Abstract
Migraine is three times as common in females as in males, and attacks may be more severe and difficult to treat in women. However, no study specifically addressed possible gender differences in response to antimigraine therapy. The objective of this study was to review the efficacy of frovatriptan vs. other triptans, in the acute treatment of migraine in subgroups of subjects classified according to gender (men vs. women) through a pooled analysis of three individual randomized Italian studies. 414 patients suffering from migraine with or without aura were randomized to frovatriptan 2.5 mg or rizatriptan 10 mg (study 1), frovatriptan 2.5 mg or zolmitriptan 2.5 mg (study 2), frovatriptan 2.5 mg or almotriptan 12.5 mg (study 3). All studies had a multicenter, randomized, double-blind, crossover design. After treating 1–3 episodes of migraine in no more than 3 months with the first treatment, patients switched to the other treatment for the next 3 months. In this analysis, traditional migraine endpoints were compared between the 66 men and 280 women of the intent-to-treat population. At baseline, long-term and debilitating migraine attacks were more frequently reported by women than men. During the observation period, the proportion of pain-free attacks at 2 h did not significantly differ between frovatriptan and the comparators in either men (32 vs. 38 %, p = NS) or women (30 vs. 33 %, p = NS). Pain relief was also similar between treatments for both genders (men: 56 % frovatriptan vs. 57 % comparators; women: 55 vs. 57 %; p = NS for both). The rate of relapse was significantly lower with frovatriptan than with the comparators in men (24 h: 10 vs. 30 %; 48 h: 21 vs. 39 %; p < 0.05) as well as in women (24 h: 14 vs. 23 %; 48 h: 28 vs. 40 %; p < 0.05). The rate of adverse drug reactions was significantly larger with comparators, irrespectively of gender. Although migraine presents in a more severe form in women, frovatriptan seems to retain its good efficacy and favorable sustained antimigraine effect regardless of the gender.
Keywords: Migraine, Gender, Frovatriptan, Rizatriptan, Zolmitriptan, Almotriptan
Introduction
Migraine is a chronic neurovascular disorder occurring in both genders, although large surveys show higher prevalence of this condition in women, with a female to male ratio in the order of 3:1 [1–4].
Although migraine acknowledges a complex pathophysiology, involving genetic and psychological factors [5], the disproportionate number of fertile women with migraine suggests that hormonal factors may indeed play an important role in the pathogenesis of migraine [6]. As a matter of fact, from adolescence migraine attacks are generally more common in women than in men, peaking during their 30 and 40 s, followed by a decline, particularly after menopause [7].This difference in migraine prevalence over a life time is mediated by the physiological fluctuation of estrogen level and consequently by its influence on cerebral vasculature, in women [8]. In addition, attacks are usually reported to be more severe and difficult to treat in women than in men [8].
The triptans, selective serotonin 5-HT1B/1D receptor agonists, are very effective acute migraine drugs; they are currently recommended as a first-line treatment for moderate to severe migraine, or for mild to moderate migraine that has not responded to adequate doses of simple analgesics [9–11]. Frovatriptan is an antimigraine agent of the triptan class, developed to provide a triptan with a clinical potential for a long duration of action and a low likelihood of side effects and drug interactions [12, 13]. Three direct comparative, prospective, double-blind, randomized, crossover studies have recently compared the efficacy and safety of frovatriptan with that of rizatriptan [14], zolmitriptan [15], and almotriptan [16]. The study showed a similar efficacy of the four triptans in the immediate treatment of migraine, but lower recurrence rates, and thus a better sustained relief, with frovatriptan. Retrospective analyses of the same studies proved the good efficacy of frovatriptan also in subgroups of female migraine patients, such as those with menstrually related migraine [17] or with oral-contraceptive menstrual migraine [18].
Although there is no reason to doubt that current drug options for migraine treatment should display a similar efficacy in male and female migraineurs, so far no study specifically addressed possible gender differences in response to triptan therapy. To this purpose, in the present paper, we report on results of a pooled analysis performed in subgroups of migraineurs classified according to gender (males vs. females) and enrolled in previous direct comparative studies of frovatriptan vs. other triptans.
