Abstract
Precisely controlling the protein-nanomaterial interactions at selective sites is crucial in engineering biomolecule composite architectures with tailored nanostructures and functions for a variety of biomedical applications. This strategy, however, is only beginning to be explored. Here, we demonstrate the facet-specific assembly of proteins, such as albumin, immunoglobulin and protamine, on {100} facets of SrTiO3 polyhedral nanocrystals, while none on {110} facets. Molecular dynamics simulations indicate the immobile surface hydration layer might play a barrier role to effectively prevent proteins adsorption on specific {110} facets. This work thus provides new insights into the fundamentally understanding of protein-nanomaterial interactions, and open a novel, general and facile route to control the selective adsorption of various proteins on various nanocrystals.
Over the past decade, the use of nanoparticles in biological applications, such as drug deliver1,2, biosensing3,4, as well as medical imaging, has been experienced an explosion of scientific interest because of their intrinsic size- and facet-dependent properties as well as functional properties3,4,5. The small size of nanoparticles confers them high specific surface areas and penetrability through many biological pathways resulting in high interaction with biological structures. When nanoparticels enter a biological fluid (for example blood, human plasma or interstitial fluid), a process of non-specific adsorption of protein onto the surface of nanoparticles immediately occurs, thus forming a nanoparticle-protein corona, which affects how nanoparticles are internalized by cells and cleared from the body6. Therefore, rational design the nanoparticles can offer tremendous opportunities in terms of engineering the nanoparticle-protein interactions7. For example, monolayer-protected metal nanoparticles, with ligand shell, can regulate cell-membrane penetration by preventing non-specific adsorption of proteins8,9. Polyethylene oxide-functionalized carbon nanotube-based biosensors have been used for selective recognition of target proteins10. In recent years, considerable efforts have been put into understanding the distribution of reacted sites for protein adsorption11,12,13. However, how to precisely and selectively engineer protein to a specific site is still a challenge.
Some progresses for site-selective adsorption of protein on flat substrate have been reached by using traditional photolithographic processes14, which are not applicable to the nonplanar objects and challenging in scaling down the objects to submicrometer size scale15. Researchers have shown that size-related surface curvature and protein structure influence protein orientation on silica nanoparticles16. Bimetallic nanowires composed of gold and nickel segments functionalized with alkanethiols with terminal hexa(ethylene glycol) groups(EG6) and palmitic acid, a 16-carbon fatty acid, respectively, have exhibited different fluorescence behavior when exposed to a fluorescently tagged protein. Intense fluorescence was only observed on the nickel segment of these wires. However, the localization of two functionalities on different regions of the nanowire cannot be easily mimicked with spherical nanoparticles17. Moreover, additional functionality means additional synthetic steps and costs, more complicated behavior and effects in vivo18. The spatial selective adsorption of protein on specific surfaces of a non-functional single nanoparticle has yet to be demonstrated. Nanocrystals with well-controlled shapes and selectively exposed facets could be a model system to better understand the mechanisms of nanoparticle-cell interactions.
Here we demonstrate the selective adsorption of proteins, such as bovine serum albumin (BSA), porcine immunoglobulin G (IgG) and salmine, on {100} facets of SrTiO3 polyhedral nanocrystals, while none on {110} facets.
Results and discussions
Shape-controlled SrTiO3 polyhedral nanocrystals are synthesized by using titanium tetrachloride (TiCl4) aqueous solution and strontium chloride (SrCl2) as the titanium precursor and strontium source, respectively, as well as 1,3-propanediol as a surfactant. Figure 1 presents scanning electron microscopy (SEM) images and the corresponding drawings of the SrTiO3 polyhedral nanocrystals synthesized by varying 1,3-propanediol concentrations (Supplementary Figure S1 shows low-magnification SEM images). By progressively increasing the concentration of 1,3-propanediol added to the reaction mixture solution (from 0 to 0.2 M, 0.6 M and 1 M), a systematic shape evolution from cube to truncated rhombic dodecahedron is successfully achieved. We consider that the mechanism behind these results can be explained by “kinetic and thermodynamic modified Wulff constructions” developed by Ringe, E. et al.19. The cube is bounded by six identical {100} facets while the truncated rhombic dodecahedron is bounded by six square {100} facets and twelve hexagonal {110} facets, which are confirmed by transmission electron microscopy (TEM) and selected-area electron diffraction (SAED) analysis (Supplementary Figure S2). The average particle sizes for the cube and truncated rhombic dodecahedron are 238 nm, 228 nm, 183 nm and 177 nm with relative standard deviations of 11%, 12%, 8% and 7%, respectively (Supplementary Figure S1).
