Abstract
BACKGROUND AND OBJECTIVE:
Improving medical regimen adherence is essential for maximizing the therapeutic potential of treatments for pediatric chronic illness. Health care providers are uniquely positioned to deliver adherence promotion interventions. However, no studies have summarized the effectiveness of health care provider-delivered adherence interventions. The objective of this study was to describe the effectiveness of health care provider-delivered adherence promotion interventions in improving adherence among children who have chronic illness. Data sources include PubMed, PsycINFO, CINAHL, and Scopus. Studies were included if they were randomized-controlled trials of pediatric interventions aiming to increase adherence to the primary regimen for a chronic illness and at least 1 health care provider delivered the intervention.
RESULTS:
A total of 35 randomized-controlled studies including 4616 children were included. Greater improvements in adherence were observed immediately after health care provider-delivered interventions (d = 0.49; 95% confidence interval, 0.32 to 0.66) than at longer-term follow-up (d = 0.32; 95% confidence interval, 0.10 to 0.54). Treatment effect sizes differed across the adherence behaviors measured. There was significant heterogeneity in treatment effects; however, no moderators of treatment effectiveness were identified. This meta-analysis focused on the published literature. In addition, the majority of studies involved children who had asthma and younger children.
CONCLUSIONS:
Health care provider-delivered interventions for children who have chronic illness can be effective in improving adherence. Gains in adherence are highest immediately after intervention. Future interventions and studies should include multiple methods of assessing adherence, include active comparators, and address long-term maintenance of adherence gains.
Keywords: medication adherence, patient compliance, pediatrics, self care, chronic disease, health personnel
Although impressive medical advances have led to the development of effective medical treatments for many pediatric chronic illnesses, these treatments are only effective if patients and families adhere to medical recommendations. Unfortunately, rates of medical regimen non-adherence are high, with ∼50% of pediatric patients and their families demonstrating non-adherence.1 Improving medical regimen adherence is of central importance for achieving better medical outcomes for chronically ill children.2 Previous meta-analyses and reviews have focused on the effectiveness of behavioral health professionals (eg, psychologists) in implementing pediatric adherence promotion interventions.3–5 However, behavioral health services to improve regimen adherence are not always available to, or necessarily wanted by, pediatric patients and families.
Health care providers (eg, physicians, nurses, dietitians) are arguably ideal individuals to implement adherence promotion interventions because of their existing relationships with families, ongoing monitoring of patient’s health outcomes, and familiarity with patient’s unique treatment needs. Indeed, there have been calls for pediatric health care providers to provide adherence promotion interventions.6,7 However, there have been no systematic reviews or meta-analyses of pediatric health care provider-delivered adherence promotion interventions. Such a review could serve as a foundation for development and dissemination of interventions, particularly in the current environment of health care reform. Thus, the primary aim of the current meta-analysis of randomized-controlled trials was to describe the effectiveness of health care provider-delivered interventions in improving pediatric adherence outcomes. The secondary, exploratory aim was to examine moderators of intervention effectiveness (eg, intervention setting). Based on existing literature,3,4 it was hypothesized that health care provider-delivered interventions would demonstrate a medium treatment effect size and that effects would be weaker at follow-up than shortly post-treatment.
Methods
In July 2012, literature searches covering all years indexed were conducted in PubMed (1996–2012), Cinahl (1937–2012), PsycINFO (1806–2012), ERIC (1966–2012), the Cochrane Library (1991–2012), and Scopus (includes Medline and Embase; 1960–2012) using combinations of relevant terms (Appendix 1). References of review articles and relevant articles were reviewed.
Study abstracts and full-length articles were screened by 2 independent reviewers trained and supervised by the first author. Studies were included if: (1) the study was a randomized-controlled trial, (2) the intervention aimed to increase patient/family adherence to the primary self-managed regimen for a chronic condition, (3) ≥1 health care provider (ie, physicians, nurses, dietitians, respiratory therapists, diabetes or asthma educators, nutritionists, occupational therapists, physical therapists, pharmacists) served as an interventionist, (4) >50% of the intervention content or time involved contact with the health care provider, (5) mean age of participants <18 years, (6) study measured adherence to behaviors targeted by the intervention, (7) study report was written in English, and (8) effect size could be calculated using data in the report or from study authors. Studies were excluded if any of the following criteria were met: (1) goal of the intervention was to change health care provider behavior, (2) intervention was delivered at schools or summer camps, (3) a mental health professional delivered >50% of the intervention, (4) study measured adherence using a health outcome (eg, hemoglobin A1c) instead of a behavior (eg, blood glucose monitoring), (5) study report and attempts to contact authors yielded insufficient information to determine whether the study met inclusion/exclusion criteria or to calculate effect sizes, and (6) study report was not in English.
Studies meeting inclusion criteria were coded by the first author for intervention content (eg, educational, behavioral) and format (eg, individual, groups), adherence behavior targeted, adherence measures used, adherence outcome data, and descriptive information about the sample and study design. Information on intervention effectiveness was coded based on study reports or information from the report authors. The second author conducted reliability coding of 20% of the included articles. Following recommendations outlined by the Cochrane Collaboration,8 risk for bias was identified across several domains (ie, sequence generation, allocation concealment, incomplete outcome data, selective outcome reporting, other sources of bias). Coding for adequate prevention of knowledge of allocated intervention was excluded because the nature of the interventions allowed participants to be aware of their allocated group (eg, assignment to psychosocial intervention for adherence versus usual medical care).