Methods
Study population and design
This pooled analysis is based on the data from three studies sharing a similar design and whose details are extensively reported in the original publications [14–16]. Overall, the studies included subjects of both genders, aged 18–65 years, with a current history of migraine with or without aura, according to International Headache Society (IHS) criteria, and with at least one, but no more than six migraine attacks per month for 6 months prior to entering the study. In this retrospective analysis, patients were classified in two subgroups according to gender (men and women).
The studies had a multicenter, randomized, double-blind, crossover design. Each patient received frovatriptan 2.5 mg or rizatriptan 10 mg [14], frovatriptan 2.5 mg or zolmitriptan 2.5 mg [15], frovatriptan 2.5 mg or almotriptan 12.5 mg [16] in a balanced computer-generated randomized sequence (1:1), where frovatriptan had to be followed by the comparator or vice versa. After treating 1–3 episodes of migraine in no more than 3 months with the first treatment, the patient had to switch to the other treatment. Subjects were encouraged to treat 1–3 attacks for a maximum period of 3 months with each study drug and to visit the center three times during the study. Subjects having no migraine episodes during one of the two observation periods were excluded from the study.
Data analysis
In this analysis, traditional migraine endpoints were compared between men and women of the intent-to-treat population, defined as all patients treating at least one attack in each treatment period. The study endpoints were qualified according to International Headache Society Guidelines [19] as: (a) the number of pain free episodes at 2 h (absence of migraine episodes at 2 h after the intake of one dose of study drug and without any rescue medication); (b) the number of pain relief episodes at 2 h (defined as a decrease in migraine intensity from severe or moderate to mild or none, after the intake of one study drug dose); (c) relapse after 24 h (namely an episode which is pain free at 2 h and headache of any severity returns within 24 h, or requires the use of rescue medication or a second dose of study drug); (d) relapse after 48 h (namely an episode which is pain free at 2 h and headache of any severity returns within 48 h, or requires the use of rescue medication or a second dose of study drug). Safety analysis was applied to the intent-to-treat population, by calculating the incidence of adverse events during the study. Continuous variables were summarized by computing average values and standard deviation (SD), while categorical variables by computing the absolute value and the frequency (as percentage). Study endpoints were separately assessed according to gender (men vs. women) and compared between attacks treated with frovatriptan and with the comparators by analysis of variance (ANOVA), in case of continuous variables, and by Chi-squared test, in case of discrete variables. A subgroup analysis was carried out in postmenopausal and fertile women. All tests were two-sided and the level of statistical significance was kept at 0.05 throughout the whole study.
Results
Demographic and migraine feature of the study population
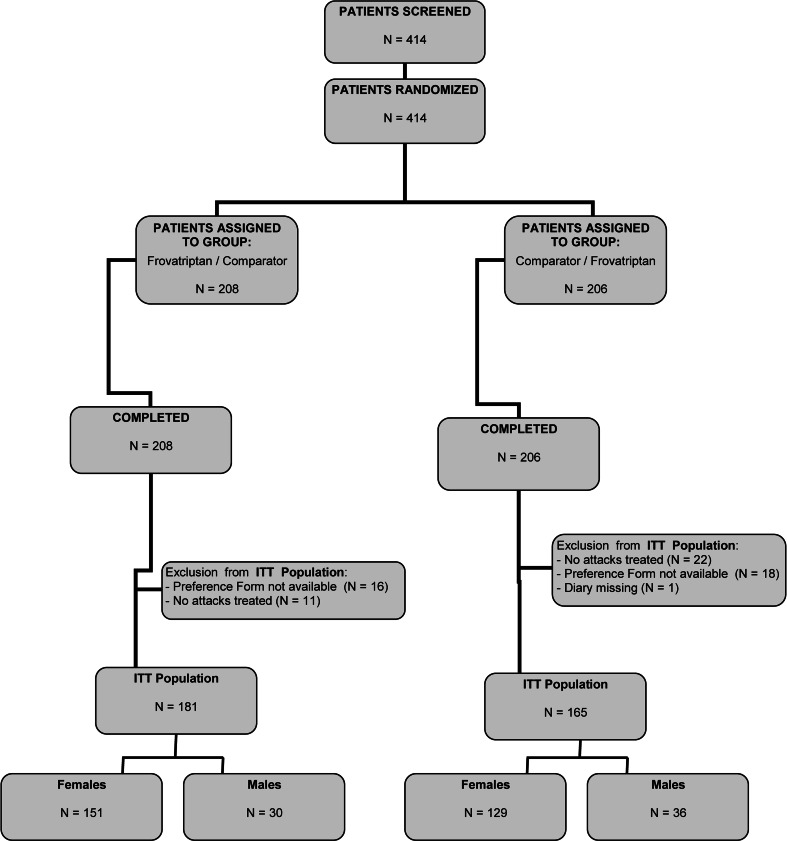
The intent-to-treat population consisted of 346 subjects, of which 66 (19 %) were men and 280 (81 %) women. A flow diagram of participants throughout the study is summarized in Fig. 1. Table 1 summarizes the main demographic and clinical characteristics of the intent-to-treat population at baseline, according to gender. The two study subgroups differed in several features at baseline, and particularly in those related to migraine severity. Women were younger, thinner and shorter than men. They reported their first attack earlier than men and the episodes were longer lasting and more debilitating (higher MIDAS score). Baseline intensity showed a statistically significant difference in the distribution of mild-moderate attacks between men and women, with attacks in women being more intense than in men.