Figure 1. Shape-controlled synthesis of SrTiO3 nanocrystals.
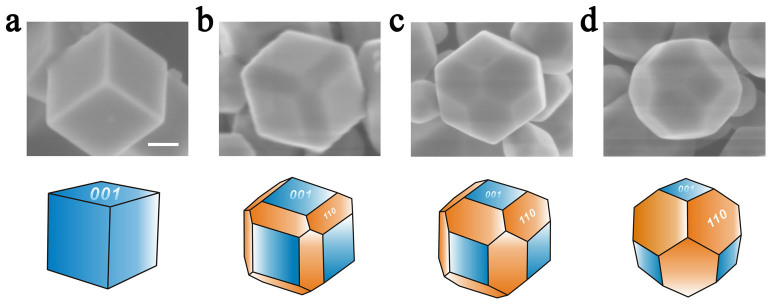
SEM images and the corresponding schematic drawings of SrTiO3 nanocrystals synthesized with morphology evolution from cube to truncated rhombic dodecahedron upon the increasing addition of 1,3-propanediol from (a) 0 ml to (b) 1 ml, (c) 3 ml and (d) 5 ml. Scale bar, 100 nm.
Supplementary Figure S3 shows X-ray diffraction (XRD) spectra of the four samples. The resulted diffraction patterns match very well with the crystal structure of cubic SrTiO3 (JCPDS No. 73-0661). Interestingly, a close examination shows that the increase of ratio of diffraction peak intensity I(220)/I(200) from 0.31 for the cube to 0.37, 0.43, and 0.47 for the series of truncated rhombic dodecahedra, indicating the increasing of the exposed {110} facets ratio20,21.
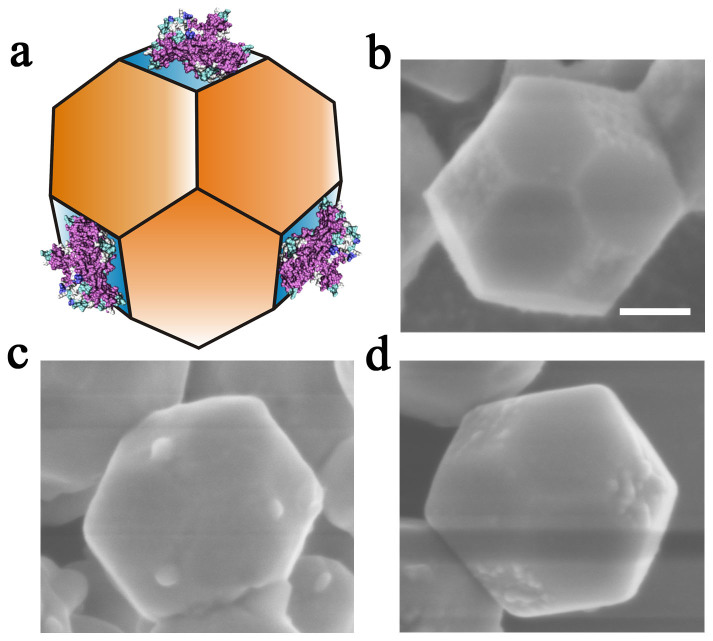
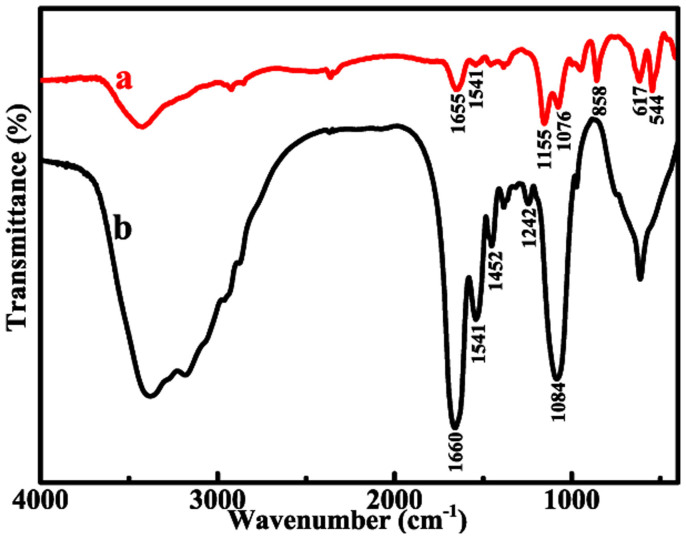
To study the adsorption behavior of proteins on SrTiO3 polyhedral nanocrystals, we put truncated rhombic dodecahedral SrTiO3 nanocrystals, which contained both {100} and {110} facets, into protein solution. Figure 2 shows SEM images of typical SrTiO3 truncated rhombic dodecahedra after treated by proteins, respectively. Surprisingly, all the proteins achieve high packing density on the {100} facets of truncated rhombic dodecahedra, while {110} facets adsorb nothing. To determine the adsorption of protein on nanocrystals, we compare the concentration of protein before/after adsorption by measuring the optical density (OD) values. In comparison of A and B in Supplementary Figure S4, the constant of OD values of the protein solution without addition of nanocrystals before/after centrifugation indicates that protein can not be centrifuged down. In contrast, the OD values of the protein solution (the supernatant after centrifugation) with addition of nanocrystals significantly decrease, which suggest the adsorption of protein on nanocrystals. Evidence for the adsorption of salmine on SrTiO3 nanocrystals is also obtained by Fourier transform infrared (FTIR) experiments. As shown in Figure 3b, the adsorption bands appeared at 1660 cm−1 (C = O stretching vibration) and 1541 cm−1 (N-H bending vibration mainly, coupled to C = O and C = C stretching) of