Abstract
Aim
Disseminated intravascular coagulation is an increasing concern for certain types of engineered nanomaterials. Recent studies have shed some light on the nanoparticle physicochemical properties contributing to this toxicity; however, the mechanisms are poorly understood. Leukocyte procoagulant activity (PCA) is a key factor contributing to the initiation of this toxicity. We have previously reported on the exaggeration of endotoxin-induced PCA by cationic dendrimers. Herein, we report an effort to discern the mechanism.
Materials & methods
Poly(amidoamine) dendrimers with various sizes and surface functionalities were studied in vitro by the recalcification test, flow cytometry and other relevant assays.
Results & conclusion
Cationic dendrimers exaggerated endotoxin-induced PCA, but their anionic or neutral counterparts did not; the cationic charge prompts this phenomenon, but different cationic surface chemistries do not influence it. Cationic dendrimers and endotoxin differentially affect the PCA complex. The inhibition of phosphoinositol 3 kinase by dendrimers contributes to the exaggeration of the endotoxin-induced PCA.
Keywords: coagulopathy, dendrimer, disseminated intravascular coagulation, leukocyte, nanoparticle, procoagulant activity, thrombosis
Disseminated intravascular coagulation (DIC), also known as consumptive coagulopathy, is a life-threatening condition characterized by massive blood clotting and abnormal bleeding (hemorrhage) [1]. DIC is a common complication in cancer and sepsis, and is also reported in patients with other pathophysiological conditions [2–5]. The initial stage of DIC is caused by simultaneous activation of the coagulation cascade and immune cells, which results in the formation of multiple thrombi depositing in tissues and causing congestion of capillaries. The massive consumption of coagulation factors occurring at this early stage alters hemostasis and is responsible for hemorrhage at a later stage [1,6]. The mechanism of DIC is complex, as it involves multiple players, including leukocytes, endothelial cells, platelets, some cancer cells and plasma coagulation factors. Various internal (e.g., inflammatory cytokines and tissue damage) and external (e.g., some cytotoxic oncology drugs and bacterial endotoxin) stimuli have been reported to be powerful factors in inducing DIC. The molecular mechanisms of DIC are not fully understood, but it is generally accepted that the presence of a phospholipid–protein complex composed of phosphatidyl serine (PS) and a tissue factor (TF; CD142), also known as procoagulant activity (PCA), on the surface of leukocytes, cancer and endothelial cells serves as a platform for the assembly of plasma coagulation factors and triggers activation of the extrinsic plasma coagulation cascade [7–10].
DIC is an increasing concern for certain types of engineered nanomaterials. For example, an intravenous bolus injection of generation (G)7 cationic poly(amidoamine) (PAMAM) dendrimers [11] and amorphous nanosilica [12] into mice triggered consumptive coagulopathy and was responsible for animal death 0.5 and 12 h after administration, respectively. Mechanistic studies conducted in our laboratory and by others revealed that cationic PAMAM dendrimers induce PCA in cancer cells in a manner similar to that described for the cytotoxic oncology drug doxorubicin, activate platelets through a mechanism involving membrane disintegration, and have a direct effect on fibrinogen [11,13–16]. Our previous studies have also found that cationic PAMAM dendrimers synergistically enhance endotoxin-induced PCA in normal human peripheral blood mononuclear cells (PBMCs) [13].
Endotoxin (a lipopolysaccharide; LPS) is a component of the outer membrane of Gramnegative bacterial cell walls. It is a very potent immunostimulant and is responsible for various pathophysiological reactions (e.g., fever, chills, hypotension and coagulopathy) observed with Gram-negative bacterial infections or following endotoxin injection into the bloodstream. Endotoxin saw early development as an anticancer therapy, but was discontinued because of overwhelming immunostimulation; this work later established its maximum tolerated dose of 50 EU/kg [17–19]. Based on these data, the US FDA and other regulatory agencies worldwide have set 5 EU/kg and 0.5 EU/ml as the limit of endotoxin allowed in drugs and medical devices, respectively [20]. Endotoxin contamination is very common in engineered nanomaterials, especially during early phases of development, because these materials are often synthesized using reagents and equipment not intended for medical use, and because nanoparticles can retain large amounts of endotoxin due to their large surface-to-volume ratio [13,21–23].
There is an increasing number of reports in literature regarding engineered nanomaterials exaggerating endotoxin-mediated inflammatory properties. For example, G6 cationic PAMAM dendrimers enhanced PCA [13], TiO2 nanobelts and carbon-based nanoparticles enhanced in vitro cytokine response and in vivo lung inflammation [24–26], and amorphous silica nanoparticles exaggerated oxidative-stress-mediated reactions [27]. Since engineered nanomaterials are becoming increasingly more prevalent in the development of drug-delivery platforms and since endotoxin contamination and its detection represent a challenge for these materials, it is imperative to understand which nanoparticle physicochemical properties are responsible for the exaggeration of endotoxin-mediated toxicities [22,23,28]. Nanoparticle charge, size and density of surface amines were found to be the key factors in PAMAM dendrimers’ induction of PCA in cancer cells, while aspect ratio was recognized to be key for TiO2 nanobelts’ exaggeration of endotoxin-mediated cytokine response [13,26].
The purpose of this study was to further understand the relationship between nanoparticle physicochemical properties and their ability to exaggerate endotoxin-mediated PCA in normal human leukocytes, as well as to gain insight into the molecular mechanisms underlying this enhancement. In continuation of our previous studies, we focused on PAMAM dendrimers. Herein, we report that the exaggeration of endotoxin-mediated PCA in normal human leukocytes is a consequence of treatment with cationic dendrimers, regardless of nanoparticle size and regardless of the source of the cationic charge (i.e., terminal group functionalization or surface chemistry); the anionic and neutral counterparts do not exhibit this effect. We further demonstrate that cationic dendrimers inhibit phosphoinositol 3 kinase (PI3K), and that this inhibition constitutes a factor contributing to the enhancement of endotoxin-mediated PCA.
Materials & methods
Reagents & cell line
Detailed list of reagents and their sources is provided in the Supplementary Materials; see online at www.futuremedicine.com/doi/suppl/10.2217/ NNM.13.137.
Isolation of PBMCs
Healthy volunteers’ blood was collected under The Frederick National Laboratory for Cancer Research Protocol OH99-C-N046. Blood was drawn into tubes containing lithium-heparin or sodium citrate. PBMCs were isolated from heparinized blood using Ficoll-Paque® PLUS (GE Healthcare, WI, USA) according to the manufacturer’s protocol. Details of platelet-free PBMC isolation can be found in the Supplementary Material.
Cell culture
PBMCs were incubated in Roswell Park Memorial Institute 1640 medium containing 10% fetal calf serum, 2 mM glutamine, 100 U/ml penicillin/streptomycin at 37°C and 5% CO2.
Dendrimers
Ethylenediamine core G3, G4, G5 and G6 PAMAM dendrimers with amine, succinamic acid and monoethanolamine terminal functionalities were purchased from Dendritech Inc. (MI, USA). Guanidinylation of PAMAM dendrimers was achieved using slight modification of previously published procedures [29,30]. Other details of dendrimer synthesis, characterization and endotoxin detection can be found in the Supplementary Material.
Leukocyte PCA
PCA analysis has been described elsewhere [13]. Brief ly, PBMCs (10.8 × 106 cells per sample) were incubated with reagents (LPS, dendrimers or medium for negative control) for 24 h. In pretreatment experiments, PBMCs were incubated with LPS or dendrimers for 6 h, following which cells were washed with phosphate-buffered saline (PBS) and reconstituted in fresh media containing cationic dendrimers or LPS for an additional 18 h. At the end of the incubation period, cells were collected, washed twice with PBS and reconstituted with prewarmed (37°C) buffer A (20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid; pH 7.4; 0.15 M NaCl; 6.6 mM CaCl2). The 0.1 ml of cells was mixed with 0.1 ml of autologous plasma, prepared by centrifugation of freshly drawn blood anticoagulated with sodium citrate at 2500 × g for 10 min. The coagulation time was measured using a StarT4 (Diagnostica Stago, Gennevilliers, France) coagulometer. Each sample was analyzed in duplicate. Neoplastine®, normal and abnormal plasma standards, from Diagnostica Stago were used for instrument validation.
Flow cytometry analysis
PBMCs in PBS containing 1% bovine serum albumin were incubated with an antibody for 30 min on ice. Following the incubation period, cells were washed twice with ice-cold PBS and samples were analyzed on a FACS Calibur (BD Biosciences, CA, USA) using CellQuest® (BD Biosciences) software. The monocyte gate was set based on CD14+ cells.
PI3K assay
Immunoprecipitation of recombinant PI3K (rPI3K) was performed according to the protocol provided by Echelon Biosciences Inc. (UT, USA), please refer to the Supplementary Material for full details. In experiments with PBMCs, cells (20 × 106 cells per sample) were treated with LPS (1 ng/ml) or cationic G6 PAMAM dendrimers (4 µg/ml) for 1 h. Wortmannin (100 nM) was used as a control for inhibition of PI3K activity [31]. Cells were lysed with buffer A containing 1% Nonidet™ P-40 (Pierce Chemical Co., IL, USA) and 1 mM phenylmethylsulfonyl fluoride on ice for 20 min. An antibody against p85 was added to the cell lysate. The next steps were similar to those described before for rPI3K.
Statistical analysis
Data were analyzed by Student’s t-test using two tails distribution and paired variance.
Results
Dendrimer characterization
Characterization of commercial dendrimers used in the present study included dynamic light scattering, zeta-potential and analysis of endotoxin levels. Amine-terminated G3, G4, G5 and G6 PAMAM dendrimers had hydrodynamic diameters of 4.1, 5.1, 6.5 and 8.3 nm, respectively; their zeta-potentials were 55.7, 61.9, 56.5 and 67.1 mV, respectively (Table 1). The hydrodynamic diameters of G5 carboxy-, hydroxy- and guanidine-terminated dendrimers were 7.7, 6.6 and 8.1 nm, respectively; their zeta-potential values were −13.3, 17.9 and 45.0 mV, respectively. Although the hydroxy-terminated dendrimers are slightly cationic, there was no biological reactivity of the hydroxy-terminated dendrimers in leukocyte PCA, and thus appeared to have no influence on the in vitro leukocyte PCA under the experimental conditions used here.
