Abstract
Chemical compounds built on a diazepine scaffold have recently emerged as potent inhibitors of the acetyl-lysine binding activity of bromodomain-containing proteins, which is required for gene transcriptional activation in cancer and inflammation. Not only have these chemical compounds validated bromodomains as attractive epigenetic drug targets, but they have also brought to the forefront another application of the diazepine, which had already been regarded as a versatile chemical scaffold in rational drug design. This article reviews the success of diazepine compounds as therapeutic agents and examines the unique chemical and geometric features of this privileged scaffold that make it an excellent template for developing potent and selective molecules that control bromodomain-related gene expression in human diseases.
Natural products extracted from plants, animals, and microbes have long been used as powerful chemical agents to treat various human diseases. While these compounds offer vast structural diversity and high potency, their methods of action against their target proteins are not always clearly elucidated, largely due to the fact that they are difficult to synthesize or isolate in large quantities – a challenging issue in the drug development process (Carlson, 2010). As such, many research laboratories and pharmaceutical companies have shifted their efforts towards synthetic molecules that are chemically engineered to interact in a specific manner with a known target protein.
As a synthetic chemistry-based drug discovery strategy matured, researchers began to notice patterns in the physiochemical qualities that make certain chemical compounds more “drug-like” and orally bioavailable than others (Lipinski, 2004). These considerations that allow for increased solubility and absorption are succinctly described as Lipinski’s “rule of five,” which states that a compound likely to possess a desired absorption/permeability profile ought have fewer than 5 hydrogen-bond donors, fewer than 10 hydrogen-bond acceptors, a molecular weight less than 500 grams per mole and a calculated LogP (cLogP) less than 5 (Lipinski et al., 1997).
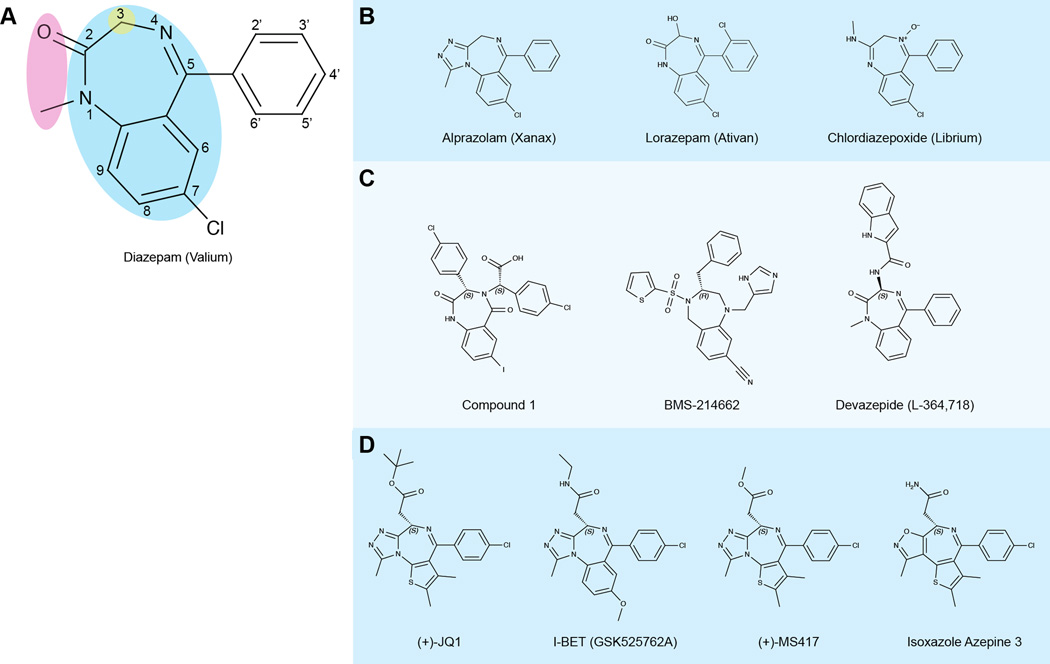
Structural patterns also emerged, as certain chemical scaffolds were found to appear more frequently than others among therapeutics that had succeeded in the clinic. These are referred to as “privileged structures,” a term first used to describe the benzodiazepine (BZD) scaffold when a compound composed of this core was being developed as a nonpeptidal antagonist of cholecystokinin (CKK) (Evans et al., 1986; Evans et al., 1988). BZDs consist of a benzene ring fused to a diazepine – a seven-membered heterocycle containing two nitrogen atoms, typically at positions 1 and 4 on the ring (Figure 1A). From a clinical perspective, the BZD is regarded as a proven privileged scaffold because it appears in many drugs that have been used for decades for anticonvulsant, sedative, and anxiolytic purposes (Bermak et al., 2007; Dubnick et al., 1983; Olkkola and Ahonen, 2008; Wang et al., 1999). Among the most widely known and prescribed members of the BZD family are diazepam, alprazolam, lorazepam, and chlordiazepoxide (Figure 1B) (Atack, 2005; Olkkola and Ahonen, 2008; Verster and Volkerts, 2004; VonVoigtlander and Straw, 1985).
Figure 1. Key structural and chemical features of diazepine-based inhibitors.
Names, structures, and targets of selected diazepine compounds are shown. If applicable, an alternate name (such as a trade name) is listed in parentheses.
(A) Pictured is the chemical structure of diazepam, a commonly prescribed benzodiazepine (BZD) drug, with the core BZD scaffold (blue) highlighted. Also highlighted are two common variable regions (pink and yellow) especially important in the development of potent and selective bromodomain inhibitors. The pink region is occupied by a triazole and the green region presents a pendant functional chain in the bromodomain inhibitors.
(B) Other diazepine compounds that target the GABAA receptor.
(C) Diazepine compounds that target various protein-protein interactions or enzymes. Compound 1 targets the HDM2/p53 interaction; BMS-214662 targets farnesyltransferase; Devazepide acts as a cholecystokinin antagonist.
(D) Diazepine compounds that target the BET bromodomains, along with the similar isoxazole azepine compound. (Citations for the structures in this figure can be found in the text.)
It is doubtful that a privileged structure appears in many clinically used drugs by chance – the structure likely has some intrinsic value that enables its success on a wide array of therapeutic targets. A privileged structure, as defined in the literature, should consist of “a single molecular framework able to provide ligands for diverse receptors (Evans et al., 1988).” Such a chemical structure provides a versatile template on which multiple functional groups can be placed or chiral centers can be generated, allowing medicinal chemists to utilize structure-based drug design techniques to tailor a compound directly to its target (Costantino and Barlocco, 2006; Horton et al., 2003; Huang and Dömling, 2010; Patchett and Nargund, 2000). The ability of the diazepine scaffold to present functional groups to many different receptors can be seen in the enzyme inhibitors (Anderson et al., 2009; McGowan et al., 2009; Nyanguile et al., 2008; Reid and Beese, 2004; Vandyck et al., 2009), GPCR receptor agonists (Joseph et al., 2008), and various other compounds with diazepine-based scaffolds that have been developed (Figure 1C).
In recent years, BZDs and related compounds with a scaffold of a diazepine fused to an isostere of benzene, thiophene (Burger, 1991; Huang and Dömling, 2010; Huang et al., 2010), have garnered considerable attention in drug discovery due to multiple published studies detailing their interactions with the bromodomains of the BET (bromodomain and extra-terminal domain) family proteins (Figure 1D). In this review article, we describe the structural importance of the diazepine ring to a variety of compounds that are built upon this core, as well as how modifications of this central ring and its chemical substituents enable the development of potent and selective chemical inhibitors of bromodomains. Such small molecule inhibitors can not only help dissect the functions of bromodomain-containing proteins and provide mechanistic insights into their role in gene transcription, but also continue to explore and validate bromodomains as potential epigenetic drug targets.
Benzodiazepines Targeting the GABAA Receptor
The therapeutic potential of the benzodiazepine scaffold was first recognized in the late 1950s when scientists at Roche submitted a previously untested compound for pharmacological evaluation. The compound was a remnant of previous research that had been conducted by the company on quinazoline N-oxides, and the scientists who submitted the compound were not anticipating favorable results. As fate would have it, this compound displayed strong sedative and muscle relaxant properties, and represented a potential improvement over the tranquilizers and hypnotics of the era. After intense investigation of this compound and similar products, Roche introduced the initial compound, chlordiazepoxide, to the market in 1960 under the brand name of Librium, and in 1963, released a similar yet more potent compound, diazepam, under the brand name of Valium (Sternbach, 1979).
