Abstract
The homeostatic balance between oxidants and antioxidants in biological systems is known as redox balance, and is regulated by complex processes. Redox balance regulates many of the known cellular pathways and disease processes. The dysregulation of redox balance can lead to acute or long-term oxidative or reductive stresses that are associated with many of the abnormalities observed in cystic fibrosis (CF). Over the past 5 decades researchers have examined contributors to redox dysregulation, their molecular products, and their impact on ion transport, cell proliferation, inflammation, bacterial killing, and the metabolism of nucleic acids, proteins, and lipids in CF. CF patients exhibit elevated markers of oxidative stress when compared to non-CF healthy controls; however, whether the reported redox imbalance is sufficient to produce pathology has been controversial. In addition, comparisons between CF and non-CF disease controls have been lacking. To better understand the mechanisms which mediate the generation of oxidants and antioxidants in CF and the importance of their balance in effecting oxidative or reductive stress, we will review the determinants of redox balance in the blood, lumen, and cellular compartments. From the perspective of methodological application, we will focus on the approaches most often used to study oxidant and antioxidants in CF, including biochemical, proteomic, metabolomic, and lipidomic studies, with a discussion of the few transcriptomic analyses that predict changes in the expression of regulators of redox. Finally, we will discuss the utility of oxidants and antioxidants as biomarkers of disease and the use of antioxidant therapy in CF.
Keywords: Oxidative stress, CFTR, Nrf2 dysfunction, Antioxidants
Introduction
The importance of the impact of oxidative stress on disease pathology has been steadily demonstrated over the past 5 decades. Increasingly, researchers have determined that a number of disease processes are very sensitive to oxidants and changes in redox balance (the homeostatic balance between oxidizing and reducing (antioxidant) equivalents, as discussed below under the Redox balance section). Even in the absence of disease, most known cellular pathways are significantly modulated (or regulated) by changes in redox balance. Cystic fibrosis is caused by mutations in a gene that codes for the cystic transmembrane conductance regulator, and is marked by abnormalities in ion transport, cell proliferation, inflammatory signaling, bacterial killing, and the metabolism of lipids, proteins, and nucleic acids. Many of these disease-causing processes are modulated by oxidants and antioxidants. Therefore, the study of oxidants, antioxidants, and the mechanisms that regulate redox balance in CF is logical.
In the context of CF, many studies have reported significant increases in the products of oxidation in patients and laboratory models since the late 1970’s. These findings have encouraged the notion of redox imbalance in CF, which was first reviewed by Winklhofer-Roob (1), and continues to be an area of interest. However, acute changes in oxidants and antioxidants are part of normal physiology, and do not necessarily entrain disease. In order to precipitate a pathological condition, such as oxidant-induced chronic inflammation, biological systems have to experience a sustained imbalance between oxidants and antioxidants. For example, oxidative stress can be caused by acute events such as infection or exposure to toxins which resolves with termination of the threat to homeostasis. In the case of progressive diseases such as chronic obstructive pulmonary disease (COPD) and CF, chronic redox imbalances favor an oxidizing environment which is hypothesized to precipitate the disease state. In the chronic state, an oxidizing environment can cause oxidation of DNA, proteins, lipids, and other metabolites, which subsequently alter signaling cascades and change the levels of oxidizing and reducing equivalents. Although these Gestalt level interactions precipitate the disease state, to improve detail and focus scope the majority of studies in CF have investigated individual molecules (oxidants, antioxidants, or products of oxidation), and have not examined the complex regulation of intracellular and extracellular redox balance. Consequently, the question of whether persistent oxidative stress exists in CF has not been definitively answered.
Traditionally, the study of oxidants and antioxidants in CF, which began in the late 1970’s, has employed biochemical approaches. More recently, the use of gene array technology has allowed for the examination of genes that regulate redox balance. A significant methodological shift in the study of CF occurred with the advent of electrospray ionization technology that allows for direct mass spectrometric examination of oxidants and antioxidants, the proteins that regulate their production, and the various targets of redox modification (nucleic acids, lipids, proteins, and metabolites). Although mass spectrometry (MS) based approaches, such as proteomics, lipidomics, and metabolomics hold much promise for studies of oxidants and antioxidants in CF, only a small number of studies have been reported. Therefore, we will review the predominantly biochemical work as well as the MS-based studies, with the aim of giving the reader a summary of the field as well as providing a solid background of areas where omics approaches can be applied. We will begin with a discussion of redox balance to provide the critical framework for the reader to understand oxidants and antioxidants in a physiological context. Moreover, because the determinants of redox balance significantly differ in different milieus, we will review mainly animal and human studies of oxidants and antioxidants in the context of three compartments; the blood, the cell (the predominant work is in airway epithelia), and the lumen.
Redox Balance
The production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) is a necessary physiological process that modulates many cellular functions. For example, both tumor necrosis factor (TNF)α (2) and interleukin (IL)-1β (3) mediated activations of NF-κB and subsequent inflammatory signaling have been shown to be hydrogen peroxide (H2O2) dependent. Peroxide enhances the phosphorylation and subsequent activation of IκB kinases leading to the increased phosphorylation of IκB, its subsequent degradation, and the increased activation of NF-κB (4). A large number of other signaling cascades, including those of AP1, MAPK, and JNK are similarly regulated. To manage signaling through these and other pathways, cells use a complex and tightly regulated balancing system to control the effects of oxidants and antioxidants. This is the system of redox balance. Normal and efficient signaling is dependent on shortly lived imbalances that result in short term oxidation or reduction of biological molecules that affect various cellular functions. However, oxidative or reductive stresses, which contribute to disease pathology, can arise when increases in the production of ROS and/or RNS are poorly balanced by antioxidants, resulting in a disruption the cellular homeostasis of oxidizing and reducing equivalents.
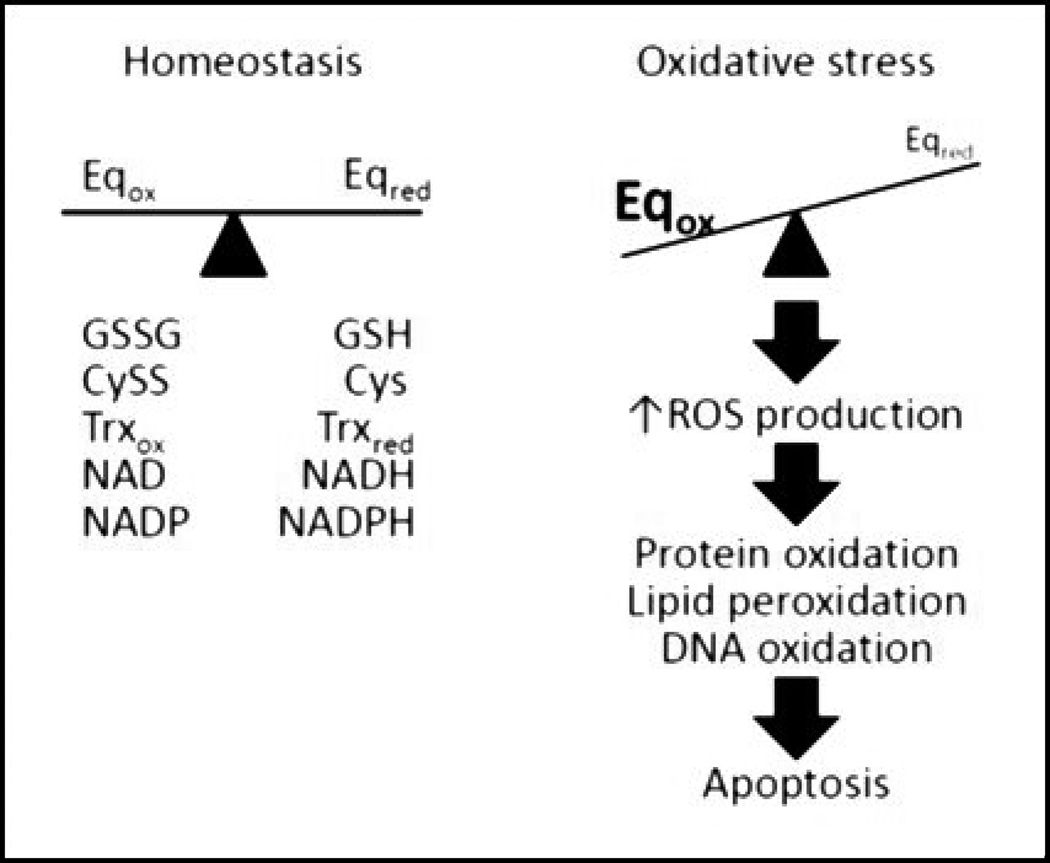
Generally, oxidizing and reducing equivalents are balanced in ratios to regulate appropriate cell function (Figure 1). Balance is achieved through antioxidant systems, enzymes and metabolic processes, which are ultimately critical to cell viability. Exposure to various environmental or pharmacological insults can alter ratios to cause an imbalance of oxidizing and reducing equivalents. This observation provides much of the rationale for more classical definition of oxidative stress that was coined nearly 35 years ago by Helmut Sies: “. . . a disturbance in the prooxidant-antioxidant balance in favor of the former” (5). There are a number of different systems in place that actively restore balance to intracellular environments during periods of oxidative imbalance, which must occur in a timely fashion to prevent cellular dysfunction and cell death. It should be noted that prolonged periods of oxidative stress require relatively high levels of mild oxidant, such as H2O2, to elicit cell death, while strong oxidants such as sodium hypochlorite (NaOCl) and hydroxyl ions (•OH) can induce cytotoxicity at low levels. Physiologically, ratiometric shifts of the reducing and oxidizing equivalent (redox) balance are much more likely to cause changes to intracellular signaling before overt cytotoxicity (Figure 2).
Figure 1.
Classic definition of oxidative stress. Cellular redox homeostasis is maintained as a balance between oxidizing and reducing equivalents (Eq). Because thiol moieties can undergo reversible oxidation/reduction that provide a mechanism to facilitate, protein function, protein-protein interactions, protein-DNA interactions and protein trafficking, many Eq couples of interest contain thiol resides including GSH/GSSG, Cys/CySS and Trxred/Trxox, but other non-thiol based redox Eq can include NADH/NAD and NADPH/NADP among others. Regulation is a consequence of cellular ROS tone, balance of reductases and oxidases and metabolic equilibrium. During periods of ROS overproduction, reduced Eq can be overwhelmed allowing oxidized Eq to predominate. In turn, increasing concentrations of ROS can augment interaction with various macromolecules, such as DNA, proteins and lipids, causing often irreversible damage. If unchecked, accumulation of damage will lead to cellular apoptosis.
