Abstract
A deficiency of mitochondrial glutathione reductase (or GR2) is capable of adversely affecting the reduction of GSSG and increasing mitochondrial oxidative stress. BCNU [1, 3-bis (2-chloroethyl)-1-nitrosourea] is an anticancer agent and known inhibitor of cytosolic GR ex vivo and in vivo. Here we tested the hypothesis that a BCNU-induced GR2 defect contributes to mitochondrial dysfunction and subsequent impairment of heart function. Intraperitoneal administration of BCNU (40 mg/kg) specifically inhibited GR2 activity by 79.8±2.7% in the mitochondria of rat heart. However, BCNU treatment modestly enhanced the activities of mitochondrial Complex I and other ETC components. The cardiac function of BCNU-treated rats was analyzed by echocardiography, revealing a systolic dysfunction associated with decreased ejection fraction, decreased cardiac output, and an increase in left ventricular internal dimension and left ventricular volume in systole. The respiratory control index of isolated mitochondria from the myocardium was moderately decreased after BCNU treatment, whereas NADH-linked uncoupling of oxygen consumption was significantly enhanced. Extracellular flux analysis to measure the fatty acid oxidation of myocytes indicated a 20% enhancement after BCNU treatment. When the mitochondria were immunoblotted with antibodies against GSH and UCP3, both protein S-glutathionylation of Complex I and expression of UCP3 were significantly up-regulated. Overexpression of SOD2 in the myocardium significantly reversed BCNU-induced GR2 inhibition and mitochondrial impairment. In conclusion, BCNU-mediated cardiotoxicity is characterized by the GR2 deficiency that negatively regulates heart function by impairing mitochondrial integrity, increasing oxidative stress with Complex I S-glutathionylation, and enhancing uncoupling of mitochondrial respiration.
Keywords: glutathione reductase, systolic dysfunction, mitochondria, oxidative stress, S-glutathionylation and Complex I
1. Introduction
Mitochondria are the major source of reactive oxygen species (ROS) in mammalian cells; the generation of •O2− and its derived oxidants acts as a redox signal in triggering the cellular events of apoptosis and necrosis in myocytes. The mitochondrial redox pool is enriched in glutathione (GSH) with a high physiological concentration (in the mM range) (1); overproduction of •O2− and •O2−-derived oxidants increases the ratio of GSSG (oxidized glutathione) to GSH, which has been hypothesized to be the major mechanism in mediating S-glutathionylation of mitochondrial Complex I (2–4).
In the animal disease model of myocardial infarction, S-glutathionylation of Complex I is enhanced in the post-ischemic heart due to an oxidative stress-induced elevated GSSG/GSH ratio and depletion of GSH in mitochondria (5). In vitro studies using isolated mitochondria also indicate that increasing Complex I S-glutathionylation is favored by the conditions of oxidative stress that raise GSSG/GSH, such as exposure to organic peroxide (6), excessive NO (6), or the thiol oxidant diamide (4). Protein S-glutathionylation of isolated Complex I can be induced by GSSG via thiol-disulfide exchange (2–4). Therefore, a marked decline in the mitochondrial GSH/GSSG ratio has emerged as the physiological setting for S-glutathionylation of Complex I in vivo.
Mitochondrial glutathione reductase (or the GR2 isoform) is a key antioxidant enzyme that recycles GSSG to GSH in an NADPH-dependent catalysis. GR2 thus regulates the mitochondrial redox status via modulation of the ratio of GSSG/GSH and protein S-glutathionylation in mitochondria. Because of this key role, we rationally hypothesize that a GR2 deficiency would affect mitochondrial function and subsequently heart function. Inhibition or ablation of GR2 activity should facilitate the major pathway of enhancement of protein S-glutathionylation mediated by GSSG or a high GSSG/GSH ratio in vivo.
BCNU (1, 3-bischloroethyl-l-nitrosourea, generic name: carmustine) has been employed as the pharmacologic inhibitor of GR in the rat or mouse model. BCNU is an anti-cancer (antineoplastic) chemotherapy drug used to treat certain types of brain tumors (Glioblastoma multiforme) and other cancers (7). The antitumor activity of BCNU originates from its function as a dialkylating agent that is capable of forming interstrand crosslinks in DNA. BCNU decomposes in situ to generate chloroethylisocyanate, an alkylating moiety that interacts with DNA, as well as a more reactive carbamyolating moiety associated with the inactivation of cellular GR (8–11). The choroethylisocyanate functions as an exogenous electrophile, attacking the susceptible cysteine thiol (Cys63) of the GR active site via carbamoylation, rendering the enzyme unable to catalyze the reduction of GSSG (11). GR inhibition with the loss of GSH indirectly reduces the peroxide-removing ability of glutathione peroxidase, leading to accumulation of H2O2, potentially augmenting cellular oxidative stress. In preclinical studies, gene therapy with AdMnSOD (or AdSOD2) has been combined with BCNU treatment to reduce tumor growth (12, 13).
It is well known that clinical use of anticancer agents (e.g., doxorubicin) is limited by a specific, cumulative, and dose-dependent cardiotoxicity, in which the toxicity is caused by impairment of mitochondrial function. Although BCNU shows effectiveness in glioblastoma multiforme chemotherapy, there is a paucity of investigations directed toward understanding the mechanism of its cardiotoxicity, the impact on post-translational S-glutathionylation, and the mitochondrial function in myocardium. Determination of the BCNU-induced pathway controlling oxidative stress and consequent Complex I S-glutathionylation is important because of the implications for cardiotoxicity in cardiovascular disease, and to understand the pathophysiological settings of mitochondrial redox. Studies were performed first in a rat model by pharmacologic inhibition of GR2 with BCNU to gain new insights into the effect on cardiac function, mitochondrial function, and S-glutathionylation of Complex I in vivo. Studies were then undertaken in HL-1 cardiac myocytes, and the effect of S-glutathionylation on Complex I was confirmed using the isolated enzyme. Finally, we validated the hypothesis of oxidative stress induced by BCNU in an SOD2 transgenic mouse animal model. The results indicate that overexpression of SOD2 in mitochondria neutralizes the deleterious effect of BCNU on the enzymatic function of GR2.
2. Materials and Methods
2.1. Animals
Male Sprague-Dawley rats (3 to 4 mo, 350 – 400 g) were purchased from Harlan (Indianapolis, IN), and the SOD2-tg mice were obtained from the Jackson Laboratory. All procedures were performed with the approval (protocol no. 12-031) of the Institutional Animal Care and Use Committee (IACUC) at Northeast Ohio Medical University (Rootstown, OH) and conformed to the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the NIH.
2.2. Reagents
BCNU, Glutathione (GSH), ammonium sulfate, diethylenetriaminepentaacetic acid (DTPA), ubiquinone-1 (Q1), sodium cholate, deoxycholic acid, rotenone, PEG-SOD (polyethylene glycol-linked superoxide dismutase), and β-nicotinamide adenine dinucleotide (reduced form, NADH) were purchased from Sigma Chemical Company (St. Louis, MO) and used as received. The anti-GSH monoclonal antibody was purchased from ViroGen (Watertown, MA). The anti-SOD2 and anti-GR polyclonal antibodies were from Santa Cruz Biotechnology, Inc. (Dallas, TX). The DMPO spin trap was purchased from Dojindo Molecular Technologies, Inc. (Rockville, MD), and stored under nitrogen at −80 °C until needed.
2.3. Analytical Methods
Optical spectra were measured on a Shimadzu 2401 UV/VIS recording spectrophotometer. The protein concentrations of mitochondrial preparations were determined by the Lowry method using BSA as a standard. The concentrations of Q1 and Q2 were determined by absorbance spectra from NaBH4 reduction using a millimolar extinction coefficient ε(275nm–290nm) = 12.25 mM−1cm−1 (14). The electron transfer activities of Complexes I–IV from the heart mitochondrial preparations were assayed by published method (15). The enzymatic activity of GR in mitochondria was assayed by measuring GSSG-mediated NADPH consumption with the absorbance decreasing at 340 nm at 25 °C. An appropriate amount of mitochondrial preparation (permeabilized by alamethicin) was added to the assay mixture (1 ml) containing 50 mM phosphate buffer (pH 7.5), 1mM EDTA, 1 mM GSSG, and 0.1 mM NADPH.