Fig. 1.
Flow diagram of the patients throughout the study
Table 1.
Demographic and clinical data of women and men and of postmenopausal and fertile women of the intent-to-treat population at the time of randomization
| Men (n = 66) | Women (n = 280) | p value | Postmenopausal women (n = 56) | Fertile women (n = 224) | p value | |
|---|---|---|---|---|---|---|
| Age (years, mean ± SD) | 40 ± 10 | 38 ± 6 | <0.05 | 52 ± 5 | 34 ± 8 | <0.0001 |
| Height (cm, mean ± SD) | 178 ± 7 | 163 ± 7 | <0.0001 | 161 ± 8 | 164 ± 5 | <0.001 |
| Weight (kg, mean ± SD) | 78 ± 13 | 59 ± 10 | <0.0001 | 60 ± 10 | 59 ± 10 | NS |
| BMI (kg/m2, mean ± SD) | 25 ± 3 | 22 ± 4 | <0.0001 | 23 ± 4 | 22 ± 4 | <0.05 |
| Age at onset of migraine (years, mean ± SD) | 20 ± 10 | 18 ± 7 | <0.05 | 22 ± 11 | 17 ± 6 | <0.01 |
| Migraine attack duration >2 days (n, %) | 4 (6) | 68 (24) | <0.01 | 16 (29) | 52 (23) | NS |
| Migraine attacks with aura (n, %) | 32 (5) | 102 (8) | NS | 39 (11) | 67 (5) | <0.0001 |
| MIDAS score (mean ± SD) | 19 ± 15 | 23 ± 18 | <0.05 | 25 ± 15 | 22 ± 19 | NS |
| Baseline migraine severity (n, %)a | ||||||
| Mild | 106 (28) | 303 (19) | <0.001 | 68 (17) | 255 (21) | NS |
| Moderate | 192 (51) | 954 (60) | 249 (62) | 698 (58) | ||
| Severe | 82 (22) | 346 (22) | 85 (21) | 248 (21) | ||
| No use of triptans in the previous 3 months (n, %) | 18 (27) | 118 (42) | NS | 22 (39) | 96 (43) | NS |
Data are shown as mean (±SD), or absolute (n) and relative frequency (%)
BMI body mass index, MIDAS migraine disability assessment
aNumbers refer to number and frequency of attacks as respect to overall number of attacks
Of the 280 women, 56 were postmenopause and 224 in fertile age. As expected, postmenopausal women were older than fertile women (Table 1). They also reported a higher rate of migraine attacks with aura and an older age at onset of the first migraine attack.
Treatment efficacy of migraine attacks
During the observation period, a total of 1,978 attacks were recorded in the 346 migraineurs of the intent-to-treat population. Of these attacks, 331 (17 %) occurred in men and 1,647 (83 %) in women, 987 were treated with frovatriptan and 991 with comparators.