The early success of these compounds brought about a widespread investigation of other benzodiazepines, as laboratories at Roche and elsewhere searched for compounds with similar neuromodulatory effects. In the following years, many analogs were produced and entered the clinic to produce various sedative, anxiolytic, or relaxant effects in patients. Even as serotonin-specific reuptake inhibitors (SSRIs) have taken the place of BZDs in the treatment of certain anxiety disorders, BZDs are still widely used, and are being actively studied in the search for selective, symptom-specific therapeutics (Griebel and Holmes, 2013; Nash and Nutt, 2007; Skolnick, 2012; Stahl, 2002).
BZDs serve as central nervous system (CNS) modulators by binding an allosteric site, aptly named the BZD site, of ionotropic GABAA receptors (Olkkola and Ahonen, 2008). GABAA receptors are heteropentamers made up of various different combinations of five subunits, taken from a pool of 19 known subunits. The BZD site arises at the junction of an α subunit (either α1, α2, α3, or α5) and a γ subunit (usually γ2) (Rudolph and Knoflach, 2011). The most common BZDs are agonists, as their binding promotes increased binding of GABA molecules to the active site of the GABAA receptor (Figures 1A and 1B). The enhanced rate of GABA binding increases the frequency of chloride channel opening, which hyperpolarizes the neuronal membrane and decreases the likelihood of an action potential. While agonists remain the most common BZDs, other members of the BZD class are inverse agonists that inhibit GABA from binding to its receptor, antagonists that mitigate effects of agonists and inverse agonists, and compounds that bind to peripheral BZD receptors (Wang et al., 1999). BZDs such as chlordiazepoxide and diazepam gained traction in the psychiatric community due to their superior efficacy and safety over barbiturates, especially as the mechanism of action of barbiturates on the GABAA receptor made overdose a much greater concern than it is with BZDs (Argyropoulos and Nutt, 1999; Atack, 2005). However, dependency, tolerance, and the potential for abuse are potential issues with the current BZDs that limit their long-term use (Rudolph and Knoflach, 2011).
Studies have shown that steric effects and the lipophilic nature of both the ligand and the binding site contribute greatly to BZD binding (Borea and Bonora, 1983; Villar et al., 1991; Wieland et al., 1992). The ligands seem to bind to regions that are quite sensitive to small additions of functional groups on the BZD scaffold, an observation that was made previously in the study of diazepine ligands that served either as CCK-A agonists or antagonists (Patchett and Nargund, 2000). Certain substitutions can increase activity against specific receptor subtypes, such as an electron-withdrawing group at position 7 of the BZD (Loew et al., 1984; Sternbach, 1979; Wang et al., 1999) (Figure 1A). A methyl group at position 1 of the BZD and modifications at positions 2’ and 4’ of the ring attached at position 5 of the BZD can affect potency as well (Sternbach, 1979). Multiple compounds, including alprazolam, have a triazole ring anchored at positions 1 and 2 of the diazepine in lieu of the carbonyl at position 2, providing activity against different receptor subtypes (Filippakopoulos et al., 2012a; VonVoigtlander and Straw, 1985) (Figure 1B). From the perspective of the ligand as a whole, it was demonstrated in the early 1980s that the closed 7-membered ring of a 1,4-benzodiazepine was essential to the ligand’s effect on the CNS, as the ring-opened form did not elicit the same activity (Fryer et al., 1982). This is not surprising, as the spatial presentation of functional groups to the receptor by the BZD scaffold would seem to be vital to activity.
The BZDs serve as important chemical tools in the site-directed mutagenesis studies that elucidate the functions of individual receptor subtypes. Many studies were launched from the finding that a single histidine residue at position 101 in the α1 subunit was vital for agonist binding, while the corresponding residue in the α6 subunit, arginine, drastically reduces ligand binding capability. When arginine was introduced to position 101 in the α1 subunit, agonist binding was lost, and moderate agonist binding was gained when histidine was introduced to the corresponding site in α6 (Wieland et al., 1992). Researchers used this information to develop genetically altered mice with histidine-to-arginine point mutations, and through the use of BZD inhibition in the altered mice and in wild type mice, were able to determine the activities controlled by each α subtype (Crestani et al., 2002; Crestani et al., 2001; Löw et al., 2000; McKernan et al., 2000; Rijnsoever et al., 2004; Rudolph et al., 1999; Rudolph and Knoflach, 2011). It is not only this conserved histidine that is important to agonist binding – receptor isoforms rely on many different residues to interact with different functional groups on BZD ligands (Amin et al., 1997; Buhr et al., 1996; Buhr et al., 1997; Kucken et al., 2000; Pritchett and Seeburg, 1991; Richter et al., 2012; Wieland and Luddens, 1994).
The SAR data collected on the BZD ligands and the growing knowledge of the functions of individual receptor subtypes provide tremendous opportunities for further research and potential drug development. The differences in receptor subtypes and the manner in which ligands make contact with different residues in the BZD binding site are growing topics of interest in the psychiatric field, as researchers seek to exploit these differences to develop anxioselective drugs (Atack, 2005; Estes, 1995; McKernan et al., 2000; Skolnick, 2012). This wide array of interactions accounts for the ability of some drugs to serve as anxiolytics, while others are sleep-inducing or muscle-relaxing (McKernan et al., 2000; Sternbach, 1979; Villar et al., 1991). However, overlapping effects among these compounds is a concern, and can bring about unintended problems. The ability to develop agents with a singular purpose (i.e. a compound that can serve as a daytime anxiolytic without inducing a sedative effect) may become an attainable goal as additional data on these interactions are obtained (Rudolph and Knoflach, 2011). In all, the GABAA receptor continues to be an intriguing target with high-impact therapeutic potential, and compounds with BZD scaffolds have the potential to be at the center of new findings, both literally and figuratively.
Versatility of the Diazepine Compounds
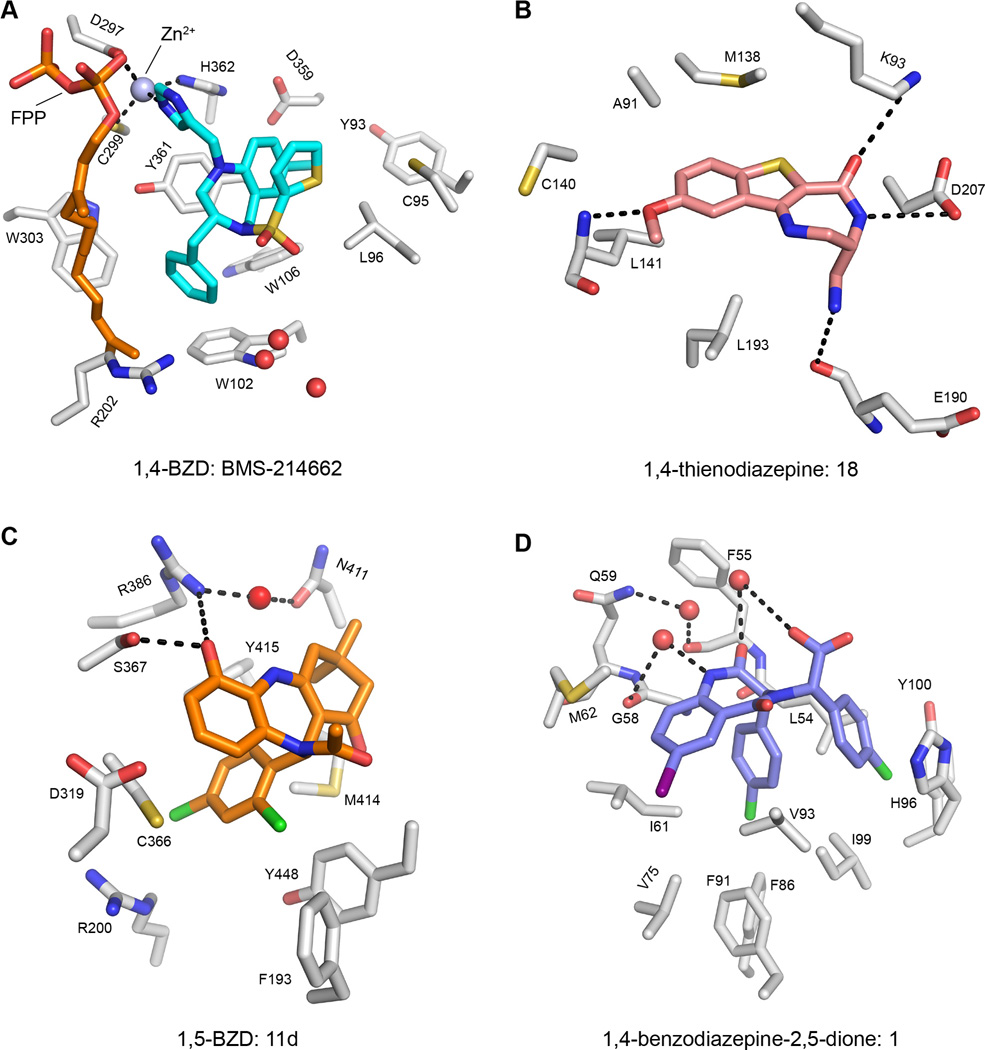
The unique physiochemical and structural qualities of diazepine compounds have led researchers to investigate their effects against a wide spectrum of drug targets beyond the GABAA receptor. For example, diazepine-based enzyme inhibitors have been reported for farnesyltransferases (FTases) (Reid and Beese, 2004) (Figure 2A), mitogen activated protein kinase-activated protein kinase 2 (MK2) (Anderson et al., 2009) (Figure 2B), Hepatitis C NS5B RNA polymerase (McGowan et al., 2009; Nyanguile et al., 2008; Vandyck et al., 2009) (Figure 2C), and histone deacetylases (HDACs) (Loudni et al., 2007). While each of these compounds contains the diazepine core, they are rather distinct from one another, placing each in a different subfamily. The FTase inhibitor is a 1,4-BZD, the MK2 inhibitor a 1,4-thienodiazepine, the HCV NS5B inhibitor a 1,5-BZD, and the HDAC inhibitor is a 1,4-benzodiazepine-2,5-dione. This series of enzyme inhibitors demonstrates the chemical diversity among diazepine scaffolds themselves, not just in the functional groups that the scaffolds present to their respective binding sites.
