Abstract
BACKGROUND:
Tobacco smoking by pregnant women is a major public health hazard with both short- and long-term effects on offspring. This study describes the presence and level of the nicotine metabolite cotinine in newborn dried blood spots (DBS) and compares it with the reported maternal smoking recorded on state birth registries. We hypothesize that cotinine in DBS may be a useful measure of newborn in utero tobacco exposure.
METHODS:
An observational, cross-sectional study of 1414 DBS obtained from California, Michigan, New York, and Washington newborn screening programs was carried out. Cotinine levels in DBS were quantified by liquid chromatography tandem mass spectrometry analysis and compared with maternal smoking as reported in vital statistics data.
RESULTS:
Cotinine ≥0.3 ng/g was detected in 35% of newborn DBS, including DBS of 29% of newborns whose mothers reportedly did not smoke cigarettes during pregnancy, some of whom were presumably exposed to environmental tobacco smoke. Twelve percent of the newborn DBS had cotinine levels that were ≥9.0 ng/g (equivalent to 6 ng/mL plasma, a level that indicates active smoking of the mother), although 41% of the mothers of these infants reportedly did not smoke.
CONCLUSIONS:
These data confirm that reported smoking during pregnancy is an imperfect measure of prenatal tobacco smoke exposure. Cotinine assessment in newborns may improve surveillance of tobacco use during pregnancy.
Keywords: cotinine, dried blood spot testing, newborn
What’s Known on This Subject:
Cotinine assays for dried blood spots have been developed but not deployed in a large sample of newborn specimens.
What This Study Adds:
Cotinine levels consistent with active maternal smoking were detectable in 12% of newborn blood spots, although 41% of the mothers reportedly did not smoke. Data confirm that reported smoking during pregnancy is an imperfect measure of prenatal tobacco smoke exposure.
Although the prevalence of smoking by pregnant women declined during the 1980s and 1990s,1,2 this proportion stabilized at ∼9% in the most recent decade.3 Thus, prenatal tobacco smoke exposure (TSE) through active maternal smoking remains a major public health hazard with both short- and long-term effects on offspring, including depressed birth weight,4 birth defects,5 neurobehavioral dysregulation,6 and asthma.7
Cotinine, the primary metabolite of nicotine, is the gold standard biomarker of tobacco exposure.8,9 However, the correlation of cotinine with cigarettes per day is moderate and depends on the genetic, environmental, and behavior characteristics of the population studied.10–13 Cotinine may be measured in plasma, saliva, hair, and meconium; however, none of these materials are systematically collected on a population-wide basis. In contrast, dried blood spots (DBS) are obtained from the vast majority of US newborn infants between 24 and 48 hours of life for testing of inborn errors of metabolism.14 Newborn DBS present an untested opportunity for both surveillance of prenatal TSE and interventions to reduce postnatal tobacco exposure.
Having first shown on a small scale that cotinine is detectable in DBS of newborn infants whose mothers smoked during pregnancy,15 we recently developed a quantitative high-throughput liquid chromatography tandem mass spectrometry method of analysis.16 In our study of both active and passive smokers, the correlation of plasma cotinine with DBS cotinine was >0.9, supporting the use of DBS cotinine as a reliable measure of TSE. To characterize DBS cotinine as a measure of prenatal TSE, we compared cotinine levels assayed in 1414 anonymous DBS to maternal smoking data as reported to birth registries.
Methods
We conducted a cross-sectional study using newborn DBS obtained from 4 state newborn screening programs (California, Michigan, New York, and Washington). DBS were requested from randomly selected singleton newborns from 2007 to 2010 for a total of 1416 subjects; 2 were subsequently excluded for having been collected past 7 days after birth. To ascertain disparities in prenatal TSE, we requested an equal number of DBS from African American and white newborns. Race was determined from birth registry data, and for the purposes of this study, newborns were eligible only if both parents were reported as African American or both parents were reported as white. DBS were linked to each state’s birth registry to produce a limited data set; the variables obtained were gender, race, birth weight, gestational age, month and year of DBS collection, age at DBS collection, and all available smoking data. For 3 states, the latter included the number of cigarettes consumed per day (CPD) in the 3 months before pregnancy and in each trimester, whereas in Michigan, the data were binary (ie, any or no use during pregnancy). This study was approved by the University of Minnesota Human Research Protection Program and those of the participating state departments of health.
