Abstract
Several critical cell functions are influenced not only by internal cellular machinery but also by external mechanical and biochemical cues from the surrounding microenvironment. Slight changes to the microenvironment can result in dramatic changes to the cell's phenotype; for example, a change in the nutrients or pH of a tumor microenvironment can result in increased tumor metastasis. While cellular fate and the regulators of cell fate have been studied in detail for several decades now, our understanding of the extracellular regulators remains qualitative and far from comprehensive. In this review, we discuss the microenvironment influence on cell fate in terms of adhesion, migration, and differentiation and focus on both developments in experimental and computation tools to analyze cellular fate.
Keywords: extracellular matrix, cell migration, cell adhesion, computational modeling, cell fate
much of the understanding of underlying mechanisms governing cell functions such as proliferation, differentiation, and migration comes as a result of research focused on cells cultured in artificial two-dimensional (2D) environments or monolayers (15, 49). While culturing of monolayers is less complex, provides repeatability, and is cost effective and less time consuming than using animal models, the monolayers lack the morphology, mechanics, and the biochemical characteristics of the cells in vivo (25,41). Recent studies have shown that three-dimensional (3D) environments, which more accurately represent physiologically relevant environment, can lead to cellular processes and fates that are substantially different from cellular behavior observed in 2D environments (10, 15, 29).
Within the last decade, 3D environments, consisting of both synthetic and nonsynthetic substrates, have been utilized to more accurately model the cell microenvironment found in vivo (75). These substrates include polymer networks (66), electrospun nanofibers (39), polydimethylsiloxane (75), matrigel (23), and collagen (27). The structure of the microenvironment is determined by the extracellular matrix (ECM), which consists of tissue-specific proteins and polysaccharides (16, 56). 2D culturing of adherent cells results in adhesion junctions with localized protein aggregates along the basal surface. In 3D, however, these proteins do not form aggregates but instead are dispersed throughout the cell and local activation results in protrusion formation (15, 24). In addition to providing structural support through the cell-ECM adhesion complexes, the ECM selectively binds and releases growth factors, allowing for temporary sequestration and controlled release of these factors (67).
Aided by advancements in computing and higher-resolution quantitative data, computational modeling has become a valuable tool providing a rapid and high-throughput method to investigate cellular fate (46). Computational models are able to bridge our understanding in processes where experimental studies are either technically or financially prohibitive. In addition, they are able to provide predictive information to guide future experiments. Models at the molecular, macromolecular, and tissue levels have provided information about cellular form and function. Historically, like experimental approaches, these studies have been focused on 2D environments, but recent years have seen developments of computational models of cellular fate in native like 3D environments (64).
In the following sections, we will cover recent developments in both experiment and modeling on microenvironmental regulation of cellular differentiation, adhesion, and migration, in both 2D and 3D environments and discuss current areas of growth and potential future directions to get new insights into cellular fate determination in native environments.
MECHANOTRANSDUCTION
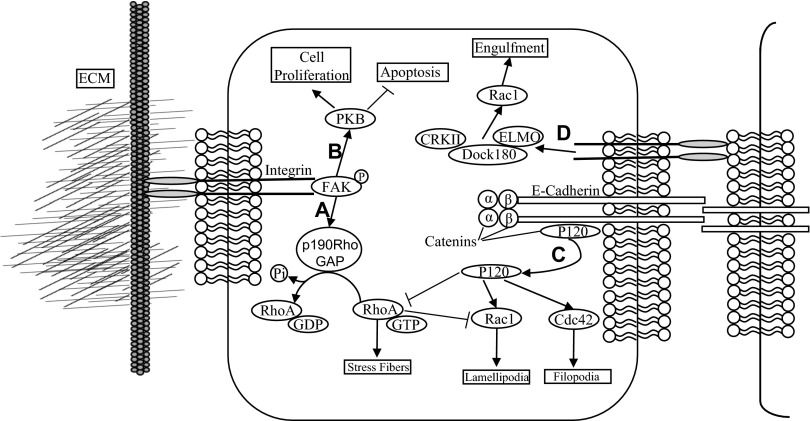
