Abstract
Increasing evidence supports a central role for ncRNA in numerous aspects of chromatin function. For instance, ncRNAs can act as a scaffold for the recruitment of certain chromatin modifying complexes to specific sites within the genome. It is easily imaginable how this can occur in cis, but examples also exist whereby targeting of complexes by ncRNA occurs in trans to the site of transcription. Moreover, association of an ncRNA with a particular locus can trigger localization of the gene to a subnuclear structure harboring a specialized transcriptional environment. In this review, we discuss new insights into the mechanisms by which ncRNAs function in trans with respect to Polycomb Group, chromatin insulator, and dosage compensation complexes in mammals and/or Drosophila.
Introduction
Noncoding RNAs (ncRNAs) play key roles in chromatin function, particularly in scaffolding and recruitment of certain chromatin modifying complexes. It has long been appreciated that ncRNAs are central components of the dosage compensation machinery, and recent work has elucidated how various ncRNAs contribute to Polycomb Group (PcG) and chromatin insulator activities. In several cases, a nascent ncRNA nucleates recruitment of a chromatin complex in cis, serving as a simple mechanism to promote binding specificity. However, ncRNAs can also stimulate targeting of chromatin complexes in trans, including nucleation of complexes at distant sites or stabilization of large nuclear structures. The likely mechanisms by which these activities occur far from the site of transcription are hardly intuitive and require further study. In this review, we focus on recent studies providing key insights regarding the function of ncRNAs in trans with respect to PcG, chromatin insulator, and dosage compensation activities in mammals and/or Drosophila.
Maintenance of cellular identity by PcG repression
The PcG proteins are required for the proper development of multicellular organisms, functioning to preserve pluripotency and/or cellular identity. Their main function is to repress the expression of genes that would otherwise promote differentiation into other cell types (Reviewed in [1]). This conserved class of proteins comprises two major subcomplexes, Polycomb Repressive Complex 1 and 2 (PRC1 and PRC2). One function of PRC2 is to methylate lysine 27 of histone H3 (H3K27) through the activity of the Ezh2 methyltransferase. Resultant H3K27me3 serves as a docking platform for the chromodomain containing Polycomb (Pc) protein of PRC1. Recruitment of PRC1 ultimately leads to stable repression through chromatin compaction and/or interference with RNA polymerase II transcription. Another property proposed to further stabilize repressive activities is the propensity of PcG complexes to concentrate at large subnuclear structures termed PcG bodies (Reviewed in [2]).
How specific recruitment of PcG complexes is achieved remains a critical outstanding question. Although a variety of DNA-binding proteins have been demonstrated to affect PcG recruitment in both Drosophila and mammals, recent studies have alternatively suggested that PcG recruitment may be promoted through interaction with RNA. Genome-wide purification approaches have identified a plethora of associated RNAs [3–6], likely due to the ability of PRC2 to bind RNA non-specifically [7]. An in-depth discussion regarding the role of ncRNAs in PcG recruitment in cis was recently presented [8]. Here we will discuss the abilities of certain ncRNAs to promote PcG activity in trans with respect to targeting of complexes to specific genomic locations as well as to subnuclear structures.
PRC2 recruitment to chromatin by HOTAIR ncRNA
The long ncRNA HOX Antisense Intergenic RNA (HOTAIR) serves as the archetypal trans acting PcG recruitment factor. HOTAIR is 2.2 kb in length and transcribed in the antisense direction with respect to the HOXC homeotic locus, preferentially expressed in distal and posterior cells of mammals [9]. HOTAIR represses the expression of the HOXD locus in trans through direct interaction and recruitment of the Suz12 and Ezh2 components of PRC2. Consistent with a key role in HOX gene regulation, targeted deletion of HOTAIR in mouse leads to skeletal malformations of the vertebrae and limbs, indicative of homeotic transformation along with extensive genome-wide loss of H3K27me3 [10].
Subsequent global analyses have determined that HOTAIR also regulates targets outside of the HOXD locus. Development of a novel method termed Chromatin Isolation by RNA Purification (ChIRP) allowed genome-wide mapping of HOTAIR binding sites in a breast cancer cell line [11]. These 832 binding sites overlap extensively with PRC2 occupancy in the same cell type, consistent with the ability of HOTAIR to serve as a recruitment factor for the complex. Whether these locations constitute natural binding sites for the ncRNA is unclear since the cell line used in this study overexpresses HOTAIR, which causes genome-wide mistargeting of PRC2 [12]. In the future, it will be important to improve the sensitivity of ChIRP and related techniques [13, 14] and apply them to different cell lines expressing natural levels of HOTAIR.
