Abstract
Murine hepatic Cyp4a mRNAs are markedly down-regulated during inflammation. Here we investigated the roles of Cyp4a10 and Cyp4a14 in the response to infection with C. rodentium. Absence of either Cyp4a gene attenuated or abrogated the changes in spleen weight, colon crypt length, hepatic cytokine and acute phase protein mRNAs, and serum acute phase proteins and cytokines caused by infection. Cyp4a10−/− mice on a low-salt diet had a similar hepatic acute phase response as those mice on a high salt diet, suggesting that hypertension associated with this genotype is not the cause of their altered inflammatory response. In contrast, wildtype, Cyp4a10−/− and Cyp4a14−/− mice showed similar responses to injected LPS. These results implicate Cyp4a10 and Cyp4a14 in the regulation of the host inflammatory response to enteropathogenic bacterial infection but not to acute aseptic inflammation. Understanding the mechanism of this role may lead to novel therapeutic approaches in some inflammatory diseases.
Keywords: Cyp4a, acute phase proteins, C. rodentium, LPS, fatty acids
INTRODUCTION
The CYP4A subfamily are cytochrome P450 (P450) fatty acid hydroxylases that catalyze the ω-hydroxylation of medium and long chain fatty acids and prostaglandins. Unlike the few other P450s that also carry out this oxidation, CYP4As preferentially target the terminal, primary C-H bonds rather than the adjacent and more easily oxidized secondary C-H bonds at the ω-1 and ω-2 carbons (1,2). This reaction initiates the degradation of long-chain fatty acids which are subsequently converted to dicarboxylic acids for use as fuel via mitochondrial or peroxisomal β-oxidation (3).
The mouse has 5 functional Cyp4a genes, of which only Cyp4a10, Cyp4a12 and Cyp4a14 have been well characterized. Humans have two CYP4A genes, CYP4A11 and CYP4A22, however only CYP4A11 encodes a functional protein (4). CYP4A subfamily proteins have important roles in several physiological pathways. In addition to the above-mentioned roles in fatty acid metabolism, Cyp4a10 and Cyp4a12 in mouse and CYP4A11 and CYP4F2 in human catalyze the ω-hydroxylation of arachidonic acid to 20-hydroxyeicosatetranoic acid (HETE)(5-7). 20- HETE appears to have opposing roles in blood pressure regulation, either promoting hypertension by vasoconstriction or anti-hypertension by natriuresis (8-11). A polymorphism of either CYP4A11 or CYP2J2 genes in humans (4,12), the introduction into mice of human CYP4F2 (13) as well as the disruption of either Cyp2j5, Cyp4a10 or Cyp4a14 in mice result in hypertension (14-16).
We have previously published that upon infection with Citrobacter rodentium, a model of human enteropathogenic Escherichia coli (EPEC) infection that causes infectious colitis, Cyp4a10 and Cyp4a14 mRNAs and Cyp4a proteins are the most sensitive P450 isoforms to down-regulation in mouse liver and kidneys both in the C57BL/6 and C3H backgrounds (17-19). This led us to postulate that selective down-regulation might indicate a role of Cyp4a enzymes in the host's response to infection and (or) the bacterium's strategy for propagation and survival. If this indeed is the case, it is reasonable to hypothesize that genetic deletion of these enzymes might be expected to either protect the host from the infection and (or) its deleterious effects, or confer an advantage to the bacterium thereby sensitizing the host to infection. The objective of this study, therefore, was to examine the effect of the deletion of Cyp4a10 or Cyp4a14 in the regulation of hepatic P450s of the mouse liver during C. rodentium infection, and to compare this to the bacterial lipopolysaccharide (LPS) model of acute inflammation.
MATERIALS AND METHODS
Unless otherwise specified, all the reagents and chemicals were obtained from Sigma–Aldrich (St. Louis, MO).
Mice and treatments
Wild-type 129X1/SvJ and 129X1/SvEvTac animals, mice were purchased at 7-8 weeks of age from The Jackson Laboratory (Bar Harbor, ME) and Taconic Farms (Germantown, NY) respectively and were acclimatized to the animal facility for at least one week before the beginning of an experiment. Cyp4a10- deficient (Cyp4a10−/−) and Cyp4a14- deficient (Cyp4a14−/−) breeders were obtained from Dr. J. Capdevila, Vanderbilt University (15) and used to establish the respective colonies. All protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Emory University.
All experiments were initiated when the mice were 9 weeks old. Mice were housed in groups of up to six to a cage. A wild-type strain of C. rodentium (#51116) was obtained from the American Type Culture Collection (Manassas, VA). Following overnight growth in Luria broth, without shaking at 37°C, the bacteria were serially diluted in sterile phosphate-buffered saline, and the nominal concentration calculated spectrophotometrically. Oral administration of C. rodentium was achieved by giving the animals an estimated 2 × 108 colony forming units (CFU) per mouse in 20% sucrose solution instead of drinking water for 24 h. To minimize differences between infections, whenever possible, all animals under treatment were infected with the same bacterial preparation. The bacterial concentrations given to each cage were determined by retrospective plating on MacConkey agar, from the volumes of liquid consumed. Infected mice were housed in a Biosafety Level-2 facility to prevent transmission of infection to other mouse colonies. Food consumption and changes in body weight were monitored daily and animals were sacrificed 7 days after administration of sucrose or bacteria. In the LPS experiment, mice in the treatment group were given an i.p. injection delivering 1mg/kg LPS (E. coli 0111:B4, ultrapure, Invivogen, San Diego, CA) dissolved in sterile saline while control mice received an i.p. injection of sterile saline alone. Food was withheld from the animals after the injection and the animals were sacrificed after 24h.
To investigate the effect of dietary sodium, breeders were switched from a standard breeder's diet containing 0.4% sodium to one with 0.24% sodium. The pups were weaned at 3-4 weeks of age and thereafter fed diets containing either 0.03 or 0.4% sodium (Lab Diet, St. Louis. MO). Infection was carried out as described above.
Tissue collection
Blood was collected from the animals at sacrifice, and allowed to clot for about 30 min. Serum was obtained by centrifugation for 10 min at 10,000g and stored at −80°C until analyzed. Liver and spleen were dissected and rinsed in cold 1.15% potassium chloride then weighed. The liver was then portioned, flash frozen then stored at −80°C for subsequent RNA or microsome preparation, or kept on ice for the determination of viable bacteria. The colon was removed and washed of fecal matter using cold 1.15% potassium chloride, sectioned, then kept on ice for the determination of viable bacteria or placed in histology cassettes and immersed in 10% formalin prior to histology analysis.