Table 1.
Summary of dendrimer characterization.
| Surface | Generation | Hydrodynamic diameter (nm) |
Zeta- potential (mV) |
Endotoxin (EU/ml/100 µg) |
|---|---|---|---|---|
| Amine (−NH2) | 3 | 4.1 ± 0.1 | 55.7 ± 0.7 | <0.05 |
| 4 | 5.1 ± 0.1 | 61.9 ± 1.5 | <0.05 | |
| 5 | 6.5 ± 0.2 | 56.5 ± 0.7 | <0.05 | |
| 6 | 8.3 ± 0.1 | 67.1 ± 2.4 | <0.05 | |
| Carboxy (−COOH) | 5 | 7.7 ± 0.3 | −13.3 ± 1.9 | 7.20 |
| Hydroxy (−OH) | 5 | 6.6 ± 0.1 | 17.9 ± 0.5 | <0.05 |
| Guanidine | 5 | 8.1 ± 2.5 | 45.0 ± 3.2 | <0.50 |
Dendrimer hydrodynamic diameter (dynamic light scattering; intensity peak), zeta-potential and endotoxin contamination were analyzed as described in the ‘Materials & methods’ section. The hydrodynamic diameter and zeta-potential data are ± standard deviation. In the limulus amebocyte lysate assay, dendrimers were tested at 1:5, 1:50 and 1:500 dilutions; shown are the data adjusted for the dilution factor.
The guanidine-terminated G5 PAMAM dendrimer was further characterized by nuclear magnetic resonance and MALDI-TOF. The 1H nuclear magnetic resonance of the guanidine product showed characteristic dendrimer proton signals with the downfield shift of the methylene group adjacent to the terminal primary amine in the starting material from δ 2.7 to δ 3.2 ppm, with appropriate integration confirming the guanidinilation (data not shown). The MALDIT-OF mass of the starting material and the product showed m/z of 26,551 and 31,480, respectively, confirming the surface modification (data not shown). This increase in mass accounts for approximately 117 average guanidine groups per dendrimer.
The level of endotoxin in all but one dendrimer sample was below the limit of detection for the assay, 0.01 EU/ml. Unlike the other samples, 7.2 EU per 100 µg of dendrimer was detected in the carboxy-terminated G5 dendrimers. Since our in vitro experiments did not involve dendrimer concentrations greater than 25 µg/ml (i.e., 1.8 EU/ml or ~0.18 ng/ml endotoxin), and since no PCA was observed by carboxy-terminated dendrimers alone or spiked with 1 ng/ml of endotoxin, these levels of contaminating endotoxin were determined to be inconsequential for these studies.
Cationic dendrimer & endotoxin have different impacts on PCA
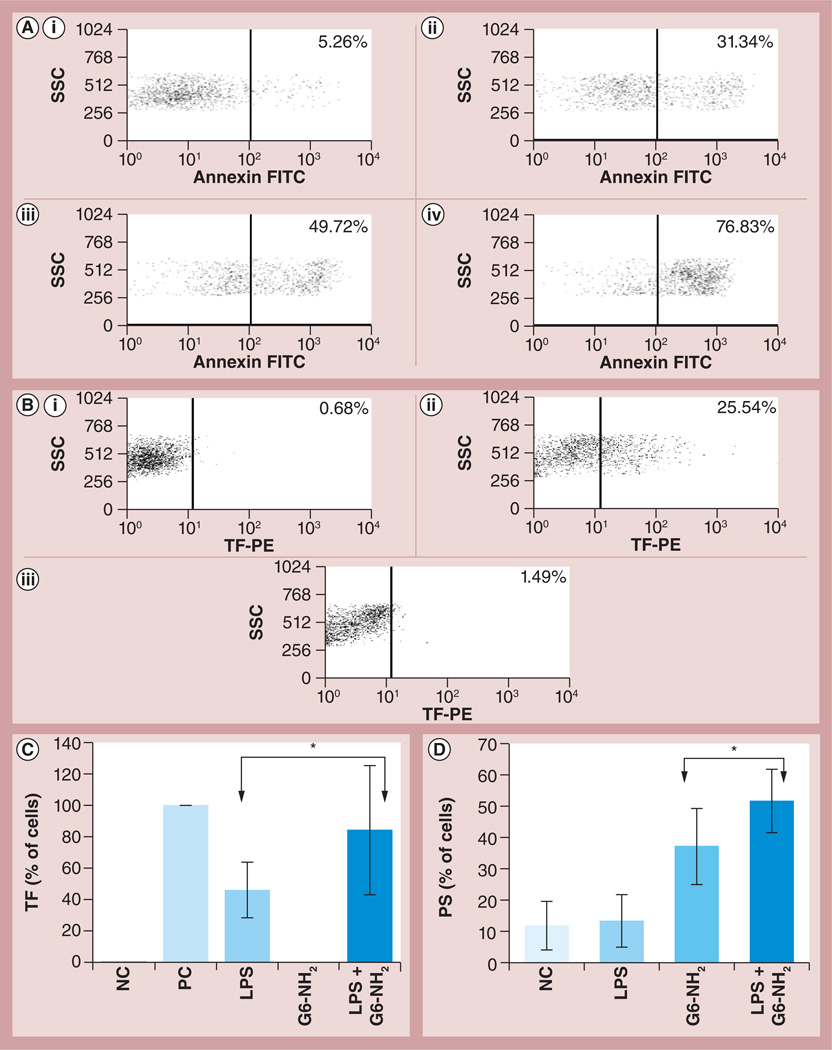
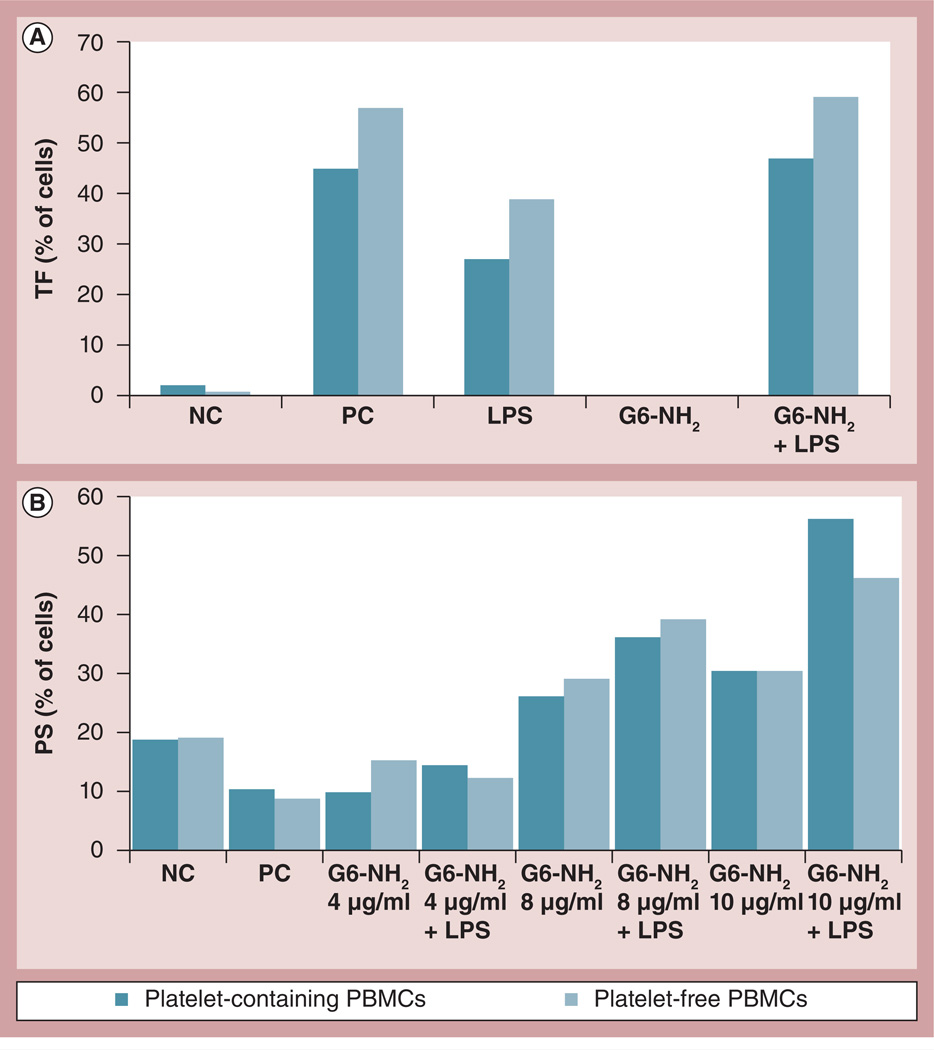
TF (CD142) and PS are generally recognized as the main components of the PCA complex [7–10]. PS is normally present in the inner layer of the cell membrane and transitions to the outer layer in response to certain stimuli. For the purpose of this article we will refer to this step of PCA formation as PS presentation. We studied TF expression and PS presentation on leukocyte surfaces in response to dendrimers, endotoxin and a combination thereof. G6 amine-terminated dendrimers (hereafter referred to as G6-NH2), were used because exaggeration of endotoxin-induced PCA in leukocytes was initially described for this nanoparticle [13]. Treatment of leukocytes with various concentrations of G6-NH2 dendrimers resulted in PS presentation on the cell surface in a dose-dependent manner; it gradually increased from 5.3% to 31.3, 49.7 and 76.8% when G6-NH2 concentrations were raised from 4.0 to 8.0, 10.0 and 25.0 µg/ml, respectively (Figure 1A). However, these particles failed to induce TF expression at a concentration of 4.0 µg/ml (Figure 1B). Evaluation of TF expression in response to higher concentrations of dendrimer was not possible due to the increased nonspecific binding of fluorescently labeled antibodies to the cell surface, as was evident by the dramatic increase in isotype control antibody staining (data not shown). LPS, on the other hand, induced TF expression but failed to significantly upregulate PS presentation. The positive response in the PS assay was validated by using a 30-min formalin treatment according to annexin V manufacturer’s instructions (data not shown). A combination of both LPS and G6-NH2 dendrimer treatments resulted in a significant increase (p < 0.05) in both TF expression and PS presentation on the cell surface (Figures 1C & 1D, respectively).