Cotinine Analysis
Cotinine in DBS was quantified by our recently developed method.16 Using that method, we previously reported that the DBS cotinine levels for both smokers and individuals exposed to secondhand tobacco smoke correlate well with plasma cotinine concentrations (r = 0.99). The mean ratio of cotinine (ng/g) in DBS to cotinine in plasma (ng/mL) was 1.49. Briefly, the method, carried out in a 96-well-plate format, was as follows: 3 punches (4.8-mm diameter) were obtained from each DBS, weighed, and cotinine extracted. The extracts were applied to solid-phase extraction columns, and the cotinine eluted from the column was analyzed by liquid chromatography tandem mass spectrometry. Standards and positive and negative controls were included as described previously. The limit of detection was 0.3 ng/g DBS (equivalent to 0.2 ng/mL plasma).
Statistical Analysis
The number and percent of subjects with detectable cotinine ≥0.3 ng/g were first described and compared using Fisher’s exact test or the χ2 test. Nonparametric methods including the Wilcoxon rank-sum test, Kruskal-Wallis test, and Spearman coefficient evaluated the amount of cotinine in DBS by each covariate. Values below the level of detection were assigned the lowest rank. Cotinine values at or above the detectable level were summarized by the median and range. After univariate analysis, we performed multivariate regression using the Tobit model,17 which is a parametric model for left-censored data assuming a normally distributed error term. Briefly, rather than assigning a fixed cotinine value to samples below the limit of detection, the Tobit model allows for uncertainty about the unobserved value. With cotinine level in the log scale as the dependent variable, we examined the main effects of any smoking during pregnancy (binary), gender, race, state, and age in days at collection of DBS. The regression coefficients and confidence intervals for the log-transformed cotinine were exponentiated such that the results could be interpreted on the original scale as the ratio of the medians of cotinine between levels of the risk factors; we’ve termed this “cotinine ratio” for convenience. Although 3 states supplied data on CPD for 4 periods before and during pregnancy, the prevalence of reported smoking during pregnancy before but not during the third trimester was too low (<2%) to conduct statistical analysis. Similarly, only 5% reported smoking in the third trimester (CPD >0) among these 3 states. Therefore, only smoking during pregnancy as a binary variable, which allowed the inclusion of 4 states’ data, was prevalent enough (10.5%) to analyze and was included in the multivariate model described earlier. We examined the association of third trimester CPD with cotinine levels using a nonparametric Spearman correlation.
For the purposes of description, we considered mothers to have shown evidence of being active smokers if cotinine in their newborn’s DBS was ≥9.0 ng/g. This was based on cut points for the classification of active smokers recommended in an analysis of an NHANES sample of nonpregnant women.18 These same cut points were used in a recent estimate of nondisclosure of smoking among pregnant women.19 The cotinine plasma concentrations used as cut points for non-Hispanic white women and non-Hispanic African American women were ≥5 ng/mL and 6 ng/mL, respectively. Since our study included both groups the higher cut point 6 ng/mL, equivalent to 9 ng/g DBS using the ratio stated above, was used.
We additionally examined whether detectable cotinine, reported smoking during pregnancy, or the combination of reported smoking during pregnancy and/or cotinine ≥9.0 ng/g was a better predictor of birth weight. We constructed 3 multivariate linear regression models with birth weight as the dependent variable and compared R2 to ascertain which best fit the data. Each examined the main effect of the measurement of maternal TSE controlling for gender, race, and state. Lastly, we examined the association between cotinine and birth weight using the Spearman correlation in both all newborns and only those with detectable cotinine.
