Abstract
BACKGROUND & AIMS
Premalignant lesions and early stage tumors contain immunosuppressive microenvironments that create barriers for cancer vaccines. KrasG12D/+;Trp53R172H/+;Pdx-1-Cre (KPC) mice, which express an activated form of Kras in pancreatic tissues, develop pancreatic intraepithelial neoplasms (PanIN) that progress to pancreatic ductal adeno-carcinoma (PDA). We used these mice to study immune suppression in PDA.
METHODS
We immunized KPC and KrasG12D/+;Pdx-1-Cre mice with attenuated intracellular Listeria monocytogenes (which induces CD4+ and CD8+ T-cell immunity) engineered to express KrasG12D (LM-Kras). The vaccine was given alone or in sequence with an anti-CD25 antibody (PC61) and cyclophosphamide, to deplete T-regulatory (Treg) cells. Survival times were measured; pancreatic and spleen tissues were collected and analyzed by histologic, flow cytometry, and immunohistochemical analyses.
RESULTS
Interferon γ-mediated, CD8+ T-cell responses were observed in KPC and KrasG12D/+;Pdx-1-Cre mice given LM-Kras, but not in unvaccinated mice. Administration of LM-Kras to KPC mice 4–6 weeks old (with early stage PanINs), depleted of Treg cells, significantly prolonged survival and reduced PanIN progression (median survival, 265 days), compared with unvaccinated mice (median survival, 150 days; P = .002), mice given only LM-Kras (median survival, 150 days; P = .050), and unvaccinated mice depleted of Treg cells (median (medium survival, 170 days; P = .048). In 8- to 12-week-old mice (with late-stage PanINs),¼LM-Kras, alone or in combination with Treg cell depletion, did not increase survival time or slow PanIN progression. The combination of LM-Kras and Treg cell depletion reduced numbers of Foxp3+CD4+ T cells in pancreatic lymph nodes, increased numbers of CD4+ T cells that secrete interleukin 17 and interferon g, and caused CD11b+Gr1+ cells in the pancreas to acquire an immunostimulatory phenotype.
CONCLUSIONS
Immunization of KPC mice with Listeria monocytogenes engineered to express KrasG12D, along with depletion of Treg cells, reduces progression of early stage, but not late-stage, PanINs. This approach increases infiltration of the lesion with inflammatory cells. It might be possible to design immuno-therapies against premalignant pancreatic lesions to slow or prevent progression to PDA.
Keywords: Pancreatic Cancer, Listeria Vaccine, Tumor Microenvironment, Immunity
Pancreatic ductal adenocarcinoma (PDA) is clinically difficult to treat owing to its late detection and resistance to conventional therapies. However, recent studies have suggested that the process of PDA development from tumor initiation to metastases requires years to decades, providing a window of opportunity for the prevention of premalignant progression during the stage when precancerous lesions, known as pancreatic intraepithelial neoplasms (PanINs), predominate.1 One potential treatment approach involves the development of immunization strategies that target the early genetic changes (driver genes) that initiate premalignant lesion formation.
Immune tolerance mechanisms to self-antigens are significant barriers to antitumor immunotherapy, especially once PDA has developed.2–4 Treatments that bypass tolerance and induce a specific antitumor immune response in the periphery still are ineffective owing to the immunosuppressive tumor microenvironment (TME), which includes T-regulatory cells (Tregs), tolerogenic antigen-presenting cells, myeloid-derived suppressor cells (MDSCs), immunoregulatory molecules, and immunosuppressive cytokines.5–8 Of these barriers, Tregs and MDSCs were shown to be increased in mouse and patient tumors,3,5,9,10 inhibiting protective CD8+ T-cell responses, and promoting PDA growth.11–13 Treatment strategies that target these suppressive populations enhance cancer-specific T-cell activation in preclinical and clinical studies.12,14 Treg depletion facilitates tumor eradication by increasing the effector T-cell to Treg ratio within the tumor and tumor-draining lymph nodes, key sites of Treg accumulation and activation.8,12,15
Genetically engineered mice that mimic the genetic induction and progression of tumors within the natural organ allow study of the immune responses to cancer and its precursor lesions in the context of natural tolerance mechanisms.10 The KrasG12D/+;Pdx-1-Cre (KC) and KrasG12D/+; Trp53R172H/+;Pdx-1-Cre (KPC) mice are programmed genetically to mimic the progression from normal tissue, through all stages of premalignant PanINs, to fully developed PDA, which genetically and histologically recapitulate human disease.16,17 Here, we report the observation that Treg infiltration occurs as early as PanIN stage 1. Given the early presence of suppressive cells at the site of tumor development, we hypothesized that immunization with an attenuated intracellular Listeria monocytogenes (LM) vaccine genetically modified to express the driver KrasG12D gene product (LM-Kras) would require concomitant modulation of one or more immune inhibitory mechanisms to effectively delay PanIN progression. We show that LM-Kras vaccination and Treg depletion slows progression to PDA when administered at the PanIN 1 stage, but not once PanIN stages 2–3 have developed. Furthermore, LM-Kras and Treg depletion alter the phenotype of CD11b+Gr-1+ cells in the pancreas and recruit T helper cell (Th)/Tc-17 type effector lymphocytes capable of halting early PanIN progression. Thus, vaccine-induced primary prevention of pancreatic cancer is feasible but requires simultaneous immune modulation.
Materials and Methods
Mice
Lox-STOP-Lox Trp53R172H/+; Lox-STOP-Lox KrasG12D/+; and Pdx-1-Cre strains on a mixed 129/SvJae/C57BL/6 background, were a gift from Dr David Tuveson (Cold Spring Harbor Laboratory, Cold Spring, NY).16,17 These mice were backcrossed to the C57BL/6 genetic background for 12 generations and interbred to obtain KC and KPC mice. Animals were kept in pathogen-free conditions and treated in accordance with Institutional Animal Care and Use Committee and American Association of Laboratory Animal Committee approved policies.
Patients and Tumor Samples
Mesothelioma biopsy specimens were collected from a subject in study ADU-CL-02, a phase I study evaluating the safety and induction of immune response of CRS-207, a LM vaccine targeting mesothelin, in combination with chemo-therapy in patients with malignant pleural mesothelioma.18 Patients provided signed informed consent after approval of the study by the institutional review board.