2.4. Measurement of Oxygen Consumption Rate (OCR) of Mitochondrial Preparations
Mitochondria were prepared from rat hearts by differential centrifugation described in the published method (16). Mitochondria were precipitated by centrifugation at 20,000 ×g for 10 min in the final step and resuspended in media (M-buffer) containing the following agents (in mM): mannitol (230), sucrose (70), EDTA (1), Trizma (1), pH 7.4. Mitochondrial respiration was measured by the polarographic method using a Clark-type oxygen electrode (Oxytherm, Hansatech Instruments, Norfolk, England) at 30 °C and reported in nmol/min. The NADH-linked respiration buffer containing malate plus glutamate included (in mM): potassium glutamate (140), NaCl (10), MgCl2 (1), EGTA (1), malate (5), Trizma (1), phosphate (2.5), and cytochrome c (0.01), and was adjusted to pH 7.4. Mitochondrial preparations were added to the respiration buffer for a final concentration of 0.5 mg/ml. OCRs were measured as follows: state 2, OCR of mitochondrial preparations with malate/glutamate; state 3, OCR stimulated by ADP (0.2 mM); state 4, OCR after the addition of oligomycin (2 μg/mL) following ADP addition; uncoupling respiration, OCR after the addition of FCCP (2.5 μM). The oxygen electrode was calibrated at 1 atm by assuming the concentration of O2 in the respiration buffer at 30 °C to be 250 μM.
2.5. Measurement of Mitochondrial •O2− Production by EPR Spin Trapping
Electron Paramagnetic Resonance (EPR) measurements were carried out on a Bruker EMX Micro spectrometer operating at 9.43 GHz with 100 kHz modulation frequency at room temperature. The reaction mixture containing the NADH-linked respiration buffer supplemented with DTPA (1 mM)/DMPO (90 mM) was mixed with a mitochondrial preparation (to a final concentration of 0.5 mg protein/mL) at 30 °C for 4 min. The reaction mixture was then transferred to a 50-μL capillary tube (Drummond Wiretrol, Broomall, PA), sealed, loaded into the EPR resonator (HS cavity, Bruker Instrument, Billerica, MA), equilibrated to 298 K, and tuned within 2 min. The scan of EPR spectra was started at exactly 6 min after the initial reaction. Parameters: center field 3360 G, sweep width 100 G, power 20 mW, receiver gain 1 × 105, modulation amplitude 1 G, conversion time 40.96 ms, time constant 163.84 ms, number of scans: 5. The spectral simulations were performed using the WinSim program developed at NIEHS by Duling (17).
2.6. Immunoblotting Analysis
The reaction mixture was mixed with Laemmli sample buffer at a ratio of 4:1 (v/v), incubated at 70 °C for 10 min, and then immediately loaded onto a 4–12 % Bis-Tris polyacrylamide gradient gel. Samples were run at room temperature for 55 min at 190 V. Protein bands were electrophoretically transferred to a nitrocellulose membrane in 25 mM Bis-Tris, 25 mM Bicine, 0.029% (w/v) EDTA, and 10% methanol. Membranes were blocked for 1 h at room temperature (R.T.) in Tris-buffered saline (TBS) containing 0.1% Tween-20 (TTBS) and 5% dry milk (BioRad, Hercules, CA). The blots were then incubated overnight with anti-GSH monoclonal antibody at 4 °C. Blots were then washed 3 times in TTBS, and incubated for 1 h with horseradish peroxidase-conjugated anti-mouse IgG in TTBS at R.T. The blots were again washed twice in TTBS and twice in TBS, and then visualized using ECL Western Blotting Detection Reagents (GE Healthcare Life Sciences, Fairfield, CT).
2.7. Cell Culture and Fluorescence Microscopy
Murine myocytes (HL-1 cell line) were grown and maintained in Claycomb medium (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum, 0.01% penicillin/streptomycin antibiotic, 2 mM glutamine, and 100 μM norepinephrine in 35 mm polystyrene tissue culture flasks at 37°C in the presence of 5% CO2 as described (18). Confluent cells with > 60% viability were used to conduct fluorescence imaging of ROS production and immunofluorescent staining with anti-GSH or Ab51 or Ab75 antibodies as described in a previous publication (19).
HL-1 myocytes were then cultured on sterile coverslips in 35-mm dishes and subjected to BCNU (25–80 μM) treatment for 4 h at 37 °C. Cells were then incubated with the ROS indicator MitoSOX™ (5 μM, Invitrogen Corporation, Carlsbad, CA) to detect ROS in living cells. MitoSOX™ emits red fluorescence (Ex/Em 510/580 nm) when oxidized by ROS. Nuclei were stained with DAPI (2 μM; Ex/Em 358/461).
2.8. Measurement of OCR of Living Myocytes
OCRs were measured by a Seahorse Bioscience extracellular flux analyzer (model XF24, North Billerica, MA) and presented in pmol/min. HL-1 myocytes were seeded at a density of 3 × 104 cells per well on the fibronectin pre-coated XF24 V7 cell culture microplate overnight. After BCNU treatment (25 μM, in supplemented Claycomb medium for 4 hrs), myocytes were equilibrated with DMEM containing glucose (10 mM) and sodium pyruvate (0.2 mM), lacking bicarbonate at 37°C without CO2 for 1 hr. Oligomycin (2 μg/mL), FCCP (0.3 μM), and antimycin A (10 μM) were sequentially injected into the microplate for the readout of ATP-linked OCR and the calculation of reserved capacity. Fatty acid oxidation (FAO) assay was conducted following the manufacturer’s protocol. In short, myocytes were equilibrated in Krebs Henseleit Buffer (KHB) containing 2.5 mM glucose at 37°C after BCNU treatment. BSA-palmitate (100 – 300 μM palmitate) was then injected for the FAO assay.
2.9. Preparations of Mitochondrial Complex I
Bovine heart mitochondrial Complex I was prepared under non-reducing conditions according to the published method by Hatefi et al. with minor modifications (20) detailed in previous publications (2, 5). Preparation of Complex I contains 4.2–4.5 nmol ubiquinone-10 and 0.2 mg of phospholipid per mg protein. The contamination by Complex III in this preparation is less than 0.5% (~0.05–0.08 nmol of heme b plus heme c1 per mg of Complex I preparation), and exhibits adequate activity to generate •O2− (determined by EPR spin-trapping with 5-(Diethoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide, DEPMPO) under the conditions of enzyme turnover (2, 5).
2.10. Data Analysis
All data were reported as group averages. Statistical analysis was performed using Origin 9.0 data analysis software. Results were presented as mean ± SEM. Comparisons between two groups (the data sets of Figs. 1, 2, 3C, 7B, and 7C) were assessed by student’s t-test to analyze the significance of differences. Comparisons among multiple groups (the data set of three groups in Fig. 4 and the data set of four groups in Fig. 7D) were assessed by one-way ANOVA followed by Tukey’s post hoc tests. For the extracellular flux analysis in measuring the effect of BCNU treatment on the fatty acid oxidation rate (Fig. 2D), raw data among five experimental groups, including control HL-1 (without palmitate), BCNU-treated HL-1 (without palmitate), HL-1 plus palmitate, BCNU-treated HL-1 plus palmitate, and KHB medium (as the background control), were analyzed with one-way ANOVA followed by Tukey’s post-hoc test to confirm significant differences among groups. Two groups of HL-1 cells plus palmitate and BCNU-treated HL-1 cells plus palmitate were then subjected to background and control corrections, and comparison was further analyzed by student’s t-test. A probability value of p < 0.05 was used to establish statistical significance.
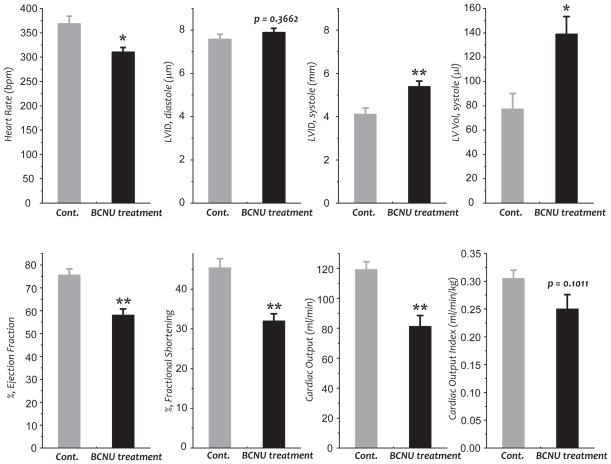
Fig. 1. Echocardiographic assessment of control (Cont.) and BCNU-treated rats.
Two-dimensional echocardiography was performed and calculations were performed offline by double-blinded reviewers using the Vevo 770/3.0 system and software (VisualSonics). (n=5 per group, *p<0.05, and **p<0.01). LVID, left ventricle internal diameter.
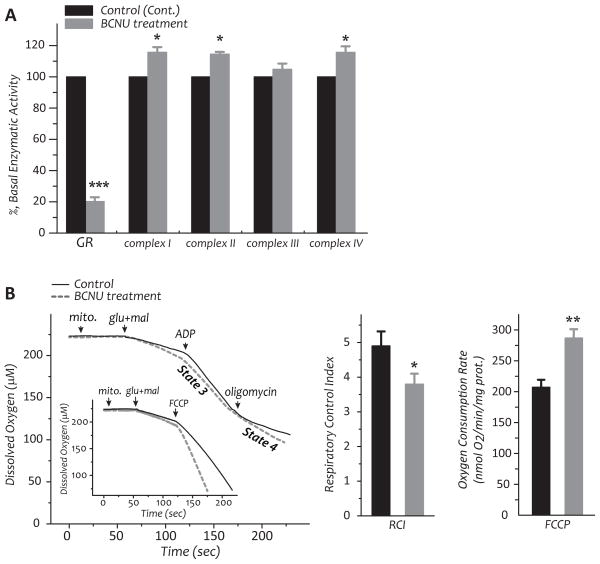
Fig. 2.