Results
All samples were assayable for cotinine. Subject characteristics and univariate comparisons of cotinine levels are presented in Table 1. Reported smoking during pregnancy was relatively infrequent at 10.5% (147 of 1396) of those with available data. Cotinine was detectable in DBS of 83.0% of infants whose mothers reportedly smoked during pregnancy and 29.0% of those who did not; however, the median level was higher among those reporting smoking (39 ng/g) compared with those who did not (1.0 ng/g).The percent of DBS with detectable cotinine and median cotinine levels differed significantly by state, race, season of collection, and day of collection, although the absolute difference in cotinine levels were relatively small. Detectable cotinine and median cotinine level did not differ by gender.
TABLE 1.
Characteristics of 1414 Newborns and Univariate Analysis of Cotinine in Their DBS
| Characteristic | n | No. (%) With Detectable Cotinine | P | Median (Range) of Detectable Cotinine (ng/g) | Pa |
|---|---|---|---|---|---|
| Reported cigarette smoking during pregnancy | <.001b | <.001c | |||
| No | 1249 | 362 (29.0) | 1.0 (0.3–218) | ||
| Yes | 147 | 122 (83.0) | 39 (0.5–247) | ||
| State | <.001b | 0.001c | |||
| California | 384 | 138 (35.9) | 1.3 (0.3–218) | ||
| Michigan | 349 | 167 (47.9) | 2.6 (0.3–226) | ||
| New York | 399 | 101 (25.3) | 4.4 (0.3–247) | ||
| Washington | 282 | 83 (29.4) | 0.7 (0.3–148) | ||
| Gender | .145b | .150c | |||
| Male | 726 | 239 (32.9) | 1.4 (0.3–247) | ||
| Female | 676 | 248 (36.7) | 1.8 (0.3–205) | ||
| Race | <.001b | .003c | |||
| African American | 694 | 274 (39.5) | 1.4 (0.3–247) | ||
| White | 708 | 213 (30.1) | 2.1 (0.3–218) | ||
| Season of collection | .009b | .001c | |||
| Spring | 155 | 61 (39.4) | 2.2 (0.3–226) | ||
| Summer | 171 | 74 (43.3) | 1.6 (0.3–178) | ||
| Fall | 253 | 80 (31.9) | 1.8 (0.3–148) | ||
| Winter | 441 | 134 (30.4) | 1.6 (0.3–247) | ||
| Collection dayd | .032b | .010 (R = –0.07)e | |||
| 0, 1 | 979 | 360 (36.8) | 1.6 (0.3–247) | ||
| 2, 3 | 401 | 118 (29.4) | 1.6 (0.3–148) | ||
| 4+ | 18 | 7 (38.9) | 0.8 (0.5–17) |
This P values in this column are derived from statistical tests that include all cotinine values, including those below the level of detection.
The P value is based on the Fisher’s exact test or the χ2 test.
The P value is based on the nonparametric Wilcoxon rank-sum test or the Kruskal-Wallis test.
Collection hour was not significantly correlated with cotinine and was missing in ∼150 cases from New York.
The P value for collection day, which ranges from 0 to 7, is based on the Spearman correlation coefficient (R).