LM Construct
The LM-Kras vaccine was constructed in the actA and inlB double-deleted strain.19 The 12 ras expression cassette was designed in silico to fuse the 25 amino acids of both V and D activating mutations (at position 12) in a synthetic gene cloned downstream of the actA promoter as described previously.19,20
Survival Experiments
LM-Kras (5 × 105 colony-forming units) in 0.2 mL phosphate-buffered saline was administered intravenously based on dose titrations for each batch of vaccine. KPC mice aged 4–6 weeks or 8–14 weeks were treated with PC61 (50 μg/ mouse)12 and cyclophosphamide (Cy) (100 mg/kg; Bristol-Myers Squibb, New York, NY) by intraperitoneal injection, 1 day before vaccine as per the experimental design. This regimen was repeated every 4 weeks and survival was monitored weekly.
Intracellular Cytokine Assays and Flow Cytometry
Splenic CD8+ T cells were negatively selected and incubated with T2Kb cells and peptides, followed by intracellular cytokine staining as previously described.15 Pancreata were prepared by incubation with 1 mg/mL collagenase and 25 mg/L hyaluronidase for 30 minutes at 37° C followed by Percoll gradient purification. Lymphocytes were stimulated with Dynabeads Mouse T-Activator CD3/CD28 (Life Technologies, Grand Island, NY) overnight at 37° C per the manufacturer's instructions. Lymphocytes from up to 3 mouse pancreata were pooled and stained as one flow cytometry sample owing to small cell numbers. Flow cytometry was performed with the specified antibodies (Supplementary Table 1) using an LSR II and analyzed using FACSDiva software (BD Biosciences, San Jose, CA).
Immunohistochemistry
Immunohistochemistry (IHC) was performed with the antibodies listed and according to standard protocols unless otherwise noted (Supplementary Materials and Methods and Supplementary Table 2).21 All slides were imaged using an Eos Rebel T2i camera (Canon USA, Melville, NY) and an Eclipse TS100 microscope (Nikon, Inc, Melville, NY).
Histologic Analysis of Pancreatic Lesions
Pancreata were formalin-fixed and paraffin-embedded, cut at 4-μm thickness, and stained using routine H&E staining protocols by the Johns Hopkins Tissue Microarray Laboratory. For analysis of vaccine and Treg depletion effect(s), 2 slides from each pancreas spaced 400-μm apart were graded based on the highest PanIN stage (or PDA) present by a pathologist blinded to treatment group.
Cytokine Array
The pancreata of KPC mice in each treatment group were pooled and Gr-1+ cells were isolated by fluorescence-activated cell sorting of the CD45+CD11b+Gr-1+ population using a FACSAria II (BD Biosciences). Cells were lysed according to instructions and lysate was blotted with the Mouse Cytokine Antibody Array, Panel A (R&D Systems, Minneapolis, MN). The resulting dot blot was scanned and analyzed using VisionWorks Q23 LS (UVP, LLC, Upland, CA) to calculate the density per pixel and fold-change in expression of the treated cells in comparison with untreated cells.
Statistical Analysis
Data were expressed as the mean ± SEM and all experiments were repeated 3 times unless otherwise stated. Statistical significance was analyzed using GraphPad Prism (GraphPad Software, Inc, La Jolla, CA) and assessed using a 2-tailed Student t test. Welch correction was used when the variance was assumed to be unequal. For survival analysis, the log-rank (Mantel–Cox) test was used.
Results
LM-Kras Induces Systematic CD8+ T-Cell Responses in KrasG12D/+- Expressing Mice
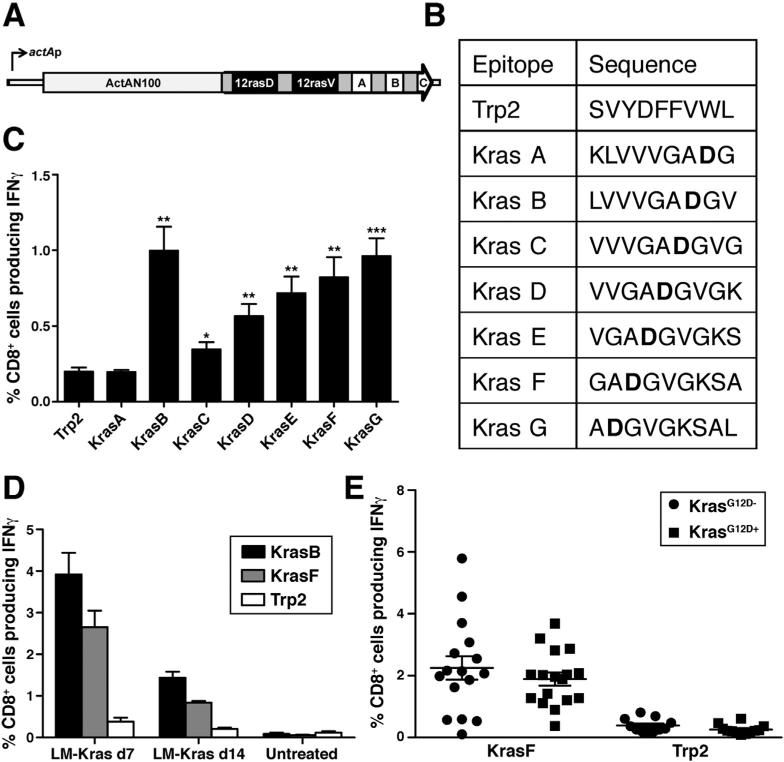
We chose mutant Kras (KrasG12D) as a target for vaccination because it is the most prevalent and earliest oncogene involved in human PDA development.22 The bacterial vector, LM, was selected because LM-based vaccines induce robust antigen-specific CD4+ and CD8+ T-cell immunity.23,24 The LM vaccine was shown to be safe and in the treatment of PDA patients.18 We first identified the specific 9 amino acid (9-mer) peptides presented by the major histocompatibility complex (MHC) I allele, H-2Kb, by screening overlapping peptides for recognition by activated immunized lymphocytes isolated from mice vaccinated with LM-Kras (Figure 1A and B).25 Vaccinated, nontolerant, MHC-matched mice harbor interferon (IFN)γ-secreting CD8+ T cells responsive to multiple mutant Kras epitopes (Kras B–G) when compared with the control tyrosinase-related protein 2 (Trp2) epitope, which also binds to MHC I Kb (Figure 1C). The LM-Kras vaccine induced Kras-specific IFNγ CD8+ T-cell responses in mice expressing the mutant KrasG12D allele whereas Krasspecific CD8+ T-cell responses were not detected in unvaccinated KC and KPC mice, suggesting that the mutation itself is not naturally immunogenic (Figure 1D and Supplementary Figure 1). Surprisingly, these studies failed to identify differences in the magnitude of systemic Krasspecific CD8+ T-cell responses in the nontolerant parental vs the transgenic mice expressing the KrasG12D mutation (Figure 1E). Thus, LM-Kras can induce a sizable systemic CD8+ T-cell response against mutant Kras in both parental and potentially tolerant KC and KPC mice.