salmine are characteristic amide I and amide II bands, respectively. The band at 1452 cm−1 can be attributed to CH2 and CH3 groups. The band at 1242 cm−1 is consistent with the canonical values for β-sheet conformation22,23. And the intense band at 1084 cm−1 can be attributed to the arginine of salmine24. By comparison with the spectra of salmine after adsorption on SrTiO3 nanocrystals (Figure 3a), the shift of amide I adsorption band to 1655 cm−1 (α-helices), which is also attributed to the carbonyl stretching frequency to interaction with the surface25, and the diminishing of the bands at 1452 cm−1 and 1242 cm−1 as well as the increase in the ratios of amide I/amide II, suggest a strong interaction of salmine with SrTiO3 nanocrystal and thus the conformational change of protein structure26. Furthermore, two apparent bands at 1155 cm−1 and 1076 cm−1 in Figure 3a, which can be assigned to asymmetrical and symmetrical stretching vibration of S = O, respectively, also reflect the interaction between salmine and SrTiO3 nanocrystal surface. The bands at 858 cm−1, 617 cm−1 (also can be assigned to amide IV of protein) and 544 cm−1, which are associated to the stretching of Ti-O-Ti of SrTiO3 nanocrystal27, are also observed in Figure 3a. We further investigate the facet-specific adsorption behavior of protein on SrTiO3 polyhedral nanocrystals by using high angle annular dark field scanning transmission electron microscopy (HAADF-STEM). The increased first then decreased signal of U and N elements of line-scan energy dispersive X-ray spectroscopy (EDS) profiles at the interfaces further verify the facet-selective adsorption of proteins on the {100} facets, while the {110} facets absorbed nothing, (see Supplementary Figure S5). The distinct proteins adsorption selectivity on various facets of SrTiO3 polyhedral nanocrystals indicates that the facet-selectivity could be a general route to control various proteins on various nanocrystals.
Figure 2. Schematic illustration and SEM images of truncated rhombic dodecahedra SrTiO3 nanocrystals after adsorption of proteins.
(a) schematic illustration of the selective adsorption of proteins on SrTiO3 {100} facets, (b) bovine serum albumin (BSA), (c) porcine immunoglobulin G (IgG) and (d) salmine. Scale bar, 100 nm.