Figure 2. Diazepine compounds and their interactions with a wide variety of targets.
(A) The farnesyl-transferase inhibitor BMS-214662, a 1,4-BZD, in complex with a farnesyl diphosphate donor, FPP (PDB: 1SA5). The ligand coordinates a catalytic zinc ion and forms van der Waals interactions with surrounding protein residues, notably stacking interactions with Y361, W102, and W106. Water molecules near the binding pocket are represented by red spheres.
(B) A 1,4-thienodiazepine, 18, in complex with MK2 (PDB: 3FYK). Selectivity for MK2 over CDK2 is conferred through interactions with the K93 and D207 side chains, as well as the backbone atoms of L141 and E190.
(C) A 1,5-BZD, 11d, in complex with the HCV NS5B RNA Polymerase (PDB: 3GOL). The ligand binds to its target through a network of interactions with water molecules (the bound water from the ‘A’ monomer of the crystal structure is depicted as a red sphere) and nearby side chains.
(D) A 1,4-benzodiazepine-2,5-dione, 1, in complex with HDM2 (PDB: 1T4E). The ligand mimics an α-helix of a p53 peptide that interacts with HDM2, displaying the benzodiazepine’s versatility. In addition to interactions with the same hydrophobic side chains as the p53 peptide, this ligand gains affinity through interactions with bound water molecules (red spheres) and the highlighted residues.
Diazepine compounds can mimic protein secondary structures, further expanding the realm of biological targets they can act upon. BZD beta-turn mimics have been used as agonists of the melanocortin system of GPCRs (Haskell-Luevano et al., 1999; Joseph et al., 2008) and ligands with affinity for the angiotensin II receptor (Rosenström et al., 2006). A 1,4-benzodiazepine-2,5-dione was also synthesized as an alpha-helix mimic and used to inhibit the HDM2 interaction with the tumor suppressor protein p53 (Figure 2D) (Grasberger et al., 2005; Leonard et al., 2006). These peptidomimetic features displayed by diazepine-based compounds reflect an expansive functional versatility, as compounds can be generated that retain their cell permeability and possess favorable pharmacokinetic and pharmacodynamic properties as compared to typical peptide-based chemical leads. It is evident that this rigid, multifaceted scaffold possesses functionality against a multitude of targets, not simply isoforms of the GABAA receptor. One way in which this knowledge can be applied practically is through the development of focused libraries of diazepine-based compounds, which could be used to screen for new inhibitors of enzymes and protein-protein interactions (Welsch et al., 2010). Lead inhibitors emerging from such a library would have a basic template known to have favorable drug-like qualities, and possess the potential to be modified in many ways (from a medicinal chemistry standpoint) to fit individual targets.
Diazepines as New Bromodomain Inhibitors
The most recently recognized protein targets for diazepine-based compounds are a group of bromodomains, which are evolutionarily conserved protein modules embedded in a large number of transcription and chromatin regulator proteins. First reported as the acetyl-lysine binding domain in the transcriptional co-activator PCAF in 1999 (Dhalluin et al., 1999), the bromodomain functions to bind acetylated lysines in histones and non-histone proteins, thereby controlling acetylation-mediated protein-protein interactions in gene transcription in chromatin (Figure 3A and 3B). The human genome contains 61 unique bromodomains and 46 bromodomain-containing proteins (Filippakopoulos et al., 2012b). Each bromodomain is approximately 110 amino acids in length and has a conserved structural fold – a left-handed four-helix bundle, with helices termed αZ, αA, αB, and αC. Two inter-helical loops (the ZA loop, connecting αZ and αA, and the BC loop, between αB and αC) form a hydrophobic pocket in which the acetylated lysine of an associated protein can bind (Figure 4A) (Dhalluin et al., 1999). The key interaction that facilitates this binding is a hydrogen bond formed between a conserved asparagine residue and the carbonyl oxygen of the acetyl group (Figure 4B) (Owen et al., 2000). In addition to this asparagine, a network of conserved water molecules and other conserved residues in the ZA and BC loops give the bromodomains the ability to bind acetylated lysine. Selectivity among binding partners within the family arises from subtle sequence variations in the two loops (Filippakopoulos et al., 2012b; Mujtaba et al., 2007).
Figure 3. Bromodomain inhibitors modulate acetylation-mediated gene transcriptional activation in chromatin.
(A) Under physiological cellular conditions, the enzymes that regulate lysine acetylation and methylation of histone tails facilitate the opening and closing of chromatin, which in turn, activates or represses gene transcription, respectively. The epigenome reader domains that recognize these marks direct the formation of multi-protein complexes containing either the transcriptional machinery or other proteins responsible for maintaining the repressive state. A bromodomain inhibitor (BrDi) can inhibit gene transcriptional activation by blocking acetylationmediated protein-protein interactions required for chromatin remodeling and transcription machinery recruitment. This effect can also result in transcriptional repression through H3 lysine 27 methylation mediated chromatin compaction.
(B) Lysine acetylation of transcription factors is functionally important to their recruitment to target gene sites and assembly into the activated transcriptional machinery through interactions with bromodomain-containing chromatin regulatory proteins (CRPs) or histone modifying enzymes such as histone acetyltransferases (HATs). Green dots refer to lysine acetylation sites on transcription proteins.
Figure 4. Molecular basis of ligand recognition by the BRD4-BrD1.
(A) Crystal structure of BRD4-BrD1 in complex with a di-acetylated histone H4 peptide, H4K12ac/H4K16ac (PDB: 3UVX). The bound water molecules are depicted as pink spheres.
(B) Crystal structure of BRD4-BrD1 bound to H4K12ac/H4K16ac peptide superimposed with BrD inhibitor MS417. The key conserved residue Asn140 responsible for recognizing the acetyllysine in the binding pocket is highlighted. The five structured water molecules at the base of the binding pocket are shown as red spheres (ligand) and pink spheres (peptide).
(C, D, E and F) Structural depictions of the BRD4-BrD1 bound to diazepine- or azepine-based BrD inhibitors, JQ1 (PDB: 3MXF), MS417 (PDB: 4F3I), I-BET (PDB: 3P5O), or Isoxazole Azepine 3 (PDB: 4LRG), respectively. Motifs unique to each individual ligand at position C3 are highlighted in yellow, and BRD4 residues important for ligand recognition are noted. D144 in BRD4-BrD1 that is not conserved in BRD4-BrD2, is highlighted in orange. The bound water molecules at the base of the binding pocket are indicated as red spheres. Orange dashed lines feature the network of hydrogen bonds in the acetyl-lysine binding pocket among the triazole ring, key protein residues, and water molecules.