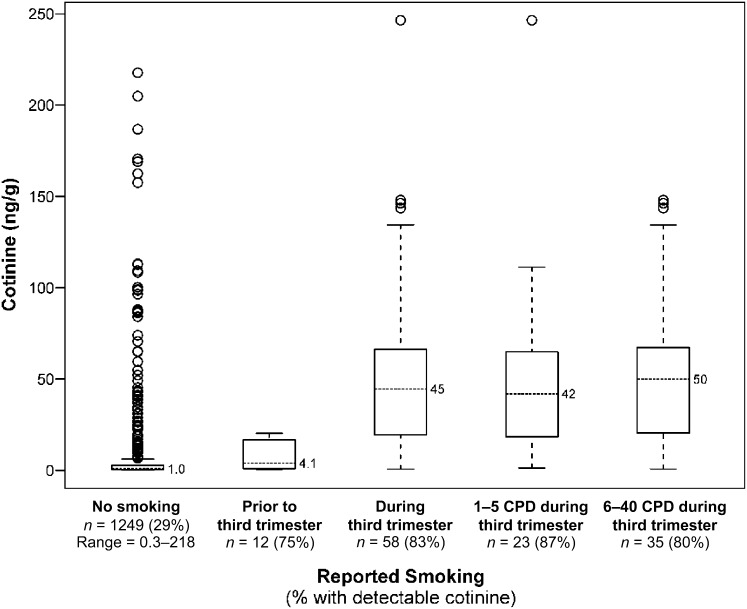
Cotinine levels in relation to reported smoking during pregnancy is shown in more detail in Fig 1. Of note, because Michigan data did not provide maternal smoking by trimester but the others states did, Michigan newborns whose mothers did not report smoking during pregnancy are included in the “No smoking” column, whereas the right 4 columns include only newborns from the 3 other states that provided smoking information by trimester and CPD. The boxplots show the median, interquartile range, and outliers (calculated as 1.5 × the 75th percentile) among newborns with cotinine detectable in their DBS. Although the percent with detectable cotinine and the median cotinine level among detectables was lowest in newborns whose mothers reportedly did not smoke during pregnancy, a substantial number of outliers are apparent. Sixty-eight newborns (5.4%) had cotinine levels ≥9.0 ng/g, suggesting their mothers were active smokers,18 even though they reported no smoking on the birth certificate. Conversely, there were 166 newborns with cotinine values ≥9.0 ng/g; 41% of these were born to mothers who reported not smoking. Among the 12 newborns whose mothers reportedly smoked during the first or second trimester but not the third, cotinine was detectable in 9 (75%), with a median of 4.1 ng/g. The DBS of 58 newborns whose mothers reportedly smoked in the third trimester of pregnancy had the highest frequency of detectable cotinine (82.8%) and far higher median cotinine (45 ng/g; Table 2). We further divided these newborns into those whose mothers smoked less than the median CPD of 5 and those who smoked 5 to 40; the range of cotinine was wider among the latter group but the median cotinine was only ∼8 ng/g higher than in the former group. The Spearman correlation between third trimester CPD and cotinine was 0.35 (P < .001).
FIGURE 1.
Percent of newborn DBS with detectable cotinine by reported cigarette exposure during pregnancy and distribution of detected cotinine values.
TABLE 2.
Comparison of Reported Maternal Smoking Versus Cotinine Detection as a Predictor of Birth Weight
| Model | Parameter Estimate (SE) | P |
|---|---|---|
| Model 1 (n = 1401) | ||
| Intercept | 3591.9 (39.4) | <.001 |
| Black race | −199.2 (29.5) | <.001 |
| Female gender | −111.7 (29.5) | <.001 |
| State | ||
| WA (reference) | 0.0 | — |
| CA | −134.7 (43.3) | .002 |
| MI | −166.0 (44.6) | <.001 |
| NY | −184.0 (43.2) | <.001 |
| Detectable cotinine | −94.6 (31.5) | .003 |
| Model 1 R2 = 0.065 (P < .001) | ||
| Model 2 (n = 1395) | ||
| Intercept | 3594.4 (38.6) | <.001 |
| Black race | −219.7 (29.3) | <.001 |
| Female gender | −122.2 (29.2) | <.001 |
| State | ||
| WA (reference) | 0.0 | — |
| CA | −145.2 (42.9) | .001 |
| MI | −145.4 (44.3) | .001 |
| NY | −154.0 (43.0) | <.001 |
| Reported smoking during pregnancy | −283.7 (48.7) | <.001 |
| Model 2 R2 = 0.081 (P < .001) | ||
| Model 3 (n = 1395) | ||
| Intercept | 3584.6 (38.6) | <.001 |
| Black race | −209.6 (29.3) | <.001 |
| Female gender | −114.0 (29.3) | <.001 |
| State | ||
| WA (reference) | 0.0 | — |
| CA | −134.4 (43.0) | .002 |
| MI | −144.0 (44.6) | .001 |
| NY | −152.0 (43.2) | <.001 |
| Reported smoking during pregnancy and/or cotinine ≥9.0 ng/g | −213.5 (41.3) | <.001 |
| Model 3 R2 = 0.076 (P < .001) |
We next modeled predictors of cotinine levels using Tobit analysis of all samples, with cotinine either above or below the detectable level (Table 3). Reported smoking during pregnancy was by far the strongest predictor of cotinine levels; the Tobit analysis indicated a 185-fold (95% confidence interval: 107.5–317.2) difference in the median cotinine levels of DBS of newborns with and without reported maternal smoking. Black race, female gender, and Michigan or California residence significantly predicted smaller differences, between 1.77- and 3.09-fold, in median cotinine versus their respective referents. Day of DBS collection was unrelated to cotinine levels.
