Abstract
Background and Objectives:
The aim of this study was to compare oncologic outcomes after laparoscopic nephroureterectomy, hand-assisted laparoscopic nephroureterectomy, and open nephroureterectomy for upper urinary tract urothelial cancer.
Methods:
Between April 1995 and August 2010, 189 patients underwent laparoscopic nephroureterectomy, hand-assisted laparoscopic nephroureterectomy, or open nephroureterectomy for upper urinary tract urothelial cancer. Of these patients, 110 with no previous or concurrent bladder cancer or any metastatic disease were included in this study. Cancer-specific survival, recurrence-free survival, and intravesical recurrence-free survival rates were analyzed by the Kaplan-Meier method and compared with the log-rank test. The median follow-up period for the cohort was 70 months (range, 6–192 months).
Results:
The 3 groups were well matched for tumor stage, grade, and the presence of lymphovascular invasion and concomitant carcinoma in situ. The estimated 5-year cancer-specific survival rates were 81.1%, 65.6%, and 65.2% for laparoscopic nephroureterectomy, hand-assisted laparoscopic nephroureterectomy, and open nephroureterectomy, respectively (P = .4179). The estimated 5-year recurrence-free survival rates were 33.8%, 10.0%, and 41.2% for laparoscopic nephroureterectomy, hand-assisted laparoscopic nephroureterectomy, and open nephroureterectomy, respectively (P = .0245). The estimated 5-year intravesical recurrence-free survival rates were 64.8%, 10.0%, and 76.2% for laparoscopic nephroureterectomy, hand-assisted laparoscopic nephroureterectomy, and open nephroureterectomy, respectively (P < .0001).
Conclusion:
Although there was no significant difference in cancer-specific survival rate among the laparoscopic nephroureterectomy, hand-assisted laparoscopic nephroureterectomy, and open nephroureterectomy groups, hand-assisted laparoscopic nephroureterectomy may be inferior to laparoscopic nephroureterectomy or open nephroureterectomy with regard to recurrence-free survival and intravesical recurrence-free survival rates.
Keywords: Nephroureterectomy, Laparoscopic, Hand-assisted laparoscopic
INTRODUCTION
Upper urinary tract urothelial cancers (UUTUCs) are uncommon and account for only 5% to 10% of urothelial carcinomas.1 Radical nephroureterectomy (RNU) with excision of the bladder cuff is the gold-standard treatment for UUTUCs, regardless of the location of the tumor in the upper urinary tract.1
Surgery for nonmetastatic UUTUC has changed considerably during the past decade. It has been feared that tumor dissection during laparoscopic procedures may be associated with a higher risk of recurrence.2 Although this remains a matter of controversy,3 one randomized controlled trial, comparing laparoscopic nephroureterectomy (LNU) with open nephroureterectomy (ONU), showed that oncologic outcomes were comparable for both groups.4 However, there has been no study of oncologic outcomes after LNU compared with hand-assisted laparoscopic nephroureterectomy (HALNU) and ONU for patients with UUTUC.5
To better understand the oncologic efficacy of less invasive surgery for nonmetastatic UUTUC, we conducted a critical retrospective review of patients with UUTUC who underwent RNU over a 15-year inclusion interval. The objective of this study was to compare the oncologic outcomes of UUTUC after LNU, HALNU, and ONU.
METHODS
We retrospectively analyzed the clinical and demographic information of 195 consecutive patients with histopathologically confirmed UUTUC treated by RNU between 1995 and 2010 at our institute. Patients with T4 disease, node-positive disease, a history of bladder cancer, or concomitant bladder cancer were excluded. Finally, a total of 108 patients with Tis-T3N0M0 UUTUC were enrolled in this study. Clinical information and follow-up data for the patients were obtained from the UUTUC database after we received their informed consent. This study was approved by the institutional review board.