Due to their interactions with a number of diazepine-based inhibitors, the bromodomains of the BET proteins are arguably the most well-characterized human bromodomains to date. All BET family proteins (BRD2, BRD3, BRD4, and BRDT) utilize tandem bromodomains (BrD1 and BrD2) to bind to acetylated histones, and an extra-terminal (ET) domain to interact with other chromatin-associated proteins (Florence and Faller, 2001). The BET bromodomains can also interact with non-histone proteins that regulate transcription, DNA replication, cell cycle, and other cellular functions (Wu and Chiang, 2007). The BET family proteins, especially BRD4, have recently been shown to play an important role in the pathways that cause multiple types of cancer, including acute myeloid leukemia (Zuber et al., 2011), mixed lineage leukemia (Dawson et al., 2011), multiple myeloma (Mertz et al., 2011), and an aggressive form of squamous carcinoma called NUT (nuclear protein in testis) midline carcinoma (Filippakopoulos et al., 2010; French et al., 2008; French et al., 2001; French et al., 2003; Reynoird et al., 2010). These clinically relevant studies have validated the BET bromodomains as attractive epigenetic cancer therapy targets, and also displayed the need for potent and selective bromodomain inhibitors (Belkina and Denis, 2012; Furdas et al., 2012; Muller et al., 2011).
The versatility of diazepine compounds makes them excellent candidates to modulate bromodomain/acetyl-lysine binding functions in gene transcription. The Mitsubishi Tanabe Pharmaceutical Corporation first reported thienotriazolodiazepines targeting the bromodomains of the BET family proteins as inhibitors of inflammation in autoimmune diseases in their patents filed in 2006 and 2008 (Adachi et al., 2006; Miyoshi et al., 2008). Increased knowledge of BET family bromodomains and their emergence as attractive drug targets led multiple research groups to build on the scaffold put forth by Mitsubishi Tanabe and develop nanomolar-affinity inhibitors of their own. One such effort yielded a thienotriazolodiazepine named JQ1, which binds tightly to BRD4 bromodomains, but not to the GABAA receptor nor to other non-BET bromodomains (Filippakopoulos et al., 2010). The BZD GSK525762A (referred to as I-BET) (Nicodeme et al., 2010) and another further improved thienotriazolodiazepine, MS417 (Miyoshi et al., 2008; Zhang et al., 2012), have also been published as potent inhibitors of BET bromodomains. The core structures of these ligands (Figure 1D) are similar to those of the clinical BZDs (Figures 1A and 1B), but various unique substitutions confer their specificity for the bromodomains.
Structural Considerations of BET Bromodomain Inhibition
The inhibitors of BET protein bromodomains bind in the acetyl-lysine binding pocket, serving as orthosteric modulators of function (Figures 4A and 4B) (Chung et al., 2011), directly contrasting with the allosteric mechanism utilized by BZDs that bind to the GABAA receptor. Within this pocket, the triazole ring found on each of the highlighted BET-specific inhibitors mimics the acetyl group of the typical biological ligand. The two nitrogen atoms of the ring that enter into the pocket interact with the network of bound water molecules and the conserved asparagine and tyrosine residues (Asn140 and Tyr97 in BRD4-BrD1). The methyl group attached to the triazole ring binds in a deep region of the pocket that is responsible for recognizing the methyl portion of the acetyl group of an acetyl-lysine. The diazepine scaffold plays a major role in directing how these inhibitors fit into the pocket. JQ1, which has a thienodiazepine scaffold, is described as having “an extraordinary shape complementarity with the KAc binding site” of both bromodomains of BET proteins (Filippakopoulos et al., 2010). The BZD scaffold of I-BET is also described as having a curvature to it that helps the ligand extend beyond the binding region that a lysine-acetylated histone would occupy, deep into a lipophilic pocket called the ZA channel (Chung et al., 2011; Nicodeme et al., 2010). These features illustrate that the triazolodiazepine inhibitors not only have the ability to mimic the important interactions that facilitate acetyl-lysine binding, but also that they sterically complement the binding site.
As alluded to above, the functional groups presented by the diazepine scaffold make important hydrophobic contacts just outside of the acetyl-lysine binding pocket in the ZA and BC loops. The WPF shelf, a tryptophan-proline-phenylalanine sequence found in all BET bromodomains (Trp81, Pro82, Phe83 in BRD4-BrD1), interacts with the pendant phenyl group of the highlighted diazepine inhibitors (Chung et al., 2011; Nicodeme et al., 2010). Access to this region is controlled by the so-called “gatekeeper” residue (Ile146 in BRD4-BrD1), which has been found to adopt a different conformation in the apo form of BRD4-BrD1 as opposed to the ligand-bound form of the domain (Filippakopoulos et al., 2012a; Prinjha et al., 2012). The gatekeeper residue is an isoleucine in the BrD1 of each of the BET proteins, while it is a valine in the BrD2. However, other bromodomains, such as those of PCAF and GCN5, have a tyrosine as their gatekeeper residue. This explains the high selectivity of this class of diazepine inhibitors for the BET protein bromodomains. The presence of a bulkier gatekeeper residue may prevent the pendant phenyl ring of these compounds from interacting with the WPF shelf, disrupting binding (Chung, 2012).
Analysis of the top BET-specific inhibitors shows that stereochemistry plays an important role in their binding modes. Potent inhibitors all have substituents in the S configuration at position C3 of their diazepine rings (blue highlights in Figures 4C and 4E). R-enantiomers of JQ1, I-BET, and MS417 were tested against BRD4-BrD1 and other BET family members, and all proved inactive due to steric clashes with Leu92 and Leu94, and in the case of MS417’s enantiomer, Tyr139 (Chung et al., 2011; Filippakopoulos et al., 2010; Zhang et al., 2012).
Aside from its stereochemistry, the moiety attached to C3 is important to take into account, especially when considering potential off-target effects of BET inhibitors. Because their scaffolds are so similar to the CNS-targeted BZDs used in the clinic, diazepine-based bromodomain inhibitors must be carefully constructed as to not induce these neurological effects in the patients who may be receiving them. For example, one of the main structural features of JQ1 is a bulky t-butyl ester group attached at C3 (Figure 4C), which is cited as the reason that JQ1 shows little activity against the BZD allosteric binding site on the GABAA receptor (Filippakopoulos et al., 2010). Indeed, past SAR studies on BZDs show that bulky groups at C3 greatly reduce the activity of a diazepine compound against its neurological target (Borea and Bonora, 1983). Additionally, recently published data indicate that BZDs targeted for the bromodomain do not interact with the GABAA receptor if certain substituents are introduced at either the meta or para positions of the pendant phenyl ring (Mirguet et al., 2013). Depending on the ligand, it appears that groups at both sites may have the potential to prevent off-target interactions. MS417 and I-BET, alternatively, seek to limit off-target effects with moieties at C3 that increase affinity for BET protein bromodomains, rather than relying on the prevention of other interactions.
MS417 contains a methyl ester at this position (Figure 4D) and I-BET contains an ethyl amine (Figure 4E), as opposed to the bulky t-butyl ester of JQ1 (Zhang et al., 2012). The crystal structure of the BRD4-BrD1/MS417 complex shows that the ligand not only binds deep in the acetyl-lysine binding pocket like its counterparts do, but also its methyl ester group makes contacts with Leu94 and Tyr139 in a hydrophobic cavity between the ZA and BC loops. While the t-butyl group of JQ1 is too large to fit into this cavity, MS417 utilizes these contacts to boost affinity for both BRD4-BrD1 and BRD4-BrD2 by 5–10-fold as compared to those of JQ1. Additionally, the BZD SAR data cited above (Borea and Bonora, 1983) shows that the methyl ester and ethyl amine may still be large enough functional groups to prevent interactions with the BZD binding site on the GABAA receptor. Therefore, MS417 and I-BET have both improved BET family affinity and a potentially similar block against off-target effects as JQ1.
The differences among diazepines designed to inhibit BET protein bromodomains and those that bind to the GABAA receptor have been further elucidated through the testing of the GABAA agonists against the BET bromodomains (Filippakopoulos et al., 2012a). The data from this paper show that alprazolam and midazolam interact with the BET bromodomains, but due to altered interactions with conserved residues and network of water molecules within the binding site, they only bind with moderate affinities. Alprazolam possesses the same triazole ring and methyl group as the BET family inhibitors, but lacks many of the other features of the designed inhibitors that contribute to binding. Midazolam binds with an imidazole instead of a triazole, potentially altering its binding with the conserved asparagine, and also lacks the other molecular features of the BET family inhibitors. On the other hand, the data show that estazolam and triazolam do not bind to members of the BET family. In the case of estazolam, this loss of affinity reinforces the importance of the interaction between the methyl group of the triazole ring and the base of the acetyl-lysine binding pocket among the BET inhibitors, as this moiety is not present in estazolam. The lack of affinity of triazolam for the acetyl-lysine binding pocket shows that a large halogen at the ortho position of the phenyl ring precludes binding. In terms of unintended effects, the BZDs may interact with the BET bromodomains only in the range of micromolar concentrations, indicating that gene transcription-related side effects from BZDs prescribed as sedatives do not appear likely to occur.