Figure 1.
Immunogenicity of LM-Kras. (A) 25mers of the mutant Kras alleles were cloned downstream and in-frame with the ActA amino terminus and were tagged with immunologic positive control T-cell epitopes for Balb/c mice (A, SYIPSAEKI) and C57BL/6 mice (B, SIINFEKL), as well as a C-terminal antibody epitope (C, Myc). (B) Sequence of epitopes used to test the immunogenicity of LM-Kras, with mutant residue 12 in bold. (C) Splenic CD8+ T-cell responses of wild-type littermates of KPC mice (n = 5) were assessed 7 days after LM-Kras by incubation with the indicated peptide and T2Kb cells and intracellular staining for IFNγ. (D) At 7 or 14 days post-vaccine, IFNγ secretion by splenic CD8+ T cells from KC mice vaccinated with LMKras or untreated was assessed as in panel C. (E) Cumulative results of 5 experiments comparing the magnitude of CD8+ T-cell responses to KrasF in mice expressing the KrasG12D mutation compared with wild-type littermates. *P < .05, **P < .01, ***P < .001.
PanIN Progression Inhibition Requires Both Treg Depletion and LM-Kras
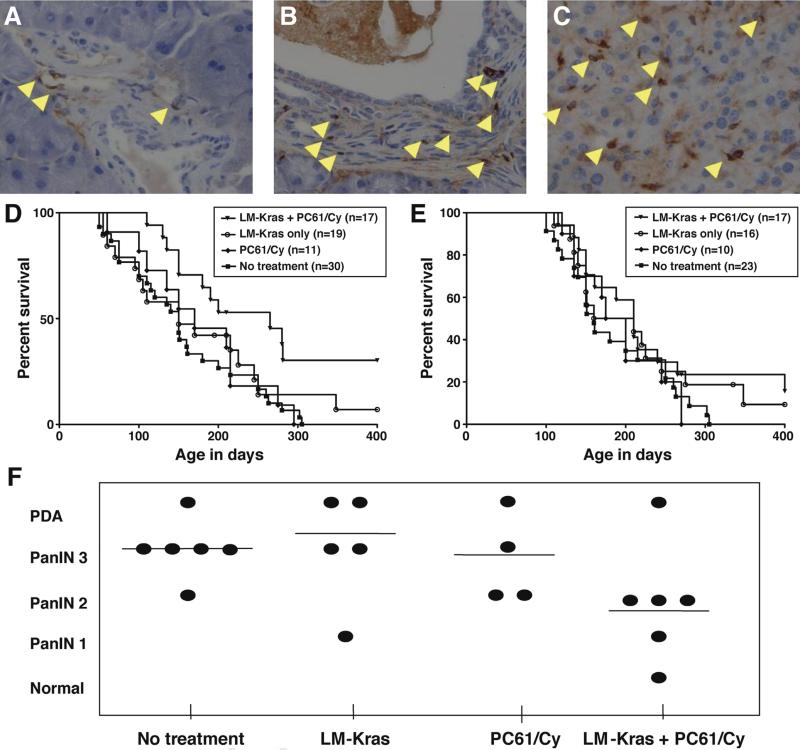
Because previous studies have reported that Tregs are present in PanINs and PDA of KPC mice,10 we further evaluated the kinetics of Treg infiltration and found that Foxp3+ cells are present even at the earliest stage of PanIN development, PanIN 1, and continue to increase with progression to malignancy (Figure 2A–C). A combination of the murine anti-CD25 antibody, PC61, and low-dose Cy, results in the most complete depletion of Tregs in PDA, when the combination is given 1 day before vaccination.12 We used KPC rather than KC mice to test the ability of LM-Kras given alone or with Treg depletion to inhibit tumors because the Trp53R172H/+ mutation produces tumor development at a predictable rate, which facilitates survival analysis. Two age groups were evaluated for survival, mice 4–6 or 8–12 weeks old, corresponding to early or late-stage PanINs, respectively. LM-Kras and Treg-depleting agents given alone did not affect survival in either age group, compared with untreated mice. However, combined LM-Kras and Treg depletion conferred a significant survival advantage to animals given treatment at the time of PanIN 1 development but not to mice beginning treatment at the time of PanINs 2 and 3 (Figure 2D and E). The systemic CD8+ T-cell response to LM-Kras did not differ significantly between 4- to 6-week-old and 8- to 12-week-old KPC mice (Supplementary Figure 2A). Furthermore, mice with early stage PanINs receiving 2 cycles of LM-Kras and Treg depletion showed fewer late-stage PanINs and PDA compared with other treatment groups (Figure 2F). Thus, the overall lower stage of PDA development in mice treated with combined LMKras and Treg depletion is consistent with the improved survival seen in Figure 2D.
Figure 2.
Effect of vaccination and Treg depletion on KPC mice. IHC for Foxp3 in the pancreas of KPC mice containing (A) early stage PanIN 1, (B) midstage PanIN 2, and (C) PDA at 40× magnification. (D) KPC mice younger than age 2 months were treated with LM-Kras, Treg depletion, the combination of LM-Kras and Treg depletion, or left untreated (no treatment). Booster vaccinations were administered monthly with or without PC61/Cy pretreatment according to group assignment. Log-rank (Mantel–Cox) analysis showed a significant improvement in survival in the combination group relative to no treatment (P = .002) and to Treg depletion or LM-Kras alone (P = .048 and P = .050, respectively). There was no statistically significant difference in survival between the untreated group and animals receiving Treg depletion or between untreated and LM-Kras. (E) Mice older than 2 months were treated and monitored as in panel D. No statistically significant difference was noted in survival between groups using Mantel–Cox analysis. (F) Four- to 6-week-old KPC mice (n = 4–6 per group) were treated as in panel C until week 5, when animals were killed and pancreatic histology was assessed.