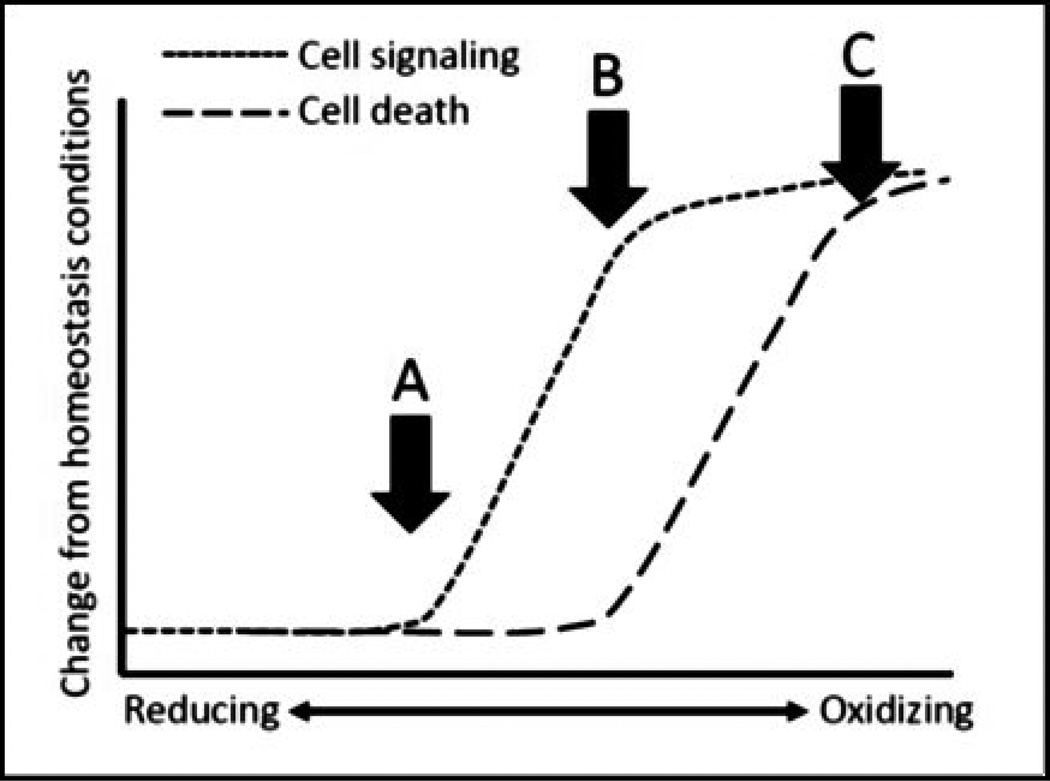
Figure 2.
The effects of reducing and oxidizing redox states on cell signaling and cell death. Redox states are a continuum ranging from normal, reducing environments to disrupted, oxidized ones. As redox states shift there is a disruption on the maintenance of normal redox signaling (arrow A). Often, redox states become sufficiently oxidized to induce a number of re-regulation systems to restore cellular redox states to optimal levels and preserve cellular function. However, in cases where redox states are not rapidly restored (arrow B), redox-sensitive elements are perturbed and thus, alter cellular function (short dotted line). When cell signaling fails to regulate important aspects of redox-related cell survival, oxidative damage occurs and there is an increase in cellular apoptosis (arrow C; long dotted line). In disease, redox states that are not sufficiently oxidizing will not promote apoptosis but rather would promote a disruption in cell signaling and function (the area in between arrows A and B).
Advances in understanding oxidative stress
Since its original definition, oxidative stress has been primarily viewed as global changes to oxidizing and reducing equivalents that evoke oxidative damage to cellular macromolecules, such as DNA, lipids and proteins. In fact, many studies have focused on biomarkers of oxidative end products to demonstrate oxidative stress including malondialdehyde, 8-hydroxyguanosine, bromotyrosine, chlorotyrosine, nitrotyrosine, 8 isoprotaglandin F2α (8 iso-PGF2α), and 2,3 dinor 8 iso-PGF2α. Many of these biomarkers are the result of sustained ROS/RNS generation promoting eventual cellular apoptosis. However, in many disease states, extremely high levels of ROS/RNS generation sufficient to cause cellular apoptosis are infrequent. It is more common to observe ROS/RNS production that induces smaller, more discrete changes to redox states and triggers specific signaling pathways or change in cellular function. Yet, this concept is somewhat incompatible with the more classic definitions of oxidative stress, where oxidative stress is defined as a more large-scale shift of all redox couples. With this view, all redox sensitive processes would be activated or deactivated during oxidative insult. The majority of reports in the literature demonstrate that ROS/RNS generation, through various signaling molecules, activates very specific pathways, and this targeted stimulation is not consistent with global activation of redox-sensitive elements. Reconciling discrete cellular signaling/functional alterations with large scale shifts in cellular oxidizing and reducing equivalents becomes difficult through the classical definition of oxidative stress leading to the need for an alternate rubric with which to examine the role of redox balance in disease.
A breakthrough in our understanding of the mechanisms by which oxidative stress may specifically alter cellular function came with the observation that cellular redox couples are not in equilibrium but are actually independently regulated. This critical finding was provided by a study of plasma redox states in people of various ages (19–85 years old) (6). In this study, glutathione (GSH) and glutathione disulfide (GSSG) were measured, and redox potentials (Eh) were determined via the Nernst equation. Data show that GSH Eh was fairly unchanged until age 45, after which it became increasingly oxidized at a rate of approximately +0.7 mV/year thereafter. Interestingly, cysteine (Cys) and cysteine (CySS) were also measured, and Cys Eh were determined. Cys Eh revealed a very different profile, where a more linear oxidation over time beginning at approximately 20 years of age, averaging nearly +0.2 mV/year over time. Differential oxidation profiles of both GSH and Cys reveal that cellular redox couples are not in equilibrium but rather are independently regulated and as such, cannot be treated as like equivalents. Thus, during periods of increased ROS production, there is not a global cellular shift of equivalents to a more oxidized state, but there are distinct shifts within specific couples. Other examples of redox couple disequilibrium include the effects of various heavy metals in toxicological studies, where specific metals (arsenic, cadmium and mercury) caused substantial oxidation of the cellular thioredoxin (Trx) Eh without significant oxidation of the cellular GSH Eh. Conversely, other metals (copper, iron and nickel) had little effect on the Trx Eh but primarily oxidized the GSH Eh (7). Redox changes during cellular differentiation of human mesenchymal stem cells (hMSCs) also support the disequilibrium of various redox couples, where oxidation profiles that occur during differentiation are specific to which differentiation pathway (i.e. bone vs. fat) is promoted (8). Thus, regulated changes to specific couples control individual signaling pathways related to individual cellular differentiation fates. These examples, and others, support the notion of differential redox control through specific redox couples (9).
A newer definition of oxidative stress
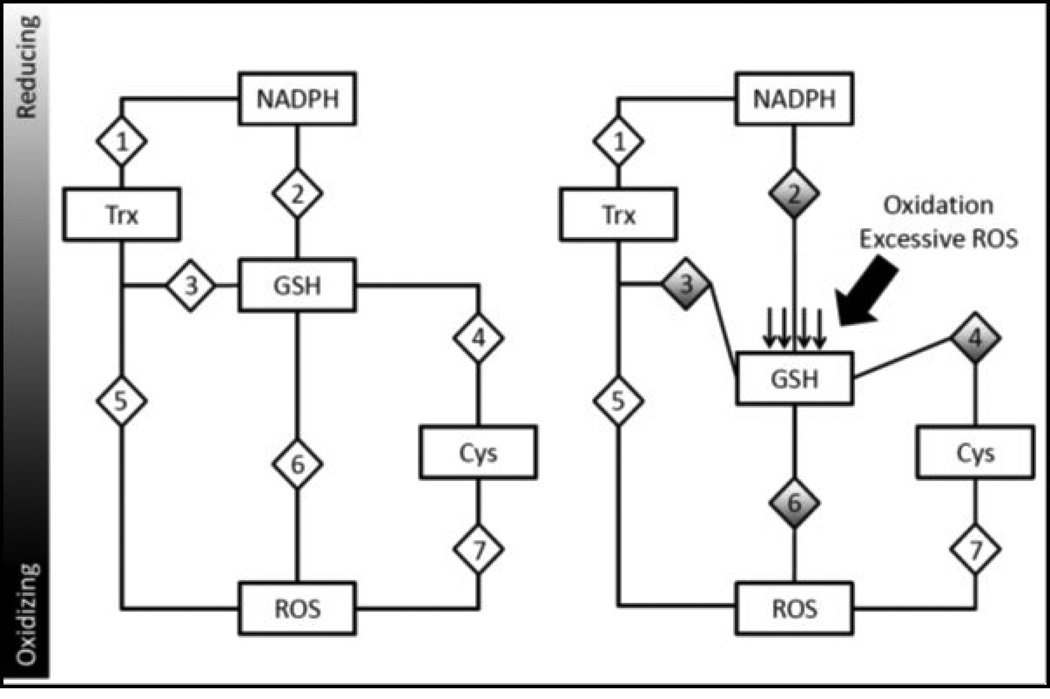
A more accurate definition of oxidative stress should take into consideration the individuality of each redox couple, its regulatory mechanisms by which it controls its own Eh and the redox-sensitive elements that are regulated through that specific couple. This newer definition has been best phrased as a ‘disruption of redox control and signaling’ where each couple controls specific targets to regulate individual activation or deactivation of relevant pathways under the control of that distinct redox couple (10). Thus, each couple has been described as an individual “node” that can act as a rheostat to fine tune redox-sensitive pathway activities (Figure 3). Couples may work in tandem to regulate specific pathways thereby avoiding global activation of redox-sensitiveelements. For proteins, the redox potential of cysteine residues can vary as a consequence of neighboring amino acids, location within the protein itself. The redox potentials of interacting molecules or residues define their role as an oxidant or antioxidant. For example, if the redox potential of a cysteine residue is lower (more reducing) than that of GSH, then GSH can act as an oxidant of that residue (Figure 3). Therefore, a molecule that is typically thought of as an antioxidant, such as GSH, can serve as an oxidant under some conditions, and this is an important concept.
Figure 3.
Redox nodes and circuitry: A mechanism of redox control and dysfunction during oxidative stress. Various redox couples are maintained at various redox states. As these couples are independently regulated, they could serve to control specific subsets of proteins. For example, a hypothetical redox-sensitive protein (diamond labeled ‘3’) may be reduced by thioredoxin but oxidized by GSH. During homeostasis (on left), this protein is maintained in a specific state. However, during periods where GSH becomes oxidized (shifting Eh to a more oxidizing state), hypothetical protein 3 would become increasingly oxidized as the influence of a more oxidizing GSH Eh would cause changes to its redox state and affect its function. Similarly, other proteins (hypothetical proteins 2, 4 and 6) under the regulation of the GSH Eh would also be affected. However, proteins that are not under the control of the GSH Eh would be unaffected, such as proteins 1, 5 and 7. This model of oxi dative stress demonstrates specificity of redox-signaling and provides rationale for ROS-mediated signal transduction.