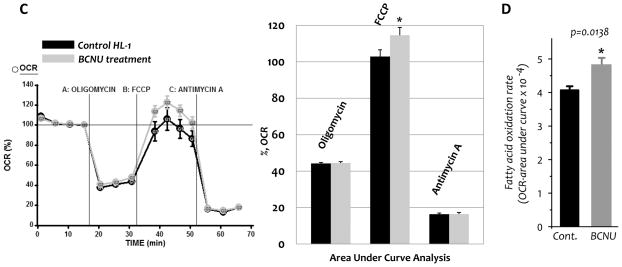
A–B, Rats were intraperitoneally injected with BCNU (10 mg/kg body weight/day) for 4 days. Rats with intraperitoneal injection of saline were used as controls. Hearts were removed and subjected to mitochondrial preparation. A, enzymatic activities of the electron transport chain and GR2 in the myocardial mitochondria of control and BCNU-treated rats. B, Measurement of oxygen consumption by mitochondria using oxygen paragraph at 30 °C (left), RCI (middle), and FCCP-mediated oxygen consumption rate (left inset and right) of mitochondrial preparations. C, The chart of extracellular flux analysis showing representative measurements of the percentage increase in OCRs and relative rates in control and BCNU-treated HL-1 myocytes in response to glucose (10 mM) and pyruvate (0.2 mM) as the substrates. D, The effect of BCNU (25 μM) on the fatty acid oxidation rate of HL-1 myocytes was measured by extracellular flux analyzer using bovine serum albumin-conjugated palmitate (0.3 mM) as the substrate (data of chart not shown). Raw data of five experimental groups as described in the Material and Methods (section 2.10) were analyzed with one-way ANOVA followed by Tukey’s post-hoc test, indicating a significant difference between any two of the five groups (p<0.001). Comparison of two groups from control HL-1 cells plus palmitate and BCNU-treated HL-1 cells plus palmitate was further subjected to control and background correction, and further analyzed with student’s t-test. *p<0.05, **p<0.01, and ***p<0.001.
Fig. 3.
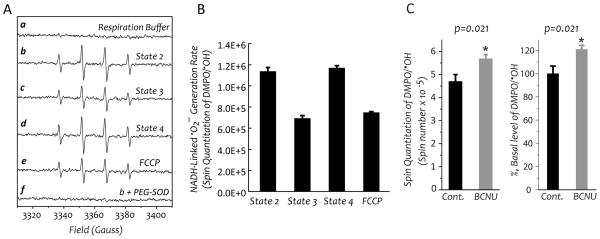
Mitochondria were prepared from the myocardium excised from the heart of control rat (in A, B, and C) and BCNU-treat rat (in C), and then subjected to measurement of oxygen consumption rates and •O2− generation. A and B, •O2− generation mediated by mitochondria in the presence of glutamate and malate (NADH-linked) was assessed by EPR spin trapping with DMPO according to the published method (19). A is the EPR spectra of SOD-sensitive DMPO/•OH adduct under the conditions of state 2 (b), state 3 (c), state 4 (d), and chemical uncoupling with FCCP (1 μM, e). B is the quantitative analysis of DMPO/•OH adducts produced under the conditions of various respiratory states in A. The spin quantitation for each spectrum was obtained by double integration of the simulation spectrum as described in a previous publication (19). C, mitochondria mediated •O2− generation of the control and BCNU-treated rat under the conditions of state 3 respiration.
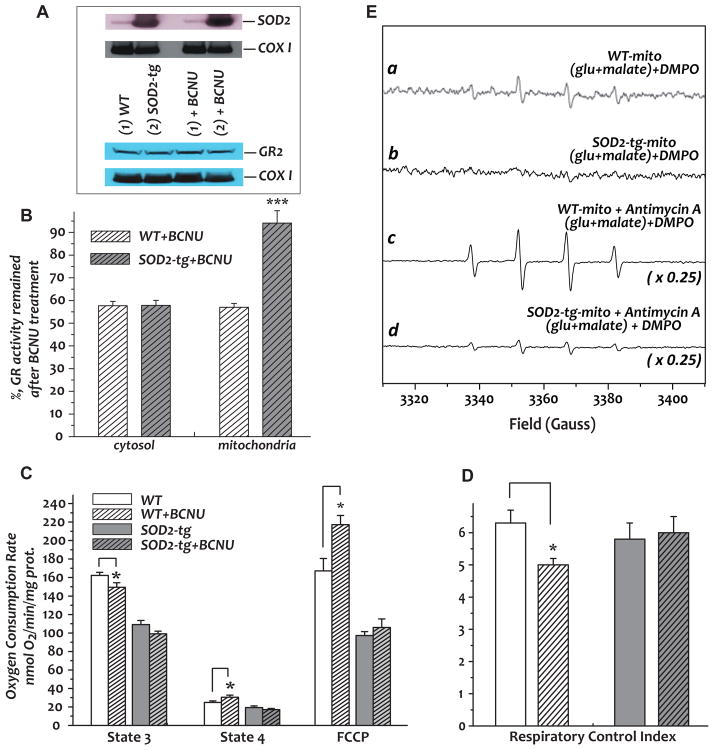
Fig. 7. Effect of BCNU treatment on the GR and mitochondrial function from SOD2-tg mice and wild type (WT) littermates.
Mice were subjected to Intraperitoneal injection of BCNU (20 mg/kg/day) for 4 days. Fractions of mitochondria and cytosol were isolated from the hearts. A, Immunoblotting analysis of mitochondria using anti-SOD2 (upper panel, titer used: 1 mg/ml, 4,000X) and anti-GR (lower panel, titer used: 1 mg/ml, 1,000X) polyclonal antibodies. B, Analysis of GR activities from the cytosolic and mitochondrial fractions. C, State 3, state 4, and FCCP-mediated OCRs of isolated mitochondria from SOD2-tg and wild type mice. D, Respiratory control index obtained from the ratio of state 3 and state 4 OCRs. E, a and b: •O2− generation mediated by mitochondria isolated from murine hearts of BCNU-treated wild type and SOD2-tg mice; c and d: the same as a and b, except that antimycin A (10 μM) was included in the system. *p<0.05, assessed by student’s t-test between WT control and BCNU-treated WT or SOD2-tg control and BCNU-treated SOD2-tg in B, C, and D. Comparison among four groups (WT, BCNU-treated WT, SOD2-tg, and BCNU-treated SOD2-tg) in D was analyzed by one-way ANOVA followed by Tukey’s post-hoc test, indicating there is no significant difference among the four groups (p = 0.16222).
Fig. 4.
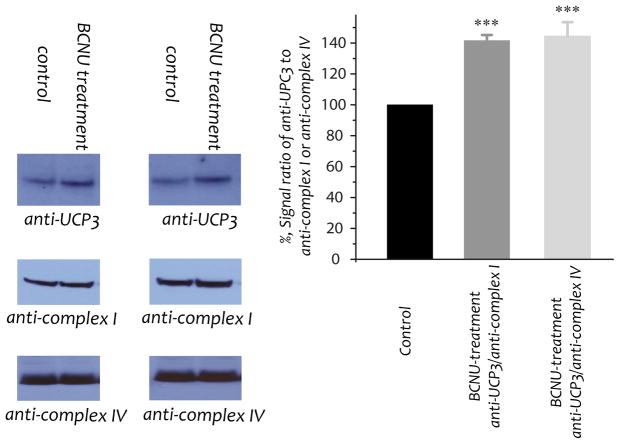
Protein expression of UCP3 in the myocardial mitochondria of control and BCNU-treated rats (n=6). Mitochondrial preparations (100 μg) were immunoblotted with anti-UCP3 antibodies (titer used: 1 mg/ml, 250X). Protein expression levels of the 51 kDa (nuclear encoded, titer used: 1 mg/ml, 6,000X) subunit of Complex I and subunit I (mitochondrial genome encoded) of Complex IV in the mitochondria were used as the loading controls. Signals were normalized to Complex I [probed with anti-complex I [(i.e., Ab51, titer used: 1 mg/ml, 2000X (5))] and Complex IV [probed with anti-complex IV (Invitrogen), titer used: 1 mg/ml, 4,000X] respectively. One-way ANOVA followed by Tukey’s post hoc analysis indicates a significant difference between the control group and the group of UCP/anti-complex I or UCP/anti-complex IV, but no difference between the UCP/anti-complex I and UCP/anti-complex IV groups. ***p<0.001.