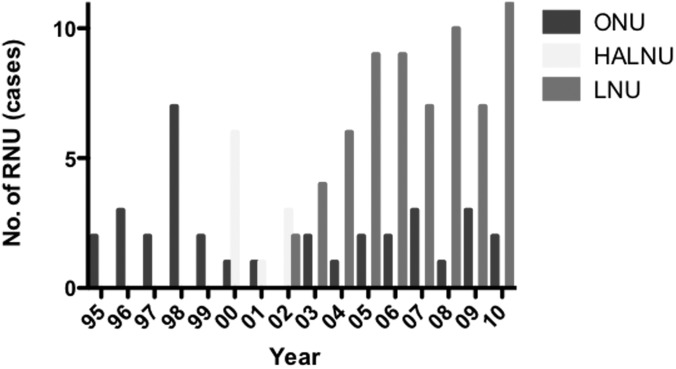
All the patients underwent ONU, HALNU, or LNU with extrafascial dissection of the kidney, as well as complete resection of the distal ureter and its orifice. ONU was performed through a flank incision combined with a lower quadrant incision or midline incision. HALNU was performed by the transperitoneal approach with a 6- to 8-cm hand incision on the right lower abdomen in right-sided HALNU and mid abdomen in left-sided HALNU and 2 trocars as previously described.6 The hand-assisted device (LAPDISC; Hakko Co, Ltd Chikuma, Japan) was applied to the wound, and the surgeon's nondominant hand was inserted into the abdomen. LNU was performed by the retroperitoneal or transperitoneal approach. The patient was placed in the lateral position. When the retroperitoneal approach was selected, a retroperitoneal working space was created with a PDB balloon (Covidien, Mansfield, Massachusetts). In both approaches, 4 trocars were inserted in the usual manner. In each procedure, the kidney, the ureter, and a bladder cuff were excised en bloc. Lymphadenectomy was performed at the surgeon's discretion. Until 1999, all patients underwent ONU. Since 2000, we have performed laparoscopic surgery in principle (HALNU in 2000–2002 and LNU in 2003 and thereafter) (Figure 1). If there was wariness about performing laparoscopic surgery (eg, because of a history of abdominal surgery or suspicion of incomplete resection of T3 upper or middle ureteral cancer), ONU was carried out. In 98 of the 108 patients (90.7%), distal ureterectomy was performed by bladder cuff excision. Endoscopic ureteral detachment (the pluck technique)6,7 was carried out in 6 of the 9 HALNUs (66.7%) and 2 of the 65 LNUs (3.1%) (Table 1).
Figure 1.
Trends for use of each procedure of RNU for stage I through stage III UUTUC.
Table 1.
Patient Characteristics
| Characteristic | RNU |
P Value | ||
|---|---|---|---|---|
| ONU | HALNU | LNU | ||
| No. of patients | 34 | 9 | 65 | |
| Median age (range) (y) | 69 (32–88) | 65 (53–71) | 70 (50–88) | .1878 |
| Conversion to ONU | — | 1 (11%) | 6 (9%) | .8566 |
| Tumor site (main) | .6423 | |||
| Renal pelvis | 20 (59%) | 4 (44%) | 35 (54%) | |
| Upper ureter | 3 (9%) | 0 (0%) | 6 (9%) | |
| Mid ureter | 4 (12%) | 1 (11%) | 5 (8%) | |
| Lower ureter | 4 (12%) | 3 (33%) | 17 (26%) | |
| Renal pelvis and ureter | 3 (9%) | 1 (11%) | 2 (3%) | |
| pT stage | .5375 | |||
| pTis | 0 (0%) | 0 (0%) | 2 (3%) | |
| pTa | 3 (9%) | 0 (0%) | 14 (22%) | |
| pT1 | 7 (21%) | 2 (22%) | 10 (15%) | |
| pT2 | 8 (24%) | 3 (33%) | 11 (17%) | |
| pT3 | 16 (47%) | 4 (44%) | 28 (43%) | |
| Grade (1973 WHOa classification) | .9203 | |||
| 1 | 1 (3%) | 0 (0%) | 2 (3%) | |
| 2 | 14 (41%) | 4 (44%) | 33 (51%) | |
| 3 | 19 (56%) | 5 (56%) | 30 (46%) | |
| Lymphovascular invasion | .1944 | |||
| Positive | 13 (38%) | 2 (22%) | 14 (22%) | |
| Negative | 21 (62%) | 7 (78%) | 51 (78%) | |
| Management of distal ureter | <.0001 | |||
| Bladder cuff | 33 (97%) | 3 (33%) | 62 (95%) | |
| Pluck technique | 0 (0%) | 6 (67%) | 2 (3%) | |
| Simple incision | 1 (3%) | 0 (0%) | 1 (2%) | |
WHO = World Health Organization.
Computed tomography (chest to pelvis), cystoscopy, and urinary cytology were performed every 3 months in the first 5 years after RNU and semiannually or annually thereafter in patients without evidence of recurrent disease. Additional radiographic and diagnostic tests were performed when clinically indicated. At the time of the retrospective analysis, the median follow-up time was 60 months (range, 6–192 months). Postoperative adverse events (AEs) were evaluated according to the Common Terminology Criteria for Adverse Events v4.0.