Figure 1. Flow cytometry analysis of tissue factor expression and phosphatidyl serine presentation on the leukocyte surface in response to endotoxin, dendrimers and the combination thereof.
Peripheral blood mononuclear cells from healthy donor volunteers were treated with LPS and G6-NH2 alone or in combination for 24 h. (A) PS presentation on the surface of cells incubated with increasing concentrations of G6-NH2 dendrimers; (i) 4, (ii) 8, (iii) 10 and (iv) 25 µg/ml. (B) TF expression on the surface of cells (i) untreated or incubated with (ii) 1 ng/ml LPS or (iii) 4 µg/ml of G6-NH2 dendrimers. (C) TF expression on the surface of cells treated with 1 ng/ml LPS or 4 µg/ml G6-NH2 dendrimers alone or in combination (LPS + G6-NH2). Shown is the mean value ± standard deviation of percentage-positive cells derived from analysis of ten donor specimens. PC was assigned a 100% value. (D) PS presentation on the surface of cells treated with 1 ng/ml LPS or 10 µg/ml G6-NH2 dendrimers alone or in combination (LPS + G6-NH2). Shown is the mean value ± standard deviation of percentage-positive cells derived from analysis of six donor specimens. *p < 0.05.
FITC: Fluorescein isothiocyanate; G6-NH2: Generation 6 amine-terminated polyamidoamine dendrimer; LPS: Lipopolysaccharide; NC: Negative control (cell culture media); PC: Positive control (10 µg/ml LPS); PE: Phycoerythrin; PS: Phosphatidyl serine; SSC: Side scatter; TF: Tissue factor.
Cationic dendrimers exaggerate endotoxin-induced PCA
Next, the role of nanoparticle size, charge and surface chemistry in the PCA enhancement phenomenon was assessed. Although G3, G4, G5 and G6 amine-terminated dendrimers all significantly enhanced LPS-induced PCA, there was no clear size-dependent difference in either TF expression or PS presentation on the cell surface (Supplementary Figure S1).
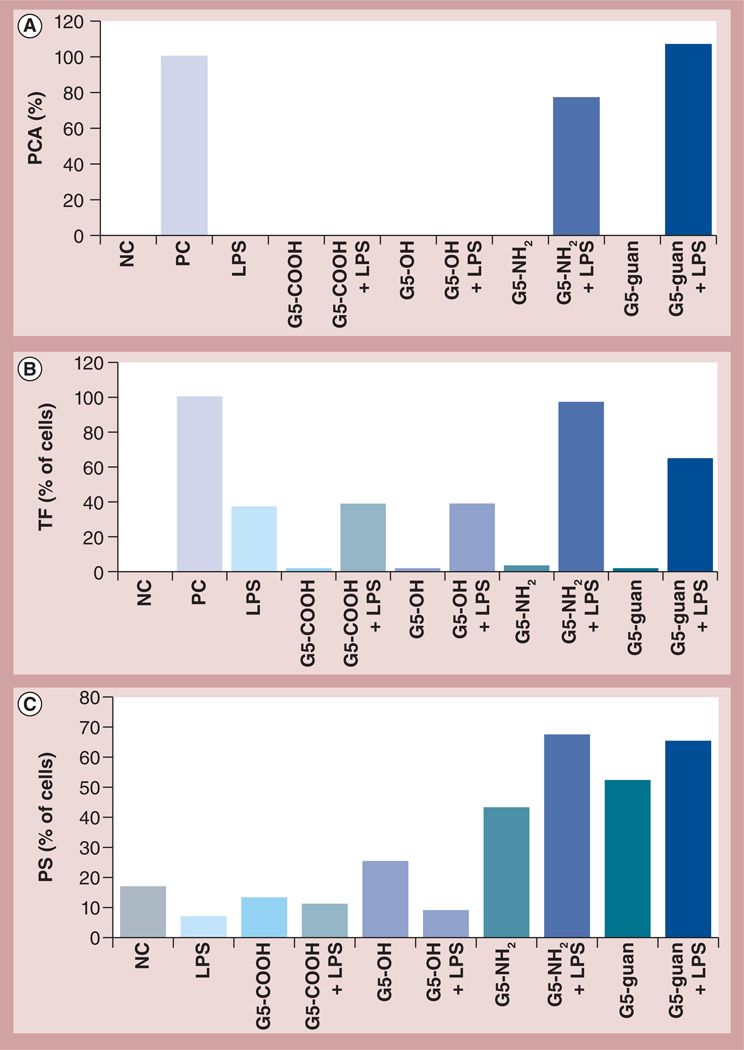
To understand the role of nanoparticle charge and surface chemistry, G5 PAMAM dendrimers with four different terminal functional groups were analyzed: succinamic acid (anionic), amidoethanol (neutral), amine (cationic) and guanidine (cationic). If nanoparticle charge played a role, a difference should be observed between dendrimers with different charges, and if the surface chemistry played a role, a difference should be observed between amineand guanidine-terminated dendrimers. Carboxyand hydroxy-terminated dendrimers did not induce PCA when used as a single treatment, nor did they enhance endotoxin-induced PCA when combined with LPS. By contrast, cationic dendrimers enhanced LPS-induced PCA, but there was no remarkable difference between nanoparticles with amine or guanidine surfaces (Figure 2A). These data correlate well with TF expression and PS presentation on the cell surface; neither charged nor neutral dendrimers induced TF on the cell surface, anionic and neutral dendrimers did not induce PS, and only cationic particles induced PS and enhanced LPS-induced PCA (Figures 2B & 2C). Since LPS is not a potent inducer of PS exposure, formalin was used as a positive control to monitor assay performance. TF expression and PS presentation were consistent between all tested donors (Supplementary Figures S2B & S2C). PCA was more variable between individual donors (Supplementary Figure S2A). Previous studies did not demonstrate PCA induction by amine-terminated dendrimers in normal human PBMCs [13]; however, the present study revealed inconsistent, donor-dependent, PCA induction by amine-terminated dendrimers (data not shown). Slight differences in donor response and nanoparticle batches are not unexpected and do not appear to influence the central biological effect in the current study (i.e., exaggeration of endotoxin-induced PCA by cationinc PAMAM dendrimers).
Figure 2. Effects of nanoparticle charge and surface chemistry on procoagulant activity.
Peripheral blood mononuclear cells from healthy donor volunteers were treated with LPS and G5 dendrimers with different surface groups alone or in combination. (A) Induction of PCA by dendrimers at 25 µg/ml and LPS at 1 ng/ml. PCA was calculated as the percentage of coagulation induced by the PC, LPS at 10 µg/ml. Shown is the mean response (n = 4) for one donor. Similar data were obtained with three additional donors (Supplementary Figure S2). (B) Flow cytometry analysis of TF expression on the surface of monocytes after treatment with various dendrimers at 4 µg/ml (G5-COOH; G5-OH; G5-NH2 and G5-guan), LPS at 1 ng/ml or a combination (G5-COOH + LPS; G5-OH + LPS; G5-NH2 + LPS and G5-guan + LPS). Shown is the percentage of positive cells from one donor relative to the PC. Similar data were obtained with two additional donors (Supplementary Figure S2). PC for this test was LPS (10 µg/ml). (C) Flow cytometry analysis of PS presentation on the surface of monocytes after treatment with various dendrimers at 12 µg/ml (G5-COOH; G5-OH; G5-NH2 and G5-guan), LPS at 1 ng/ml or a combination (G5-COOH + LPS; G5-OH + LPS; G5-NH2 + LPS and G5-guan + LPS). Shown is the percentage of positive (i.e., PS presenting) cells. Similar data were obtained with two additional donors (Supplementary Figure S2). PC for this test was 30 min treatment with formalin (not shown).
G5: Generation 5; guan: Guanidine; LPS: Lipopolysaccharide; NC: Negative control; PC: Positive control; PCA: Procoagulant activity; PS: Phosphatidyl serine; TF: Tissue factor.
Exaggeration of LPS-induced PCA is not a result of interaction between LPS & dendrimers
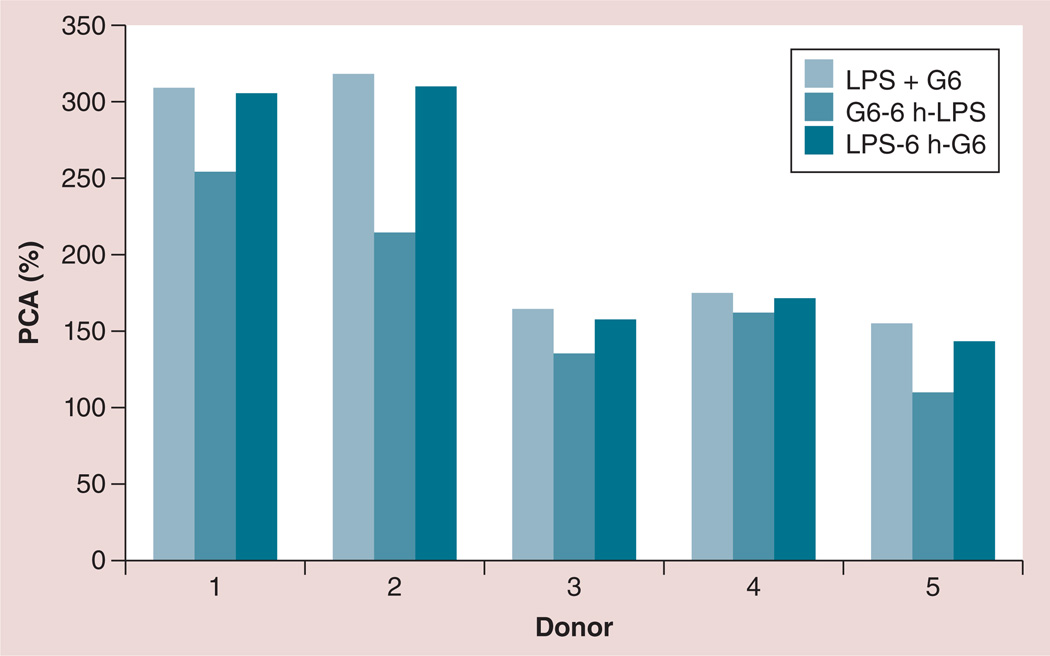
Due to their cationic nature, amine-terminated dendrimers are likely to bind to negatively charged LPS. This interaction could facilitate LPS presentation to, and interaction with, the Toll-like receptor 4 receptor complex on the cell surface. To verify whether electrostatic interactions between the dendrimers and LPS were responsible for the exaggeration of LPS-induced PCA, cells were treated with LPS alone for 6 h, excess LPS was removed and cells were treated with dendrimers for another 12–18 h. Similarly, cells were treated with dendrimers alone for 6 h, then washed and challenged with LPS for an additional 12–18 h. Cells challenged with LPS and dendrimer concurrently were also included in the same experiment for comparison. There was no significant difference in the induction of PCA between cells treated concurrently with the LPS and dendrimer and cells sequentially treated with LPS before dendrimer addition. In both cases, the induction of PCA was stronger than that achieved by LPS treatment alone (Figure 3). By contrast, PCA in cells sequentially treated with dendrimers before being challenged with LPS was lower than that observed in the LPS–dendrimer combination treatment (Figure 3).