Cells are capable of converting mechanical cues into biochemical signals by mechanotransduction systems (6, 60). This capability is due to the interaction between integrins and several adaptor proteins, including focal adhesion kinase (FAK), p130Cas, paxillin, talin, and vinculin. Cardiosphere-derived cells (CDCs) sense the stiffness of the surrounding ECM through p190RhoGAP, a guanosine triphosphatase-activating protein for RhoA, which mediates endothelial differentiation of CDCs (Fig. 1A). A global decrease in the presence of p190RhoGAP, in addition to a local decrease in the lamellipodia, leads to a change in cell morphology to a more rounded cell shape, which, when coupled with decreased integrin abundance, can reduce cell contact with the ECM resulting in spheroid-like aggregates. These tight cell aggregates require E-cadherin and p120-catenin in addition to an increased nuclear localization of Yes-associated protein (YAP) (48). The yorkie homologs YAP and TAZ act as the transcription regulators independently of the NF2/Hippo/LATS pathway (19). In microvascular epithelial cells, p190RhoGAP sequesters TFII-I in the cytoplasm. Once released TFII-I results in transcription and upregulation of vascular endothelial growth factor receptor 2 (VEGFR2), leading to angiogenesis (53). The composition and stiffness of the ECM are not the only factors that can result in mechanotransduction signaling. The nanotopography of the ECM is capable of influencing differentiation, migration, and proliferation (38, 39). The nanotopography of the ECM alters the focal adhesions and cytoskeletal organization of human mesenchymal stem cells (78). This likely occurs through the FAK pathway and could result in YAP/TAZ transcriptional regulation to dictate cell lineage (73).
Fig. 1.
Cell-extracellular matrix (ECM) and cell-cell interaction mediated by integrins and E-cadherin. Integrin-mediated focal adhesion kinase (FAK) phosphorylation results in activation of p190RhoGAP leading to a reduction in stress fibers (A) and activation of protein kinase B (PKB) leading to cell survival (B). Disassociation of p120 catenin from the E-cadherin-catenin complex (C) results in activation of Rac1 and Cdc42 as well as inhibition of RhoA activity. Engulfment of adjacent apoptotic cells (D) is induced by Rac1 activation mediated by integrin.
MICROENVIRONMENTAL REGULATION OF CELL DIFFERENTIATION
Regulation of stem cell self-renewal and differentiation is dependent on the ECM, growth factors, morphogenic factors, small molecules, and cytokines that are secreted by the stem cells and the surrounding cells that make up the niche (47, 69, 79). The stiffness of the ECM has a direct effect on the differentiation of several stem cell lineages. When cultured in matrigel, adult neural stem cells differentiated into glial cells when the stiffness of the ECM was between 1 and 10 kPa and differentiated into neurons when the stiffness was between 100 and 500 Pa. Upon decreasing the stiffness of the matrigel to 10 Pa, the adult neural stem cells were no longer able to self-renew or differentiate (66). The influence of ECM stiffness on differentiation also occurs for naïve mesenchymal stem cells. When cultured in ECM with stiffness of 1, 10, or 100 kPa, the cells undergo neurogenesis, myogenesis, or osteogenesis, respectively. These three stiffness levels correspond with the stiffness normally found in brain, muscle, and bone tissue, respectively (21).
In separate experiments, mesenchymal stem cells cultured on 2D environments displayed a correlation between cell type commitment and morphology of the stem cell. However, investigators have determined that, in 3D culture environments, it is not the morphology that correlates with the cell type commitment but the stiffness of the environment. Softer environments between 2.5 and 5 kPa result in adipogenic commitment, while firmer environments between 11 and 30 kPa result in osteogenic commitment (32).
Skeletal muscle differentiation requires cell-ECM interactions (58). Muscle stem cells that naturally reside in adult tissues rapidly proliferate in vivo but do not have this rapid proliferation when cultured on rigid plastic dishes that have a stiffness of 106 kPa. However, if the cells are cultured in matrigel, with a stiffness of 12 kPa, that mimics the stiffness of muscle, the muscle stem cells will continue to self-renew in vitro. Furthermore, these cells can be transplanted back into mice and significantly aid in the regeneration of muscle (9). ECM stiffness regulates chondrocyte differentiation through ROCK signaling and upregulation of the TGF-β. Chondrocyte differentiation requires Smad3 phosphorylation, which is optimized at the same ECM stiffness that induces chondrocyte gene expression (1).