Histological analysis
Liver and distal colon sections were fixed in 10% formalin and embedded in paraffin. Sections (5 μm) were cut and stained with hematoxylin and eosin. The samples were coded and the observations made in a blinded fashion to avoid bias in the evaluation process. The heights of three well-oriented crypts were measured in the distal colon for each mouse, using a Zeiss 200M microscope (Thornwood, NY) with a 20X NA1.4 lens, and Slidebook (Intelligent Imaging Innovations, Denver CO. Stained liver tissue sections were scored under blinded conditions for the presence of inflammation, as indicated by the presence of neutrophils, macrophages, and hepatocyte necrosis using the following scale: 0, normal; 1, minimal; 2, mild; 3, moderate; 4, severe.
Determination of Tissue Bacterial Loads
The number of viable bacteria was determined from organ homogenates. Liver and colon were weighed and homogenized at low speed with a tissuemizer (IKA Works, Inc., Wilmington, NC) in 1ml of phosphate buffered saline. Liver homogenate, blood or serial dilutions of the colon homogenates were plated onto MacConkey's agar, on which C. rodentium forms small pink colonies. The number of CFU were determined after overnight incubation at 37°C and the results reported per gram of tissue or 50μL of blood.
RNA extraction, cDNA synthesis and quantitative RT-PCR
Total liver and hepatocyte RNA were prepared using RNA-Bee isolation reagent (Tel-Test, Friendswood TX), according to the manufacturer's instructions. RNA concentration was determined spectrophotometrically by measuring absorbance at 260 nm while RNA purity and integrity were confirmed by formaldehyde-agarose gel electrophoresis followed by visualization with ethidium bromide.
To synthesize cDNA, purified total RNA was reverse-transcribed using the SuperScript First-Strand Synthesis System kit (Invitrogen, Carlsbad, CA), according to the manufacturer's protocol. PCR primers were custom-synthesized by MWGBiotech, Inc. (High Point, NC) or Operon Biotechnologies, Inc. (Huntsville, AL) and are listed in Supplemental Table 1. The relative expression of mouse liver mRNAs was measured by real-time RT-PCR using the ABI PRISM 7000 Sequence Detection System and SYBR® Green Master Mix reagent (Applied Biosystems, Bedford, MA) as described previously (17,20). To normalize the inter-sample variation in quality inherently associated with RNA preparation, the transcription level of the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was quantified for all samples. The value obtained from each target gene was then normalized using the GAPDH value to calculate the relative target gene mRNA levels in comparison with the corresponding control groups by the ΔΔCt method described by Livak and Schmittgen (21). The expression level in control samples was arbitrarily set at 1.
Serum Analytes
Using a customized Milliplex™ mouse cytokine/chemokine kit (Millipore Corporation, Billerica, MA) and following the manufacturer's protocol, serum samples were assayed for the cytokines interleukin (IL)-2 (IL2), IL3, IL4, IL6, IL7, IL9, IL10, IL17, interferon-γ (IFNγ) and tumor necrosis factor-α (TNFα) and the chemokines CC ligand 5 (CCL5), CXC motif ligand 1 (CXCL1), CXCL10 , macrophage inflammatory protein-1 alpha (MIP-1α), monocyte chemotactic protein-1 (MCP-1) and granulocyte colony-stimulating factor (G-CSF). The Milliplex™ mouse acute phase magnetic bead panel 2 kit (Millipore Corporation, Billerica, MA) was used to measure levels of α1-acid glycoprotein (AGP), C-reactive protein (CRP), haptoglobin (HAPT) and serum amyloid P component (SAP). Data was analyzed using MasterPlex software1.2 (Hitachi Software Engineering America, Ltd., San Francisco, CA) and the concentrations are expressed in picograms or nanograms per milliliter (pg/ml).
Statistical Analysis
All data are presented as mean values ± standard error of the mean (S.E.M.) unless otherwise designated. Data were analyzed by two-way ANOVA followed by Holm-Sidak's multiple comparison test, unless otherwise indicated. For statistical analysis to allow comparison of the size of the effect of infection among genotypes, each continuous variable was normalized to the uninfected group mean within each genotype. Liver pathology scores are discontinuous variables, and were analyzed by a nonparametric Kruskal-Wallace test followed by Dunn's multiple comparison test.
RESULTS
Comparison of Wild-Type Animals
In the ensuing studies, we used 129X1/SvJ mice as the WT strain, as indicated in Holla et al (18) and Quigley et al. (22). However, we subsequently discovered that the correct 129 strain might in fact be 129X1/SvEvTac, which were the source of the embryonic stem cells used to construct the Cyp4a14−/− line (18). We therefore compared the effect of infection on wild-type female 129X1/SvJ (Jax) and 129X1/SvEvTac (Tac) mice. While there were no significant differences, the colony counts in the blood and liver but not the colon tended to be higher in Tac than in Jax animals (data not shown). As expected, infection down-regulated hepatic Cyp3a11, Cyp 3a25, Cyp4a10 and Fmo3 mRNAs while up-regulating Cyp 2d9, Cyp 4f18 and various cytokine mRNAs (Fig. 1). The effects of infection on hepatic mRNAs (Fig. 1A, B) and serum cytokines (Fig. 1C) were similar and therefore it was concluded that either 129X1/SvJ or 129X1/SvEvTac animals were appropriate WT controls for our study model.
Figure 1.
Comparison of the effect of oral C. rodentium infection in wildtype 129X1/SvJ (Jax) and 129X1/SvEvTac (Tac) mice. Over 24 h, female mice were allowed to drink either sucrose solution (Control) or C. rodentium in sucrose (Infected) then sacrificed after 7 days. Livers were harvested for measurement of mRNAs by real-time RT-PCR, and serum was prepared for analysis of serum cytokines A) Hepatic P450 and Fmo3 mRNAs. B) Hepatic cytokine and acute phase protein mRNAs. C) Serum cytokines. mRNA levels are expressed as relative levels of mRNA expression after normalization to GAPDH, with sucrose-treated (Control) group set to 1. Values represent mean ± S.E.M, (n = 6). *, significantly different from control mice of same genotype; #, significantly different from Jax LPS-treated mice, p < 0.05.
Effects of Cyp4a genotype on responses to infection
Colony Counts
Female Cyp4a10−/− mice, and male Cyp4a10−/− and Cyp4a14−/− mice had significantly lower C. rodentium colonization of their colons than did WT mice (Supplemental Fig. 1). There was a similar trend in Cyp4a14−/− females, but it was not significant. Interestingly Cyp4a10−/− males or females trended towards lower bacterial levels in the blood also. There were no significant effects of genotype on levels of bacteria in the liver (Supplemental Fig. 1). The mean doses of bacteria ingested by the three groups were similar (Supplemental Table 2), although the exposure of the WT mice was slightly lower than that of the two Cyp4a-null genotypes.