Cancer-specific survival (CSS), recurrence-free survival (RFS), and intravesical recurrence-free survival (ivRFS) rates were estimated by the Kaplan-Meier method and evaluated with the use of the log-rank test. Clinicopathologic factors in each group were compared by use of the χ2 or Kruskal-Wallis test. Statistical significance was set at P < .05, and statistical tests were performed with JMP software, version 10.0.0 (SAS Institute, Cary, North Carolina).
RESULTS
The characteristics of the patients who underwent ONU, HALNU, and LNU are shown in Table 1. There was no significant difference in age, tumor site, pT stage, tumor grade, or lymphovascular invasion among the 3 groups. The pluck technique was more frequently selected in HALNU patients, followed by LNU patients in this order (P < .0001).
Table 2 shows perioperative data, including the operative time, blood loss, time to discharge, and postoperative AEs, for each surgical approach. There was no significant difference in operative time among the 3 groups. The amount of blood loss was significantly smaller in LNU and HALNU patients than in ONU patients (P < .0001). The patients who underwent LNU had a shorter hospital stay than those who underwent ONU or HALNU (P < .0001). There was no significant difference in the incidence of postoperative AEs among the groups. In the ONU group, 3 cases of wound dehiscence, 1 case of ileus, and 1 postoperative hemorrhage greater than grade 3 were observed. The AEs of grade 3 or greater in the HALNU group were 1 kidney infection and 1 case of hypotension. In the LNU group, 5 patients had postoperative AEs of grade 3 or greater, including 3 cases of wound dehiscence, 1 postoperative hemorrhage, 1 kidney infection, 1 lung infection, and 1 increase in creatinine level.
Table 2.
Perioperative Data
| Variable | RNU |
P Value | ||
|---|---|---|---|---|
| ONU (n = 34) | HALNU (n = 9) | LNU (n = 65) | ||
| Median operative time (range) (min) | 286 (130–577) | 325 (237–390) | 327 (168–601) | .4450 |
| Median blood loss (range) (mL) | 475 (100–3670) | 250 (50–880) | 220 (0–2500) | <.0001 |
| Median time to discharge (range) (d) | 14.5 (5–36) | 17 (9–24) | 10 (4–62) | <.0001 |
| Postoperative AEs of grade 3 or greater | .5908 | |||
| Yes | 5 (15%) | 2 (22%) | 7 (11%) | |
| No | 29 (85%) | 7 (78%) | 58 (89%) | |
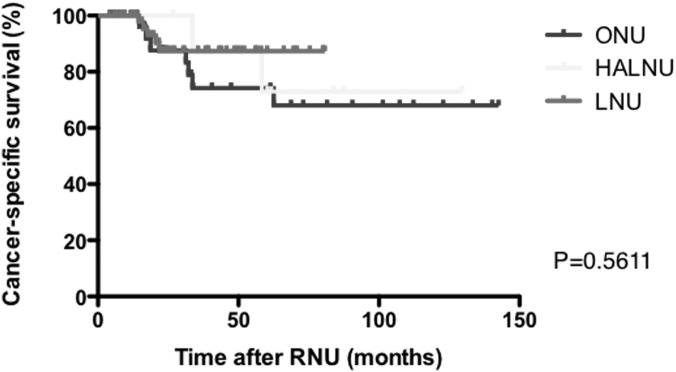
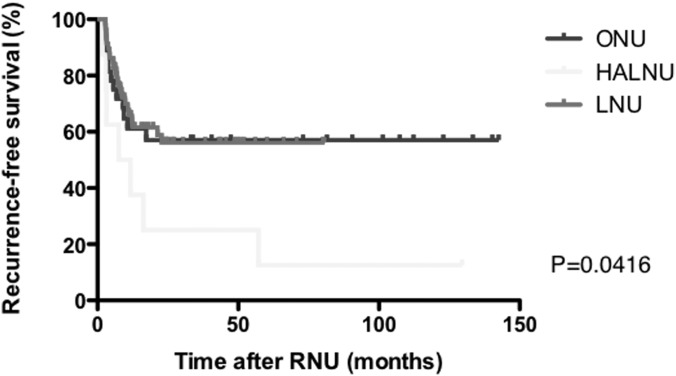
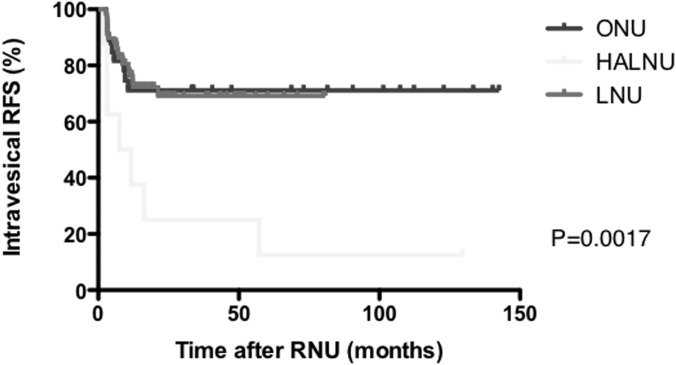
Figures 2, 3, and 4 present Kaplan-Meier estimates of CSS, RFS, and ivRFS, respectively, in each group. The 5-year CSS rates of patients who underwent ONU, HALNU, and LNU were 74.2%, 72.9%, and 87.4%, respectively (P = .5611). The 5-year RFS rates were 57.1% in the ONU group, 12.5% in the HALNU group, and 69.2% in the LNU group (P = .0416). The 5-year ivRFS rates of patients who underwent ONU, HALNU, and LNU were 71.1%, 12.5%, and 69.2%, respectively (P = .0017). Multivariate Cox regression analysis showed that the operative technique (HALNU) and tumor grade (grade 3) were independent factors for intravesical recurrence (Table 3).
Figure 2.