3. RESULTS
3.1. BCNU-induced GR deficiency in the myocardium results in systolic dysfunction and mitochondrial dysfunction
To test the hypothesis that BCNU-induces cardiotoxicity through uncoupling mitochondrial function, studies were performed to explore the role of BCNU-induced GR2 defects in cardiac function and mitochondrial function. Rats were subjected to intraperitoneal injection of BCNU (10 mg/kg/day) for 4 consecutive days (21, 22), and myocardial function was then analyzed by echocardiography. As indicated in Fig. 1, BCNU treatment significantly decreased the parameters of heart rate, ejection fraction (decreased from 75.6±2.7% to 58.0±1.8%, n=5), fractional shortening, and cardiac output (decreased from 119.3±5.2 to 81.2±7 ml/min, n=5), indicating impairment of cardiac function and cardiotoxicity from the chemotherapeutic agent (23, 24). Furthermore, rat hearts exhibited a significant increase in left ventricular internal diameter (LVID, increased from 4.1±0.3 to 5.4±0.3 mm, n=5) and left ventricular volume at systole (increased from 77.4± 12.8 to 139.1±14.3 μl, n=5), but no significant difference in LVID at diastole, suggesting that a systolic dysfunction was induced by BCNU treatment. In support of this result, a chronic effect of decreased systolic pressure on rat cardiac function has been reported by a single higher dosage treatment in vivo (20 mg BCNU/kg) (25).
Mitochondria were then isolated from rat hearts, and subjected to an assay of enzymatic activities. The GR2 activity declined to 20.2±2.7% (n=11, p<0.001, Fig. 2A) after BCNU treatment, consistent with the hypothesis that BCNU induces mitochondrial GR inactivation in vivo. However, the enzymatic activity of selenium-containing glutathione peroxidase (GPx) was slightly up-regulated to 112.6±4.4 % (n=11). We have further detected a marginal enhancement in the enzymatic activities of Complex I (15.6% enhancement in Fig. 2A) and other ETC complexes (5%–17% enhancement in Fig. 2A). There are no significant alterations in protein expression of the mitochondrial GR and ETC components(data not shown).
Fig. 2B (the first chart on left side) shows a typical profile of oxygen consumption in mitochondria from the myocardium of control and BCNU-treated rats. State 3 (ADP-dependent) and state 4 (ADP-independent) respiration of mitochondrial preparations was measured by oxygraph. After BCNU treatment, state 3 OCR was slightly decreased from 204.9±15.26 to 191.3±22.9 (in nmol O2/min/mg protein, n=5), whereas state 4 OCR was marginally increased from 42.8±6.7 to 50.7±6.2 (n=5). Therefore, the respiratory control index (RCI, a ratio of state 3/state 4) significantly declined from 4.9±0.4 to 3.8±0.3 after BCNU treatment (n=5, p<0.05, Fig. 2B). To access the maximal ETC activity of mitochondria, FCCP was added to uncouple the mitochondria and maximize the respiratory capacity and OCR. We observed that FCCP uncoupling of O2 consumption rate was significantly enhanced from 220.3±11.7 to 297.4±13.6 (n=5, p<0.01, Fig. 2B and inset) after BCNU treatment, supporting the data showing marginal enhancement of Complex I and other ETC activities (in Fig. 2B).
The above results were further confirmed with isolated myocytes from the HL-1 cell line (18) using an extracellular flux analyzer (Seahorse Bioscience, Billerica, MA). Incubation of BCNU (25 μM) with HL-1 cells at 37°C for 4 h resulted in GR inhibition in both cytosol (23.3±5.4% activity remaining, n=3) and mitochondria (11.3±0.9% activity remaining, n=3). An increase in the FCCP uncoupling reserve bioenergetic capacity in myocytes occurred as a result of BCNU treatment, shown by extracellular flux analysis in Fig. 2C. Extracellular flux analysis also indicated the extracellular acidification rate (ECAR, an indication of lactate production) by HL-1 cells was not affected by BCNU treatment, thus eliminating the possible effect of glycolysis. Further extracellular flux analysis using palmitate as a substrate showed a significant enhancement of the fatty acid oxidation rate in BCNU-treated HL-1 cells (p < 0.05, Fig. 2D).
3.2 The •O2− mediated by mitochondria
The production of •O2− mediated by isolated mitochondria at different respiratory states was induced by glutamate/malate (NADH-linked), and measured by EPR spin-trapping with DMPO (26). An SOD-dependent four-line spectrum of DMPO/•OH was detected, indicating that •O2− generation was mediated by the mitochondria under the conditions of state 2 respiration (Fig. 3A, b and f). Addition of ADP (state 3 respiration) diminished mitochondria-mediated •O2− generation by ~40%, a significant decrease that indicates that coupling of enhanced O2 consumption with oxidative phosphorylation for ATP synthesis decreased the e− leakage to molecular oxygen (Fig. 3A, c; and Fig. 3B). In the presence of oligomycin A (state 4 respiration), mitochondria-mediated •O2− generation induced by glutamate/malate was restored to the level of state 2 respiration. The •O2− generation under the conditions of state 4 respiration was not affected by the addition of ADP due to an inhibitory effect of oligomycin on F1F0-ATPase (Fig. 3A, d; and Fig. 3B). The proton gradient (ΔpH) and electrochemical gradient (Δp) was gradually restored under the conditions of state 4 respiration. However, the addition of FCCP (1 μM) to dissipate Δp and ΔpH significantly decreased the •O2− production to the level of state 3 conditions (Fig. 3A, e; and Fig. 3B). These results support the concept that increasing electrochemical gradients and proton back pressure drive a portion of the e− leakage for •O2− production.
The effect of BCNU treatment on mitochondria-mediated •O2− generation was determined by EPR spin trapping with DMPO under the conditions of state 3 respiration. In the presence of ADP and glutamate/malate, the level of SOD-dependent DMPO/•OH adducts mediated by the mitochondria isolated from BCNU-treated rat hearts was marginally increased to 121±3.4% (n=6, p=0.021, Fig. 3C), due to increased electron leakage for •O2− generation caused by impaired mitochondrial integrity after BCNU treatment (Fig. 2B).
3.3. A BCNU-induced GR2 deficiency results in up-regulation of UCP3 in mitochondria from the myocardium
Uncoupling proteins (UCPs) are mitochondrial transporters of the inner membrane; they function to mediate proton conductance and control the level of respiration coupling. UCP3 is mainly expressed in skeletal muscles. It has been reported that •O2− activates mitochondrial UCPs (27), and the physiological function of UCP3 has been linked to attenuated ROS production. The levels of UCP3 in our mitochondrial preparations were assessed by Western blot using a polyclonal antibody against UCP3 (Santa Cruz biotechnology, San Diego, CA). The 51 kDa FMN-binding protein of Complex I (nuclear-encoded, anti-complex I in Fig. 4) and subunit I of Complex IV (mitochondrial DNA-encoded, anti-complex IV Ab in Fig. 4) were used as the internal protein loading controls. As a result of a BCNU-induced GR2 deficiency, the expression of UCP3 in heart mitochondria was increased to 141.5±3.7% (based on Complex I) and 144.3±9.2% (based on Complex IV) as shown in Fig. 4. The results support the data of increased FCCP-mediated uncoupling OCR by the mitochondria isolated from BCNU-treat rat (in Fig. 2B).
3.4. Redox alterations in the GSH pool and S-glutathionylation of Complex I
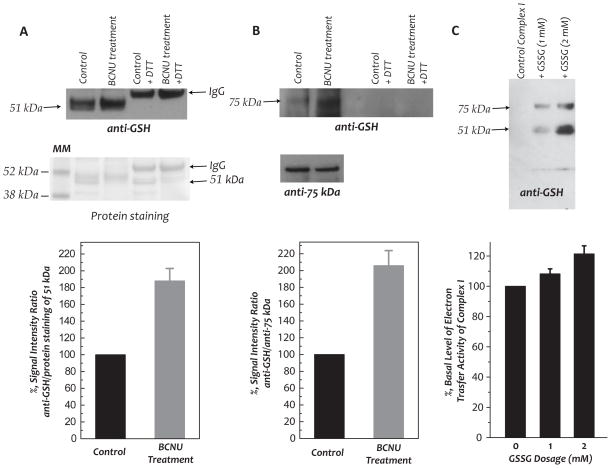
The BCNU-induced GR deficiency resulted in significantly enhanced oxidation of GSH to GSSG in both the cytosol and mitochondrial fractions. We determined GSH and GSSG levels quantitatively by the enzymatic recycling method, observing (i) an elevation of GSSG levels in both the cytosol (increased from 0.4±0.06 nmol/mg protein to 0.78±0.11 nmol/mg protein, p<0.05 by student’s t-test, n=3) and the mitochondrial preparation (increased from 0.27±0.02 to 0.36±0.03 nmol/mg protein, p<0.05 by student’s t-test, n=3), (ii) a slight depression in the GSH level of the mitochondria (from 4.74±0.25 to 4.53±0.03 or 100% to 95.7±3.1%), and (iii) a significant decrease in the ratio of GSH/GSSG in mitochondria after BCNU treatment (18.0 vs 12.6). These results were basically consistent with a modest increase in the oxidative stress in the myocardium and mitochondria after BCNU treatment. A depression in the GSH/GSSG ratio was expected to increase the status of S-glutathionylation of the Complex I. To test this hypothesis, we used polyclonal antibodies against Complex I, Ab51 and Ab75 (5, 28), to immunoprecipitate the 51 kDa- and 75 kDa- subunits of Complex I from mitochondrial preparations, followed by immunoblotting with an anti-GSH monoclonal antibody. As indicated in Fig. 5, the detected Complex I-derived S-glutathionylation on the 51 kDa and 75 kDa subunits of Complex I was enhanced to 188±14.3% and 206±14.7% respectively, after BCNU treatment.