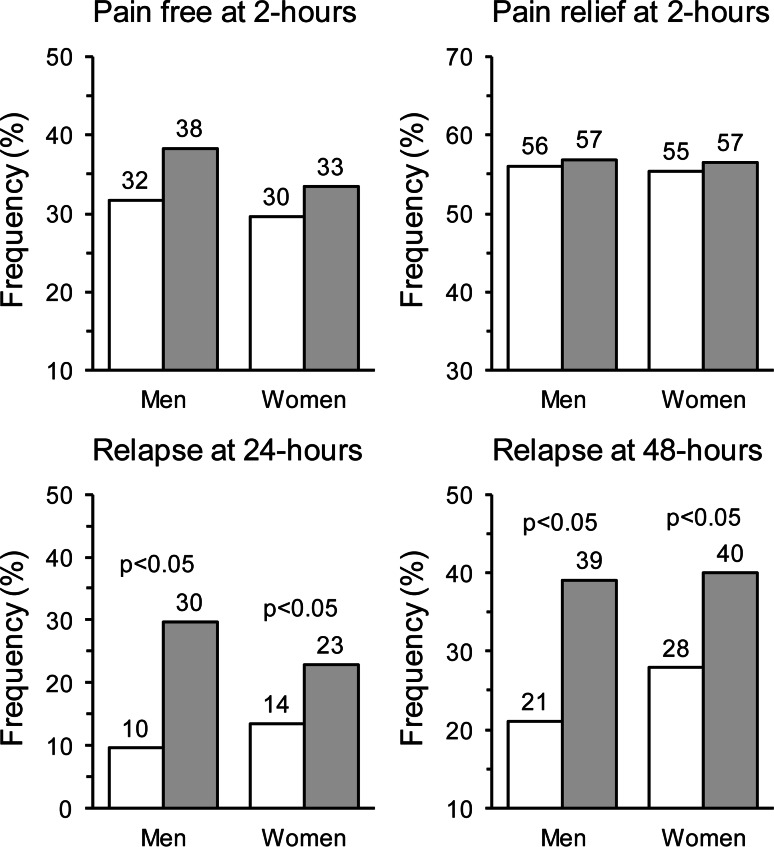
As shown in Fig. 2, at 2-h rate of pain-free episodes was not significantly different between frovatriptan and comparators, either in men (32 vs. 38 %; p = NS) or women (30 vs. 33 %; p = NS). Pain relief episodes at 2 h were also similarly distributed between the two treatments and both genders (men: 56 % frovatriptan vs. 57 % comparators; women: 55 vs. 57 %; p = NS for both). Conversely, relapse at 24 h was significantly (p < 0.05) less likely to be reported in frovatriptan than in comparator-treated patients, with no between-gender difference (men: 10 vs. 30 %; women: 14 vs. 23 %). This was the case also for rate of relapse after 48 h (men: 21 vs. 39 % and women: 28 vs. 40 %, p < 0.05 between treatments).
Fig. 2.
Proportion (%) of pain free at 2 h, pain relief at 2 h and relapse at 24 and 48 h in the 66 men and 280 women with migraine of the intent-to-treat population. Data are separately shown for frovatriptan- (white bars) and comparator-treated patients (gray bars). The p value refers to the statistical significance of the between-treatment difference
No statistically significant differences were ever observed between men and women in response to study treatments, for all the considered endpoints.
The efficacy of frovatriptan and comparators on pain free and pain relief at 2 h was identical in the subgroup of postmenopausal and fertile women (Table 2). However, the risk of relapse at 24 and 48 h was higher in women treated with the comparators, with a statistically significant between-treatment difference for women of fertile age.
Table 2.
Study endpoints in men and subgroups of postmenopausal and fertile women of the intent-to-treat population treated with frovatriptan or the comparators
| Men (n = 66) | Postmenopausal women (n = 56) | Fertile women (n = 224) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Frovatriptan | Comparators | p value | Frovatriptan | Comparators | p value | Frovatriptan | Comparators | p value | |
| Pain free at 2 h (%) | 52/164 (32) | 64/167 (38) | NS | 56/173 (32) | 64/180 (36) | NS | 188/650 (29) | 211/644 (33) | NS |
| Pain relief at 2 h (%) | 62/123 (56) | 70/123 (57) | NS | 76/137 (56) | 82/148 (55) | NS | 294/531 (55) | 300/528 (57) | NS |
| Relapse at 24 h (%) | 5/52 (10) | 19/64 (30) | <0.05 | 9/56 (16) | 18/64 (28) | NS | 24/188 (13) | 45/211 (21) | <0.05 |
| Relapse at 48 h (%) | 11/52 (21) | 25/64 (39) | <0.05 | 20/56 (36) | 24/64 (38) | NS | 48/188 (26) | 86/211 (41) | <0.01 |
Data are shown as absolute values (number of attacks with the event and total number of attacks evaluated) and relative frequencies (%)
Safety
A total of 133 adverse events were recorded in 2,033 treated attacks in the safety population (1,088 under frovatriptan and 945 under the other triptans), of which 55 occurred in men and 78 in women. The proportion of subjects with an adverse event was significantly (p < 0.05) lower with frovatriptan than with the comparators in both men (2 vs. 7 %) or in women (6 vs. 9 %).
