. e9. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weis VG, Sousa JF, Lafleur BJ, et al. Heterogeneity in mouse spasmolytic polypeptide-expressing metaplasia lineages identifies markers of metaplastic progression. Gut. 2012 doi: 10.1136/gutjnl-2012-302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshizawa N, Takenaka Y, Yamaguchi H, et al. Emergence of spasmolytic polypeptide-expressing metaplasia in Mongolian gerbils infected with Helicobacter pylori. Lab Invest. 2007;87:1265–1276. doi: 10.1038/labinvest.3700682. [DOI] [PubMed] [Google Scholar]
- 6.Nam KT, Lee HJ, Mok H, et al. Amphiregulin-deficient mice develop spasmolytic polypeptide expressing metaplasia and intestinal metaplasia. Gastroenterology. 2009;136:1288–1296. doi: 10.1053/j.gastro.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldenring JR, Nam KT, Wang TC, et al. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology. 2010;138:2207–2210. doi: 10.1053/j.gastro.2010.04.023. 2210 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meining A, Morgner A, Miehlke S, et al. Atrophy-metaplasia-dysplasia-carcinoma sequence in the stomach: a reality or merely an hypothesis? Best Pract Res Clin Gastroenterol. 2001;15:983–998. doi: 10.1053/bega.2001.0253. [DOI] [PubMed] [Google Scholar]
- 9.El-Zimaity HM, Ota H, Graham DY, et al. Patterns of gastric atrophy in intestinal type gastric carcinoma. Cancer. 2002;94:1428–1436. doi: 10.1002/cncr.10375. [DOI] [PubMed] [Google Scholar]
- 10.Goldenring JR, Ray GS, Coffey RJ, et al. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology. 2000;118:1080–1093. doi: 10.1016/s0016-5085(00)70361-1. [DOI] [PubMed] [Google Scholar]
- 11.Nomura S, Yamaguchi H, Ogawa M, et al. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrindeficient mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G362–G375. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- 12.Wang TC, Dangler CA, Chen D, et al. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 13.Houghton J, Stoicov C, Nomura S, et al. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 14.Varon C, Dubus P, Mazurier F, et al. Helicobacter pylori infection recruits bone marrow-derived cells that participate in gastric preneoplasia in mice. Gastroenterology. 2012;142:281–291. doi: 10.1053/j.gastro.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 15.Fox JG, Blanco M, Murphy JC, et al. Local and systemic immune responses in murine Helicobacter felis active chronic gastritis. Infect Immun. 1993;61:2309–2315. doi: 10.1128/iai.61.6.2309-2315.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth KA, Kapadia SB, Martin SM, et al. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J Immunol. 1999;163:1490–1497. [PubMed] [Google Scholar]
- 17.Chow A, Brown BD, Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol. 2011;11:788–798. doi: 10.1038/nri3087. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Demitrack ES, Keeley TM, et al. IFNgamma contributes to the development of gastric epithelial cell metaplasia in Huntingtin interacting protein 1 related (Hip1r)-deficient mice. Lab Invest. 2012;92:1045–1057. doi: 10.1038/labinvest.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva MT. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J Leukoc Biol. 2010;87:93–106. doi: 10.1189/jlb.0809549. [DOI] [PubMed] [Google Scholar]
- 20.Daley JM, Thomay AA, Connolly MD, et al. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 21.Ferenbach DA, Sheldrake TA, Dhaliwal K, et al. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int. 2012;82:928–933. doi: 10.1038/ki.2012.207. [DOI] [PubMed] [Google Scholar]
- 22.van Amerongen MJ, Harmsen MC, van Rooijen N, et al. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol. 2007;170:818–829. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins SJ, Ruckerl D, Cook PC, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoger JL, Gijbels MJ, van der Velden S, et al. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis. 2012;225:461–468. doi: 10.1016/j.atherosclerosis.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Quiding-Jarbrink M, Raghavan S, Sundquist M. Enhanced M1 macrophage polarization in human helicobacter pylori-associated atrophic gastritis and in vaccinated mice. PLoS One. 2010;5:e15018. doi: 10.1371/journal.pone.0015018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verreck FA, de Boer T, Langenberg DM, et al. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-gamma- and CD40L-mediated costimulation. J Leukoc Biol. 2006;79:285–293. doi: 10.1189/jlb.0105015. [DOI] [PubMed] [Google Scholar]