LM-Kras and Treg Depletion Recruit Effector T Cells to the Premalignant TME
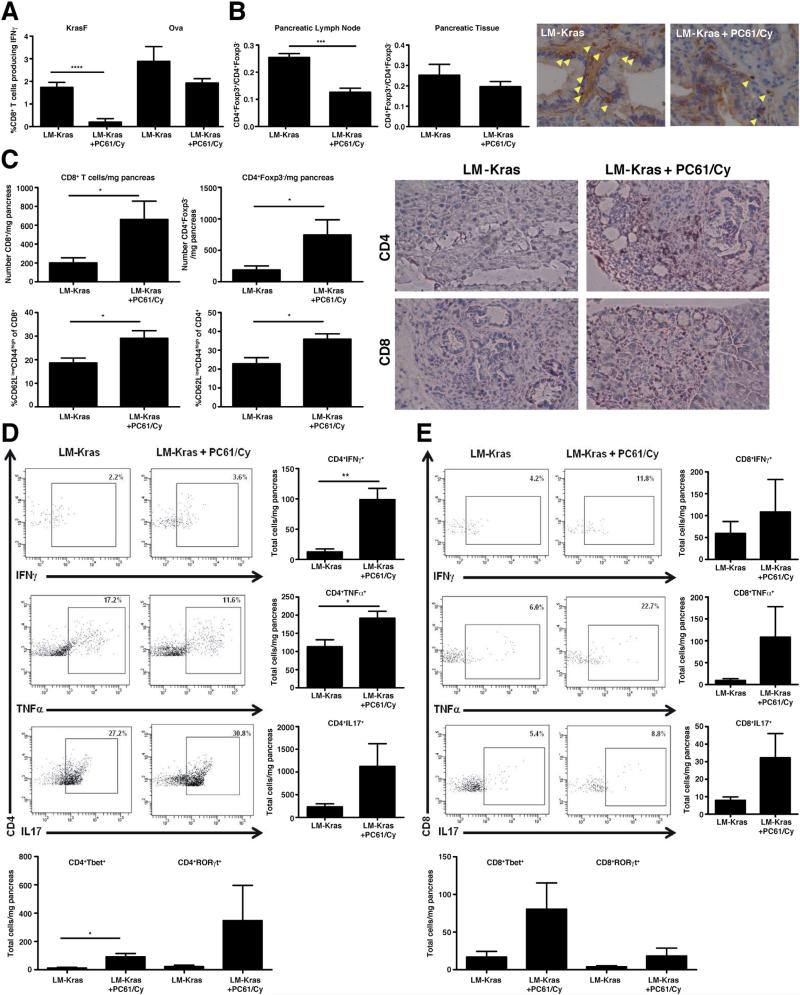
The systemic immune response to one mutated Kras peptide, KrasF, and to the ovalbumin H-2Kb–binding peptide, SIINFEKL (Ova), was assessed 1 week after 2 cycles of immunotherapy (the LM-Kras construct contains the immunogenic Ova peptide to confirm vaccine potency). Treg depletion 1 day before LM-Kras decreased the systemic response to KrasF peptide when compared with LM-Kras alone (Figure 3A). In contrast, there was not a significant difference between splenocyte CD8+ T-cell responses measured against Ova in mice receiving LM-Kras and Treg depletion vs LM-Kras (Figure 3A). Although total numbers of systemic CD8+ and CD4+ responders were decreased with LM-Kras and Treg depletion compared with LM-Kras alone, this was not significant, suggesting that this could not be attributed entirely to generalized deletion of T cells by PC61 and Cy and that Kras-targeted CD8+ T cells in the periphery specifically were affected by Treg depletion (Supplementary Figure 3). Flow cytometric analysis of the CD4+ T-cell populations showed a statistically significant decrease in CD4+Foxp3- T cells relative to CD4+Foxp3- T cells in the pancreas-draining lymph nodes and a trend in fewer CD4+Foxp3+ T cells within the pancreata of mice with early stage PanINs receiving LM-Kras and Treg depletion vs LM-Kras alone (Figure 3B). IHC for Foxp3 showed fewer Tregs in pancreata of KPC mice receiving LM-Kras and Treg depletion (Figure 3B).
Figure 3.
Systemic and local T-cell responses with Treg depletion and LM-Kras. KPC mice were treated with beginning at age 4–6 weeks and harvested after 2 cycles of treatment with LM-Kras with or without Treg depletion. (A) The percentage of splenic CD8+ cells producing IFNγ in response to KrasF and Ova peptides were normalized for response to negative control peptide (Trp2) (n = 6-9 per treatment group). (B) The ratio of CD4+Foxp3+ to CD4+Foxp3- cells in the pancreatic lymph node and pancreas was measured by flow cytometry (n = 5–10 per treatment group) and corresponds with Foxp3 staining in KPC pancreata (magnification, 40× ). (C) The total number of CD8+ and CD4+Foxp3- cells per milligram of pancreas and percentage of CD44highCD62Llow was measured by flow cytometry (n = 7–8 per group). IHC for CD4 and CD8 is shown at 20× magnification. (D and E) Pancreatic lymphocytes were restimulated with CD3/CD28 activation beads overnight and gated on CD3+ and CD4+ or CD8+ (n = 4 samples per group). Representative flow cytometry plots as well as total numbers of each population are shown. Number of total Tbet+ and RORγt+ CD4+ and CD8+ cells were measured by flow cytometry. All data are representative of 2 or more independent experiments. Populations of cells positive for cell surface markers and cytokines were gated using isotype controls and nonstimulated controls for each sample. *P < .05, **P < .01, ***P < .001.