Figure 3. Interaction between lipopolysaccharide and dendrimers are not responsible for the enhancement of lipopolysaccharide-induced procoagulant activity by the dendrimers.
Peripheral blood mononuclear cells from healthy donor volunteers were treated with 1 ng/ml of LPS and 25 µg/ml G6-NH2 poly(amidoamine) dendrimers under the following conditions: LPS and G6-NH2 were added to cells concurrently (LPS + G6); cells were treated with G6-NH2 for 6 h, then washed with phosphate-buffered saline and treated with LPS for an additional 12–18 h (G6-6 h-LPS); or cells were treated with LPS for 6 h, then washed with phosphate-buffered saline and treated with G6-NH2 for an additional 12–18 h (LPS-6 h-G6). PCA was assessed 24 h after addition of the first treatment. PCA was calculated as a percentage relative to that induced by the positive control LPS at 10 µg/ml.
G6: Generation 6; LPS: Lipopolysaccharide; PCA: Procoagulant activity.
Cationic charge per se & Ca2+ influx are not responsible for the enhancement of LPS-induced PCA by cationic dendrimers
To probe whether positive charge per se was responsible for the enhancement of LPS-induced PCA, different cationic molecules were also included in the PCA assay. Ca2+ ionophore (CI), the cationic peptide polymyxin B (PMB), and linear polymer poly-l-lysine (PLL) added to the cells alone or in combination with LPS and PCA were assessed. Concentrations of CI, PLL and PMB were selected based on their most common use in experimental immunology [32–34]. CI and PLL were used at equimolar concentrations of approximately 1 µM, while PMB was used at approximately 8 µM. PMB is known to ‘neutralize’ the inflammatory properties of LPS due to its cationic charge [35]; therefore, to avoid neutralization of these LPS effects these two treatments were separated. The cells were first treated with LPS for 6 h. The excess LPS was then washed away and the cells were challenged with PMB. CI and PLL treatments were also assessed in this separated manner (data not shown), but no significant difference was noted versus CI and PLL cotreatment with LPS.
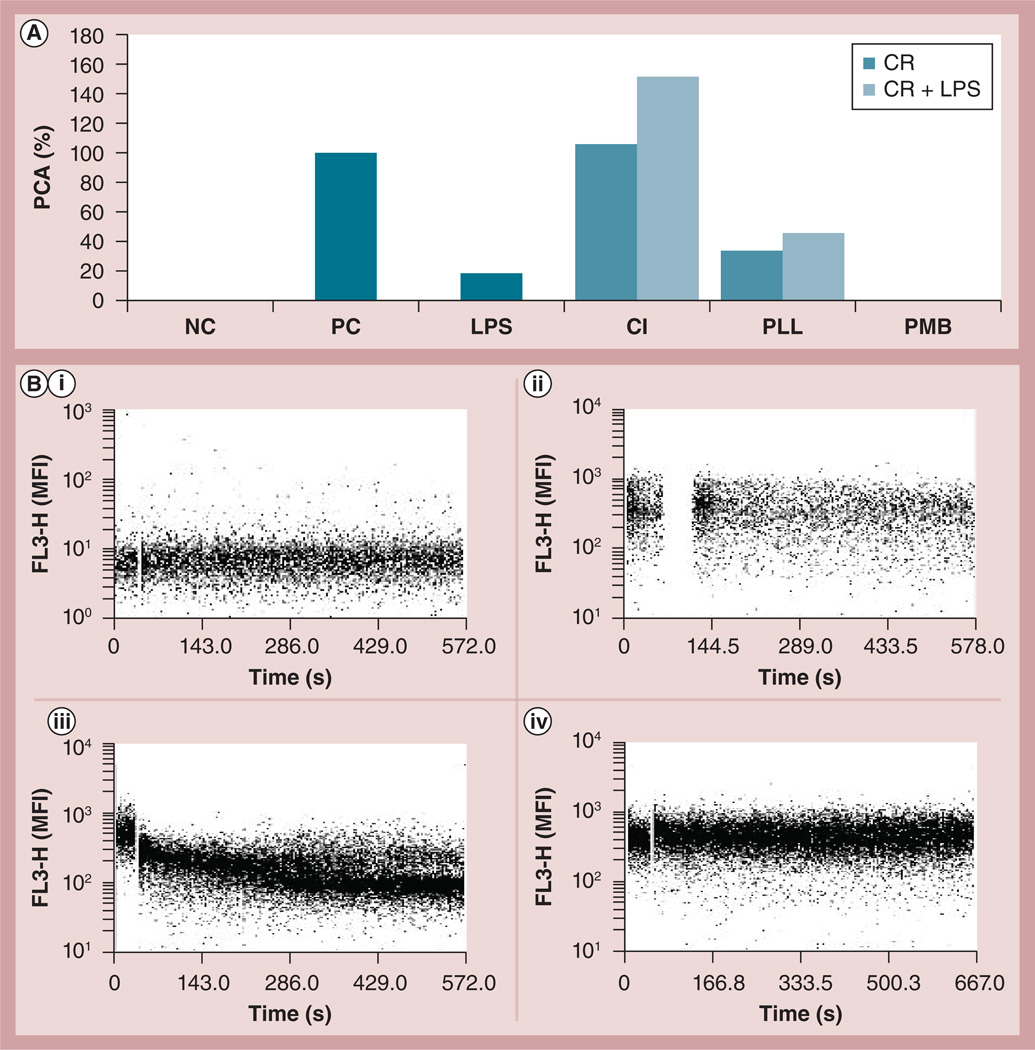
The various cationic molecules exhibited different effects on PCA and on LPS-induced PCA (Figure 4A). While LPS induced PCA, both PMB treatment-alone and in combination with LPS failed to induce PCA. Although both CI and PLL induced PCA when used as lone treatments, CI induction was approximately 2.5-times more potent than PLL (~100 vs ~40% PCA, respectively). The PCA induced by PLL and LPS cotreatment was not significantly stronger than that induced by PLL alone, and was approximately equivalent to the sum of PCA induced by the PLL and LPS single treatments. CI cotreatment, however, significantly enhanced LPS-induced PCA, and the CI effect on LPS-induced PCA was similar to that observed with the dendrimer-LPS combination treatment. Therefore, we evaluated the hypothesis that dendrimers could induce Ca2+ influx into cells, which in turn could be responsible for the enhancement of LPS-induced PCA. To assess this, intracellular Ca2+ levels were measured by flow cytometry in response to G6-NH2 treatment. In contrast to CI, which was used as a positive control in this case, there were no changes in Ca2+ levels detected in cells treated with G6-NH2 at various concentrations up to 1 mg/ml (Figure 4B). We also used EDTA as a calcium chelator to verify the role of Ca2+ flux in the studied phenomenon. In agreement with the data shown in Figure 4B the results of this experiment did not support involvement of Ca2+ (data not shown).
Figure 4. Understanding the role of cationic charge and Ca2+ influx in the enhancement of lipopolysaccharide-induced procoagulant activity.
(A) Peripheral blood mononuclear cells from healthy donor volunteers were treated with different cationic reagents: poly-l-lysine at 25 µg/ml (~1 µM), Ca+ ionophore at 1 µM; or PMB at 10 µg/ml (~8 µM). Peripheral blood mononuclear cells were treated with the CR alone or in combination with 1 ng/ml of LPS (CR + LPS) for 24 h. To avoid possible neutralizing effects of PMB on LPS, the cells in the PMB + LPS group were first treated with LPS for 6 h, washed with PBS, then challenged with PMB for an additional 12–18 h. PCA activity was measured 24 h after the first treatment. PCA was calculated as a percentage relative to that induced by the positive control LPS at 10 µg/ml. Similar results were obtained with two additional donors (Supplementary Figure S3). (B) Flow cytometry analysis of Ca2+ influx. Unloaded cells were treated with (i) Ca2+ ionophore as control; (ii) peripheral blood mononuclear cells loaded with Fura Red® (Molecular Probe, OR, USA) were untreated or (iii) treated with 1 µM Ca2+ ionophore or (iv) 25 µg/ml generation 6 amine-terminated polyamidoamine dendrimer. The fluorescence emission of Fura Red decreases with the increase of the intracellular Ca2+ (see manufacturer’s instructions for details).
CR: Cationic reagent; FL3-H: Fluorescent channel 3; LPS: Lipopolysaccharide; MFI: Mean fluorescent intensity; PCA: Procoagulant activity; PLL: Poly-l-lysine; PMB: Polymyxin B.