Alternatives Abound, but a Bright Future
A prime argument for using privileged scaffolds in drug development is their ability to work on multiple targets. However, lead compounds built on a core scaffold with potential activity for multiple targets could also be difficult to optimize into a selective drug with minimal off-target effects. As discussed above in this review, as it pertains to JQ1 and the GABAA receptor, the diazepine-based bromodomain inhibitors are tailored to target bromodomains via added functional groups in an attempt to mitigate this risk. But short of testing these compounds in human subjects, there is no way to state this with certainty.
The promise yet potential limitation of diazepine-based bromodomain inhibitors have inspired the development of other classes of small molecule compounds with distinct scaffolds as potent and selective bromodomain inhibitors. For instance, a 1,3,4-benzotriazepine, BzT-7, shows sub-micromolar affinity for the BET family, and displays the same binding mode as the other BET family inhibitors (Filippakopoulos et al., 2012a). Constellation Pharmaceuticals recently published binding, crystallographic, and pharmacokinetic/pharmacodynamic (PK/PD) data related to an isoxazole azepine compound, which takes on a nearly identical binding mode as the diazepine inhibitors described above (Figure 4F) (Gehling et al., 2013). In the case of this ligand, an isoxazole ring serves as the mimic of the acetylated lysine instead of a triazole ring, and the group at position C3 of the azepine ring is a primary carboxamide. Additional compounds with cycloheptene and azepine scaffolds have also been tested for their efficacy by Constellation, but their precise target-binding and crystallographic data have not been released (Albrecht et al., 2012).
Several groups have also utilized structure-guided fragment-based drug design or virtual screening to discover and develop BET family inhibitors that do not have a seven-membered ring scaffold (Bamborough et al., 2012; Chung et al., 2012; Hewings et al., 2011; Lucas et al., 2013; Picaud et al., 2013a; Seal et al., 2012). Some of these compounds have a quinolone scaffold, while others are still fragments based off of a phenyl group, and are in the early stages of development. As is evident from Constellation’s recent work, new ways to mimic the acetylated lysine are also being explored, as researchers are attempting to determine if inhibitors with an isoxazole ring as the acetyl-lysine mimic are better candidates for future development than the existing family of compounds that utilize the triazole ring (Albrecht et al., 2012; Bamborough et al., 2012; Dawson et al., 2011; Hewings et al., 2011; Mirguet et al., 2012; Seal et al., 2012).
Even as the efficacy and selectivity of alternative scaffolds are actively being tested, the story of the triazolodiazepine family of compounds remains incomplete due to their lack of selectivity within the BET family. The current triazolodiazepines can be further developed using structure-based rational drug design into compounds selective for an individual protein among the BET family members, or for one of the tandem bromodomains of a single BET family member. For the latter, the potential for success lies in the subtle variations between the tandem domains of the BET proteins, such as Asp144 in BRD4-BrD1 and its counterpart His433 in BRD4-BrD2 (orange highlights in Figures 4C–4F) (Zhang et al., 2012). One quinazolone compound, RVX-208, shows selectivity for BrD2s of the BET family members, but lacks the potency of the diazepine compounds (Picaud et al., 2013b). As currently constructed, the diazepine scaffold has the space to accept additional functionality, allowing for the potential development of a new compound that may favor one BrD of a BET family member over the other, while maintaining the potency of the current series of diazepine BET family inhibitors. With such inhibitors in hand, researchers could address how the two tandem bromodomains of BET protein may function differentially in control of gene transcription in chromatin.
The diazepine’s potential for inhibiting non-BET bromodomains remains unclear. JQ1, MS417, and I-BET were all tested against other bromodomains, including ones that have been referred to as “druggable” (Vidler et al., 2012), yet each showed selectivity only limited to the BET bromodomains (Filippakopoulos et al., 2010; Nicodeme et al., 2010; Zhang et al., 2012). Both the conserved structural fold among members of the bromodomain family and the diazepine’s ability to house numerous functional groups could contribute to the construction of diazepine-based compounds as inhibitors of other bromodomains. For example, a fragmentbased approach akin to the one used in the search for alternative BET bromodomain inhibitors could show that a diazepine binds deep within the binding pocket of a non-BET bromodomain, making it a suitable scaffold to build upon. The functional groups of JQ1, MS417, and I-BET may have previously prevented such an interaction from being detected, yet starting with a scaffold and building an inhibitor in a rational manner to match an individual bromodomain may prove to be successful.
Inhibitors of bromodomain-containing proteins are a part of the extensive pipeline of epigenetic modulators that has the potential to bring advanced, targeted treatments to patients in the near future (Arrowsmith et al., 2012; Dawson et al., 2012; DeWoskin and Million, 2013; Kelly et al., 2010). One of the triazolodiazepines featured in this review, I-BET (GSK525762; GlaxoSmithKline) has recently entered into a clinical trial for NUT midline carcinoma. Other notable bromodomain inhibitors are in the clinical development process for the treatment of different diseases – TEN-010 (Tensha Therapeutics) for NUT midline carcinoma, the quinazolone RVX-208 (Resverlogix) for atherosclerosis and type 2 diabetes, OTX015 (Oncoethix) for haematological malignancies, and CPI-0610 (Constellation Pharmaceuticals) for progressive lymphoma (http://clinicaltrials.gov). Aside from RVX-208, the bromodomain inhibitors being clinically evaluated are diazepine-based compounds.
Recent studies detail additional potential breakthrough uses of these compounds. JQ1 has been shown to cross the hemato-testicular barrier, and is being explored as an inhibitor of BRDT for male contraception (Gaucher et al., 2012; Matzuk et al., 2012). Data shows that JQ1 can cross the blood-brain barrier, as well (Matzuk et al., 2012), leading researchers to test JQ1 against glioblastoma tumors, both in vitro and in an in vivo mouse model (Cheng et al., 2013). They found that JQ1 altered the levels of multiple genes important to glioblastoma growth, and that JQ1 slowed tumor growth and increased survival time in mice with tumors. With the ability of the bromodomain-inhibiting diazepine compounds to cross the blood-brain barrier and not have an effect on neurological function, further testing of this nature should be in order.
Conclusion
Privileged chemical scaffolds such as the diazepine play an important role in drug design for new, targeted therapeutic treatment for human disease. These scaffolds possess favorable physiochemical and versatile structural qualities that make them excellent templates for medicinal chemists to develop lead inhibitors to modulate molecular functions of drug targets. In the case of the diazepine scaffold, the unique geometry of its seven-membered ring allows it to fit tightly in a binding site while serving as a steady framework for the addition of functional groups necessary to interact selectively with amino acid residues in a target protein, improving both affinity and selectivity. This core structure fits the definition of a “privileged structure” in drug design, as it forms the centerpiece of small molecule chemical ligands that are constructed to target many different receptors including GABAA, multiple enzymes, and most recently, the epigenome-reading bromodomains. The recent research into diazepine-based compounds as bromodomain inhibitors further highlights the great potential for this functionally versatile scaffold to serve as a solid fundamental chemical foundation upon which novel therapeutic agents are being developed as new epigenetic therapies to treat a wide range of human diseases including cancer and inflammation.
Acknowledgments
We wish to thank Dr. Jamel Meslamani for discussion. This work was supported in part by the research grants from the National Institutes of Health (M.-M.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests statement
The authors declare no competing financial interests.