Because CD8+ T cells are excluded from pancreata in untreated KPC mice,10 we evaluated whether Treg depleting therapy given with LM-Kras preferentially recruited effector T cells to the premalignant pancreas. In support of this hypothesis, we observed a significant increase in the number of CD8+ and CD4+Foxp3- T cells within the pancreata of mice treated with LM-Kras and Treg depletion compared with LM-Kras alone–treated mice as measured by flow cytometry and confirmed by IHC (Figure 3C). A higher percentage of the CD4þFoxp3- and CD8+ T cells found within PanINs of mice treated with Treg-depleting therapy and LM-Kras were CD62LlowCD44high, indicating an antigen-experienced phenotype when compared with the vaccine alone–treated mice (Figure 3C). Although attempted, the small number of cells in the pancreas limited the ability to assess the T-cell response to KrasF directly ex vivo. However, anti-CD3/CD28 beads were used to stimulate lymphocytes isolated from the pancreata of KPC mice first vaccinated at 4–6 weeks of age, allowing for evaluation of the cytokine profile of all PanIN-infiltrating T cells. Both LMKras and combined LM-Kras and Treg depletion resulted in the production of effector cytokines including IFNγ, tumor necrosis factor α, and interleukin (IL)17 in infiltrating CD4+ and CD8+ T cells, correlating with expression of Tbet and RORγt. Importantly, mice receiving LM-Kras and Treg depletion had greater total numbers of cytokine-producing CD4+ and CD8+ T cells (Figure 3D and E). We verified that both CD8+ T cells and IL17-secreting cells were important for delaying PanIN progression because the protective effect was abrogated when CD8+ cells were depleted and when IL17 was neutralized during LM-Kras and Treg depletion (Supplementary Figure 4).
To further test our hypothesis regarding the induction of Th17 phenotypes with a Listeria vaccine, we evaluated tissue from a single patient in a clinical trial of CRS-207, a vaccine using the same attenuated LM vector to target mesothelin, without Treg depletion therapy.18 Staining for RORγt showed enhanced staining at the post-LM time point compared with a pre-LM biopsy (Supplementary Figure 5A). Staining of serial sections from each time point with anti-CD3 showed that the intranuclear RORγt staining correlated with CD3+ cells in the post-LM biopsy, whereas CD3+ cells in the pre-LM biopsy were negative for RORγt (Supplementary Figure 5B), suggesting that IL17 expression in tumor-infiltrating lymphocytes may be driven by the use of a Listeria vector.
LM-Kras and Treg Depletion Enhances Recruitment of Pancreatic Gr-1Cells
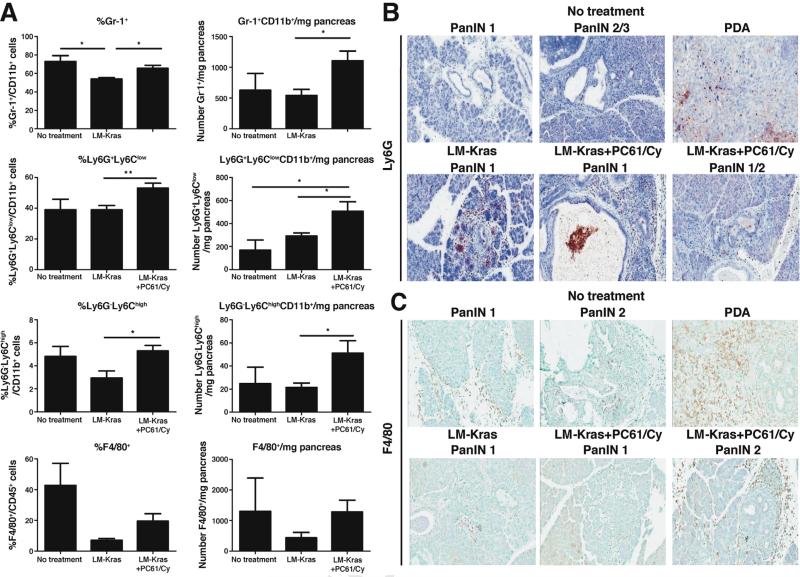
MDSCs were shown previously to infiltrate PanINs and PDA in untreated KPC mice, promoting tumorigenesis and inhibiting effector T cells.10,11 We found that the total number of Gr-1+ cells (Ly6G-Ly6Chigh and Ly6G+Ly6Clow), were increased in mice given LM-Kras and Treg depletion when compared with LM-Kras alone or untreated mice of the same age (Figure 4A). Ly6G+Ly6Clow cells were the predominant Gr-1+ population identified by flow cytometry. IHC analysis showed that Ly6G+ cells begin infiltrating in midstage PanINs, increase with progression to PDA in untreated KPC mice, and are abundant regardless of treatment group (Figure 4B). F4/80+ macrophages were less numerous, but showed similarly increased infiltration with progression from early stage PanIN to PDA in treated and untreated KPC mice (Figure 4A and C).
Figure 4.
Myeloid cell infiltration into untreated and treated PanINs and PDA. (A) Pancreata from KPC mice treated with 2 cycles of LM-Kras with or without PC61/Cy starting at age 4-6 weeks or left untreated for the same timeframe were analyzed by flow cytometry (n = 4–6 mice per group). Representative of 2 experiments. *P < .05, **P < .01. Pancreata were harvested from untreated KPC mice of various ages or from 4- to 6-week-old KPC mice treated with 2 cycles of LM-Kras with or without Treg depletion, and slides were stained with (B) Ly6G or (C) F4/80 antibodies. (B and C) Magnification, 20×.