Plasma
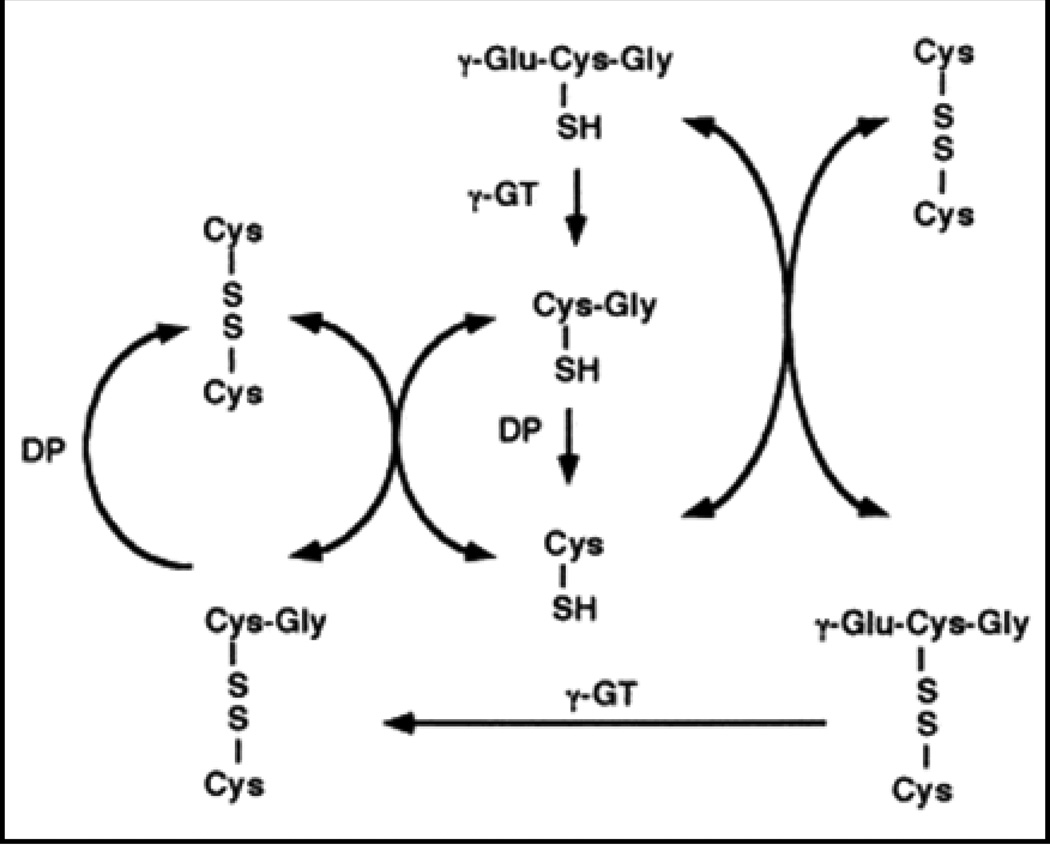
While redox states have been revealed to control cell function and signaling, only recently has it been appreciated that the extracellular redox compartment plays a role in these processes. Because it is the largest extracellular compartment, plasma can reflect an organism’s redox status. Glutathione turnover in cells begins with its transport across the plasma membrane into the extracellular space, including into the plasma. There, it can serve to move throughout the body to various destinations. However, extracellularly, GSH concentrations are very low by comparison to intracellular levels (2–10 mM intracellular vs. 1–5 μM extracellular). Rather, the primary extracellular small biothiol in the plasma is the sulfur containing amino acid, cysteine (Cys). Total pools of Cys can exist in either a reduced or oxidized state, either Cys or cysteine (CySS), respectively, and thereby constitute a unique node of redox control that functions independently from GSH or thioredoxin-1 redox states (11). While capable of controlling specific redox-sensitive elements, extracellular Cys pools are interconnected to GSH as Cys levels are largely a consequence of GSH efflux and breakdown from cells into the plasma. In the extracellular space, GSH can be cleaved to the dipeptide, Cys-Gly, by the enzyme γ-glutamyltranspeptidase (GGT). Cys-Gly is cleaved again by dipeptidases to yield Cys (Figure 4). Free Cys forms a redox equilibrium forming cysteine (CySS) and together constitute the largest small biothiol in the plasma, where total Cys/CySS levels (~250–300 μM) are much higher than GSH levels in the plasma. Reported findings show that Cys Eh in the plasma are approximately -80 mV (6). The Cys Eh is associated with aging, macular degeneration and regulation of intracellular signaling (6,12–14). The role of extracellular Cys redox states in CF pathology remains unstudied, yet previous work unrelated to CF may suggest that this couple regulates multiple facets of CF disease states.
Figure 4.
Extracellular metabolism of glutathione (GSH). Glutathione is the largest pool of intracellular, non-protein small biothiols and can be transported to extracellular spaces (i.e. plasma, airway surface liquid, etc.). Once transported, GSH is metabolized by γ-glutamyltranspeptidase (γGT) to yield glutamate and the dipeptide of cysteine-glycine (Cys-Gly). Dipeptidases (DP) can digest Cys-Gly to single amino acids. As such, GSH concentrations are relatively low in extracellular compartments compared to intracellular stores. Conversely, Cys levels are much higher and constitute the primary small biothiol. The free Cys forms an equilibrium to produce its oxidized form, cystine (CySS).
A number of studies have shown that oxidative extracellular Cys redox states are associated with an increase in intracellular ROS generation (14,15). Oxidizing extracellular redox states regulate the redox state of extracellular sulfhydryl protein residues on the extracellular plasma membrane (Figure 5). While the exact targets of extracellular Cys-mediated redox control are unknown, studies show that oxidative alteration of these residues are important in the regulation of intracellular ROS generation (16). However, extracellular redox states regulate intracellular ROS generation that originates from the mitochondria not at the plasma membrane (12,16). This is of particular interest to CF as mitochondrial dysfunction was an observation made nearly 40 years ago (discussed below under the Intracellular compartment section. Because mitochondrial GSH levels are low in CF, extracellular-mediated intracellular ROS generation originating from the mitochondria may play an even greater role in changes to metabolism and signaling.
Figure 5.
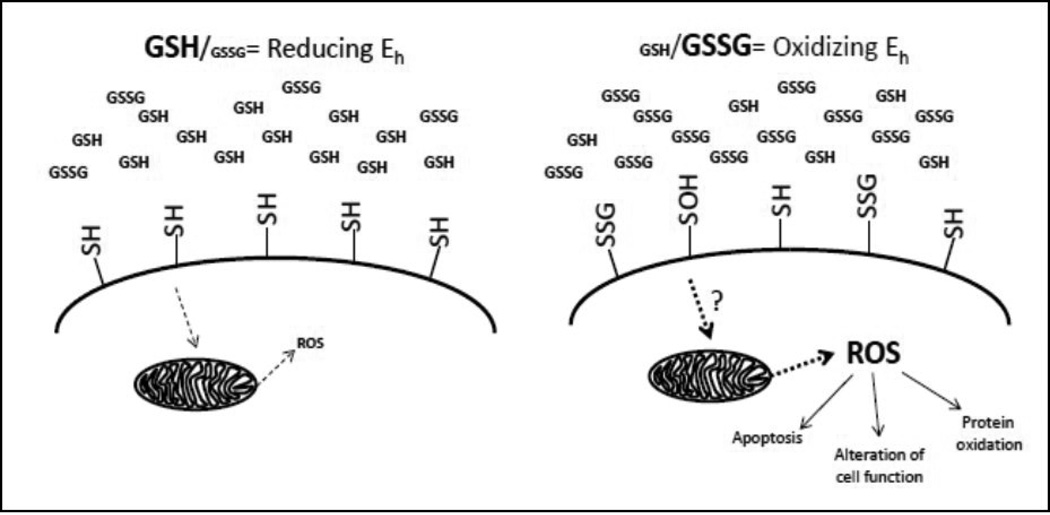
Extracellular redox regulation of membrane protein thiols via GSH redox potential and control intracellular signaling and function. Extracellular GSH exists primarily in its reduced form (left). Redox potentials can be tightly regulated through cellular ROS production, GSH export and Cys transporting. In a reducing extracellular environment, protein thiols are reduced. In an oxidizing extracellular environment (increase in GSSG concentrations), alterations to extracellular thiols can occur to yield sulfenic acid formation (−SOH) or S-glutathionylated (−SSG) modifications. Although the mechanism but which this occurs remains unknown, an outcome of these modifications is the intracellular production of mitochondria-derived ROS, which can contribute to changes in cell function, signaling and protein redox states and prime cells to become apoptotic. Thus, crosstalk between extracellular and intracellular environments has profound effects of cellular physiology and may contribute to cellular pathophysiology.
Nearly 40 years ago, the earliest studies on antioxidants in CF patient plasma described deficiency in vitamin E (17,18) and selenium (18–20). Interestingly, these deficiencies were found to correlate with an increased risk of cancer (21,22). A large study of 101 CF patients reported significant increases in lipid peroxidation and protein oxidation, when vitamin E deficiency was observed, although no clinical signs were correlated with these findings (23). Other lipid markers of oxidation are elevated in CF plasma. For example, 8 iso-PGF2α plasma levels in CF patients were double those measured in non-CF controls, while levels of vitamins A and E, and β-carotene were all significantly decreased in CF (24). Furthermore, during pulmonary exacerbations deficiencies in antioxidants correlated with increases in oxidants. In the largest study of oxidants and antioxidants conducted to date in CF patients (n = 312), Lagrange-Puget M and colleagues found significant decreases in the plasma antioxidants vitamin A, vitamin E, carotenoids, and GSH, while lipid peroxidation, a maker of oxidation, , significantly increased (25). As briefly reviewed here, alterations in vitamin A, vitamin E, carotenoids, GSH, and lipid peroxidation in plasma of CF patients have been documented across a number of independent studies suggesting that these are good markers of CF pathology.
In terms of GSH, seminal findings by Roum et al. (26) showed a systemic deficiency in GSH in patients with CF. Healthy and CF patients showed a sharp discrepancy in plasma GSH concentrations with a 40% decrease below normal controls in the CF patients. Total plasma GSH concentrations for healthy controls were 4.5 μM while GSH concentrations were only 2.7 μM in CF subjects. The portion of the total GSH concentration that was reduced also differed significantly between healthy controls and CF patients (4.3 μM and 2.6 μM, respectively). Conversely, GSSG concentration were not statistically different in CF (0.1 μM) and healthy (0.4 μM) patients. While not measured in the original study, redox potentials (Eh) can be measured by inputting these values into the Nernst equation. There is not a substantial difference in GSH Eh in the plasma between CF and healthy individuals, measuring −139 and −138 mV, respectively. While the GSH Eh is similar between CF and healthy individuals, lower concentrations would suggest an impaired ability to deal with exogenous oxidative insults. Equally potent systemic oxidative insults would likely affect the GSH Eh to a greater degree in CF than in healthy subjects. Other mediators to redox states probably include protein thiols. Most notably, albumin and alpha1 antitrypsin, which are cysteine rich are believed to significantly contribute to the regulation of plasma redox states but this has not been studied in the context of CF.
Although the mechanism for total plasma GSH concentration differences in CF is not entirely understood, but the cystic fibrosis transmembrane conductance regulator (CFTR), has been implicated in the transport of GSH (discussed below under Involvement of CFTR section). The plasma constitutes the largest extracellular compartment in the body, and moreover, plasma redox states are likely to regulate numerous aspects of endothelial function. Regardless of the mechanisms by which GSH is exported to the extracellular space, the observations that GSH levels are depleted in CF are consistent, suggesting that these may serve as an avenue of potential therapy or biomarker discovery and are an important area for further study.