Figure 3. FT-IR spectra of SrTiO3 nanocrystals after adsorption of salmine (a) and salmine itself (b).
According to the previous investigations of protein-nanoparticle adsorption, some factors, such as surface atomic structure, chemisorbed functional groups and adsorption conditions, affect the adsorption behavior28,29,30. Among these factors, functionalization of nanoparticles with organic molecules, such as poly(ethylene glycol) (PEG), is a popular way to reduce protein adsorption31,32. In this work, 1,3-propanediol, an alcohol molecules, is used during synthesis process. Infrared spectrum analysis shows that the final products have no organic residuals after calcination process. Therefore, the influence of alcohol molecules on protein-nanocrystal interaction could be ruled out. Additionally, the selective adsorption of proteins on the {100} facets of SrTiO3 nanocrystals is almost not affected by other protein treatment conditions, such as temperature and solvent, based on the comparative experiments carried out at different temperature and using water as the solvent, respectively.
Since the above mentioned factors are not responsible for the distinct protein adsorption selectivity on {100} and {110} facets of SrTiO3 polyhedral nanocrystals, it is reasonable for us to point the origin of the selectivity is the facet-selective protein adsorption, which is related to intrinsic difference of the surface atomic structures of the {100} and {110} facets of SrTiO3. Chiu et al. reported that facet-selective binding peptides as regulating agents for the synthesis of Pt nanocrystals with selectively exposed facets33. When conjugated with a-chymotrypsin (ChT), the estimated surface coverage of Au nanocube with {100} facets is 1–2 times more than that of Au nanooctahedra with {111} facets34. These previous reports have large difference from the present results that the protein fully cover {100} facets of SrTiO3 nanocrystals, but none for {110} facets. Furthermore, fundamental understanding of protein-nanoparticle interaction mechanism at the molecular-level is largely incomplete and under considerable debate. Our Molecular Dynamic (MD) simulations of BSA adsorption on {100} and {110} facets of SrTiO3 only predict that former has higher protein coverage probability than the latter, which does not describe the fact of no protein-coverage on {110} facets. Some researchers have suggested that the formation of structured or tightly bound water at the interface may play a critical role in determining surfaces for their ability of adsorption of proteins from solution because the interfacial water molecules provide a physical barrier preventing direct contact between protein and surface35,36,37,38. Theoretical modeling for the structure of interfacial water molecules onto surfaces also helps better understanding of protein adsorption behavior39,40,41. To gain insight into the facet-selective adsorption of proteins onto the facets of SrTiO3 nanocrystals, we carried out atomistic MD simulations to explore the structures of the interfacial water molecules onto the {100} and {110} facets of SrTiO3.
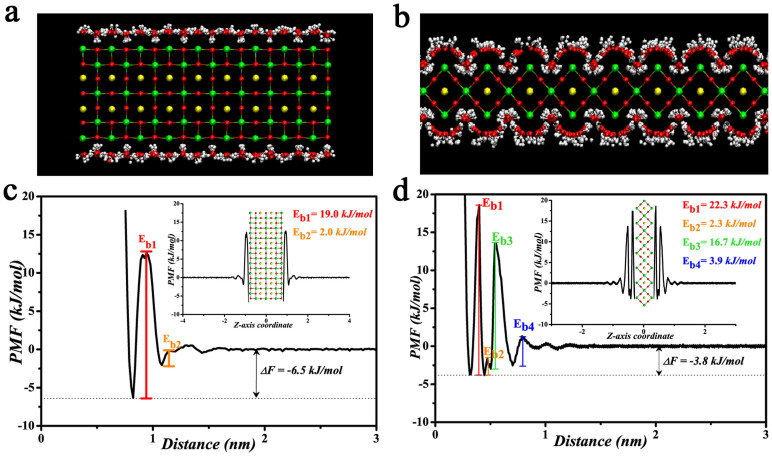
Figure 4a, b show the interfacial water structures on the {100} and {110} facets of SrTiO3 according to the trajectory of the last nanosecond in each system. The interfacial water structures are highly influenced by the underlying nanocrystal's atomic structures. For the {100} facet, a random arrangement of interfacial water molecules on the surface is observed, as shown in Figure 4a. On the other hand, for {110} facet, water molecules are capable of entering the lattice structure of SrTiO3 by occupying the Sr atomic vacancies with highly ordered orientation and forming a water “shell” on the {110} facet of SrTiO3, as illustrated in Figure 4b. The distinct difference in the behavior of the SrTiO3 water interface can be attributed to the difference of surface atomic structure of the {100} and {110} facets. Interestingly, the