Abstract
Changes in cerebrospinal fluid (CSF) biomarkers are representative of biochemical changes in the brain. Collection of CSF by lumbar puncture (LP) is essential for biomarker analysis, which is important for research in neurodegenerative disorders. However, LP for research purposes has been controversial due to a reported high incidence of severe LP headache when using standard 18g or 20g Quincke needles with a beveled cutting tip. A procedural safety analysis was performed using the database of a multicenter, 13-week study of CSF cholinesterase activity. A 24g Sprotte atraumatic needle was used to collect CSF at baseline and at Week 13 from 63 older patients with mild to moderate Alzheimer's disease. There was a < 2% LP headache incidence, and a favorable safety profile was reported. In conclusion, LP performed with a 24g Sprotte atraumatic needle (blunt, “bullet” tip) was a well-tolerated procedure, with good acceptability.
Keywords: Alzheimer's disease, cerebrospinal fluid (CSF), lumbar puncture, lumbar puncture headache, cholinesterase inhibitors
INTRODUCTION
Cerebrospinal fluid (CSF) is in direct contact with the extracellular space of the brain. Thus, changes in CSF biomarkers may be considered representative of biochemical changes occurring in the brain. This is of particular importance for research in Alzheimer's disease (AD) and other neurodegenerative disorders that involve specific brain neurochemical changes.
The collection of CSF by lumbar puncture (LP) is essential for the analysis of these biomarkers. However, the use of LP for research purposes has been controversial, as there is no immediate benefit to the patient and the procedure is perceived as invasive, with a substantial risk of adverse events (AEs).
The most prevalent AEs associated with LP procedures are mild-moderate headache and backache. However, more severe post-LP headaches are considered the primary safety concern. These occur in as many as 30% of patients using the 18g or 20g Quincke spinal needle in the standard LP kit, and can cause severe headache that may be accompanied by nausea and vomiting and last for as long as a week if left untreated. Other complications occur very rarely, and include parasthesias, nerve root trauma and CNS infection.
Despite these concerns, previous studies involving specialized LP procedures in elderly and cognitively impaired individuals have suggested that they can be well-tolerated with a low rate of clinically significant AEs [1, 2]. The primary objective of this analysis was to evaluate the safety and tolerability of LP procedures in a recent study of CSF cholinesterase activity in elderly patients with mild to moderate AD.
METHODS
This was a prospective, randomized, parallel-cohort, multicenter 13-week, open-label comparative study of the effects of rivastigmine (Exelon®, Novartis), donepezil (Aricept®, Pfizer) and galantamine (Reminyl/Razadyne®, Janssen) on CSF cholinesterase activity [3]. Patients aged 50-85, with a diagnosis of mild to moderate AD [4, 5] and MMSE scores of 10-20 were randomized to treatment. For this procedural safety analysis the focus was on the overall study population, rather than individual randomized treatment groups.
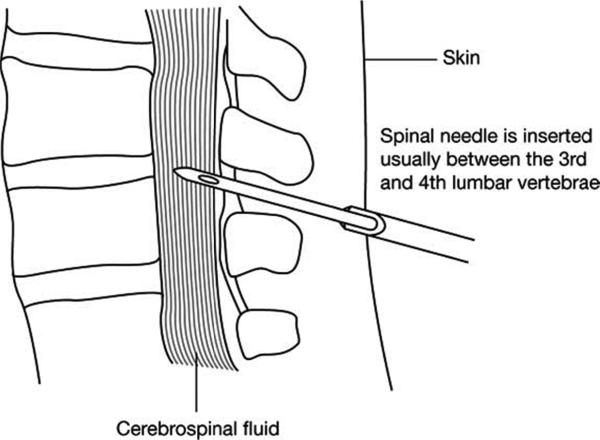
CSF samples were collected by LP at baseline and at Week 13. LPs were carried out in the L3-4 or L4-5 interspace using a 20g spinal introducer needle and a 24g Sprotte atraumatic spinal needle (Fig. 1) approximately 4-6 hours after the morning meal. Following puncture of the dura, the stylet was removed and a total of 10 ml of CSF was collected under negative pressure. Patients remained supine for a brief period, and were encouraged to drink fluids. Fluoroscopic guidance was utilized if deemed necessary by the administering physician.
Figure. (1).
An illustration of the lumbar puncture procedure (not to scale). The sharp 20-guage introducer needle is used to pierce the skin, and the Sprotte 24-guage atraumatic spinal needle passed through the introducer and into the spinal column (spinal cord not shown). The Sprotte needle has a blunt “bullet” tip to minimize damage to nerve endings, with the opening located on the side of the needle just below the tip.
Safety and tolerability were monitored throughout the study by recording all AEs using an AE case report form (CRF), and followed up as appropriate. AEs thought to be associated with the LP procedure were recorded separately on an LP Follow-up CRF.
Safety analyses were performed on the safety population, defined as all randomized patients who received at least one dose of study medication.
RESULTS
Of the 63 patients comprising the safety population (mean age 74.9 years, 70% female), 10 patients discontinued the study; 8 due to treatment-related AEs. One discontinuation due to LP-related AEs was reported.
Out of the 116 LP procedures performed throughout the study, 1 patient reported a typical ‘post-LP headache’, 5 reported non-specific headaches, and 4 reported back pain (Table 1). Aside from 2 incidences of dizziness, no other LP-related AE occurred more than once, no serious AEs such as CNS infection or nerve-root damage were reported, and no deaths occurred.