Analyses
Effect size calculation and other analyses were based on procedures outlined by the Cochrane Collaboration8 and Lipsey and Wilson.9 All analyses were completed by using Comprehensive Meta-Analysis10 and SPSS. Cohen’s κ (κ = 0.74) and Pearson’s correlation (r > 0.9) were used to examine coding reliability. The primary summary effect size measure was Cohen’s d. Statistical independence within studies was maintained in the following ways: (1) multiple measures of adherence within studies were averaged so that each study contributed 1 effect size for the overall analyses, and (2) if a study’s results were presented in more than 1 publication, the effect sizes were averaged. Effect sizes for adherence promotion interventions were examined in several ways. First, the overall effectiveness of interventions was examined across studies at post-treatment (ie, mean pre- to post-treatment (mo): 7.3; SD = 6.4, range, 1–24) and follow-up (mean post-treatment to follow-up (mo): 14.1; SD = 20.9, range, 2–72). Follow-up analyses summarized the effectiveness of interventions after excluding those involving a behavioral health provider and interventions reportedly based on theory versus those that were not. Second, intervention effectiveness was examined by types of adherence behavior (eg, completing medication regimen, making environmental modifications) separately at post-treatment and follow-up. The random effects model was used because it was expected that the treatment effects would differ across studies owing to differences in study design, intervention content, and participants. Heterogeneity in the treatment effects was examined by using the Q-statistic. Pre-specified subgroup analyses compared treatment effects by intervention type (education only versus other types), number of health care providers delivering the intervention (ie, 1 provider vs >1), setting (clinic versus other settings), format (individual child/family versus group), and adherence outcome measure (self- or parent-report versus other measures). Rosenthal’s fail-safe n was calculated to address the potential for sampling or publication bias.
Results
Participants and Study Design
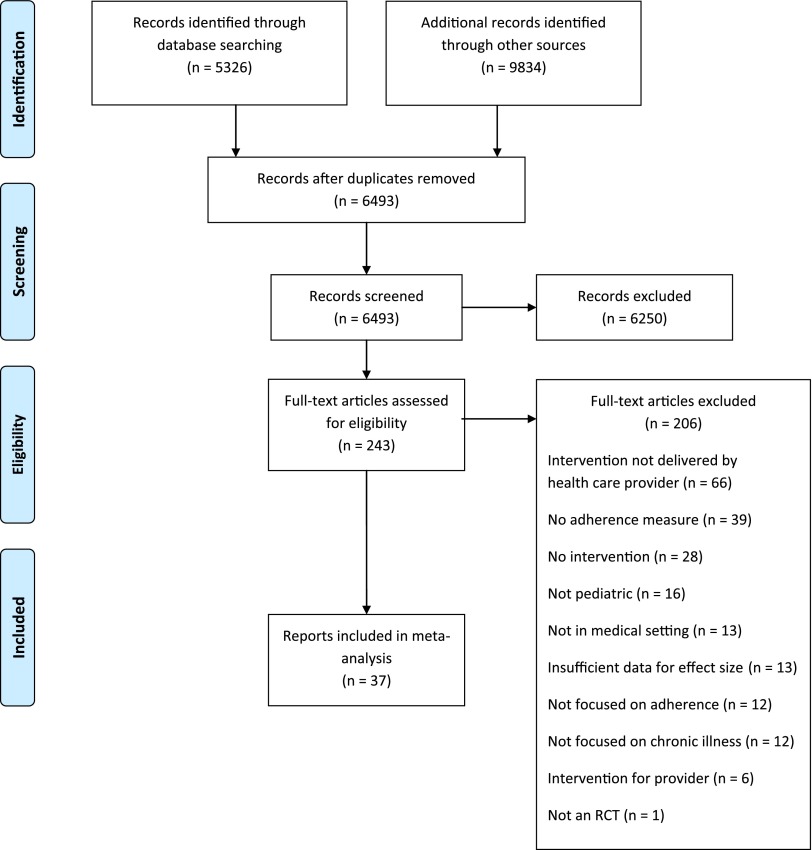
In total, 37 reports representing 35 randomized-controlled studies were included (Fig 1). Across all studies, there were 4616 participants (Msample size = 125; SD = 113). The majority of reports included youth who had asthma (n = 23; 62.1%). Others focused on youth who had diabetes (n = 7; 18.9%), obesity (n = 2; 5.4%), eczema (n = 2; 5.4%), or other conditions (n = 3; 8.1%; juvenile rheumatoid arthritis, HIV, sickle cell disease). Fifty-six percent of youth participants were male and their average age using reported central tendencies was 7.0 years. In the reports, the primary adherence intervention of interest was compared with treatment as usual (n = 25; 67.6%), an alternative active intervention (n = 11; 29.7%), or an attention placebo (n = 1; 2.7%). Alternative interventions typically included provision of education in a way that placed greater responsibility on patients and their families and involved less interaction with health care providers (eg, education booklet).
FIGURE 1.
PRISMA flow sheet.
