Abstract
Objective
Treatment responses of placebo groups in addiction medicine trials have important implications for research methodology and clinical practice, however, studies examining placebo group responses in addiction medicine is scarce. Extant data suggests the importance of early treatment responsiveness for long-term outcomes. Among methamphetamine (MA) dependent individuals randomized to placebo pill plus behavioral support conditions in pharmacotherapy development trials, we hypothesized that immediate abstinence would be a necessary but insufficient predictor for end-of-trial (EOT) abstinence.
Methods
The study is a secondary analysis of participants (N=184; 36% female) in the placebo condition of three randomized, placebo-controlled methamphetamine dependence pharmacotherapy trials. Receiver operating characteristic (ROC) curve analyses assessed the predictive power of initial abstinence, assessed by thrice weekly urine samples, for EOT abstinence.
Results
Sixty percent of individuals with complete abstinence in the first two weeks of treatment were abstinent at EOT while 18% of people who failed to meet this standard were abstinent at EOT. Early response was related to retention at EOT and 12 month follow-up. Findings suggested that the inability to achieve at least three MA negative screenings in the first two weeks is associated with greater than 90% likelihood of treatment failure. A third week of screening added minimally to the prediction of EOT outcomes. The prediction of treatment failure was more precise than the prediction of treatment success.
Conclusion
The absence of a clinical response in the first two weeks of treatment among participants in the placebo group signals high risk of treatment failure. The vast majority of information regarding response in the placebo group from a12-week trial is obtained in early in the trial.
Keywords: methamphetamine dependence, placebo effects, early response, abstinence, treatment
Introduction
In randomized double-blind placebo-controlled pharmacotherapy trials for substance dependence, the control condition determines the cumulative effect of non-medication salutary factors against which a putative medicine may be compared. Addiction medicine trials typically feature control conditions that include an inert ‘placebo’ pill and an evidence-based behavioral intervention such as cognitive behavioral therapy or contingency management. Thus, responses in the placebo group represent the combined effects of a behavioral treatment, pill-taking effects and other non-specific factors. Although responses of placebo groups have important implications for addiction research methodology and clinical practice, research examining placebo group responses in addiction medicine is virtually non-existent. Relevant data are found for other psychiatric conditions, notably, depression. Walsh, Seidman, Sysko and Gould (2002) documented an association between publication year and placebo group responses in pharmacotherapy trials for major depression whereby more recent trials featured larger placebo effects. The magnitude of placebo group responding is strongly implicated in the failure to detect medication effects (Merlo-Pich, Alexander, Fava, & Gomeni, 2010) and the size of placebo group responses appear to vary across psychiatric disorders (Khan et al., 2005). It is unclear if clinical response in the placebo condition of addiction medicine trials is similar to other psychiatric disorders. In order to harness the positive effects of the placebo response, minimize its negative effects, and develop methodologies optimally suited for medication development, the role of placebo group response warrants additional study.
The present study assessed the timing of clinical response in the placebo condition of three MA dependence pharmacotherapy trials. Within the addiction field, evidence suggests that early responsiveness is critical for distal outcomes for both efficacious medications and for placebo group responses. Plebani, Kampman and Lynch (2009) assessed the predictive value of early abstinence among 407 cocaine dependent patients, most with comorbid alcohol dependence, who received either putative medications or placebo. As there were no observed medication effects, analyses collapsed the medication and placebo groups. Abstinence during the first two weeks of treatment was related to end-of-treatment (EOT) abstinence and to the proportion of cocaine-negative screens. Kenford et al. (1994) similarly identified early responsiveness as critical in smoking cessation pharmacotherapy. The particular importance of early response in placebo groups is suggested by the mechanisms mediating the effects of placebos. Expectancy effects and classical conditioning are the two dominant models explaining placebo effects (Stewart-Williams & Podd, 2004). These effects are likely to be evident at the start of pill-taking behavior and a delayed onset for the effects of expectancies or conditioning effects is unlikely. The present study assesses the hypothesis that a response in the early weeks among placebo participants is critically important for EOT abstinence. The study uses data from three randomized double-blind, placebo controlled trials (12 weeks of treatment) to determine the relationship between abstinence in the first weeks of treatment and abstinence at EOT and follow-up. We used receiver operating characteristic (ROC) analyses to estimate the optimal number of MA-positive urines screens in the early weeks of a clinical trial for predicting EOT outcomes and determined how many weeks of treatment are required to obtain highly precise estimates of EOT outcomes.