Kaplan-Meier estimates of CSS.
Figure 3.
Kaplan-Meier estimates of RFS.
Figure 4.
Kaplan-Meier estimates of ivRFS.
Table 3.
Univariate and Multivariate Cox Regression Analyses for Prediction of Intravesical Recurrence After Radical Nephroureterectomy
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| HRa | 95% CIa | P Value | HR | 95% CI | P Value | |
| Age | 1.00 | 0.97–1.04 | .9078 | |||
| Sex | 1.42 | 0.68–3.37 | .3639 | |||
| Operative technique | ||||||
| ONU | 1.00 | Reference | 1.00 | Reference | ||
| HALNU | 4.72 | 1.77–12.4 | .0026 | 5.52 | 2.05–14.6 | .0011 |
| LNU | 1.08 | 0.49–2.52 | .8582 | 1.10 | 0.50–2.57 | .8207 |
| Architecture | 1.51 | 0.76–3.13 | .2401 | |||
| Multiplicity | 1.29 | 0.62–2.54 | .4853 | |||
| Tumor size | 1.35 | 0.69–2.64 | .3726 | |||
| Grade (3 vs 1 or 2) | 2.12 | 1.08–4.36 | .0292 | 2.41 | 1.21–5.02 | .0118 |
| pT stage | ||||||
| Ta/Tis | 1.00 | Reference | ||||
| T1 | 1.36 | 0.43–4.59 | .5991 | |||
| T2 | 1.43 | 0.49–4.67 | .5134 | |||
| T3 | 1.04 | 0.40–3.23 | .9342 | |||
| Lymphovascular invasion | 0.77 | 0.32–1.62 | .5060 | |||
CI = confidence interval; HR = hazard ratio.
DISCUSSION
In this study there was no significant difference in CSS, RFS, and ivRFS rates between LNU and ONU. To date, many studies have reported that LNU is comparable with ONU with regard to oncologic outcomes.2,8–14 A recent cumulative analysis including 21 studies showed that LNU offered oncologic efficacy and reliable perioperative safety comparable with ONU, although most of the included studies were retrospective.15 The Cochrane Collaboration's review using comprehensive searches concluded that oncologic outcomes of organ-confined disease were comparable for LNU and ONU.5 Only one prospective randomized study showed that LNU was superior to ONU in terms of the perioperative outcomes and comparable with ONU for oncologic outcomes in patients with organ-confined disease.4 On the basis of these reports, the European Association of Urology Guideline Group considers that ONU and LNU are equivalent in terms of efficacy in a grade B recommendation.1
Meanwhile, we found that HALNU was comparable with ONU and LNU with regard to CSS but was inferior to ONU and LNU for RFS and ivRFS. There are studies reporting benefits of HALNU such as less intraoperative blood loss and a shorter hospital stay with an equivalent intermediate-term oncologic outcome compared with ONU.16,17 In a meta-analysis of studies comparing HALNU with LNU, there were no significant differences in the operative time, the length of stay, or the risks of perioperative transfusion or complications, but HALNU was associated with significantly less operative blood loss and risk of open conversion than LNU.18 Another study with a small series and a short-term follow-up period showed no difference in oncologic outcomes between LNU and HALNU.19 Thus most studies reported the equivalence or superiority of HALNU compared with ONU or LNU. However, a large multicenter study showed that HALNU was an independent risk factor for intravesical recurrence compared with LNU,20 like this study.
