Abstract
Smoking has been reported to increase the risk of periodontal disease by disrupting the balance of immune responses and tissue repair processes; however, this risk varies among smokers. Cotinine levels in saliva are routinely used to measure the level of smoking, and reflect the quantity of nicotine, and other smoking related xenobiotics that challenge host systems. This study delineated characteristics of inflammatory mediators in saliva and serum antibody responses to both periodontal pathogens and commensal bacteria in smokers as they related to cotinine levels. This case-control study (n=279) examined salivary inflammatory mediator responses (IL-1ß, IL-10, PGE2, MPO, PAI-1) and serum IgG antibody responses to 3 periodontal pathogens (A. actinomycetemcomitans, P. gingivalis, T. denticola) and 5 commensal oral microorganisms (V. parvula, S. sanguis, P. loescheii, A. naeslundii, C. ochracea). The patients were stratified into health (n=30), gingivitis (n=55) and periodontitis (n=184); cotinine levels correlated with reported smoking habits in health, less so with gingivitis, and were not correlated in periodontitis. Of the inflammatory mediators/acute phase proteins, only IL-1ß levels were positively associated (p<0.001) with the pack years and cotinine levels. As might be predicted, patients with periodontitis smoked more (p<0.001) and had higher levels of cotinine. IL-1ß and antibody to Aa, Pg, and Td were significantly higher in the periodontitis patients than either gingivitis or healthy patients. Generally antibody to the pathogens and commensals was lower with decreased cotinine levels. Smoking exacerbated differences in both inflammatory mediators and antibody in periodontal disease compared to healthy subjects.
Keywords: smoking, cotinine, periodontitis, inflammation
INTRODUCTION
Periodontal diseases have afflicted man since before the dawn of recorded history. They collectively are of great public health importance since periodontal disease the leading cause of tooth loss and may be associated with other systemic chronic diseases (1-7). Plaque-induced periodontal diseases are a group of related conditions that result from interaction between oral bacteria and the host inflammatory response. Plaque-induced periodontal diseases have traditionally been divided into two general categories based on whether connective attachment loss has occurred; gingivitis and periodontitis. Following cessation of normal oral hygiene methods, virtually all individuals will develop a clinically observable condition known as gingivitis (8). Gingivitis is a reversible inflammation of the gum tissue (i.e., gingiva) caused by the presence of a biofilm that forms on the tooth surface and resolves rather quickly after the reinstitution of oral hygiene procedures. Most individuals will experience at least mild and transient gingivitis at some time in their life (9, 10).
Periodontitis characterized by persistent gingival inflammation, breakdown of the connective tissue (i.e., attachment apparatus sourrounding teeth), and destruction of alveolar bone. Unlike gingivitis, bacteria are necessary, but not sufficient, to cause periodontitis. That is, the presence of periodontal pathogens will not predictably relate to disease in all infected individuals (11, 12) suggesting the concept of differential susceptibility to periodontitis (13, 14). Differential susceptibility is a characteristic of other chronic diseases such as cardiovascular disease and various types of cancers and is the rationale for the clinical importance of risk assessment in clinical practice.
The microbial ecology of the subgingival environments of periodontally healthy and periodontally diseased sites is quite distinct (15-17). Microorganisms that colonize the oral soft and hard tissues and maintain a symbiotic relationship with the host, are referred to as commensal bacteria (18). Pathogenic bacteria are those that are harmful to the host (15, 17). In sites colonized by pathogen-dominated biofilms, the inflammatory response results in destruction of connective tissue and alveolar bone, the classic features of periodontitis. Various environmental factors affect the microbial composition in the oral cavity, as well as the host response. Smoking has also been demonstrated to be a major risk factor for developing periodontitis (19-22). Although smoking is a well-recognized risk factor for periodontal attachment loss, smokers often exhibit less gingival bleeding than would be predicted (23). This is likely due to effects of the toxic cigarette chemicals on the local vascular functions (23, 24). Through mechanisms that are not entirely understood, tobacco smoke amplifies the response to the microbial challenge. Recent data by Matthews and colleagues have provided some insight into these potential mechanisms by demonstrating the impact of smoking products on increasing oxidative stress reactions, but apparently decreasing the capacity of neutrophils to produce reactive oxygen species in response to microbial stimuli (25, 26). An epidemiological study using NHANES III data for periodontal risk factors, found a population attributable risk (PAR) for current or former smoking was approximately 50% (27). Although smoking confers significant risk for periodontitis and tooth loss, not all individuals are equally susceptible. It is not known why some individuals do not develop significant disease, although they continue to smoke. It is likely that some of this risk differential could be explained by enzymes that degrade components of tobacco smoke (28, 29).
The immune system is comprised of both innate and adaptive immune responses that are used to manage bacterial infections. The innate immune and inflammatory responses to the microbial ecology juxtaposed to the subgingival tissues vary with respect to the quantity and quality of microbial species in the biofilms. The accumulation of oral microbial biofilms elicits a robust inflammatory response consisting of polymorphonuclear leukocytes (PMNs), monocytes and macrophages. These cells release pro-inflammatory molecules that are known to initiate and perpetuate inflammation that manifests clinically as gingival redness and edema (14, 30-32). These various biomolecules associated with this response have been investigated in gingival crevicular fluid, included pro-inflammatory molecules such as IL-1ß and PGE2, anti-inflammatory cytokines, such as IL-10, and various acute phase response reactants (33), including plasminogen activator inhibitor-1 (PAI-1;, and myeloperoxidase (MPO) that generates reactive oxygen species (ROS), which can contribute to antibacterial responses (34). Based upon these observations, the tissue destruction in periodontal disease is now considered to actually result from the host response elicited by the pathogens, rather than direct toxic/noxious actions of the bacterial virulence factors (35).
Beyond innate immune and inflammatory responses, substantial literature documents the production of specific antibodies in local tissues and systemically to oral bacteria (32, 36, 37). These antibody levels increase significantly to selected oral bacteria with periodontitis and decrease following therapy (14, 38). However, it remains unclear how the immune system differentiates commensals that are important to maintain for health from those bacteria with greater virulence capabilities (17, 18, 39, 40). Thus, minimal data are available to compare the potential effect of smoking on the immune system discrimination of commensals from pathogenic oral bacteria.
This investigation addressed the relationship between smoking and systemic inflammatory and adaptive immune responses. We evaluated the data to address these responses related to characteristics of periodontal health/disease and related to various smoking parameters, particularly cotinine levels as an objective marker of the extent of current smoking activity. It was hypothesized that the level of smoking would not only contribute to the expression of periodontal disease, but also would alter local and systemic inflammatory and adaptive immune responses.