The mechanism of HOTAIR targeting of PcG is further complicated by its involvement in targeting a second chromatin modifying complex. HOTAIR harbors two independent interaction modules and can interact simultaneously with PRC2 and the H3K4 lysine demethylase LSD1 [15]. Consistent with the possibility that HOTAIR acts as a scaffold to coordinate both activities, HOTAIR knockdown leads to loss of both Suz12 and LSD1 chromatin association at shared sites. However, HOTAIR also affects LSD1 recruitment at non-PcG sites, suggesting that HOTAIR also harbors PcG-independent functions. Assuming then that HOTAIR alone cannot specify PcG recruitment, other factors may be required to act in concert with HOTAIR. This possibility is also supported by the recent finding that the affinity of PRC2 association with HOTAIR is no higher than its affinity for non-specific RNA [7]. In fact, HOTAIR binding sites are enriched for a GA-rich polypurine motif [11], which could indicate interaction with a sequence-specific DNA-binding factor to assist in targeting.
Recruitment of PcG by ncRNA to distinct nuclear structures
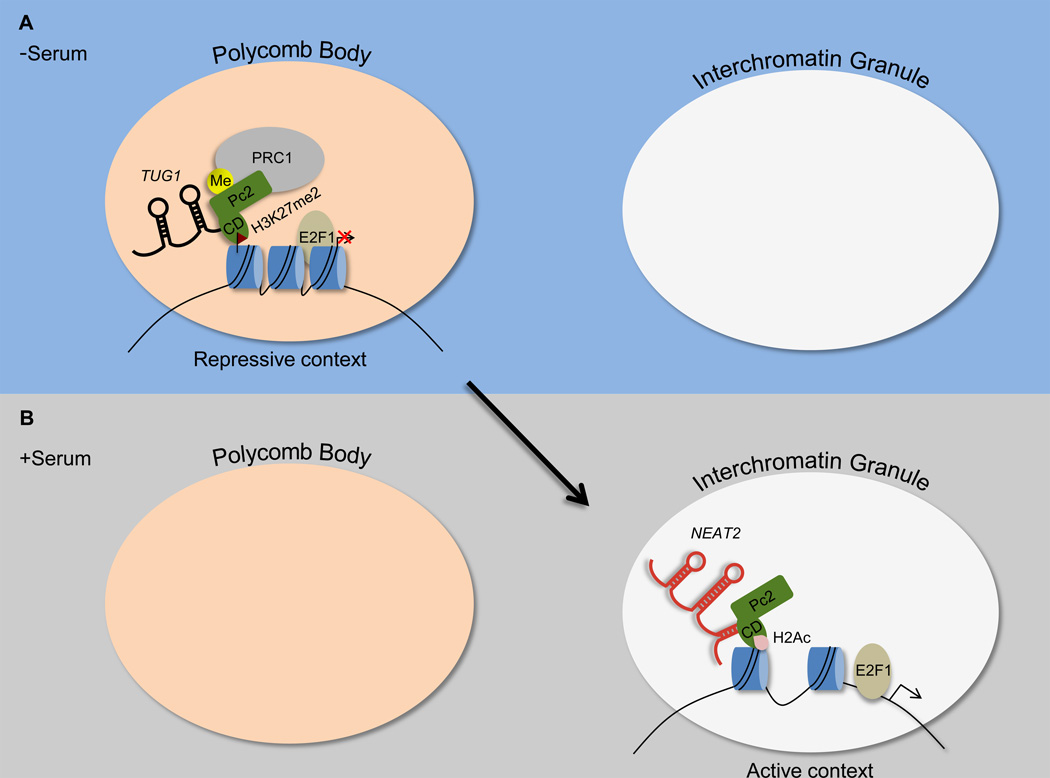
Two different Pc-associated ncRNAs can target genes to subnuclear compartments associated with either transcriptional activation or repression. Under serum starvation conditions, E2F1-regulated growth genes are rendered inactive and are associated with Pc2/CBX4 that is methylated on lysine 191 [16] (Figure 1). Furthermore, Pc2K191me2 interacts with the ncRNA TUG1, which is required for the localization of these genes to PcG bodies. Upon serum stimulation, Pc2 is demethylated and associates with the ncRNA NEAT2/MALAT1. These events are coincident with the relocalization of growth genes from the repressive PcG body to an interchromatin granule (ICG), which is associated with active transcription.