Body weights, Organ weights, colon and liver pathology
In female animals, there were no significant differences in body weight gain among any of the groups (not shown). The body weights of male mice are shown in Fig. 2. After the initial loss of weight on day 1 (due to withdrawal of food), all groups quickly regained weight by day 2. Body weights of infected male mice were not significantly different from those of uninfected mice of the same genotype at any time point, except for infected WT mice which lost less weight than their uninfected counterparts on day 1, and infected Cyp4a14−/− mice which gained more weight than uninfected on day 2. In contrast to all of the other groups, however, infected WT did not gain weight on days 4-7, so that body weights were significantly higher in the infected Cyp4a10−/− and Cyp4a14−/− mice than in the infected WT animals.
Figure 2.
Body weight changes in control and infected mice. Over 24 h, male mice were allowed to drink either sucrose solution (Control) or C. rodentium in sucrose (Infected). Body weights were monitored, and expressed as a percentage of the original body weight for each animal. Most groups had n= 5 or 6 animals, but data are missing for some animals on days 2-6. Values represent mean ± S.E.M. *, significantly different from control mice of same genotype; #, significantly different from WT infected mice, p < 0.05.
In WT male mice infection resulted in a significantly increased spleen weight, which was attenuated in the Cyp4a10−/− or Cyp4a14−/− males (Table 1). In female mice, the splenomegaly observed in WT mice was not significant in the Cyp4a10−/− or Cyp4a14−/− mice, although the mean values for Cyp4a14−/− were not greatly less than in WT. There was no significant effect of infection on liver weight in males of any genotype. In females, however, liver weight was increased by 24%, and this change was no longer observed in the Cyp4a-null strains (Table 1).
Table 1.
Crypt heights, liver and spleen weights of control and infected mice.
| Liver (% of body weight) | Spleen (% of body weight) | Crypt heights (μm) | ||||
|---|---|---|---|---|---|---|
| Control | Infected | Control | Infected | Control | Infected | |
| WT (M) | 4.17 ± 0.076 (6) | 4.73 ± 0.258 (6) | 0.309 ± 0.017 (6) | 0.786 ± 0.115 (6)* | 164 ± 5.10 (17) | 282 ± 9.01 (16)* |
| Cyp4a10−/− (M) | 3.80 ± 0.147 (5) | 3.85 ± 0.188 (5) | 0.352 ± 0.062 (5) | 0.442 ± 0.028 (5)# | 219 ± 6.68 (13) | 305 ± 18.4 (12)*# |
| Cyp4a14−/− (M) | 3.52 ± 0.123 (6) | 3.96 ± 0.195 (6) | 0.308 ± 0.065 (6) | 0.501 ± 0.109 (6) | 210 ± 7.65 (14) | 266 ± 7.17 (15)*# |
| WT (F) | 3.48 ± 0.049 (6) | 4.31 ± 0.235 (6)* | 0.370 ± 0.025 (6) | 0.882 ± 0.177 (6)* | 205 ± 7.75 (17) | 287 ± 11.8 (17)* |
| Cyp4a10−/− (F) | 3.40 ± 0.223 (5) | 3.67 ± 0.143 (6) | 0.313 ± 0.022 (5) | 0.472 ± 0.041 (6) | 218 ± 9.37 (15) | 301 ± 12.2 (17)* |
| Cyp4a14−/− (F) | 3.37 ± 0.193 (5) | 3.44 ± 0.152 (5) | 0.349 ± 0.073 (5) | 0.716 ± 0.071 (5) | 208 ± 11.32 (15) | 236 ± 5.49 (12)# |
Values are the mean ± S.E.M. The number of animals is given in parentheses.
p < 0.05 compared to uninfected mice of same genotype
p < 0.05 compared to infected WT mice.
As expected, infection caused an increase in colonic crypt height (a measure of colonic hypertrophy) of the WT animals of both sexes (Table 1). In male mice, the magnitude of this increase was attenuated in both the Cyp4a10−/− or Cyp4a14−/− strains. In female mice, only the absence of Cyp4a14 had this effect, whereas Cyp4a10−/− mice had the same degree of hypertrophy as WT (Table 1)
Infection of WT mice with C. rodentium induced a minimal to mild degree of liver inflammation, as judged by the density of inflammatory foci, and was more pronounced in WT males than in females (Fig. 3). No male Cyp4a10−/− mice exhibited histologically detectable liver inflammation (p<0.05), and minimal inflammation was detected in only one Cyp4a14−/− animal (p=0.058). Female mice showed the same trends, but the differences were not significant.
Figure 3.
Liver pathology during C. rodentium infection wildtype (WT), Cyp4a10-deficient (Cyp4a10−/−) and Cyp4a14-deficient (Cyp4a14−/−) mice. Liver sections were stained with hematoxylin and eosin and scored for the presence of inflammation using the following scale: 0, normal; 1, minimal; 2, mild; 3, moderate; 4, severe. Points on the graph are livers from individual mice. All livers for uninfected mice had a score of zero, and the data are not shown. *, significantly different from WT group, p<0.05 by Kruskal-Wallace test followed by Dunn's multiple comparison test.