There have been many computational models designed to predict differentiation of stems cells depending on soluble factors; however, models designed to take into account the physical conditions and makeup of the microenvironment are lacking (75).
MICROENVIRONMENTAL REGULATION OF ADHESION AND MIGRATION
Cell adhesion and migration have been and continue to be a major area of investigation of microenvironmental regulation of cellular form and function (7). Much work has been done in understanding molecular and macromolecular regulators and signaling cascades affected by microenvironmental properties. In particular, integrins and their interactions with the ECM have been studied extensively over the years in their role in maintaining homeostasis and aiding the progression of many diseases (51,52). Integrins are heterodimeric cell surface receptors made up of α- and β-subunits that bind to specific ECM components and are responsible for the activation of a variety of cell survival and cell motility pathways (70). Following integrin ligation, FAK becomes autophosphorylated at Y397, which in turn provides a binding site for the p85 subunit of phosphatidylinositide 3-kinase (PI3K), causing downstream activation of protein kinase B (PKB), inhibiting apoptosis, and promoting cell proliferation (Fig. 1B) (11). When this pathway is intact, the loss of adhesion to the ECM results in a decrease of PI3K activity resulting in anoikis (44). Integrins are involved not only in cell-ECM signaling but in cell-cell signaling as well. Apoptotic cells displaying phosphatidylserine on their cell membranes are identified by integrins in other cells. This triggers a pathway activating the Dock180, CRKII, ELMO complex and then activating Rac1, triggering the engulfment of the adjacent apoptotic cell (30,37,43).
Cancer cell metastasis requires the cell to undergo epithelial-to-mesenchymal transition (EMT) and acquire anoikis resistance (55). Cells that undergo EMT can be identified by the loss of the epithelial proteins β-catenin, γ-catenin, and E-cadherin, in addition to increased expression of the mesenchymal proteins vimentin, fibronectin, and N-cadherin, which are characteristic of EMT (36). E-cadherin forms a complex with p120, α-, and β-catenins that is critical for cell-cell adhesion. Dissociation of this complex results in p120-catenin's no longer being localized to the membrane, allowing p120-catenin to activate Rac1 and Cdc42 while inhibiting RhoA activity, potentially leading to lamellipodia and filopodia formation (Fig. 1C) (14). Matrix metalloproteases (MMPs) degrade ECM and cleave E-cadherin at the cell surface, releasing a soluble 80 kDa cleavage product (57). This soluble cleavage product of E-cadherin upregulates MMP2, MMP9, and MMP14, resulting in further cell invasion and anoikis resistance (56). Inhibition of MMP activity results in reduced speed and persistence of cell migration in 3D collagen matrices (39). These experimental results are described by modeling the movement of cells through a matrix, accounting for modulus, viscosity, and ligand density of the matrix, as well as the cell geometry and receptor density of the cells (28).
The microenvironment of solid tumors is more acidic than normal (pH 6.5–6.9 compared with pH 7.2–7.4) due to poor perfusion and increased anaerobic metabolism (22). This acidic environment further increases the degradation of the ECM by MMPs and results in increased secretion of VEGF and angiogenesis (22). Other proteolytic enzymes, including urokinase-type plasminogen activator (uPA), and their effects on cancer cell invasion of neighboring tissue have been modeled to occur as a result of both chemotactic and haptotactic reactions of cancer cells to the spatio-temporal effects of the uPA system (2).
The core physiological properties of the majority of cells within a tumor remain identical even though the phenotypes of these cells may vary dramatically. Models coupling tumor cell proliferation, cell death, angiogenesis, and blood flow predict that the tumor will initially develop a necrotic core at its center and later an area with high microvascular density at the periphery. This is because of the lack of nutrients delivered to the center of the tumor and the increased demand for nutrients of the rapidly dividing cells located at the periphery (12).
In these harsh environments containing low oxygen and nutrient levels, invasive cells are predicted to be responsible for the growth of the primary tumor as they selected due to their invasiveness. However, small changes in the oxygen and nutrient levels may cause a separate subpopulation of the tumor cells to be preferentially selected and account for the growth of the primary tumor. Furthermore, the model predicts that branches composed of tumor cells will be formed, which has been experimentally confirmed (34).