Figure 1. E2F1-responsive genes localize to a Polycomb body or interchromatin granule structure in the absence or presence of serum, respectively, dependent on the methylation status of Pc2.
(A) Under serum starvation conditions, Pc2 is methylated and interacts with TUG1 through its chromodomain (CD), which results in a higher affinity toward binding of H3K27me2. As a result, E2F1-responsive genes localize to a Polycomb body, which provides an environment to reinforce transcriptional repression.
(B) After serum stimulation, Pc2 is demethylated and interacts with NEAT2, which promotes association of the Pc2 CD with acetylated histone H2. As a result, E2F1-responsive genes localize to an interchromatin granule, which harbors a transcriptionally permissive environment.
Of key mechanistic importance, association of Pc2 with either TUG1 or NEAT2 affects the specificity of Pc2 interaction with modified histones. In vitro binding assays showed that the Pc2 chromodomain preferentially binds H3K9me3 in the absence of RNA; however, in the presence of TUG1, Pc2 binding specificity is altered toward modifications associated with repression such as H3K27me2. In contrast, addition of NEAT2 alters Pc2 specificity toward that of acetylated H2A peptides, which are associated with active transcription. Previous work showed that the chromodomain of another Pc homolog, CBX7, can bind the ANRIL cis-acting ncRNA simultaneously using an alternate face needed for H3K27me3 interaction [17]. However, at high concentrations the two interactions are competitive, suggesting feedback between the two domains. Since Pc2K191 methylation occurs outside of the chromodomain [16], it will be important to determine the potential structural crosstalk between these two domains. Collectively, these results provide new mechanistic insights into how PcG-associated ncRNAs can influence binding specificity as well as the spatial positioning of genes towards the appropriate transcriptional environment.
Chromatin insulators demarcate independent transcriptional domains
Chromatin insulators are multiprotein-DNA complexes capable of preventing inappropriate communication between adjacent cis-regulatory elements. For example, insulators can prevent the spread of PcG repression into an active region or block communication of an enhancer and promoter when placed in between the two elements (Reviewed in [18]). Insulators minimally consist of a DNA-binding protein specificity factor, as well as a module to mediate interactions between insulator complexes in order to promote long-distance chromatin looping. The conserved zinc finger containing CCCTC-binding factor (CTCF) is the only insulator protein thus far identified in mammals. In Drosophila, there exist a variety of insulator complexes, such as the CTCF/CP190 and the gypsy insulators, defined by CTCF and Suppresor of Hairy wing (Su(Hw)) proteins respectively. Both complexes include the Centrosomal Protein 190 kDa (CP190) protein. Like PcG complexes, Drosophila insulator complexes coalesce within the nucleus at sites termed insulator bodies. Evidence exists suggesting that these bodies consist of DNA-bound insulator complexes that may form higher order chromatin domains. Although mammalian CTCF does not localize to an equivalent structure, it has previously been suggested that tethering of CTCF to the nucleolus promotes insulator activity [19].
The conserved p68 RNA helicase influences insulator activity in Drosophila and mammals
The steroid receptor RNA activator (SRA) ncRNA was identified as the first RNA regulator of chromatin insulator activity. SRA is stably associated with the p68 DEAD-box RNA helicase, and together these factors promote CTCF insulator activity, not by affecting its targeting to DNA, but by stimulating recruitment of the cofactor cohesin at a variety of sites [20]. On a genome-wide level, binding of p68 overlaps extensively with CTCF, but it is not known if SRA is also present at these sites. An SRA counterpart does not exist in Drosophila; however, the p68 homolog Rm62 was previously reported to act as a negative regulator of gypsy insulator enhancer blocking activity and insulator body localization [21]. Interestingly, mutation of Rm62 does not affect CTCF/CP190 insulator activity [22], suggesting conservation of components yet divergence of mechanisms of insulator regulation.