- 28.Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Transl Med. 2011;9:216. doi: 10.1186/1479-5876-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 30.Chang NC, Hung SI, Hwa KY, et al. A macrophage protein, Ym1, transiently expressed during inflammation is a novel mammalian lectin. J Biol Chem. 2001;276:17497–17506. doi: 10.1074/jbc.M010417200. [DOI] [PubMed] [Google Scholar]
- 31.Nair MG, Gallagher IJ, Taylor MD, et al. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect Immun. 2005;73:385–394. doi: 10.1128/IAI.73.1.385-394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smythies LE, Waites KB, Lindsey JR, et al. Helicobacter pylori-induced mucosal inflammation is Th1 mediated and exacerbated in IL-4, but not IFN-gamma, gene-deficient mice. J Immunol. 2000;165:1022–1029. doi: 10.4049/jimmunol.165.2.1022. [DOI] [PubMed] [Google Scholar]
- 33.Mombaerts P, Iacomini J, Johnson RS, et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 34.Appelmelk BJ, Simoons-Smit I, Negrini R, et al. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect Immun. 1996;64:2031–2040. doi: 10.1128/iai.64.6.2031-2040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tu SP, Quante M, Bhagat G, et al. IFN-gamma inhibits gastric carcinogenesis by inducing epithelial cell autophagy and T-cell apoptosis. Cancer Res. 2011;71:4247–4259. doi: 10.1158/0008-5472.CAN-10-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang W, Rathinavelu S, Samuelson LC, et al. Interferon gamma induction of gastric mucous neck cell hypertrophy. Lab Invest. 2005;85:702–715. doi: 10.1038/labinvest.3700260. [DOI] [PubMed] [Google Scholar]
- 37.Mueller A, Merrell DS, Grimm J, et al. Profiling of microdissected gastric epithelial cells reveals a cell type-specific response to Helicobacter pylori infection. Gastroenterology. 2004;127:1446–1462. doi: 10.1053/j.gastro.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 38.Syu LJ, El-Zaatari M, Eaton KA, et al. Transgenic expression of interferongamma in mouse stomach leads to inflammation, metaplasia, and dysplasia. Am J Pathol. 2012;181:2114–2125. doi: 10.1016/j.ajpath.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kozol R, Domanowski A, Jaszewski R, et al. Neutrophil chemotaxis in gastric mucosa. A signal-to-response comparison. Dig Dis Sci. 1991;36:1277–1280. doi: 10.1007/BF01307522. [DOI] [PubMed] [Google Scholar]
- 40.Ismail HF, Zhang J, Lynch RG, et al. Role for complement in development of Helicobacter-induced gastritis in interleukin-10-deficient mice. Infect Immun. 2003;71:7140–7148. doi: 10.1128/IAI.71.12.7140-7148.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evans DJ, Jr, Evans DG, Takemura T, et al. Characterization of a Helicobacter pylori neutrophil-activating protein. Infect Immun. 1995;63:2213–2220. doi: 10.1128/iai.63.6.2213-2220.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mai UE, Perez-Perez GI, Wahl LM, et al. Soluble surface proteins from Helicobacter pylori activate monocytes/macrophages by lipopolysaccharide-independent mechanism. J Clin Invest. 1991;87:894–900. doi: 10.1172/JCI115095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blaser MJ. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology. 1992;102:720–727. doi: 10.1016/0016-5085(92)90126-j. [DOI] [PubMed] [Google Scholar]
- 44.Wroblewski LE, Peek RM, Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hashimoto D, Chow A, Noizat C, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beuscher HU, Gunther C, Rollinghoff M. IL-1 beta is secreted by activated murine macrophages as biologically inactive precursor. J Immunol. 1990;144:2179–2183. [PubMed] [Google Scholar]
- 47.Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut. 1998;42:227–234. doi: 10.1136/gut.42.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tu S, Bhagat G, Cui G, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaynagetdinov R, Sherrill TP, Polosukhin VV, et al. A critical role for macrophages in promotion of urethane-induced lung carcinogenesis. J Immunol. 2011;187:5703–5711. doi: 10.4049/jimmunol.1100558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oshima H, Hioki K, Popivanova BK, et al. Prostaglandin E(2) signaling and bacterial infection recruit tumor-promoting macrophages to mouse gastric tumors. Gastroenterology. 2011;140:596–607. e7. doi: 10.1053/j.gastro.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Oshima H, Popivanova BK, Oguma K, et al. Activation of epidermal growth factor receptor signaling by the prostaglandin E(2) receptor EP4 pathway during gastric tumorigenesis. Cancer Sci. 2011;102:713–719. doi: 10.1111/j.1349-7006.2011.01847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fehlings M, Drobbe L, Moos V, et al. Comparative analysis of the interaction of Helicobacter pylori with human dendritic cells, macrophages, and monocytes. Infect Immun. 2012;80:2724–2734. doi: 10.1128/IAI.00381-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.