References
- Adachi K, Hikawa H, Hamada M, Endoh J-i, Ishibuchi S, Fujie N, Tanaka M, Sugahara K, Oshita K, Murata M. Thienotriazolodiazepine Compound and Medicinal Use Thereof. Japan: Mitsubishi Tanabe Pharma Corporation; 2006. [Google Scholar]
- Albrecht BK, Audia JE, Cote A, Gehling VS, Harmange J-C, Hewitt MC, LeBlanc Y, Naveschuk CG, Taylor AM, Vaswani RG. Bromodomain Inhibitors and Uses Thereof. USA: Constellation Pharmaceuticals, Inc.; 2012. [Google Scholar]
- Amin J, Brooks-Kayal A, Weiss DS. Two Tyrosine Residues on the α Subunit Are Crucial for Benzodiazepine Binding and Allosteric Modulation of γ-Aminobutyric AcidA Receptors. Mol Pharmacol. 1997;51:833–841. doi: 10.1124/mol.51.5.833. [DOI] [PubMed] [Google Scholar]
- Anderson DR, Meyers MJ, Kurumbail RG, Caspers N, Poda GI, Long SA, Pierce BS, Mahoney MW, Mourey RJ. Benzothiophene inhibitors of MK2. Part 1: Structure-activity relationships, assessments of selectivity and cellular potency. Bioorg Med Chem Lett. 2009;19:4878–4881. doi: 10.1016/j.bmcl.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Argyropoulos SV, Nutt DJ. The use of benzodiazepines in anxiety and other disorders. Euro Neuropsychopharmacol. 1999;9:S407–S412. doi: 10.1016/s0924-977x(99)00052-8. [DOI] [PubMed] [Google Scholar]
- Arrowsmith CH, Bountra C, Fish PV, Lee K, Schapira M. Epigenetic protein families: a new frontier for drug discovery. Nat Rev Drug Disc. 2012;11:384–400. doi: 10.1038/nrd3674. [DOI] [PubMed] [Google Scholar]
- Atack JR. The benzodiazepine binding site of GABAA receptors as a target for the development of novel anxiolytics. Expert Opin Investig Drugs. 2005;14:601–618. doi: 10.1517/13543784.14.5.601. [DOI] [PubMed] [Google Scholar]
- Bamborough P, Diallo H, Goodacre JD, Gordon L, Lewis A, Seal JT, Wilson DM, Woodrow MD, Chung C-w. Fragment-Based Discovery of Bromodomain Inhibitors Part 2: Optimization of Phenylisoxazole Sulfonamides. J Med Chem. 2012;55:587–596. doi: 10.1021/jm201283q. [DOI] [PubMed] [Google Scholar]
- Belkina AC, Denis GV. BET domain co-regulators in obesity, inflammation and cancer. Nat Rev Cancer. 2012;12:465–477. doi: 10.1038/nrc3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermak J, Johnstone T, Gee K. Encyclopedia of Stress. New York, NY: 2007. Benzodiazepines; pp. 310–316. [Google Scholar]
- Borea PA, Bonora A. Brain Receptor Binding and Lipophilic Character of Benzodiazepines. Biochemical Pharmacology. 1983;32:603–607. doi: 10.1016/0006-2952(83)90482-3. [DOI] [PubMed] [Google Scholar]
- Buhr A, Baur R, Malherbe P, Sigel E. Point Mutations of the α1β2γ2 γ-Aminobutyric AcidA Receptor Affecting Modulation of the Channel by Ligands of the Benzodiazepine Binding Site. Mol Pharmacol. 1996;49:1080–1084. [PubMed] [Google Scholar]
- Buhr A, Schaerer MT, Baur R, Sigel E. Residues at Positions 206 and 209 of the α1 Subunit of γ-Aminobutyric AcidA Receptors Influence Affinities for Benzodiazepine Binding Site Ligands. Mol Pharmacol. 1997;52:676–682. doi: 10.1124/mol.52.4.676. [DOI] [PubMed] [Google Scholar]
- Burger A. Isosterism and bioisosterism in drug design. Prog Drug Res. 1991;37:287–371. doi: 10.1007/978-3-0348-7139-6_7. [DOI] [PubMed] [Google Scholar]
- Carlson EE. Natural Products as Chemical Probes. ACS Chem Biol. 2010;5:639–653. doi: 10.1021/cb100105c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Gong Y, Ma Y, Lu K, Lu X, Pierce LA, Thompson RC, Muller S, Knapp S, Wang J. Inhibition of BET Bromodomain Targets Genetically Diverse Glioblastoma. Clinical Cancer Research. 2013;19:1–12. doi: 10.1158/1078-0432.CCR-12-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C-w. Small Molecule Bromodomain Inhibitors: Extending the Druggable Genome. Prog Med Chem. 2012;51:1–55. doi: 10.1016/B978-0-12-396493-9.00001-7. [DOI] [PubMed] [Google Scholar]
- Chung C-w, Coste H, White JH, Mirguet O, Wilde J, Gosmini RL, Delves C, Magny SM, Woodward R, Hughes SA, et al. Discovery and Characterization of Small Molecule Inhibitors of the BET Family Bromodomains. J Med Chem. 2011;54:3827–3838. doi: 10.1021/jm200108t. [DOI] [PubMed] [Google Scholar]
- Chung C-w, Dean AW, Woolven JM, Bamborough P. Fragment-Based Discovery of Bromodomain Inhibitors Part 1: Inhibitor Binding Modes and Implications for Lead Discovery. J Med Chem. 2012;55:576–586. doi: 10.1021/jm201320w. [DOI] [PubMed] [Google Scholar]
- Costantino L, Barlocco D. Privileged Structures as Leads in Medicinal Chemistry. Curr Med Chem. 2006;13:65–85. [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy J-M, Benke D, Vogt K, Prut L, Bluethmann H, Möhler H, Rudolph U. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc Natl Acad Sci USA. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Löw K, Keist R, Mandelli M-J, Möhler H, Rudolph U. Molecular Targets for the Myorelaxant Action of Diazepam. Mol Pharmacol. 2001;59:442–445. doi: 10.1124/mol.59.3.442. [DOI] [PubMed] [Google Scholar]
- Dawson MA, Kouzarides T, Huntly BJP. Targeting Epigenetic Readers in Cancer. N Engl J Med. 2012;367:647–657. doi: 10.1056/NEJMra1112635. [DOI] [PubMed] [Google Scholar]
- Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan W-I, Robson SC, Chung C-w, Hopf C, Savitski MM, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478:529–533. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWoskin VA, Million RP. The epigenetics pipeline. Nat Rev Drug Disc. 2013;12:661–662. doi: 10.1038/nrd4091. [DOI] [PubMed] [Google Scholar]
- Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou M-M. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399:491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- Dubnick B, Lippa AS, Klepner CA, Coupet J, Greenblatt EN, Beer B. The Separation of 3H-Benzodiazepine Binding Sites in Brain and of Benzodiazepine Pharmacological Properties. Pharmacology Biochemistry & Behavior. 1983;18:311–318. doi: 10.1016/0091-3057(83)90385-4. [DOI] [PubMed] [Google Scholar]
- Estes JW. The Road to Tranquility: The Search for Selective Anti-Anxiety Agents. Synapse. 1995;21:10–20. doi: 10.1002/syn.890210103. [DOI] [PubMed] [Google Scholar]
- Evans BE, Bock MG, Rittle KE, DiPardo RM, Whitter WL, Veber DF, Anderson PS, Freidinger RM. Design of potent, orally effective, nonpeptidal antagonists of the peptide hormone cholecystokinin. Proc Natl Acad Sci USA. 1986;83:4918–4922. doi: 10.1073/pnas.83.13.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BE, Rittle KE, Bock MG, DiPardo RM, Freidinger RM, Whitter WL, Lundell GF, Veber DF, Anderson PS, Chang RSL, et al. Methods for Drug Discovery: Development of Potent, Selective, Orally Effective Cholecystokinin Antagonists. J Med Chem. 1988;31:2235–2246. doi: 10.1021/jm00120a002. [DOI] [PubMed] [Google Scholar]
- Filippakopoulos P, Picaud S, Fedorov O, Keller M, Wrobel M, Morgenstern O, Bracher F, Knapp S. Benzodiazepines and benzotriazepines as protein interaction inhibitors targeting bromodomains of the BET family. Bioorg Med Chem. 2012a;20:1878–1886. doi: 10.1016/j.bmc.2011.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippakopoulos P, Picaud S, Mangos M, Keates T, Lambert J-P, Barsyte-Lovejoy D, Felletar I, Volkmer R, Muller S, Pawson T, et al. Histone Recognition and Large-Scale Structural Analysis of the Human Bromodomain Family. Cell. 2012b;149:214–231. doi: 10.1016/j.cell.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florence B, Faller DV. You BET-Cha: A Novel Family of Transcriptional Regulators. Frontiers in Bioscience. 2001;6:1008–1018. doi: 10.2741/florence. [DOI] [PubMed] [Google Scholar]
- French C, Ramirez C, Kolmakova J, Hickman T, Cameron M, Thyne M, Kutok J, Toretsky J, Tadavarthy A, Kees U, et al. BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008;27:2237–2242. doi: 10.1038/sj.onc.1210852. [DOI] [PubMed] [Google Scholar]