Jpx ncRNA as an anti-scaffold for CTCF
In contrast, ncRNA can also negatively regulate CTCF activity, by competing with its ability to bind to DNA. In mammals, inactivation of one X chromosome in females requires expression of an ncRNA termed Xist, which coats the entire chromosome and recruits PcG to promote silencing [8]. Xist transcription is positively activated by Jpx, an ncRNA located 10 kb upstream of Xist that is transcribed in antisense orientation [23]. Recent work showed that during differentiation, Jpx expression results in loss of CTCF binding at the Xist promoter, permitting activation of the gene by an unknown mechanism [24]. CTCF and Jpx interact directly in vitro, and this association can compete with CTCF binding to Xist promoter DNA. Therefore, it was suggested that association of Jpx can titrate CTCF away from its chromatin binding site. Intriguingly, Jpx can induce Xist expression when overexpressed in trans from an autosomal transgene at high levels [23, 24], and CTCF association is reduced at the Xist promoter but not several other CTCF binding sites tested. Although the specificity of this disruptive effect should be explored in more detail, this result suggests that the higher sensitivity of CTCF to titration effects at this particular site results from some unique property of the Xist promoter, such as the presence of a specific factor or a specialized conformation.
Noncoding functions for mRNA in promoting gypsy insulator activity
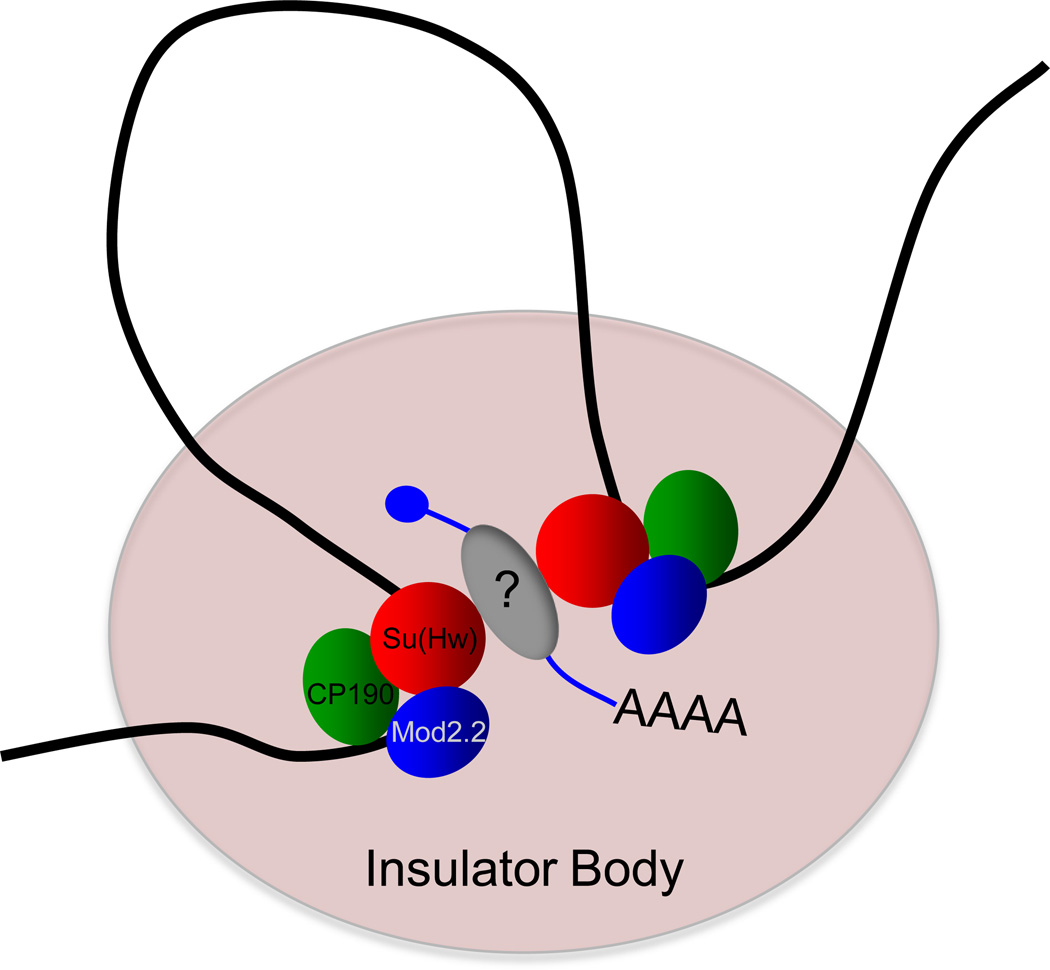
Recent work in Drosophila revealed that instead of ncRNA, certain mRNAs are stably associated with insulator complexes. Native tandem immunoaffinity purification of gypsy insulator complexes followed by high-throughput sequencing identified association of mature su(Hw) and Cp190 mRNAs [25]. In order to evaluate the biological importance of these associated transcripts, untranslatable versions of su(Hw) and Cp190 RNAs were overexpressed in vivo using T7 polymerase. Ectopic expression of these transcripts resulted in an increase of the number of insulator bodies per nucleus and improvement of enhancer blocking activity. Several additional mRNA transcripts coding for chromatin-associated proteins were also identified in these purifications, suggesting the possible generality of bifunctional