Methods
Design
The data analyzed here are from the placebo groups in three randomized, double-blind, placebo-controlled trials of sertraline, bupropion and modafinil. Methodological details are provided in the original reports (Heinzerling et al., 2010; Shoptaw et al., 2008; Shoptaw et al., 2006). Briefly, participants (N=184) had MA dependence verified by the Structured Clinical Interview for DSM-IV-TR and no dependence on alcohol or illicit drugs. Those with serious medical or psychiatric disorders including psychotic and bipolar disorders were excluded, as were individuals with psychopathology requiring pharmacologic or behavioral intervention. A two-week non-treatment screening period was followed by 12 weeks of active treatment. In addition to medication or matching placebo, participants received cognitive behavioral therapy at least weekly and most (N=129; 70%) received abstinence-based contingency management (CM). Fifty-five participants did not receive CM as the sertraline study randomized participants in a four-group design to medication vs. placebo and CM vs. no CM. Participants visited the clinic thrice-weekly to provide urine samples, to undergo monitoring for medication adherence and assessment of participant safety, to complete study measures and to receive behavioral treatments. Urine samples were analyzed onsite for MA metabolites using radioimmunoassay (Phamatech, Inc., San Diego, CA or Branan Medical Corp., Irvine, CA). Research activities were overseen by the Institutional Review Boards of UCLA and Friends Research Institute and the trials were registered with clinicaltrials.gov.
Data Analysis
MA abstinence was defined on the basis of thrice-weekly urine drug screens for MA-metabolites during the first two weeks and final two weeks of treatment. Early abstinence was defined as no MA positive urine drug screens during the first two weeks and no more than 2 of the six possible urine drug screens during the first two weeks missing. EOT abstinence was defined using the same criteria during the final two weeks of treatment. The comparative predictive value of this variable was assessed was assessed in logistic regression models against baseline MA use, educational attainment, and gender – variables evidencing association with outcomes in previous studies. Models also examined if the behavioral condition (CM vs. no CM) moderated the relationship between early treatment response and EOT abstinence. As the sertraline study featured a 12-month follow-up, outcomes at later time points were modeled similarly. Although traditionally used to classify disease states based on a particular diagnostic marker, receiver operating characteristic (ROC) analysis effectively summarizes important information about the relation of early treatment response and EOT outcomes. ROC analysis determined the extent of drug use in the first two weeks of treatment that optimally predicted persistent drug use at EOT, based on the Youden’s (1950) index. Thus, the number of MA positive/missing urine drug screens served as the classification variable with persistent MA use at EOT as the disease state variable. Two additional ROC analyses assessed the predictive accuracy of one week and three weeks of MA screening for EOT outcomes. The area under the curve (AUC) between the three ROC curves was compared to determine the extent to which predictive power increases with each additional week of data. As clinical care often utilizes weekly assessments rather than the thrice-weekly assessments featured in these clinical trials, the predictive power of a single screening in each of the first three weeks was also assessed. AUC comparisons were calculated according to the recommendations of DeLong, DeLong and Clarke-Pearson (1988). Summary statistics including AUC, sensitivity, specificity and positive and negative predictive values characterized different dimensions of predictive validity. Hosmer and Lemeshow (2000) have offered the following guidelines for interpreting AUC: .70–.80 is acceptable discrimination, .80–.90 is excellent and AUCs exceeding .90 are outstanding.
Results
Participants (N=184) had a mean age of 33.7 (SD = 8.9), 36% were female, and were predominantly Caucasian (66%) and Hispanic (30%). The mean number of days of MA use in the month preceding study screening was 14 (SD=10). Sixty-six patients (35.9%) achieved MA abstinence during the first two weeks of treatment. Fifty-six patients (30.4%) achieved MA abstinence in the final two weeks of treatment. EOT abstinence did not differ significantly between men (33% abstinent) and women (26% abstinent). Behavioral treatment condition (CBT plus CM vs. CBT without CM) did not interact with early abstinence and was included as a covariate. A logistic regression model assessed the relationship between early abstinence and EOT abstinence, controlling for gender, age, baseline MA use, behavioral treatment condition and educational attainment. MA abstinence during the first two weeks of treatment was a significant predictor of EOT abstinence conferring an increased odds of abstinence of 5.7 (95% CI = 2.7, 11.9; p<.001). When excluding early abstinence from the equation, higher baseline MA use was associated with lower probability of EOT abstinence, but this variable was no longer significantly associated with EOT abstinence when early abstinence was included in the model. None of the covariates were significantly related to EOT abstinence. Early abstinence was also strongly associated with treatment retention adjusting for covariates (OR=12.4; 95% CI = 5.3, 28.9; p<.001). One of the placebo conditions (N=110) included a one-year follow-up screening. Early abstinence was unrelated to abstinence at the 12-month follow-up, controlling for previous covariates but was associated with study retention at 12 months (OR=2.9; 95% CI = 1.1, 7.4; p<.05).