In our study the difference in ivRFS among the 3 surgical techniques was larger than that for all RFS because the first recurrence site was the bladder in all patients who underwent HALNU and had a recurrence during follow-up. We excluded patients with unresectable or metastatic tumors because minimally invasive surgery is not recommended for advanced UUTUC.3 To avoid contamination causing recurrence of bladder cancer itself other than intravesical recurrence of UUTUC, we also excluded patients with a history of bladder cancer or concomitant bladder cancer. In these circumstances, the HALNU technique was associated with a high risk of intravesical recurrence. Because early ligation of the ureter7 was not performed during RNU in our series, even in ONU and LNU patients, we think that the hand manipulation during HALNU might release cancer cells into the urinary tract and those cells might be seeded in the bladder mucosa. As shown in Figure 1, we performed HALNU before introducing LNU. This may mean that inexperience in using laparoscopic procedures was associated with a higher incidence of intravesical recurrence. We suggest that HALNU should not be performed during training for unskilled laparoscopic surgeons.
There were several limitations to this study. First, this was a retrospective study with selection biases and a small number of patients, especially in the HALNU group, at a single institute. To determine the differences in the incidences of intravesical recurrence among ONU, HALNU, and LNU patients, a prospective randomized trial is needed. Second, RNU was performed by various surgeons over a long period. Finally, early ligation of the ureter was not performed during RNU.
We found that there was no significant difference in CSS among ONU, HALNU, and LNU patients but that RFS and ivRFS in HALNU patients were inferior to those in ONU and LNU patients. LNU and HALNU were associated with diminished blood loss, which is compatible with the results of previous studies. Patients who underwent LNU had a shorter hospital stay than those undergoing ONU or HALNU. Thus, we suggest that LNU is the most appropriate technique, with a balance between lesser invasiveness and favorable oncologic outcomes for patients with organ-confined disease and without a history of bladder cancer.
CONCLUSION
Although there was no significant difference in CSS among LNU, HALNU, and ONU patients, HALNU may be inferior to LNU or ONU in terms of RFS and ivRFS. LNU is a less invasive procedure with comparable long-term oncologic outcomes in comparison with ONU.
Contributor Information
Hiroshi Kitamura, Department of Urology, Sapporo Medical University School of Medicine, Sapporo, Japan..
Toshihiro Maeda, Department of Urology, Sapporo Medical University School of Medicine, Sapporo, Japan..
Toshiaki Tanaka, Department of Urology, Sapporo Medical University School of Medicine, Sapporo, Japan..
Fumimasa Fukuta, Department of Urology, Sapporo Medical University School of Medicine, Sapporo, Japan..
Ko Kobayashi, Department of Urology, Sapporo Medical University School of Medicine, Sapporo, Japan..
Naotaka Nishiyama, Department of Urology, Sapporo Medical University School of Medicine, Sapporo, Japan..
Satoshi Takahashi, Department of Urology, Sapporo Medical University School of Medicine, Sapporo, Japan..
Naoya Masumori, Department of Urology, Sapporo Medical University School of Medicine, Sapporo, Japan..