MATERIALS and METHODS
Patient Population
Serum from a venipuncture blood sample was obtained from a group of 269 smokers (age 21-65). The protocol for this study was approved by the University of Kentucky Institutional Review Board and all participants signed an appropriate consent form. A comprehensive oral and periodontal examination was completed to assess the periodontal health. Inclusion/exclusion criteria for participating in the study: must be smokers, able to complete a questionnaire and sign a consent form, have a minimum of 20 teeth, willing to have blood drawn, whole saliva collected, and have a full periodontal evaluation. The cohort included 30 periodontally healthy subjects (M:F – 7:23), 55 gingivitis patients (M:F – 14:41), and 184 periodontitis patients (M:F – 73:111). A non-smoking control group was not included, since the study design was specifically addressing differences in local inflammatory responses and antibody responses to commensal versus pathogenic bacteria related to periodontal disease and cotinine levels.
Clinical Parameters
Full-mouth mean pocket depth (PD), measured in millimeters (mm) and bleeding on probing, measured by percentage of sites in the mouth that bleed were determined at 6 sites per tooth excluding third molars (36). The measurements were taken and recorded by a single examiner. Patients were classified as healthy with BOP <30% and mean PD ≤3 mm (<1% pockets ≥4 mm). Gingivitis patients had BOP >30% and mean PD ≤3 mm (<5% 4 mm pockets, no pockets ≥ 5 mm). Periodontitis patients had BOP >30% and mean PD >3 mm (>5% pockets ≥4 mm).
Serum Analyses
The serum samples were stored at −80°C until the assays were performed. An array of oral microorganisms were used in the assays, cultivated under standard conditions, and prepared for antigens as described previously (37). The bacteria included proposed periodontal pathogens: Aggregatibacter actinomycetemcomitans (Aa) strain JP2, Porphyromonas gingivalis (Pg) ATCC 33277, Treponema denticola (Td) ATCC 35405, and a group of oral commensal bacteria that included Streptococcus sanguis (Ss) ATCC 10556, Actinomyces naeslundii (An) ATCC 49340, Prevotella loescheii (Pl) ATCC 15930, Veillonella parvula (Vp) ATCC 10790, Capnocytophaga ochracea (Co) ATCC 33596. An ELISA was used to determine the level of IgG antibody to the bacteria (36). Purified human IgG was bound to the plate to produce a standard curve. Sample data was extrapolated from this curve, using a four parameter logistic curve fit (41). Serum was also analyzed for IL-1β, IL-10, MPO and PAI-1 by Luminex (Millipore, Billerica, MA) and PGE2 levels were evaluated by a high sensitivity PGE2 ELISA (Assay Design, Ann Arbor, MI). The working range for the assays was: IL-1ß and IL-10 (0.64-1,000 pg/mL); MPO (0.024-100 ng/ml); PAI-1(0.0096-150 ng/ml) and PGE2 (39.1-2,500 ng/mL).
Salivary Analyses
Saliva was collected by unstimulated expectoration from each individual in the sample population. Each sample was centrifuged at 3000rpm and frozen at −80°C until needed for data collection. Cotinine levels were measured for each sample using a standard procedure with the Salimetrics’ High Sensitivity Salivary Cotinine Quantitative EIA Kit.
Statistical Analyses
Analyses of any differences among inflammatory mediators and IgG antibody levels, was conducted via a Kruskal-Wallis ANOVA with post hoc testing of paired groups using a Dunn's method (SigmaStat, Systat Software, Inc., Richmond, CA). Evaluation of the significance of correlation data was performed using the Spearman Correlation test. Data with an alpha of <0.05 (after being adjusted for the multiple comparisons) were accepted as statistically significant.
RESULTS
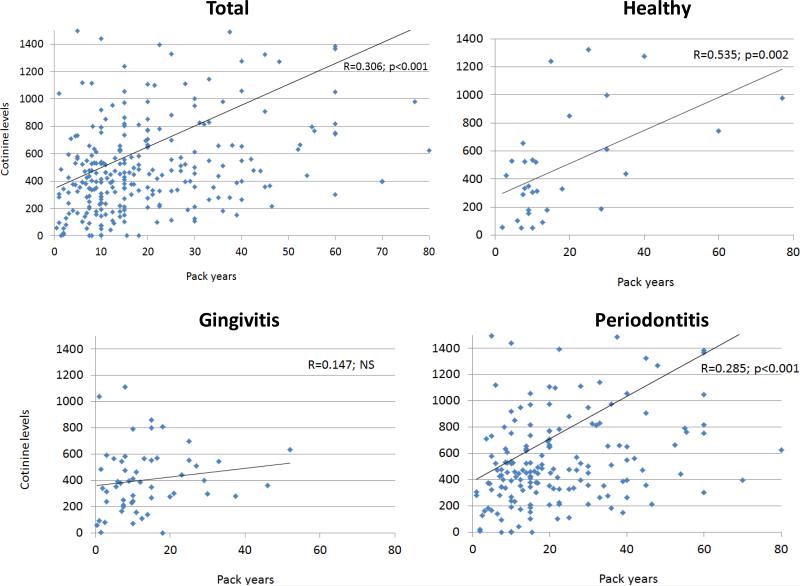
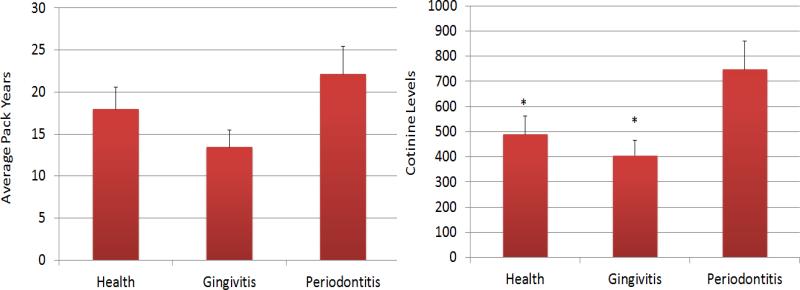
The results in Figure 1 demonstrate the relationship of smoking pack years as reported by the participants and the actual levels of salivary cotinine measured at the onset of the study. The results show a significant correction in the overall population, as well as in the periodontally healthy and periodontitis patients. We then compared both smoking pack years and cotinine levels as related to the periodontal health/disease of the subjects. The results in Figure 2 show no particular relationship with pack years and disease; however, the periodontitis patients demonstrated significantly elevated cotinine levels compared to the healthy and gingivitis patients.
Figure 1.
Correlation analyses for salivary cotinine levels and smoking pack years as reported by the patients. Data provided for the total population, and for subsets based upon periodontal disease characteristics. Each point denotes a patient.
Figure 2.
Smoking parameters defined as average pack years or salivary cotinine levels determined in patients stratified by periodontal disease characteristics. The bars denote group means and the vertical brackets signify 1 SD. The asterisk (*) denotes significantly different from periodontitis group at least at p<0.05.