water structures with highly ordered orientation are tightly bounded on {110} facet, i.e. forming a stable surface hydration layer, which is supported by the potential of mean force (PMF) for stability calculations for water molecules transferring from SrTiO3 surface into bulk water (Figure 4c, d). The calculation results show that the free energy (ΔF) for bulk water molecules transferring to the surface of {100} facet (−6.5 kJ/mol) is comparable to that of {110} facet (−3.8 kJ/mol), which suggests that thermodynamical stability of the water layers on the {100} and {110} facets has no significant difference. Surprisingly, the energy barriers (Eb) relevant to the {100} and {110} facets exhibit great difference. There are four energy barriers for the water molecules transferring from SrTiO3 {110} facet into bulk water and the barrier height (Eb1, 22.3 kJ/mol; Eb2, 2.3 kJ/mol; Eb3, 16.7 kJ/mol; Eb4, 3.9 kJ/mol) is remarkably higher than that from {100} facet (Eb1, 19.0 kJ/mol; Eb2, 2.0 kJ/mol) (Figure 4c, d), which indicates that the interfacial water structures on the {110} facet is far more still than that on the {100} facet. According to the experimental observations by Chou et al.42 and the theoretical calculations by Jiang et al.43, the immobile surface hydration layer plays a role of barrier to effectively prevent protein adsorption, which can explain much stronger protein adsorption affinity for the {100} facet in our experimental results, selective protein adsorption on the {100} facet, but none on the {110} facet. The MD simulations provide detailed insights into the role of the interfacial water in determining the protein adsorption behavior.
Figure 4. MD simulations of the interfacial water structures on two facets and their calculated potential mean force (PMF).
(a) {100} facet, (b) {110} facet, (c), (d) the corresponding PMF. The insets show the full profiles.
Conclusions
In summary, the proteins, such as bovine serum albumin (BSA), porcine immunoglobulin G (IgG) and salmine, attain high packing density on the {100} facets of truncated rhombic dodecahedra, while {110} facets adsorb nothing. The distinctly different proteins adsorption behavior between the {100} and {110} facets of SrTiO3 might be attributed to the distinctly different behavior of water molecules-binding properties of these two facets. Molecular dynamics (MD) simulations indicate the immobile surface hydration layer might play a role of barrier to effectively prevent protein adsorption on specific {110} facet. The distinct proteins adsorption selectively on the facets of SrTiO3 polyhedral nanocrystals indicates that the facet-selectivity could be a general route to control other proteins on the other nanocrystals.
Methods
Synthesis of SrTiO3 nanocrystals
In a typical synthesis, 0.265 ml of TiCl4 (aladdin, 99%) solution is dropwised into 25 ml of the 1,3-propanediol solution that is cooled in an ice bath. After stirring for 5 min, 30 ml of 3 M LiOH (aladdin, 98%)solution and 10 ml of 0.24 M SrCl2 (aladdin, 99.5%) solution are added subsequently. After stirring for another 30 min, the resulting solution is transferred to a Teflon-lined autoclave and heat at 180°C for 48 h. After the reaction, the resulted precipitate is centrifuged and rinsed using water and ethanol, then dried at 70°C for 12 h. Finally, the dried samples are heated at 600°C to remove organic residue.
Adsorption of proteins on SrTiO3 nanocrystals
We selected simple cubic perovskite oxide SrTiO3 nanocrystals as model, since its surface atomic-level and electronic structure has attracted continuing interest44,45, and thus can facilitate our understanding the facet-dependent protein adsorption behavior. To investigate the adsorption of proteins to the facets of SrTiO3 nanocrystals, 1 ml of 5 mg/ml SrTiO3 nanocrystals solution is added to 5 ml 1 g/l protein solution in phosphate buffered saline (PBS, pH 7.4) at 37°C for 12 h. The collected sample is centrifuged and then dried at ambient air.