coding/noncoding transcripts. The majority of these transcripts do not correspond to gypsy insulator binding sites, suggesting that these RNAs act in trans, perhaps to promote scaffolding of insulator complexes (Figure 2). An obvious question raised by this finding is whether separate pools of a given mRNA are either retained in the nucleus or exported to the cytoplasm for translation, or alternately, if each mRNA can serve both functions. Recent work identified a novel RNA-binding protein in negative regulation of gypsy insulator activity [26]; however, it is unknown what factor(s) mediate the interaction between these mRNAs and insulator complexes.
Figure 2. Model for mRNA acting as a scaffold for gypsy chromatin insulator complex formation.
Specific mRNAs associate with the gypsy insulator complex, comprised of Su(Hw), CP190, and Mod(mdg4)2.2, promoting insulator-insulator interaction and loop formation, possibly in the context of an insulator body. Interaction of the mRNA and insulator complex is mediated by an unknown RNA-binding protein.
Remodeling of ncRNAs involved in Drosophila dosage compensation
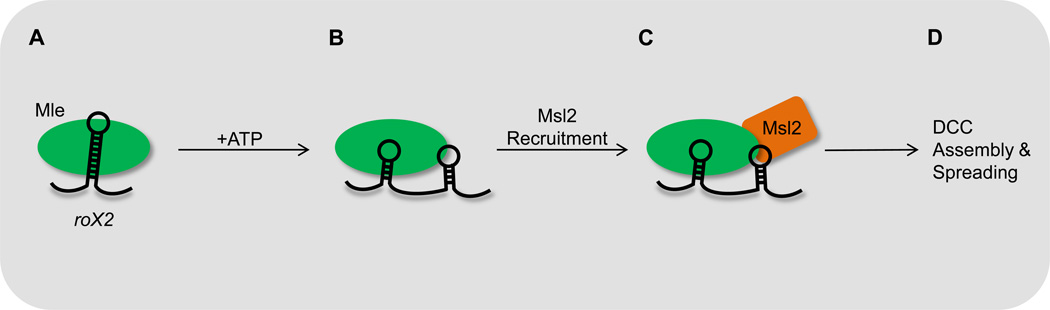
In Drosophila males, X chromosome dosage is normalized by upregulation of genes through specific recruitment of a transcription activation complex termed the dosage compensation complex (DCC). Transcribed from the X chromosome, the roX1 and roX2 noncoding RNAs serve redundant roles as scaffolds for DCC, despite little sequence similarity (Reviewed in [27]). In addition to the sites of roX transcription, DCC complex formation is nucleated at hundreds of additional chromatin entry sites along the X chromosome. A major advance in understanding DCC assembly came recently with the elucidation of secondary structures for several critical stem-loop structures in each RNA using chemical probing and nuclease sensitivity methods [28, 29]. Importantly, association of the Mle RNA helicase alters the conformation of the stem loop structure in an ATP-dependent manner, and this remodeling step stimulates the binding of the Msl2 protein, which triggers formation of the DCC (Figure 3). Incorporation of a maturation step at the site of action provides a potential mechanism by which a trans-acting ncRNA is prevented from acting before licensed to do so.