Cells migrate at a rate dependent on several factors including the strength and number of cell-cell and cell-ECM adhesions in addition to the stiffness of the ECM. During migration lamellipodia form focal adhesions between the cell and the ECM. When cultured in 2D the cell tugs against these focal adhesions as a means to test the stiffness of the ECM via the FAK-phosphopaxillin-vinculin pathway, and it is this tugging that allows the cell to identify the stiffness of the ECM and continue to migrate in that direction (62). The advent of microfluidics has enabled scientists to more accurately mimic the 3D physiological environment in terms of structure and soluble factors (26, 63, 80). Microfluidic technology involves the manipulation of nanoliters of liquid within channels of microdevices (45). This allows for small quantities of soluble factors to be controlled and delivered to small environments (18). Microfluidic systems utilizing an ECM gradient consisting of either collagen I, laminin, or fibronectin result in migration of cells along the gradient. In the case of fibroblasts these ECM gradients are sensed by the signaling proteins FAK, neural Wiskott-Aldrich syndrome protein (N-WASP), and Cdc42. While increasing the ECM gradient does increase the likelihood of a cell migrating in that direction it does not increase the speed of migration. N-WASP and activated Cdc42 signaling result in the directional migration toward stiffer ECM, while the FAK signaling increases the persistence of the migration (65).
In addition to experimental studies, computational and mathematical modeling of cell adhesion and migration, in both 2D and 3D, has provided new insights into cellular fate. Computational modeling of focal adhesion dynamics, actin motor activity, lamellipodia protrusion, and remodeling of nuclear and cellular membranes predicts the increase in the rate of cell migration by the anchorage of the cell's trailing edge with actin stress fibers (42). Experimental findings that the geometry of the cell microenvironment determines the orientation and location of the contractile stress fibers and the force application sites support this predictive model. When no ECM is available for cell adhesion, large stress fibers form along the cell edges to confine the cell, resulting in traction force applied at cell apices. Epithelial cells, which have high levels of contractility, are observed in either disorganized multicellular structures or detaching from each other as determined by measuring the degree of contraction of a cell by micropatterning of poly-acrylamide gels (74). When considering cell-cell and cell-ECM adhesions in addition to substrate gradients, computational models rooted in the biophysical laws of conservation of mass, cell migration, and cell survival predict that the cells with more aggressive phenotypes will migrate up the substrate gradient beyond the tumor mass, resulting in a loss of cell adhesion and an increase in anoikis resistance (8). Creating a hybrid of a random-walk model to control for cell migration and interaction, as well as a second model to determine chemotaxis, enables us to determine the extent of proliferation, death, and cell-cell adhesion at the individual cell level. Simulations using this model predicts conditions such as hypoxia and a heterogeneous ECM increase selection in the tumor cell population, resulting in an invasive tumor containing primarily a few clones with aggressive traits. At a more physiological pH and homogeneous ECM conditions these same clones with aggressive traits are expected to coexist with nonaggressive phenotypes resulting in a more heterogeneous tumor (3, 4). An off-lattice version of the hybrid model decreases the effects of anisotropy on both cell migration and tumor morphology and addresses the mechanical and physical aspects of cell behavior in more detail. This off-lattice hybrid model predicts that while tumors consisting of cells with multiple phenotypes eventually form a spherical shape, they will initially develop asymmetric tumor morphology. This is due to the more aggressive cells that leave the tumor being distributed nonuniformly throughout the tumor (33).
CONCLUSIONS AND FUTURE DIRECTIONS
Over the last several decades we have made tremendous progress in understanding cellular form, function, and the underlying machinery that governs cellular fate and processes. Yet, most of our understanding has been either qualitative, rooted in experiments that have been carried out in artificial environments far from in vivo, or blind to the surrounding microenvironment. Fortunately, the last few years have seen a change in our approach. This review highlights recent developments in both computational and experimental approaches (Table 1) focused on understanding cellular fate as an integrated system, where both the cell and the matrix interact synergistically.
Table 1.