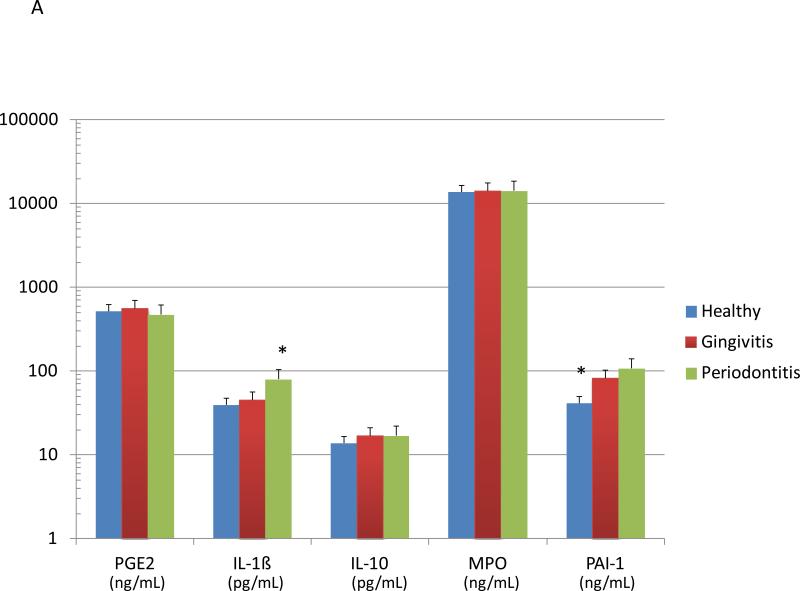
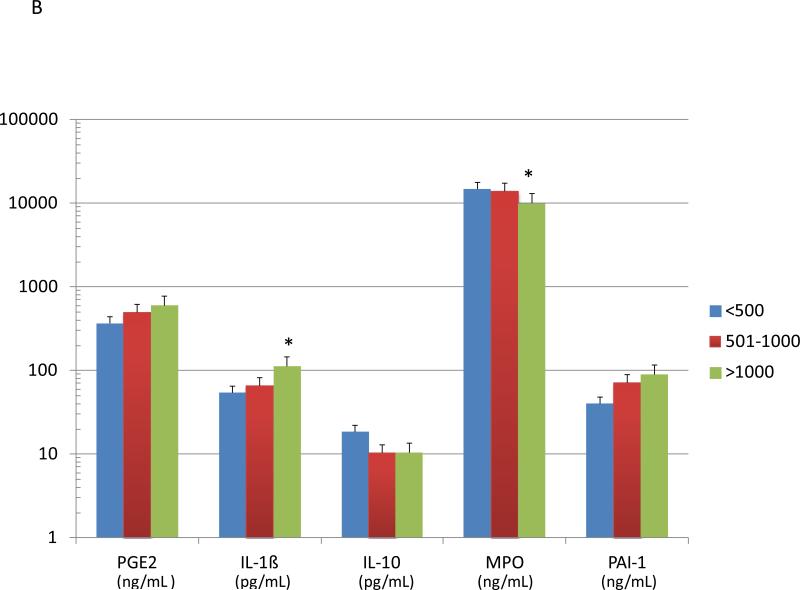
Figure 3A summarizes the levels of various systemic inflammatory biomarkers in smokers, stratified based upon periodontal health/disease. Serum IL-1ß levels were significantly elevated in the periodontitis patients, while PAI-1 (plasminogen activator inhibitor-1) was significantly decreased in the serum of the periodontally healthy smokers. The patients were also stratified based upon salivary cotinine levels (Figure 3B). The data demonstrated significant elevations in IL-1ß and significantly decreased serum MPO in the high cotinine, with elevated IL-10 levels in the low cotinine group.
Figure 3.
Levels of serum inflammatory biomolecules in smoking patients stratified by periodontal disease characteristics (A) or salivary continine levels (B). The bars denote group means and the vertical brackets signify 1 SD. The asterisk (*) denotes significantly different than the other groups at least at p<0.05.
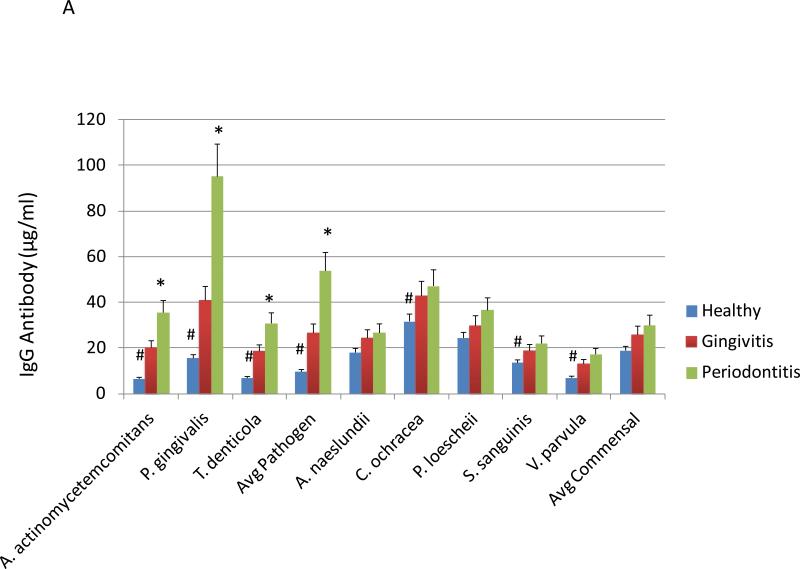
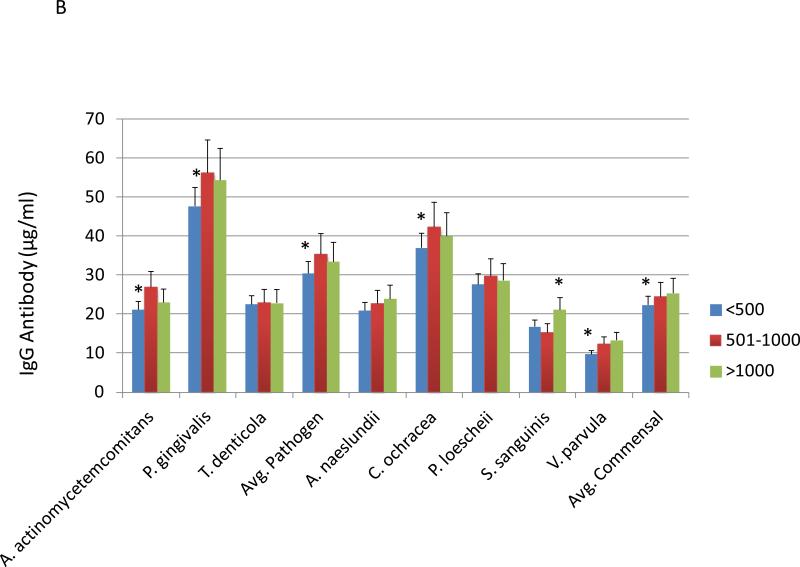
Figure 4A provides the results of antibodies levels to pathogenic and commensal bacteria in smokers related to periodontal health/disease. Elevated levels of serum IgG antibody was detected in periodontitis patients to the oral pathogens, A. actinomycetemcomitans, P. gingivalis, and T. denticola. Furthermore, the healthy smokers had significantly lower antibody levels to these pathogens as well as the oral commensal bacteria, C. ochracea, S. sanguinis, and V. parvula. Figure 4B stratifies the patients based upon salivary cotinine levels. In this view, patients with the lowest cotinine levels had significantly decreased antibody to both pathogens and commensal bacteria.