Discussion
In the present pooled analysis of three double-blind, randomized, crossover studies, acute treatment of male and female migraineurs with frovatriptan and with other triptans (rizatriptan, zolmitriptan and almotriptan) resulted in similar proportions of pain-free and pain relief episodes at 2 h, with no between-gender differences. However, frovatriptan showed a more sustained effect on relief of migraine symptoms than comparators, an effect that did not differ between men and women and such a finding is clinically relevant because it has been shown that female patients have a twofold greater risk than male patients of experiencing headache return following pain-free response [20]. The particular pharmacokinetics of frovatriptan, characterizing its slower onset of action and longer duration as compared to other triptans, may explain its greater efficacy in preventing headache recurrence [12].
From adolescence women experience more frequent, long-lasting and more painful headaches as compared to men, and are potentially less responsive to specific migraine treatment than men [4, 7]. For instance, in a study of risk factors for headache recurrence after oral and subcutaneous sumatriptan, recurrence following oral sumatriptan was more frequent in female patients [21]. In our retrospective analysis, we showed for the first time that no difference in response to triptan treatment exists between the two genders. Notably, such a lack of difference occurred even despite the fact that women presented at enrolment with more severe migraine symptoms than men.
Our results obtained in women also confirm other retrospective analyses performed on the same pooled study sample. In 187 of the 346 women who treated at least one episode of menstrually related migraine, rate of recurrence was significantly lower with frovatriptan than with comparators, either after 24 or 48 h [17]. Frovatriptan showed a more sustained relieving effect on migraine, with lower headache relapses over 24 h and even more so over 48 h, also in a subgroup of 35 women with oral-contraceptive-induced menstrual migraine [18].
Our study also provides some additional matters for discussion. We replicated the evidence from previous large observational studies that the prevalence of migraine is much higher in women than in men, and that in the female gender attacks tend to present in a more severe and debilitating fashion than in men [1–4, 7]. The effect of frovatriptan on relapses was more consistent when the subgroup of fertile women was considered, suggesting that this more numerous group of migraine patients may particularly benefit from frovatriptan treatment. Notably, the tolerability profile of frovatriptan was better than that of the comparators, irrespective of the gender, adding a further positive feature to the good efficacy pattern of the tested drug.
Despite the interesting results, we must recognize the limitation of the post hoc nature of our analysis: we performed a retrospective analysis on a subgroup of patients which were not originally selected for a gender-related study. However, to our knowledge, our report stands as the first large comparative systematic analysis of head-to-head trials of frovatriptan vs. other triptans in male and female migraineurs. In addition, we did not evaluate plasma levels of the different triptans, which act as substrates for different enzymes (see Table 3): some of these enzymes (e.g. CYP3A4 and CYP2D6) are differently expressed in men and women [22] suggesting that plasma levels of the various types of triptans may differ in the two sexes [23–30]. We think that all these limitations of our study are at least partially contrasted by the large number of subjects and migraine attacks included in the analysis.
Table 3.
Enzymes involved in triptan metabolism, reported in order of importance
| Triptan | Enzyme involved in the metabolism |
|---|---|
| Almotriptan [23, 24] | MAO-A, CYP3A4, CYP2D6 |
| Frovatriptan [25, 26] | CYP1A2 |
| Rizatriptan [27, 28] | MAO-A |
| Zolmitriptan [29, 30] | CYP1A2, MAO-A |
MAO monoamine oxidase, CYP3A4 cytochrome P3A4, CYP2D6 cytochrome P2D6, CYP1A2 cytochrome P1A2
In conclusion, the results of our combined analysis of individual data of three double-blind, randomized, crossover trials provide strong evidence that, in both men and women, frovatriptan seems to offer the advantage of a lower risk of recurrence as compared to other triptans. Our results might be helpful to stimulate the design and implementation of larger direct comparative randomized clinical trials evaluating triptan efficacy separately in men and women suffering from migraine.