Analyses evaluated associations between drug use during a two-week baseline screening period, early response, and EOT abstinence. Less frequent MA use during screening associated with both early treatment response (p<.01) and EOT abstinence (p<.01). A logistic regression model examined the comparative predictive power of MA abstinence during the screening period and early treatment response. Inclusion of in-screening use did not improve model fit when adjusting for early treatment response. Models excluding all participants who were completely abstinent during the baseline screening period did not alter the predictive power of early abstinence for EOT abstinence.
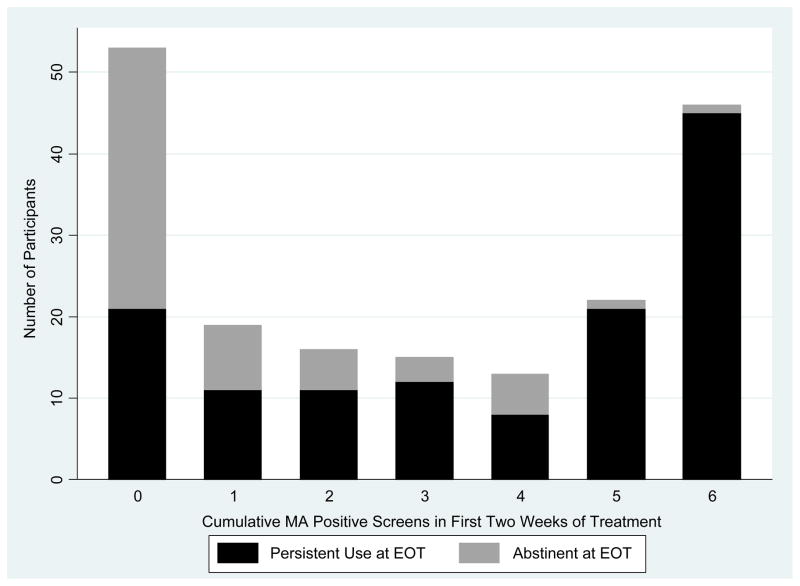
We next identified empirically-derived standards for predicting EOT outcomes by estimating the number of MA positive urine samples that would optimally predict persistent EOT MA use. Figure 1 shows that 60% of individuals with complete abstinence in the first two weeks of treatment were abstinent at EOT while 18% of people who failed to meet this standard were abstinent at EOT. ROC curve analysis showed that three MA positive samples within the first two weeks of treatment optimally predicted persistent EOT MA use. As there were six samples during this period, the ROC result suggested that the inability to achieve at least three MA negative samples is associated with greater than 90% likelihood of treatment failure. Testing whether the addition of CM diluted the relationship between early response and EOT abstinence, the AUC for those in the CM condition (N=129) was .726 and was .837 for those not receiving CM (N=55), estimates that were not statistically distinguishable (p=.15).
Figure 1.