Figure 4.
Levels of serum IgG antibody in smoking patients stratified by periodontal disease characteristics (A) or salivary continine levels (B). The asterisk (*) and hashtag (#) denotes significantly different than the other groups at least at p<0.05. Avg. Pathogen/Commensal represents the average value for 3 pathogens and 5 commensals determined for each individual patient in the group.
Table 1 summarizes the relationship between the various measures of smoking in this population and both systemic inflammatory biomolecules and antibody responses to the oral bacteria. The results demonstrated that serum PGE2 and IL-1ß were positively correlated with various smoking parameters, while PAI-1 was negatively correlated. Generally, the antibody levels were not well correlated with the smoking parameters at the individual patient level.
Table 1.
| Smoking Variable | Serum Analyte | Avg. Ab Path | Avg. Ab Comm | ||||
|---|---|---|---|---|---|---|---|
| PGE2 | IL-1ß | IL-10 | MPO | PAI-1 | |||
| Cigarettes/day | 0.1974* | 0.1337 | 0.0490 | 0.0367 | −0.1441* | 0.1323 | −0.0008 |
| Total Yrs. Smoked | 0.0629 | 0.2381* | −0.0323 | −0.0268 | −0.0377 | 0.0308 | 0.0227 |
| Pack Years | 0.1863* | 0.2151* | 0.0143 | 0.0184 | −0.1816* | 0.0418 | −0.0068 |
| Salivary Cotinine | 0.2123* | 0.2514* | −0.1000 | −0.1601 | −0.1298 | 0.1242 | 0.0671 |
| <500 | −0.1283 | −0.0491 | −0.1175 | −0.1450 | −0.1481 | 0.0041 | −0.1472 |
| 501-1000 | −0.0082 | 0.1182 | −0.0207 | 0.0639 | −0.0867 | 0.0027 | 0.2284* |
| >1000 | −0.0070 | 0.3469 | −0.0785 | −0.0453 | 0.1467 | 0.0096 | 0.1135 |
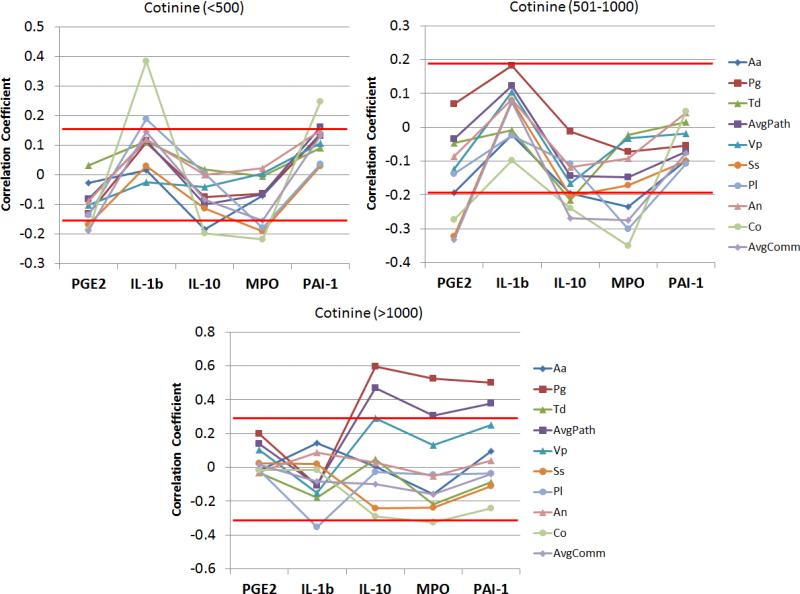
Figure 5 provides an overview of the relationship of serum levels of the inflammatory analytes with antibody responses to the oral pathogens and commensals. The patients were stratified into low, moderate, and high salivary cotinine levels and correlations between the range of variables determined. It appears that in the low-moderate cotinine subset of smokers, antibody levels to the commensal bacteria were significantly negatively correlated with levels of PGE2 and MPO. In contrast, the high level cotinine group showed antibodies to the pathogenic oral bacteria that were significantly positively correlated with IL-10, MPO, and PAI-1 levels in the serum.
Figure 5.
Correlation coefficients comparing levels of each inflammatory mediator and each antibody specificity in patients stratified based upon salivary cotinine levels. The red horizontal lines denote coefficients above or below which the p-value was <0.05 for the individual group.
Discussion
The literature is clear that smoking has substantial and numerous negative effects on general and oral health (20-22, 42-44). The role of smoking as a major modifiable environmental risk factor in the extent and severity of periodontal disease was reported in epidemiological longitudinal studies (45-48). It has been reported that smokers are 3-6 times more likely to have periodontitis than non-smokers (47-49), as importantly is the negative effect of xenobiotics derived from smoking on response to periodontal therapy (20, 50). However, it is also evident from studies of periodontitis and smoking that a subset of individuals with extensive smoking history and activity remain periodontally healthy or only develop gingivitis (51).
While it might be expected that inflammation in the oral cavity related to gingivitis and periodontitis could have an effect on the ability to measure cotinine levels, our findings examining cotinine levels and pack years suggested generally similar correlation patterns irrespective of the oral condition of the individual. Nevertheless, in stratifying the patients based upon periodontal health or disease, the periodontitis patients had significantly higher cotinine levels versus the other groups, while overall average pack years were not different, although trended higher in the periodontitis group. This was not unexpected based upon the literature that demonstrates the ability of salivary cotinine levels to more accurately represent current smoking activities. Another facet of the broader literature was that a subset of smokers appeared periodontally healthy even with long term smoking (ie. pack years) and substantial continine levels. Thus, some features of either the microbial challenge or characteristics of the host response profile appear to afford these patients an enhanced capacity to retain tissue homeostasis in the oral cavity. Similar observations have been summarized in a recent report by Genco and Borgnakke (52).