Hepatic Cytokine and Acute Phase Protein mRNAs
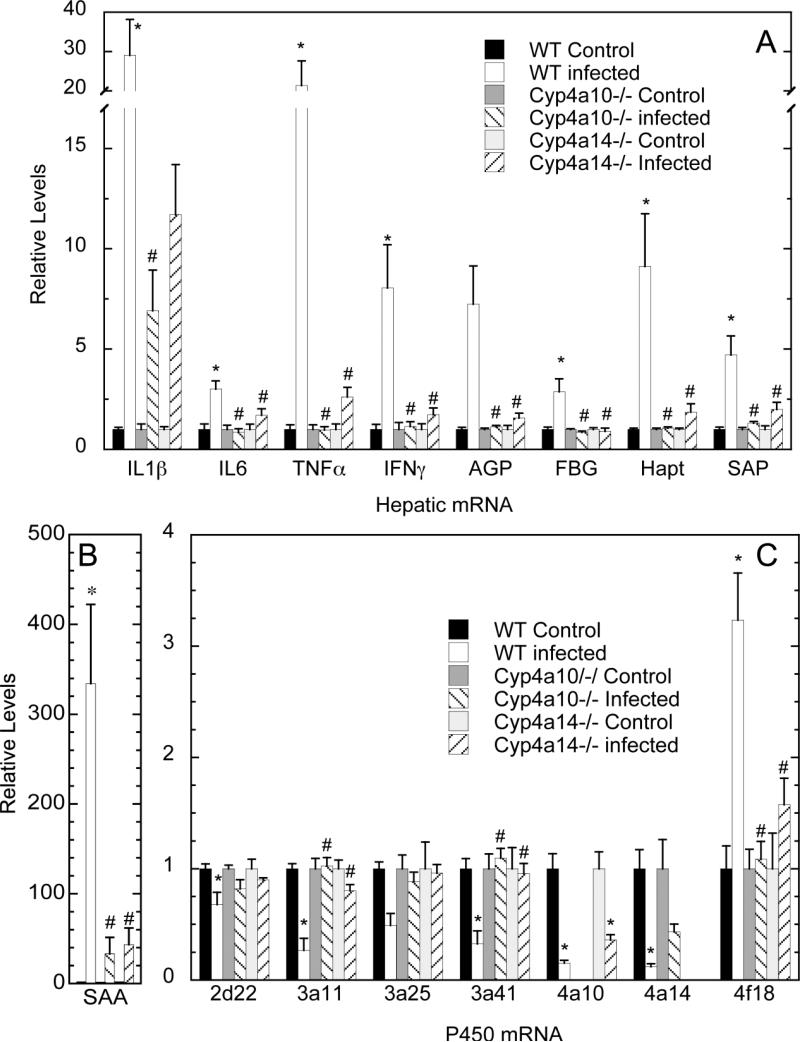
In male animals, the induction of hepatic cytokine and acute phase protein (APP) mRNAs caused by infection were either attenuated or abolished in Cyp4a10−/− and Cyp4a14−/− mice (Figure 4A and B). This phenotype appeared to be more robust in the Cyp4a10−/− than the Cyp4a14−/− mice. Thus, the mRNA levels of IL1β, IL6, TNFα, IFNγ, AGP, FBG, HAPT, SAA and SAP were all induced by infection in WT mice, whereas none were significantly affected by infection in the two Cyp4a-null strains. Basal expression of IL1β, AGP, FBG, HAPT, SAA and SAP was also significantly reduced in the Cyp4a10−/− and Cyp4a14−/− mice, with IL6, TNFα and IFNγ trending towards the same pattern (Supplemental Fig. 2).
Figure 4.
Effect of oral C. rodentium infection on hepatic mRNA expression in wildtype (WT), Cyp4a10-deficient (Cyp4a10−/−) and Cyp4a14-deficient (Cyp4a14−/−) mice. Over 24 h, male mice were allowed to drink either sucrose solution (Control) or C. rodentium in sucrose (Infected) then sacrificed after 7 days and livers harvested for measurement of mRNA by real-time RT-PCR. Cytokines and acute phase proteins (A and B); P450s (C). Values are expressed as relative levels of mRNA expression after normalization to GAPDH, with sucrose-treated (Control) group set to 1. Values represent mean ± S.E.M, (n = 5 or 6). *, significantly different from control mice of same genotype; #, significantly different from WT infected mice, p < 0.05.
For most cytokine and APP mRNAs, the pattern of regulation seen in female animals was similar to that in males, though several of the effects caused by infection in WT males failed to reach significance in female mice (Supplemental Fig. 3A, B). In notable contrast to males, however, the induction of SAA by infection was potentiated in the female Cyp4a10−/− and Cyp4a14−/− mice.
Hepatic P450 mRNAs
In both female and male animals, the relative constitutive levels of most P450 mRNAs were generally higher in uninfected wildtype, WT (Con), than in uninfected Cyp4a10−/− or Cyp4a14−/− mice (Supplemental Fig. 4) with some exceptions. Cyp3a41 mRNA expression was higher than WT levels in Cyp4a10−/− and Cyp4a14−/− females but not in males. Cyp4a14 mRNA expression was elevated 2-3 fold in both genders of the Cyp4a10−/− mice. Basal expression of Cyp4f18 (Supplemental Fig. 4), and of flavin monooxygenase 3 (Fmo3) and peroxisome proliferator-activated receptor-α(Pparα) (Supplemental Fig. 2) was not different among the genotypes.
In WT male mice, infection down-regulated Cyp2d22, Cyp3a11, Cyp3a41, Cyp4a10 and Cyp4a14 to differing degrees, and up-regulated Cyp4f18 (Fig. 4C). Cyp3a25 also trended towards down-regulation. Of these P450 mRNAs, none were significantly different from uninfected controls in the Cyp4a10−/− and Cyp4a14−/− strains, except for Cyp4a10, whose down-regulation by infection was not different from the WT mice (Fig. 4C). The mRNAs for Cyp1a2, Cyp2a4/5, Cyp2b9, Cyp2c29, Cyp2d9, Cyp2e1, Cyp3a13, Cyp4a12, Fmo3 and Pparα were not significantly affected by infection in WT mice and are not shown.
As for the cytokine and APP mRNAs, the responses of WT female hepatic P450 mRNAs to infection were generally smaller than those seen the WT males (Supplemental Fig. 3C). Similarly, the impact of the Cyp4a10 and Cyp4a14 deletions on the responses to infection were more muted than observed in males. In female animals of both genotypes, infection did not have significant effects on mRNA levels of Cyp1a2, 2b9, 2e1, 3a13 and 4a12. Cyp3a25 and Fmo3 were down-regulated similarly in all three strains. Cyp4a10 and Cyp4a14 down-regulations were unaffected in the Cyp4a14−/− and Cyp4a10−/− mice, respectively. The changes caused by infection in WT female animals were attenuated (did not reach significance) in the Cyp4a10−/− and Cyp4a14−/− strains for Cyp3a11, 3a25, 2d9 and 4f18 (Supplemental Fig. 3C).
Serum Analytes
Serum cytokine, chemokine and APP profiles of the male animals are shown in Fig. 5. Serum levels of IL2, IL3, IL4, IL7, IL9, IL10, IL17, CXCL1, MIP-1α and CCL5 were not significantly changed by infection in any strain, and are not shown. Serum levels of IFNγ, TNFα, IL6, CXCL10 and G-CSF were all significantly increased by infection in WT male mice (Fig. 5A, B), and again there was a tendency for most of these responses to be attenuated or blocked in the Cyp4a10−/− and Cyp4a14−/− animals. Thus, no significant effect of infection was observed for any of these analytes (compared to uninfected controls of the same genotype) in the Cyp4a10−/− and Cyp4a14−/− mice. When the magnitudes of changes due to infection were compared via ANOVA (# symbols), Cyp4a10−/− mice were significantly different from WT for all six analytes whereas Cyp4a14−/− mice were significantly different from WT with respect to IL6, CXCL10 and G-CSF. Serum APPs showed a similar pattern (Fig. 5C). The increases in serum AGP and SAP were abolished in the Cyp4a10−/− and Cyp4a14−/− mice, whereas the increase in HAPT was significantly attenuated.
Figure 5.