MD simulations of the interfacial water structures on {100} and {110} facets
Considering that the atomic surface structures of SrTiO3 nanocrystals are synthesis dependent46, we use spherical aberration (Cs) corrected TEM to determine the surface atomic structures of SrTiO3 (001) and (110) facets (see Supplementary Figure S6). The results indicate that the surface compositions of the atomic layer of (100) and (110) surfaces are TiO2 double-layer46 and a Sr deficient TiO layer47, respectively. A 20-ns MD simulation is performed for each SrTiO3 facet in water molecules system. The facets modeled slabs are set parallel to the x-y plane. Each system is immersed in a rectangular box. The box dimensions in the x and y directions fit the crystal parameters of the facets, thus creating infinite slabs in the x-y plane40. The boxes are filled with water molecules and the facets are frozen during the simulations. All simulations are performed in the canonical (NVT) ensemble with the Gromacs program package48. The SrTiO3 parameters are supplemented, in which the Lennard-Jones parameters of Sr, Ti and O atoms are obtained from fitting to the Buckingham potentials (Supplementary Table S1)49. For the water molecules, the SPC/E (extended simple point charge model) model is employed50. The particle mesh Ewald method is used to describe the long-range electrostatic interactions with a cutoff of 12 Å51. Lennard-Jones interactions are truncated between 10 Å and 11 Å with a smooth switching of the potential. The temperature is maintained at 310 K by using a Nose-Hoover thermostat52. A time step of 2 fs is used and the trajectories are saved every 10 ps for analysis. To calculate the potential of mean force (PMF) of water molecules, the probability distribution of water molecules (approximately represented by the oxygen atoms) g(ξ) along z-axis direction is calculated using Eq. (1): g(ξ) = <ρ(ξ)>/<ρ>, where <ρ(ξ)> is the local density of the water molecules at the z-axis coordinate ξ, and <ρ> is the bulk density of water molecules. Then PMF(ξ) is calculated based on g(ξ) using Eq.(2): PMF(ξ) = −RT ln g(ξ).
Author Contributions
W.H., W.W. and L.D. conceived and designed the experiments. L.D. and K.C. carried out the experiments. L.D., K.C., W.W. and W.H. analyzed the data. Q.L., H.S. and Q.W. performed theoretical simulations. L.D., Q.L. and W.H. wrote the manuscript. All authors commented on the manuscript.
Supplementary Material
SUPPORTING INFORMATION-Facet-Specific Assembly of Proteins on SrTiO3 Polyhedral Nanocrystals
Acknowledgments
This work was supported by the National Basic Research Program of China (973 project, 2012CB933600), the National Natural Science Foundation of China (Grant No. 51072178, 51272228, 81071258 and 21273200). W.H. thanks the support from the Project of the Ningbo 3315 International Team. L.D acknowledges the contribution of Dr. Minmin Mao and Dr. Zhenju Shen of the Center of Electron Micoscope, Zhejiang University, for their assistance in characterization and discussion of SrTiO3 surface atomic structure.