Fig. 5.
In vivo protein S-glutathionylation of the 51 kDa and 75 kDa subunits of Complex I in myocardial mitochondria, the effect of BCNU-induced GR2 inhibition (A–B), and in vitro S-glutathionylation of Complex I by GSSG (C). Mitochondria preparation (1 mg protein used per IP) was subjected to immunoprecipitation (IP) with Ab51 and Ab75 (Ab against 51 kDa and 75 kDa in (5, 28)) respectively, and subsequently subjected to SDS-PAGE and immunoblotted with anti-GSH monoclonal Ab (upper panel, titer used: 1 mg/ml, 500X). In A, the nitrocellulose membrane was stained with Ponceau S to quantitate the protein of the 51 kDa subunit. In B, blottings on the membrane were probed with Ab75 to quantitate the protein of the 75 kDa subunit. The density ratios of the signals were quantitated by the software NIH Image J (n=3). C, In vitro S-glutathionylation of isolated complex I was induced by incubating enzyme preparation (2.8 mg/ml) in PBS with GSSG (0–2 mM) at 25 °C for 1 h, and confirmed with immunoblotting with anti-GSH antibody. The reaction mixture was then subjected to dialysis against PBS at 4 °C to remove excess GSSG. The protein concentration of dialysate containing complex I or S-glutathionylated complex I was determined by Lowry method prior to enzymatic activity assay.
3.5. Effect of S-glutathionylation on the electron transfer activity of Complex I in vitro
Protein S-glutathionylation has been implicated as a mechanism to regulate the functions of the protein (29, 30). Therefore, significant changes in the electron transfer activity of Complex I is expected due to site-specific S-glutathionylation at 51 kDa and 75 kDa subunits. S-glutathionylation of isolated Complex I was induced with different dosage of GSSG (1 mM and 2 mM), and confirmed by Western blotting using anti-GSH monoclonal antibody (Fig. 5C, upper panel). The S-glutathionylated Complex I was further subjected to analysis of the electron transfer activity. The result indicated S-glutathionylation moderately enhanced the electron transfer activity of Complex I (Fig. 5C, lower panel). The results basically support the data from in vivo S-glutathionylation of Complex I as induced by BCNU.
3.6. BCNU Induces oxidative stress and S-glutathionylation of Complex I in the myocytes of HL-1 cells
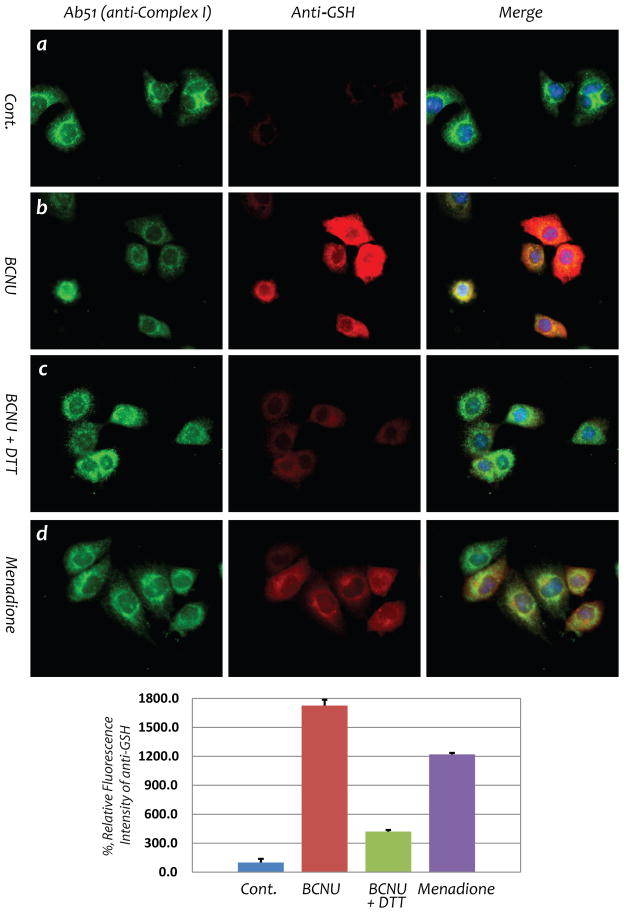
Further experiments were performed to determine whether protein S-glutathionylation of Complex I occurs in myocytes following BCNU treatment using the HL-1 cell line. Cellular S-glutathionylation of Complex I as induced by menadione was employed as a positive control (Fig. 6, d) (19). In HL-1 cells treated with BCNU (25 μM for 4 hrs), fluorescence microscopy with an anti-GSH monoclonal antibody showed a marked increase in cellular S-glutathionylation that subsequently decreased upon the addition of DTT (75% reduction at a dosage of 1 mM DTT) as shown in Fig. 6 (b and c). Fluorescence microscopy with a polyclonal antibody against the 51 kDa subunit (Ab51) further demonstrated a marked increase in cellular S-glutathionylation that co-localized with Complex I.
Fig. 6. BCNU enhances S-glutathionylation of Complex I 51 kDa subunit from HL-1 myocyte.
Immunofluorescent detection of S-glutathionylation in HL-1 using an anti-GSH monoclonal antibody was carried out according to the published method (19). Images a and b are control HL-1 myocytes with or without BCNU (25 μM) treatment, demonstrating drastic enhancement of cellular S-glutathionylation by BCNU. Image c is HL-1 treated with BCNU in the presence of DTT (1 mM) in culture, confirming reversible S-glutathionylation induced by BCNU. Image d is the positive control showing increased cellular S-glutathionylation from HL-1 under oxidative stress induced by menadione (40 μM) (19).
3.7. Overexpression of SOD2 in the myocardium prevents the mitochondria from undergoing BCNU-induced GR inhibition and oxidative impairment
GR inhibition was further tested in a model of cardiac-specific SOD2 transgenic mice (SOD2-tg, strain: FVB-Tg (Myh6-SOD2, Tyr)3Pne/J, from Jackson Labs). The SOD2-tg hemizygotes exhibit a phenotype that is viable, fertile, and normal in size. Transgenic expression of SOD2 is specific to the heart, and localized to the mitochondria. Western analysis indicated enhanced SOD2 expression in the mitochondria of murine hearts up to 7-fold (Fig. 7A, upper panel, with subunit I of Complex IV (COX I) as the loading control, n=6). Intraperitoneal injection of BCNU (20 mg/kg/day) for 4 days (total dosage is 80 mg/kg) resulted in GR inhibition in both the cytosol (42.6±1.7% inhibition, n=7) and mitochondria (42.2±1.5% inhibition, n=7) from the myocardium of the wild type control mice, and in the cytosol from the SOD2-tg mice (43.1±2.1% inhibition, n=7), as indicated in Fig. 7B. However, the BCNU-induced GR inhibition was significantly lower in the mitochondrial fraction of the myocardium from SOD2-tg mice (93.2±5.2% GR activity remaining or 6±5.2% inhibition, n=7, Fig. 7B). No significant alteration was seen in the GR expression in mitochondria (indicated as GR2 in the lower panel of Fig. 7A) or cytosol (data not shown) after BCNU treatment. As measured by echocardiography, the hearts of SOD2-tg exhibited enhanced and supernormal cardiac function with an increased ejection fraction (89.3%±1.6% in SOD2-tg versus 74.1%±1.6% in wild type littermate). Furthermore, the cardiac function of SOD2-tg mice was protected from BCNU treatment in which no significant change was observed in the ejection fraction 89.3%±1.6% versus 86.7±2.4%), whereas the cardiac function of wild type mice was significantly impaired by BCNU treatment (74.1%±1.6% versus 63.5%±3.0%, n=4, p<0.05 by student’s t-test).