We have previously demonstrated serum antibody levels to selected oral pathogens and commensal bacteria related to race/ethnicity, age, and periodontal disease extent in a population of smokers (53). This study extended these findings by examining both serum antibodies and inflammatory molecules related to smoking parameters, and periodontal condition to identify the impact of active smoking on these responses. In this regard, we evaluated systemic inflammatory and immune responses in this population of smokers stratified based upon periodontal disease or cotinine levels to identify characteristic responses within the various subpopulations. Serum levels of IL-1ß were significantly elevated in the periodontitis patients. IL-1ß has been identified to be a crucial molecule in chronic inflammatory responses, both locally and systemically (54). Kornman and colleagues (55) have demonstrated a relationship between specific IL-1ß genetic polymorphisms, IL-1ß responses to stimuli, and risk for periodontitis. These polymorphisms of inflammatory mediator genes (56) appear to predispose to expression of periodontitis and have been linked to serum levels of C-reactive protein and together with altered IL-6 production extended to playing a role in periodontitis in type 2 diabetes mellitus (57). Our findings in smokers with periodontitis with the highest cotinine levels demonstrating elevated serum IL-1ß provides additional data regarding the importance of this pro-inflammatory mediator in periodontitis.
In addition to cytokines, a number of pro-inflammatory mediators are derived via the arachidonic acid pathway, including PGE2 (58, 59), which has been implicated in the pathogenesis of gingival inflammation and periodontitis (60, 61). The results in this study demonstrated no differences in serum PGE2 levels when patients were classified as periodontally healthy, gingivitis or periodontitis. While no significant differences were observed related to periodontal disease, there was an pattern of increasing PGE2 when patients were categorized based upon salivary cotinine levels. As the noxious materials from smoking are environmental stressors on cells that they contact, the elevated cotinine may reflect greater stress to the cells and associated responses, such as PGE2 production.
There is also some evidence that anti-inflammatory cytokines, such as IL-10, may play a role in modulating the chronic inflammatory responses in periodontitis. Berglundh and co-workers (62, 63) have identified a genetic polymorphism associated with IL-10 that is associated with severe chronic periodontitis and IL-10 or its mRNA has been detected in gingival crevicular fluid and gingival tissue specimens (64). Our results with this population of smokers did not identify that IL-10 levels in serum varied either based upon oral disease or cotinine levels.
Additional biomarkers of inflammatory responses in the systemic circulation are components of the acute phase response (33). This array of molecules serves a range of functions with the goal of reestablishing systemic homeostasis following noxious challenge. Increased levels of plasminogen activator inhibitor-1 (PAI-1) have been detected in inflamed gingival and periodontal tissues (65, 66). Moreover, treatment of periodontal disease has resulted in decreased levels of inflammatory biomarkers like PAI-1 and myeloperoxidase (MPO) (67, 68) (69). PAI-1 primarily functions to inhibit tissue plasminogen activator and urokinase that are involved in fibrinolysis, and has been identified to inhibit the activity of matrix metalloproteinases that are involved in tissue remodeling, but also tissue destruction during chronic inflammation (70, 71). Polymorphisms of the PAI-1 gene and/or smoking have been previously associated with increased levels and activity of PAI-1 (65, 66). Alterations in levels of PAI-1 have been suggested to play a role in systemic inflammatory conditions such as obesity and metabolic syndrome (72), asthma (73), and septic shock (74). Thus, our findings of lower levels of this biomolecule in orally healthy smokers are consistent with these observations.
During the chronic inflammatory response reactive oxygen species (ROS) and free radicals (e.g., NO and NO2) are generated by neutrophils and macrophages. Myeloperoxidase (MPO) is an enzyme stored in azurophilic granules and released by neutrophils and macrophages and is released into extracellular fluid at sites of inflammatory. This includes roles in producing bactericidal reactive intermediates (75, 76) and the potential that release of these ROS in chronic inflammation can lead to tissue destruction through oxidizing proteins and lipids. Rudolph et al. (77) have shown elevated MPO in smokers, apparently resulting from nicotine stimulation of neutrophils, which results in higher levels of oxidative stress to various tissues. Both endogenous (eg. superoxide dismutase, catalase, glutathione) and exogenous (eg. carotenoids, vitamins A, C, and E) antioxidants are critical in mitigating damage from ROS. Smoking appears to not only elevate ROS, but decreases these protective antioxidants (78).
Serum IgG antibody levels to a group of putative periodontal pathogens and oral commensal bacteria were also evaluated. As would be expected antibody to the periodontal pathogens were significantly elevated in the periodontitis smokers and significantly decreased in the periodontally healthy smokers. Responses to a number of the commensals were also significantly lower in the healthy individuals. However, a very different profile of responses was observed when the patients were stratified by cotinine levels. Antibody levels to the pathogens and commensals were significantly lower in the low smoking subjects, compared to those with higher continine, eg. greater smoking. These findings differed from the inflammatory mediator patterns and suggested that the antibody responses to the oral bacteria were not directly related to smoking level, but required the presence of a disease process. Substantial literature has demonstrated local and systemic adaptive immune responses to oral bacteria related to the presence, extent/severity, and therapeutic outcomes of periodontitis (32). A report by Apatzidou et al. (79) examined antibody levels to multiple oral bacteria in smokers compared to non-smokers with periodontitis. The results showed that generally serum IgG antibody levels were lower in the smokers. Schenkein and colleagues (80) have also demonstrated the impact of smoking on both chronic and aggressive periodontitis related to levels of serum antibody to oral pathogens, as well as skewing of IgG subclasses for the antibody. We have found similar types of response differences in smokers related to the oral health of the individual; however, we extended the available literature by demonstrating a racial/ethnic contribution to these responses, as well as a difference in response profile to pathogenic compared to commensal oral bacteria (53).
These differences in response were also obvious in demonstrating significant correlations of serum PGE2, IL-1ß, and PAI-1 with various smoking parameters. In contrast, the serum antibodies lacked these relationships. We subsequently examined the direct relationship between the individual serum inflammatory mediators and the adaptive immune responses in subsets of patients stratified by salivary cotinine levels. The results demonstrated that in the subsets with lower cotinine levels, antibodies to a number of the commensal bacteria were significantly negatively correlated with serum PGE2 and MPO levels. In contrast, in the high cotinine group, antibody levels to the pathogens, and particularly P. gingivalis were significantly positively correlated with some of the inflammatory mediators. Thus, while antibodies were not directly correlated with cotinine and smoking levels, there appeared to be some interface between the systemic inflammatory and adaptive responses related to the interactions of smoking and periodontal disease.
Whether there are molecules in the xenobiotic mix of smoking products that trigger unique host response profiles, or this relationship reflects a general noxious challenge to host tissues and cells that results in some specificity for eliciting the inflammatory and adaptive responses remains to be determined.
Summary.
This study identifies relationships in quantitative aspects of smoking with host response biomolecules in saliva and serum.
Acknowledgements
This work was supported by a USPHS grant from the NIH/NCRR P20 RR020145 and the services of the Center for Clinical and Translational Sciences at the University of Kentucky (NCRR and the National Center for Advancing Translational Sciences, NIH UL1 RR033173).
Source of Funding: This work was supported by U.S.P.H.S. grant P20 GM103538 and UL1 TR000117 from the National Institutes of Health.
Footnotes
Conflict of Interest: None of the authors have a financial interest related to the conduct of this research.
