Abstract
There are evidences for exposure to vehicular emissions and adverse cardiopulmonary health effects. This study attempted to further explore these effects on elderly. This study monitored personal PM2.5 concentrations and ambulatory electrocardiograms continuously for 24 h on 1 working day in 3 separate weeks for 11 school crossing guards. Spirometry was also performed before and after the morning shift. The traffic at each work location was video recorded during one of the three morning shifts. The increases in the average personal PM2.5 concentrations (baseline PM2.5 was subtracted) of 1.2–87 and 1.1–98 μg/m3 were observed during the 1-h morning (ΔPM2.5-ave-m) and afternoon shift (ΔPM2.5-ave-a), respectively. Traffic count was not a significant predictor of the ΔPM2.5-ave-m (P=0.78). Mean heart rate variability (HRV), measured as 5-min standard deviation of normal-to-normal (SDNN) beats during the 10-min rest periods, decreased 18–26% (P<0.02) 15 min, 2 and 4 h after the morning shift, but changes in SDNN (ΔSDNN) were insignificant post-afternoon exposure (−0.3 to −7% with P>0.53). ΔSDNN were negatively associated with ΔPM2.5-ave-m, with the strongest association at 2 h after the morning shift (P<0.01) but insignificant 4 h after the morning exposure. The peak PM2.5 concentration (ΔPM2.5-peak, baseline PM2.5 was subtracted) was not a significant predictor for ΔSDNN, and no clear effect of PM2.5 exposure on heart rate was observed. There was no effect of PM exposure on lung function (P>0.16), either. In conclusion, acute exposure to the PM2.5 resulting from mobile sources can cause acute decline in HRV in healthy older adults, suggesting one of the biological mechanisms for the adverse cardiovascular health effects associated with traffic-related air pollution. Traffic count may not be an appropriate surrogate measure of acute personal exposure to vehicular emission in traffic congested areas.
Keywords: PM2.5, vehicular emission, heart rate variability, heart rate, lung function, traffic count
Introduction
Chronic exposure to vehicular emissions has been associated with cardiopulmonary mortality and morbidity (Brauer et al., 2002; Buckeridge et al., 2002; Hoek et al., 2002; Lin et al., 2002; Finkelstein et al., 2004; Kim et al., 2004; Slaughter et al., 2005; Wellenius et al., 2005). Pollutants that are related to vehicular emissions, especially fine particulate matter (PM2.5), have been studied for the mechanisms underlying the observed associations between chronic exposure to vehicular emissions and cardiopulmonary health effects. As summarized by Rowan et al.(2007), numerous studies have reported reduced heart rate variability (HRV) in association with exposure to PM2.5, which may represent autonomic nervous system changes that could mediate some of the cardiovascular effects of PM2.5. Changes in heart rate (HR) in association with exposure to PM2.5 air pollution were also observed, although inconsistent findings were reported (Peters et al., 1999; Pope et al., 1999; Ibald-Mulli et al., 2004; Adar et al., 2007). As opposed to other sources, some studies showed a stronger association between particles originating from traffic and cardiovascular endpoints (Laden et al., 2000; Gold et al., 2005; Park et al., 2005; Adar et al., 2007). Furthermore, the elderly appear to be more susceptible to the cardiopulmonary effects of PM exposure (Tsuji et al., 1994; Laden et al., 2000; Gold et al., 2005; Park et al., 2005; Schwartz et al., 2005; Adar et al., 2007).
Exposure to vehicular emissions, however, often includes short-term (minutes to hours) exposure to relatively high concentrations of air pollutants, such as exposure during commuting (Sabin et al., 2005; Adar et al., 2007). Many health effect studies that examined exposure to traffic-related PM used the 24- or 48-h average concentrations of PM2.5 measured at central monitoring sites distant from traffic to represent personal PM2.5 exposure levels (Tsuji et al., 1994; Liao et al., 1999; Pope et al., 1999, 2004; Gold et al., 2000; Laden et al., 2000). Given the dramatic decreases in concentrations and rapid changes in physical and chemical properties of PM2.5 with time and distance from vehicular emissions (Zhu et al., 2002; Marr et al., 2004), integrated measurements of PM2.5 obtained at distant central monitoring sites may underestimate the acute exposure as well as the potential health effects due to being near traffic sources.
The present study investigated the effects resulting from acute exposure to PM2.5 freshly generated by vehicles on the cardiopulmonary health of older adults — school crossing guards. School crossing guards are likely to experience elevated PM2.5 concentrations generated by both school buses and other vehicles given their very close proximity to traffic during their morning and afternoon work shifts. Moreover, many crossing guards are elderly, and may therefore be more susceptible to the cardiopulmonary effects of air pollutants than the general population.
