Abstract
Aims
Botulinum neurotoxin serotype A (BoNT/A) has emerged as an effective treatment of urinary bladder overactivity. Intravesical lipotoxin (BoNT/A delivery using liposomes), which may target the urothelium, is effective in blocking acetic acid induced hyperactivity in animals. The objective of this study was to assess the possible site of toxin action within the urothelium.
Methods
We examined expression of the toxin receptor (SV2) and its cleavage targets (SNAP-25 and SNAP-23) within urothelium as well as effects of the toxin on mechanically evoked release of ATP from cultured rat urothelial cells. ATP release was measured using the luciferin-luciferase assay; we examined expression of SNAP-23 and -25 in urothelial cells and mucosa of rat and human bladders.
Results
BoNT/A (1.5U; 1–3 hr) blocked hypotonic evoked release of urothelial ATP, without affecting morphology. The expression of protein targets for BoNT/A binding (SV2) was detected in human and rat bladder mucosa and catalytic action (SNAP-23, -25) in urothelial cells and mucosa (differed in intensity) fromrat and human bladder. Incubation of cultured (rat) urothelial cells with BoNT/A decreased expression levels of both SNAP-23 (44%) and SNAP-25 (80%).
Conclusions
Our findings reveal that the bladder urothelium expresses the intracellular targets and the binding protein for cellular uptake of BoNT/A; and that the toxin is able to suppress the levels of these targets as well as hypotonic-evoked ATP release. These data raise the possibility that intravesical treatment with BoNT/A suppresses bladder reflex and sensory mechanisms by affecting a number of urothelial functions including release of transmitters.
Keywords: SNAP-25, SNAP-23, bladder mucosa, exocytosis
INTRODUCTION
The intramural injection of BoNT/A into the urinary bladder has proven to be a useful therapeutic approach for the treatment of various bladder disorders including refractory idiopathic detrusor overactivity as well as neurogenic detrusor overactivity.1,2 Following uptake into nerve terminals BoNT/A is thought to affect bladder function by targeting intracellular proteins involved in exocytosis and then suppressing the release of transmitters from intramural nerves.
Though most of the studies on mechanism of action have focused on bladder nerves and change in neural control of the smooth muscle, other cellular targets may also be involved in the therapeutic effects of the toxin. Recent evidence has suggested that BoNT/A may act in part by targeting afferent mechanisms.3,4 This may be due to a direct effect on afferent nerves or an indirect effect to block release of transmitters from the epithelial cells that line the bladder wall (i.e., the urothelium). Transmitters released from the urothelium are likely to alter the activity of underlying bladder afferent nerves or spontaneous activity of smooth muscle to alter bladder sensations.5
In motor neurons, BoNT/A cleaves the SNARE protein, SNAP-25, which plays a role in release of acetylcholine, thereby suppressing neurotransmission and preventing muscle contraction. 6 While there is also evidence for an effect on urothelial-release of mediators,1,7 there are conflicting histological/ anatomical results regarding the expression of protein targets for this toxin in the urothelium.8 The purpose of this study was to assess the effect of BoNT/A on mechanically evoked ATP release from isolated urothelial cells and to examine the expression of its binding site (SV2) and SNARE proteins, SNAP-23 and SNAP-25, in human as well as rodent mucosa.
METHODS
Tissue and Urothelial Cell Preparation
All procedures were conducted in accordance and approval by both Institutional Animal Care and Use Committee and Institutional Review Board Committee Policies at the University of Pittsburgh. For immunoblot preparation, urinary bladder urothelium/lamina propria or “mucosa” (mechanically stripped from the underlying connective and smooth muscle tissues) from Harlan Sprague Dawley rats, aged 2–4 months, or de-identified superficial human biopsies from asymptomatic subjects were homogenized in HBSS (5mM KCl, 0.3mM KH2PO4, 138mM NaCl, 4mM NaHCO3, 0.3mM Na2HPO4, 5.6mM glucose and 10mM HEPES, pH 7.4 containing complete protease inhibitor cocktail, 1 tablet/10 ml, Roche, Indianapolis, IN) and phosphatase inhibitor cocktail (Sigma, St. Louis, MO, 1:100). After centrifugation (13,000g, 15 min), the membrane protein fraction was prepared by suspending the membrane pellets in lysis buffer containing 0.3M NaCl, 50mM Tris-HCl (pH 7.6) and 0.5% Triton X-100 and the same concentration of protease inhibitor as above. The suspensions were incubated on ice and centrifuged (13,000g; 15 min at 4°C) and protein concentrations were determined (Pierce BCA protein assay, Thermo Scientific, Rockford, IL).
Rat or human urothelial cell cultures were prepared as previously described.9,10 Urinary bladders were removed and placed in cold MEM (Invitrogen, Carlsbad, CA) with HEPES (2.5 g/L, Sigma) and penicillin/streptomycin/fungizone (PSF; 1%; Invitrogen). The bladder was cut open to expose the urothelium, and incubated in dispase (2.0mg/ml, Invitrogen) overnight at 4°C. Urothelial cells were gently scraped from the underlying tissue, placed in trypsin (0.25% w/v; 5–15 min, Invitrogen) triturated, suspended in MEM containing 10% FBS (Invitrogen) and centrifuged (416g; 15min). For human biopsies, cultured cells were prepared according to previously published methods. 10 In brief, following dissociation in trypsin and resuspension in MEM (same as above), the supernatant was removed, and cells (rat or human) were suspended in PCT bladder epithelium medium (CNT-16, CELLnTEC, Bern, Switzerland). The cells were centrifuged again, resuspended in the same medium, plated on collagen-coated glass coverslips at 20 × 104 cells/ml and utilized within 18–72 hr following plating.