TABLE 3.
Multivariate Regression of Newborn DBS Characteristics and Cotinine Levels Using Tobit Analysis
| Cotinine Ratio | 95% Confidence Interval | P | |
|---|---|---|---|
| Smoked during pregnancy | 184.6 | 107.5–317.2 | <.001 |
| Black race | 2.3 | 1.6–3.3 | <.001 |
| Female gender | 1.8 | 1.2–2.5 | .002 |
| Collection day (1-d increase) | 0.9 | 0.7, 1.3 | .602 |
| State | <.001 | ||
| Washington (reference) | 1.0 | — | |
| California | 2.4 | 1.4–4.2 | |
| Michigan | 3.1 | 1.8–5.3 | |
| New York | 0.7 | 0.4–1.4 |
We lastly compared the fit of 3 multivariate models of birth weight, newborn characteristics, and three measures of prenatal TSE. Detectable cotinine predicted depression of birth weight by 94.6 g, any reported smoking during pregnancy by 283.7 g, and any reported smoking during pregnancy and/or cotinine ≥9.0 ng/g by 213.5 g. R2 was 0.065, 0.081, and 0.076, respectively, for the models. Thus, although reported smoking during pregnancy provided the best model fit, none of the models explained >10% of the variability in birth weight in this data set. The Spearman correlation between birth weight and cotinine as a continuous variable was –0.13 (P < .001) for all subjects (n = 1403) and –0.21 (P < .001) for those with detectable cotinine (n = 513). Because cotinine as a continuous variable showed little association with birth weight in univariate analysis, it also would be unlikely to improve the prediction of birth weight.
Discussion
We report here on the application of a sensitive, high-throughput assay for cotinine to a large series of newborn DBS. A major finding was the detection of cotinine at levels ≥0.3 ng/g (equivalent to 0.2 ng/mL plasma) in 35% of newborns, including 29% of newborns whose mothers reportedly did not smoke cigarettes during pregnancy, some of whom were presumably exposed to environmental tobacco smoke. Equally striking is the outcome that 12% of the newborns had cotinine levels that are observed in active smokers,18 although 41% of the mothers of these infants reportedly did not smoke. These data confirm that reported smoking during pregnancy is an imperfect measure of TSE.
Several factors influence the level of cotinine detectable in DBS, including the amount of tobacco exposure, the route of exposure (maternal active vs passive smoking), the time since maternal exposure, interindividual variation in nicotine metabolism of both mother and child, and the time interval between delivery and obtaining the DBS. A limitation of our cross-sectional study design is the inability to account for these factors to estimate prenatal TSE.
DBS are typically obtained at least 24 hours after birth, but this may vary,20 and the mean duration of labor is 6 to 8 hours in uncomplicated deliveries21; thus, we presume that cotinine detected in newborn DBS is from maternal cigarette smoke exposure at least 30 hours earlier. However, this assumes that mothers did not smoke during labor (which has been reported in the past22) and that breastfeeding is not an additional route of exposure. Although both nicotine and cotinine have been detected in the breast milk of smokers,23 there are few data that address whether this is also true for the colostrum that expresses in the first 48 hours after delivery. Some data suggest that nicotine and cotinine clearance is markedly faster in women in late pregnancy,24,25 whereas that in newborns appears comparable to (nonpregnant) adults.26 Lastly, there is substantial variation in cotinine disposition both within and between European- and African American populations.27–29 These many variables preclude the ability to establish a cutoff in newborn DBS that distinguishes active maternal smoking from passive TSE. However, for purposes of discussion, we used a cotinine level of 9 ng/g DBS as an indication of smoking by the mother. This level is equivalent to the 6 ng/mL suggested as a sensitive and selective cut point for distinguishing smokers from nonsmokers in the 1999–2006 NHANES18 and due to the many variables discussed earlier, we believe it is a conservative estimate of smoking by the mother.