Effect of oral C. rodentium infection on serum cytokine and acute phase protein concentrations in male wildtype (WT), Cyp4a10-deficient (Cyp4a10−/−) and Cyp4a14-deficient (Cyp4a14−/−) mice. Mice were treated as described in Fig. 2 then sacrificed after 7 days, at which time blood was collected for measurement of serum cytokines, chemokines and APPs. IL2, IL3, IL4, IL7, IL9, IL10, IL17, CCL5, CXCL1, MIP1α did not change with infection and are not shown. Values represent mean ± S.E.M, (n = 5 or 6). *, significantly different from control mice of same genotype; #, significantly different magnitude of response from that of WT infected mice, p < 0.05.
In contrast to the males, when the basal levels of cytokines and chemokines of uninfected female animals were compared, markedly higher serum levels of IFNγ, IL6, IL17, G-CSF, CRP and HAPT were observed in Cyp4a14−/− than in WT (Fig. 6). This was only true for CRP and HAPT in the Cyp4a10−/− mice. In females, the significant increases of serum TNFα, IL17, CXCL10, and G-CSF due to infection in WT animals were either attenuated or abrogated in the Cyp4a10−/− and Cyp4a14−/− animals (Fig. 6A, B). Infection did not cause significant elevations of any APPs in the WT females, but did significantly up-regulate AGP in Cyp4a10−/− mice, and HAPT in both Cyp4a-null strains.
Figure 6.
Effect of oral C. rodentium infection on serum cytokine and acute phase protein concentrations in female wildtype (WT), Cyp4a10-deficient (Cyp4a10−/−) and Cyp4a14-deficient (Cyp4a14−/−) mice. Mice were treated as described in Fig. 2 then sacrificed after 7 days, at which time blood was collected for measurement of serum cytokines, chemokines and APPs. IL2, IL3, IL4, IL7, IL9, IL10, IL17, CCL5, CXCL1, MIP1α did not change with infection and are not shown. Values represent mean ± S.E.M, (n = 5 or 6). *, significantly different from control mice of same genotype; #, significantly different magnitude of response from that of WT infected mice, p < 0.05.
Effect of dietary sodium, during infection, on Hepatic APP mRNA
Since Cyp4a10−/− mice exhibit salt-sensitive hypertension (16), we next asked whether the impact of the Cyp4a10 gene deletion on the response to infection is also related to this phenotype. Therefore, we compared the responses in WT and Cyp4a10-deficient male mice fed diets containing either 0.03 or 0.4% sodium. In blood, liver and colon, there were no significant differences in bacterial counts among the different treatment groups (data not shown.
Among the animals fed the 0.4% Na diet, the up-regulation, by infection, of acute phase mRNAs observed in WT mice was either attenuated or abrogated in the KO animals (Fig. 7). Though some of the effects in WT mice were not significant (p > 0.05), the pattern of regulation in WT and Cyp4a-null animals fed 0.03% Na was similar to that seen at the 0.4% Na level with the exceptions that infection did not affect α-fibrinogen (FBG) in WT animals on the low salt diet (Fig. 7).
Figure 7.
Effect of dietary sodium on hepatic acute phase response to oral C. rodentium infection in wildtype (WT) and Cyp4a10-deficient (Cyp4a10−/−) mice. Over 24 h, male mice on diets containing either 0.03 or 0.4% sodium were allowed to drink either sucrose solution (Control) or C. rodentium in sucrose (Infected) then sacrificed after 7 days and livers harvested for measurement of mRNA by real-time RT-PCR. Values are expressed as relative levels of mRNA expression after normalization to GAPDH, with sucrose-treated (Control) group set to 1. Values represent mean ± S.E.M, (n = 5 or 6). *, significantly different from control mice of same genotype; #, significantly different from WT infected mice, p < 0.05.
Effect of Cyp4a gene deletion on the response to acute, sterile inflammation
The effect of acute, sterile inflammation caused by injection of LPS on hepatic P450 mRNA levels was investigated in male mice. Unlike the selective effects of infection, LPS administration resulted in the decrease of almost all P450 mRNAs 24 h after a 1mg/kg i.p. injection (Fig. 8A). Similarly, all APP mRNAs measured were induced (Fig. 8B). There were no significant differences among the genotypes in these responses to LPS.
Figure 8.
Effect of LPS injection on hepatic P450 and acute phase protein mRNAs in wildtype (WT), Cyp4a10-deficient (Cyp4a10−/−) and Cyp4a14-deficient (Cyp4a14−/−) mice. Male mice were injected i.p. with either saline (Control) or 1 mg/kg LPS (LPS), and livers harvested after 24 h for measurement of mRNA expression by real-time RT-PCR. Values are expressed as relative levels of mRNA expression after normalization to GAPDH with saline-treated group set to 1. Values represent mean ± S.E.M, (n = 5 or 6). *, significantly different from control mice of same genotype; #, significantly different from WT infected mice, p < 0.05.
Essentially the same results were found in female animals (not shown), except that Cyp2d9 mRNA was up-regulated in female mice (Supplemental Fig. 5). This induction occurred to the same extent in all three genotypes as well.
DISCUSSION
In agreement with our hypothesis that Cyp4a10 or Cyp4a14 ablation would affect the host response to C. rodentium infection, many of the hepatic and humoral inflammatory responses of both Cyp4a-deficient strains to C. rodentium infection were attenuated or abolished compared to WT mice. These effects of Cyp4a deletion were particularly pronounced for hepatic APP and cytokine mRNAs. APPs are synthesized primarily by hepatocytes and are induced and secreted as part of the acute phase response to inflammation or infection. APPs are involved in homeostasis as well as restraint of microbial growth before an acquired immunity is established (23). APP concentrations may be correlated with the severity of a disorder and thus may be valuable biomarkers of the inflammatory response (24). In this study, infection induced the expression of most hepatic APP and cytokine mRNAs. The absence of Cyp4a10 or Cyp4a14, especially in male animals greatly attenuated or obviated this induction (Fig. 4). Liver inflammation observed histologically was significantly attenuated in Cyp4a10−/− males and similar trends were seen in the Cyp4a10−/− males as well as the females of each genotype. This suggests that both Cyp4a10 and Cyp4a14 are involved in the innate response of the host liver to the infection. In WT mice, the effects of infection on the liver were more pronounced in males than in females. Thus, the impact of the Cyp4a10−/− and Cyp4a14−/− genotypes were more clearly observed in males although females followed the same pattern.