Acknowledgments
The present study was supported by Istituto Lusofarmaco d’Italia S.p.A.. Authors gratefully thank Dr. Stephen Pawsey of Vernalis Ltd. for his valuable suggestions which helped to improve the quality of the manuscript.
Conflict of interest
All authors have occasionally served as scientific consultants for manufacturers of frovatriptan, rizatriptan, zolmitriptan or almotriptan. Deborha Pezzola and Dario Zava are employees of the manufacturer of frovatriptan.
References
- 1.Rasmussen BK, Jensen R, Schroll M, Olesen J. Epidemiology of headache in a general population—a prevalence study. J Clin Epidemiol. 1991;44:1147–1157. doi: 10.1016/0895-4356(91)90147-2. [DOI] [PubMed] [Google Scholar]
- 2.Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology. 1999;53:537–542. doi: 10.1212/WNL.53.3.537. [DOI] [PubMed] [Google Scholar]
- 3.Lipton Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 4.Stewart WF, Wood C, Reed ML, Roy J, Lipton RB; AMPP Advisory Group Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28:1170–1178. doi: 10.1111/j.1468-2982.2008.01666.x. [DOI] [PubMed] [Google Scholar]
- 5.Peterlin LB, Calhoun AH, Balzac F. Men, women and migraine: the role of sex, hormones, obesity and PTSD. J Fam Pract. 2012;61(4):7–11. [PubMed] [Google Scholar]
- 6.Martin VT, Lipton RB. Epidemiology and biology of menstrual migraine. Headache. 2008;48(3):S124–S130. doi: 10.1111/j.1526-4610.2008.01310.x. [DOI] [PubMed] [Google Scholar]
- 7.MacGregor EA, Rosenberg JD, Kurth T. Sex-related differences in epidemiological and clinical-based headache studies. Headache. 2011;51:843–859. doi: 10.1111/j.1526-4610.2011.01904.x. [DOI] [PubMed] [Google Scholar]
- 8.Brandes JL. The influence of estrogen on migraine: a systematic review. JAMA. 2006;295:1824–1830. doi: 10.1001/jama.295.15.1824. [DOI] [PubMed] [Google Scholar]
- 9.Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754–762. doi: 10.1212/WNL.55.6.754. [DOI] [PubMed] [Google Scholar]
- 10.Evers S, Afra J, Frese A, Goadsby PJ, Linde M, May A, Sándor PS. European Federation of Neurological Societies. EFNS guideline on the drug treatment of migraine—revised report of an EFNS task force. Eur J Neurol. 2009;16:968–981. doi: 10.1111/j.1468-1331.2009.02748.x. [DOI] [PubMed] [Google Scholar]
- 11.Worthington I, Pringsheim T, Gawel MJ, Canadian Headache Society Acute Migraine Treatment Guideline Development Group et al. Canadian Headache Society Guideline: acute drug therapy for migraine headache. Can J Neurol Sci. 2013;40(5 (Suppl 3)):S1–S80. [PubMed] [Google Scholar]
- 12.Kelman L. Review of frovatriptan in the treatment of migraine. Neuropsychiatr Dis. 2008;4:49–54. doi: 10.2147/NDT.S1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanford M. Frovatriptan: a review of its use in the acute treatment of migraine. CNS Drugs. 2012;26:791–811. doi: 10.2165/11209380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Savi L, Omboni S, Lisotto C, Zanchin G, Ferrari MD, Zava D, Pinessi L. A double-blind, randomized, multicenter, Italian study of frovatriptan versus rizatriptan for the acute treatment of migraine. J Headache Pain. 2011;12:219–226. doi: 10.1007/s10194-010-0243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tullo V, Allais G, Ferrari MD, Curone M, Mea E, Omboni S, Benedetto C, Zava D, Bussone G. Frovatriptan versus zolmitriptan for the acute treatment of migraine: a double-blind, randomized, multicenter. Italian study. Neurol Sci. 2010;31(1):S51–S54. doi: 10.1007/s10072-010-0273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartolini M, Giamberardino MA, Lisotto C, Martelletti P, Moscato D, Panascia B, Savi L, Pini LA, Sances G, Santoro P, Zanchin G, Omboni S, Ferrari MD, Brighina F, Fierro B. A double-blind, randomized, multicenter, Italian study of frovatriptan versus almotriptan for the acute treatment of