Intracellular compartment
Within cells, redox balance is regulated mainly by the levels of GSH/GSSG, with a significant contribution from TRX1/TRX1s-s in nuclei and TRX2/TRX2s-s in mitochondria. The mitochondrion, the most significant source of ROS in the cell, has been the subject of a number of CF focused studies since the late 1970’s. Early studies by Shapiro and colleagues (27,28) in fibroblasts reported significant increases in mitochondrial oxygen consumption in CF patients versus non-CF control subjects. Later studies of epithelial cells from nasal polyps demonstrated a ~50% increase in oxygen consumption in CF versus non-CF controls, and found that this increase was reversed by ouabain treatment, suggesting that increased energy demands by CF cells were due to increased Na+ K+ ATPase activity (29). While abnormalities in Na+ K+ ATPase activity in CF have not been widely reported, findings of increased oxygen consumption by polarographic oxygen electrode measurement in different CF cells have been consistent (27–29). If CF cells consume more oxygen then CF mitochondria may be the source of increased superoxide (O2−) and peroxide (H2O2) levels (30).. Importantly, this suggested increase in O2− and H2O2 in CF cells does not necessarily indicate a redox imbalance in CF cells if they exhibit an increase in antioxidant capacity. In fact, elegant studies by Schwarzer and colleagues in non-polarized nasal epithelial cell lines did not find any significant differences in the redox potential of the mitochondria of CF (homozygous for F508del) versus wildtype CFTR-corrected controls (31). These studies employed organelle-targeted redox sensitive GFP, and also showed no difference in redox potential in the endoplasmic reticulum (ER), at the cell surface or in the cytosol of CF versus non-CF cells. Notably, redox sensing molecules, such as the GFP used in these studies, can themselves affect the redox environment by acting as a reductant. Nevertheless, the Schwarzer et al. studies remain to be the most global examination of intracellular redox balance in CF epithelial cells.
Despite some controversy over the presence of steady state intracellular redox imbalance or oxidative stress in CF, investigators have provided a range of evidence that collectively suggests increased oxidant stress in CF. Significant increases in urinary 8-hydroxydeoxyguanosine in CF patients versus controls indicate DNA oxidation and damage, although these data did not correlate with disease severity (32). Later studies by Velsor and colleagues found significant increases in DNA oxidation and lipid peroxidation in lungs from Cftr knockout mice versus wildtype controls (33). The investigators found no difference in GSH levels in CF versus control lungs, a finding which supports similar observations made earlier by Gao and colleagues (34) in human epithelial cell lines, and by Mangione and colleagues in erythrocytes (35). However, follow up studies by Velsor and colleagues in Cftr knockout mice and human epithelial cell lines revealed a significant decrease in mitochondrial GSH coupled with significant increases in mitochondrial DNA oxidation and decreased aconitase activity (usually resulting from the oxidation of aconitase) in CF versus non-CF controls (36). The importance of mitochondrial GSH was further demonstrated when treatment with a mitochondria permeable GSH analog, glutathione monoethylester (GSH-EE), restored aconitase activity and reduced ROS production (37). These findings highlight the importance of examining cellular compartments such as the cytosol, mitochondrion, and nucleus separately.
Redox regulation is of much importance in inflammatory cells, which play a pivotal role in CF. The production of ROS by neutrophils and macrophages is essential to their bactericidal functions. As early as 1977, oxidase activity in granulocytes from 50 CF patients was found to be elevated compared to controls by colorimetric nitro blue tetrazolium analysis (38). Importantly, these studies found no correlation of this increase with bacterial killing or clinical status. More recent studies suggest that CF phagocytes may actually exhibit decreased bactericidal function despite of increased ROS production due to the lack of chloride transport in the CF phagosome (39). These studies highlight the importance of chloride as a substrate of myeloperoxidase (MPO)-mediated production of hypochlorous acid (HOCl), and the importance of chloride transport in bacterial killing. In addition to MPO, the NADPH/Dual oxidase (NOX/DUOX) family of oxidases play a significant role in ROS production by neutrophils and macrophages, as well as epithelial cells. However, no significant examination of the differential behavior of these oxidases in CF versus non-CF has been performed.
Involvement of CFTR
There is some evidence that CFTR may serve as an efflux channel for GSH. CFTR shares a structural similarity to ABCC proteins, including multidrug resistant proteins (MRPs), which are believed to export glutathione and/or glutathione-S-conjugates (40,41). Using current recordings from excised membrane patches, GSH was shown to be permeant in CFTR (42). Utilization of inhibitors, such as glibenclamide and dinitrostilbene-2,2’-disfulonate (DNDS), prevented GSH efflux. Furthermore, in other studies, inside-out membrane vesicles were used to evaluate CFTR-mediated GSH efflux (43). Vesicles expressing CFTR demonstrated an increased uptake in radiolabeled GSH ([35S] GSH) compared to vesicles lacking CFTR. In this same report, mutant CFTR (R347D) showed a decreased ability to traffic GSH compared to wild-type. While these studies suggest GSH export through CFTR, reproducibility of these results and inconsistencies have been problematic for the notion that CFTR is a GSH transporter. In terms of its primary function the inhibition of CFTR mediated chloride transport in primary blood neutrophils and a neutrophil cell line was linked to significant decreases of neutrophil phagosome HOCl that result in the decrease of Pseudomonas aeruginosa protein chlorination (44). Follow up studies demonstrated the importance of chloride transport to both oxidant dependent and independent killing of bacteria in primary blood neutrophils from CF patients and non-CF controls (39).
CFTR appears to be susceptible to redox regulation. A direct impact of redox potential on CFTR was not demonstrated until the 1990’s (45). Stutts and colleagues studied CFTR sensitivity to acute variations in the ratio of NADPH/NADP+ in whole cell patch experiments. Under these conditions, treatment with NADP+ (oxidized) significantly increased basal and forskolin stimulated Cl− currents through CFTR. Treatment with the reduced couple, NADPH, inhibited the channel. Similar results were observed for other oxidants. However, this phenomenon may not occur physiologically as concentrations 6–7 times the normal cellular levels were used in the study. Nevertheless, a sensitivity of CFTR to redox potential was clearly demonstrated. Concordantly, in studies of inside out patches and single channel recording, Harrington and colleagues (46) found that oxidants slow channel kinetics and increase channel open probability, while high level reducing agents speed up channel gating kinetics and decrease open probability. Interestingly, these studies were also the first to demonstrate that CFTR channel function can be modulated by S-nitrosylation reagents and that this oxidation increases open probability. Later studies by Zaman and colleagues further demonstrated that S-nitrosylation not only increases the expression of CFTR, but also its maturation by modifying interacting chaperones (47,48). Finally, some studies indicate that oxidant generated signaling molecules, such as 8-iso-prostaglandin E(2), induce CFTR channel function (49,50).
Oxidation and oxidative stress have also been shown to suppress CFTR channel function. The channel can be reversibly inhibited by directly glutathionylation, albeit at levels of GSSG that are 10 times physiological concentrations (51). Although others of the 18 cysteine residues in CFTR may be susceptible to glutathionylation, mutant channels lacking cys-1344 are largely resistant to inhibition by oxidation, highlighting the importance of this residue in redox modulation of the channel. Interestingly, these studies also demonstrate a decrease in channel function following treatment with proposed therapeutic levels of S-nitrosylation reagents. Cantin and colleagues observed a decrease in CFTR message stability following cell exposure to oxidants, which translated into decreased protein expression (52). This decrease in CFTR was concomitant with an increase in γ-glutamylcysteine synthase (the rate limiting enzyme in GSH synthesis), and GSH. This finding suggested that decreased CFTR protein expression was a cellular adaptive response to decrease GSH efflux and increase intracellular levels to address the exposure to oxidants, suggesting a regulatory link between redox balance and CFTR expression. Interestingly, some bacteria may also exploit CFTR’s redox susceptibility. For example, Pseudomonas aeruginosa produces pyocyanin, a molecule that inhibits cellular peroxidases and disrupts the redox balance of nicotinamide nucleotide, and thus acts as an oxidant. Exposure of CF and non-CF human bronchial epithelial cell lines to clinically relevant levels of pyocyanin increased depleted GSH and ATP levels in both CF and non-CF cells (53). No impact on Cl− current was observed in treated CF cells. In non-CF cells pyocyanin slightly increased basal Cl− current, but markedly inhibited forskolin stimulated current, indicating a significant inhibition of CFTR. Taken together, the evidence points to interplay between the regulation of CFTR and redox regulation, and suggests that CFTR can be both activated and inhibited by oxidation.
Nrf2 dysregulation in CF
Nuclear factor erythroid 2 [NF-E2] - related factor 2 (Nrf2) is the central transcription factor of the antioxidant response element (ARE) pathway (54). The ARE is the cell’s chief protective response to changes in redox balance. Under conditions of increased oxidant production, the Nrf2 inhibitory protein Keap1 is oxidized and dissociates from Nrf2 allowing for subsequent transcriptional activation that can entrain the expression of over 200 antioxidant/protective proteins. Many of these proteins are primary regulators of redox balance and oxidative damage repair, and include: γ-glutamylcysteine synthase (the rate limiting enzyme in glutathione synthesis), Trx1 (the predominant regulator of nuclear redox balance), and catalase (the chief cellular peroxidase). Importantly, Nrf2 regulated proteins play an active role in suppressing inflammatory signaling and are antagonistic to pro-inflammatory transcription factors, such as NF-κB. Because of this antioxidant / anti-inflammatory role, Nrf2 dysfunction has been linked to disease progression and pathology in a number of inflammatory lung diseases, including COPD.
There is some evidence that Nrf2 is dysfunctional in CF airway epithelial cells. Targeted proteomic studies in human primary bronchial epithelia and airway epithelial cell lines revealed a significant decrease in the expression of a number of Nrf2 regulated antioxidant proteins and a significant increase in steady intracellular H2O2 in CF versus non-CF cells (55). Furthermore, a large decrease (~70%) in Nrf2 basal transcriptional activity that was further exacerbated by inflammatory stimulation was observed in CF versus non-CF cells, and could also be produced by inhibition of CFTR, suggesting a link between Nrf2 dysregulation and cellular adaptive responses to the loss of CFTR function (55). Further examinations revealed that in CF cells, the interaction of Nrf2 with CREB binding protein (CBP) that is necessary for maximal transcriptional activation is diminished by a cAMP-dependent mechanism (56).
Nrf2 regulation in non-epithelial CF cells may differ. For example, the expression of heme oxygenase 1 (HO-1), a Nrf2 regulated gene, is significantly elevated in CF patient macrophages versus non-CF controls (57). However, this same study reported that the CF IB3 epithelial cell line fails to increase HO-1 expression in response to stimuli, supporting our findings on Nrf2. Furthermore, the investigators demonstrated that transfection with and over expression of an HO-1 construct protected against effects of Pseudomonas aeruginosa infection, suggesting that manipulations that compensate for Nrf2 dysfunction in CF can be beneficial. Further studies are required to definitively define Nrf2 behavior in CF, but a report that CF patients, versus controls, do not exhibit Nrf2 activation in response to the conventional Nrf2 activator sulforaphane (58) supports the notion of Nrf2 dysregulation.