Platelets do not contribute to enhancement of LPS-induced PCA by cationic dendrimers
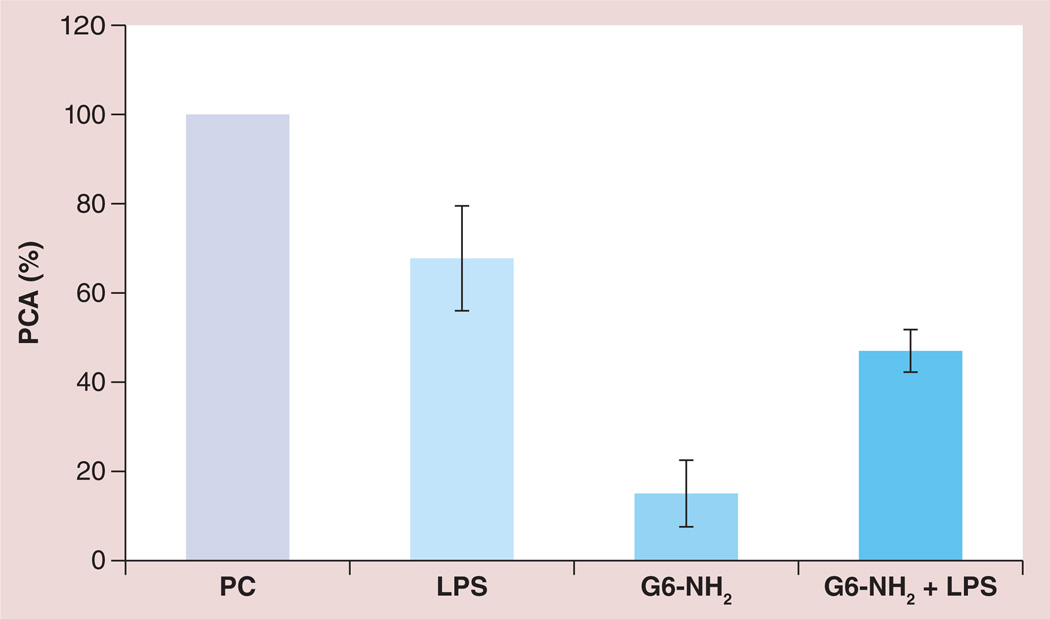
Since monocytes are the primary cells in the PBMC fraction responsible for PCA [8], we tested whether the monocytic cell line MM-6, known to possess many features of primary monocytes [36,37], could be used as a surrogate for PBMC cultures in the PCA assay. Although LPS alone induced PCA in MM-6 cells similar to that in PBMCs, in contrast to the PBMC cultures, this PCA was not enhanced by the addition of G6-NH2 (Figure 5; see also Figure 2 in [13]).
Figure 5. Procoagulant activity of MM-6 cells in response to lipopolysaccharide, cationic dendrimers and the combination thereof.
MM-6 cells were treated with 1 ng/ml LPS, 25 µg/ml amine-terminated generation 6 poly(amidoamine) dendrimers, or a combination of both LPS and G6-NH2 (G6-NH2 + LPS). PCA is calculated as a percentage of that induced by the positive control. Shown is the mean response ± standard deviation of three independent experiments (n = 3).
G6-NH2: Generation 6 amine-terminated polyamidoamine dendrimer; LPS: Lipopolysaccharide; PC: Positive control (lipopolysaccharide; 10 µg/ml); PCA: Procoagulant activity.
This finding, coupled with previous findings by us [14] and others [16] describing instantaneous platelet activation by large cationic dendrimers, and the fact that traditional Ficoll-Paque-separated PBMC fractions contain trace numbers of platelets, prompted us to probe whether platelet degranulation caused by cationic dendrimers was responsible for the enhancement of LPS-induced PCA of monocytes in PBMC cultures. To evaluate this, PBMCs were purified from the same donor using two different techniques: traditional Ficoll-Paque gradient centrifugation, referred to as ‘platelet-containing PBMCs’; and OptiPrep™ (Axis-Shield, Oslo, Norway) gradient centrifugation followed by centrifugation through 100% fetal bovine serum, referred to as ‘platelet-free PBMCs’. The depletion of platelets using the OptiPrep technique was confirmed by microscopy (Supplementary Figure S4).
PCA analysis, in this case, would require prohibitively high volumes of blood from the same donor due to the large number of cells needed for this in vitro assay; therefore, since TF expression and PS presentation correlated with PCA (Figure 1), these were used as the end point to assess the role of platelets. The level of TF expression was higher using platelet-free PBMCs versus platelet-containing PBMCs (Figure 6A). The effects of platelet depletion on PS presentation, however, varied between donors (Figure 6B & Supplementary Figure S5).
Figure 6. Role of platelets in enhancement of endotoxin-induced procoagulant activity by amine-terminated dendrimers.
Platelet-containing and platelet-free PBMCs from healthy donor volunteers were treated with 1 ng/ml of LPS or G6-NH2 alone (LPS and G6-NH2, respectively) or in combination (LPS + G6-NH2) for 24 h. (A) TF expression and (B) phosphatidyl serine presentation were analyzed by flow cytometry. TF was assessed using 4 µg/ml G6-NH2. Phosphatidyl serine was assessed using 4, 8 and 10 µg/ml G6-NH2. Shown is the mean percentage of positive cells. Shown are data from a single donor. Data from additional donors are provided in Supplementary Figure S5.
LPS: Lipopolysaccharide; G6-NH2: Generation 6 amine-terminated polyamidoamine dendrimer; NC: Negative control; PBMC: Peripheral blood mononuclear cell; PC: Positive control; TF: Tissue factor.
Amine-terminated G6 dendrimers inhibit PI3K in vitro
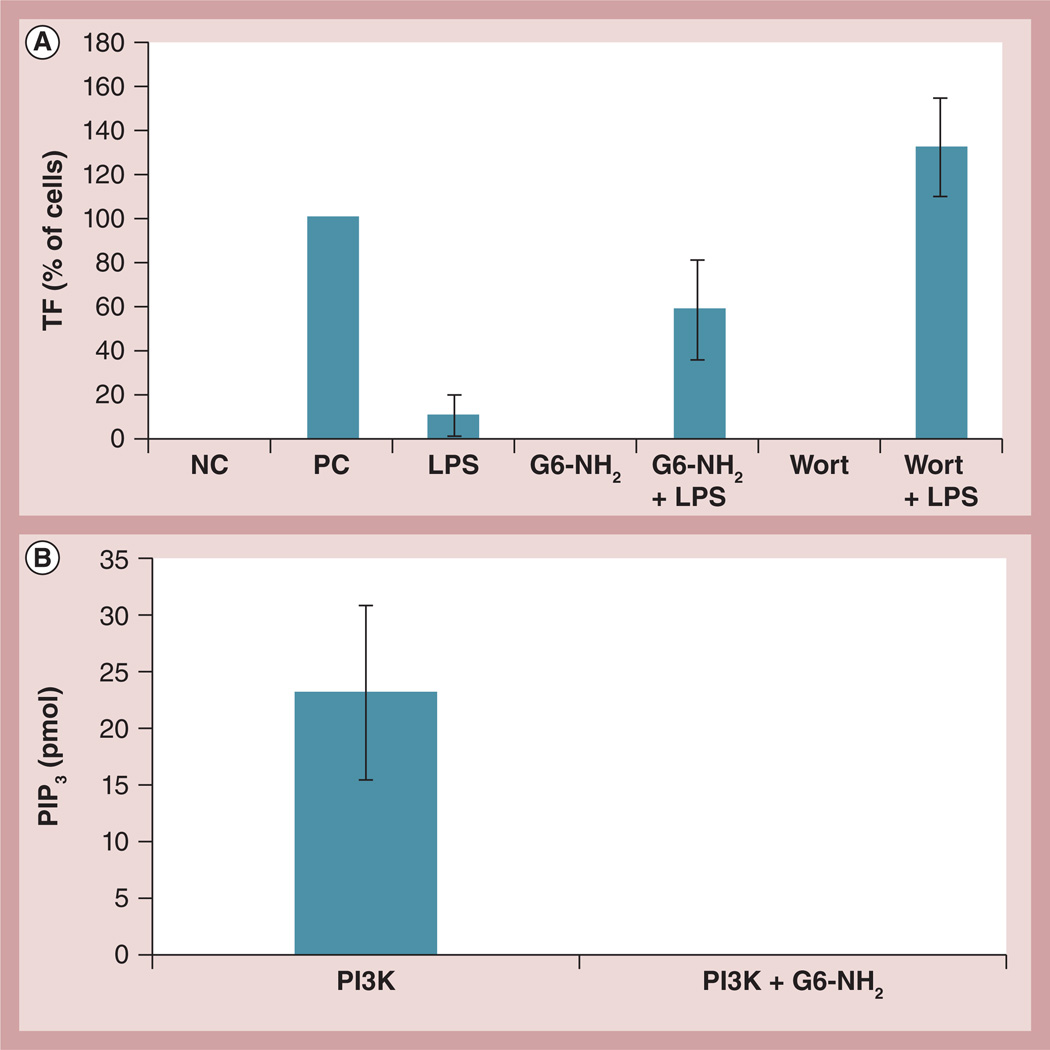
A recent study described the enhancement of TNF-induced TF expression by the cationic small molecule caffeine and attributed this phenomenon to the inhibition of PI3K by caffeine [38]. This prompted us to investigate the role of PI3K in the enhancement of LPS-induced TF expression by cationic dendrimers. First, to assess whether inhibition of PI3K in our experimental system resulted in an increase in the LPS-induced TF expression, cells were treated with wortmannin, a known pharmacological inhibitor of PI3K, alone or in combination with LPS. While wortmannin alone did not change TF expression, it significantly enhanced LPS-induced TF expression (Figure 7A).
Figure 7. Probing the role of phosphoinositol 3 kinase in the enhancement of lipopolysaccharide-induced procoagulant activity by cationic dendrimers.
(A) Peripheral blood mononuclear cells from healthy donor volunteers were treated for 24 h with 1 ng/ml of LPS, 4 µg/ml of G6-NH2, or the combination (LPS + G6-NH2). Cells were also treated with 100 nM Wort alone or in combination with LPS (Wort + LPS). Tissue factor expression on monocyte surfaces was measured by flow cytometry. Shown is the mean percentage of positive cells relative to the positive control. Media was used as the NC and 10 µg/ml of LPS was used as the PC. (B) Recombinant human PI3K was immunoprecipitated with anti-p85 antibody followed by several washes; the beads with isolated PI3K were then incubated with 12.5 µg/ml G6-NH2. After eight washes to remove excess dendrimer, the kinase activity was assessed by incubation with phosphatidylinositol (4,5)-biphosphate substrate. Shown is the mean amount of PIP3 generated from the kinase reaction ± standard deviation (n = 3).