Antibodies
The SNAP-25 antibodies were from Covance (Emeryville, CA; SMI-81), Research and Diagnostic Antibodies (Las Vegas, NV; MC-6050), and Synaptic Systems (Gottingen, Germany). The rabbit polyclonal antibody to SNAP-23 and immunizing peptides for SNAP-23 and -25 were from Synaptic Systems. The mouse monoclonal antibody to SV2 (developed by Dr. Kathleen M. Buckley) was obtained from the Developmental Studies using a Hybridoma Bank under the auspices of the NICHD and maintained by the University of Iowa, Iowa City, IA. Secondary goat anti-rabbit HRP and goat anti-mouse antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA), and Becton Dickinson (Franklin Lakes, NJ), respectively. Loading control, rabbit anti-β-actin was from Abcam (Cambridge, MA). Z0–1 antibody was from Invitrogen, DAPI from EMD Millipore (Billerica, MA), DAPI from Molecular Probes (Eugene, OR) and cytokeratin antibodies from Dako Cytomation (Carpinteria, CA); fluorochrome-labeled secondary antibodies were obtained from Abcam.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated from intact rat bladder mucosa by homogenization in Trizol (Invitrogen). RNA purification was performed using Qiagen RNeasy mini kit including an on column DNase Digestion step. RNA samples were reverse transcribed (RT) with an oligo (dT) primer by Superscript II (Invitrogen). RT-controls were performed for each sample by omission of the enzyme. Polymerase chain reaction (PCR) amplification was conducted using 1.25 units of platinum Taq DNA Polymerase (Life Technologies) using primer pairs for the following genes: synaptosomal-associated protein 23 (SNAP-23), synaptosomal-associated protein 25 (SNAP-25) and isoforms of the synaptic vesicle glycoprotein 2 (SV2-A, SV2-B, and SV2-C).Primer sequence; product size; and gene accession numbers were:
SNAP-23: forward: caccaacaagaatcgcattg; reverse: cccttctcgcacagtctttc; 150 bp; NM_022689
SNAP-25: forward: caaagatgctgggaagtggt; reverse: gggggtgactgactctgtgt; 181 bp; NM_030991
SV2-A: forward: tttgggggagtcagtattgc; reverse: cccacaaaggacgtgaagat; 155 bp; NM_057210
SV2-B: forward: agctgtatcccaccaaccag; reverse: agagaagcagcagccagaag; 145 bp; NM_057207
SV2-C: forward: gtacaaggaccgcagagagc; reverse: taacacaaagcccaccacaa; 181 bp; NM_031593
Amplification products were visualized on a 1.3% agarose gel with ethidium bromide. Empty RT − lanes confirmed purity of the RNA isolation (absence of genomic DNA).
Immunoblot (SNAP-23, SNAP-25, and SV2)
Lysates from each sample were denatured in SDS–PAGE sample buffer (all except SV2 were treated at 100°C for 5 min) and separated on an SDS–PAGE gel using a standard Western protocol. Proteins were transferred to polyvinylidene fluoride membranes, blocked with 5% Milk TBS-T (1 hr), rinsed in TBS-T, and incubated (overnight at 4°C) with primary antibody diluted in 5% Milk TBS-T (SV2 and SNAP-23) or 2% normal goat serum TBS-T (SNAP-25). The membranes were then incubated with secondary antibody for 1 hr in 5% Milk TBS-T, developed with ECL Plus (Amersham, Piscataway, NJ) and exposed to film. The volume of each band was determined using a Personal Densitometer SI (Molecular Probes, Carlsbad, CA). The membranes were stripped (membrane recycling kit from Alpha Diagnostic International, San Antonio, TX) and reprobed overnight with rabbit anti-β-actin as a loading control. Bands for SNAP-23 (23 kDa) and SNAP-25 (75 kDa) were blocked by incubation with the immunizing peptide (1 mg/ml). A single immunoreactive band was observed for SV2 (95 kDa) and bactin (43 kDa); a competing peptide was not commercially available for these antibodies.