Lung lumen
Unlike other extracellular compartments, the lumen of the lung possesses a unique redox environment. Dissimilar to the plasma, where the predominate small biothiol is Cys, GSH concentrations far outweigh those for Cys, where total concentrations are estimated to be approximately 120 μM and a Eh of nearly −110 mV (59). Generally, total GSH concentrations in the airway surface liquid (ASL) have been estimated to be 275–500 μM in healthy patients (26,60,61) and are comparable to some mouse models where total GSH is approximately 600 μM (36). Most of this pool is reduced and is representative of approximately 80–90% of the total pool. With these measurements, GSH Eh potentials can be generated to show that in healthy patients are relatively reduced compared to the plasma, ranging from −175 to −210 mV (approximately −130 to −140 mV in plasma) (62). The mechanism by which extracellular GSH redox states are regulated is currently unknown, but may occur through GSH breakdown by enzymes such as gamma-glutamyltranspeptidase (GGT), subsequent Cys uptake by cells (Figure 4), and GSH resynthesis and secretion. While a better understanding of extracellular GSH redox state regulation is needed, minimal research has been conducted, especially in the airway.
In CF patients, total GSH concentrations in ASL are greatly diminished and are representative of only a fraction of that in healthy individuals. Total GSH concentrations are only 92 μM, constituting only 20–30% of totals in a healthy pulmonary ASL (26). Glutathione in the CF ASL was measured to be 78 μM and are significantly lower than healthy lung ASL of 257 μM. However, GSSG concentrations were not significantly different with GSSG in the healthy lung ASL at 14 μM and in the CF lung ASL at 2 μM. Significant loss of GSH appears to dictate changes to GSH Eh in the ASL. Normal ASL GSH Eh is approximately −175 mV but in the CF ASL, GSH Eh increased by over +45 mV to −129 mV (26). Interestingly, CF mouse models also demonstrate severe loss of GSH as well, where in CFTR deficient mice, ASL GSH concentrations were approximately 35% of that in normal mice. In gut corrected CFTR-deficient mice (expressing human CFTR in the gut epithelium), GSH concentrations demonstrated a similar loss of GSH (63).
Other antioxidants in the lung lumen may be important in CF. The antioxidant lipid lipoxin A4 is significantly decreased in CF lung ASL (64). Furthermore, administration of exogenous lipoxin A4 to CF mouse lungs significantly decreased neutrophilia and infection severity. Thiocyanate, which was speculated to be transported by CFTR based on studies of CF patient sweat in the 1960s (65), serves as an antioxidant in the ASL. In the Cftr knockout mice, thiocyanate levels are significantly decreased and, interestingly, hypertonic saline treatment significantly increased the levels of both GSH and thiocyanate (66). In non-CF mice, thiocyanate helps maintain levels of GSH in the lung and protects against HOCl damage during infection (67). No exhaustive analysis have been conducted in human CF lungs, although a very brief report shows a ~20% decrease in the thiocyanate levels in CF patient saliva compared with non-CF controls (68). Collectively, these data demonstrate significant decreases in antioxidant levels in CF ASL and suggest that oxidant buffering capacity may be diminished in the CF lung lumen.
Increases of oxidants in the CF lung lumen have been reported since the mid-1990s. Most notably, a study by Witko-Sarsat and colleagues of CF patient sputum revealed significant increases in active MPO levels (69). MPO mainly derives from the neutrophil in the lung, catalyzes the H2O2-mediated production of a number of damaging oxidants, and has been reproducibly found to be elevated in CF sputum (70) and bronchoalveolar lavage fluid (BALF) (71). Later studies would discover that a polymorphism in MPO tracks with CF disease severity (72). MPO also catalyzes the halogenation (oxidation) of proteins at tyrosine residues, and thus investigators have examined the levels of chloro- and bromotyrosine. Most recently, researchers examining early CF pathology found significant increases in MPO and halogenated tyrosine that correlated with infection status (73).
Large-scale changes to extracellular redox states have a multitude of implications to lung physiology and to CF pathology. Physiological impacts of increased oxidation have been found to be elevated in the CF airway, including significant increases in general protein oxidation (74), and more specifically nitration (75) and halogenation (73). As for the exact role of GSH in the lung ASL, it is not entirely understood, but may revolve around (1) scavenging ROS, (2) regulating redox states of membrane bound proteins, (3) regulating viscosity of mucus, and/or (4) controlling aspects of the pulmonary immune response (76). Reducing the oxidative environment in the CF airway may serve to alleviate many of the oxidative features of the CF lung. For that reason, GSH (or related compounds) supplementation has been of interest as a potential therapy in CF.
Oxidant and antioxidant therapy in CF
Antioxidant therapy has been proposed in CF since the early 1980’s. The seminal study by Ratjen and colleagues explored N-acetylcysteine (NAC) because of interest in its mucolytic properties, and delivered the drug orally in a double-blinded 12 week trial with 36 CF patients in Germany (77). The studies showed no differences in clinical outcomes in NAC-treated patients versus placebo-treated controls. However, due to deterioration in placebo-treated controls that was not observed in NAC-treated patients, significant differences in lung function and air trapping were observed. NAC was revisited as a therapeutic for CF in Europe and North America for the subsequent 3 decades. A Danish study of the effects of oral NAC treatment for 3 months in 41 CF patients found significant, but slight, improvements in FEV1 in the 3 months following the treatment (78). In a follow up study, the investigators examined the effect of alternating treatment with oral placebo or NAC for two 3-month periods (79). They studied 52 CF patients with chronic Pseudomonas aeruginosa infection, and examined clinical measures that included sputum bacteriology and titers of antimicrobial antibodies, as well as changes in lung function. In this study, no significant differences in clinical responses were observed, but NAC treatment of the sickest CF patients (treated during the autumn) significantly improved FEV1 versus same subject placebo pre-treatment. This finding suggested that any NAC benefit may be due to its antioxidant / anti-inflammatory properties rather than its mucolytic properties.
More recently, investigators used high dose oral NAC (600−1000 mg three times daily for 4 weeks) in a phase I study of 18 CF patients and 9 non-CF controls (80). Significant increases in blood cell GSH concentrations and diminished pulmonary neutrophil counts were observed following treatment. ASL GSH concentrations were not determined, so it is unclear whether decreases in neutrophils were an outcome of redox shifts occurring in the lung lumen. No significant improvement in FEV1 was observed, perhaps due to the short duration of the study. Other investigators examined the effect of oral NAC dose in 21 CF patients, with 11 receiving a low dose (700 mg/daily) and 10 receiving a high dose (2800 mg/daily) over a 12 week period (81). In this study ASL GSH was measured and found to significantly increase with treatment, but no clinical or FEV1 improvement was observed following treatment with either dose of NAC. A follow up to the original high dose studies increased the number of CF patients to 70 and the duration of study to 6 months in a phase IIB trial (82). The study was completed in late 2011 and safety criteria for this study were met. However, data on efficacy has not yet been published.
Direct GSH supplementation has been given to CF patients with varied results. Studies by Griese and colleagues have shown that GSH inhalation in CF patients increases GSH (83) and lymphocyte number (84) in the airway, while decreasing prostaglandin E(2) (84). Interestingly, airway levels of the oxidative stress markers 8-isoprostane, ascorbic acid, uric acid, and MPO were either not changed or trended towards increases (84). Most recently, a randomized, double-blind, placebo-controlled trial was completed in Germany where nebulized GSH (646 mg) was given twice a day for 6 months. This treatment regimen did not show any significant changes in FEV1, number of pulmonary exacerbations or a reduction in biomarkers of oxidative damage to proteins and lipids (85). Interestingly, at 3 months, there was a significant FEV1 improvement in the GSH treatment group which was lost at 6 months. While in a smaller pilot trial peak expiratory flow did show improvement with GSH treatments, again FEV1 did not (86). In mouse models, oral GSH supplementation (a single oral dose of 300 mg/kg) was given to various CF mouse models. In wild-type mice possessing functional CFTR, GSH in ASL, lung and plasma increased dramatically following GSH administration (63). In the ASL, GSH concentrations increased by nearly 5-fold from basal levels after only 60 min of initial treatment. Cftr knockout mice expressing human CFTR in the gut also showed an increase in these same compartments albeit to a much lower extent. Specific to the ASL, GSH levels increased by just over 2-fold compared to baseline concentrations. However, GSH oral supplementation did not show any significant increase in CFTR knockout mice that do not have the gut correction. These data suggest that with oral administration of GSH, CFTR is an essential facilitator of proper trafficking of GSH, especially into the lumen of the lung. Therefore, oral administration of GSH may be problematic in restoring ASL redox states in CF patients.
Other strategies for antioxidant therapy in CF have focused on diet supplementation. Selenium supplementation results in a significant decrease in CF patient plasma lipid peroxidation (87). Long-term oral β-carotene supplementation in CF patients resulted in a ~50% decrease in plasma lipid peroxidation, as well as an increase in plasma α-tocopherol and retinol (88). Supplementation with 10 grams of whey protein twice daily for 3 months increased lymphocyte GSH levels by ~47%, but failed to improve FEV1 (89). Zinc supplementation for 1 year in CF patients decreased the number of days on antibiotics only in trial subjects with inadequate zinc concentrations, but did not significantly decrease inflammatory cytokine levels in plasma (90). As with NAC and GSH treatment, studies on supplementation with antioxidants have produced mixed results, and it is important to note that almost all NAC, GSH, and supplement studies have failed to demonstrate significant improvement in lung function, as assessed by FEV1. Nevertheless, most antioxidants therapies have been very well tolerated and have shown some benefits.
Although many antioxidant therapies have failed to show significant benefit in CF, some proposals of oxidant / antioxidant therapies remain promising. Treatments with the oxidant nitric oxide (NO) or donors of NO such as S-Nitrosoglutathione (GSNO), both of which are decreased in CF, hold promise for improved bacterial killing, as well as maximal activation of CFTR (91). Work in non-CF models that indicates airway delivery of thiocyanate (SCN−) improves infection outcomes (67) holds the promise that deficiencies of SCN− in the CF airway (92) may be corrected. SCN− spontaneously reacts with H2O2 to form hypothiocyanate (OSCN−), a bacteriocidal molecule, and water. Finally, activating Nrf2 may be more beneficial than the use of high turnover antioxidant molecules. Studies with a potent activator of Nrf2, the synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO), report significant decrease in lung neutrophilia and inflammatory signaling following simulation with Pseudomonas aeruginosa LPS or flagellum (93). However, another triterpenoid, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid methyl (CDDO-Me) has been reported to increase mortality in patients with stage 4 chronic kidney disease (94), although this outcome has not been clearly linked to Nrf2 activation. Therefore, while therapeutically targeting Nrf2 may address one of the underlying causes of the dysregulation of redox balance and inflammation in CF, a better understanding of drug mechanisms is necessary.