Current experimental and computation models of the microenvironment influence on cell fate
| Type of Model | Characteristic Being Tested | Results Influenced by the Characteristic | Reference |
|---|---|---|---|
| Experimental | ECM stiffness | lineage dependent on stiffness | 9, 21, 32, 65 |
| cocultured cells | lineage dependent on cocultured cells | 66, 67 | |
| MMP activity | inhibition of MMP activity results in reduced speed and persistence of cell movement | 40 | |
| Computational | MMP activity | inhibition of MMP activity results in reduced speed and persistence of cell movement | 28 |
| uPA activity | cell invasion result of chemotactic and haptotactic reactions | 2 | |
| proliferation, angiogenesis | necrotic core within tumor, high microvascular density at periphery | 12 | |
| proliferation, migration | cancer cells with aggressive phenotypes preferentially migrate | 8 | |
| migration, chemotaxis | hypoxia and heterogeneous ECM result in more aggressive tumor cells | 3, 4 | |
| oxygen, nutrient levels | subpopulation preferentially accounts for tumor growth | 34 |
ECM, extracellular matrix; MMP, matrix metalloproteinase; uPA, urokinase-type plasminogen activator.
The system level thinking of the synergistic interaction between cell and matrix is of paramount importance in the fields of drug discovery, cancer research, and tissue regeneration because of the influence of the microenvironment on cell properties. 3D cell culture has already started to become adopted in drug discovery where high content screening is heavily used (35, 50). Furthermore, cancer treatments that have appeared promising when in monolayers have ended up being less effective when used in 3D or animal models (13). In several cases drug ineffectiveness has been attributed to cell-ECM interactions (5, 16, 59, 68). The effects of the microenvironment on adhesion and migration explain the importance of the microenvironment on tumor progression by identifying not only the structure of solid tumors but also microenvironmental factors contributing to invasion. Modeling has been beneficial in these aspects as various models have been able to predict the characteristics of solid tumors, in terms of a necrotic core and high amounts of microvascular on the periphery as well as the methods of local invasion (7, 36, 39, 56, 57). Given these validated models, consideration should be given to link between hypoxia and other harsh microenvironments to tumor metastasis in future research. Modeling itself can greatly aid future research by providing a rapid, inexpensive, high-throughput method that can be used for both prediction and explanation of experimental results. Ultimately computational modeling enables the complex system of the cell to be broken down into key pathways, predicts how these pathways affect the properties of the cell, and can be very valuable when resources are limited. The relative dearth of computational models predicting ECM stiffness-dependent lineage specification is likely due to the limited understanding of precisely how cells perceive the surrounding microenvironment and how this mechanochemical signal is transduced into the cell. Identifying possible key components to the mechanotransduction system could greatly benefit the creation of computation models for ECM stiffness-dependent lineage specification.
Despite recent developments, much more needs to be done in developing a systems-level, integrated, and quantitative understanding of microenvironmental regulation of cellular fate. This requires development of new tools, accessible to researchers in both basic and applied sciences, with which to analyze how changes in microenvironmental structure, stiffness, mechanics, or biochemical makeup influence cellular decisions. Microfluidic systems have shown initial promise, but their use has not penetrated widely. Research in tissue regeneration and stem cell differentiation can potentially benefit by adopting 3D culturing approaches as the microenvironment influences the lineage that the stem cell differentiates into (10, 17, 25, 31, 61). In addition to the effects on differentiation, the microenvironment influences the migration of cells; both processes are vital for wound healing, and research in this field would be greatly aided by adoption of 3D culture systems.
Perhaps at a more fundamental level, it is the importance of using quantitative tools and gathering quantitative information that needs to be better appreciated for our understanding of cellular fate. A better appreciation for tools that can give precise and quantitative information will not only aid in a superior fundamental understanding of the key processes, but also aid in developing more realistic and predictive models of cellular fate, form, and function in native environments. This requires not only improvement in tool development but also creation of intellectual bridges between modelers, engineers, biologists, and clinicians to overcome the inherent unease and distrust that may have plagued truly collaborative interactions. Fortunately, many of those barriers have started to fall, yet more needs to be done to make long-lasting impact on our fundamental and applied knowledge of cellular fate in complex native environments.
GRANTS
The authors thank the National Institutes of Health (U01-CA-177799) for its generous support.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.B.B. and M.H.Z. conception and design of research; A.B.B. prepared figures; A.B.B. drafted manuscript; A.B.B. and M.H.Z. edited and revised manuscript; A.B.B. and M.H.Z. approved final version of manuscript.