Reported maternal smoking in birth data are not itself a gold standard measurement of cigarette use,30,31 and none of the states contributing to this study collect data on environmental tobacco smoke. Consequently, we did not calculate the sensitivity and specificity of DBS cotinine compared with reported maternal smoking. Indeed, the newborns of 68 of 1249 (5.4%) mothers who reportedly did not smoke during pregnancy had cotinine ≥9.0 ng/g, a level that clearly indicates active maternal smoking. This level of nondisclosure is similar to that reported recently by Dietz et al, who examined serum cotinine among pregnant women in the 1999–2006 NHANES.19 Among 904 pregnant women who did not report current smoking at interview, 33 (3.7%) were classified as active smokers using the cut points previously mentioned. Additionally, we found that 17% of newborns whose mothers reportedly smoked during the third trimester had no detectable cotinine.
Although reported smoking during pregnancy was by far the strongest predictor of DBS cotinine in multivariate analysis, we also detected significantly higher levels in African Americans. The percentage of newborns with detectable cotinine was greater among African Americans than whites, but among newborns with detectable cotinine, the median level was lower in African Americans than whites. In interpreting these data, it is important to remember that we are quantifying cotinine in infants with 2 types of TSE: exposure from an actively smoking mother or from environmental TSE to a nonsmoking mother. The relative proportion of these 2 exposure groups may vary by ethnicity. Fewer African American women, pregnant or not, than white women report smoking,32 and nondisclosure of smoking among pregnant women is similar between the 2 races.19 This is consistent with the maternal data on reported smoking in our study; among African Americans, 56 of 689 (8.1%) reportedly smoked during pregnancy whereas 91 of 707 (12.9%) whites did. In addition, the mean plasma cotinine level in African Americans exposed to environmental tobacco smoke is higher than the mean level in whites.18,33 In our study, this might have lead to the detection of cotinine in a higher number of African American newborns with environmental TSE.
A significant difference in cotinine levels in newborn DBS was also observed by gender and state. Variability of cotinine levels by state generally reflect state-specific smoking rates; however, oversampling of African Americans may account for higher-than-expected levels in California.34 The reason for the observed differences in cotinine in newborns by gender is not known.
We examined whether birth weight was better predicted by reported maternal smoking or DBS cotinine. In previous research, the relationship between third-trimester smoking and birth weight is closer to L-shaped, rather than linear, meaning that the steepest drop in birth weight occurs with the first few CPD; the drop in birth weight plateaus at ∼300 g at ≥10 CPD.8 In our data, detectable DBS cotinine, reported maternal smoking, and reported maternal smoking and/or DBS cotinine ≥9.0 ng/g predicted drops of ∼95, ∼284, and ∼214 g of birth weight, respectively. Reported smoking most closely approximated the expected birth weight depression and in addition provided the best model fit. However, all of the models had R2 <0.10 and thus explained relatively little of the variance in birth weight.
There are several strengths and limitations to our study. Strengths include the large, population-based sample and the use of a newly developed, highly sensitive assay for cotinine in DBS. Our study thus represents an accurate snapshot of cotinine in newborns of 4 states. However, the study design was cross-sectional and lacked detailed information on maternal smoking in the days before DBS collection. Moreover, women who used nicotine replacement therapy or smokeless tobacco would also have tested positive for cotinine, although we had no self-reported data on the use of such products.