Intervention Characteristics
Most adherence promotion interventions were delivered by a single health care provider (n = 23, 62%), whereas the remaining were delivered by 2 or more health care providers (n = 14; 38%). Most commonly, nurses delivered interventions (n = 23; 62%), followed by physicians (n = 11; 30%), psychologists (n = 6; 16%), health educators (n = 5; 14%), dieticians (n = 3; 8%), nutritionists (n = 2; 5%), social workers (n = 2; 5%), case managers/coordinators (n = 2; 5%), respiratory therapists (n = 1; 3%), and exercise therapists (n = 1; 3%). Interventions targeted a variety of adherence-related behaviors: taking medication (n = 24; 65%), symptom monitoring (n = 9; 24%), dietary changes (n = 6; 16%), environmental modifications (n = 5; 14%), insulin administration (n = 4; 11%), physical activity changes (n = 2; 5%), topical treatments (n = 2; 5%), and other health behaviors (eg, refilling medication, attending appointments; n = 3; 8%).
Intervention content varied across studies. Behavioral interventions (eg, providing families with specific strategies to manage the regimen, such as increasing parental supervision of regimen completion) were most common (n = 19; 51%). Educational interventions were next most common (n = 15; 40%) and included providing basic information to families about the patient’s illness (eg, etiology, course) and the importance of adherence. Approximately one-third (n = 12; 32%) of reports tested interventions that aimed to improve patient adherence through health care provider-initiated actions, such as simplifying the treatment regimen or increasing contact with families. Organizational interventions, such as introducing pillboxes or calendars for self-monitoring, were also used (n = 5; 14%). Other interventions included facilitating discussion with caregivers about their child’s illness and supporting effective caregiver-health care provider interactions (n = 4; 11%).
On average, interventions were comprised of 7 sessions or contacts between the health care provider(s) and patients and families (SD = 4 sessions; range, 4–16). Interventions took place in a variety of settings: clinic (n = 16; 43%), patient’s home (n = 10; 27%), phone (n = 8; 22%), inpatient (n = 1; 3%), emergency department (n = 2; 5.4%), and other (eg, postal mail, university research center; n = 8; 21.6%). The vast majority of interventions (n = 28; 76%) involved both youths and their families. Some interventions targeted only caregivers (n = 6; 16%) or only youths (n = 3, 8%). Most interventions were delivered to individual patients or families (n = 25; 68%), some in a group format (n = 6; 16%), and others comprised both formats (n = 6; 16%). The majority of interventions were not explicitly grounded in a theoretical framework (n = 22; 60%; n = 4, unclear whether grounded in theoretical framework). Thirty percent of interventions (n = 11) were grounded in a theoretical framework, such as the Health Belief Model,11 social cognitive learning theory,12 and stages of change model.13
Adherence Outcome Measurement
Adherence outcomes were measured by using patient- or parent-report (n = 30; 81%), pharmacy records (n = 6; 16%), electronic monitoring (n = 5; 14%), direct observation (n = 3; 8%), physician rating (n = 3; 8%), biological assay (n = 1; 3%), and other methods (eg, blood glucometer download) (n = 2; 5%).
Effect Sizes
Figure 2 contains a forest plot of post-treatment effect sizes. At post-treatment, there was a medium effect such that participants receiving the health care provider intervention had higher adherence than participants in the control condition (d = 0.49; 95% CI, 0.32 to 0.66; n = 35). The effect size after excluding studies that included a behavioral health provider was 0.54 (95% CI, 0.33 to 0.75; n = 28). The effect size for interventions based on theory was 0.42 (95% CI, 0.31 to 0.53; n = 11) whereas those not explicitly based on theory was 0.37 (95% CI, 0.33 to 0.42; n = 24). At follow-up, the treatment effect was small (d = 0.32; 95% CI, 0.10 to 0.54; n = 10). Effect sizes differed across the adherence behaviors measured (Table 1). The largest effect sizes were for medical regimen completion (d = 0.57), medication refills (d = 0.51), and composite adherence measures (d = 0.61). In contrast, other adherence behaviors, including appointment-making (d = 0.24), dietary changes (d = 0.19), and symptom monitoring (d = 0.17) were smaller in size.
FIGURE 2.
Forest plot.
TABLE 1.
Effect Sizes
| Post-Treatment | Follow-Up | |||||
|---|---|---|---|---|---|---|
| Number of Effect Sizes | Mean Effect Size | 95% CI | Number of Effect Sizes | Mean Effect Size | 95% CI | |
| Adherence methodology | 5 | 0.34 | −0.07 to 0.75 | — | — | — |
| Appointment-making | 1 | 0.24 | −0.31 to 0.78 | — | — | — |
| Adherence composite | 4 | 0.61 | 0.34 to 0.88 | — | — | — |
| Dietary change | 9 | 0.19 | 0.02 to 0.36 | 4 | 0.32 | 0.17 to 0.48 |
| Environmental change | 9 | 0.35 | −0.03 to 0.73 | 3 | −0.03 | −0.30 to 0.24 |
| Medical regimen completion | 36 | 0.57 | 0.30 to 0.84 | 10 | 0.32 | −0.06 to 0.69 |
| Physical activity | 2 | 0.11 | −0.11 to 0.32 | — | — | — |
| Refill | 8 | 0.51 | 0.24 to 0.78 | 3 | 0.11 | −0.07 to 0.29 |
| Sedentary behavior | 3 | 0.24 | −0.05 to 0.53 | 1 | 0.00 | −0.31 to 0.31 |
| Symptom monitoring | 10 | 0.17 | −0.14 to 0.49 | 2 | 0.18 | −0.35 to 0.72 |
—, No follow-up data available.