References:
- 1. Roupret M, Zigeuner R, Palou J, et al. European guidelines for the diagnosis and management of upper urinary tract urothelial cell carcinomas: 2011 update. Eur Urol. 2011;59(4):584–594 [DOI] [PubMed] [Google Scholar]
- 2. Ariane MM, Colin P, Ouzzane A, et al. Assessment of oncologic control obtained after open versus laparoscopic nephroureterectomy for upper urinary tract urothelial carcinomas (UUT-UCs): results from a large French multicenter collaborative study. Ann Surg Oncol. 2012;19(1):301–308 [DOI] [PubMed] [Google Scholar]
- 3. Roupret M, Smyth G, Irani J, et al. Oncological risk of laparoscopic surgery in urothelial carcinomas. World J Urol. 2009;27(1):81–88 [DOI] [PubMed] [Google Scholar]
- 4. Simone G, Papalia R, Guaglianone S, et al. Laparoscopic versus open nephroureterectomy: perioperative and oncologic outcomes from a randomised prospective study. Eur Urol. 2009;56(3):520–526 [DOI] [PubMed] [Google Scholar]
- 5. Rai BP, Shelley M, Coles B, Biyani CS, El-Mokadem I, Nabi G. Surgical management for upper urinary tract transitional cell carcinoma. Cochrane Database Syst Rev. 2011(4):CD007349. [DOI] [PubMed] [Google Scholar]
- 6. Munver R, Del Pizzo JJ, Sosa RE. Hand-assisted laparoscopic nephroureterectomy for upper urinary-tract transitional-cell carcinoma. J Endourol. 2004;18(4):351–358 [DOI] [PubMed] [Google Scholar]
- 7. Kurzer E, Leveillee RJ, Bird VG. Combining hand assisted laparoscopic nephroureterectomy with cystoscopic circumferential excision of the distal ureter without primary closure of the bladder cuff—is it safe? J Urol. 2006;175(1):63–67 [DOI] [PubMed] [Google Scholar]
- 8. Capitanio U, Shariat SF, Isbarn H, et al. Comparison of oncologic outcomes for open and laparoscopic nephroureterectomy: a multi-institutional analysis of 1249 cases. Eur Urol. 2009;56(1):1–9 [DOI] [PubMed] [Google Scholar]
- 9. Favaretto RL, Shariat SF, Chade DC, et al. Comparison between laparoscopic and open radical nephroureterectomy in a contemporary group of patients: are recurrence and disease-specific survival associated with surgical technique? Eur Urol. 2010;58(5):645–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greco F, Wagner S, Hoda RM, Hamza A, Fornara P. Laparoscopic vs open radical nephroureterectomy for upper urinary tract urothelial cancer: oncological outcomes and 5-year follow-up. BJU Int. 2009;104(9):1274–1278 [DOI] [PubMed] [Google Scholar]
- 11. Roupret M, Hupertan V, Sanderson KM, et al. Oncologic control after open or laparoscopic nephroureterectomy for upper urinary tract transitional cell carcinoma: a single center experience. Urology. 2007;69(4):656–661 [DOI] [PubMed] [Google Scholar]
- 12. Manabe D, Saika T, Ebara S, et al. Comparative study of oncologic outcome of laparoscopic nephroureterectomy and standard nephroureterectomy for upper urinary tract transitional cell carcinoma. Urology. 2007;69(3):457–461 [DOI] [PubMed] [Google Scholar]
- 13. Waldert M, Remzi M, Klingler HC, Mueller L, Marberger M. The oncological results of laparoscopic nephroureterectomy for upper urinary tract transitional cell cancer are equal to those of open nephroureterectomy. BJU Int. 2009;103(1):66–70 [DOI] [PubMed] [Google Scholar]
- 14. Walton TJ, Novara G, Matsumoto K, et al. Oncological outcomes after laparoscopic and open radical nephroureterectomy: results from an international cohort. BJU Int. 2011;108(3):406–412 [DOI] [PubMed] [Google Scholar]
- 15. Ni S, Tao W, Chen Q, et al. Laparoscopic versus open nephroureterectomy for the treatment of upper urinary tract urothelial carcinoma: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2012;61(6):1142–1153 [DOI] [PubMed] [Google Scholar]
- 16. Raman JD, Palese MA, Ng CK, et al. Hand-assisted laparoscopic nephroureterectomy for upper urinary tract transitional cell carcinoma. JSLS. 2006;10(4):432–438 [PMC free article] [PubMed] [Google Scholar]
- 17. Hsueh TY, Huang YH, Chiu AW, Shen KH, Lee YH. A comparison of the clinical outcome between open and hand-assisted laparoscopic nephroureterectomy for upper urinary tract transitional cell carcinoma. BJU Int. 2004;94(6):798–801 [DOI] [PubMed] [Google Scholar]
- 18. Silberstein J, Parsons JK. Hand-assisted and total laparoscopic nephrectomy: a comparison. JSLS. 2009;13(1):36–43 [PMC free article] [PubMed] [Google Scholar]
- 19. Landman J, Lev RY, Bhayani S, et al. Comparison of hand assisted and standard laparoscopic radical nephroureterectomy for the management of localized transitional cell carcinoma. J Urol. 2002;167(6):2387–2391 [PubMed] [Google Scholar]
- 20. Kamihira O, Hattori R, Yamaguchi A, et al. Laparoscopic radical nephroureterectomy: a multicenter analysis in Japan. Eur Urol. 2009;55(6):1397–1407 [DOI] [PubMed] [Google Scholar]