DBS cotinine adds to maternal smoking data obtained from birth data as a measure of prenatal TSE, in our study identifying nearly 5% of infants as having cotinine levels suggestive of active maternal smoking despite no self-reported cigarette use. Incorporating DBS cotinine into routine newborn screens will improve population surveillance of prenatal TSE and could be used to identify children at high risk of sequelae for monitoring or prevention efforts.
Glossary
- CPD
cigarettes consumed per day
- DBS
dried blood spots
- TSE
tobacco smoke exposure
Footnotes
Dr Spector assisted with study design, acquired samples and data, directed analysis, and drafted the manuscript; Dr Murphy developed the dried blood spot cotinine assay, assisted with study design, directed laboratory analyses, assisted with interpretation of results, and edited the manuscript; Ms Wickham conducted cotinine assays, assisted with interpretation, and edited the manuscript; Mr Lindgren assisted with study design, conducted analysis, assisted with interpretation, and edited the manuscript; Dr Joseph, as principal investigator of the study, oversaw all aspects of study design, data collection, analysis, interpretation, and drafting of the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by National Heart, Lung and Blood Institute grant 1RC2HL10140. Liquid chromatography tandem mass spectrometry was carried out in the Analytical Biochemistry Shared Resource of the Masonic Cancer Center supported in part by CA-77598. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Ebrahim SH, Floyd RL, Merritt RK, II, Decoufle P, Holtzman D. Trends in pregnancy-related smoking rates in the United States, 1987–1996. JAMA. 2000;283(3):361–366 [DOI] [PubMed] [Google Scholar]
- 2.Tong VT, Jones JR, Dietz PM, D’Angelo D, Bombard JM, Centers for Disease Control and Prevention (CDC) . Trends in smoking before, during, and after pregnancy—Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000–2005. MMWR Surveill Summ. 2009;58(4):1–29 [PubMed] [Google Scholar]
- 3.Zhao G, Ford ES, Tsai J, et al. Trends in health-related behavioral risk factors among pregnant women in the United States: 2001–2009. J Womens Health (Larchmt). 2012;21(3):255–263 [DOI] [PubMed] [Google Scholar]
- 4.England LJ, Kendrick JS, Gargiullo PM, Zahniser SC, Hannon WH. Measures of maternal tobacco exposure and infant birth weight at term. Am J Epidemiol. 2001;153(10):954–960 [DOI] [PubMed] [Google Scholar]
- 5.Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Hum Reprod Update. 2011;17(5):589–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornelius MD, Day NL. Developmental consequences of prenatal tobacco exposure. Curr Opin Neurol. 2009;22(2):121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke H, Leonardi-Bee J, Hashim A, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129(4):735–744 [DOI] [PubMed] [Google Scholar]
- 8.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18(2):188–204 [DOI] [PubMed] [Google Scholar]
- 9.Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009(192):29–60 [DOI] [PMC free article] [PubMed]
- 10.Pickett KE, Rathouz PJ, Kasza K, Wakschlag LS, Wright R. Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy. Paediatr Perinat Epidemiol. 2005;19(5):368–376 [DOI] [PubMed] [Google Scholar]
- 11.Mustonen TK, Spencer SM, Hoskinson RA, Sachs DP, Garvey AJ. The influence of gender, race, and menthol content on tobacco exposure measures. Nicotine Tob Res. 2005;7(4):581–590 [DOI] [PubMed] [Google Scholar]
- 12.Muscat JE, Stellman SD, Caraballo RS, Richie JP, Jr. Time to first cigarette after waking predicts cotinine levels. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3415–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pérez-Stable EJ, Benowitz NL, Marín G. Is serum cotinine a better measure of cigarette smoking than self-report? Prev Med. 1995;24(2):171–179 [DOI] [PubMed] [Google Scholar]
- 14.Therrell BL, Jr, Schwartz M, Southard C, Williams D, Hannon WH, Mann MY, PEAS Organizing and Working Groups . Newborn Screening System Performance Evaluation Assessment Scheme (PEAS). Semin Perinatol. 2010;34(2):105–120 [DOI] [PubMed] [Google Scholar]