Figure 3. Remodeling of roX2 acts a structural switch to provide a platform for Msl2 recruitment and subsequent DCC assembly.
(A) The helicase Mle is recruited to the roX2 ncRNA.
(B) Mle helicase activity remodels roX2 conformation in an ATP-dependent manner.
(C) Remodeled roX2 serves as a platform for Msl2 recruitment.
(D) Recruitment of Msl2 triggers DCC assembly and spreading across the X chromosome.
Conclusions
Several key concepts have emerged based on the various examples of trans-acting chromatin-associated ncRNAs discussed here. Due to their length, long ncRNAs can harbor multiple binding sites for different protein complexes and act as a scaffold for their interaction or coordinated recruitment to a particular site. Next, the binding of an ncRNA with a protein can alter the specificity of interaction of the protein with other factors. Therefore, association of an ncRNA could act as a switch to trigger either assembly or disassembly of specific complexes. This capacity could also affect targeting of the complex to a specific location within the genome or nucleus. When this complex is associated with a particular locus, the ncRNA could thereby promote association of that locus to a subnuclear structure to enforce a specific transcriptional environment. Finally, to control the timing of its activity, the ncRNA itself can be regulated through a change in secondary structure by a helicase, which can affect its interaction specificity. This remodeling step would allow the ncRNA to diffuse from its site of transcription and function only at the appropriate location and context.
A major difficulty in studying long ncRNAs is that their structures are complex and difficult to predict based on sequence. As can be seen in recent DCC studies, detailed RNA analysis approaches are needed to elucidate secondary and eventually tertiary structures of these mysterious RNAs. Moreover, improvements to methods such as ChIRP will promote our understanding of ncRNA function on a genome-wide level, while application of single transcript in situ and single molecule tracking techniques (reviewed in [30]) should help elucidate the relationship between ncRNAs and specialized nuclear structures. Further insight into the mechanisms of ncRNA function in chromatin will certainly be obtained using a combination of these biochemistry, molecular biology, and cell biology approaches.
Acknowledgements
We would like to thank R. Dale, V. Sartorelli, and members of the Lei laboratory for comments on the manuscript. This work was funded by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
of special interest
of outstanding interest
- 1.Simon JA, Kingston RE. Occupying chromatin: Polycomb mechanisms for getting to genomic targets, stopping transcriptional traffic, and staying put. Mol. Cell. 2013;49:808–824. doi: 10.1016/j.molcel.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pirrotta V, Li H-B. A view of nuclear Polycomb bodies. Curr. Opin. Genet. Dev. 2012;22:101–109. doi: 10.1016/j.gde.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Morales DR, Thomas K, Presser A, Bernstein BE, Oudenaarden A van, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc. Natl. Acad. Sci. 2009;106:11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao J, Ohsumi TK, Kung JT, Ogawa Y, Grau DJ, Sarma K, Song JJ, Kingston RE, Borowsky M, Lee JT. Genome-wide identification of polycomb-associated RNAs by RIP-seq. Mol. Cell. 2010;40:939–953. doi: 10.1016/j.molcel.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guil S, Soler M, Portela A, Carrère J, Fonalleras E, Gómez A, Villanueva A, Esteller M. Intronic RNAs mediate EZH2 regulation of epigenetic targets. Nat. Struct. Mol. Biol. 2012;19:664–670. doi: 10.1038/nsmb.2315. [DOI] [PubMed] [Google Scholar]
- 6.Kaneko S, Son J, Shen SS, Reinberg D, Bonasio R. PRC2 binds active promoters and contacts nascent RNAs in embryonic stem cells. Nat. Struct. Mol. Biol. 2013 doi: 10.1038/nsmb.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davidovich C, Zheng L, Goodrich KJ, Cech TR. Promiscuous RNA binding by Polycomb repressive complex 2. Nat. Struct. Mol. Biol. 2013 doi: 10.1038/nsmb.2679. The authors performed a quantitative analysis of PRC2 affinity for RNA and found comparable affinities for target ncRNAs compared to irrelevant transcripts. By integrating data sets from ChIP-seq, RIP-seq and GRO-seq the authors suggest that such promiscuous binding could represent a way to track and silence nascent transcripts that have escaped repression.