Cumulative Methamphetamine Positive Urine Drug Screens in First Two Weeks of Treatment for Those Abstinent and Persisting in MA Use at end-of-treatment (EOT) among placebo group participants
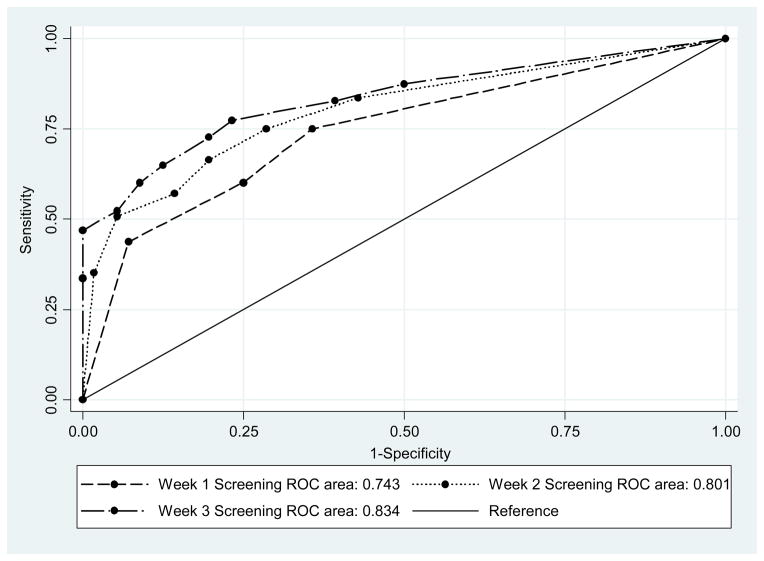
Analyses assessed the comparative predictive power of using urine toxicology results during the first three weeks of treatment to predict EOT. Results showed that for week 1, week 2 and week 3, the AUCs were .743, .801, and .834, respectively, all of which are significantly better than a non-informative variable which has an AUC of 0.5. Data for the optimal cut-points established for the ROC analyses are shown in Table 2. In pairwise comparisons of these three AUCs, each additional week captured valuable predictive information, and the AUCs were each statistically distinguished from the other (p<.01). Although statistically different, the inclusion of week 3 data contributes minimally to the prediction of treatment failures beyond data from the first two weeks. It is relevant that positive predictive values only increased from 88.5% to 89.2% % with the additional third week of data. Although the focus of this paper was on placebo group responses, we conducted an analysis where the predictive power of early abstinence was compared for placebo and active medication groups. Each active medication condition evidenced similar relationships between early abstinence and EOT response.
Table 2.
Comparative Predictive Power 1, 2, and 3 Weeks of Thrice Weekly Screening Data for persistent MA use at EOT*
| Weeks of Screening Data | Optimal Cut-Point (Number MA Positive Screens) | AUC | SN | SP | PPV | NPV |
|---|---|---|---|---|---|---|
| One Week | >=1 | 0.743 | 75 | 64 | 82.8 | 52.9 |
| Two Weeks | >=3 | 0.801 | 66 | 80 | 88.5 | 51.1 |
| Three Weeks | >=4 | 0.834 | 72 | 80 | 89.2 | 55.5 |
ROC analysis assessed number of MA positive/missing urine drug screens to predict MA use at EOT. SN=sensitivity; SP=specificity; PPV=positive predictive value; NPV=negative predictive value; AUC= area under ROC curve
Next, the predictive power of only the first sample of each week was examined – a standard more closely approximating clinical settings. ROC analysis suggested that the failure to produce a single clean sample in the first three weekly visits confers very high risk of treatment failure (positive predictive value=95%). The ability to predict treatment successes was substantially less precise. Having only one positive sample in the first three weeks was associated with a negative predictive value of .52. Analyses with the first two weekly samples suggested that failure to produce at least one clean urine also represented high risk of treatment failure (positive predictive value=91%).
Last, ROC curves for each of the first ten weeks of treatment screening data were compared to determine if EOT outcomes could be precisely predicted with fewer weeks of treatment data. Results suggested that each week of data contributed to predictive efficiency with statistically significant differences observed for all comparisons. For weeks 4 through 10, the AUCs were, respectively, .859, .880. .892, .904, .919, .932, and .945. Of note, by Week 6, the AUC approached .900, suggesting that the vast majority of information about response in the placebo group is captured in the first 6 weeks of a trial.
Discussion
Abstinence in the first two weeks of placebo treatment with behavioral support was associated with EOT abstinence and treatment retention when adjusting for variables potentially related to MA outcomes. For participants where longer-term follow-up was included, early abstinence predicted study retention at 12-month follow-up but was not associated with abstinence at follow-up. While a significant proportion of individuals establishing early abstinence were using MA at EOT, the majority of placebo-treated participants who were abstinent in the first two weeks of treatment were abstinent at EOT. The fact that this variable appears relevant for proximal and distal outcomes suggests that pre-treatment disposition and immediate treatment receptivity is critically important for outcomes. These results cohere with previous findings of pill-taking (placebo or ineffective candidate medications) combined with behavioral support for cocaine dependence where early abstinence predicted later abstinence (Plebani et al., 2009). Similar findings for purely behavioral interventions have also been reported for cocaine dependence (Weinstock, Rash, & Petry, 2010). In the methamphetamine literature, Hillhouse et al. (2007) did not examine early treatment reponse specifically, but found that three consecutive MA-free urine samples during treatment predicted abstinence at EOT, 6-month and 12-month follow-up. We recently examined moderate users of methamphetamine from two clinical trials of bupropion – a subgroup in which the medication has demonstrated preliminary efficacy. Results were consistent with the findings reported here suggesting the possibility that immediate responsiveness may be critically important for a range of addiction treatments (Brensilver, Heinzerling, Swanson, & Shoptaw, 2012).