LM-Kras and Treg Depletion Repolarize PanIN-Infiltrating Gr-1+ Cells
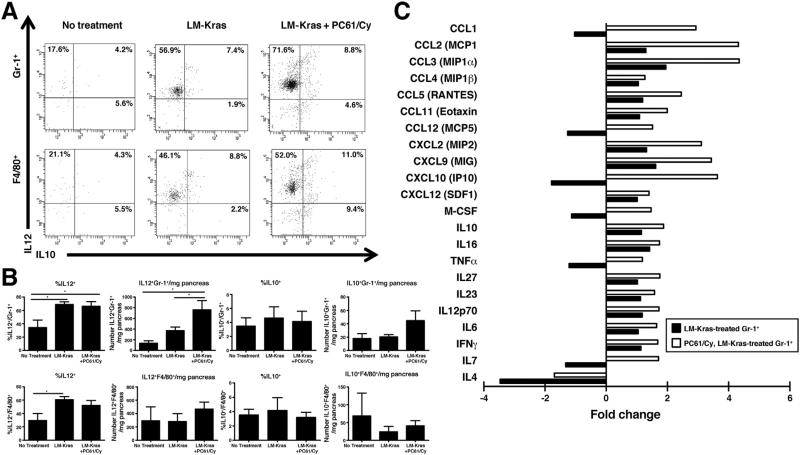
Because the number of Gr-1+ myeloid cells and F4/80+ macrophages in PanINs was not decreased with immunotherapy, we hypothesized that cells with these surface markers represent less-suppressive populations in mice Q28 given LM-Kras and Treg depletion.26 We investigated IL12 and IL10 production by CD11b+Gr-1+ and F4/80+ cells from the pancreata of mice treated with 2 cycles of therapy, and found that LM-Kras given with or without Treg depletion induced IL12 secretion by Gr-1+ cells, resulting in an increased total number of IL12-secreting Gr-1+ cells in pancreata of mice given LM-Kras and Treg depletion (Figure 5A and B). PanIN-infiltrating macrophages treated with LM-Kras or LM-Kras and Treg depletion also produced increased amounts of IL12 (Figure 5A and B). There was no significant difference in IL10 production by F4/80+ macrophages or Gr-1+ cells (Figure 5B). CD11b+Gr-1+ cells from the pancreata of treated mice secreted higher levels of many immunostimulatory cytokines than untreated mice, including chemokines responsible for recruiting myeloid and lymphoid cells and M1/N1-associated cytokines responsible for promoting IFNγ and IL17 responses in T cells, with the highest levels being produced by Gr-1+ cells from mice given LM-Kras and Treg depletion (Figure 5C).
Figure 5.
Phenotype of innate populations in pancreata of KPC mice. (A) Representative flow cytometry plots are shown for mice treated at age 4–6 weeks with LM-Kras with or without Treg depletion or left untreated. (B) Mean percentages and total numbers of IL12- and IL10-expressing Gr-1+ or F4/80+ cells per mg of pancreas are shown from same experiments as in panel A (n = 4–6 mice per group). Representative of 2 experiments. *P < .05. (C) Cytokine array data using cell lysate from pooled CD11b+Gr-1+ cells is shown by fold change in expression for treated mice compared with untreated mice (n = 2 technical replicates per group).
Discussion
We report here a new model for developing immuno-therapy to prevent premalignant progression to cancer that targets the earliest driver gene in PDA, mutant Kras. Inactivating mutant Kras reverses dysplasia in early stage Pan-INs and causes apoptosis of more advanced PanINs and PDA in KPC mice, providing further rationale for targeting this mutation in PDA.27 In addition, we show that a Listeria vaccine can enhance early infiltrating inflammatory cells, altering the premalignant microenvironment in favor of inhibiting lesion progression, when given in combination with agents to modify the TME. Furthermore, we have identified CD8+ and Th17 T-cell populations as critical effectors of PDA prevention.
Cancer progressed when KPC mice were treated with LM-Kras alone, even though the vaccine induced a significant peripheral CD8+ T-cell response directed against epitopes from the mutant Kras allele, showing that the induction of systemic antigen-specific T cells does not constitute effective antimalignancy immunity. These data are consistent with previous findings that increased levels of systemic T-cell responses to a viral human papilloma virus antigen encoded by LM vaccines do not correlate with efficacy in an orthotopic model of cervical cancer28 despite a well-documented requirement for CD8+ T cells for efficacy of the vaccine.23 Even when CD8+ T cells are able to infiltrate tumors, the influx of antitumor effector cells can turn on mechanisms of immune tolerance, including recruitment of Tregs.29
The KPC mouse model mimics naturally developing human PDA, characterized by hypovascularity and a dense fibrotic stromal response.30,31 Unlike transplantable PDA, spontaneously developing PDA in mice is resistant to gemcitabine unless the stroma is inhibited.32,33 PanINs and PDA in KPC mice also may differ in susceptibility to penetration by other chemotherapeutic agents, including Cy, explaining the statistically significant depletion of Tregs in the draining lymph nodes but not the tumor. It is possible that enhanced tumor depletion of Tregs would have augmented the effects of LM-Kras and Treg depletion further. Alternatively, tumor-draining lymph nodes are a crucial site for orchestrating tumor antigen tolerance against developing tumors8 and depletion of Tregs in the draining lymph node may be sufficient for CD8+ and CD4+ effector T-cell activation and infiltration into the pancreas. The role of IL17-secreting cells in the premalignant microenvironment remains controversial. In a transplantable melanoma model, adoptively transferred Th17 CD4+ T cells were superior to Th1 CD4+ T cells in tumor eradication, possibly owing to the ability of Th17 cells to persist and develop into other Th subsets in vivo.34,35 Increasing evidence supports the concept of plasticity among the different CD4+ T-cell subsets and the existence of pathogenic and nonpathogenic populations even within the same Th subset.36,37 Tc1 and Tc17 cells both have been shown to be effective in mouse tumor models; Tc1 directly through the expression of tumor-cytotoxic IFNγ, and Tc17 through the capability to secrete IFNg and IL17 as well as maintain a central memory phenotype.38–40 Although IL17 has been implicated in the recruitment of neutrophils and MDSCs, which can promote cancer progression, IL17-recruited neutrophils also may promote anticancer responses, depending on the context and tumor type.41,42 Cells of both neutrophil and macro phage lineages accumulate with increasing frequency with PanIN progression, underscoring the concept that inflammation caused by acute and chronic pancreatitis is associated with pancreatic cancer development.43,44 Surprisingly, we found that LM-Kras and Treg depletion administered in early stage PanINs actually enhanced the prevalence of CD11b+Gr-1+ cells, including both granulocytic Ly6G+Ly6Clow and monocytic Ly6G-Ly6Chigh cells. However, we also found that the addition of LM-Kras, with or without Treg depletion, resulted in a higher percentage of Gr-1+ cells producing IL12 and other anticancer cytokines in the premalignant microenvironment. One potential explanation for this change in function is less immunosuppressive signals from Tregs and more proinflammatory cytokines secreted after infection of antigen-presenting cells by LM, including IL12.24 IL12 and Cy co-administration has been shown to switch the phenotype of cells infiltrating tumors from MDSCs to less-suppressive immature myeloid cells.45 In our study, although there was a mix of proin-flammatory and anti-inflammatory cytokines and chemokines secreted by the Gr-1+ population in LM-Kras and PC61/Cy-treated mice, the overall trend was toward a more immunostimulatory phenotype. Polarization of these cells toward an antitumor phenotype should serve as a therapeutic goal in the treatment of PDA, both in the early and late stages.