Omics-based studies
One development that can potentially lead to improved antioxidant therapy in CF is the emerging application of omics technologies. Transcriptomics, proteomics, and metabolomics have been the most widely used approaches, with lipidomics gaining much interest in recent years. Although few studies have been conducted, findings demonstrate the utility and promise of applying these approaches to the study of redox in CF. Given the knowledgebase of redox regulation in CF, omics approaches can significantly improve our understanding of the universe of oxidants and antioxidants in CF. Furthermore, omics technologies such as mass spectrometry can very efficiently and rapidly examine markers of redox regulation on a broad basis, allowing for more complete analyses of individual samples. Table 1 summarizes markers of redox regulation that are amenable to analysis by omic approaches. These molecules can be detected by conventional proteomic (95), metabolomic (96), and lipidomic (97) methodologies.
Table 1.
List of oxidants and antioxidants that are amenable to omics analysis.
| Molecule | Species |
|---|---|
| Glutathione; GSH | Peptide |
| Glutathione disulfide; GSSG | Peptide |
| Cysteine; Cys | Amino acid |
| Cystine; Cys-s | Amino acid |
| Reduced nicotinamide adenine dinucleotide phosphate; NADPH | Nucleotide |
| Oxidzed nicotinamide adenine dinucleotide phosphate; NADP+ | Nucleotide |
| Reduced nicotinamide adenine dinucleotide; NADH | Nucleotide |
| Reduced nicotinamide adenine dinucleotide; NAD+ | Nucleotide |
| Malondialdehyde | Aldehyde |
| 8-hydroxydeoxyguanosine | Nucleotide |
| Thioredoxin; Trx 1, 2, and 3 | Protein |
| γ-glutamyl cysteine synthase; γ-GCS | Protein |
| Peroxiredoxin; PRDX 1, 2, 3, 5, and 6 | Protein |
| 8-iso-prostaglandin E(2); 8-IsoPGE2 | Isoprostane lipid |
| 8-iso-prostaglandin F(2α); 8-IsoPGF2α | Isoprostane lipid |
| 2,3 dinor 8 isoprotaglandin F2α; 2,3 dinor 8-lsoPGF2α | Isoprostane lipid |
| Chlorotyrosine | Amino acid |
| Nitrotyrosine | Amino acid |
| Bromotyrosine | Amino acid |
| Pyocyanin | Bacterial toxin |
| Protein carbonyls | Protein |
Gene array studies have alluded to redox dysregulation in F508del mutant Cftr mice versus non-CF controls (98). Investigators glimpsed potential evidence of antioxidant abnormalities in CF mutant mice that exhibited significantly lower expression of many antioxidant genes, including glutathione S transferase (GST) m2, peroxiredoxin (PRDX) 2, and a number of lipoxygenases. In an elegant study by Wright and colleagues, gene expression was examined in the nasal respiratory epithelia of CF patients with either mild or severe lung disease (99). Significant abnormalities in the expression of genes involved in lipid metabolism, ubiquination, and mitochondrial / whole-cell redox regulation were present and segregated based on disease severity. Although transcriptomics studies are powerful and can point to the presence of redox imbalance, it is important to note that gene expression levels do not always correlate with protein levels, and the majority of accepted markers of oxidative or reductive stress are proteins, lipids, or other metabolites.
Electrospray and matrix-assisted laser desorption ionization mass spectrometry-based techniques have been applied to the study of biological molecules. These approaches now allow for rapid, sensitive, and accurate analysis of proteins. Extensive proteomic analysis of CF sputum discovered a significant relationship between MPO and pulmonary inflammation (100), which has been validated in BALF (73). When the proteome of CF patient nasal epithelia was examined, a significant decrease in the expression of a number of antioxidant proteins, including GST pi and PRDX6 was revealed (101). These studies were closely followed by independent proteomic studies that also observed significant decreases in PRDX2 (102) in CF epithelial cell lines, and GST mu, GST pi, PRDX1, 3, and 6 in CF primary epithelial cells (55,56). In concert, proteomic studies on oxidants and antioxidants in a number of different CF airway epithelia strongly agree on the observation that a number of important antioxidant proteins are down regulated in CF versus non-CF controls. Subsequent studies based on proteomic studies of CF identified one mechanism for antioxidant down regulation in CF, namely Nrf2 dysfunction (55,56). Importantly, analysis of the CF redox proteome has yet to be performed, but this application of proteomics would be beneficial for a better global understanding of the determinants of redox balance in CF.
High throughput metabolomic analyses of human primary airway epithelia measured significant decreases in GSH, GSSG, S-lactoylglutathione (a metabolite of GSNO), and ophthalmate in CF versus non-CF cells (103). This study highlights glutathione synthesis as the most impacted pathway related to oxidants and antioxidants in CF. Importantly, the ratio of GSH / GSSG was significantly diminished in CF cells, strongly suggesting the presence of oxidative stress. Interestingly, metabolomic analysis of non-fasted CF patients revealed a decrease in β-oxidation of fatty acids, a marker of mitochondrial dysfunction (104), but did not detect significant differences in markers of oxidative stress in the blood between CF and non-CF cohorts. Similarly, lipidomic analyses of CF patient plasma (105,106) failed to detect differences in prostanes, lipids that are classically associated with CF plasma oxidative stress (24). However, CF plasma lipidomic studies have detected significant decreases in thiol-containing lipids (105). In CF sputum, a metabolomic / lipidomic analysis by Yang and colleagues identified a number of redox modulated lipids (oxylipins), previously not identified in the CF lung (107). These studies also found a significant decrease in lipoxin A4 in CF lungs, which had previously been identified and validated by other approaches (64). The fact that these broad analysis studies also accurately detected the known lipoxin A4 deficiency in CF increases confidence in lipidomic analyses of redox balance in CF. The precise details and potential promise of large broad scale analyses in CF have been recently reviewed (108).
Although omics approaches hold much promise for the study of oxidants and antioxidants in CF, it is important to note that mass spectrometry-based analyses are semi-quantitative, and therefore it is essential that observations made in proteomic, metabolomic, and lipidomic studies are validated by other approaches. This is especially true for high-throughput analyses that are non-targeted. In addition, the levels of proteins, metabolites, and lipids in patient tissues are very sensitive to diet. The potential influence of diet is a particularly important consideration studies that examine samples from non-fasted animal or human subjects. Nevertheless, continuing advances in the reliability and sensitivity of mass spectrometers now make proteomic, metabolomic, and lipidomic approaches very informative and these cutting edge approaches may lead to additional avenues of discovery and redox balance focused treatment in CF.
Conclusion
Redox state depends on the global balance of oxidant and antioxidants, which is regulated differently in the plasma, cells, and ASL. Many studies have reported significant abnormalities in CF in these compartments, and there may be links between these and the disease pathophysiology. There have been repeated reports about CFTR interplay with redox, and the presence of significant differences in oxidants and antioxidants in CF plasma, cells, and ASL. However, definitive mechanistic evidence of the links between abnormalities in redox balance and the primary defect in CF, as well as evidence of the benefits of oxidant and/or antioxidant therapies, have yet to be demonstrated. Contributors to this state of the field include the complexity of studying oxidants and antioxidants, and the variety of mechanisms that may give rise to abnormalities. Furthermore, the use of different cell culture and animal models, and methodologies complicate the issue. A good approach is to study abnormalities that have been found in patient samples in models that are amenable to mechanistic examination, such as primary cells and cell lines. Once some mechanisms are gleamed, they should be validated in patients, before further study of potential therapies. Many oxidants and antioxidants, as well as markers of redox imbalance, are amenable to sensitive and accurate analysis by high throughput or targeted omics technologies. The use of omics strategies can help provide the global analyses necessary for a better understating of redox status in CF and the regulatory mechanisms involved, which is necessary for the development of more efficacious treatments.
Acknowledgements
This work was funded by the NHLBI (1R01HL109362-01 (Ziady) and the Cystic Fibrosis Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI, the NIH, or the CF Foundation.
Abbreviations
- GSH
glutathione
- GSSG
glutathione disulfide
- Cys
cysteine
- Cys-Cys
cystine
- CFTR
Cystic fibrosis transmembrane conductance regulator
- Nrf2
nuclear factor erythroid 2 - related factor 2
- Trx
Thioredoxin
- PRDX
Peroxiredoxin
- H2O2
hydrogen peroxide
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Winklhofer-Roob BM. Acta Paediatr. Suppl. 1994;83:49–57. doi: 10.1111/j.1651-2227.1994.tb13229.x. [DOI] [PubMed] [Google Scholar]