State 3 and State 4 respiration and the respiratory control index (RCI) were also measured (Fig. 7C–7D), showing that BCNU treatment significantly impaired the mitochondrial integrity of wild type littermates (RCI, 6.3±0.4 vs 5.0±0.2, n=8, p<0.05, Fig. 7D), but had no effect on the mitochondria of SOD2-tg mice (RCI, 5.7±0.5 vs 6.0±0.5, n=8, p=0.76, Fig. 7D). Furthermore, BCNU treatment enhanced FCCP uncoupling of O2 consumption in the mitochondria of wild type littermates, but had no significant effect on the mitochondria of SOD2-tg mice (Fig. 7C, n=8). Therefore, overexpression of SOD2 in the myocardium protected mitochondrial GR from BCNU-induced inhibition, subsequently preventing mitochondria from suffering oxidative impairment induced by BCNU treatment. EPR spin trapping analysis further showed no detectable •O2− generation by the mitochondria isolated from BCNU-treated SOD2-tg mice (Fig. 7E, lower panel, b vs a) and significantly lower sensitivity to antimycin A treatment (Fig. 7E, lower panel, d vs c).
3.8. BCNU increases oxygen-free radical generation in cardiac HL-1 myocytes
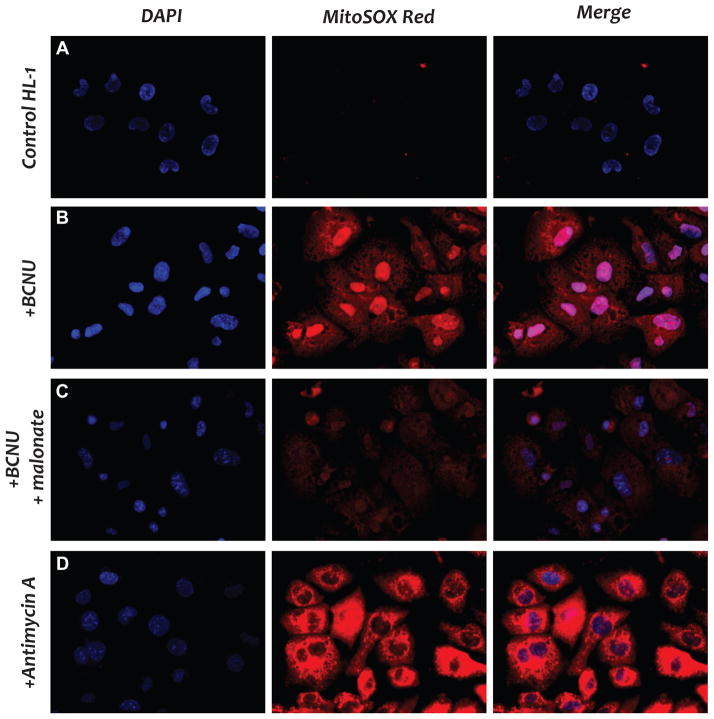
The hypothesis of BCNU-induced oxidative stress was further demonstrated in living cells. We used fluorescent microscopy with MitoSOX to detect ROS in the mitochondria of HL-1 myocytes, and malonate was employed to confirm the state of FADH2-linked ROS generation. HL-1 cells were treated with antimycin A as a positive control experiment (Fig. 8D). When the HL-1 cells were treated with BCNU, a strong oxidized MitoSOX red fluorescence was detected (Fig. 8B), indicating that BCNU stimulated ROS production from the HL-1 mitochondria. Treatment of HL-1 cells with malonate, which suppressed the FADH2 formation of Complex II, dramatically inhibited the red fluorescence of oxidized MitoSOX (Fig. 8C), thus confirming that BCNU stimulated ROS that were mediated by the Complexes II and III of mitochondria.
Fig. 8. Imaging of ROS generation induced by BCNU in HL-1 myocytes using MitoSOX probe.
A, fluorescent microscopic measurement of ROS from the culture of HL-1 without treatment. The assay was conducted as described previously (33). B, same as A, except that HL-1 was incubated with BCNU (25 μM) at 37 °C for 4 h. C, same as B, except that the competitive inhibitor of Complex II, malonate (1 mM), was included in the cell culture. D, same as A, except that HL-1 myocytes were incubated with antimycin A (10 μM) for 10 min as the positive control.
4. DISCUSSION
4.1. BCNU mediates in vivo S-glutathionylation of Complex I
This study explains how the redox signaling pathway of thiol-sulfide exchange mediates S-glutathionylation of mitochondrial Complex I, which has never been demonstrated in vivo. In addition, we demonstrate how BCNU activation underlies this redox pathway. The use of BCNU via intraperitoneal injection has been proven to be an effective way to inhibit mitochondrial GR in vivo in the rat (heart). However, a higher dosage is required to inhibit the GR activity of mouse liver (31) or mouse heart (this study) in vivo, which is likely due to a higher basal metabolic rate in mouse. We expected that BCNU-induced GR2 inhibition would result in oxidative stress, leading to increased S-glutathionylation of Complex I through the mechanism of thiol-disulfide exchange in vivo, thereby altering mitochondrial function, and impairing myocardial function as it did. Because in vitro S-glutathionylation of Complex I, mediated by higher GSSG/GSH ratio or low dosage of GSSG (1–2 mM in the Fig. 5C) via thiol-disulfide exchange reaction can modestly enhance its electron transfer activity, we can explain the marginal increase in the enzymatic activity of Complex I of the mitochondria isolated from BCNU-treated rats (Fig. 2A) as being due to enhanced protein S-glutathionylation induced by GR2 deficiency (Fig. 5). Reversal of S-glutathionylation with thiol reductants normalized Complex I activity to the basal level (data not shown), suggesting increasing formation of Complex I S-glutathionylation in vivo by the BCNU was not likely involved in the oxidative damage to the enzyme. In vitro S-glutathionylation of Complex I with a low dosage of GSSG also leads to a decrease in the electron leakage (2). However, in vitro S-glutathionylation of Complex I with a very high dosage of GSSG (up to 20 mM) can impair Complex I activity and enhance •O2− production (6), but this was not likely to occur under the conditions of the in vivo studies. Therefore, an increase in Complex I S-glutathionylation in vivo by BCNU is likely an important mechanism in preventing oxidative damage to the enzyme, which can include forming the thiyl radicals or sulfenic acids on the reactive cysteines of Complex I (4, 29, 30). That is, in vivo S-gluathionylation of Complex I should play a minor role in •O2− production by mitochondria. Similar results have been observed in the S-glutathionylation of Complex II during myocardial ischemia and reperfusion injury (32). Analysis of OCR further confirmed these results, showing higher NADH-linked FCCP-mediated OCR and FAO-mediated OCR in the mitochondria of BCNU-treated rats and BCNU-treated myocytes, respectively (Fig. 2B–2D).
4.2. BCNU mediates cardiac mitochondrial ROS generation, oxidative injury, and consequent systolic dysfunction
The impaired mitochondrial integrity from BCNU-induced GR2 deficiency in vivo is due to a decreased state 3 OCR and an increased state 4 OCR. The consequence of these processes likely mediated a decreased oxidative phosphorylation in state 3 respiration, and modest enhancement of mitochondria-mediated •O2− generation and oxidative stress (Fig. 3C) due to an elevated mitochondrial uncoupling in vivo. This increased oxidative stress was further confirmed by (i) the protective effect of SOD2 overexpression in the SOD2-tg mouse model (Fig. 7), and (ii) the MitoSOX staining of BCNU-treated HL-1 myocytes showing increased ROS generation that could be inhibited by malonate through blocking the FADH2-linked electron flow of the mitochondrial ETC (33) as indicated in Fig. 8. The result strongly implicated a major role of Complex III and a minor role of S-glutathionylated Complex I in the mitochondrial ROS production induced by BCNU treatment.
Oxidative stress-induced injury of mitochondrial function is involved in the pathogenesis of myocardial infarction, heart failure, and other cardiovascular disease. The heart requires a constant supply of energy in order to support contractile activity. Excess mitochondrial ROS production from the BCNU-induced GR2 defects led to detrimental effects on myocytes through mitochondrial dysfunction, leading to impairment of excitation-contraction coupling and contributing to systolic dysfunction (Fig. 1). The clinical use of anticancer agents in chemotherapy is limited by a specific, cumulative, dose-dependent cardiotoxicity. Impairment of mitochondrial function and increased ROS production plays a major role in this toxicity. Therefore, BCNU-induced systolic dysfunction of the rat heart was linked to mitochondrial dysfunction mediated by BCNU treatment and GR2 inhibition. Clinically, BCNU infusion was reported to induce myocardial ischemia (34). The incidence and mechanism of such an adverse effect is likely caused by increasing both uncoupling oxygen consumption (Fig. 2B) and •O2− generation (Fig. 3C), as demonstrated in this investigation. Vascular injury is likely the other factor involved in the mechanism. As reported previously, BCNU has been demonstrated to induce GR inhibition ex vivo, leading to S-glutathionylation of eNOS, and impairing endothelium-dependent vessel relaxation (35).