The regulation of most P450 enzymes followed the same trends in both male and female animals; that is, their down-regulation by infection was attenuated in Cyp4a14−/− mice. This is consistent with the reduced hepatic inflammatory response inferred from the hepatic APP and cytokine mRNAs as well as from histological analysis. Down-regulation of Cyp2d22, Cyp3a11 and the female-dominant Cyp3a41 were abolished in male but not female Cyp4a10−/− and Cyp4a14−/− mice, consistent with the APP and cytokine data above. Conversely, the absence of Cyp4a10, and to a lesser extent Cyp4a14 attenuated the up-regulation of the male dominant Cyp2d9 in females whereas it had no effect on Cyp2d9 in males of either strain. Interestingly, the down-regulation of Cyp4a10 was not attenuated in Cyp4a14−/−mice, nor was the down-regulation of Cyp4a14 attenuated in Cyp4a10−/−, supporting evidence from our previous work that these enzymes are regulated by different mechanisms than the drug-metabolizing P450s in this disease model (18,25).
Consistent with the hepatic cytokine and APP mRNAs, serum cytokine and APP responses to infection in male mice were also attenuated in the Cyp4a10−/− and Cyp4a14−/− animals. However, uninfected female Cyp4a14−/− (but not males or Cyp4a10−/− mice) had very high serum levels of IFNγ, IL1, IL3, IL4, IL6, IL9, IL10, IL17, as well as chemokines CXCL1, MIP-1α, CCL5 and G-CSF and the APPs CRP and HAPT (Fig. 6 and data not shown). This suggests that female Cyp4a14−/− have constitutive inflammation. However, hepatic cytokine or APP mRNAs tended to be reduced in uninfected Cyp4a14−/− mice. This suggests that the elevated APPs in sera of female Cyp4a14−/− mice may be of extrahepatic origin. In any case, the blunted responses of these analytes to infection could be due to the already high basal levels in Cyp4a14−/− females.
Colon crypt lengths are a measure of mucosal inflammation and hypertrophy caused by infection. The magnitude of the increase in crypt length caused by infection was significantly reduced in Cyp4a14−/− males and females (Table 1), whereas in the Cyp4a10−/− mice this phenotype was only observed in the females. Conversely, the bacterial counts in colons of Cyp4a10−/− and Cyp4a14−/− trended towards being lower than WT animals, an effect that was more pronounced in males than in female Cyp4a14−/− mice. Attenuated inflammatory responses are also indicated by the fact that Cyp4a10−/− and Cyp4a14−/− mice were resistant to hepatomegaly and splenomegaly in response to the infection, again most apparent in male mice.
The above findings are consistent with the hypothesis that Cyp4a14 down-regulation during infection could be a homeostatic response to help moderate or terminate inflammation before it becomes deleterious. Alternatively, the reduced inflammation might help the invading organism to thrive. Since colonic C. rodentium counts were, if anything, lower in the Cyp4a – null strains than in WT mice, the former explanation seems more likely. It should also be considered that, since expression of other P450 enzymes in the liver was generally lower in the uninfected Cyp4a-null mice compared to WT, this altered expression may have also contributed to the reduced inflammatory responses observed in the Cyp4a10−/− and Cyp4a14−/− mice.
With some exceptions noted above, the impacts of the Cyp4a10−/− and Cyp4a14−/− genotypes on the responses to C. rodentium infection were remarkably similar. This indicates that the functions of these enzymes in regulating the inflammatory response are not redundant. This could be because the enzymes catalyze different steps in a common pathway. Alternatively, the substrates they metabolize, or the products they produce, may be different. By analogy, both Cyp4a10−/− and Cyp4a14−/− mice are hypertensive, but the mechanisms by which hypertension is elicited are quite different. The Cyp4a14−/− hypertensive phenotype is gender (androgen)-dependent and insensitive to dietary sodium (15). It is related to elevated Cyp4a12 expression and higher rates of 20-hydroxyeicosatetraenoic acid (20-HETE) formation in the kidneys. Disruption of the Cyp4a10 gene results in salt-sensitive hypertension that is not gender-dependent (16). Thus, Cyp4a10−/− mice fed on diets containing 0.3 or 8% sodium were severely hypertensive, and became normotensive when fed 0.05% sodium diets (16).
The hypertensive phenotype per se does not appear to be the cause of the attenuated inflammation in the Cyp4a-null animals. As shown in Fig. 7, reduced inflammation as assessed by APP regulation was no different in animals consuming high-salt or low-salt diets. While we did not measure the blood pressure, it has been reported that in WT animals of the same strain, even 8% NaCl had no significant effects on the blood pressure, while Cyp4a10–/– mice on 0.3% or 8% but not 0.05% sodium were severely hypertensive (16)). Although both hypertension and the diminished responses to inflammation are both more prevalent in male than female Cyp4a14−/− mice, the tendency to observe a reduced impact of Cyp4a gene deletion on inflammation in females appears to be mainly because WT females have a smaller response to infection compared to males. The female Cyp4a-null strains themselves showed little if any gender differences in the response to infection.
The mechanisms by which Cyp4a10 and Cyp4a14 deletions cause resistance to inflammation can only be speculated upon. If 20-HETE were involved in the effects observed here, one would expect it to be pro-inflammatory (since eliminating Cyp4a enzymes that produce it is anti-inflammatory). To the contrary, a synthetic agonistic analogue of 20-HETE was reported to reduce renal activation of NF-κB and elevation of serum TNF in LPS-induced septic shock in rats (26). 20-HETE is also a potent agonist of PPAR (27), which can exert anti-inflammatory effects via inhibition of NF-κB (28). Also, recombinant Cyp4a12a and Cyp4a12b are about 100 times more active than Cyp4a10 in 20-HETE formation from arachidonic acid, whereas Cyp4a14 is essentially inactive (29). Accordingly, Cyp4a12 appears to be the important 20-hydroxylase in the mouse kidney (15). Thus, the roles of Cyp4a10 and Cyp4a14 in this disease model may be to regulate the levels of fatty acids or other substrates that are precursors of anti-inflammatory molecules. Alternatively, this phenomenon could be related to the fact that CYP4A enzymes are catalytically uncoupled (30), resulting in production of reactive oxygen species. Oxidative stress caused by induction of Cyp4a10 has been implicated in hepatic neoplasia (31).
Christmas et al (32) identified Cyp4f18 as the functional orthologue of human CYP4F3A, a critical protein in the inactivation of leukotriene B4 (LTB4), a potent mediator of inflammation and chemoattractant for polymorphonuclear leukocytes. The abolition of Cyp4f18 induction in response to infection in Cyp4a10−/− and Cyp4a14−/− mice seems to belie the hypothesis that the absence these P450s is beneficial for the resolution of the infection, at least in terms of LTB4 action. It would be interesting to find out if these animals have defective LTB4 synthesis and therefore do not need to increase their production of the LTB4- ω-hydroxylase Cyp4f18.