- 2.Li Q, Spencer NY, Oakley FD, Buettner GR, Engelhardt JF. Antioxid. Redox. Signal. 2009;11:1249–1263. doi: 10.1089/ars.2008.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Engelhardt JF. J. Biol. Chem. 2006;281:1495–1505. doi: 10.1074/jbc.M511153200. [DOI] [PubMed] [Google Scholar]
- 4.Kamata H, Manabe T, Oka S, Kamata K, Hirata H. FEBS Lett. 2002;519:231–237. doi: 10.1016/s0014-5793(02)02712-6. [DOI] [PubMed] [Google Scholar]
- 5.Cadenas E, Sies H. Adv. Enzyme Regul. 1985;23:217–237. doi: 10.1016/0065-2571(85)90049-4. [DOI] [PubMed] [Google Scholar]
- 6.Jones DP, Mody VC, Jr, Carlson JL, Lynn MJ, Sternberg P., Jr Free Radic. Biol. Med. 2002;33:1290–1300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 7.Hansen JM, Zhang H, Jones DP. Free Radic. Biol. Med. 2006;40:138–145. doi: 10.1016/j.freeradbiomed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Imhoff BR, Hansen JM. Cell Mol. Biol. Lett. 2011;16:149–161. doi: 10.2478/s11658-010-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nkabyo YS, Gu LH, Jones DP, Ziegler TR. J. Nutr. 2006;136:1242–1248. doi: 10.1093/jn/136.5.1242. [DOI] [PubMed] [Google Scholar]
- 10.Jones DP. Antioxid. Redox. Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 11.Jones DP, Go YM, Anderson CL, Ziegler TR, Kinkade JM, Jr, Kirlin WG. FASEB J. 2004;18:1246–1248. doi: 10.1096/fj.03-0971fje. [DOI] [PubMed] [Google Scholar]
- 12.Imhoff BR, Hansen JM. Biochem. J. 2009;424:491–500. doi: 10.1042/BJ20091286. [DOI] [PubMed] [Google Scholar]
- 13.Jones DP. Rejuvenation. Res. 2006;9:169–181. doi: 10.1089/rej.2006.9.169. [DOI] [PubMed] [Google Scholar]
- 14.Jiang S, Moriarty-Craige SE, Orr M, Cai J, Sternberg P, Jr, Jones DP. Invest Ophthalmol. Vis. Sci. 2005;46:1054–1061. doi: 10.1167/iovs.04-0949. [DOI] [PubMed] [Google Scholar]
- 15.Nkabyo YS, Go YM, Ziegler TR, Jones DP. Am. J. Physiol Gastrointest. Liver Physiol. 2005;289:G70–G78. doi: 10.1152/ajpgi.00280.2004. [DOI] [PubMed] [Google Scholar]
- 16.Go YM, Park H, Koval M, Orr M, Reed M, Liang Y, Smith D, Pohl J, Jones DP. Free Radic. Biol. Med. 2010;48:275–283. doi: 10.1016/j.freeradbiomed.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrell PM, Bieri JG, Fratantoni JF, Wood RE, di Sant'Agnese PA. J. Clin. Invest. 1977;60:233–241. doi: 10.1172/JCI108760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castillo R, Landon C, Eckhardt K, Morris V, Levander O, Lewiston N. J. Pediatr. 1981;99:583–585. doi: 10.1016/s0022-3476(81)80262-4. [DOI] [PubMed] [Google Scholar]
- 19.Hubbard VS, Barbero G, Chase HP. J. Pediatr. 1980;96:421–422. doi: 10.1016/s0022-3476(80)80685-8. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd-Still JD, Ganther HE. Pediatrics. 1980;65:1010–1012. [PubMed] [Google Scholar]
- 21.Stead RJ, Redington AN, Hinks LJ, Clayton BE, Hodson ME, Batten JC. Lancet. 1985;2:862–863. doi: 10.1016/s0140-6736(85)90127-8. [DOI] [PubMed] [Google Scholar]
- 22.Braganza JM. Lancet. 1985;2:1238. doi: 10.1016/s0140-6736(85)90761-5. [DOI] [PubMed] [Google Scholar]
- 23.Dominguez C, Gartner S, Linan S, Cobos N, Moreno A. Biofactors. 1998;8:149–153. doi: 10.1002/biof.5520080125. [DOI] [PubMed] [Google Scholar]
- 24.Collins CE, Quaggiotto P, Wood L, O'Loughlin EV, Henry RL, Garg ML. Lipids. 1999;34:551–556. doi: 10.1007/s11745-999-0397-1. [DOI] [PubMed] [Google Scholar]
- 25.Lagrange-Puget M, Durieu I, Ecochard R, Abbas-Chorfa F, Drai J, Steghens JP, Pacheco Y, Vital-Durand D, Bellon G. Pediatr. Pulmonol. 2004;38:43–49. doi: 10.1002/ppul.20041. [DOI] [PubMed] [Google Scholar]
- 26.Roum JH, Buhl R, McElvaney NG, Borok Z, Crystal RG. J. Appl. Physiol. 1993;75:2419–2424. doi: 10.1152/jappl.1993.75.6.2419. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro BL, Feigal RJ, Lam LF. Proc. Natl. Acad. Sci. U. S. A. 1979;76:2979–2983. doi: 10.1073/pnas.76.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feigal RJ, Shapiro BL. Nature. 1979;278:276–277. doi: 10.1038/278276a0. [DOI] [PubMed] [Google Scholar]
- 29.Stutts MJ, Knowles MR, Gatzy JT, Boucher RC. Pediatr. Res. 1986;20:1316–1320. doi: 10.1203/00006450-198612000-00026. [DOI] [PubMed] [Google Scholar]
- 30.Turrens JF, Freeman BA, Levitt JG, Crapo JD. Arch. Biochem. Biophys. 1982;217:401–410. doi: 10.1016/0003-9861(82)90518-5. [DOI] [PubMed] [Google Scholar]
- 31.Schwarzer C, Illek B, Suh JH, Remington SJ, Fischer H, Machen TE. Free Radic. Biol. Med. 2007;43:300–316. doi: 10.1016/j.freeradbiomed.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown RK, McBurney A, Lunec J, Kelly FJ. Free Radic. Biol. Med. 1995;18:801–806. doi: 10.1016/0891-5849(94)00172-g. [DOI] [PubMed] [Google Scholar]
- 33.Velsor LW, van HA, Day BJ. Am. J. Physiol Lung Cell Mol. Physiol. 2001;281:L31–L38. doi: 10.1152/ajplung.2001.281.1.L31. [DOI] [PubMed] [Google Scholar]
- 34.Gao L, Kim KJ, Yankaskas JR, Forman HJ. Am. J. Physiol. 1999;277:L113–L118. doi: 10.1152/ajplung.1999.277.1.L113. [DOI] [PubMed] [Google Scholar]
- 35.Mangione S, Patel DD, Levin BR, Fiel SB. Chest. 1994;105:1470–1473. doi: 10.1378/chest.105.5.1470. [DOI] [PubMed] [Google Scholar]
- 36.Velsor LW, Kariya C, Kachadourian R, Day BJ. Am. J. Respir. Cell Mol. Biol. 2006;35:579–586. doi: 10.1165/rcmb.2005-0473OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly-Aubert M, Trudel S, Fritsch J, Nguyen-Khoa T, Baudouin-Legros M, Moriceau S, Jeanson L, Djouadi F, Matar C, Conti M, Ollero M, Brouillard F, Edelman A. Hum. Mol. Genet. 2011;20:2745–2759. doi: 10.1093/hmg/ddr173. [DOI] [PubMed] [Google Scholar]
- 38.Berry DH, Brewster MA. Ann. Allergy. 1977;38:316–319. [PubMed] [Google Scholar]
- 39.Painter RG, Bonvillain RW, Valentine VG, Lombard GA, LaPlace SG, Nauseef WM, Wang G. J. Leukoc. Biol. 2008;83:1345–1353. doi: 10.1189/jlb.0907658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ballatori N, Hammond CL, Cunningham JB, Krance SM, Marchan R. Toxicol. Appl. Pharmacol. 2005;204:238–255. doi: 10.1016/j.taap.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Keppler D, Leier I, Jedlitschky G. Biol. Chem. 1997;378:787–791. [PubMed] [Google Scholar]
- 42.Linsdell P, Hanrahan JW. Am. J. Physiol. 1998;275:C323–C326. doi: 10.1152/ajpcell.1998.275.1.C323. [DOI] [PubMed] [Google Scholar]
- 43.Kogan I, Ramjeesingh M, Li C, Kidd JF, Wang Y, Leslie EM, Cole SP, Bear CE. EMBO J. 2003;22:1981–1989. doi: 10.1093/emboj/cdg194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Painter RG, Valentine VG, Lanson NA, Jr, Leidal K, Zhang Q, Lombard G, Thompson C, Viswanathan A, Nauseef WM, Wang G, Wang G. Biochemistry. 2006;45:10260–10269. doi: 10.1021/bi060490t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stutts MJ, Gabriel SE, Price EM, Sarkadi B, Olsen JC, Boucher RC. J. Biol. Chem. 1994;269:8667–8674. [PubMed] [Google Scholar]
- 46.Harrington MA, Gunderson KL, Kopito RR. J. Biol. Chem. 1999;274:27536–27544. doi: 10.1074/jbc.274.39.27536. [DOI] [PubMed] [Google Scholar]
- 47.Zaman K, McPherson M, Vaughan J, Hunt J, Mendes F, Gaston B, Palmer LA. Biochem. Biophys. Res. Commun. 2001;284:65–70. doi: 10.1006/bbrc.2001.4935. [DOI] [PubMed] [Google Scholar]
- 48.Zaman K, Carraro S, Doherty J, Henderson EM, Lendermon E, Liu L, Verghese G, Zigler M, Ross M, Park E, Palmer LA, Doctor A, Stamler JS, Gaston B. Mol. Pharmacol. 2006;70:1435–1442. doi: 10.1124/mol.106.023242. [DOI] [PubMed] [Google Scholar]
- 49.Soodvilai S, Jia Z, Yang T. Am. J. Physiol Renal Physiol. 2007;293:F1571–F1576. doi: 10.1152/ajprenal.00132.2007. [DOI] [PubMed] [Google Scholar]
- 50.Joy AP, Cowley EA. Am. J. Respir. Cell Mol. Biol. 2008;38:143–152. doi: 10.1165/rcmb.2006-0295OC. [DOI] [PubMed] [Google Scholar]
- 51.Wang W, Oliva C, Li G, Holmgren A, Lillig CH, Kirk KL. J. Gen. Physiol. 2005;125:127–141. doi: 10.1085/jgp.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cantin AM, Bilodeau G, Ouellet C, Liao J, Hanrahan JW. Am. J. Physiol Cell Physiol. 2006;290:C262–C270. doi: 10.1152/ajpcell.00070.2005. [DOI] [PubMed] [Google Scholar]
- 53.Schwarzer C, Fischer H, Kim EJ, Barber KJ, Mills AD, Kurth MJ, Gruenert DC, Suh JH, Machen TE, Illek B. Free Radic. Biol. Med. 2008;45:1653–1662. doi: 10.1016/j.freeradbiomed.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang DD. Drug Metab Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 55.Chen J, Kinter M, Shank S, Cotton C, Kelley TJ, Ziady AG. PLoS. One. 2008;3:e3367. doi: 10.1371/journal.pone.0003367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ziady AG, Sokolow A, Shank S, Corey D, Myers R, Plafker S, Kelley TJ. Am. J. Physiol Lung Cell Mol. Physiol. 2012;302:L1221–L1231. doi: 10.1152/ajplung.00156.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou H, Lu F, Latham C, Zander DS, Visner GA. Am. J. Respir. Crit Care Med. 2004;170:633–640. doi: 10.1164/rccm.200311-1607OC. [DOI] [PubMed] [Google Scholar]
- 58.Chmiel J, Ziady A, Cantin AM, Comhair S, Hazen SL, Schluchter M, Margevicius C, Bucur P, Campbell PW, III, Konstan M. Pediatr. Pulmonol. Suppl. 2012;35:250. [Google Scholar]