G6-NH2: Generation 6 amine-terminated polyamidoamine dendrimer; LPS: Lipopolysaccharide; NC: Negative control; PC: Positive control; PI3K: Phosphoinositol 3 kinase; PIP3: Phosphatidylinositol (3,4,5)-triphosphate; Wort: Wortmannin.
Next, dendrimer effects on PI3K kinase activity were investigated. Since nanoparticle interference with in vitro assays is a common problem [39], potential dendrimer interference with the immunoprecipitation of PI3K from cell lysates and potential interference with the kinase reaction was first assessed using rPI3K. The rPI3K was immunoprecipitated using the anti-p85 PI3K subunit antibody conjugated to agarose beads. The beads with precipitated PI3K were incubated with cationic dendrimers; after several washes to remove excess dendrimers, we proceeded with the kinase reaction. The results demonstrate that G6-NH2 dendrimers completely abrogate PI3K activity (Figure 7B).
PI3K activity was also tested in PBMCs from several donors. PBMCs were stimulated with LPS and G6-NH2 alone or in combination. After stimulation with respective agonists for 1 h, PI3K was pulled down by immunoprecipitation and the kinase activity was assessed. Although various degrees of inhibition of PI3K activity were detected in different donors, the inhibition detected was less prominent than that observed in the experiment with rPI3K (Supplementary Figure S6).
Discussion & conclusion
The purpose of this work was to investigate the mechanisms involved in the enhancement of LPS-induced PCA by PAMAM dendrimers and to understand how particle charge and surface functionalization play a role. Herein, we show that inhibition of PI3K by cationic PAMAM dendrimers contributes to exaggeration of endotoxin (LPS)-induced PCA.
Endotoxin contamination is very common in engineered nanomaterials [21,22], yet its detection and elimination are often challenged by nanoparticle interferences and nanoparticles are frequently incompatible with standard depyrogenation procedures [22,23,39,40]. Understanding the mechanism for exaggeration of LPS-mediated toxicities by nanomaterials is very important for both environmental toxicology and nanomedicine. Several in vivo studies have reported induction of consumptive coagulopathy and DIC-like toxicities following intravenous administration of certain types of nanoparticles, particularly cationic PAMAM dendrimers and amorphous silica [11,12]. We and others have shown the procoagulant properties of PAMAM dendrimers stem from their interaction with platelets, resulting in alterations in membrane integrity, induction of leukocyte PCA and interaction with fibrinogen, and that these effects are determined by dendrimer size, charge and density of the surface amines [13–16].
Exaggeration of endotoxin-induced PCA by G6-NH2 dendrimers was described earlier [13]. The current study assesses how dendrimer size and charge influence this property. As anticipated, amine-terminated dendrimers exaggerate LPS-induced PCA in normal human PBMCs (Figure 2A); hydroxy- and carboxy-terminated dendrimers do not. However, unlike our previous observation in platelets and the HL-60 model, there is no direct correlation between dendrimer size and enhancement of LPS-induced PCA in leukocytes. Comparable elevation of LPS-induced PCA was detected in the presence of G3, G4, G5 and G6 dendrimers with an amine surface (Supplementary Figure S1). We also studied whether this property was unique to amine-terminated PAMAM dendrimers, or if it was shared by other cationic PAMAM dendrimers. Guanidine-terminated dendrimers have a comparable hydrodynamic size and zeta-potential with the amine-terminated dendrimers, but the cationic charge stems from different surface functionalizations. Dendrimers with both amine and guanidine surfaces resulted in enhancement of LPS-induced PCA. None of the studied particles (amine-, guanidine-, carboxy- or hydroxy-terminated) resulted in consistent PCA when used as single treatments (Figure 2).
Since the main components of PCA are TF and PS, effects on TF expression and PS presentation on the cell surface were analyzed following dendrimer and LPS treatment. Flow cytometry data correlated with PCA data such that enhancement of both TF and PS was detected on the surface of cells costimulated with LPS and cationic dendrimers (Figures 1C, 1D, 2B & 2C). This validates the premise that accelerated plasma coagulation – detected in vitro when cells costimulated with dendrimer and LPS were added to plasma – is not due to an artifact, but rather is determined by expression of the PCA complex (TF + PS) on the cell surface. Surprisingly, single treatments of cationic dendrimer and LPS had different impacts on PCA components. Cationic dendrimers alone did not induce TF expression but did induce PS, whereas LPS alone stimulated only TF and had no effect on PS presentation expression were consistent between donors for both the amine- and guanidine-terminated dendrimers, while PS presentation showed slight variability between donors with the guanidine-terminated dendrimers (Figure 2 & Supplementary Figure S2).
We propose two hypothetical mechanisms that could explain dendrimer-mediated PS presentation observed with the cationic dendrimers. The first suggests an alteration in the integrity of the anionic cellular membrane via interaction between the cell surface and the cationic dendrimers. Dendrimer-mediated membrane damage, extraction of lipids and intercalation into the lipid bilayer have been described by several earlier studies [41–44]. The second proposed mechanism is the initiation of apoptosis through disruption of lysosome integrity. Induction of apoptosis by cationic PAMAM dendrimers has also been described before [45]. Due to their charge, cationic dendrimers may act as proton ‘sponges’ and result in an increase in proton influx through proton pumps. These changes may lead to osmotic swelling and disruption of the lysosomal membrane. The release of lysosomal content leads to damage of the mitochondrial outer membrane, which in turn results in the induction of apoptosis. Lysosomal dysfunction by nanoparticles and its relationship to cell toxicity and death have been reviewed elsewhere [46]. We further hypothesize that these two mechanisms are activated by different concentrations of cationic dendrimers: disruption of the cellular membrane through electrostatic interactions (the first mechanism) takes place when cationic PAMAM dendrimers are used at low concentrations, while an increase in the dendrimer concentration and/or extension in treatment time activates apoptosis through lysosomal dysfunction (the second mechanism).
The presence of PS on the cell surface is essential for PCA. PS is thought to serve as a platform for the interaction with and assembly of coagulation factors, ultimately leading to the initiation of the extrinsic plasma coagulation cascade [7,10]. It has also been suggested that PS participates in the decryption of TF [47]. TF (CD142) is a 47 kDa cellular transmembrane protein which, upon interaction with factor VIIa, triggers the extrinsic coagulation cascade [48]. TF is constantly expressed on fibroblasts, smooth muscle cells and pericytes, but not on endothelial cells or monocytes. Expression and activation of TF is triggered by inflammatory stimuli (e.g., LPS, TNF and IL-1) in normal endothelial cells, normal monocytes [38,49,50], leukemia cells [51–53] and by some cytotoxic oncology drugs (e.g., daunorubicin and doxorubicin) in malignant leukocytes [54,55]. The ability of platelets to express TF is doubtful and controversial [8]. The expression of TF on the surface alone is not enough to initiate the coagulation cascade. It has been suggested that TF exists in two functionally different states: encrypted (inactive) and decrypted (active) [47]. Factors responsible for the conversion of the encrypted form into the decrypted form are not completely understood [47,56]. Lipid asymmetry and PS transition from the inner to the outer part of the lipid bilayer, transformation of the TF protein from a dimer to a monomer, Ca2+ influx, and disulfide bond switching are currently considered among factors triggering the activation of TF [47]. For this reason, it is generally agreed upon that the detection of TF expression per se is insufficient and should be confirmed by coagulation tests. The enhancement of LPS-induced PCA observed in our study was confirmed at both levels: plasma coagulation and TF/PS expression on the cell surface.
In efforts to probe the mechanism involved in the enhancement of LPS-induced PCA by cationic dendrimers, we first assessed whether interactions between anionic LPS and cationic dendrimers were leading to improved LPS presentation to the endotoxin receptor complex. Since there was no difference between particle size and surface chemistry of cationic particles, only G6-NH2 dendrimers were used for the mechanistic experiments. There was no difference, however, in PCA induction between cultures costimulated with LPS and dendrimers, and cultures treated with LPS and then restimulated with dendrimers after LPS removal (Figure 3). We next assessed whether cationic charge per se was essential for the enhancement of LPS-induced PCA. Several cationic molecules including PMB, PLL and CI were tested, since cationic molecules may trigger change in the intracellular Ca2+ levels and calcium is a very potent secondary messenger also known to regulate coagulation [57]. However, only CI enhanced LPS-induced PCA in a fashion similar to that of cationic dendrimers. The other cationic molecules, PLL and PMB, used at equivalent or greater molar concentrations, respectively, did not have this effect (Figure 4A), suggesting that cationic charge per se was not essential for the exaggeration of the LPS-induced PCA, but leaving the possibility that Ca2+ influx could be a contributing factor. Although Ca2+ influx was confirmed in cells treated with CI, no such effect was detected in cells treated with cationic PAMAM dendrimers (Figure 4B), thus ruling out the role of Ca2+ influx as a potential mechanism. We also studied the role of platelets as a potential mechanistic contributor of PCA enhancement, since disruption of the cellular membrane and degranulation of platelets occurs almost instantaneously after exposure to amine-terminated PAMAM dendrimers [14,16], and because platelet contamination is common in PBMC cultures isolated using traditional Ficoll-Paque gradient centrifugations [58]. Furthermore, the fact that no exaggeration of LPS-induced PCA by G6-NH2 was observed in the MM-6 cell line (Figure 5) also supported the hypothesis that platelets may contribute to the enhancement phenomenon. However, to our surprise, removal of platelets from PBMC cultures did not eliminate the enhancement phenomenon (Figure 6). Therefore, platelets do not appear to contribute to the exaggeration of endotoxin-induced PCA by cationic PAMAM dendrimers. These data, coupled with results demonstrating the lack of TF induction by single dendrimer treatment (Figures 1 & 2), and the dearth of direct interaction between LPS and dendrimers (Figure 3), prompted us to investigate pathways involved in TF induction by LPS.