References
- 1.Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2007;13(Suppl 4):3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 2.Belstrom D, Damgaard C, Nielsen CH, Holmstrup P. Does a causal relation between cardiovascular disease and periodontitis exist? Microbes and infection / Institut Pasteur. 2012;14:411–418. doi: 10.1016/j.micinf.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Jimenez M, Krall EA, Garcia RI, Vokonas PS, Dietrich T. Periodontitis and incidence of cerebrovascular disease in men. Ann Neurol. 2009;66:505–512. doi: 10.1002/ana.21742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinane D, Bouchard P. Periodontal diseases and health: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35:333–337. doi: 10.1111/j.1600-051X.2008.01278.x. [DOI] [PubMed] [Google Scholar]
- 5.Preshaw PM, Alba AL, Herrera D, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia. 2012;55:21–31. doi: 10.1007/s00125-011-2342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bobetsis YA, Barros SP, Offenbacher S. Exploring the relationship between periodontal disease and pregnancy complications. J Am Dent Assoc. 2006;137(Suppl 2):7S–13S. doi: 10.14219/jada.archive.2006.0403. [DOI] [PubMed] [Google Scholar]
- 7.Noble JM, Borrell LN, Papapanou PN, Elkind MS, Scarmeas N, Wright CB. Periodontitis is associated with cognitive impairment among older adults: analysis of NHANES-III. J Neurol Neurosurg Psychiatry. 2009;80:1206–1211. doi: 10.1136/jnnp.2009.174029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loe H, Theilade E, Jensen SB. Experimental Gingivitis in Man. J Periodontol. 1965;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- 9.Eke PI, Thornton-Evans G, Dye B, Genco R. Advances in surveillance of periodontitis: the Centers for Disease Control and prevention periodontal disease surveillance project. J Periodontol. 2012;83:1337–1342. doi: 10.1902/jop.2012.110676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ. Cdc Periodontal Disease Surveillance workgroup: James Beck GDRP. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 2012. 91:914–920. doi: 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- 11.Zarco MF, Vess TJ, Ginsburg GS. The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral Dis. 2012;18:109–120. doi: 10.1111/j.1601-0825.2011.01851.x. [DOI] [PubMed] [Google Scholar]
- 12.Teles FR, Teles RP, Uzel NG, et al. Early microbial succession in redeveloping dental biofilms in periodontal health and disease. J Periodontal Res. 2012;47:95–104. doi: 10.1111/j.1600-0765.2011.01409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cobb CM. Microbes, inflammation, scaling and root planing, and the periodontal condition. J Dent Hyg. 2008;82(Suppl 3):4–9. [PubMed] [Google Scholar]
- 14.Kinane DF, Bartold PM. Clinical relevance of the host responses of periodontitis. Periodontol 2000. 200743:278–293. doi: 10.1111/j.1600-0757.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 15.Kumar PS, Leys EJ, Bryk JM, Martinez FJ, Moeschberger ML, Griffen AL. Changes in periodontal health status are associated with bacterial community shifts as assessed by quantitative 16S cloning and sequencing. J Clin Microbiol. 2006;44:3665–3673. doi: 10.1128/JCM.00317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paster BJ, Dewhirst FE. Molecular microbial diagnosis. Periodontol 2000. 2009;51:38–44. doi: 10.1111/j.1600-0757.2009.00316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000. 2006;42:80–87. doi: 10.1111/j.1600-0757.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 18.Edelman SM, Kasper DL. Symbiotic commensal bacteria direct maturation of the host immune system. Curr Opin Gastroenterol. 2008;24:720–724. doi: 10.1097/MOG.0b013e32830c4355. [DOI] [PubMed] [Google Scholar]
- 19.Van Dyke TE, Sheilesh D. Risk factors for periodontitis. J Int Acad Periodontol. 2005;7:3–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Chambrone L, Preshaw PM, Rosa EF, et al. Effects of smoking cessation on the outcomes of non-surgical periodontal therapy: a systematic review and individual patient data meta-analysis. J Clin Periodontol. 2013;40:607–615. doi: 10.1111/jcpe.12106. [DOI] [PubMed] [Google Scholar]
- 21.Thomson WM, Sheiham A, Spencer AJ. Sociobehavioral aspects of periodontal disease. Periodontol 2000. 201260:54–63. doi: 10.1111/j.1600-0757.2011.00405.x. [DOI] [PubMed] [Google Scholar]
- 22.Filoche SK, Cornford E, Gaudie W, Wong M, Heasman P, Thomson WM. Smoking, chronic periodontitis and smoking cessation support: reviewing the role of dental professionals. The New Zealand dental journal. 2010;106:74–77. [PubMed] [Google Scholar]
- 23.Dietrich T, Bernimoulin JP, Glynn RJ. The effect of cigarette smoking on gingival bleeding. J Periodontol. 2004;75:16–22. doi: 10.1902/jop.2004.75.1.16. [DOI] [PubMed] [Google Scholar]
- 24.Dietrich T, Hoffmann K. A comprehensive index for the modeling of smoking history in periodontal research. J Dent Res. 2004;83:859–863. doi: 10.1177/154405910408301107. [DOI] [PubMed] [Google Scholar]
- 25.Matthews JB, Chen FM, Milward MR, et al. Effect of nicotine, cotinine and cigarette smoke extract on the neutrophil respiratory burst. J Clin Periodontol. 2011;38:208–218. doi: 10.1111/j.1600-051X.2010.01676.x. [DOI] [PubMed] [Google Scholar]
- 26.Matthews JB, Chen FM, Milward MR, Ling MR, Chapple IL. Neutrophil superoxide production in the presence of cigarette smoke extract, nicotine and cotinine. J Clin Periodontol. 2012;39:626–634. doi: 10.1111/j.1600-051X.2012.01894.x. [DOI] [PubMed] [Google Scholar]
- 27.Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol. 2000;71:743–751. doi: 10.1902/jop.2000.71.5.743. [DOI] [PubMed] [Google Scholar]
- 28.Palmer RM, Wilson RF, Hasan AS, Scott DA. Mechanisms of action of environmental factors--tobacco smoking. Journal of clinical periodontology. 2005;32(Suppl 6):180–195. doi: 10.1111/j.1600-051X.2005.00786.x. [DOI] [PubMed] [Google Scholar]
- 29.Yeh HL, Kuo LT, Sung FC, Chiang CW, Yeh CC. GSTM1, GSTT1, GSTP1, and GSTA1 genetic variants are not associated with coronary artery disease in Taiwan. Gene. 2013 doi: 10.1016/j.gene.2013.02.052. [DOI] [PubMed] [Google Scholar]