- 59.Iyer SS, Ramirez AM, Ritzenthaler JD, Torres-Gonzalez E, Roser-Page S, Mora AL, Brigham KL, Jones DP, Roman J, Rojas M. Am. J. Physiol Lung Cell Mol. Physiol. 2009;296:L37–L45. doi: 10.1152/ajplung.90401.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moss M, Guidot DM, Wong-Lambertina M, Ten HT, Perez RL, Brown LA. Am. J. Respir. Crit Care Med. 2000;161:414–419. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- 61.Cantin AM, North SL, Hubbard RC, Crystal RG. J. Appl. Physiol. 1987;63:152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 62.Moriarty SE, Shah JH, Lynn M, Jiang S, Openo K, Jones DP, Sternberg P. Free Radic. Biol. Med. 2003;35:1582–1588. doi: 10.1016/j.freeradbiomed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 63.Kariya C, Leitner H, Min E, van HC, van HA, Day BJ. Am. J. Physiol Lung Cell Mol. Physiol. 2007;292:L1590–L1597. doi: 10.1152/ajplung.00365.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, Yang R, Uddin J, Guggino WB, Atabani SF, Belkaid Y, Xu Y, Whitsett JA, Accurso FJ, Wills-Karp M, Petasis NA. Nat. Immunol. 2004;5:388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- 65.Gibbs GE, HUTCHINGS RH. Proc. Soc. Exp. Biol. Med. 1961;106:368–369. doi: 10.3181/00379727-106-26341. [DOI] [PubMed] [Google Scholar]
- 66.Gould NS, Gauthier S, Kariya CT, Min E, Huang J, Brian DJ. Respir. Res. 2010;11:119. doi: 10.1186/1465-9921-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chandler JD, Min E, Huang J, Nichols DP, Day BJ. Br. J. Pharmacol. 2013;169:1166–1177. doi: 10.1111/bph.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Minarowski L, Sands D, Minarowska A, Karwowska A, Sulewska A, Gacko M, Chyczewska E. Folia Histochem. Cytobiol. 2008;46:245–246. doi: 10.2478/v10042-008-0037-0. [DOI] [PubMed] [Google Scholar]
- 69.Witko-Sarsat V, Delacourt C, Rabier D, Bardet J, Nguyen AT, Descamps-Latscha B. Am. J. Respir. Crit Care Med. 1995;152:1910–1916. doi: 10.1164/ajrccm.152.6.8520754. [DOI] [PubMed] [Google Scholar]
- 70.van d, V, Nguyen MN, Shigenaga MK, Eiserich JP, Marelich GP, Cross CE. Am. J. Physiol Lung Cell Mol. Physiol. 2000;279:L537–L546. doi: 10.1152/ajplung.2000.279.3.L537. [DOI] [PubMed] [Google Scholar]
- 71.Kettle AJ, Chan T, Osberg I, Senthilmohan R, Chapman AL, Mocatta TJ, Wagener JS. Am. J. Respir. Crit Care Med. 2004;170:1317–1323. doi: 10.1164/rccm.200311-1516OC. [DOI] [PubMed] [Google Scholar]
- 72.Reynolds WF, Sermet-Gaudelus I, Gausson V, Feuillet MN, Bonnefont JP, Lenoir G, Descamps-Latscha B, Witko-Sarsat V. Mediators. Inflamm. 2006;2006:36735. doi: 10.1155/MI/2006/36735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thomson E, Brennan S, Senthilmohan R, Gangell CL, Chapman AL, Sly PD, Kettle AJ, Balding E, Berry LJ, Carlin JB, Carzino R, de KN, Douglas T, Foo C, Garratt LW, Hall GL, Harrison J, Kicic A, Laing IA, Logie KM, Massie J, Mott LS, Murray C, Parsons F, Pillarisetti N, Poreddy SR, Ranganathan SC, Robertson CF, Robins-Browne R, Robinson PJ, Skoric B, Stick SM, Sutanto EN, Williamson E. Free Radic. Biol. Med. 2010;49:1354–1360. doi: 10.1016/j.freeradbiomed.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 74.Starosta V, Rietschel E, Paul K, Baumann U, Griese M. Chest. 2006;129:431–437. doi: 10.1378/chest.129.2.431. [DOI] [PubMed] [Google Scholar]
- 75.Jones KL, Hegab AH, Hillman BC, Simpson KL, Jinkins PA, Grisham MB, Owens MW, Sato E, Robbins RA. Pediatr. Pulmonol. 2000;30:79–85. doi: 10.1002/1099-0496(200008)30:2<79::aid-ppul1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 76.Galli F, Battistoni A, Gambari R, Pompella A, Bragonzi A, Pilolli F, Iuliano L, Piroddi M, Dechecchi MC, Cabrini G. Biochim. Biophys. Acta. 2012;1822:690–713. doi: 10.1016/j.bbadis.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 77.Ratjen F, Wonne R, Posselt HG, Stover B, Hofmann D, Bender SW. Eur. J. Pediatr. 1985;144:374–378. doi: 10.1007/BF00441781. [DOI] [PubMed] [Google Scholar]
- 78.Stafanger G, Garne S, Howitz P, Morkassel E, Koch C. Eur. Respir. J. 1988;1:161–167. [PubMed] [Google Scholar]
- 79.Stafanger G, Koch C. Eur. Respir. J. 1989;2:234–237. [PubMed] [Google Scholar]
- 80.Tirouvanziam R, Conrad CK, Bottiglieri T, Herzenberg LA, Moss RB, Herzenberg LA. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4628–4633. doi: 10.1073/pnas.0511304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dauletbaev N, Fischer P, Aulbach B, Gross J, Kusche W, Thyroff-Friesinger U, Wagner TO, Bargon J. Eur. J. Med. Res. 2009;14:352–358. doi: 10.1186/2047-783X-14-8-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tirouvanziam R, Lymp J, Thompson V, Chatfield B, Nichols DP, Clancy JP, Vender RL, Egan ME, Quittell L, Michelson PH, Anthony V, Spahr J, Rubenstein RC, Herzenberg LA, Conrad CK. Pediatr. Pulmonol. Suppl. 2011;34:280–281. [Google Scholar]
- 83.Griese M, Ramakers J, Krasselt A, Starosta V, van KS, Fischer R, Ratjen F, Mullinger B, Huber RM, Maier K, Rietschel E, Scheuch G. Am. J. Respir. Crit Care Med. 2004;169:822–828. doi: 10.1164/rccm.200308-1104OC. [DOI] [PubMed] [Google Scholar]
- 84.Hartl D, Starosta V, Maier K, Beck-Speier I, Rebhan C, Becker BF, Latzin P, Fischer R, Ratjen F, Huber RM, Rietschel E, Krauss-Etschmann S, Griese M. Free Radic. Biol. Med. 2005;39:463–472. doi: 10.1016/j.freeradbiomed.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 85.Griese M, Kappler M, Eismann C, Ballmann M, Junge S, Rietschel E, van Koningsbruggen-Rietschel S, Staab D, Rolinck-Werninghaus C, Mellies U, Kohnlein T, Wagner T, Konig S, Teschler H, Heuer HE, Kopp M, Heyder S, Hammermann J, Kuster P, Honer M, Mansmann U, Beck-Speier I, Hartl D, Fuchs C, Hector A. Am. J. Respir. Crit Care Med. 2013;188:83–89. doi: 10.1164/rccm.201303-0427OC. [DOI] [PubMed] [Google Scholar]
- 86.Bishop C, Hudson VM, Hilton SC, Wilde C. Chest. 2005;127:308–317. doi: 10.1378/chest.127.1.308. [DOI] [PubMed] [Google Scholar]
- 87.Portal B, Richard MJ, Coudray C, Arnaud J, Favier A. Clin. Chim. Acta. 1995;234:137–146. doi: 10.1016/0009-8981(94)05991-z. [DOI] [PubMed] [Google Scholar]
- 88.Winklhofer-Roob BM, Schlegel-Haueter SE, Khoschsorur G, van't Hof MA, Suter S, Shmerling DH. Pediatr. Res. 1996;40:130–134. doi: 10.1203/00006450-199607000-00022. [DOI] [PubMed] [Google Scholar]
- 89.Grey V, Mohammed SR, Smountas AA, Bahlool R, Lands LC. J. Cyst. Fibros. 2003;2:195–198. doi: 10.1016/S1569-1993(03)00097-3. [DOI] [PubMed] [Google Scholar]
- 90.Abdulhamid I, Beck FW, Millard S, Chen X, Prasad A. Pediatr. Pulmonol. 2008;43:281–287. doi: 10.1002/ppul.20771. [DOI] [PubMed] [Google Scholar]
- 91.Zaman K, Bennett D, Fraser-Butler M, Greenberg Z, Getsy P, Sattar A, Smith L, Corey D, Sun F, Hunt J, Lewis SJ, Gaston B. Biochem. Biophys. Res. Commun. 2014 doi: 10.1016/j.bbrc.2013.12.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Conner GE, Wijkstrom-Frei C, Randell SH, Fernandez VE, Salathe M. FEBS Lett. 2007;581:271–278. doi: 10.1016/j.febslet.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nichols DP, Ziady AG, Shank SL, Eastman JF, Davis PB. Am. J. Physiol Lung Cell Mol. Physiol. 2009;297:L828–L836. doi: 10.1152/ajplung.00171.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de ZD, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM. N. Engl. J. Med. 2013;369:2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ziady AG, Kinter M. Methods Mol. Biol. 2009;544:325–341. doi: 10.1007/978-1-59745-483-4_21. [DOI] [PubMed] [Google Scholar]
- 96.Gowda GA, Zhang S, Gu H, Asiago V, Shanaiah N, Raftery D. Expert. Rev. Mol. Diagn. 2008;8:617–633. doi: 10.1586/14737159.8.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Isaac G, Jeannotte R, Esch SW, Welti R. Genet. Eng (N. Y) 2007;28:129–157. doi: 10.1007/978-0-387-34504-8_8. [DOI] [PubMed] [Google Scholar]
- 98.Xu Y, Liu C, Clark JC, Whitsett JA. J. Biol. Chem. 2006;281:11279–11291. doi: 10.1074/jbc.M512072200. [DOI] [PubMed] [Google Scholar]
- 99.Wright JM, Merlo CA, Reynolds JB, Zeitlin PL, Garcia JG, Guggino WB, Boyle MP. Am. J. Respir. Cell Mol. Biol. 2006;35:327–336. doi: 10.1165/rcmb.2005-0359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sloane AJ, Lindner RA, Prasad SS, Sebastian LT, Pedersen SK, Robinson M, Bye PT, Nielson DW, Harry JL. Am. J. Respir. Crit Care Med. 2005;172:1416–1426. doi: 10.1164/rccm.200409-1215OC. [DOI] [PubMed] [Google Scholar]
- 101.Roxo-Rosa M, da CG, Luider TM, Scholte BJ, Coelho AV, Amaral MD, Penque D. Proteomics. 2006;6:2314–2325. doi: 10.1002/pmic.200500273. [DOI] [PubMed] [Google Scholar]
- 102.Pollard HB, Eidelman O, Jozwik C, Huang W, Srivastava M, Ji XD, McGowan B, Norris CF, Todo T, Darling T, Mogayzel PJ, Zeitlin PL, Wright J, Guggino WB, Metcalf E, Driscoll WJ, Mueller G, Paweletz C, Jacobowitz DM. Mol. Cell Proteomics. 2006;5:1628–1637. doi: 10.1074/mcp.M600091-MCP200. [DOI] [PubMed] [Google Scholar]
- 103.Wetmore DR, Joseloff E, Pilewski J, Lee DP, Lawton KA, Mitchell MW, Milburn MV, Ryals JA, Guo L. J. Biol. Chem. 2010;285:30516–30522. doi: 10.1074/jbc.M110.140806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Joseloff E, Sha W, Bell SC, Wetmore DR, Lawton KA, Milburn MV, Ryals JA, Guo L, Muhlebach MS. Pediatr. Pulmonol. 2013 doi: 10.1002/ppul.22859. [DOI] [PubMed] [Google Scholar]
- 105.Guerrera IC, Astarita G, Jais JP, Sands D, Nowakowska A, Colas J, Sermet-Gaudelus I, Schuerenberg M, Piomelli D, Edelman A, Ollero M. PLoS. One. 2009;4:e7735. doi: 10.1371/journal.pone.0007735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ollero M, Astarita G, Guerrera IC, Sermet-Gaudelus I, Trudel S, Piomelli D, Edelman A. J. Lipid Res. 2011;52:1011–1022. doi: 10.1194/jlr.P013722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang J, Eiserich JP, Cross CE, Morrissey BM, Hammock BD. Free Radic. Biol. Med. 2012;53:160–171. doi: 10.1016/j.freeradbiomed.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eiserich JP, Yang J, Morrissey BM, Hammock BD, Cross CE. Ann. N. Y. Acad. Sci. 2012;1259:1–9. doi: 10.1111/j.1749-6632.2012.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]