References
- Goldberg M. S. et al. Nanoparticle-mediated delivery of sirna targeting parp1 extends survival of mice bearing tumors derived from brca1-deficient ovarian cancer cells. Proc. Natl Acad. Sci. USA 108, 745–750 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan B. et al. Gold nanoparticle platforms as drug and biomacromolecule delivery systems. J. Controlled Release 148, 122–127 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. et al. Antibiofouling polymer-coated gold nanoparticles as a contrast agent for in vivo x-ray computed tomography imaging. J. Am. Chem. Soc. 129, 7661–7665 (2007). [DOI] [PubMed] [Google Scholar]
- von Maltzahn G. et al. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 69, 3892–3900 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.-H. et al. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 8, 331–336 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nel A. E. et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 8, 543–557 (2009). [DOI] [PubMed] [Google Scholar]
- Gagner J. E. et al. Engineering nanomaterials for biomedical applications requires understanding the nano-bio interface: A perspective. J. Phys. Chem. lett. 3, 3149–3158 (2012). [DOI] [PubMed] [Google Scholar]
- Verma A. et al. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat. Mater. 7, 588–595 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. M. et al. Spontaneous assembly of subnanometre-ordered domains in the ligand shell of monolayer-protected nanoparticles. Nat. Mater. 3, 330–336 (2004). [DOI] [PubMed] [Google Scholar]
- Chen R. J. et al. Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc. Natl Acad. Sci. USA 100, 4984–4989 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakem I. F. et al. Understanding ligand distributions in modified particle and particlelike systems. J. Am. Chem. Soc. 132, 16593–16598 (2010). [DOI] [PubMed] [Google Scholar]
- Mullen D. G. et al. A quantitative assessment of nanoparticle-ligand distributions: Implications for targeted drug and imaging delivery in dendrimer conjugates. Acs Nano 4, 657–670 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meder F. et al. Controlling protein-particle adsorption by surface tailoring colloidal alumina particles with sulfonate groups. Acta Biomater. 9, 5780–5787 (2013). [DOI] [PubMed] [Google Scholar]
- Wong L. S. et al. Selective covalent protein immobilization: Strategies and applications. Chem. Rev. 109, 4025–4053 (2009). [DOI] [PubMed] [Google Scholar]
- Chen H.-Y. et al. Colloids with high-definition surface structures. Proc. Natl Acad. Sci. USA 104, 11173–11178 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava S. et al. Position-specific chemical modification and quantitative proteomics disclose protein orientation adsorbed on silica nanoparticles. Nano. Lett. 12, 1583–1587 (2012). [DOI] [PubMed] [Google Scholar]
- Birenbaum N. S. et al. Selective noncovalent adsorption of protein to bifunctional metallic nanowire surfaces. Langmuir 19, 9580–9582 (2003). [Google Scholar]
- Cheng Z. et al. Multifunctional nanoparticles: Cost versus benefit of adding targeting and imaging capabilities. Science 338, 903–910 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringe E. et al. Kinetic and thermodynamic modified wulff constructions for twinned nanopaticles. J. Phys. Chem. C 117, 15859–15870 (2013). [Google Scholar]
- Huang W.-C. et al. Synthesis of CuO2 nanocrystals from cubic to rhombic dodecahedral structures and their comparative photocatalytic activity. J. Am. Chem. Soc. 134, 1261–1267 (2012). [DOI] [PubMed] [Google Scholar]
- Huang M. H. et al. Facet-dependent properties of polyhedral nanocrystals. Chem. Commun. 50, 1634–1644 (2014). [DOI] [PubMed] [Google Scholar]
- Susi H. et al. Infrared spectroscopy-Conformation. Methods Enzymol. 26, 445–472 (1972). [DOI] [PubMed] [Google Scholar]
- Miyazawa T. et al. Infrared spectra of polypeptides in various conformations: amide I and II bands. J. Am. Chem. Soc. 83, 712–719 (1961). [Google Scholar]
- Awotwe-Otoo D. et al. Physicochemical Characterization of Complex Drug Substances: Evaluation of Structural Similarities and Differences of Protamine Sulfate from Various Sources. AAPS. J. 14, 619–626 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey B. W. et al. The conformation of adsorbed blood proteins by infrared bound fraction measurements. J. Coll. Inferf. Sci. 46, 152–164 (1974). [Google Scholar]