Rodent CYP4As are induced via activation of the nuclear receptor PPARα in fasting and starvation commensurate with their roles in fatty acid metabolism (33). The hepatic expression of Cyp4a10 and 4a14 were higher in wildtype compared to PPARα-null mice suggesting a role of the nuclear receptor in the basal expression of Cyp4a enzymes (34). The down regulation of Cyp4as by C. rodentium infection however is not due to changes in PPARα mRNA levels as these are not significantly different when control and infected animals are compared (Supplemental Fig. 2A and data not shown).
P450 mRNAs are selectively modulated during Citrobacter rodentium infection in a pattern that is different from the LPS model. Infection down-regulates Cyp4a10 and Cyp4a14 in the liver and kidney of female mice (17-19). LPS treatment induces these enzymes in the kidney (20) as well as liver of CD-1 mice (35) but down-regulates Cyp4a10 in the liver of 129/SvJ and C57BL/6J mice (18,34). In contrast to the sub-chronic inflammation caused by C. rodentium infection, LPS injection is a model of sterile acute inflammation. Here we report that LPS caused the down-regulation of P450s and up-regulation of APPs in a pattern that was similar in all three genotypes of both sexes, hence there was no evidence of the involvement of Cyp4a10 or Cyp4a14 in the LPS response. These results indicate that the presence or absence of Cyp4a10 and Cyp4a14 affects hepatic and humoral inflammatory responses to enteral C. rodentium infection but not to acute exposure to endotoxin. Unresolved questions include mechanisms by which Cyp4a10 and Cyp4a14 affect the inflammatory response to infection, and whether it is applicable to other bacterial, viral or parasitic infections.
Supplementary Material
Table 2.
Liver and spleen weights of control and infected mice.
| Liver (% of body weight) | Spleen (% of body weight) | |||
|---|---|---|---|---|
| Control | Infected | Control | Infected | |
| Jax | 3.89 ± 0.178 (6) | 4.78 ± 0.133 (6)* | 0.336 ± 0.024 (6) | 0.852 ± 0.098 (6)* |
| Tac | 3.90 ± 0.184 (6) | 4.28 ± 0.238 (6) | 0.319 ± 0.040 (6) | 0.667 ± 0.108 (6)* |
Wildtype female 129X1/SvJ (Jax) and 129X1/SvEvTac (Tac) mice were allowed to drink either sucrose solution or C. rodentium in sucrose then sacrificed after 7 days. Values are the mean ± S.E.M. The number of animals is given in parentheses.
p < 0.05, compared to control, t test
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant R01 DK072372]. We are grateful to Dr. Jorge Capdevila, Vanderbilt University for providing the Cyp4a-deficient mice that were used to establish our breeding colonies. The technical assistance of Mr. William Watkins, Dr. Matthew Merrell and Dr. Choon-Myung Lee is gratefully acknowledged. We wish to thank Dr. Dean P. Jones and Mr. Yongliang Liang (Clinical Biomarkers Laboratory, Department of Medicine, Emory University) for access to equipment used to quantify serum cytokines.
Footnotes
Conflict of Interest. The authors declare that they have no conflict of interest.
REFERENCES
- 1.Hardwick JP. Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochemical Pharmacology. 2008;75:2263–2275. doi: 10.1016/j.bcp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Johnson EF, Palmer CN, Griffin KJ, Hsu MH. Role of the peroxisome proliferator-activated receptor in cytochrome P450 4A gene regulation. FASEB journal. 1996;10:1241–1248. doi: 10.1096/fasebj.10.11.8836037. [DOI] [PubMed] [Google Scholar]
- 3.Sanders RJ, Ofman R, Valianpour F, Kemp S, Wanders RJ. Evidence for two enzymatic pathways for omega-oxidation of docosanoic acid in rat liver microsomes. Journal of Lipid Research. 2005;46:1001–1008. doi: 10.1194/jlr.M400510-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Gainer JV, Bellamine A, Dawson EP, Womble KE, Grant SW, Wang Y, Cupples LA, Guo CY, Demissie S, O'Donnell CJ, Brown NJ, Waterman MR, Capdevila JH. Functional variant of CYP4A11 20-hydroxyeicosatetraenoic acid synthase is associated with essential hypertension. Circulation. 2005;111:63–69. doi: 10.1161/01.CIR.0000151309.82473.59. [DOI] [PubMed] [Google Scholar]
- 5.Heng YM, Kuo CS, Jones PS, Savory R, Schulz RM, Tomlinson SR, Gray TJ, Bell DR. A novel murine P-450 gene, Cyp4a14, is part of a cluster of Cyp4a and Cyp4b, but not of CYP4F, genes in mouse and humans. Biochemical Journal. 1997;325(Pt 3):741–749. doi: 10.1042/bj3250741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powell PK, Wolf I, Jin R, Lasker JM. Metabolism of arachidonic acid to 20-hydroxy-5,8,11, 14-eicosatetraenoic acid by P450 enzymes in human liver: involvement of CYP4F2 and CYP4A11. Journal of Pharmacology and Experimental Therapeutics. 1998;285:1327–1336. [PubMed] [Google Scholar]
- 7.Lasker JM, Chen WB, Wolf I, Bloswick BP, Wilson PD, Powell PK. Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of Cyp4F2 and Cyp4A11. Journal of Biological Chemistry. 2000;275:4118–4126. doi: 10.1074/jbc.275.6.4118. [DOI] [PubMed] [Google Scholar]
- 8.McGiff JC, Quilley J. 20-HETE and the kidney: resolution of old problems and new beginnings. American Journal of Physiology. 1999;277:R607–623. doi: 10.1152/ajpregu.1999.277.3.R607. [DOI] [PubMed] [Google Scholar]
- 9.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. Journal of Smooth Muscle Research. 2005;41:175–193. doi: 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- 10.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiological Reviews. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 11.Sarkis A, Roman RJ. Role of cytochrome P450 metabolites of arachidonic acid in hypertension. Current Drug Metabolism. 2004;5:245–256. doi: 10.2174/1389200043335603. [DOI] [PubMed] [Google Scholar]