Immunohistochemistry/Immunocytochemistry (SNAP-23; SNAP-25; Z0–1)
Excised bladders were embedded in optimum cutting temperature (OCT) compound (Tissue-Tek OCT, Sakura Finetek, CA), snap frozen and stored at −80°C. Serial cryosections (6µm) were mounted onto microscope slides, post-fixed with 4% paraformaldehyde and washed in phosphate buffered saline (PBS). Primary cultured urothelial cells, grown on glass coverslips, were fixed with 2% paraformaldehyde and washed in PBS. Tissue sections or cells were incubated with permeabilizing/blocking solution (0.5%Triton X-100 and 10% goat serumin PBS) before incubation (24–48 hr at 4°C) with primary antibodies in blocking solution (10% goat serum in PBS). Cells were incubated with monoclonal anti-cytokeratin 17 (basal urothelial cell marker; Dako) and polyclonal anti-zona occludens-1 (ZO-1; a tight junctional marker; EMD Millipore) at a 1:200 dilution. Tissue sections were incubated with rabbit polyclonal primary (SNAP-23 or SNAP-25, 1:100, Synaptic Systems) antibodies; control experiments were done using antibodies blocked by incubation with the immunizing peptide (1mg/ml). Co-localization studies were also conducted using monoclonal anticytokeratin 17 (1:500). Tissue and cells were washed with PBS before incubation with the appropriate secondary antibodies. Cells were incubated (2 hr at room temperature) with fluorophore-tagged secondary antibodies: Alexa Fluor 488 Donkey Anti-Mouse and (green) and Alexa Fluor 555 Donkey Anti-Mouse (red) (1:700; Invitrogen). Tissue sections were incubated with donkey anti-rabbit biotin (1:1,000, Abcam) for 2 hr, washed, and incubated with Neutravidin FITC (1:1,000, Abcam). Nuclei were counterstained with DAPI (4′,6-diamidino-2-phenylindole, dilactate; Molecular Probes, Eugene, OR). Tissue or cells were treated with ProLong Gold antifade reagent (Invitrogen) and imaged on a BX-62 Olympus upright fluorescent microscope using CImaging software (Hamamatsu Photonics, Sewickley, PA). Background immunofluorescence was assessed in the absence of primary antibodies and secondary only.
ATP Release Assay
ATP release was conducted as per previously published methods.9 In brief, a perfusate of 100µl of HBSS (5mM KCl, 0.3mM KH2PO4, 138mM NaCl, 4mM NaHCO3, 0.3mM Na2HPO4, 5.6mM Glucose, 2mM CaCl2, 1mM MgCl2, and 10mM HEPES, pH 7.4) was collected every 30 sec. Background release of ATP from the cultures was measured for 15–20 min prior to the test stimulus (HBSS with 50% reduction of NaCl). ATP measurements were calculated based on the luciferin-luciferase reaction using a standard curve (Adenosine Triphosphate Assay Kit, Sigma; Glomax 20/20 Luminometer, Promega). Data were analyzed by calculating the area under the curve during each portion of the experiment.
Statistical Analysis
Data were graphed in GraphPad Prism 5 and analyzed by a paired Students t-test. Data were considered significantly different when P<0.05.
RESULTS
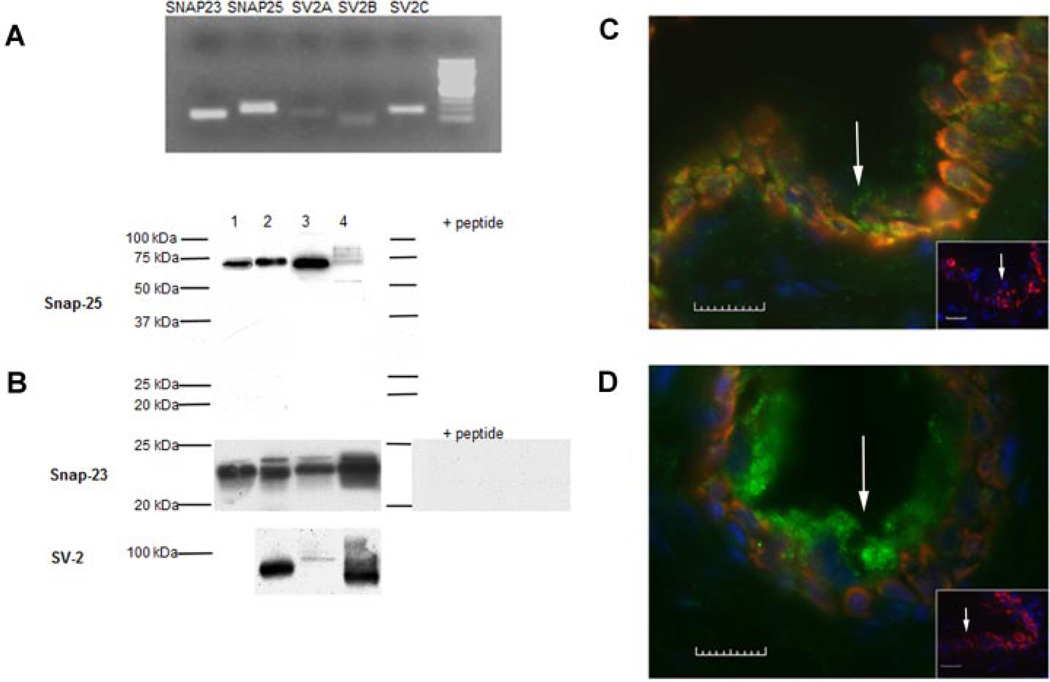
Expression of the BoNT/A receptor, SV2, as well as the SNARE proteins, SNAP-23 and SNAP-25, was detected in urinary bladder mucosa (ex vivo). Using RNA extracted from rat mucosal tissue and gel electrophoresis, positive bands were obtained for SNAP-23, SNAP-25, and all three SV2 isoforms (SV2-A, SV2-B, SV2-C) with the SV2C isoform exhibiting the greatest expression (Fig. 1A). Protein expression for SV2, SNAP-23, and SNAP-25 was also evident in rat mucosa and in human mucosal biopsies taken from asymptomatic controls. Positive bands for SNAP-23 and SNAP-25 were also observed in proteins extracted from urothelial cells isolated from rat as well as human urinary bladder and SV2 in rat mucosa (Fig. 1B). We also have observed a difference in the immunoblot intensity between mucosal samples and cultured urothelial cells, though the reasoning for these differences are not known. The protein band for both SNAP-23 and SNAP-25 for mucosa and cells was completely blocked by incubation of the antibodies with the immunizing peptide, demonstrating specificity of the antibodies.