REFERENCES
- 1.Allen J, Cooke M, Alliston T. ECM stiffness primes the TGFβ pathway to promote chondrocyte differentiation. Mol Biol Cell 23: 3731–3742, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andasari V, Gerisch A, Lolas G, South AP, Chaplain MA. Mathematical modeling of cancer cell invasion of tissue: biological insight from mathematical analysis and computational simulation. J Math Biol 63: 141–171, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Anderson ARA. A hybrid mathematical model of solid tumour invasion: the importance of cell adhesion. Math Med Biol 22: 163–186, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Anderson ARA, Weaver AM, Cummings PT, Quaranta V. Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell 127: 905–915, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Aoudjit F, Vuori K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene 20: 4995–5004, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Arnadóttir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu Rev Biophys 39: 111–137, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci 125: 3015–3024, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bearer E, Lowengrub J, Frieboes H. Multiparameter computational modeling of tumor invasion. Cancer Res 69: 4493–4501, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329: 1078–1081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro–a growing case for three-dimensional (3D) culture systems. Semin Cancer Biol 15: 405–412, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Boudreau NJ, Jones PL. Extracellular matrix and integrin signalling: the shape of things to come. Biochem J 339: 481–488, 1999 [PMC free article] [PubMed] [Google Scholar]
- 12.Cai Y, Xu S, Wu J, Long Q. Coupled modelling of tumour angiogenesis, tumour growth and blood perfusion. J Theor Biol 279: 90–101, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Castells M, Thibault B, Delord JP, Couderc B. Implication of tumor microenvironment in chemoresistance: tumor-associated stromal cells protect tumor cells from cell death. Int J Mol Sci 13: 9545–9571, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christofori G. New signals from the invasive front. Nature 441: 444–450, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science 294: 1708–1712, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Dangi-Garimella S, Krantz SB, Barron MR, Shields MA, Heiferman MJ, Grippo PJ, Bentrem DJ, Munshi HG. Three-dimensional collagen I promotes gemcitabine resistance in pancreatic cancer through MT1-MMP-mediated expression of HMGA2. Cancer Res 71: 1019–1028, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Lullo GA, Sweeney SM, Korkko J, Ala-Kokko L, San Antonio JD. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J Biol Chem 277: 4223–4231, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Domachuk P, Tsioris K, Omenetto FG, Kaplan DL. Bio-microfluidics: biomaterials and biomimetic designs. Adv Mater 22: 249–260, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature 474: 179–183, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Earls JK, Jin S, Ye K. Mechanobiology of human pluripotent stem cells. Tissue Eng 19: 420–430, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engler A, Sen S, Sweeney H, Discher D. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Estrella V, Chen T, Lloyd M, Wojtkowiak J, Cornnell HH, Ibrahim-Hashim A, Bailey K, Balagurunathan Y, Rothberg JM, Sloane BF, Johnson J, Gatenby RA, Gillies RJ. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res 73: 1524–1535, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fessart D, Begueret H, Delom F. Three-dimensional culture model to distinguish normal from malignant human bronchial epithelial cells. Eur Respir J 42: 1345–1356, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Fraley SI, Feng Y, Krishnamurthy R, Kim DH, Celedon A, Longmorev Wirtz D. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat Cell Biol 12: 598–604, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 7: 211–224, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Gupta K, Kim DH, Ellison D, Smith C, Kundu A, Tuan J, Suhc KY, Levchenko A. Lab-on-a-chip devices as an emerging platform for stem cell biology. Lab Chip 10: 2019–2031, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Hadjipanayi E, Brown RA, Mudera V. Interface integration of layered collagen scaffolds with defined matrix stiffness : implications for sheet-based tissue engineering. J Tissue Eng Regen Med 3: 230–241, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Harjanto D, Zaman MH. Computational study of proteolysis-driven single cell migration in a three-dimensional matrix. Ann Biomed Eng 38: 1815–1825, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Harjanto D, Zaman MH. Matrix mechanics and receptor-ligand interactions