LPS is a very potent immunostimulant and monocytes respond to LPS by production of inf lammatory cytokines. Some cytokines, specifically TNF and IL-1, are known to induce TF expression [38,50]. In our earlier studies, we observed an enhancement of both TNF and IL-1 by amine-terminated PAMAM dendrimers [Dobrovolskaia MA, Potter T, McNeil SE, Unpublished Data]. This, taken together with the enhancement of PCA and elevated expression of the PCA complex components on the cell surface, led us to hypothesize that perhaps dendrimers alter negative regulation of inflammation. One such negative loop involved in the regulation of inflammation is the activation of PI3K [59]. Although the pathways connecting PI3K activation and inhibition of LPS-mediated inflammation are still debatable, it has been demonstrated that the inhibition of PI3K results in an increase in TF expression [60]. Therefore, we tested whether dendrimers could inhibit PI3K, such that in the absence of negative regulation, LPS-induced inflammatory reactions including PCA expression could continue to propagate. A recent study by Gebhard et al., describing enhancement of TNF-mediated TF expression by the cationic small molecule caffeine, further fueled this hypothesis and led us to study dendrimer effects on PI3K [38]. We found that cationic PAMAM dendrimers completely abrogated kinase activity of rPI3K (Figure 7). This inhibition was not due to an in vitro artifact, as we first immobilized the rPI3K on agarose beads carrying anti-p85 antibody and then incubated it with the amine-terminated dendrimer (Figure 7). However, the dendrimer effect on PI3K immunoprecipitated from cell lysates was less prominent and varied between donors as well as between different days using the same donor (Supplementary Figure S6 and data not shown). This difference suggested that dendrimer effects on PI3K were probably due to the unspecific electrostatic interactions between cationic groups on the dendrimer surface and anionic repeats in the protein. When only PI3K and cationic dendrimers were present in the test tube (as in the case of rPI3K experiments) the interaction was optimal and easily detectable by the in vitro kinase assay. However, when cells were treated with dendrimers, their penetration into a cell and the intracellular concentration of nanoparticles are less controlled. Presumably, the intracellular concentrations of both PI3K and internalized dendrimer are less than that used in vitro. In addition, the activation state of the native cellular protein is transient, while the recombinant protein is designed to be constitutively active. The sensitivity of the in vitro kinase assay to the transient activation state of the native protein is a priori lower than that of the constitutive activation state of the recombinant protein, thus making the former less reliable. Electrostatic interaction between cationic dendrimer and anionic moiety in carboxy-terminal region of p85 subunit of PI3K is also predicted by molecular dynamics modeling (Supplementary Figure S7).
In summary, exaggeration of endotoxin-induced PCA by dendrimers is determined by particle charge and is a property of cationic surface functionalities regardless of the nature of functional groups delivering this cationic charge. The mechanism underlying this phenomenon includes, but is not limited to, inactivation of PI3K by cationic dendrimers.
Supplementary Material
Future perspective.
While our study has demonstrated that exaggeration of endotoxin-mediated PCA is not unique to amine-terminated PAMAM dendrimers and is also observed in PAMAM dendrimers with other cationic surface functionalities, it will be interesting to see if the same applies to cationic dendrimers with different composition (e.g., triazin, PLL and polyethylene-imine dendrimers). Although we show that inhibition of PI3K contributes to the observed exaggeration phenomenon, it would also be interesting to find other pathways and intracellular molecules affected by the cationic dendrimers. Another question that would be interesting to follow-up is the investigation of the role of PI3K in other nanoparticle-mediated enhancements of endotoxin-triggered immunotoxicites.
Executive summary.
-
▪
Cationic poly(amidoamine) dendrimers exaggerate lipopolysaccharide (LPS)-induced procoagulant activity (PCA) in peripheral blood mononuclear cells, but their anionic or neutral counterparts do not.
-
▪
The role of nanoparticle size in the enhancement of LPS-induced PCA is negligible; there was no clear difference between generation (G)3, G4, G5 and G6 amine-terminated dendrimers.
-
▪
A cationic charge is necessary for enhancement of LPS-induced PCA by dendrimers; however, there was no difference between cationic dendrimers with amine and guanidine surfaces.
-
▪
Enhancement of LPS-induced PCA by dendrimers correlates well with the enhancement of tissue factor expression and phosphatidyl serine presentation on the cell surface; however, single dendrimer and single LPS treatments had different impacts on the PCA components: dendrimers had more of a prominent impact on phosphatidyl serine presentation, while LPS induced only tissue factor expression.
-
▪
Interactions between LPS and dendrimers, Ca2+ influx, and platelets are not responsible for the enhancement of LPS-induced PCA by cationic dendrimers.
-
▪
Inhibition of phosphoinositol 3 kinase activity by cationic dendrimers contributes to the enhancement of LPS-induced PCA.
Acknowledgements
We thank T Potter and J Rodriguez for excellent technical assistance.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, NIH, under contract HHSN261200800001E.
Footnotes
Disclosure
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
- 1.Levi M. Disseminated intravascular coagulation. Crit. Care Med. 2007;35(9):2191–2195. doi: 10.1097/01.ccm.0000281468.94108.4b. [DOI] [PubMed] [Google Scholar]
- 2.Levi M, Schultz M, van der Poll T. Disseminated intravascular coagulation in infectious disease. Semin. Thromb. Hemost. 2010;36(4):367–377. doi: 10.1055/s-0030-1254046. [DOI] [PubMed] [Google Scholar]
- 3.Franchini M, Di Minno MN, Coppola A. Disseminated intravascular coagulation in hematologic malignancies. Semin. Thromb. Hemost. 2010;36(4):388–403. doi: 10.1055/s-0030-1254048. [DOI] [PubMed] [Google Scholar]
- 4.Lippi G, Cervellin G. Disseminated intravascular coagulation in trauma injuries. Semin. Thromb. Hemost. 2010;36(4):378–387. doi: 10.1055/s-0030-1254047. [DOI] [PubMed] [Google Scholar]
- 5.Montagnana M, Franchi M, Danese E, Gotsch F, Guidi GC. Disseminated intravascular coagulation in obstetric and gynecologic disorders. Semin. Thromb. Hemost. 2010;36(4):404–418. doi: 10.1055/s-0030-1254049. [DOI] [PubMed] [Google Scholar]
- 6.Furie B, Furie BC. Mechanisms of thrombus formation. N. Engl. J Med. 2008;359(9):938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 7.Morrissey JH, Davis-Harrison RL, Tavoosi N, et al. Protein-phospholipid interactions in blood clotting. Thromb. Res. 2010;125(Suppl. 1):S23–S25. doi: 10.1016/j.thromres.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osterud B, Bjorklid E. Tissue factor in blood cells and endothelial cells. Front. Biosci. (Elite Ed.) 2012;4:289–299. doi: 10.2741/e376. [DOI] [PubMed] [Google Scholar]
- 9.Bach RR. Tissue factor encryption. Arterioscler. Thromb. Vasc. Biol. 2006;26(3):456–461. doi: 10.1161/01.ATV.0000202656.53964.04. [DOI] [PubMed] [Google Scholar]
- 10.Shaw AW, Pureza VS, Sligar SG, Morrissey JH. The local phospholipid environment modulates the activation of blood clotting. J. Biol. Chem. 2007;282(9):6556–6563. doi: 10.1074/jbc.M607973200. [DOI] [PubMed] [Google Scholar]
- 11.Greish K, Thiagarajan G, Herd H, et al. Size and surface charge significantly influence the toxicity of silica and dendritic nanoparticles. Nanotoxicology. 2012;6:713–723. doi: 10.3109/17435390.2011.604442. [DOI] [PubMed] [Google Scholar]
- 12.Nabeshi H, Yoshikawa T, Matsuyama K, et al. Amorphous nanosilicas induce consumptive coagulopathy after systemic exposure. Nanotechnology. 2012;23(4):045101. doi: 10.1088/0957-4484/23/4/045101. [DOI] [PubMed] [Google Scholar]
- 13.Dobrovolskaia MA, Patri AK, Potter TM, Rodriguez JC, Hall JB, McNeil SE. Dendrimer-induced leukocyte procoagulant activity depends on particle size and surface charge. Nanomedicine (Lond.) 2012;7(2):245–256. doi: 10.2217/nnm.11.105. [DOI] [PubMed] [Google Scholar]
- 14.Dobrovolskaia MA, Patri AK, Simak J, et al. Nanoparticle size and surface charge determine effects of PAMAM dendrimers on human platelets in vitro. Mol. Pharm. 2012;9(3):382–393. doi: 10.1021/mp200463e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones CF, Campbell RA, Brooks AE, et al. Cationic PAMAM dendrimers aggressively initiate blood clot formation. ACS Nano. 2012;6(11):9900–9910. doi: 10.1021/nn303472r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones CF, Campbell RA, Franks Z, et al. Cationic PAMAM dendrimers disrupt key platelet functions. Mol. Pharm. 2012;9(6):1599–1611. doi: 10.1021/mp2006054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang M, Tracey KJ. Endotoxin and cancer. In: Brade H, editor. Endotoxin in Health and Disease. NY, USA: Marcel Dekker; 1999. pp. 915–926. [Google Scholar]
- 18.Engelhardt R, Otto F, Mackensen A, Mertelsmann R, Galanos C. Endotoxin (Salmonella abortus equi) in cancer patients. Clinical and immunological findings. Prog. Clin. Biol. Res. 1995;392:253–261. [PubMed] [Google Scholar]
- 19.Engelhardt R, Mackensen A, Galanos C. Phase I trial of intravenously administered endotoxin (Salmonella abortus equi) in cancer patients. Cancer Res. 1991;51(10):2524–2530. [PubMed] [Google Scholar]