Although this report did not explicitly examine the mechanisms by which placebo pills combined with behavioral support conferred its benefit, some suggestions are offered. The robustness of early response challenges the assumption that skills developed later in the psychosocial treatment process are critical for good outcomes and affirms previous findings in the behavioral treatment of depression (Ilardi & Craighead, 1994). Instead, present data are more consistent with the interpretation that unmeasured baseline differences presage response in the placebo condition. Immediate treatment response is a marker of participant characteristics predisposing the individual to more favorable outcomes. Previous analyses have highlighted baseline MA use as a predictor of treatment outcome (Dean et al., 2009). However, that behavioral level of explanation does not provide clear pharmacological targets. Pharmacogenetics (Haile, Kosten, & Kosten, 2009) and brain imaging (Wang et al., 2012) hold promise for identifying moderating variables. At present, immediate treatment response efficiently categorizes a sample of putative responders.
These findings have implications for researchers and clinical trial design. In clinical trials for major depressive disorder, placebo group responses are strongly implicated in the failure to detect medication effects (Merlo-Pich, et al., 2010). In addiction medicine trials, participants immediately establishing abstinence may create statistical noise that obscures a medication signal. Trial designs where early non-responders are randomized to a second condition may also have utility in stimulant dependence medication development efforts. Alternatively, a brief placebo lead-in, as is common in trials for depression, or behavioral treatment delivered to the entire sample before randomization could provide an important variable to be included in a stratified randomized design (Bisaga et al., 2005). The present findings suggest that two weeks of placebo pills and behavioral treatment is adequate to identify a subgroup of non-responders. However, substantially more time, perhaps six weeks, would be required to confidently predict the subgroup of responders. The substantial early treatment response in the placebo condition – 36% abstinent for the first two weeks – makes it challenging to detect medication effects during the period when statistical power is greatest. Efforts to understand the role of placebo response for addiction medication signal detection are likely to be important in the development of efficacious treatments. Research designs in addiction medicine might consider strategies implemented in antidepressant medication trials to improve signal detection (Mallinckrodt, Meyers, Prakash, Faries, & Detke, 2007; Mallinckrodt, Tamura, & Tanaka, 2011)
Results were consistent across behavioral treatment conditions suggesting immediate treatment response is similarly important for those receiving CM and CBT as for those receiving CBT only. We expected that CM would weaken the relationship between early response and EOT outcome as the reinforcement might induce abstinence in individuals with marginal capacity for sustained abstinence. This hypothesis was unsupported.
In future trials where medication effects are observed, examining the role of early treatment responsiveness may suggest interesting hypotheses regarding the putative mechanism of action. With an efficacious medication, immediate response will likely still be important as evidenced from findings in nicotine dependence (Kenford et al., 1994) and depression (Szegedi et al., 2009). However, some mid-trial responders after the medication has reached its peak effect are likely. Comparing the timing of onset of abstinence between placebo and an efficacious medication could suggest interesting hypotheses about the putative mechanism of action.
The power of early treatment data to predict treatment failures is substantially better than in the prediction of treatment successes. Robust early response is critically important if success is to be expected. It is important to note that the high positive predictive values (predicting treatment failure) are partially dependent on the high level of treatment failure −70% were classified as EOT failures. Nevertheless, the finding that predicting treatment success is more difficult than predicting treatment failure echoes previous findings (Dean et al., 2009). We found that two weeks of thrice-weekly samples provide enough data to confidently predict treatment failure. For individuals receiving pill placebo, the inability of a patient to produce at least three MA-negative urine samples during the first two weeks of treatment signals the need for a new clinical approach – perhaps increasing the intensity of behavioral support or considering inpatient treatment. As many clinical settings feature weekly meetings, it is relevant that the inability to produce one clean MA urine sample in the first two weeks of treatment is similarly associated with high likelihood of treatment failure.