- 30.Benakanakere M, Kinane DF. Innate cellular responses to the periodontal biofilm. Frontiers of oral biology. 2012;15:41–55. doi: 10.1159/000329670. [DOI] [PubMed] [Google Scholar]
- 31.Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol. 2008;79:1585–1591. doi: 10.1902/jop.2008.080183. [DOI] [PubMed] [Google Scholar]
- 32.Ebersole JL, Dawson DR, 3rd, Morford LA, Peyyala R, Miller CS, Gonzalez OA. Periodontal disease immunology: ‘double indemnity’ in protecting the host. Periodontol 2000. 2013;62:163–202. doi: 10.1111/prd.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebersole JL, Cappelli D. Acute-phase reactants in infections and inflammatory diseases. Periodontol 2000. 2000;23:19–49. doi: 10.1034/j.1600-0757.2000.2230103.x. [DOI] [PubMed] [Google Scholar]
- 34.Malik A, Batra JK. Antimicrobial activity of human eosinophil granule proteins: involvement in host defence against pathogens. Critical reviews in microbiology. 2012;38:168–181. doi: 10.3109/1040841X.2011.645519. [DOI] [PubMed] [Google Scholar]
- 35.Garlet GP. Destructive and protective roles of cytokines in periodontitis: a reappraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89:1349–1363. doi: 10.1177/0022034510376402. [DOI] [PubMed] [Google Scholar]
- 36.Ebersole JL, Holt SC, Hansard R, Novak MJ. Microbiologic and immunologic characteristics of periodontal disease in Hispanic americans with type 2 diabetes. J Periodontol. 2008;79:637–646. doi: 10.1902/jop.2008.070455. [DOI] [PubMed] [Google Scholar]
- 37.Ebersole JL. Humoral immune responses in gingival crevice fluid: local and systemic implications. Periodontol 2000. 2003;31:135–166. doi: 10.1034/j.1600-0757.2003.03109.x. [DOI] [PubMed] [Google Scholar]
- 38.Papapanou PN, Neiderud AM, Disick E, Lalla E, Miller GC, Dahlen G. Longitudinal stability of serum immunoglobulin G responses to periodontal bacteria. J Clin Periodontol. 2004;31:985–990. doi: 10.1111/j.1600-051X.2004.00599.x. [DOI] [PubMed] [Google Scholar]
- 39.Vaughan AT, Gorringe A, Davenport V, Williams NA, Heyderman RS. Absence of mucosal immunity in the human upper respiratory tract to the commensal bacteria Neisseria lactamica but not pathogenic Neisseria meningitidis during the peak age of nasopharyngeal carriage. J Immunol. 2009;182:2231–2240. doi: 10.4049/jimmunol.0802531. [DOI] [PubMed] [Google Scholar]
- 40.Singer RE, Moss K, Beck JD, Offenbacher S. Association of systemic oxidative stress with suppressed serum IgG to commensal oral biofilm and modulation by periodontal infection. Antioxid Redox Signal. 2009;11:2973–2983. doi: 10.1089/ars.2009.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cappelli D, Steffen MJ, Holt SC, Ebersole JL. Periodontitis in pregnancy: clinical and serum antibody observations from a baboon model of ligature-induced disease. J Periodontol. 2009;80:1154–1165. doi: 10.1902/jop.2009.080199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rocchietta I, Nisand D. A review assessing the quality of reporting of risk factor research in implant dentistry using smoking, diabetes and periodontitis and implant loss as an outcome: critical aspects in design and outcome assessment. J Clin Periodontol. 2012;39(Suppl 12):114–121. doi: 10.1111/j.1600-051X.2011.01829.x. [DOI] [PubMed] [Google Scholar]
- 43.Snider TN, Cottrell D, Batal H. Summary of current consensus on the effect of smoking on implant therapy. Journal of the Massachusetts Dental Society. 2011;59:20–22. [PubMed] [Google Scholar]
- 44.Huttunen R, Heikkinen T, Syrjanen J. Smoking and the outcome of infection. J Intern Med. 2011;269:258–269. doi: 10.1111/j.1365-2796.2010.02332.x. [DOI] [PubMed] [Google Scholar]
- 45.Ismail AI, Burt BA, Eklund SA. Epidemiologic patterns of smoking and periodontal disease in the United States. J Am Dent Assoc. 1983;106:617–621. doi: 10.14219/jada.archive.1983.0137. [DOI] [PubMed] [Google Scholar]
- 46.Machtei EE, Dunford R, Hausmann E, et al. Longitudinal study of prognostic factors in established periodontitis patients. J Clin Periodontol. 1997;24:102–109. doi: 10.1111/j.1600-051x.1997.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 47.Haber J. Smoking is a major risk factor for periodontitis. Current opinion in periodontology. 1994:12–18. [PubMed] [Google Scholar]
- 48.Bolin A, Lavstedt S, Frithiof L, Henrikson CO. Proximal alveolar bone loss in a longitudinal radiographic investigation. IV. Smoking and some other factors influencing the progress in individuals with at least 20 remaining teeth. Acta Odontol Scand. 1986;44:263–269. doi: 10.3109/00016358609004732. [DOI] [PubMed] [Google Scholar]
- 49.Grossi SG, Skrepcinski FB, DeCaro T, Zambon JJ, Cummins D, Genco RJ. Response to periodontal therapy in diabetics and smokers. J Periodontol. 1996;67:1094–1102. doi: 10.1902/jop.1996.67.10s.1094. [DOI] [PubMed] [Google Scholar]
- 50.Fiorini T, Musskopf ML, Oppermann RV, Susin C. Is There a Positive Effect of Smoking Cessation on Periodontal Health? A Systematic Review. J Periodontol. 2013 doi: 10.1902/jop.2013.130047. [DOI] [PubMed] [Google Scholar]
- 51.Scott DA, Singer DL. Suppression of overt gingival inflammation in tobacco smokers - clinical and mechanistic considerations. Int J Dent Hyg. 2004;2:104–110. doi: 10.1111/j.1601-5037.2004.00079.x. [DOI] [PubMed] [Google Scholar]
- 52.Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol 2000. 2013;62:59–94. doi: 10.1111/j.1600-0757.2012.00457.x. [DOI] [PubMed] [Google Scholar]
- 53.Hayman L, Steffen MJ, Stevens J, et al. Smoking and periodontal disease: discrimination of antibody responses to pathogenic and commensal oral bacteria. Clin Exp Immunol. 2011;164:118–126. doi: 10.1111/j.1365-2249.2010.04314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graves DT, Oates T, Garlet GP. Review of osteoimmunology and the host response in endodontic and periodontal lesions. Journal of oral microbiology. 2011;3 doi: 10.3402/jom.v3i0.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kornman KS. Interleukin 1 genetics, inflammatory mechanisms, and nutrigenetic opportunities to modulate diseases of aging. The American journal of clinical nutrition. 2006;83:475S–483S. doi: 10.1093/ajcn/83.2.475S. [DOI] [PubMed] [Google Scholar]