Moderator Analyses
To explore the heterogeneity in treatment effects at post-treatment (Q[34] = 446.1; P < .001) and follow-up (Q[9] = 57.8; P < .001), analyses were conducted to examine a priori, potential moderators of treatment effects. Intervention type, number of health care providers delivering the intervention, intervention setting, intervention format (individual child or family versus group), and adherence outcome measure did not significantly account for heterogeneity in treatment effects (all P values <.02 for Q-within moderator groups).
Risk for Bias
Potential risk for bias in the studies included in the meta-analysis was identified by using Cochrane Collaboration’s recommendations8 (Table 2): adequate allocation generation (89.2%; n = 33), adequate allocation concealment (27.0%; n = 10), adequate explanation of incomplete outcome data (70.3%; n = 26), study reports free of suggestion of selective outcome reporting (59.5%; n = 22), and study free of other problems that could put it at high risk for bias (67.6%; n = 25).
TABLE 2.
Risk for Bias for Included Studies
| Adequate Sequence Generation | Allocation Concealment | Incomplete Outcome Data Addressed | Free of Selective Reporting | Free of Other Bias | |
|---|---|---|---|---|---|
| Anderson (1989)31 | ? | ? | + | ? | ? |
| Berkovitch (1998)32 | ? | ? | + | ? | ? |
| Berrien (2004)33 | + | + | + | + | + |
| Bloomfield (1990)34 | ? | ? | ? | ? | ? |
| Bonner (2002)36 | + | ? | + | ? | + |
| Brown (2002)35 | + | + | + | + | + |
| Butz (2005)40 | + | ? | — | ? | ? |
| Butz (2010)39 | + | ? | — | ? | ? |
| Burkhart (2002)37 | + | ? | ? | + | + |
| Burkhart (2007)38 | + | ? | + | + | + |
| Chan (2003)41 | + | ? | ? | ? | ? |
| DeBar (2012)42 | + | ? | + | + | + |
| Deforche (2005)43 | — | ? | + | ? | + |
| Ducharme (2011)44 | + | + | + | + | + |
| Elamin (1993)45 | + | ? | ? | + | + |
| Farber (2004)46 | + | + | + | + | + |
| Gorelick (2006)47 | + | + | + | + | + |
| Gray (1998)48 | + | ? | ? | + | + |
| Hederos (2005)27 | + | ? | + | ? | + |
| Hederos (2009)63 | + | ? | + | ? | + |
| Howe (2005)49 | + | ? | — | ? | ? |
| Hughes (1991)50 | + | ? | + | ? | ? |
| La Roche (2006)28 | + | ? | + | ? | + |
| Lawson (2005)51 | + | + | + | + | + |
| LeBaron (1985)52 | + | ? | ? | + | + |
| McNabb (1994)53 | + | ? | + | ? | — |
| Mitchell (1986)54 | + | ? | + | + | ? |
| Moore (2009)55 | + | + | + | + | + |
| Otsuki (2009)56 | + | + | + | + | + |
| Rapoff (2002)57 | + | ? | + | + | + |
| Smith (1986)58 | + | ? | + | + | + |
| Staab (2002)59 | + | ? | + | + | + |
| Teach (2006)60 | + | + | + | + | + |
| Wilson (1996)30 | + | ? | + | ? | + |
| Van Es (2001)61 | + | + | ? | + | ? |
| Watson (2009)62 | + | ? | — | + | + |
+, Low risk for bias; —, high risk for bias; ?, unclear risk for bias.
Publication Bias
Based on Rosenthal’s fail-safe n, 2643 unpublished studies reporting null results would be needed to reduce the overall effect size across studies (ie, d = 0.49) to 0.
Discussion
The current meta-analysis indicates that health care provider-delivered interventions for children who have chronic illness can be effective in improving adherence when compared with treatment as usual or alternative interventions. Treatment effects are strongest immediately after intervention and adherence improvements dissipate over time. Intervention effectiveness also differed depending on the adherence behavior measured. Intervention effects were larger when medical regimen completion (eg, ingesting medications) was assessed compared with when dietary change was the outcome. These findings suggest that health care provider-delivered interventions may be more effective for certain adherence behaviors. Other adherence behaviors, such as making dietary and physical activity changes, may require more intensive interventions or interventions delivered by multidisciplinary providers (eg, nutritionists, psychologists specializing in adherence behavior change).