This study should be interpreted in light of its limitations. The clinical trials sampled MA dependent individuals who may not be representative of the MA dependent population as serious psychiatric disorder and dependence on alcohol or other drugs of abuse were exclusion criteria. Analyses were conducted retrospectively although results cohere closely with previous work on cocaine (Plebani, et al., 2009). An additional limitation stems from the fact that the definition of EOT abstinence is a measure of both retention and MA abstinence as participants who dropped out prematurely are counted as non-abstinent. As a result, the association between achieving early MA abstinence and EOT success may be driven by treatment retention especially if participants expecting an immediate medication effect disproportionately drop out. Efforts to improve retention in MA dependence trials and to investigate medications that are likely to have an immediate effect may address this problem. The present analyses assessed response to pill placebo and the behavioral treatment, which represents the standard combination of interventions in the control condition of addiction medicine trials. However, it is unclear how the present results generalize to pill placebo without behavioral treatment or a purely behavioral treatment condition. Results from the COMBINE study for alcohol dependence suggest that the addition of a placebo pill and medical management to a behavioral treatment improves drinking outcomes and treatment engagement (Weiss, O’Malley, Hosking, LoCastro, & Swift, 2008). Future research should attempt to disambiguate the therapeutic effects of placebo pill taking from the effects of behavioral treatment.
Despite these limitations, these data highlight the importance of early onset of abstinence in placebo pill and behavioral treatment for methamphetamine dependence and suggests methodological considerations relevant to the discovery of efficacy medications.
Figure 2.
ROC Curves for 1-Week, 2-Week and 3-Week of Thrice Weekly Urine Drug Screens for Predicting Persistent Methamphetamine Use at End-of-Treatment
Table 1.
Predictive power of the number of MA-positive thrice weekly urine drug screens during first two weeks of treatment for persistent MA use at end-of-treatment (EOT) among placebo group participants*
| MA Positive Screens | Sensitivity | Specificity | PPV | NPV | LR+ | LR− |
|---|---|---|---|---|---|---|
| =6 | 35.2 | 98.2 | 98 | 39 | 19.69 | 0.66 |
| >=5 | 50.8 | 94.6 | 96 | 46 | 9.48 | 0.52 |
| >=4 | 57.0 | 85.7 | 90 | 47 | 3.99 | 0.50 |
| >=3 | 66.4 | 80.4 | 89 | 51 | 3.38 | 0.42 |
| >=2 | 75.0 | 71.4 | 86 | 56 | 2.63 | 0.35 |
| >=1 | 83.6 | 57.1 | 82 | 60 | 1.95 | 0.29 |
ROC analysis assessed number of MA positive/missing urine drug screens to predict MA use at EOT. Bolded is optimal Youden (1950) predictor; PPV=positive predictive value; NPV=negative predictive value; LR+ = positive likelihood ratio; LR− = negative likelihood ratio
Acknowledgments
This work was supported by grants from the National Institute on Drug Abuse at the National Institutes of Health (P50 DA18185, T32 DA026400). The National Institute on Drug Abuse had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report or in the decision to submit the paper for publication.
The authors wish to thank the research staff for their careful work collecting the trial data.
Footnotes
All authors have contributed meaningfully to this research and approve the submission of the manuscript.
All authors declare no conflict of interest.