The mitochondrial DNA (mtDNA) encodes 13 subunits of the ETC complexes and F1F0-ATPase. It is worth noting that possible alkylation of mtDNA by chloroethyl isocyanate metabolites should not be ruled out in BCNU-induced mitochondrial dysfunction. Alkylation of mtDNA can block DNA replication, mRNA transcription, and protein translation, resulting in mitochondrial dysfunction. However, current studies have concluded no significant changes in the ETC protein expression after BCNU treatment, suggesting oxidant stress caused by GR2 inhibition was more efficient than mtDNA alkylation to induce mitochondrial dysfunction and the subsequent phenotype of systolic dysfunction.
4.3. BCNU mediates UCP3 up-regulation to increase electron transport activity
Up-regulation of UCP3 in the mitochondria could represent the other novel finding of the current study. Together with an enhancement of Complex I S-glutathionylation, UCP3 up-regulation functions as an antioxidant defense in response to oxidative stress induced by GR2 defect in vivo. Our results indicated increased physiological uncoupling as induced by BCNU treatment (Fig. 4), which might directly or indirectly result in an increase in state 4 respiration and moderate depreciation of the RCI of mitochondria (Fig. 2B). The physiological role of UCP3 has been linked to the regulation of fatty acid metabolism (36, 37). Thus, increased UCP3 expression may up-regulate FAO-mediated OCR in BCNU-treated myocytes (Fig. 2C), allowing continuous reoxidation of coenzymes that are essential to the metabolic pathways in BCNU-treated myocytes. Our experimental data clearly supports this conclusion. Because ROS and elevated oxidative stress have been shown to activate UCP3 (27, 38), we thus surmise that enhanced mitochondrial ROS production from BCNU treatment increased proton conductance through up-regulation of UCP3, providing a negative feedback loop to limit further mitochondrial ROS production. In essence, the proton pumps of the electron transport chain respond to uncoupling with increased proton pumping and electron flow to maintain the electrochemical gradient. We can postulate that BCNU treatment increased proton conductance through UCP3-mediated uncoupling of oxidative phosphorylation in mitochondria, leading to increased electron transport activity and subsequent oxygen consumption rate.
4.4. Conclusion
Taken together, the present studies provide insights regarding the molecular mechanism of systolic dysfunction resulting from pharmacological inhibition of GR2 by BCNU in vivo. The underlying mechanism was characterized by GR2 inhibition, increased associated S-glutathionylation of Complex I, increased mitochondrial oxidative stress, mitochondrial dysfunction, up-regulation of UCP3, and increased uncoupling OCR as illustrated in the diagram of Fig. 9. Pharmacological treatment with BCNU inhibits GR2 activity in mitochondria, which would favor a redox environment of a higher GSSG/GSH ratio and S-glutathionylation of Complex I via the mechanism of thiol-disulfide exchange (2, 19). A dramatic reduction of GR2 activity potentially increases oxidative stress in mitochondria. Elevation of oxidative stress triggers the impairment of mitochondrial integrity and decreased ATP synthesis, conditions that would favor increased electron leakage for excessive •O2 − production by Complex III (NADH-linked in this study). The S-glutathionylated Complex I likely plays a minor role in the •O2− production in vivo. Increased oxidative stress also triggers up-regulation of UCP3, which dissipates the electrochemical gradient (Δp) and enhances electron transport activity and O2 consumption rate.
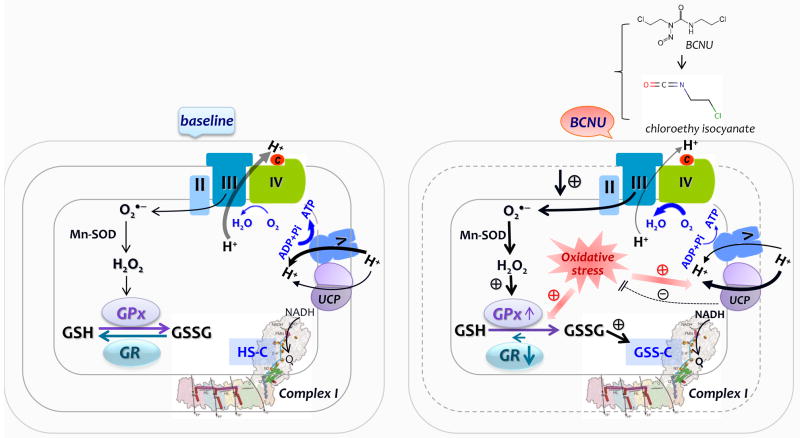
Fig. 9. Diagram showing the effect of BCNU treatment on the mitochondrial function of the rat heart.
In comparison to the baseline conditions, BCNU treatment induces GR inhibition via chloroethyl isocyanate that was decomposed from the BCNU in vivo, which would decrease recycling of GSSG, leading to elevation of mitochondrial oxidative stress, which might be negatively regulated by modest enhancement of GPx activity. Increased oxidative stress impairs the coupling of electron transport with oxidative phosphorylation for ATP synthesis, which would further increase electron leakage for ROS production mainly from the Complex III (denoted by III). Inhibition of GR also facilitates S-glutathionylation of Complex I (denoted by I) via thiol-disulfide exchange, which moderately enhances Complex I-mediated electron transport activity from NADH to ubiquinone (Q). Increased ROS production activates UCP up-regulation, which contributes to decreased ATP synthesis by Complex V (or F0F1ATPase, denoted by V), elevated uncoupling oxygen consumption rate by the ETC, and a negative feedback to limit further ROS production.
The mechanism addressed here provides a useful concept for understanding the fundamental mechanism of how mitochondrial dysfunction and related thiol redox alteration mediate BCNU-induced cardiotoxicity. Briefly, 1) mitochondria use the redox thiols of the GSH pool and Complex I to address the situation of oxidative stress mediated by BCNU-induced GR2 defect, and 2) mitochondria modulate the conditions under which BCNU promotes ROS generation— through up-regulation of uncoupling. Recognition of this mechanism is valuable in understanding the fundamental basis of chemotherapy-induced cardiotoxicity.
Acknowledgments
This work was supported by National Institutes of Health Grant HL83237 (Y-RC). We thank Dr. Denise M. Inman (Department of Pharmaceutical Sciences, Northeast Ohio Medical University) for critical review of the manuscript.
Abbreviations
- GR
glutathione reductase
- NQR
NADH ubiquinone reductase, or mitochondrial Complex I
- •O2−
superoxide anion radical
- ETC
electron transport chain
- OCR
oxygen consumption rate
- RCI
respiratory control index
- Q1
ubiquinone-1
- DMPO
5,5-dimethyl pyrroline N-oxide
- FMN
flavin mononucleotide
- BCNU
1,3-bischloroethyl-l-nitrosourea
- SOD-2 or MnSOD
manganese-containing superoxide dismutase
- PEG-SOD
polyethylene glycol-linked superoxide dismutase
- FCCP
carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone
- GSH
glutathione
- Ab
antibody
- SDS-PAGE
SDS polyacrylamide gel electrophoresis
- EPR
electron paramagnetic resonance
Footnotes
Conflicts of Interest Statement
The authors declare that there are no conflicts of interest.