Health care provider-delivered adherence promotion interventions hold great promise and have a number of characteristics that support sustainability and feasibility. First, the overall results (ie, medium-sized treatment effects) are on par with those described in previous meta-analyses of pediatric adherence promotion interventions, the majority of which were delivered by mental health professionals.3,4 The use of behavioral interventions may be driving this treatment effect given that interventions incorporating behavioral strategies are typically most effective at improving adherence3,4 and comprised the majority of studies included in the current meta-analysis. Second, interventions focused on changing both child and family member’s behavior, which is consistent with the family-centered approach in pediatrics.14 Third, health care providers have established relationships with patients and families, which could improve families’ willingness to engage in adherence promotion interventions. Interventions that improve communication between providers and families will be critical.6,7 Fourth, health care providers often already monitor patient’s medical information related to adherence and health outcomes (eg, for patients who have type 1 diabetes, HbA1c), and thus can provide patients with real-time feedback on the consequences of their adherence. Consensus statements for the management of certain pediatric illnesses (eg, asthma), in fact, encourage health care providers to provide adherence or self-management support.15 Fifth, interventions in the current meta-analysis were delivered in settings that maximized feasibility for patients and families or that capitalized on existing medical system structures. Continued efforts should be made to develop, test, and disseminate health care provider-delivered adherence promotion interventions. If families do not receive adherence promotion interventions from health care providers, a primary alternative would be a referral to mental health professionals (eg, psychologist, social worker). Although these referrals can be useful and can lead to improved medical regimen adherence for some families,16,17 many families do not pursue such referrals because of perceived stigma of meeting with a mental health professional, insurance coverage,18,19 and, based on our clinical experience, the hassles of attending “extra” appointments.
Given the studies included in this meta-analysis, there are several limitations with implications for future health care provider-delivered adherence promotion interventions. Most studies focused on children who have asthma and younger children. Increased focus on testing interventions for other chronic illnesses associated with high health care costs, low health-related quality of life, and high rates of morbidity, such as type 1 diabetes, is needed.20–22 In addition, it will be important to address the unique adherence issues of older youth, including adolescents and young adults. Because of the common developmental transitions during adolescence and young adulthood (eg, increased independence, living apart from their families of origin), adolescents and young adults require tailored interventions that address these ongoing transitions. Related to intervention and study design, most interventions were not grounded in a broader theoretical approach, which may have limited their comprehensiveness and examination of mechanisms of change underlying adherence improvements. Fewer than half of the interventions were delivered by >1 multidisciplinary provider. However, given ongoing health care reform and broader movements toward providing multidisciplinary care in pediatrics, it will be important to test multidisciplinary interventions in the future. Most studies compared interventions with treatment as usual, which typically does not control for adherence gains owing to the more frequent or lengthy interactions with providers that patients experience in the active intervention group. Adherence outcomes were also often assessed by using patient- and parent-report, which is typically an overestimate.1 Future studies should consider including an attention control or active comparison intervention and adherence assessments other than self-report.23
The current results should be interpreted with several limitations in mind. This meta-analysis focused on the published literature across several specialties, including medicine, nursing, and nutrition. Future studies could seek to replicate the current results using unpublished work. There was wide variability in illness populations targeted and treatment effectiveness across adherence behaviors. Although this reflects the current state of the broader literature on medical regimen adherence, future reviews focused on particular illness populations and adherence behaviors will be essential for advancing the field. For example, adherence may differ across chronic conditions owing to factors such as the symptoms associated with the illness, perceived illness severity, and regimen complexity, and thus each illness may necessitate unique interventions. Because of the number of studies identified for this meta-analysis, a limited number of moderator analyses could be conducted and those that were conducted used aggregated categories. As the literature in this area grows, future reviews will be able to conduct more moderator analyses comparing interventions and interventions delivered by particular provider types or multidisciplinary teams. Also, the length of time between pre- and post-treatment varied between studies, which may have added variability to the treatment effects. Although approximately one-third of the studies assessed adherence at a follow-up period, the lengths of these follow-up periods ranged widely. The long-term effectiveness of health care provider-delivered adherence promotion interventions may differ when a greater number of studies with follow-up are included.
The current findings hold several implications for next steps in developing health care provider-delivered interventions for adherence. Regarding the content and delivery of interventions, it will be important to design programs that are grounded in a theoretical model. In addition, to facilitate the dissemination of adherence interventions into clinical practice, it will be helpful for future interventions to include clear criteria and algorithms for incorporating adherence assessment and intervention into clinical decision-making. Future adherence interventions should also address maintenance of adherence gains beyond the intervention period (eg, addition of booster sessions for families who need them).
Next steps could then include optimizing adherence and health outcomes by tailoring the intervention content or delivery based on cost and patient need. For example, families could benefit from interventions that address comorbid mental health conditions that inhibit effective self-management.24 Given the trend toward multidisciplinary care for chronic diseases, it will be important for future intervention studies to quantify the degree of contact and collaboration between providers. Once effective interventions are further developed within illness conditions, a modular approach could be used whereby patients who have different illnesses or specific barriers to adherence receive only the specific “modules” they need.25,26 When implementing this research, studies should include active comparators (eg, attention control group, alternative intervention), assess adherence using multiple methods beyond self-report alone,23 and examine other potential moderators of treatment effectiveness (eg, family-provider communication, patient/family characteristics impacting adherence such as psychological functioning and problem-solving abilities). In addition, studies could examine other important outcomes, including the clinical impact adherence improvements have on health and the cost-effectiveness of implementing adherence promotion interventions.27–30
Conclusions
Health care provider-delivered interventions for children who have chronic illness and their families appear to be effective at improving adherence to prescribed regimens. The findings suggest that clinicians who want to integrate adherence promotion into their practice could consider a number of intervention formats (eg, in-person and/or phone interventions delivered by multidisciplinary teams) and content (eg, combination of educational and behavioral strategies), which could help to structure provider’s interactions with families and add flexibility to care delivery. Adherence interventions that target medication-taking and refills are more likely to be effective, and ongoing monitoring and booster sessions will be needed to sustain improved adherence. Continuing to develop and deliver health care provider-led adherence promotion interventions could lead to increased patient and family access to needed adherence interventions, better pediatric health outcomes, and health care cost savings.