- McClellan S. J. & Franses E. I. Adsorption of bovine serum albumin at solid/aqueous interfaces. Colloid Surf. A-Physicochem. Eng. Asp. 260, 265–275 (2005). [Google Scholar]
- Jiang Y. J. et al. Preparation of protamine-titania microcapsules through synergy between layer-by-layer assembly and biomimetic mineralization. Adv. Funct. Mater. 19, 150–156 (2009). [Google Scholar]
- Mahmoudi M. et al. Protein-nanoparticle interactions: Opportunities and challenges. Chem. Rev. 111, 5610–5637 (2011). [DOI] [PubMed] [Google Scholar]
- Lynch I. & Dawson K. A. Protein-nanoparticle interactions. Nano Today 3, 40–47 (2008). [Google Scholar]
- Shemetov A. A. et al. Molecular interaction of proteins and peptides with nanoparticles. Acs Nano 6, 4585–4602 (2012). [DOI] [PubMed] [Google Scholar]
- Zheng M. et al. Ethylene glycol monolayer protected nanoparticles for eliminating nonspecific binding with biological molecules. J. Am. Chem. Soc. 125, 7790–7791 (2003). [DOI] [PubMed] [Google Scholar]
- Larson T. A. et al. Preventing protein adsorption and macrophage uptake of gold nanoparticles via a hydrophobic shield. Acs Nano 6, 9182–9190 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C.-Y. et al. Platinum nanocrystals selectively shaped using facet-specific peptide sequences. Nat. Chem. 3, 393–399 (2011). [DOI] [PubMed] [Google Scholar]
- Gagner J. E. et al. Effect of gold nanoparticle structure on the conformation and function of adsorbed proteins. Biomaterials 33, 8503–8516 (2012). [DOI] [PubMed] [Google Scholar]
- Chapman R. G. et al. Surveying for surfaces that resist the adsorption of proteins. J. Am. Chem. Soc. 122, 8303–8304 (2000). [Google Scholar]
- Raschke T. M. Water structure and interactions with protein surfaces. Curr. Opin. Struct. Biol. 16, 152–159 (2006). [DOI] [PubMed] [Google Scholar]
- Kane R. S. et al. Kosmotropes form the basis of protein-resistant surfaces. Langmuir 19, 2388–2391 (2003). [Google Scholar]
- Kitano H. et al. Structure of water in the vicinity of phospholipid analogue copolymers as studied by vibrational spectroscopy. Langmuir 19, 10260–10266 (2003). [Google Scholar]
- Israelachvili J. & Wennerstrom H. Role of hydration and water structure in biological and colloidal interactions. Nature 379, 219–225 (1996). [DOI] [PubMed] [Google Scholar]
- Kang Y. et al. On the mechanism of protein adsorption onto hydroxylated and nonhydroxylated TiO2 surfaces. J. Phys. Chem. C 114, 14496–14502 (2010). [Google Scholar]
- Skelton A. A. et al. Interplay of sequence, conformation, and binding at the peptide-titania interface as mediated by water. ACS Appl. Mater. Interfaces 1, 1482–1491 (2009). [DOI] [PubMed] [Google Scholar]
- Leung B. O. et al. Role of interfacial water on protein adsorption at cross-linked polyethylene oxide interfaces. Langmuir 28, 5724–5728 (2012). [DOI] [PubMed] [Google Scholar]
- He Y. et al. Origin of repulsive force and structure/dynamics of interfacial water in oeg-protein interactions: A molecular simulation study. Phys. Chem. Chem. Phys. 10, 5539–5544 (2008). [DOI] [PubMed] [Google Scholar]
- Enterkin J. A. et al. A homologous series of structure on the surface of SrTiO3 (110). Nat. Mater. 9, 245–248 (2010). [DOI] [PubMed] [Google Scholar]
- Kienzle D. M. et al. Surface transmission electron diffraction for SrTiO3 surfaces. CrystEngComm 14, 7833–7839 (2012). [Google Scholar]
- Lin Y. Y. et al. Synthesis-dependent atomic surface structure of oxide nanoparticles. Phys. Rev. Lett. 111, 156101 (2013). [DOI] [PubMed] [Google Scholar]
- Biswas A. et al. Universal ti-rich termination of atomically flat SrTiO3 (001), (110), and (111) surfaces. Appl. Phys. Lett. 98, 051904 (2011). [Google Scholar]
- Van der Spoel D. et al. Gromacs: Fast, flexible, and free. J. Comput. Chem. 26, 1701–1718 (2005). [DOI] [PubMed] [Google Scholar]
- Wohlwend J. L. et al. Molecular dynamics simulations of SrTiO3 thin-film growth from cluster deposition. J. Phys.- Condens. Matter 22, (2010). [DOI] [PubMed] [Google Scholar]
- Berendsen H. J. C. et al. Interaction models for water in relation to protein hydration. In: Intermolecular forces. D. Reidel Publishing Company Dordrecht 331–342 (1981). [Google Scholar]
- Darden T. et al. Particle mesh ewald - an n.Log(n) method for ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993). [Google Scholar]
- Nose S. A unified formulation of the constant temperature molecular-dynamics methods. J. Chem. Phys. 81, 511–519 (1984). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION-Facet-Specific Assembly of Proteins on SrTiO3 Polyhedral Nanocrystals