Fig. 1.
The synaptosomal-associated protein-23 (SNAP-23), synaptosomal-associated protein-25 (SNAP-25) and synaptic vesicle protein 2 (SV2) are expressed in urinary bladder urothelium. A: Electrophoresis of PCR product on a 1.3% agarose gel with ethidium bromide staining showing positive mucosal expression in the rat of SNAP-23, SNAP-25 and synaptic vesicle (SV) glycoprotein isoforms: 2A, 2B, and 2C (SV2-A, SV2-B, SV2-C). B: Using immunoblot analysis is the protein expression for SNAP-23 (23 kDa), SNAP-25 (75 kDa), and SV2 (95 kDa). Each lane represents samples from the same source: human urothelial cells (lane 1), human mucosa (lane 2), rat urothelial cells (lane 3) and rat mucosa (lane 4). The protein expression for both SNAP-23 and SNAP-25 was completely blocked by the immunizing peptide. C,D: Rat bladder cross-sections showing SNAP-25-immunoreactivity (IR, panel C, expressed throughout urothelium) and for SNAP-23-IR (panel D, expressed mainly in superficial or apical cells). Arrows in each panel indicate the epithelial surface. In both C and D inset panels show lack of SNAP-IR in controls following incubation with the corresponding immunizing peptide (calibration bar = 10 µm).
Immunocytochemical studies revealed expression of both SNARE proteins within the rat urothelium. Co-localization experiments for urothelial cytokeratin 17 (basal cells) revealed that SNAP-25 was observed throughout the urothelium (Fig. 1C) while SNAP-23 was expressed mainly within the superficial or apical layer (Fig. 1D). Omission of primary antibodies from the incubation buffer as well as incubation of the antibodies with the immunizing peptide completely attenuated secondary antibody labeling (data not shown).
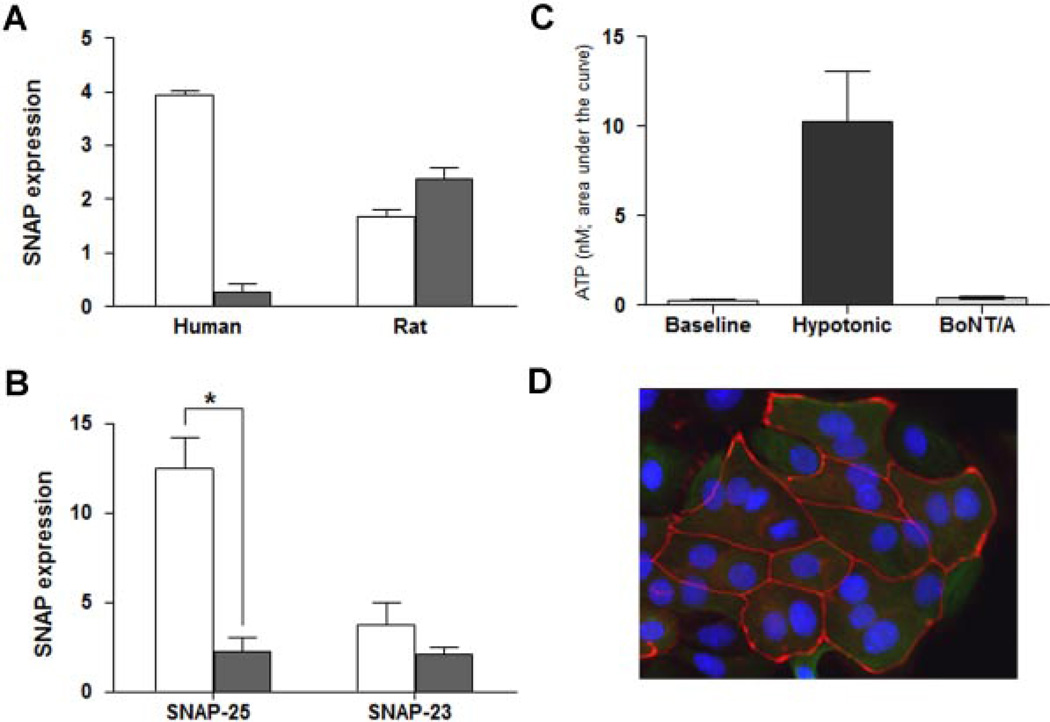
To explore the relative expression levels for both SNAP-23 and SNAP-25 in the mucosa (in human and rat), we stripped each blot and compared the relative expression patterns (normalized to β-actin; Fig. 2A). The experiments revealed a distinct difference in distribution in tissue from rat and human. SNAP-25 was by far the predominant protein in human tissue (93% of total) but the minor component (40%) in rat tissue. SNAP-23 was approximately 10-fold higher in rat versus human tissue and SNAP-25 was approximately 2.5-fold higher in human tissue.
Fig. 2.