References
- 1.Costa NJ, Dahm CC, Hurrell F, Taylor ER, Murphy MP. Interactions of mitochondrial thiols with nitric oxide. Antioxid Redox Signal. 2003;5:291–305. doi: 10.1089/152308603322110878. [DOI] [PubMed] [Google Scholar]
- 2.Chen CL, Zhang L, Yeh A, Chen CA, Green-Church KB, Zweier JL, Chen YR. Site-specific S-glutathiolation of mitochondrial NADH ubiquinone reductase. Biochemistry. 2007;46:5754–5765. doi: 10.1021/bi602580c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beer SM, Taylor ER, Brown SE, Dahm CC, Costa NJ, Runswick MJ, Murphy MP. Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant DEFENSE. J Biol Chem. 2004;279:47939–47951. doi: 10.1074/jbc.M408011200. [DOI] [PubMed] [Google Scholar]
- 4.Hurd TR, Requejo R, Filipovska A, Brown S, Prime TA, Robinson AJ, Fearnley IM, Murphy MP. Complex I within oxidatively stressed bovine heart mitochondria is glutathionylated on Cys-531 and Cys-704 of the 75-kDa subunit: potential role of CYS residues in decreasing oxidative damage. J Biol Chem. 2008;283:24801–24815. doi: 10.1074/jbc.M803432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Chen C, Rawale S, Chen C, Zweier J, Kaumaya P, Chen Y. Peptide-based antibodies against glutathione-binding domains suppress superoxide production mediated by mitochondrial complex I. J Biol Chem. 2010;285:3168–3180. doi: 10.1074/jbc.M109.056846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor ER, Hurrell F, Shannon RJ, Lin TK, Hirst J, Murphy MP. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J Biol Chem. 2003;278:19603–19610. doi: 10.1074/jbc.M209359200. [DOI] [PubMed] [Google Scholar]
- 7.Lea M. Effects of carbamoylating agents on tumor metabolism. Crit Rev Oncol Hematol. 1987;7:329–371. doi: 10.1016/s1040-8428(87)80004-5. [DOI] [PubMed] [Google Scholar]
- 8.Becker K, Schirmer R. 1,3-Bis(2-chloroethyl)-1-nitrosourea as thiol-carbamoylating agent in biological systems. Methods Enzymol. 1995;251:173–188. doi: 10.1016/0076-6879(95)51120-2. [DOI] [PubMed] [Google Scholar]
- 9.Babson J, Reed D. Inactivation of glutathione reductase by 2-chloroethyl nitrosourea-derived isocyanates. Biochem Biophys Res Commun. 1978;83:754–762. doi: 10.1016/0006-291x(78)91053-7. [DOI] [PubMed] [Google Scholar]
- 10.Arscott LD, Gromer S, Schirmer RH, Becker K, Williams CH., Jr The mechanism of thioredoxin reductase from human placenta is similar to the mechanisms of lipoamide dehydrogenase and glutathione reductase and is distinct from the mechanism of thioredoxin reductase from Escherichia coli. Proc Natl Acad Sci U S A. 1997;94:3621–3626. doi: 10.1073/pnas.94.8.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rice KP, Penketh PG, Shyam K, Sartorelli AC. Differential inhibition of cellular glutathione reductase activity by isocyanates generated from the antitumor prodrugs Cloretazine™ and BCNU. Biochemical Pharmacology. 2005;69:1463–1472. doi: 10.1016/j.bcp.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Sun WG, Weydert CJ, Zhang Y, Yu L, Liu J, Spitz DR, Cullen JJ, Oberley LW. Superoxide Enhances the Antitumor Combination of AdMnSOD Plus BCNU in Breast Cancer. Cancers (Basel) 2010;2:68–87. doi: 10.3390/cancers2010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Darby Weydert CJ, Smith BB, Xu L, Kregel KC, Ritchie JM, Davis CS, Oberley LW. Inhibition of oral cancer cell growth by adenovirusMnSOD plus BCNU treatment. Free Radic Biol Med. 2003;34:316–329. doi: 10.1016/s0891-5849(02)01245-5. [DOI] [PubMed] [Google Scholar]
- 14.Redfearn ER, Whittaker PA. The determination of the oxidation-reduction states of ubiquinone (coenzyme Q) in rat-liver mitochondria. Biochim Biophys Acta. 1966;118:413–418. doi: 10.1016/s0926-6593(66)80050-4. [DOI] [PubMed] [Google Scholar]
- 15.Yeh S, Lee H, Aune S, Chen C, Chen Y, Angelos M. Preservation of mitochondrial function with cardiopulmonary resuscitation in prolonged cardiac arrest in rats. J Mol Cell Cardiol. 2009;47:789–797. doi: 10.1016/j.yjmcc.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H, Chen C, Yeh S, Zweier J, Chen Y. Biphasic modulation of the mitochondrial electron transport chain in myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2012;302:H1410–1422. doi: 10.1152/ajpheart.00731.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duling DR. Simulation of multiple isotropic spin-trap EPR spectra. J Magn Reson B. 1994;104:105–110. doi: 10.1006/jmrb.1994.1062. [DOI] [PubMed] [Google Scholar]
- 18.White S, Constantin P, Claycomb W. Cardiac physiology at the cellular level: use of cultured HL-1 cardiomyocytes for studies of cardiac muscle cell structure and function. Am J Physiol Heart Circ Physiol. 2004;286:H823–829. doi: 10.1152/ajpheart.00986.2003. [DOI] [PubMed] [Google Scholar]
- 19.Kang P, Zhang L, Chen C, Chen J, Green K, Chen Y. Protein thiyl radical mediates S-glutathionylation of complex I. Free Radical in Biology and Medicine. 2012;53:962–973. doi: 10.1016/j.freeradbiomed.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galante YM, Hatefi Y. Purification and molecular and enzymic properties of mitochondrial NADH dehydrogenase. Arch Biochem Biophys. 1979;192:559–568. doi: 10.1016/0003-9861(79)90126-7. [DOI] [PubMed] [Google Scholar]
- 21.Jacot W, Gerlotto-Borne MC, Thezenas S, Pouderoux S, Poujol S, About M, Romieu G. Carmustine and methotrexate in combination after whole brain radiation therapy in breast cancer patients presenting with brain metastases: a retrospective study. BMC Cancer. 2010;10:257. doi: 10.1186/1471-2407-1110-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenblum ML, Dougherty DA, Wilson CB. Rational planning of brain tumour therapy based on laboratory investigations: comparison of single- and multiple-dose BCNU schedules. Br J Cancer Suppl. 1980;4:253–254. [PMC free article] [PubMed] [Google Scholar]
- 23.Pai V, Nahata M. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000;22:263–302. doi: 10.2165/00002018-200022040-00002. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan G, Chaudhary V, Al-Janadi A, Ramanarayanan J, D’Silva K. BCNU toxicity presenting with a large pericardial and pleural effusion. Ann Transplant. 2008;13:44–47. [PubMed] [Google Scholar]
- 25.Rawson C, Larson R. Cardiovascular performance in anesthetized rats pretreated with 1,3-bis(2-chloroethyl)-1-Nitrosourea (BCNU) Res Commun Chem Pathol Pharmacol. 1989;64:407–419. [PubMed] [Google Scholar]
- 26.Chen YR, Zweier JL. Cardiac mitochondria and reactive oxygen species generation. Circ Res. 2014;114:524–537. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Echtay K, Roussel D, St-Pierre J, Jekabsons M, Cadenas S, Stuart J, Harper J, Roebuck S, Morrison A, Pickering S, Clapham J, Brand M. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 28.Kang P, Yun J, Kaumaya P, Chen Y. Design and use of peptide-based antibodies decreasing superoxide production by mitochondrial complex I and complex II. Biopolymer: Peptide Science. 2011;96:207–220. doi: 10.1002/bip.21457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallogly MM, Mieyal JJ. Mechanisms of reversible protein glutathionylation in redox signaling and oxidative stress. Curr Opin Pharmacol. 2007;7:381–391. doi: 10.1016/j.coph.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Kehrer J. The effect of BCNU (carmustine) on tissue glutathione reductase activity. Toxicol Lett. 1983;17:63–68. doi: 10.1016/0378-4274(83)90036-x. [DOI] [PubMed] [Google Scholar]
- 32.Chen YR, Chen CL, Pfeiffer DR, Zweier JL. Mitochondrial Complex II in the Post-ischemic Heart: OXIDATIVE INJURY AND THE ROLE OF PROTEIN S-GLUTATHIONYLATION. J Biol Chem. 2007;282:32640–32654. doi: 10.1074/jbc.M702294200. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Chen C, Alevriadou B, Zweier J, Chen Y. Excess no predisposes mitochondrial succinate-cytochrome c reductase to produce hydroxyl radical. Biochim Biophys Acta. 2011;1807:491–502. doi: 10.1016/j.bbabio.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanj S, Sharara A, Shpall E, Jones R, Peters W. Myocardial ischemia associated with high-dose carmustine infusion. Cancer. 1991;68:1910–1912. doi: 10.1002/1097-0142(19911101)68:9<1910::aid-cncr2820680911>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 35.Chen C, Wang T, Varadharaj S, Reyes L, Hemann C, Talukder M, Chen Y, Druhan L, Zweier J. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harper M, Dent R, Monemdjou S, Bezaire V, Van Wyck L, Wells G, Kavaslar G, Gauthier A, Tesson F, McPherson R. Decreased mitochondrial proton leak and reduced expression of uncoupling protein 3 in skeletal muscle of obese diet-resistant women. Diabetes. 2002;51:2459–2466. doi: 10.2337/diabetes.51.8.2459. [DOI] [PubMed] [Google Scholar]
- 37.Clapham J, Arch J, Chapman H, Haynes A, Lister C, Moore G, Piercy V, Carter S, Lehner I, Smith S, Beeley L, Godden R, Herrity N, Skehel M, Changani K, Hockings P, Reid D, Squires S, Hatcher J, Trail B, Latcham J, Rastan S, Harper A, Cadenas S, Buckingham J, Brand M, Abuin A. Mice overexpressing human uncoupling protein-3 in skeletal muscle are hyperphagic and lean. Nature. 2000;406:415–418. doi: 10.1038/35019082. [DOI] [PubMed] [Google Scholar]
- 38.Gustafsson H, Soderdahl T, Jonsson G, Bratteng J, Forsby A. Insulin-like growth factor type 1 prevents hyperglycemia-induced uncoupling protein 3 down-regulation and oxidative stress. J Neurosci Res. 2004;77:285–291. doi: 10.1002/jnr.20142. [DOI] [PubMed] [Google Scholar]