in cell adhesion. Org Biomol Chem 8: 299–304, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Hsu TY, Wu YC. Engulfment of apoptotic cells in C. elegans is mediated by integrin alpha/SRC signaling. Curr Biol 20: 477–486, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Huang NF, Li S. Regulation of the matrix microenvironment for stem cell engineering and regenerative medicine. Ann Biomed Eng 39: 1201–1214, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huebsch N, Arany PR, Mao AS, Shvartsman D, Alil OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater 9: 518–526, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeon J, Quaranta V, Cummings PT. An off-lattice hybrid discrete-continuum model of tumor growth and invasion. Biophys J 98: 37–47, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiao Y, Torquato S. Emergent behaviors from a cellular automaton model for invasive tumor growth in heterogeneous microenvironments. PLoS Comput Biol 7: e1002314, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Justice BA, Badr NA, Felder RA. 3D cell culture opens new dimensions in cell-based assays. Drug Discov Today 14: 102–107, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420–1428, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kato S, Shiratsuchi A, Nagaosa K, Nakanishi Y. Phosphatidylserine- and integrin-mediated phagocytosis of apoptotic luteal cells by macrophages of the rat. Dev Growth Differ 47: 153–161, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Kim DH, Provenzano PP, Smith CL, Levchenko A. Matrix nanotopography as a regulator of cell function. J Cell Biol 197: 351–360, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim H, Guo TW, Wu AP, Wells A, Gertler FB, Lauffenburger DA. Epidermal growth factor - induced enhancement of glioblastoma cell migration in 3D arises from an intrinsic increase in speed but an extrinsic matrix- and proteolysis-dependent increase in persistence. Mol Biol 19: 4249–4259, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim HN, Jiao A, Hwang NS, Kim MS, Kang DH, Kim DH, Suh KY. Nanotopography-guided tissue engineering and regenerative medicine. Adv Drug Deliv Rev 65: 536–558, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim JB. Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol 15: 365–77, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Kim MC, Neal DM, Kamm RD, Asada HH. Dynamic modeling of cell migration and spreading behaviors on fibronectin coated planar substrates and micropatterned geometries. PLoS Comput Biol 9: e1002926, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim S, Park SY, Kim SY, Bae DJ, Pyo JH, Hong M, In-San Kim IS. Cross talk between engulfment receptors stabilin-2 and integrin αvβ5 orchestrates engulfment of phosphatidylserine-exposed erythrocytes. Mol Cell Biol 32: 2698–2708, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim YN, Koo KH, Sung JY, Yun UJ, Kim H. Anoikis resistance: an essential prerequisite for tumor metastasis. Int J Cell Biol 2012: 306879, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobel S, Lutolf MP. Biomaterials meet microfluidics: building the next generation of artificial niches. Curr Opin Biotechnol 22: 690–697, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Kolitz SE, Lauffenburger DA. Measurement and modeling of signaling at the single-cell level. Biochemistry 51: 7433–7443, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kshitiz, Kim DH, Beebe DJ, Levchenko A. Micro- and nanoengineering for stem cell biology: the promise with a caution. Trends Biotechnol 29: 399–408, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kshitiz, Hubbi ME, Ahn EH, Downey J, Afzal J, Kim DH, Rey S, Chang C, Kundu A, Semenza GL, Abraham RM, Levchenko A. Matrix rigidity controls endothelial differentiation and morphogenesis of cardiac precursors. Sci Signal 5: ra41, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kshitiz, Park J, Kim P, Helen W, Engler AJ, Levchenko A, Kim DH. Control of stem cell fate and function by engineering physical microenvironments. Integr Biol 4: 1008, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar N, Zaman MH, Kim HD, Lauffenburger DA. A high-throughput migration assay reveals HER2-mediated cell migration arising from increased directional persistence. Biophys J 91: L32–L34, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai Y, Asthana A, Kisaalita WS. Biomarkers for simplifying HTS 3D cell culture platforms for drug discovery: the case for cytokines. Drug Discov Today 16: 293–297, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Liu S, Leask A. Integrin β1 is required for dermal homeostasis. J Invest Dermatol 133: 899–906, 2013 [DOI] [PubMed] [Google Scholar]