Acknowledgments
We thank Noelle Bergman, Sarah Williams, Bridget Crippen, Youngeun Lee, Sarah Bidwell, Avelina Padin, and Emily Shultz for assistance with literature searches and data entry.
Glossary
- CI
confidence interval
- HbA1c
Hemoglobin A1c
APPENDIX 1.
Exemplar Literature Search Strategy
| Search | Search Term |
|---|---|
| 1 | Adherence OR compliance |
| 2 | Intervention OR treatment |
| 3 | Physician OR nurse OR dietitian OR respiratory therapist OR nutritionist OR school nurse OR occupational therapist OR physical therapist OR pharmacist |
| 4 | Chronic illness OR asthma OR diabetes OR cystic fibrosis OR cancer OR hematology OR oncology OR sickle cell disease OR obesity OR epilepsy OR overweight OR transplant OR gastrointestinal disorder OR inflammatory bowel disease OR colitis OR irritable bowel OR pain disorder OR juvenile rheumatoid arthritis OR infectious diseases OR tuberculosis OR HIV OR cardiovascular disease OR abdominal pain OR burn |
| 5 | Pediatric OR child OR adolescent |
| 6 | 1 AND 2 AND 3 AND 4 AND 5 |
Footnotes
Dr Wu conceptualized and designed the study, supervised data collection, conducted analyses, drafted the initial manuscript, and revised the manuscript; Dr Pai supervised the conceptualization and design of the study, consulted on issues related to data collection and analyses, and critically reviewed the manuscript; and both authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This work was supported by a training grant from the National Institutes of Health supporting Dr Wu (grant T32HD068223). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Rapoff M. Adherence to Pediatric Medical Regimens. 2nd ed. New York: Springer Science+Business Media; 2010 [Google Scholar]
- 2.World Health Organization Adherence to Long-Term Therapies: Evidence for Action. World Health Organization; 2003. Available at: http://whqlibdoc.who.int/publications/2003/9241545992.pdf. Accessed April 9, 2014 [Google Scholar]
- 3.Graves M, Roberts M, Rapoff M, Boyer A. The efficacy of adherence interventions for chronically ill children: a meta-analytic review. J Pediatr Psychol. 2010;35(4):368–382 [DOI] [PubMed] [Google Scholar]
- 4.Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33(6):590–611 [DOI] [PubMed] [Google Scholar]
- 5.Kripalani S, Yao X, Haynes R. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167(6):540–550 [DOI] [PubMed] [Google Scholar]
- 6.Drotar D. Physician behavior in the care of pediatric chronic illness: association with health outcomes and treatment adherence. J Dev Behav Pediatr. 2009;30(3):246–254 [DOI] [PubMed] [Google Scholar]
- 7.Goode M, Harrod M, Wales S, Crisp J. The role of specialist nurses in improving treatment adherence in children with a chronic illness. Aust J Adv Nurs. 2004;21(4):41–45 [PubMed] [Google Scholar]
- 8.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. West Sussex: John Wiley & Sons; 2008 [Google Scholar]
- 9.Lipsey M, Wilson D. Practical Meta-Analysis. Thousand Oaks, CA: Sage; 2001 [Google Scholar]
- 10.Comprehensive Meta-Analysis [computer program]. Englewood, NJ: Biostat
- 11.Janz N, Becker M. The health belief model: a decade later. Health Educ Behav. 1984;11(1):1–47 [DOI] [PubMed] [Google Scholar]
- 12.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31(2):143–164 [DOI] [PubMed] [Google Scholar]
- 13.Prochaska J, Velicer W. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12(1):38–48 [DOI] [PubMed] [Google Scholar]
- 14.Kuhlthau K, Bloom S, Van Cleave J, Knapp A, Romm D, Klatka K, et al. Evidence for family-centered care for children with special health care needs: a systematic review. Acad Pediatr. 2011;11(2):136–143.e138 [DOI] [PubMed]
- 15.Bacharier L, Boner A, Carlsen K, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63(1):5–34 [DOI] [PubMed] [Google Scholar]
- 16.Herzer M, Ramey C, Rohan J, Cortina S. Incorporating electronic monitoring feedback into clinical care: a novel and promising adherence promotion approach. Clin Child Psychol Psychiatry. 2012;17(4):505–518 [DOI] [PubMed] [Google Scholar]
- 17.Hilliard ME, Ramey C, Rohan JM, Drotar D, Cortina S. Electronic monitoring feedback to promote adherence in an adolescent with Fanconi anemia. Health Psychol. 2011;30(5):503–509 [DOI] [PubMed] [Google Scholar]
- 18.Sabbeth B, Stein R. Mental health referral: a weak link in comprehensive care of children with chronic physical illness. J Dev Behav Pediatr. 1990;11(2):73–78 [PubMed] [Google Scholar]
- 19.Inkelas M, Raghavan R, Larson K, Kuo A, Ortega A. Unmet mental health need and access to services for children with special health care needs and their families. Ambul Pediatr. 2007;7(6):431–438 [DOI] [PubMed] [Google Scholar]