- 20.US Pharmacopeia 30 National Formulary 25. MD, USA: US Pharmacopeial Convention; 2007. US Pharmacopeial Convention. Bacterial endotoxin test. [Google Scholar]
- 21.Vallhov H, Qin J, Johansson SM, et al. The importance of an endotoxin-free environment during the production of nanoparticles used in medical applications. Nano Lett. 2006;6(8):1682–1686. doi: 10.1021/nl060860z. [DOI] [PubMed] [Google Scholar]
- 22.Dobrovolskaia MA, McNeil SE. Endotoxin and engineered nanomaterials. In: Yarmush ML, Shi D, editors. Handbook of Immunological Properties of Engineered Nanomaterials. Singapore: World Scientific Publishing; 2012. pp. 77–115. [Google Scholar]
- 23.Jones CF, Castner DG, Grainger DW. Surface adsorbates on nanomaterial sand. Their possible roles in host inflammatory and toxocological processing. In: Yarmush ML, Shi D, editors. Handbook of Immunological Properties of Engineered Nanomaterials. Singapore: World Scientific Publishing; 2012. pp. 117–143. [Google Scholar]
- 24.Inoue K. Promoting effects of nanoparticles/ materials on sensitive lung inflammatory diseases. Environ. Health Prev. Med. 2011;16(3):139–143. doi: 10.1007/s12199-010-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue K, Takano H, Koike E, et al. Effects of pulmonary exposure to carbon nanotubes on lung and systemic inflammation with coagulatory disturbance induced by lipopolysaccharide in mice. Exp. Biol. Med. (Maywood) 2008;233(12):1583–1590. doi: 10.3181/0805-RM-179. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton RF, Wu N, Porter D, Buford M, Wolfarth M, Holian A. Particle lengthdependent titanium dioxide nanomaterials toxicity and bioactivity. Part. Fibre Toxicol. 2009;6:35. doi: 10.1186/1743-8977-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi Y, Yadav S, Wang F, Wang H. Endotoxin promotes adverse effects of amorphous silica nanoparticles on lung epithelial cells in vitro. J. Toxicol. Environ. Health A. 2010;73(11):748–756. doi: 10.1080/15287391003614042. [DOI] [PubMed] [Google Scholar]
- 28.Duncan R, Gaspar R. Nanomedicine(s) under the microscope. Mol. Pharm. 2011;8(6):2101–2141. doi: 10.1021/mp200394t. [DOI] [PubMed] [Google Scholar]
- 29.Bernatowicz MS, Wu YL, Matsueda GR. 1H-pyrazole-1-carboxamidine hydrochloride - an attractive reagent for guanylation of amines and its application to peptidesynthesis. J. Org. Chem. 1992;57(8):2497–2502. [Google Scholar]
- 30.Tziveleka LA, Psarra AM, Tsiourvas D, Paleos CM. Synthesis and characterization of guanidinylated poly(propylene imine) dendrimers as gene transfection agents. J. Control. Release. 2007;117(1):137–146. doi: 10.1016/j.jconrel.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem. J. 1993;296(Pt 2):297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henriksson CE, Klingenberg O, Hellum M, et al. Calcium ionophore-induced deencryption of tissue factor in monocytes is associated with extensive cell death. Thromb. Res. 2007;119(5):621–630. doi: 10.1016/j.thromres.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Nicolaou G, Goodall AH, Erridge C. Diverse bacteria promote macrophage foam cell formation via Toll-like receptor-dependent lipid body biosynthesis. J. Atheroscler. Thromb. 2012;19(2):137–148. doi: 10.5551/jat.10249. [DOI] [PubMed] [Google Scholar]
- 34.Chu AJ, Rauci M, Nwobi OI, Mathews ST, Beydoun S. Novel anticoagulant activity of polyamino acid offsets bacterial endotoxininduced extrinsic hypercoagulation: downregulation of monocytic tissue factordependent FVII activation. J. Cardiovasc. Pharmacol. 2003;42(4):477–483. doi: 10.1097/00005344-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Lynn WA, Golenbock DT. Lipopolysaccharide antagonists. Immunol. Today. 1992;13(7):271–276. doi: 10.1016/0167-5699(92)90009-V. [DOI] [PubMed] [Google Scholar]
- 36.Ziegler-Heitbrock HW, Thiel E, Futterer A, Herzog V, Wirtz A, Riethmuller G. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int. J. Cancer. 1988;41(3):456–461. doi: 10.1002/ijc.2910410324. [DOI] [PubMed] [Google Scholar]
- 37.Drexler HG, Quentmeier H, MacLeod RA. Malignant hematopoietic cell lines in vitro models for the study of MLL gene alterations. Leukemia. 2004;18(2):227–232. doi: 10.1038/sj.leu.2403236. [DOI] [PubMed] [Google Scholar]
- 38.Gebhard C, Holy EW, Camici GG, et al. Caffeine induces endothelial tissue factor expression via phosphatidylinositol 3-kinase inhibition. Thromb. Haemost. 2012;107(5):884–894. doi: 10.1160/TH11-09-0624. [DOI] [PubMed] [Google Scholar]
- 39.Dobrovolskaia MA, McNeil SE. In vitro assays for monitoring nanoparticle interaction with components of the immune system. In: Yarmush ML, Shi D, editors. Handbook of Immunological Properties of Engineered Nanomaterials. Singapore: World Scientific Publishing; 2012. pp. 581–634. [Google Scholar]
- 40.Jones CF, Grainger DW. In vitro assessments of nanomaterial toxicity. Adv. Drug Deliv. Rev. 2009;61(6):438–456. doi: 10.1016/j.addr.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong S, Bielinska AU, Mecke A, et al. Interaction of poly(amidoamine) dendrimers with supported lipid bilayers and cells: hole formation and the relation to transport. Bioconjug. Chem. 2004;15(4):774–782. doi: 10.1021/bc049962b. [DOI] [PubMed] [Google Scholar]
- 42.Hong S, Leroueil PR, Janus EK, et al. Interaction of polycationic polymers with supported lipid bilayers and cells: nanoscale hole formation and enhanced membrane permeability. Bioconjug. Chem. 2006;17(3):728–734. doi: 10.1021/bc060077y. [DOI] [PubMed] [Google Scholar]
- 43.Mecke A, Majoros IJ, Patri AK, Baker JR, Jr, Holl MM, Orr BG. Lipid bilayer disruption by polycationic polymers: the roles of size and chemical functional group. Langmuir. 2005;21(23):10348–10354. doi: 10.1021/la050629l. [DOI] [PubMed] [Google Scholar]
- 44.Lee H, Larson RG. Molecular dynamics simulations of PAMAM dendrimer-induced pore formation in DPPC bilayers with a coarse-grained model. J. Phys. Chem. B. 2006;110(37):18204–18211. doi: 10.1021/jp0630830. [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee SP, Lyng FM, Garcia A, Davoren M, Byrne HJ. Mechanistic studies of in vitro cytotoxicity of poly(amidoamine) dendrimers in mammalian cells. Toxicol. Appl. Pharmacol. 2010;248(3):259–268. doi: 10.1016/j.taap.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 46.Stern ST, Adiseshaiah PP, Crist RM. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part. Fibre Toxicol. 2012;9:20. doi: 10.1186/1743-8977-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rao LV, Kothari H, Pendurthi UR. Tissue factor: mechanisms of decryption. Front. Biosci. (Elite Ed.) 2012;4:1513–1527. doi: 10.2741/477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vadivel K, Bajaj SP. Structural biology of factor VIIa/tissue factor initiated coagulation. Front. Biosci. (Landmark Ed.) 2012;17:2476–2494. doi: 10.2741/4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Egorina EM, Sovershaev MA, Bjorkoy G, et al. Intracellular and surface distribution of monocyte tissue factor: application to intersubject variability. Arterioscler. Thromb. Vasc. Biol. 2005;25(7):1493–1498. doi: 10.1161/01.ATV.0000168413.29874.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bevilacqua MP, Pober JS, Majeau GR, Cotran RS, Gimbrone MA Jr. Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J. Exp. Med. 1984;160(2):618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norris LA, Weldon S, Nugent A, Roche HM. LPS induced tissue factor expression in the THP-1 monocyte cell line is attenuated by conjugated linoleic acid. Thromb. Res. 2006;117(4):475–480. doi: 10.1016/j.thromres.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 52.Chu AJ, Wang ZG, Walton MA, Seto A. Involvement of MAPK activation in bacterial endotoxin-inducible tissue factor upregulation in human monocytic THP-1 cells. J. Surg. Res. 2001;101(1):85–90. doi: 10.1006/jsre.2001.6271. [DOI] [PubMed] [Google Scholar]
- 53.Kubota T, Andoh K, Sadakata H, Tanaka H, Kobayashi N. Tissue factor released from leukemic cells. Thromb. Haemost. 1991;65(1):59–63. [PubMed] [Google Scholar]
- 54.Langer F, Amirkhosravi A, Loges S, et al. An in vitro study on the mechanisms of coagulation activation in acute myelogenous leukemia (AML): role of tissue factor regulation by cytotoxic drugs and GM-CSF. Thromb. Haemost. 2004;92(5):1136–1146. doi: 10.1160/TH04-04-0215. [DOI] [PubMed] [Google Scholar]
- 55.Boles JC, Williams JC, Hollingsworth RM, et al. Anthracycline treatment of the human monocytic leukemia cell line THP-1 increases phosphatidylserine exposure and tissue factor activity. Thromb. Res. 2012;129(2):197–203. doi: 10.1016/j.thromres.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 56.Osterud B. The role of platelets in decrypting monocyte tissue factor. Dis. Mon. 2003;49(1):7–13. doi: 10.1053/shem.2001.29508b. [DOI] [PubMed] [Google Scholar]
- 57.Bach R, Rifkin DB. Expression of tissue factor procoagulant activity: regulation by cytosolic calcium. Proc. Natl Acad. Sci. USA. 1990;87(18):6995–6999. doi: 10.1073/pnas.87.18.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Invest. 1968;97:77–89. [PubMed] [Google Scholar]
- 59.Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J. Biol. Chem. 2002;277(35):32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 60.Steffel J, Latini RA, Akhmedov A, et al. Rapamycin, but not FK-506, increases endothelial tissue factor expression: implications for drug-eluting stent design. Circulation. 2005;112(13):2002–2011. doi: 10.1161/CIRCULATIONAHA.105.569129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.