- French CA, Miyoshi I, Aster JC, Kubonishi I, Kroll TG, Cin PD, Vargas SO, Perez-Atayde AR, Fletcher JA. BRD4 Bromodomain Gene Rearrangement in Aggressive Carcinoma with Translocation t(15;19) American Journal of Pathology. 2001;159:1987–1992. doi: 10.1016/S0002-9440(10)63049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French CA, Miyoshi I, Kubonishi I, Grier HE, Perez-Atayde AR, Fletcher JA. BRD4-NUT Fusion Oncogene: A Novel Mechanism in Aggressive Carcinoma. Cancer Research. 2003;63:304–307. [PubMed] [Google Scholar]
- Fryer RI, Leimgruber W, Trybulski EJ. Quinazolines and 1,4-Benzodiazepines. 90. Structure-Activity Relationship between Substituted 2-Amino-N-(2-benzoyl-4-chlorophenyl)acetamides and 1,4-Benzodiazepinones. J Med Chem. 1982;25:1050–1055. doi: 10.1021/jm00351a009. [DOI] [PubMed] [Google Scholar]
- Furdas SD, Carlino L, Sippl W, Jung M. Inhibition of bromodomain-mediated protein-protein interactions as a novel therapeutic strategy. Med Chem Commun. 2012;3:123–134. [Google Scholar]
- Gaucher J, Boussouar F, Montellier E, Curtet S, Buchou T, Bertrand S, Hery P, Jounier S, Depaux A, Vitte A-L, et al. Bromodomain-dependent stage-specific male genome programming by Brdt. EMBO J. 2012;31:3809–3820. doi: 10.1038/emboj.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehling VS, Hewitt MC, Vaswani RG, Leblanc Y, Cote A, Nasveschuk CG, Taylor AM, Harmange J-C, Audia JE, Pardo E, et al. Discovery, Design, and Optimization of Isoxazole Azepine BET Inhibitors. ACS Med Chem Lett. 2013 doi: 10.1021/ml4001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasberger BL, Lu T, Schubert C, Parks DJ, Carver TE, Koblish HK, Cummings MD, LaFrance LV, Milkiewicz KL, Calvo RR, et al. Discovery and Cocrystal Structure of Benzodiazepinedione HDM2 Antagonists That Activate p53 in Cells. J Med Chem. 2005;48:909–912. doi: 10.1021/jm049137g. [DOI] [PubMed] [Google Scholar]
- Griebel G, Holmes A. 50 years of hurdles and hope in anxiolytic drug discovery. Nat Rev Drug Disc. 2013;12:667–687. doi: 10.1038/nrd4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell-Luevano C, Rosenquist A, Souers A, Khong KC, Ellman JA, Cone RD. Compounds That Activate the Mouse Melanocortin-1 Receptor Identified by Screening a Small Molecule Library Based upon the β-Turn. J Med Chem. 1999;42:4380–4387. doi: 10.1021/jm990190s. [DOI] [PubMed] [Google Scholar]
- Hewings DS, Wang M, Philpott M, Fedorov O, Uttarkar S, Filippakopoulos P, Picaud S, Vuppusetty C, Marsden B, Knapp S, et al. 3,5-Dimethylisoxazoles Act As Acetyl-lysine-mimetic Bromodomain Ligands. J Med Chem. 2011;54:6761–6770. doi: 10.1021/jm200640v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton DA, Bourne GT, Smythe ML. The Combinatorial Synthesis of Bicyclic Privileged Structures or Privileged Substructures. Chem Rev. 2003;103:893–930. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]
- http://clinicaltrials.gov. ClinicalTrials.gov.
- Huang Y, Dömling A. 1,4-Thienodiazepine-2,5-diones via MCR (II): Scaffold Hopping by Gewald and Ugi-Deprotection-Cyclization Strategy. Chem Biol Drug Des. 2010;76:130–141. doi: 10.1111/j.1747-0285.2010.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Wolf S, Bista M, Meireles L, Camacho C, Holak TA, Dömling A. 1,4-Thienodiazepine-2,5-diones via MCR (I): Synthesis, Virtual Space and p53-Mdm2 Activity. Chem Biol Drug Des. 2010;76:116–129. doi: 10.1111/j.1747-0285.2010.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph CG, Wilson KR, Wood MS, Sorenson NB, Phan DV, Xiang Z, Witek RM, Haskell-Luevano C. The 1,4-Benzodiazepine-2,5-dione Small Molecule Template Results in Melanocortin Receptor Agonists with Nanomolar Potencies. J Med Chem. 2008;51:1423–1431. doi: 10.1021/jm701303z. [DOI] [PubMed] [Google Scholar]
- Kelly TK, Carvalho DDD, Jones PA. Epigenetic modifications as therapeutic targets. Nat Biotech. 2010;28:1069–1078. doi: 10.1038/nbt.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucken AM, Wagner DA, Ward PR, Teissere JA, Boileau AJ, Czajkowski C. Identification of Benzodiazepine Binding Site Residues in the γ2 Subunit of the γ-Aminobutyric AcidA Receptor. Mol Pharmacol. 2000;57:932–939. [PubMed] [Google Scholar]
- Leonard K, Marugan JJ, Raboisson P, Calvo R, Gushue JM, Koblish HK, Lattanze J, Zhao S, Cummings MD, Player MR, et al. Novel 1,4-benzodiazepine-2,5-diones as Hdm2 antagonists with improved cellular activity. Bioorg Med Chem Lett. 2006;16:3463–3468. doi: 10.1016/j.bmcl.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discovery Today: Technologies. 2004;1:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced Drug Delivery Reviews. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Loew GH, Nienow JR, Poulsen M. Theoretical Structure-Activity Studies of Benzodiazepine Analogues: Requirements for Receptor Affinity and Activity. Mol Pharmacol. 1984;26:19–34. [PubMed] [Google Scholar]
- Loudni L, Roche J, Potiron V, Clarhaut J, Bachmann C, Gesson J-P, Tranoy-Opalinski I. Design, synthesis and biological evaluation of 1,4-benzodiazepine-2,5-dione-based HDAC inhibitors. Bioorg Med Chem Lett. 2007;17:4819–4823. doi: 10.1016/j.bmcl.2007.06.067. [DOI] [PubMed] [Google Scholar]
- Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, Fritschy J-M, Rülicke T, Bluethmann H, Möhler H, et al. Molecular and Neuronal Substrate for the Selective Attenuation of Anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Lucas X, Wohlwend D, Hugle M, Schmidtkunz K, Gerhardt S, Schule R, Jung M, Einsle O, Gunther S. 4-Acyl Pyrroles: Mimicking Acetylated Lysines in Histone Code Reading. Angew Chem Int Ed. 2013;52:14055–14059. doi: 10.1002/anie.201307652. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, McKeown MR, Filippakopoulos P, Li Q, Ma L, Agno JE, Lemieux ME, Picaud S, Yu RN, Qi J, et al. Small-Molecule Inhibition of BRDT for Male Contraception. Cell. 2012;150:673–684. doi: 10.1016/j.cell.2012.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan D, Nyanguile O, Cummings MD, Vendeville S, Vandyck K, Broeck WVd, Boutton CW, Bondt HD, Quirynen L, Amssoms K, et al. 1,5-Benzodiazepine inhibitors of HCV NS5B polymerase. Bioorg Med Chem Lett. 2009;19:2492–2496. doi: 10.1016/j.bmcl.2009.03.035. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nature Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA, Bergeron L, III, RJS Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci USA. 2011;108:16669–16674. doi: 10.1073/pnas.1108190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirguet O, Gosmini R, Toum J, Clement CA, Barnathan M, Brusq J-M, Mordaunt JE, Grimes RM, Crowe M, Pineau O, et al. Discovery of Epigenetic Regulator IBET762: Lead Optimization to Afford a Clinical Candidate Inhibitor of the BET Bromodomains. J Med Chem. 2013;56:7501–7515. doi: 10.1021/jm401088k. [DOI] [PubMed] [Google Scholar]
- Mirguet O, Lamotte Y, Donche Fdr, Toum Jrm, Gellibert Fo, Bouillot A, Gosmini R, Nguyen V-L, Delannée D, Seal J, et al. From ApoA1 upregulation to BET family bromodomain inhibition: Discovery of I-BET151. Bioorg Med Chem Lett. 2012;22:2963–2967. doi: 10.1016/j.bmcl.2012.01.125. [DOI] [PubMed] [Google Scholar]
- Miyoshi S, Ooike S, Iwata K, Hikawa H, Sugahara K. Antitumor Agent. Japan: Mitsubishi Tanabe Pharma Corporation; 2008. [Google Scholar]
- Mujtaba S, Zeng L, Zhou M-M. Structure and acetyl-lysine recognition of the bromodomain. Oncogene. 2007;26:5521–5527. doi: 10.1038/sj.onc.1210618. [DOI] [PubMed] [Google Scholar]
- Muller S, Filippakopoulos P, Knapp S. Bromodomains as therapeutic targets. Expert Rev Mol Med. 2011;13:1–21. doi: 10.1017/S1462399411001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash JR, Nutt DJ. Pharmacotherapy of Anxiety. In: Sibley DR, Hanin I, Kuhar M, Skolnick P, editors. Handbook of Contemporary Neuropharmacology. John Wiley & Sons, Inc.; 2007. pp. 59–91. [Google Scholar]
- Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung C-w, Chandwani R, Marazzi I, Wilson P, Coste H, et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468:1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyanguile O, Pauwels F, Broeck WVd, Boutton CW, Quirynen L, Ivens T, Helm Lvd, Vandercruyssen G, Mostmans W, Delouvroy F, et al. 1,5-Benzodiazepines, a Novel Class of Hepatitis C Virus Polymerase Nonnucleoside Inhibitors. Antimicrob Agents Chemother. 2008;52:4420–4431. doi: 10.1128/AAC.00669-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkkola KT, Ahonen J. Midazolam and Other Benzodiazepines. Handb Exp Pharmacol. 2008;182:335–360. doi: 10.1007/978-3-540-74806-9_16. [DOI] [PubMed] [Google Scholar]
- Owen DJ, Ornaghi P, Yang J-C, Lowe N, Evans PR, Ballario P, Neuhaus D, Filetici P, Travers AA. The structural basis for the recognition of acetylated histone H4 by the bromodomain of histone acetyltransferase Gcn5p. EMBO J. 2000;19:6141–6149. doi: 10.1093/emboj/19.22.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchett AA, Nargund RP. Privileged Structures - An Update. Annual Reports in Medicinal Chemistry. 2000;35:289–298. [Google Scholar]
- Picaud S, Costa DD, Thanasopoulou A, Filippakopoulos P, Fish PV, Philpott M, Fedorov O, Brennan P, Bunnage ME, Owen DR, et al. PFI-1, a Highly Selective Protein Interaction Inhibitor, Targeting BET Bromodomains. Cancer Research. 2013a;73:3336–3346. doi: 10.1158/0008-5472.CAN-12-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picaud S, Wells C, Felletar I, Brotherton D, Martin S, Savitsky P, Diez-Dacal B, Philpott M, Bountra C, Lingard H, et al. RVX-208, an inhibitor of BET transcriptional regulators with selectivity for the second bromodomain. Proc Natl Acad Sci. 2013b doi: 10.1073/pnas.1310658110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinjha RK, Witherington J, Lee K. Place your BETs: the therapeutic potential of bromodomains. Trends in Pharmacological Sciences. 2012;33:146–153. doi: 10.1016/j.tips.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Seeburg PH. γ-Aminobutyric acid type A receptor point mutation increases the affinity of compounds for the benzodiazepine site. Proc Natl Acad Sci USA. 1991;88:1421–1425. doi: 10.1073/pnas.88.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid TS, Beese LS. Crystal Structures of the Anticancer Clinical Candidates R115777 (Tipifarnib) and BMS-214662 Complexed with Protein Farnesyltransferase Suggest a Mechanism of FTI Selectivity. Biochemistry. 2004;43:6877–6884. doi: 10.1021/bi049723b. [DOI] [PubMed] [Google Scholar]
- Reynoird N, Schwartz BE, Delvecchio M, Sadoul K, Meyers D, Mukherjee C, Caron C, Kimura H, Rousseaux S, Cole PA, et al. Oncogenesis by sequestration of CBP/p300 in transcriptionally inactive hyperacetylated chromatin domains. EMBO J. 2010;29:2943–2952. doi: 10.1038/emboj.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter L, Graaf Cd, Sieghart W, Varagic Z, Morzinger M, Esch IJPd, Ecker GF, Ernst M. Diazepam-bound GABAA receptor models identify new benzodiazepine binding-site ligands. Nature Chem Bio. 2012;8:455–464. doi: 10.1038/nchembio.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijnsoever Cv, Tauber M, Choulli MK, Keist R, Rudolph U, Mohler H, Fritschy J-M, Crestani F. Requirement of α5-GABAA Receptors for the Development of Tolerance to the Sedative Action of Diazepam in Mice. J Neurosci. 2004;24:6785–6790. doi: 10.1523/JNEUROSCI.1067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenström U, Sköld C, Lindeberg G, Botros M, Nyberg F, Karlen A, Hallberg A. Design, Synthesis, and Incorporation of a β-Turn Mimetic in Angiotensin II Forming Novel Pseudopeptides with Affinity for AT1 and AT2 Receptors. J Med Chem. 2006;49:6133–6137. doi: 10.1021/jm051222g. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy J-M, Martin JR, Bluethmann H, Möhler H. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Knoflach F. Beyond classical benzodiazepines: novel therapeutic potential of GABA(A) receptor subtypes. Nat Rev Drug Disc. 2011;10:685–697. doi: 10.1038/nrd3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal J, Lamotte Y, Donche F, Bouillot A, Mirguet O, Gellibert F, Nicodeme E, Krysa G, Kirilovsky J, Beinke S, et al. Identification of a novel series of BET family bromodomain inhibitors: Binding mode and profile of I-BET151 (GSK1210151A) Bioorg Med Chem. 2012;22:2968–2972. doi: 10.1016/j.bmcl.2012.02.041. [DOI] [PubMed] [Google Scholar]
- Skolnick P. Anxioselective anxiolytics: on a quest for the Holy Grail. Trends in Pharmacological Sciences. 2012;33:611–620. doi: 10.1016/j.tips.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM. Don't Ask, Don't Tell, but Benzodiazepines Are Still the Leading Treatments for Anxiety Disorder. J Clin Psychiatry. 2002;63:756–757. doi: 10.4088/jcp.v63n0901. [DOI] [PubMed] [Google Scholar]
- Sternbach LH. The Benzodiazepine Story. J Med Chem. 1979;22:1–7. doi: 10.1021/jm00187a001. [DOI] [PubMed] [Google Scholar]
- Vandyck K, Cummings MD, Nyanguile O, Boutton CW, Vendeville S, McGowan D, Devogelaere B, Amssoms K, Last S, Rombauts K, et al. Structure-Based Design of a Benzodiazepine Scaffold Yields a Potent Allosteric Inhibitor of Hepatitis C NS5B RNA Polymerase. J Med Chem. 2009;52:4099–4102. doi: 10.1021/jm9005548. [DOI] [PubMed] [Google Scholar]
- Verster JC, Volkerts ER. Clinical Pharmacology, Clinical Efficacy, and Behavioral Toxicity of Alprazolam: A Review of the Literature. CNS Drug Reviews. 2004;10:45–76. doi: 10.1111/j.1527-3458.2004.tb00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidler LR, Brown N, Knapp S, Hoelder S. Druggability analysis and structural classification of bromodomain acetyl-lysine binding sites. J Med Chem. 2012;55:7346–7359. doi: 10.1021/jm300346w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar HO, Davies MF, Loew GH, Maguire PA. Molecular Models for Recognition and Activation at the Benzodiazepine Receptor: A Review. Life Sciences. 1991;48:593–602. doi: 10.1016/0024-3205(91)90533-h. [DOI] [PubMed] [Google Scholar]
- VonVoigtlander PF, Straw RN. Alprazolam: Review of Pharmacological, Pharmacokinetic, and Clinical Data. Drug Development Research. 1985;6:1–12. [Google Scholar]
- Wang Q, Han Y, Xue H. Ligands of the GABAA Receptor Benzodiazepine Binding Site. CNS Drug Reviews. 1999;5:125–144. [Google Scholar]
- Welsch ME, Snyder SA, Stockwell BR. Privileged scaffolds for library design and drug discovery. Curr Opin Chem Biol. 2010;14:347–361. doi: 10.1016/j.cbpa.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland HA, Luddens H. Four Amino Acid Exchanges Convert a Diazepam-Insensitive, Inverse Agonist-Preferring GABAA Receptor into a Diazepam-Preferring GABAA Receptor. J Med Chem. 1994;37:4576–4580. doi: 10.1021/jm00052a019. [DOI] [PubMed] [Google Scholar]
- Wieland HA, Luddens H, Seeburg PH. A Single Histidine in GABAA Receptors Is Essential for Benzodiazepine Agonist Binding. J Biol Chem. 1992;267:1426–1429. [PubMed] [Google Scholar]
- Wu S-Y, Chiang C-M. The Double Bromodomain-containing Chromatin Adaptor Brd4 and Transcriptional Regulation. J Biol Chem. 2007;282:13141–13145. doi: 10.1074/jbc.R700001200. [DOI] [PubMed] [Google Scholar]
- Zhang G, Liu R, Zhong Y, Plotnikov AN, Zhang W, Zeng L, Rusinova E, Gerona-Navarro G, Moshkina N, Joshua J, et al. Down-regulation of NF-κB Transcriptional Activity in HIV-associated Kidney Disease by BRD4 Inhibition. J Biol Chem. 2012;287:28840–28851. doi: 10.1074/jbc.M112.359505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]