- 20.Daneman D. Type 1 diabetes. Lancet. 2006;367(9513):847–858 [DOI] [PubMed] [Google Scholar]
- 21.Ingerski L, Modi A, Hood K, et al. Health-related quality of life across pediatric chronic conditions. J Pediatr. 2010;156(4):639–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang G, Dietz W. Economic burden of obesity in youths aged 6 to 17 years: 1979–1999. Pediatrics. 2002;109(5). Available at: www.pediatrics.org/cgi/content/full/109/5/e81 [DOI] [PubMed] [Google Scholar]
- 23.Quittner A, Modi A, Lemanek K, Ievers-Landis C, Rapoff M. Evidence-based assessment of adherence to medical treatments in pediatric psychology. J Pediatr Psychol. 2008;33(9):916–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGrady M, Hood K. Cognitive-behavioral therapy for adolescents with Type 1 diabetes and subclinical depressive symptoms. Diabetes Management. 2013;3(3):207–215 [Google Scholar]
- 25.Chorpita BF, Daleiden EL, Weisz JR. Identifying and selecting the common elements of evidence based interventions: a distillation and matching model. Ment Health Serv Res. 2005;7(1):5–20 [DOI] [PubMed] [Google Scholar]
- 26.Kendall PC, Beidas RS. Smoothing the trail for dissemination of evidence-based practices for youth: flexibility within fidelity. Prof Psychol Res Pr. 2007;38(1):13–20 [Google Scholar]
- 27.Hederos C, Janson S, Hedlin G. Group discussions with parents have long-term positive effects on the management of asthma with good cost-benefit. Acta Paediatr. 2005;94(5):602–608 [DOI] [PubMed] [Google Scholar]
- 28.La Roche M, Koinis-Mitchell D, Gualdron L. A culturally competent asthma management intervention: a randomized controlled pilot study. Ann Allergy Asthma. 2006;96(1):80–85 [DOI] [PubMed] [Google Scholar]
- 29.McGrady M, Hommel K. Medication adherence and health care utilization in pediatric chronic illness: a systematic review. Pediatrics. 2013;132(4):730–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson S, Latini D, Starr N, et al. Education of parents of infants and very young children with asthma: a developmental evaluation of the Wee Wheezers program. J Asthma. 1996;33(4):239–254 [DOI] [PubMed] [Google Scholar]
- 31.Anderson B, Wolf F, Burkhart M, Cornell R, Bacon G. Effects of peer-group intervention on metabolic control of adolescents with IDDM. Randomized outpatient study. Diabetes Care. 1989;12(3):179–183 [DOI] [PubMed] [Google Scholar]
- 32.Berkovitch M, Papadouris D, Shaw D, Onuaha N, Dias C, Olivieri N. Trying to improve compliance with prophylactic penicillin therapy in children with sickle cell disease. Br J Clin Pharmacol. 1998;45(6):605–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berrien V, Salazar J, Reynolds E, McKay K. Adherence to antiretroviral therapy in HIV-infected pediatric patients improves with home-based intensive nursing intervention. AIDS Patient Care STDS. 2004;18(6):355–363 [DOI] [PubMed] [Google Scholar]
- 34.Bloomfield S, Calder J, Chisholm V, et al. A project in diabetes education for children. Diabet Med. 1990;7(2):137–142 [DOI] [PubMed] [Google Scholar]
- 35.Brown J, Bakeman R, Celano M, Demi A, Kobrynski L, Wilson S. Home-based asthma education of young low-income children and their families. J Pediatr Psychol. 2002;27(8):677–688 [DOI] [PubMed] [Google Scholar]
- 36.Bonner S, Zimmerman B, Evans D, Irigoyen M, Resnick D, Mellins R. An individualized intervention to improve asthma management among urban Latino and African-American families. J Asthma. 2002;39(2):167–179 [DOI] [PubMed] [Google Scholar]
- 37.Burkhart P, Dunbar-Jacob J, Fireman P, Rohay J. Children’s adherence to recommended asthma self-management. Pediatr Nurs. 2002;28(4):409–414 [PubMed] [Google Scholar]
- 38.Burkhart P, Rayens M, Oakley M, Abshire D, Zhang M. Testing an intervention to promote children’s adherence to asthma self-management. J Nurs Scholarsh. 2007;39(2):133–140 [DOI] [PubMed] [Google Scholar]
- 39.Butz A, Kub J, Donithan M, et al. Influence of caregiver and provider communication on symptom days and medication use for inner-city children with asthma. J Asthma. 2010;47(4):478–485 [DOI] [PubMed] [Google Scholar]
- 40.Butz A, Syron L, Johnson B, Spaulding J, Walker M, Bollinger M. Home-based asthma self-management education for inner city children. Public Health Nurs. 2005;22(3):189–199 [DOI] [PubMed] [Google Scholar]
- 41.Chan D, Callahan C, Sheets S, Moreno C, Malone F. An Internet-based store-and-forward video home telehealth system for improving asthma outcomes in children. Am J Health Syst Pharm. 2003;60(19):1976–1981 [DOI] [PubMed] [Google Scholar]