The effect of BoNT/A on SNAP-25, SNAP-23 expression and ATP release. A: Summary of Western blot analysis showing protein levels of SNAP 25 (open bars) versus SNAP-23 (solid bars) in both human and rat mucosa. SNAP-25 levels in rat mucosa are less than half of the levels in human mucosa; while SNAP-23 levels in rat mucosa are 10-fold higher as compared to human. Three independent experiments were analyzed and are normalized to beta-actin. B: Levels of SNAP-25 and SNAP-23 protein in control rat urothelial cells (open bars) and urothelial cells treated with BoNT/A (1.5U, for 1–3 hr, solid bars). BoNT/A significantly decreased the expression of SNAP-25 (P<0.05 as compared by unpaired Student’s t-test). C: BoNT/A inhibits hypotonic-evoked release of ATP. Summary graph showing baseline release of ATP, the effect of hypotonic stimuli alone, and hypotonic-evoked release following treatment of cells with BoNT/ A (1.5 U; 1–3 hr). Data are expressed as nM of ATP and were obtained from cultures prepared from at least 6 rats. Experiments were performed on at least n = 6 independent cultures from each animal. P<0.05 as compared by unpaired Student’s t-test. D: Prolonged exposure to BoNT/A does not induce deleterious changes in urothelial cell morphology. Shown is a representative fluorescent image of cultured rat urothelial cells 24 hr following incubation with BoNT/A. The cells were fixed (4% paraformaldehyde) and stained with the junctional marker ZO-1 (shown as red; primary rabbit and secondary antibody Cy3 goat-antirabbit IgG) and counter-stained with the nuclear marker, DAPI (shown as blue).
We next used cultured urothelial cells from rat urinary bladder to examine the effect of botulinum toxin A (BoNT/A) on expression levels of SNAP-23 and SNAP-25 proteins as well as on release of ATP evoked by exposure to hypotonic solution. Incubation with BoNT/A (1.5 U/ml; for 1–3 hr) decreased the expression of both SNAP-23 (44% decrease as compared with control) and SNAP-25 (80% decrease as compared with control) (Fig. 2B).
Cultured urothelial cells released a low level of ATP in the absence of stimulation and exposure to hypotonic solution elicited a large increase in ATP (10.4±2.6 nM; Fig. 2C). Incubation with BoNT/A (1.5 U; for 1–3 hr) completely blocked the hypotonic-evoked ATP release (Fig. 2C). Depletion of ATP stores by BoNT/A is unlikely to be the cause of the suppression of ATP release because mechanical disruption (lysis) of the cells at the end of the experiment resulted in ATP release which was not significantly different in treated and untreated cells (data not shown). Incubation of urothelial cells with the toxin for 24 hr did not result in any visible changes in cell morphology such as cell blebbing or loss of tight junction proteins (Fig. 2D).
DISCUSSION
By blocking release of multiple transmitters and/or reducing receptor expression (i.e., muscarinic, P2×3, TRPV1, EP4),11–12 botulinum toxin A (BoNT/A) has the potential for relieving symptoms of overactive bladder as well as the hyperalgesia associated with other lower urinary tract disorders.12–15 Though bladder afferent and efferent nerves have been proposed4,8,16 as sites of action for BoNT/A and SV2 and SNAP-25 are expressed in these nerves, the mechanism of toxin action is likely to be more complex involving other cells within the bladder wall. Our findings reveal that urinary bladder urothelium expresses the synaptosomal proteins SNAP-25 and SNAP-23. The former is involved in release of transmitters from nerve cells while the latter is thought to be involved in non-neuronal secretory processes and is expressed in tissues rich in epithelial cells. The protein receptor for BoNT/A has been shown to be SV2 (in this study expressed in mucosa and rat UT cells) and some studies have indicated that specific isoforms (such as SV2C in phrenic nerves17) may be involved in mediating toxin uptake. Though all three SV2 isoforms are expressed in mucosa (with SV2-C at the highest levels), it is not yet known which SV2 isoforms are involved in the uptake of BoNT/A within urothelium. Though a previous study has reported the expression of SNAP-23 within the apical urothelial layer of rat urinary bladder,18 to the best of our knowledge our results are the first to also show evidence of both SNAP-25 and SNAP-23 in both rat and human bladder urothelium as well as a relative difference in expression levels. Though the observed band size for SNAP 25 (75 kDa) in our preparations differs from the predicted value, one explanation for this difference includes the formation of SNAP25 containing protein complexes. Studies have shown the presence of multiple SNAP-protein complexes (identified from 40 to 120 kDa) that can form teterodimers and heterotrimers in various conditions in a number of (tissue and cellular) preparations including hippocampal slices.19,20 Further, these types of complexes are likely to migrate at a different position than the predicted molecular weight of SNAP-25 (25 kDa) would suggest, and this could also reflect a number of protein changes (i.e., glycosylation or palmitolyation). Our findings were confirmed using urothelial cells as well as mucosa from rat and human biopsies.
SV2 as well as SNAP-25 is expressed in parasympathetic and sympathetic (VAChT and TH positive) efferent as well as afferent (CGRP positive) bladder nerves.8 By inhibiting SNARE-dependent exocytotic processes, BoNT/A can prevent the release of transmitters (i.e., acetylcholine, norepinephrine, ATP) from both types of efferent nerves or release of neuropeptides (i.e., substance P, CGRP) from afferent nerves as well as suppress the translocation of various receptors and channels (TRPV1, P2X3) to the plasma membrane.1,3,18,21,22 In addition, recent studies have shown that BoNT/A injected in the bladder wall of the mouse urinary bladder can suppress afferent activity evoked by mechanical or chemical stimulation.4 There is also evidence that BoNT/A treatment (in vitro) can normalize neuronal SNAP-25 expression that is altered in different pathologies.23 There is ample precedent for expression levels of a large number of proteins (including SNAP) rapidly changing in a number of cell types. For example, it has been shown in motoneurons24 that SNAP-25 levels decrease 1 hr after exposure to BoNT/A, which correlates and supports our findings that both SNAP-23 and SNAP-25 expression levels are significantly decreased in urothelial cells hours after exposure to the toxin. In parallel experiments, we also demonstrate that BoNT/A inhibits the release of ATP from cultured urothelial cells; a finding previously reported using intact urinary bladder.7 Urothelial-release of ATP can stimulate purinergic receptors on nearby bladder afferents that can convey information about bladder filling or irritation to central nervous system.25 The sensitivity of trafficking processes involving translocation of receptors,26 membrane,27,28 and other putative urothelial transmitters29 during bladder filling remains to be elucidated.