References
- Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, Nunes EV. Utility of lead-in period in cocaine dependence pharmacotherapy trials. Drug and Alcohol Dependence. 2005;77:7–11. doi: 10.1016/j.drugalcdep.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Brensilver M, Heinzerling KG, Swanson AN, Shoptaw SJ. A retrospective analysis of two randomized trials of bupropion for methamphetamine dependence: Suggested guidelines for treatment discontinuation/augmentation. Drug and Alcohol Dependence. 2012 doi: 10.1016/j.drugalcdep.2012.03.027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AC, London ED, Sugar CA, Kitchen CM, Swanson AN, Heinzerling KG, Shoptaw S. Predicting adherence to treatment for methamphetamine dependence from neuropsychological and drug use variables. Drug and Alcohol Dependence. 2009;105:48–55. doi: 10.1016/j.drugalcdep.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- Haile CN, Kosten TR, Kosten TA. Pharmacogenetic treatments for drug addiction: cocaine, amphetamine and methamphetamine. The American Journal of Drug and Alcohol Abuse. 2009;35:161–177. doi: 10.1080/00952990902825447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzerling KG, Swanson AN, Kim S, Cederblom L, Moe A, Ling W, Shoptaw S. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug and Alcohol Dependence. 2010;109:20–29. doi: 10.1016/j.drugalcdep.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse MP, Marinelli-Casey P, Gonzales R, Ang A, Rawson RA the Methamphetamine Treatment Project Corporate A. Predicting in-treatment performance and post-treatment outcomes in methamphetamine users. Addiction. 2007;102:84–95. doi: 10.1111/j.1360-0443.2007.01768.x. [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S. Applied logistic regression. Vol. 354. Wiley-Interscience; 2000. [Google Scholar]
- Ilardi SS, Craighead WE. The Role of Nonspecific Factors in Cognitive-Behavior Therapy for Depression. Clinical Psychology: Science and Practice. 1994;1:138–155. [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. JAMA: The Journal of the American Medical Association. 1994;271:589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- Khan A, Kolts RL, Rapaport MH, Rama Krishnan KR, Brodhead AE, Brown WA. Magnitude of placebo response and drug-placebo differences across psychiatric disorders. Psychological Medicine. 2005;35:743–749. doi: 10.1017/s0033291704003873. [DOI] [PubMed] [Google Scholar]
- Mallinckrodt CH, Meyers AL, Prakash A, Faries DE, Detke MJ. Simple options for improving signal detection in antidepressant clinical trials. Psychopharmacology bulletin. 2007;40:101–114. [PubMed] [Google Scholar]
- Mallinckrodt CH, Tamura RN, Tanaka Y. Recent developments in improving signal detection and reducing placebo response in psychiatric clinical trials. Journal of Psychiatric Research. 2011;45:1202–1207. doi: 10.1016/j.jpsychires.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Merlo-Pich E, Alexander R, Fava M, Gomeni R. A New Population-Enrichment Strategy to Improve Efficiency of Placebo-Controlled Clinical Trials of Antidepressant Drugs. Clinical Pharmacology & Therapeutics. 2010;88:634–642. doi: 10.1038/clpt.2010.159. [DOI] [PubMed] [Google Scholar]
- Plebani JG, Kampman KM, Lynch KG. Early abstinence in cocaine pharmacotherapy trials predicts successful treatment outcomes. Journal of Substance Abuse Treatment. 2009;37:313–317. doi: 10.1016/j.jsat.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson AN, Ling W. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug and Alcohol Dependence. 2008;96:222–232. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Huber A, Peck J, Yang X, Liu J, Jeff D, Ling W. Randomized, placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug and Alcohol Dependence. 2006;85:12–18. doi: 10.1016/j.drugalcdep.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Stewart-Williams S, Podd J. The placebo effect: dissolving the expectancy versus conditioning debate. Psychological Bulletin. 2004;130:324–340. doi: 10.1037/0033-2909.130.2.324. [DOI] [PubMed] [Google Scholar]
- Szegedi A, Jansen WT, Van Willigenburg A, van der Meulen E, Stassen HH, Thase ME. Early improvement in the first 2 weeks as a predictor of treatment outcome in patients with major depressive disorder: a meta-analysis including 6562 patients. The Journal of clinical psychiatry. 2009;70:344–353. doi: 10.4088/jcp.07m03780. [DOI] [PubMed] [Google Scholar]
- Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression. JAMA: The Journal of the American Medical Association. 2002;287:1840–1847. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- Wang G, Smith L, Volkow N, Telang F, Logan J, Tomasi D, Alia-Klein N. Decreased dopamine activity predicts relapse in methamphetamine abusers. Molecular Psychiatry. 2012 doi: 10.1038/mp.2011.86. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock J, Rash CJ, Petry NM. Contingency management for cocaine use in methadone maintenance patients: When does abstinence happen? Psychology of Addictive Behaviors. 2010;24:282–291. doi: 10.1037/a0017542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, O’Malley SS, Hosking JD, LoCastro JS, Swift R. Do patients with alcohol dependence respond to placebo? Results from the COMBINE Study. Journal of studies on alcohol and drugs. 2008;69:878–884. doi: 10.15288/jsad.2008.69.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youden W. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]