Listeria vaccines represent an effective strategy for inducing immune responses to tumor antigens. CRS-207, a LM vaccine targeting mesothelin, has been shown to be both safe and capable of inducing mesothelin-specific CD8+ T cells in patients with PDA.18 Although the interpretation of our data regarding the induction of Th17/Tc17-type cells in human cancer was limited by the availability of samples and the use of the LM vaccine alone without additional immunotherapy agents in this particular clinical trial, it suggests that LM vaccines may be particularly efficacious in inducing potent anticancer phenotypes in tumor-infiltrating lymphocytes. Despite effective induction of immune responses in KPC mice with LM-Kras, the vaccine alone was not sufficient to protect against PDA and delay progression of PanINs, even at an age representative of the earliest PanIN stages. One of the limitations of the KPC model is that the induction of mutated Kras and p53 is spontaneous and therefore somewhat heterogeneous, so we had to generalize the PanIN stage by age (in weeks) based on previous characterizations of untreated KPC mice. We cannot ascertain if other factors related to age (outside of the systemic CD8+ T-cell responses to Kras, which were not significantly different) may have contributed to the differences in PanIN progression in the early and late stage PanIN groups. However, it was evident that early intervention with combined LM-Kras and Treg depletion was necessary to redirect a potentially pro-oncogenic inflammatory response to an effective antitumor immune response. Future vaccine strategies therefore will require incorporating agents designed to alter the inflammatory milieu within the developing tumor's microenvironment, even when designed for prophylactic treatment of patients with premalignant lesions or with a genetic predisposition for cancer development.
Supplementary Material
Acknowledgments
The authors thank T. Cornish (Johns Hopkins University School of Medicine, Baltimore, MD) for providing the software for analysis of pancreas histopathology and E. Sugar (Johns Hopkins School of Public Health, Baltimore, MD) for providing advice regarding statistical testing and data analysis. The authors also thank L. Zheng and A. Wu (Johns Hopkins University School of Medicine) for technical assistance with immunohistochemistry and A. Tam and L. Blosser for assistance with flow cytometry (Johns Hopkins SKCCC Flow Cytometry Core, Baltimore, MD).
Bridget Keenan, Yvonne Saenger, Michel Kafrouni, Peter Lauer, Dirk Brockstedt, Thomas Dubensky Jr, Raffit Hassan, Todd Armstrong, and Elizabeth Jaffee were responsible for the study concept and design; Bridget Keenan, Yvonne Saenger, Michel Kafrouni, Ashley Leubner, Agnieszka Rucki, Andrew Gunderson, and Todd Armstrong were responsible for the acquisition of data; Bridget Keenan, Yvonne Saenger, Michel Kafrouni, Anirban Maitra, Agnieszka Rucki, Andrew Gunderson, Lisa Coussens, Todd Armstrong, and Elizabeth Jaffee were responsible for the analysis and interpretation of data; Bridget Keenan, Yvonne Saenger, Todd Armstrong, and Elizabeth Jaffee drafted the manuscript; Bridget Keenan, Todd Armstrong, and Elizabeth Jaffee were responsible for critical revision of the manuscript for important intellectual content; Bridget Keenan, Yvonne Saenger, and Michel Kafrouni were responsible for the statistical analysis; and Elizabeth Jaffee obtained funding and was responsible for study supervision.
Funding
Supported by grants from the National Cancer Institute, National Institutes of Health (P50CA062924, R01CA122081, and U19CA113341 to E.M.J.), and supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Abbreviations used in this paper
- Cy
cyclophosphamide
- IFN
interferon
- IHC
immunohistochemistry
- IL
interleukin
- LM
Listeria monocytogenes
- LM-Kras
Listeria monocytogenes bacterial vaccine targeting mutated Kras
- KC
KrasG12D/D, Pdx-1-Cre
- KPC
KrasG12D/D, Trp53R172H/D, Pdx-1-Cre
- MDSC
myeloid-derived suppressor cell
- MHC
major histocompatibility complex
- PanIN
pancreatic intraepithelial neoplasm
- PDA
pancreatic ductal adenocarcinoma
- RORγt
__________________________
- TME
tumor microenvironment
- Treg
T-regulatory cell
- Trp2
tyrosinase-related protein 2
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2014.02.055.
Conflicts of interest
These authors disclose the following: Peter Lauer, Dirk Brockstedt, and Thomas Dubensky Jr are all paid employees of Aduro BioTech and hold stock in the company; Peter Lauer, Dirk Brockstedt, and Thomas Dubensky Jr are inventors on multiple patents and patent applications that apply to the Listeria monocytogenes–based vaccines described in this article; and through a licensing agreement between Johns Hopkins University and Aduro Biotech, Elizabeth Jaffee has the potential to receive royalties on the future sale of human Listeria vaccines. The remaining authors disclose no conflicts.