migraine. J Headache Pain. 2011;12:361–368. doi: 10.1007/s10194-011-0325-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allais G, Tullo V, Omboni S, Benedetto C, Sances G, Zava D, Ferrari MD, Bussone G. Efficacy of frovatriptan versus other triptans in the acute treatment of menstrual migraine: pooled analysis of three double-blind, randomized, crossover, multicenter studies. Neurol Sci. 2012;33(1):S65–S69. doi: 10.1007/s10072-012-1044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allais G, Tullo V, Omboni S, Pezzola D, Zava D, Benedetto C, Bussone G. Frovatriptan vs. other triptans for the acute treatment of oral contraceptive-induced menstrual migraine: pooled analysis of three double-blind, randomized, crossover, multicenter studies. Neurol Sci. 2013;34(1):S83–S86. doi: 10.1007/s10072-013-1393-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Headache Classification Subcommittee of the International Headache Society The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 20.Sheftell F, Almas M, Weeks R, Mathew NT, Pitman V, Lipton RB. Quantifying the return of headache in triptan-treated migraineurs: an observational study. Cephalalgia. 2010;30:838–846. doi: 10.1177/0333102409354390. [DOI] [PubMed] [Google Scholar]
- 21.Visser WH, de Vriend RH, Jaspers NH, Ferrari MD. Sumatriptan-nonresponders: a survey in 366 migraine patients. Headache. 1996;36:471–475. doi: 10.1046/j.1526-4610.1996.3608471.x. [DOI] [PubMed] [Google Scholar]
- 22.Franconi F, Brunelleschi S, Steardo L, Cuomo V. Gender differences in drug responses. Pharmacol Res. 2007;55:81–95. doi: 10.1016/j.phrs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 23.McEnroe JD, Fleishaker JC. Clinical pharmacokinetics of almotriptan, a serotonin 5-HT(1B/1D) receptor agonist for the treatment of migraine. Clin Pharmacokinet. 2005;44:237–246. doi: 10.2165/00003088-200544030-00002. [DOI] [PubMed] [Google Scholar]
- 24.Salva M, Jansat JM, Martinez-Tobed A, Palacios JM. Identification of the human liver enzymes involved in the metabolism of the antimigraine agent almotriptan. Drug Metab Dispos. 2003;31:404–411. doi: 10.1124/dmd.31.4.404. [DOI] [PubMed] [Google Scholar]
- 25.Balbisi EA. Frovatriptan: a review of pharmacology, pharmacokinetics and clinical potential in the treatment of menstrual migraine. Ther Clin Risk Manag. 2006;2:303–308. doi: 10.2147/tcrm.2006.2.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Negro A, Lionetto L, Casolla B, Lala N, Simmaco M, Martelletti P. Pharmacokinetic evaluation of frovatriptan. Expert Opin Drug Metab Toxicol. 2011;7:1449–1458. doi: 10.1517/17425255.2011.622265. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg MR, Sciberras D, De Smet M, Lowry R, Tomasko L, Lee Y, Olah TV, Zhao J, Vyas KP, Halpin R, Kari PH, James I. Influence of beta-adrenoceptor antagonists on the pharmacokinetics of rizatriptan, a 5-HT1B/1D agonist: differential effects of propranolol, nadolol and metoprolol. Br J Clin Pharmacol. 2001;52:69–76. doi: 10.1046/j.0306-5251.2001.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sternieri E, Coccia CP, Pinetti D, Ferrari A. Pharmacokinetics and interactions of headache medications, part I: introduction, pharmacokinetics, metabolism and acute treatments. Expert Opin Drug Metab Toxicol. 2006;2:961–979. doi: 10.1517/17425255.2.6.961. [DOI] [PubMed] [Google Scholar]
- 29.Lionetto L, Casolla B, Mastropietri F, D’Alonzo L, Negro A, Simmaco M, Martelletti P. Pharmacokinetic evaluation of zolmitriptan for the treatment of migraines. Expert Opin Drug Metab Toxicol. 2012;8:1043–1050. doi: 10.1517/17425255.2012.701618. [DOI] [PubMed] [Google Scholar]
- 30.Kalanuria AA, Peterlin BL. A review of the pharmacokinetics, pharmacodynamics and efficacy of zolmitriptan in the acute abortive treatment of migraine. Clin Med: Thera. 2009;1:397–413. [Google Scholar]