- 12.King LM, Gainer JV, David GL, Dai D, Goldstein JA, Brown NJ, Zeldin DC. Single nucleotide polymorphisms in the CYP2J2 and CYP2C8 genes and the risk of hypertension. Pharmacogenetics and Genomics. 2005;15:7–13. doi: 10.1097/01213011-200501000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Wu J, Liu H, Lai G, Zhao Y. Disturbed ratio of renal 20-HETE/EETs is involved in androgen-induced hypertension in cytochrome P450 4F2 transgenic mice. Gene. 2012;505:352–359. doi: 10.1016/j.gene.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 14.Athirakul K, Bradbury JA, Graves JP, DeGraff LM, Ma J, Zhao Y, Couse JF, Quigley R, Harder DR, Zhao X, Imig JD, Pedersen TL, Newman JW, Hammock BD, Conley AJ, Korach KS, Coffman TM, Zeldin DC. Increased blood pressure in mice lacking cytochrome P450 2J5. FASEB Journal. 2008;22:4096–4108. doi: 10.1096/fj.08-114413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holla VR, Adas F, Imig JD, Zhao X, Price E, Jr., Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5211–5216. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakagawa K, Holla VR, Wei Y, Wang WH, Gatica A, Wei S, Mei S, Miller CM, Cha DR, Price E, Jr., Zent R, Pozzi A, Breyer MD, Guan Y, Falck JR, Waterman MR, Capdevila JH. Salt-sensitive hypertension is associated with dysfunctional Cyp4a10 gene and kidney epithelial sodium channel. Journal of clinical Investigation. 2006;116:1696–1702. doi: 10.1172/JCI27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaluvadi MR, Kinloch RD, Nyagode BA, Richardson TA, Raynor MJ, Sherman M, Antonovic L, Strobel HW, Dillehay DL, Morgan ET. Regulation of hepatic cytochrome P450 expression in mice with intestinal or systemic infections of citrobacter rodentium. Drug Metabolism and Disposition. 2009;37:366–374. doi: 10.1124/dmd.108.024240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyagode BA, Lee CM, Morgan ET. Modulation of hepatic cytochrome P450s by Citrobacter rodentium infection in interleukin-6- and interferon-{gamma}-null mice. Journal of Pharmacology and Experimental Therapeutics. 2010;335:480–488. doi: 10.1124/jpet.110.171488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson TA, Sherman M, Antonovic L, Kardar SS, Strobel HW, Kalman D, Morgan ET. Hepatic and renal cytochrome p450 gene regulation during citrobacter rodentium infection in wild-type and toll-like receptor 4 mutant mice. Drug Metabolism and Disposition. 2006;34:354–360. doi: 10.1124/dmd.105.007393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richardson TA, Morgan ET. Hepatic cytochrome P450 gene regulation during endotoxin-induced inflammation in nuclear receptor knockout mice. Journal of Pharmacology and Experimental Therapeutics. 2005;314:703–709. doi: 10.1124/jpet.105.085456. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Quigley R, Chakravarty S, Zhao X, Imig JD, Capdevila JH. Increased renal proximal convoluted tubule transport contributes to hypertension in Cyp4a14 knockout mice. Nephron Physiology. 2009;113:p23–28. doi: 10.1159/000235774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cray C, Zaias J, Altman NH. Acute phase response in animals: a review. Comparative Medicine. 2009;59:517–526. [PMC free article] [PubMed] [Google Scholar]
- 24.Murata H, Shimada N, Yoshioka M. Current research on acute phase proteins in veterinary diagnosis: an overview. Veterinary Journal. 2004;168:28–40. doi: 10.1016/S1090-0233(03)00119-9. [DOI] [PubMed] [Google Scholar]
- 25.Kinloch RD, Lee CM, van Rooijen N, Morgan ET. Selective role for tumor necrosis factor-alpha, but not interleukin-1 or Kupffer cells, in down-regulation of CYP3A11 and CYP3A25 in livers of mice infected with a noninvasive intestinal pathogen. Biochemical Pharmacology. 2011;82:312–321. doi: 10.1016/j.bcp.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tunctan B, Korkmaz B, Sari AN, Kacan M, Unsal D, Serin MS, Buharalioglu CK, Sahan-Firat S, Cuez T, Schunck WH, Falck JR, Malik KU. 5,14-HEDGE, a 20-HETE mimetic, reverses hypotension and improves survival in a rodent model of septic shock: contribution of soluble epoxide hydrolase, CYP2C23, MEK1/ERK1/2/IKKbeta/IkappaB-alpha/NF-kappaB pathway, and proinflammatory cytokine formation. Prostaglandins and Other Lipid Mediators. 2013;102-103:31–41. doi: 10.1016/j.prostaglandins.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng VY, Huang Y, Reddy LM, Falck JR, Lin ET, Kroetz DL. Cytochrome P450 eicosanoids are activators of peroxisome proliferator-activated receptor alpha. Drug Metabolism and Disposition. 2007;35:1126–1134. doi: 10.1124/dmd.106.013839. [DOI] [PubMed] [Google Scholar]
- 28.Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ, Fruchart JC, Tedgui A, Haegeman G, Staels B. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. Journal of Biological Chemistry. 1999;274:32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- 29.Muller DN, Schmidt C, Barbosa-Sicard E, Wellner M, Gross V, Hercule H, Markovic M, Honeck H, Luft FC, Schunck WH. Mouse Cyp4a isoforms: enzymatic properties, gender- and strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formation. Biochemical Journal. 2007;403:109–118. doi: 10.1042/BJ20061328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dierks EA, Davis SC, Ortiz de Montellano PR. Glu-320 and Asp-323 are determinants of the CYP4A1 hydroxylation regiospecificity and resistance to inactivation by 1-aminobenzotriazole. Biochemistry. 1998;37:1839–1847. doi: 10.1021/bi972458s. [DOI] [PubMed] [Google Scholar]
- 31.Xu Z, Chen L, Leung L, Yen TS, Lee C, Chan JY. Liver-specific inactivation of the Nrf1 gene in adult mouse leads to nonalcoholic steatohepatitis and hepatic neoplasia. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4120–4125. doi: 10.1073/pnas.0500660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christmas P, Tolentino K, Primo V, Berry KZ, Murphy RC, Chen M, Lee DM, Soberman RJ. Cytochrome P-450 4F18 is the leukotriene B4 omega-1/omega-2 hydroxylase in mouse polymorphonuclear leukocytes: identification as the functional orthologue of human polymorphonuclear leukocyte CYP4F3A in the down-regulation of responses to LTB4. Journal of Biological Chemistry. 2006;281:7189–7196. doi: 10.1074/jbc.M513101200. [DOI] [PubMed] [Google Scholar]
- 33.Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Molecular and Cellular Biology. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barclay TB, Peters JM, Sewer MB, Ferrari L, Gonzalez FJ, Morgan ET. Modulation of cytochrome P-450 gene expression in endotoxemic mice is tissue specific and peroxisome proliferator-activated receptor-alpha dependent. Journal of Pharmacology and Experimental Therapeutics. 1999;290:1250–1257. [PubMed] [Google Scholar]
- 35.Pan J, Xiang Q, Ball S, Scatina J, Kao J, Hong JY. Lipopolysaccharide-mediated modulation of cytochromes P450 in Stat1 null mice. Drug Metabolism and Disposition. 2003;31:392–397. doi: 10.1124/dmd.31.4.392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.