- 8.Brockdorff N. Noncoding RNA and Polycomb recruitment. RNA. 2013;19:429–442. doi: 10.1261/rna.037598.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, et al. Functional Demarcation of Active and Silent Chromatin Domains in Human HOX Loci by Noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li L, Liu B, Wapinski OL, Tsai M-C, Qu K, Zhang J, Carlson JC, Lin M, Fang F, Gupta RA, et al. Targeted Disruption of Hotair Leads to Homeotic Transformation and Gene Derepression. Cell Rep. 2013;5:3–12. doi: 10.1016/j.celrep.2013.09.003. The authors generated targeted and conditional knockouts of the ncRNA HOTAIR in mouse. Knockout of HOTAIR derepresses HoxD genes and a substantial number of imprinted loci, resulting in homeotic transformation of the spine and malformation of the wrist. Loss of HOTAIR also results in an increase of H3K4 and, to a lower extent, a decrease of H3K27me3 at target loci.
- 11. Chu C, Qu K, Zhong FL, Artandi SE, Chang HY. Genomic Maps of Long Noncoding RNA Occupancy Reveal Principles of RNA-Chromatin Interactions. Mol. Cell. 2011;44:667–678. doi: 10.1016/j.molcel.2011.08.027. The authors developed the Chromatin Isolation by RNA Purification (ChIRP) assay to allow in vivo genome-wide mapping of ncRNA binding sites. The authors evaluate three different ncRNAs including HOTAIR and roX2.
- 12.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai M-C, Hung T, Argani P, Rinn JL, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simon MD, Wang CI, Kharchenko PV, West JA, Chapman BA, Alekseyenko AA, Borowsky ML, Kuroda MI, Kingston RE. The genomic binding sites of a noncoding RNA. Proc. Natl. Acad. Sci. U.S.A. 2011;108:20497–20502. doi: 10.1073/pnas.1113536108. The authors developed the Capture Hybridization Analysis of RNA Targets (CHART) assay to allow in vivo genome-wide mapping of ncRNA binding sites. The authors evaluate three different ncRNAs including roX2 and NEAT2/MALAT1.
- 14. Simon MD, Pinter SF, Fang R, Sarma K, Rutenberg-Schoenberg M, Bowman SK, Kesner BA, Maier VK, Kingston RE, Lee JT. High-resolution Xist binding maps reveal two-step spreading during X-chromosome inactivation. Nature. 2013 doi: 10.1038/nature12719. The authors used CHART-Seq to generate high-resolution maps of Xist RNA binding on the X chromosome throughout a developmental time course.
- 15.Tsai M-C, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long Noncoding RNA as Modular Scaffold of Histone Modification Complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang L, Lin C, Liu W, Zhang J, Ohgi KA, Grinstein JD, Dorrestein PC, Rosenfeld MG. ncRNA-and Pc2 Methylation-Dependent Gene Relocation between Nuclear Structures Mediates Gene Activation Programs. Cell. 2011;147:773–788. doi: 10.1016/j.cell.2011.08.054. The authors showed that methylation/demethylation of Pc2, a PcG protein associated with E2F1-bound growth-control genes, contributes to the localization of these genes to PcG bodies, in which their expression is repressed, and ICGs, in which their expression is activated. The differential methylation status of Pc2 licenses its ability to interact with two different ncRNAs, NEAT2 and TUG1, that in turn promote its localization to either PcG bodies or ICGs, respectively.
- 17. Yap KL, Li S, Muñoz-Cabello AM, Raguz S, Zeng L, Mujtaba S, Gil J, Walsh MJ, Zhou M-M. Molecular Interplay of the Noncoding RNA ANRIL and Methylated Histone H3 Lysine 27 by Polycomb CBX7 in Transcriptional Silencing of INK4a. Mol. Cell. 2010;38:662–674. doi: 10.1016/j.molcel.2010.03.021. The authors provide molecular and structural insight into how the Pc protein CBX7 interacts with H3K27me and the ncRNA ANRIL. Disruption of either interaction impairs the ability of CBX7 to maintain INK4b/ARF/INK4a silencing.