- 42.DeBar L, Stevens V, Perrin N, et al. A primary care-based, multicomponent lifestyle intervention for overweight adolescent females. Pediatrics. 2012;129(3). Available at: www.pediatrics.org/cgi/content/full/129/3/e611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deforche B, De Bourdeaudhuij I, Tanghe A, Debode P, Hills A, Bouckaert J. Post-treatment phone contact: a weight maintenance strategy in obese youngsters. Int J Obes. 2005;29(5):543–546 [DOI] [PubMed] [Google Scholar]
- 44.Ducharme F, Zemek R, Chalut D, et al. Written action plan in pediatric emergency room improves asthma prescribing, adherence, and control. Am J Respir Crit Care Med. 2011;183(2):195–203 [DOI] [PubMed] [Google Scholar]
- 45.Elamin A, Eltayeb B, Hasan M, Hofvander Y, Tuvemo T. Effect of dietary education on metabolic control in children and adolescents with type-I diabetes-mellitus. Diabetes Nutr Metab. 1993;6(4):223–229 [Google Scholar]
- 46.Farber H, Oliveria L. Trial of an asthma education program in an inter-city pediatric emergency department. Pediatr Asthma Aller. 2004;17(2):107–115 [Google Scholar]
- 47.Gorelick M, Meurer J, Walsh-Kelly C, et al. Emergency department allies: a controlled trial of two emergency department-based follow-up interventions to improve asthma outcomes in children. Pediatrics. 2006;117:S127–S134 [DOI] [PubMed] [Google Scholar]
- 48.Grey M, Boland E, Davidson M, Yu C, Sullivan-Bolyai S, Tamborlane W. Short-term effects of coping skills training as adjunct to intensive therapy in adolescents. Diabetes Care. 1998;21(6):902–908 [DOI] [PubMed] [Google Scholar]
- 49.Howe C, Jawad A, Tuttle A, et al. Education and telephone case management for children with type 1 diabetes: a randomized controlled trial. J Pediatr Nurs. 2005;20(2):83–95 [DOI] [PubMed] [Google Scholar]
- 50.Hughes DM, McLeod M, Garner B, Goldbloom RB. Controlled trial of a home and ambulatory program for asthmatic children. Pediatrics. 1991;87(1):54–61 [PubMed] [Google Scholar]
- 51.Lawson M, Cohen N, Richardson C, Orrbine E, Pham B. A randomized trial of regular standardized telephone contact by a diabetes nurse educator in adolescents with poor diabetes control. Pediatr Diabetes. 2005;6(1):32–40 [DOI] [PubMed] [Google Scholar]
- 52.LeBaron S, Zeltzer L, Ratner P, Kniker W. A controlled study of education for improving compliance with cromolyn sodium (Intal): the importance of physician-patient communication. Ann Allergy. 1985;55(6):811–818 [PubMed] [Google Scholar]
- 53.McNabb W, Quinn M, Murphy D, Thorp F, Cook S. Increasing children’s responsibility for diabetes self-care: the In Control study. Diabetes Educ. 1994;20(2):121–124 [DOI] [PubMed] [Google Scholar]
- 54.Mitchell E, Ferguson V, Norwood M. Asthma education by community child health nurses. Arch Dis Child. 1986;61(12):1184–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore E, Williams A, Manias E, Varigos G, Donath S. Eczema workshops reduce severity of childhood atopic eczema. Australas J Dermatol. 2009;50(2):100–106 [DOI] [PubMed] [Google Scholar]
- 56.Otsuki M, Eakin M, Rand C, et al. Adherence feedback to improve asthma outcomes among inner-city children: a randomized trial. Pediatrics. 2009;124(6):1513–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rapoff M, Belmont J, Lindsley C, Olson N, Morris J, Padur J. Prevention of nonadherence to nonsteroidal anti-inflammatory medications for newly diagnosed patients with juvenile rheumatoid arthritis. Health Psychol. 2002;21(6):620–623 [DOI] [PubMed] [Google Scholar]
- 58.Smith N, Seale J, Ley P, Shaw J, Bracs P. Effects of intervention on medication compliance in children with asthma. Med J Aust. 1986;144(3):119–122 [DOI] [PubMed] [Google Scholar]
- 59.Staab D, von Rueden U, Kehrt R, et al. Evaluation of a parental training program for the management of childhood atopic dermatitis. Pediatr Allergy Immunol. 2002;13(2):84–90 [DOI] [PubMed] [Google Scholar]
- 60.Teach S, Crain E, Quint D, Hylan M, Joseph J. Improved asthma outcomes in a high-morbidity pediatric population: results of an emergency department-based randomized clinical trial. Arch Pediatr Adolesc Med. 2006;160(5):535–541 [DOI] [PubMed] [Google Scholar]
- 61.Van Es S, Nagelkerke A, Colland V, Scholten R, Bouter L. An intervention programme using the ASE-model aimed at enhancing adherence in adolescents with asthma. Patient Educ Couns. 2001;44(3):193–203 [DOI] [PubMed] [Google Scholar]
- 62.Watson W, Gillespie C, Thomas N, et al. Small-group, interactive education and the effect on asthma control by children and their families. CMAJ. 2009;181(5):257–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hederos C, Janson S, Hedlin G. Six-year follow-up of an intervention to improve the management of preschool children with asthma. Acta Paediatr. 2009;98(12):1939–1944 [DOI] [PubMed] [Google Scholar]