The mechanism of action of BoNT/A is thought to involve binding to the membrane followed by internalization into the cytosol and finally inhibition of cellular processes involving exocytosis. In neurons, the toxin is thought to bind with vesicular proteins such as SV2 when vesicular membrane is exposed to the extracellular compartment during exocytosis. 1,30 In the urothelium a similar mechanism may mediate endocytosis of BoNT/A. We have evidence as also reported by others31,32 that this type of binding does not elicit any adverse effects on cellular structure or function. However while the internalized toxin may remain in the terminals of neurons (though retrograde axonal transport to the CNS may also occur),33,34 in polarized epithelial cells internalized toxin may distribute throughout the entire cell and be present within vesicles that mediate transcellular transport. In the epithelium of the small intestine the neurotoxin does not passively diffuse but can undergo transcytosis across cells to eventually reach the circulation and elicit systemic effects such as block of transmission at the neuromuscular junction.35 In the urinary bladder, this type of transport may provide a way for the toxin to “escape” the lumen to reach cells underlying the urothelium thereby broadening its effects within the bladder wall and extending its duration of action. In this regard, the duration, formulation as well as the concentration of BoNT/A instillation may lead to changes in penetration (or trafficking) that could influence other cell types in the bladder wall.
Although previous studies have failed to detect the expression of SNAP-25 within the bladder mucosa,8 the present experiments revealed the expression of SNAP-25 and SNAP-23 in rat and human urothelium as well as SV2 in rat and human mucosa. A difference in the immunoblot intensity between mucosal samples and cultured urothelial cells was observed, though the reasoning for these differences are not known. Though mucosal samples could contain other sources of SV2 and SNAP-25 protein (i.e., afferent and efferent nerve terminals), these sources should not exhibit positive signals using RTPCR. While SNAP-23 is expressed in both neuronal and nonneuronal cells, it is possible that the difficulty in detection of non-neuronal SNAP-25 may be due to low expression levels, as reported for pancreatic endocrine cells.36 While we did not examine SNAP-25 cleavage products in the present study, a number of variables may influence cleaved SNAP-25 content. Studies have shown that in some cases limited amount of SNAP-25 may actually be cleaved (in nerves innervating the detrusor) and identification of cleavage product may also be precluded by sensitivity of the detection.37 Further, the use of SNAP-25 (for detection of neurotoxin activity) has been based on the endopeptidase activity of the L-chain of BoNT/A that enables the toxin to degrade the SNAP-25 protein in nerves. There is no information as to the activities of the toxin on exocytotic processes (including transmitter release) from urothelial cells. Consistent with previous studies17 we also found that SNAP-23 is expressed mainly within the superficial or apical urothelium that may in part explain the higher expression of SNAP-23 in rat mucosa compared to isolated urothelial cells (containing mainly basal cells in the preparation). Though in some cell types the roles for specific fusion events have been linked to specific components of the SNARE machinery, it is unclear why urothelial cells express both SNAP- 25 and SNAP-23 (which is also sensitive to BoNT/A)38 and also how these SNAREs regulate urothelial fusion events. While SNAP-25 and SNAP-23 may be functionally equivalent, there is evidence that these two SNARE proteins may play a role in exocytotic processes having different calcium thresholds.39 Thus it is possible that SNAP-25 may be linked with a higher rate of fusion events. In contrast, SNAP-23 may be involved in a constitutive release pathway in epithelial cells, involving docking and fusion of vesicles that occur at low or resting calcium concentrations.
CONCLUSIONS
Taken together, our findings suggest that the mechanism of action of BoNT/A in the urinary bladder may be more complex and likely to involve urothelial sites of action. Support for this also comes from a Phase I/II study in which clinical improvement was seen following intravesical instillation of BoNT/A with DMSO40 and a promising effect on irritant-induced bladder hyperactivity noted in animal models using intravesical instillation of BoNT/A encapsulated in liposomes.41 It is likely that BoNT/A inhibition of transmitter release and other exocytotic processes in urothelial cells may be complex. This may involve additional mechanisms such as inactivation of proteins (i.e., synaptotagmins)42 that regulate exocytotic events.
ACKNOWLEDGEMENTS
This work was supported in part by NIH grants R37 DK54824 and R01 DK57284 (L.A.B.), K01 DK080184 (A.H.M.) and R01 DK083323 (M.B.C.).