- 53.Lukashev ME, Werb Z. ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol 8: 437–441, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Mammoto A, Connor KM, Tadanori Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LEH, Ingber DE. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature 457: 1103–1108, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mammoto T, Mammoto A, Ingber DE. Mechanobiology and developmental control. Annu Rev Cell Dev Biol 29: 27–61, 2013 [DOI] [PubMed] [Google Scholar]
- 56.Nagaprashantha LD, Vatsyayan R, Lelsani PCR, Awasthi S, Singhal SS. The sensors and regulators of cell-matrix surveillance in anoikis resistance of tumors. Int J Cancer 128: 743–752, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nawrocki-Raby B, Gilles C, Polette M, Bruyeel E, Laronze JY, Bonnet N, Jean-Michel Foidart JM, Mareel M, Birembaut P. Upregulation of MMPs by soluble E-cadherin in human lung tumor cells. Int J Cancer 105: 790–795, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Noë V, Fingleton B, Jacobs K, Crawford HC, Vermeulen S, Steelant W, Bruyneel E, Matrisian LM, Mareel M. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci 114: 111–118, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Osses N, Brandan E. ECM is required for skeletal muscle differentiation independently of muscle regulatory factor expression. Am J Physiol Cell Physiol 282: C383–C394, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Paraiso KHT, Smalley KSM. Fibroblast-mediated drug resistance in cancer. Biochem Pharmacol 85: 1033–1041, 2013 [DOI] [PubMed] [Google Scholar]
- 61.Pedersen JA, Swartz MA. Mechanobiology in the third dimension. Ann Biomed Eng 33: 1469–1490, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Plotnikov SV, Pasapera AM, Sabass B, Waterman CM. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 151: 1513–1527, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Polacheck WJ, Li R, Uzel SGM, Kamm RD. Microfluidic platforms for mechanobiology. Lab Chip 13: 2252–2267, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rangarajan R, Zaman MH. Modeling cell migration in 3D: status and challenges. Cell Adh Migr 2: 106–109, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rhoads DS, Guan JL. Analysis of directional cell migration on defined FN gradients: role of intracellular signaling molecules. Exp Cell Res 313: 3859–3867, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saha K, Keung A, Irwin E, Li Y, Little L. Substrate modulus directs neural stem cell behavior. Biophys J 95: 4426–4438, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 17: 153–162, 2009 [DOI] [PubMed] [Google Scholar]
- 68.Serebriiskii I, Castelló-Cros R, Lamb A, Golemis EA, Cukierman E. Fibroblast-derived 3D matrix differentially regulates the growth and drug-responsiveness of human cancer cells. Matrix Biol 27: 573–585, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spiegel A, Kalinkovich A, Shivtiel S, Kollet O, Lapidot T. Stem cell regulation via dynamic interactions of the nervous and immune systems with the microenvironment. Cell Stem Cell 3: 484–492, 2008 [DOI] [PubMed] [Google Scholar]
- 70.Stupack DG. Get a ligand, get a life: integrins, signaling and cell survival. J Cell Sci 115: 3729–3738, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676, 2006 [DOI] [PubMed] [Google Scholar]
- 72.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Teo BKK, Wong ST, Lim CK, Kung TYS, Yap CH, Yamini Ramagopal Y, Romer LH, Yim EKF. Nanotopography modulates mechanotransduction of stem cells and induces differentiation through focal adhesion kinase. ACS Nano 7: 4785–4598, 2013 [DOI] [PubMed] [Google Scholar]
- 74.Tseng Q, Wang I, Duchemin-Pelletier E, Azioune A, Carpi N, Gao J, Filhol O, Piel M, Thery M, Martial Balland M. A new micropatterning method of soft substrates reveals that different tumorigenic signals can promote or reduce cell contraction levels. Lab Chip 11: 2231–2240, 2011 [DOI] [PubMed] [Google Scholar]
- 75.Viswanathan S, Zandstra PW. Towards predictive models of stem cell fate. Cytotechnology 41: 75–92, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Watt FM, Huck WTS. Role of the extracellular matrix in regulating stem cell fate. Nat Rev Mol Cell Biol 14: 467–473, 2013 [DOI] [PubMed] [Google Scholar]
- 77.Wang PY, Tsai WB, Voelcker NH. Screening of rat mesenchymal stem cell behaviour on polydimethylsiloxane stiffness gradients. Acta Biomater 8: 519–530, 2012 [DOI] [PubMed] [Google Scholar]
- 78.Yim EKF, Darling EM, Kulangara K, Guilak F, Leong KW. Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials 31: 1299–1306, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang H, Dai S, Bi J, Liu KK. Biomimetic three-dimensional microenvironment for controlling stem cell fate. Interface Focus 1: 792–803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Z, Nagrath S. Microfluidics and cancer: are we there yet? Biomed Microdevices 15: 595–609, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]