Table 1.
A Summary of All Adverse Events (AEs) Suspected of Being Related to Lumbar Puncture (LP), Presented by Patient and Procedure
| Adverse event | Procedures resulting in post-LP AEs n (%) | Patients reporting post-LP AEs n (%) |
|---|---|---|
| All headaches* | 8 (6.9) | 7 (11.1) |
| Lumbar puncture headache | - | 1 (16) |
| Migraine | - | 1 (16) |
| Non-specific headache | - | 5 (7.9) |
| Back pain | 5 (4.3) | 4 (6.4) |
| Dizziness | 2 (1.7) | 2 (3.2) |
| Muscle tightness | 1 (0.9) | 1 (16) |
| Procedural pain | 1 (0.9) | 1 (16) |
| Anxiety† | 1 (0.9) | 1 (16) |
| Fatigue† | 1 (0.9) | 1 (16) |
| Neck injury† | 1 (0.9) | 1 (16) |
| Nausea† | 1 (0.9) | 1 (16) |
| Vomiting† | 1 (0.9) | 1 (16) |
Safety population
A total of 116 LP procedures were performed on the safety population (n = 63). Twelve procedures (10.3%) resulted in one or more AEs, and 11 patients (17.5%) reported at least one AE; some patients reported more than one AE each.
Includes all severities of headache, from the mildest to the most severe. The type of headache (e.g. LP headache, migraine) was not specified
These AEs were all reported by a single patient following the baseline LP procedure
All LP-related AEs occurred within 24 hours of the procedure, with the exception of 1 case of lower back pain and 1 case of nausea and vomiting that occurred on the second day following the procedure. One patient reporting a headache at their baseline assessment also reported a “worsening headache” for 4 days afterwards.
DISCUSSION
The controversy surrounding the use of LP procedures stems from the high incidence of severe LP headache reported when using standard 18g or 20g Quincke needles with a beveled cutting tip [6]. These data demonstrate that LP performed with a 24g Sprotte atraumatic needle (blunt, “bullet” tip) is a well-tolerated procedure in older patients with mild to moderate AD (< 2% LP headache incidence), with a favorable safety profile. This is consistent with previous studies of the safety of modern LP procedures in older and cognitively impaired patients [1, 2, 7]. In a previous study of the safety and acceptability of research lumbar puncture using the Sprotte 24-gauge spinal needle, anxiety and pain ratings were obtained immediately following LP in young, middle-aged, and older normal persons and patients with MCI and AD. Although all pain and anxiety ratings were low, younger patients reported slightly but significantly higher pain and anxiety, but no increased risk of post-LP headache compared to the other subject groups [1]. Thus, despite previous perceptions, these results suggest that LPs may in fact be ethically employed as clinical research and diagnostic tools in AD patients. The ability to analyze CSF biomarkers could allow more accurate determination of the onset and progression of AD, as well as response to treatment.
In conclusion, research LP using the Sprotte 24-gauge atraumatic spinal needle may be performed with a low rate of clinically significant AEs, and with good acceptability in older patients with mild to moderate AD.
Acknowledgements
This study was supported by Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA. Alpha-Plus Medical Communications Ltd (UK) provided editorial assistance with the production of the manuscript.
Footnotes
Disclosure/Conflicts of Interest
This study was sponsored by Novartis Pharmaceuticals Corporation. Professor Peskind was involved as a consultant for methods of CSF collection, processing, and storage prior to study initiation and was not involved in collection or analysis of study data. Professors Nordberg and Darreh-Shori were responsible for the enzyme analysis in the primary CSF study, and received research sponsorship from Novartis. Professor Soininen was a study investigator; her institutes received research sponsorship from Novartis.
REFERENCES
- 1.Peskind ER, Riekse R, Quinn JF, Kaye J, Clark CM, Farlow MR, et al. Safety and acceptability of the research lumbar puncture. Alzheimer Dis Assoc Disord. 2005;19:220–225. doi: 10.1097/01.wad.0000194014.43575.fd. [DOI] [PubMed] [Google Scholar]
- 2.Blennow K, Wallin A, Hager O. Low frequency of post-lumbar puncture headache in demented patients. Acta Neurol Scand. 1993;88:221–223. doi: 10.1111/j.1600-0404.1993.tb04221.x. [DOI] [PubMed] [Google Scholar]
- 3.Nordberg A, Darreh-Shori T, Peskind E, Soininen H, Mousavi M, Eagle G, et al. Different cholinesterase inhibitor effects on CSF cholinesterases in AD patients. Curr Alzheimer Res. 2009;6:3–13. doi: 10.2174/156720509787313961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Psychiatric Association . Diagnostic Criteria from DSMIV. American Psychiatric Association; Washington DC: 1994. [Google Scholar]
- 5.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 6.Vilming ST, Kloster R, Sandvik L. The importance of sex, age, needle size, height and body mass index in post-lumbar puncture headache. Cephalalgia. 2001;21:738–743. doi: 10.1046/j.1468-2982.2001.00200.x. [DOI] [PubMed] [Google Scholar]
- 7.Darreh-Shori T, Almkvist O, Guan ZZ, Garlind A, Strandberg B, Svensson AL, et al. Sustained cholinesterase inhibition in AD patients receiving rivastigmine for 12 months. Neurology. 2002;59:563–572. doi: 10.1212/wnl.59.4.563. [DOI] [PubMed] [Google Scholar]