- 56.Brett PM, Zygogianni P, Griffiths GS, et al. Functional gene polymorphisms in aggressive and chronic periodontitis. J Dent Res. 2005;84:1149–1153. doi: 10.1177/154405910508401211. [DOI] [PubMed] [Google Scholar]
- 57.Javed F, Al-Askar M, Al-Hezaimi K. Cytokine profile in the gingival crevicular fluid of periodontitis patients with and without type 2 diabetes: a literature review. J Periodontol. 2012;83:156–161. doi: 10.1902/jop.2011.110207. [DOI] [PubMed] [Google Scholar]
- 58.Tsai CC, Hong YC, Chen CC, Wu YM. Measurement of prostaglandin E2 and leukotriene B4 in the gingival crevicular fluid. J Dent. 1998;26:97–103. doi: 10.1016/s0300-5712(96)00084-x. [DOI] [PubMed] [Google Scholar]
- 59.Tanaka H, Tanabe N, Shoji M, et al. Nicotine and lipopolysaccharide stimulate the formation of osteoclast-like cells by increasing macrophage colony-stimulating factor and prostaglandin E2 production by osteoblasts. Life Sci. 2006;78:1733–1740. doi: 10.1016/j.lfs.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 60.Salvi GE, Yalda B, Collins JG, et al. Inflammatory mediator response as a potential risk marker for periodontal diseases in insulin-dependent diabetes mellitus patients. J Periodontol. 1997;68:127–135. doi: 10.1902/jop.1997.68.2.127. [DOI] [PubMed] [Google Scholar]
- 61.Lamster IB, Novak MJ. Host mediators in gingival crevicular fluid: implications for the pathogenesis of periodontal disease. Critical reviews in oral biology and medicine : an official publication of the American Association of Oral Biologists. 1992;3:31–60. doi: 10.1177/10454411920030010501. [DOI] [PubMed] [Google Scholar]
- 62.Larsson L, Thorbert-Mros S, Rymo L, Berglundh T. Interleukin-10 genotypes of the -1087 single nucleotide polymorphism influence sp1 expression in periodontitis lesions. J Periodontol. 2011;82:1376–1382. doi: 10.1902/jop.2011.100623. [DOI] [PubMed] [Google Scholar]
- 63.Berglundh T, Donati M, Hahn-Zoric M, Hanson LA, Padyukov L. Association of the -1087 IL 10 gene polymorphism with severe chronic periodontitis in Swedish Caucasians. J Clin Periodontol. 2003;30:249–254. doi: 10.1034/j.1600-051x.2003.10274.x. [DOI] [PubMed] [Google Scholar]
- 64.Lappin DF, MacLeod CP, Kerr A, Mitchell T, Kinane DF. Anti-inflammatory cytokine IL-10 and T cell cytokine profile in periodontitis granulation tissue. Clin Exp Immunol. 2001;123:294–300. doi: 10.1046/j.1365-2249.2001.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buduneli N, Buduneli E, Kardesler L, Lappin D, Kinane DF. Plasminogen activator system in smokers and non-smokers with and without periodontal disease. J Clin Periodontol. 2005;32:417–424. doi: 10.1111/j.1600-051X.2005.00694.x. [DOI] [PubMed] [Google Scholar]
- 66.Kinnby B. The plasminogen activating system in periodontal health and disease. Biological chemistry. 2002;383:85–92. doi: 10.1515/BC.2002.008. [DOI] [PubMed] [Google Scholar]
- 67.Taylor BA, Tofler GH, Carey HM, et al. Full-mouth tooth extraction lowers systemic inflammatory and thrombotic markers of cardiovascular risk. J Dent Res. 2006;85:74–78. doi: 10.1177/154405910608500113. [DOI] [PubMed] [Google Scholar]
- 68.Taylor B, Tofler G, Morel-Kopp MC, et al. The effect of initial treatment of periodontitis on systemic markers of inflammation and cardiovascular risk: a randomized controlled trial. Eur J Oral Sci. 2010;118:350–356. doi: 10.1111/j.1600-0722.2010.00748.x. [DOI] [PubMed] [Google Scholar]
- 69.Behle JH, Sedaghatfar MH, Demmer RT, et al. Heterogeneity of systemic inflammatory responses to periodontal therapy. J Clin Periodontol. 2009;36:287–294. doi: 10.1111/j.1600-051X.2009.01382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Strongin AY. Proteolytic and non-proteolytic roles of membrane type-1 matrix metalloproteinase in malignancy. Biochimica et biophysica acta. 2010;1803:133–141. doi: 10.1016/j.bbamcr.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghosh AK, Vaughan DE. PAI-1 in tissue fibrosis. J Cell Physiol. 2012;227:493–507. doi: 10.1002/jcp.22783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jankun J, Al-Senaidy A, Skrzypczak-Jankun E. Can inactivators of plasminogen activator inhibitor alleviate the burden of obesity and diabetes? Int J Mol Med. 2012;29:3–11. doi: 10.3892/ijmm.2011.810. (Review) [DOI] [PubMed] [Google Scholar]
- 73.Ma Z, Paek D, Oh CK. Plasminogen activator inhibitor-1 and asthma: role in the pathogenesis and molecular regulation. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2009;39:1136–1144. doi: 10.1111/j.1365-2222.2009.03272.x. [DOI] [PubMed] [Google Scholar]
- 74.Paulus P, Jennewein C, Zacharowski K. Biomarkers of endothelial dysfunction: can they help us deciphering systemic inflammation and sepsis? Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. 2011;16(Suppl 1):S11–21. doi: 10.3109/1354750X.2011.587893. [DOI] [PubMed] [Google Scholar]
- 75.Klebanoff SJ. Myeloperoxidase. Proceedings of the Association of American Physicians. 1999;111:383–389. doi: 10.1111/paa.1999.111.5.383. [DOI] [PubMed] [Google Scholar]
- 76.Lau D, Baldus S. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacology & therapeutics. 2006;111:16–26. doi: 10.1016/j.pharmthera.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 77.Rudolph TK, Rudolph V, Baldus S. Contribution of myeloperoxidase to smoking-dependent vascular inflammation. Proceedings of the American Thoracic Society. 2008;5:820–823. doi: 10.1513/pats.200807-063TH. [DOI] [PubMed] [Google Scholar]
- 78.Buduneli N, Kardesler L, Isik H, et al. Effects of smoking and gingival inflammation on salivary antioxidant capacity. J Clin Periodontol. 2006;33:159–164. doi: 10.1111/j.1600-051X.2006.00892.x. [DOI] [PubMed] [Google Scholar]
- 79.Apatzidou DA, Riggio MP, Kinane DF. Impact of smoking on the clinical, microbiological and immunological parameters of adult patients with periodontitis. J Clin Periodontol. 2005;32:973–983. doi: 10.1111/j.1600-051X.2005.00788.x. [DOI] [PubMed] [Google Scholar]
- 80.Tangada SD, Califano JV, Nakashima K, et al. The effect of smoking on serum IgG2 reactive with Actinobacillus actinomycetemcomitans in early-onset periodontitis patients. J Periodontol. 1997;68:842–850. doi: 10.1902/jop.1997.68.9.842. [DOI] [PubMed] [Google Scholar]