Contract grant sponsor: NIH; Grant numbers: R37 DK54824; R01 DK57284; K01 DK080184; R01 DK083323
References
- 1.Chancellor MB, Fowler CJ, Apostolidis A, et al. Drug insight: Biological effects of botulinum toxin A in the lower urinary tract. Nat Clin Pract Urol. 2008;5:319–328. doi: 10.1038/ncpuro1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dowson C, Khan MS, Dasgupta P, et al. Repeat botulinum toxin-A injections for treatment of adult detrusor overactivity. Nat Rev Urol. 2010;7:661–667. doi: 10.1038/nrurol.2010.187. [DOI] [PubMed] [Google Scholar]
- 3.Apostolidis A, Popat R, Yiangou Y. Decreased sensory receptors P2 × 3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005;174:977–982. doi: 10.1097/01.ju.0000169481.42259.54. [DOI] [PubMed] [Google Scholar]
- 4.Ikeda Y, Zabbarova IV, Birder LA, et al. Botulinum neurotoxin serotype A suppresses neurotransmitter release from afferent as well as efferent nerves in the urinary bladder. Eur Urol. 2012;62:1157–1164. doi: 10.1016/j.eururo.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birder LA, de Groat WC. Mechanisms of disease: Involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol. 2007;4:46–54. doi: 10.1038/ncpuro0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith CP, Chancellor MB. Emerging role of botulinum toxin in the management of voiding dysfunction. J Urol. 2004;171:2128–2137. doi: 10.1097/01.ju.0000127725.48479.89. [DOI] [PubMed] [Google Scholar]
- 7.Smith CP, Gangitano DA, Munoz A, et al. Botulinum toxin type A normalized alterations in urothelial ATP and NO release induced by chronic spinal cord injury. Neurochem Int. 2008;52:1068–1075. doi: 10.1016/j.neuint.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coelho A, Dinis P, Pinto R, et al. Distribution of the high-affinity binding site and intracellular target of botulinum toxin type A in the human bladder. Eur Urol. 2010;57:884–890. doi: 10.1016/j.eururo.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Chopra B, Barrick SR, Meyers S, et al. Expression and function of bradykinin B1 and B2 receptors in normal and inflamed rat urinary bladder urothelium. J Physiol. 2005;562:859–871. doi: 10.1113/jphysiol.2004.071159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birder LA, Wolf-Johnston AS, Sun Y, et al. Alteration in TRPV1 and muscarinic (M3) receptor expression and function in idiopathic overactive bladder urothelial cells. Acta Physiol. 2013;207:123–129. doi: 10.1111/j.1748-1716.2012.02462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuang YC, Yoshimura N, Huang CC, et al. Intravesical botulinum toxin A administration inhibits COX-2 and EP4 expression and suppresses bladder hyperactivity in cyclophosphamide-induced cystitis in rats. Eur Urol. 2009;56:159–166. doi: 10.1016/j.eururo.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Datta SN, Roosen A, Pullen A, et al. Immunohistochemical expression of muscarinic receptors in the urothelium and suburothelium of neurogenic and idiopathic overactive bladders, and changes with botulinum neurotoxin administration. J Urol. 2010;184:2578–2585. doi: 10.1016/j.juro.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 13.Yokoyama T, Chancellor MB, Ogama K, et al. Botulinum toxin type A for the treatment of lower urinary tract disorders. Int J Urol. 2012;19:202–215. doi: 10.1111/j.1442-2042.2011.02946.x. [DOI] [PubMed] [Google Scholar]
- 14.Nitti VW, Dmochowski R, Herschorn S, et al. Onabotulinum toxin A for the treatment of patients with overactive bladder and urinary incontinence: Results of a phase 3 randomized placebo-controlled trial. J Urol. 2013;189:2186–2193. doi: 10.1016/j.juro.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Pinto R, Lopes T, Frias B, et al. Trigonal injection of botulinum toxin A in patients with refractory bladder pain syndrome/interstitial cystitis. Eur Urol. 2012;58:360–365. doi: 10.1016/j.eururo.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi R, Yunoki T, Naoto S, et al. Differential effects of botulinum neurotoxin A on bladder contractile responses to activation of efferent nerves, smooth muscles and afferent nerves in rats. J Urol. 2012;188:1993–1999. doi: 10.1016/j.juro.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Mahrhold S, Rummel A, Bigalke H, et al. The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Lett. 2006;580:2011–2014. doi: 10.1016/j.febslet.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 18.Born M, Pahner I, Hilger-Ahnert G, et al. The maintenance of the permeability barrier of bladder facet cells requires a continuous fusion of discoid vesicles with the apical plasma membrane. Eur J Cell Biol. 2003;82:343–350. doi: 10.1078/0171-9335-00326. [DOI] [PubMed] [Google Scholar]
- 19.Braun J, Madison DV. A novel SNAP-25-caveolin complex correlates with the onset of persistent synaptic potentiation. J Neurosci. 2000;20:5997–6006. doi: 10.1523/JNEUROSCI.20-16-05997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honer WG, Falkai P, Bayer TA, et al. Abnormalities of SNARE mechanism proteins in anterior frontal cortex in severe mental illness. Cerebral Cortex. 2002;12:549–556. doi: 10.1093/cercor/12.4.349. [DOI] [PubMed] [Google Scholar]
- 21.Lucioni A, Bales GT, Lotan TL, et al. Botulinum toxin type A inhibits sensory neuropeptide release in rat bladder models of acute injury and chronic inflammation. BJU Int. 2008;101:366–370. doi: 10.1111/j.1464-410X.2007.07312.x. [DOI] [PubMed] [Google Scholar]