References
- 1.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laheru D, Jaffee EM. Immunotherapy for pancreatic cancer–science driving clinical progress. Nat Rev Cancer. 2005;5:459–467. doi: 10.1038/nrc1630. [DOI] [PubMed] [Google Scholar]
- 3.Morse MA, Hall JR, Plate JM. Countering tumor-induced immunosuppression during immunotherapy for pancreatic cancer. Expert Opin Biol Ther. 2009;9:331–339. doi: 10.1517/14712590802715756. [DOI] [PubMed] [Google Scholar]
- 4.Houghton AN, Guevara-Patino JA. Immune recognition of self in immunity against cancer. J Clin Invest. 2004;114:468–471. doi: 10.1172/JCI22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleeff J, Beckhove P, Esposito I, et al. Pancreatic cancer microenvironment. Int J Cancer. 2007;121:699–705. doi: 10.1002/ijc.22871. [DOI] [PubMed] [Google Scholar]
- 6.Schmitz-Winnenthal FH, Volk C, Z'Graggen K, et al. High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res. 2005;65:10079–10087. doi: 10.1158/0008-5472.CAN-05-1098. [DOI] [PubMed] [Google Scholar]
- 7.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 8.Munn DH, Mellor AL. The tumor-draining lymph node as an immune-privileged site. Immunol Rev. 2006;213:146–158. doi: 10.1111/j.1600-065X.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 9.Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 10.Clark CE, Hingorani SR, Mick R, et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 11.Bayne LJ, Beatty GL, Jhala N, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21:822–835. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leao IC, Ganesan P, Armstrong TD, et al. Effective depletion of regulatory T cells allows the recruitment of mesothelin-specific CD8 T cells to the antitumor immune response against a mesothelin-expressing mouse pancreatic adenocarcinoma. Clin Transl Sci. 2008;1:228–239. doi: 10.1111/j.1752-8062.2008.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pylayeva-Gupta Y, Lee KE, Hajdu CH, et al. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laheru D, Lutz E, Burke J, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss VL, Lee TH, Song H, et al. Trafficking of high avidity HER-2/neu-specific T cells into HER-2/neu-expressing tumors after depletion of effector/memory-like regulatory T cells. PLoS One. 2012;7:e31962. doi: 10.1371/journal.pone.0031962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 17.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Le DT, Brockstedt DG, Nir-Paz R, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res. 2012;18:858–868. doi: 10.1158/1078-0432.CCR-11-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brockstedt DG, Giedlin MA, Leong ML, et al. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc Natl Acad Sci U S A. 2004;101:13832–13837. doi: 10.1073/pnas.0406035101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauer P, Chow MY, Loessner MJ, et al. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J Bacteriol. 2002;184:4177–4186. doi: 10.1128/JB.184.15.4177-4186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeNardo DG, Brennan DJ, Rexhepaj E, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh R, Paterson Y. Listeria monocytogenes as a vector for tumor-associated antigens for cancer immunotherapy. Expert Rev Vaccines. 2006;5:541–552. doi: 10.1586/14760584.5.4.541. [DOI] [PubMed] [Google Scholar]
- 24.Brockstedt DG, Dubensky TW. Promises and challenges for the development of Listeria monocytogenes-based immunotherapies. Expert Rev Vaccines. 2008;7:1069–1084. doi: 10.1586/14760584.7.7.1069. [DOI] [PubMed] [Google Scholar]
- 25.Uram JN, Black CM, Flynn E, et al. Nondominant CD8 T cells are active players in the vaccine-induced antitumor immune response. J Immunol. 2011;186:3847–3857. doi: 10.4049/jimmunol.1000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Youn JI, Nagaraj S, Collazo M, et al. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins MA, Bednar F, Zhang Y, et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122:639–653. doi: 10.1172/JCI59227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunn GR, Zubair A, Peters C, et al. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167:6471–6479. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 29.Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sofuni A, Iijima H, Moriyasu F, et al. Differential diagnosis of pancreatic tumors using ultrasound contrast imaging. J Gastroenterol. 2005;40:518–525. doi: 10.1007/s00535-005-1578-z. [DOI] [PubMed] [Google Scholar]
- 31.Pandol S, Edderkaoui M, Gukovsky I, et al. Desmoplasia of pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2009;7:S44–S47. doi: 10.1016/j.cgh.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin-Orozco N, Muranski P, Chung Y, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei S, Zhao E, Kryczek I, et al. Th17 cells have stem cell-like features and promote long-term immunity. Oncoimmunology. 2012;1:516–519. doi: 10.4161/onci.19440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lanca T, Silva-Santos B. The split nature of tumor-infiltrating leukocytes: implications for cancer surveillance and immunotherapy. Oncoimmunology. 2012;1:717–725. doi: 10.4161/onci.20068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee Y, Awasthi A, Yosef N, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu Y, Cho HI, Wang D, et al. Adoptive transfer of Tc1 or Tc17 cells elicits antitumor immunity against established melanoma through distinct mechanisms. J Immunol. 2013;190:1873–1881. doi: 10.4049/jimmunol.1201989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saxena A, Desbois S, Carrie N, et al. Tc17 CD8+ T cells potentiate Th1-mediated autoimmune diabetes in a mouse model. J Immunol. 2012;189:3140–3149. doi: 10.4049/jimmunol.1103111. [DOI] [PubMed] [Google Scholar]
- 40.Tajima M, Wakita D, Satoh T, et al. IL-17/IFN-gamma double producing CD8+ T (Tc17/IFN-gamma) cells: a novel cytotoxic T-cell subset converted from Tc17 cells by IL-12. Int Immunol. 2011;23:751–759. doi: 10.1093/intimm/dxr086. [DOI] [PubMed] [Google Scholar]
- 41.Zhuang Y, Peng LS, Zhao YL, et al. CD8(+) T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology. 2012;143:951–962. e8. doi: 10.1053/j.gastro.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Hernandez Mde L, Hamada H, Reome JB, et al. Adoptive transfer of tumor-specific Tc17 effector T cells controls the growth of B16 melanoma in mice. J Immunol. 2010;184:4215–4227. doi: 10.4049/jimmunol.0902995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carriere C, Young AL, Gunn JR, et al. Acute pancreatitis markedly accelerates pancreatic cancer progression in mice expressing oncogenic Kras. Biochem Biophys Res Commun. 2009;382:561–565. doi: 10.1016/j.bbrc.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitcomb DC, Pogue-Geile K. Pancreatitis as a risk for pancreatic cancer. Gastroenterol Clin North Am. 2002;31:663–678. doi: 10.1016/s0889-8553(02)00004-3. [DOI] [PubMed] [Google Scholar]
- 45.Medina-Echeverz J, Fioravanti J, Zabala M, et al. Successful colon cancer eradication after chemoimmunotherapy is associated with profound phenotypic change of intratumoral myeloid cells. J Immunol. 2011;186:807–815. doi: 10.4049/jimmunol.1001483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.