- 15.Spector LG, Hecht SS, Ognjanovic S, Carmella SG, Ross JA. Detection of cotinine in newborn dried blood spots. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1902–1905 [DOI] [PubMed] [Google Scholar]
- 16.Murphy SE, Wickham KM, Lindgren BR, Spector LG, Joseph A. Cotinine and trans 3′-hydroxycotinine in dried blood spots as biomarkers of tobacco exposure and nicotine metabolism. J Expo Sci Environ Epidemiol. 2013;23(5):513–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tobin J. Estimation of relationships for limited dependent variables. Econometrica. 1958;26(1):24–36 [Google Scholar]
- 18.Benowitz NL, Bernert JT, Caraballo RS, Holiday DB, Wang J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169(2):236–248 [DOI] [PubMed] [Google Scholar]
- 19.Dietz PM, Homa D, England LJ, et al. Estimates of nondisclosure of cigarette smoking among pregnant and nonpregnant women of reproductive age in the United States. Am J Epidemiol. 2011;173(3):355–359 [DOI] [PubMed] [Google Scholar]
- 20.American Academy of Pediatrics Newborn Screening Authoring Committee . Newborn screening expands: recommendations for pediatricians and medical homes—implications for the system. Pediatrics. 2008;121(1):192–217 [DOI] [PubMed] [Google Scholar]
- 21.Albers LL. The duration of labor in healthy women. J Perinatol. 1999;19(2):114–119 [DOI] [PubMed] [Google Scholar]
- 22.Latulippe LG, Marcoux S, Fabia J, Weber JP, Tennina S. Smoking during labour. Can J Public Health. 1992;83(3):184–187 [PubMed] [Google Scholar]
- 23.Llaquet H, Pichini S, Joya X, et al. Biological matrices for the evaluation of exposure to environmental tobacco smoke during prenatal life and childhood. Anal Bioanal Chem. 2010;396(1):379–399 [DOI] [PubMed] [Google Scholar]
- 24.Dempsey D, Jacob P, III, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301(2):594–598 [DOI] [PubMed] [Google Scholar]
- 25.Klein J, Blanchette P, Koren G. Assessing nicotine metabolism in pregnancy—a novel approach using hair analysis. Forensic Sci Int. 2004;145(2–3):191–194 [DOI] [PubMed] [Google Scholar]
- 26.Dempsey D, Jacob P, III, Benowitz NL. Nicotine metabolism and elimination kinetics in newborns. Clin Pharmacol Ther. 2000;67(5):458–465 [DOI] [PubMed] [Google Scholar]
- 27.Berg JZ, Mason J, Boettcher AJ, Hatsukami DK, Murphy SE. Nicotine metabolism in African Americans and European Americans: variation in glucuronidation by ethnicity and UGT2B10 haplotype. J Pharmacol Exp Ther. 2010;332(1):202–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Signorello LB, Cai Q, Tarone RE, McLaughlin JK, Blot WJ. Racial differences in serum cotinine levels of smokers. Dis Markers. 2009;27(5):187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bloom J, Hinrichs AL, Wang JC, et al. The contribution of common CYP2A6 alleles to variation in nicotine metabolism among European-Americans. Pharmacogenet Genomics. 2011;21(7):403–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srisukhumbowornchai S, Krikov S, Feldkamp ML. Self-reported maternal smoking during pregnancy by source in Utah, 2003–2007. Birth Defects Res A Clin Mol Teratol. 2012;94(12):996–1003 [DOI] [PubMed] [Google Scholar]
- 31.Vinikoor LC, Messer LC, Laraia BA, Kaufman JS. Reliability of variables on the North Carolina birth certificate: a comparison with directly queried values from a cohort study. Paediatr Perinat Epidemiol. 2010;24(1):102–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention (CDC) . Current cigarette smoking among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(44):889–894 [PubMed] [Google Scholar]
- 33.Pirkle JL, Bernert JT, Caudill SP, Sosnoff CS, Pechacek TF. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988–2002. Environ Health Perspect. 2006;114(6):853–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention (CDC) . Smoking prevalence among women of reproductive age—United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57(31):849–852 [PubMed] [Google Scholar]