- 22.Smith CP, Franks ME, McNeil BK, et al. Effect of botulinum toxin A on the autonomic nervous system of the rat lower urinary tract. J Urol. 2003;169:1896–1900. doi: 10.1097/01.ju.0000049202.56189.54. [DOI] [PubMed] [Google Scholar]
- 23.Mika J, Rojewska E, Makuch W, et al. The effect of botulinum neurotoxin A on sciatic nerve injury-induced neuroimmunological changes in rat dorsal root ganglia and spinal cord. Neuroscience. 2011;175:358–366. doi: 10.1016/j.neuroscience.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 24.Kiris E, Nuss JE, Burnett JC, et al. Embryonic stem cell-derived motoneurons provide a highly sensitive cell culture model for botulinum neurotoxin studies, with implications for high-throughput drug discovery. Stem Cell Res. 2001;6:195–205. doi: 10.1016/j.scr.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ford AP. In pursuit of P2 × 3 antagonists: Novel therapeutics for chronic pain and afferent sensitization. Purinergic Signal. 2012;8:S3–S26. doi: 10.1007/s11302-011-9271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prakasam HS, Herrington H, Roppolo JR, et al. Modulation of bladder function by luminal adenosine turnover and A1 receptor activation. Am J Physiol. 2012;303:F279–F292. doi: 10.1152/ajprenal.00566.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu W, Khanderwal P, Apodaca G. Distinct apical and basolateral membrane requirements for stretch-induced membrane traffic at the apical surface of bladder umbrella cells. Mol Biol Cell. 2009;20:282–295. doi: 10.1091/mbc.E08-04-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khanderwal P, Ruiz WG, Apodaca G. Compensatory endocytosis in bladder umbrella cells occurs through an integrin-regulated and RhoA- and dynamin-dependent pathway. EMBO J. 2010;29:1961–1975. doi: 10.1038/emboj.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang EC, Lee JM, Ruiz WG, et al. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest. 2005;115:2412–2422. doi: 10.1172/JCI24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong M, Yeh F, Tepp WH, et al. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006;312:592–596. doi: 10.1126/science.1123654. [DOI] [PubMed] [Google Scholar]
- 31.Haferkamp A, Schurch B, Reitz A, et al. Lack of ultrastructural detrusor changes following endoscopic injection of botulinum toxin type A in overactive neurogenic bladder. Eur Urol. 2004;46:784–791. doi: 10.1016/j.eururo.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Kessler TM, Khan S, Panicker JN, et al. In the human urothelium and suburothelium, intradetrusor botulinum neurotoxin type A does not induce apoptosis: Preliminary results. Eur Urol. 2010;57:879–883. doi: 10.1016/j.eururo.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 33.Matak I, Riederer P, Lackovic Z. Botulinum toxin’s axonal transport from periphery to the spinal cord. Neurochem Int. 2012;61:236–239. doi: 10.1016/j.neuint.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Restani L, Novelli E, Bottari D, et al. Botulinum neurotoxin A impairs neurotransmission following retrograde transynaptic transport. Traffic. 2012;13:1083–1089. doi: 10.1111/j.1600-0854.2012.01369.x. [DOI] [PubMed] [Google Scholar]
- 35.Maksymowych AB, Simpson LL. Structural features of the botulinum neurotoxin molecule that govern binding and transcytosis across polarized human intestinal epithelial cells. J Pharm Exp Ther. 2004;310:633–641. doi: 10.1124/jpet.104.066845. [DOI] [PubMed] [Google Scholar]
- 36.Sadoul K, Lang J, Montecucco C, et al. SNAP-25 is expressed in islets of langerhans and is involved in insulin release. J Cell Biol. 1995;128:1019–1028. doi: 10.1083/jcb.128.6.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulte-Baukloh H, Zurawski TH, Knispel HH, et al. Persistence of the synaptosomal-associated protein-25 cleavage product after intradetrusor botulinum toxin A injections in patients with myelomeningocele showing an inadequate response to treatment. BJU Int. 2007;100:1075–1080. doi: 10.1111/j.1464-410X.2007.07137.x. [DOI] [PubMed] [Google Scholar]
- 38.Banerjee A, Li G, Alexander EA, et al. Role of SNAP-23 in trafficking of H+ - ATPase in cultured inner medullary collecting duct cells. Am J Physiol Cell Physiol. 2001;280:C775–C781. doi: 10.1152/ajpcell.2001.280.4.C775. [DOI] [PubMed] [Google Scholar]
- 39.Chieregatti E, Chicka MC, Chapman ER, et al. SNAP-23 functions in docking/ fusion of granules at low Ca2+ Mol Biol Cell. 2004;15:1928–1930. doi: 10.1091/mbc.E03-09-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petrou SP, Parker AS, Crook JE, et al. Botulinum A toxin/dimethyl sulfoxide bladder instillations for women with refractory idiopathic detrusor overactivity: A phase 1/2 study. Mayo Clin Proc. 2009;84:702–706. doi: 10.4065/84.8.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chuang Y-C, Tyagi P, Huang C-C. Urodynamic and immunohistochemical evaluation of intravesical botulinum toxin A delivery using liposomes. J Urol. 2009;182:786–792. doi: 10.1016/j.juro.2009.03.083. [DOI] [PubMed] [Google Scholar]
- 42.O’Connor V, Lee AG. Synaptic vesicle fusion and synaptotagmin: 2B or not 2B? Nat Neurosci. 2002;5:823–824. doi: 10.1038/nn0902-823. [DOI] [PubMed] [Google Scholar]