- 18.Matzat LH, Lei EP. Surviving an identity crisis: A revised view of chromatin insulators in the genomics era. Biochim. Biophys. Acta. 2013 doi: 10.1016/j.bbagrm.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yusufzai TM, Tagami H, Nakatani Y, Felsenfeld G. CTCF tethers an insulator to subnuclear sites, suggesting shared insulator mechanisms across species. Mol. Cell. 2004;13:291–298. doi: 10.1016/s1097-2765(04)00029-2. [DOI] [PubMed] [Google Scholar]
- 20.Yao H, Brick K, Evrard Y, Xiao T, Camerini-Otero RD, Felsenfeld G. Mediation of CTCF transcriptional insulation by DEAD-box RNA-binding protein p68 and steroid receptor RNA activator SRA. Genes Dev. 2010;24:2543–2555. doi: 10.1101/gad.1967810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei EP, Corces VG. RNA interference machinery influences the nuclear organization of a chromatin insulator. Nat. Genet. 2006;38:936–941. doi: 10.1038/ng1850. [DOI] [PubMed] [Google Scholar]
- 22.Moshkovich N, Nisha P, Boyle PJ, Thompson BA, Dale RK, Lei EP. RNAi-independent role for Argonaute2 in CTCF/CP190 chromatin insulator function. Genes Dev. 2011;25:1686–1701. doi: 10.1101/gad.16651211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian D, Sun S, Lee JT. The Long Noncoding RNA, Jpx, Is a Molecular Switch for X Chromosome Inactivation. Cell. 2010;143:390–403. doi: 10.1016/j.cell.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sun S, Del Rosario BC, Szanto A, Ogawa Y, Jeon Y, Lee JT. Jpx RNA Activates Xist by Evicting CTCF. Cell. 2013;153:1537–1551. doi: 10.1016/j.cell.2013.05.028. The authors showed that during the onset of X chromosome inactivation, the ncRNA Jpx interacts directly with CTCF. As a consequence, Jpx titrates away CTCF from binding the Xist promoter thus allowing Xist transcription activation.
- 25. Matzat LH, Dale RK, Lei EP. Messenger RNA is a functional component of a chromatin insulator complex. EMBO Rep. 2013;14:916–922. doi: 10.1038/embor.2013.118. The authors showed that certain mRNAs are functional components of a chromatin insulator complex. By expressing untranslatable versions, the authors found that these transcripts are able to modulate enhancer-blocking activity and insulator body localization, suggesting a noncoding function.
- 26.Matzat LH, Dale RK, Moshkovich N, Lei EP. Tissue-specific regulation of chromatin insulator function. PLoS Genet. 2012;8:e1003069. doi: 10.1371/journal.pgen.1003069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conrad T, Akhtar A. Dosage compensation in Drosophila melanogaster: epigenetic fine-tuning of chromosome-wide transcription. Nat. Rev. Genet. 2011;13:123–134. doi: 10.1038/nrg3124. [DOI] [PubMed] [Google Scholar]
- 28. Maenner S, Müller M, Fröhlich J, Langer D, Becker PB. ATP-dependent roX RNA remodeling by the helicase maleless enables specific association of MSL proteins. Mol. Cell. 2013;51:174–184. doi: 10.1016/j.molcel.2013.06.011. The authors demonstrated that the helicase Mle remodels the structure of the ncRNA roX2 using biochemical and enzymatic approaches. This change in roX2 conformation stimulates binding of the Msl2 protein, which in turn promotes DCC assembly.
- 29. Ilik IA, Quinn JJ, Georgiev P, Tavares-Cadete F, Maticzka D, Toscano S, Wan Y, Spitale RC, Luscombe N, Backofen R, et al. Tandem stem-loops in roX RNAs act together to mediate X chromosome dosage compensation in Drosophila. Mol. Cell. 2013;51:156–173. doi: 10.1016/j.molcel.2013.07.001. The authors performed UV crosslinking and immunoprecipitation (iCLIP) and found that the major RNA targets of Mle and Msl2 in vivo are roX1 and roX2. The interaction between roX1 and roX2 with Mle and Msl2 takes place at discrete domains rich in repetitive tandem stem-loops. Furthermore, SHAPE and PARS were used to analyze secondary structures of roX1 and roX2. Using transgenic rescue constructs, the authors found that these stem-loops are functionally redundant.
- 30.Armitage BA. Imaging of RNA in live cells. Curr. Opin. Chem. Biol. 2011;15:806–812. doi: 10.1016/j.cbpa.2011.10.006. [DOI] [PubMed] [Google Scholar]