Materials and methods
Subject Information
This study was conducted on school crossing guards from Paterson, the third largest city in New Jersey (NJ). Research protocols and consent forms were approved by the Institutional Review Board of the University of Medicine and Dentistry of New Jersey (UMDNJ) and all the subjects signed the consent form before their participation in this study. Subject recruitment was conducted with the assistance of the Paterson Police Department. Traffic information about the locations where the crossing guards worked was initially provided by the police department. Site visits were conducted and the locations with a high volume of traffic were selected for inclusion in the study. Subsequently, study information was distributed by the police department to the crossing guards who worked at the selected locations. Eleven crossing guards volunteered to participate in the study. The demographic information of the subjects is presented in Table 1. The mean age of the crossing guards was 61 years. All subjects were currently non-smokers and generally healthy by self-reports. One subject reported a previous diagnosis of asthma by a physician but was asymptomatic during participation in the study. No subjects took β-blocker medications or other medications that are likely to affect cardiac function measurement. No subjects had diabetic or hypertensive diseases. Except one subject, all participants completed the 3 days of measurements and complied fully with the study protocols.
Table 1.
Characteristics of study subjects (n=11).
| Characteristics | |
|---|---|
| Age (years) | 61.2±13.7 |
| <50 | 2 |
| 50–69 | 5 |
| >70 | 4 |
| Sex | |
| Male | 6 |
| Female | 5 |
| Race/ethnicity | |
| Black | 6 |
| Hispanic | 5 |
No reports of bronchitis, respiratory illness, diabetics, or prior myocardial infarction. No subject taking β-blockers.
Study Protocols
All of the school crossing guards had the same working schedule: 0730–0830 hours for the morning shift and 1430–1530 hours for the afternoon shift. Thus, personal PM2.5 concentration and electrocardiogram (ECG) monitoring started at ~0700 hours, a half-hour before crossing guards went on-duty in the morning, to ~0700 hours the following day, for a total of 24 h. Each subject was monitored on 1 working day in 3 separate weeks. PM2.5 concentration was expected to differ among the 3 sampling days at each location and between locations given the day-to-day variation in meteorological conditions and different traffic volume between locations. Each subject was monitored on the same day of the week and at the same time of day to reduce the possible confounding effects of temporal changes in physiological and activity-related variables. The study was conducted from February to May 2005. All tests were conducted on days with no precipitation to avoid suppression of PM2.5 concentrations. Temperature, humidity, and other meteorological data were obtained from Teterboro airport located 10 miles southwest of Paterson. The 24-h average temperature ranged from −11°C to 28°C and relative humidity ranged from 32% to 78%. During each monitoring period, subjects were instructed not to participate in vigorous physical activities, such as playing sports, bicycling, running, and so on. The subjects were asked to complete baseline (including demographic information, general information about living conditions, health status, medications, etc.), activity, and health symptom questionnaires.
Personal PM2.5 Concentrations
The 24-h personal PM2.5 mass concentrations (μg/m3) for each subject were continuously monitored using a real-time personal PM2.5 monitor (Side-Pak, Model AM510-1D11; TSI Inc., Shoreview, MN, USA). The monitor was placed either in a pouch or in a back pack so that it was easy for the subject to wear. The inlet of the sampling tube was clipped to the shirt within the participant’s breathing zone. The PM2.5 monitor has a dynamic measurement range of 1 μg/m3 to 20 mg/m3 and was fitted with an impactor to collect PM2.5. The monitor was programmed to record sequential 1-min PM2.5 measurements.
Traffic Count
The intersection was video recorded with a Sony Digital Video recorder (model GV-D200) for ~15 min during one morning shift for each subject but two locations were recorded twice. The 12 videos were subsequently transcribed using Virtual Timing Device software (Sama Sama Consulting). The standard template was modified to enable investigators to record the type of vehicles (bus, car, large truck, small truck or other) observed in the intersection. The traffic count by vehicle type and observation time was recorded for each video.
Electrocardiogram (ECG)
The ECG of each subject was recorded continuously for 24 h using a five-lead Holter monitor (Model Dynacord 3 Channel Model 423; Raytel Cardiac Services, Windsor, CT, USA). A trained field technician placed the electrodes on the subjects in the subjects’ homes before his/her morning shift began. The protocol for the ECG monitoring included 10 min rest periods, during which the subjects were instructed to sit quietly in a chair, beginning at ~15 min before, and at 15 min, 2 and 4 h after the morning and afternoon shifts. A timer was given to the subjects to remind them to sit still for the designated time intervals, and the technician or subjects recorded the actual time interval on a sampling sheet. The tapes were sent to Raytel Cardiac Services for analysis of 5-min standard deviation of normal-to-normal (SDNN) intervals, a measure of overall HRV, by trained professionals.
Lung Function Measurement
The lung function of each subject was measured with a portable spirometer (Micro Inc., CT, USA) according to the quality criteria of the American Thoracic Society (American Thoracic Society, 1995), and the measurement was only performed before and right after the morning shift. Lung function was measured after the 10-min rest period for the ECG recording. The lung function parameters included forced vital capacity (FVC), forced expired volume in the first second (FEV1.0), and peak expired flow (PEF). The expiratory maneuver was repeated until the three FEV1.0 measurements within 10% of the maximum value were obtained. Due to the age of the subjects (mean of 61 years), a 10% variation quality criterion among repetitive measurements was used to consider the lung function measurement valid. Parameter values from these three trials were used for analysis.
Data Analysis
Descriptive statistics and mixed effect models (SAS 9.1) were used to examine (1) whether there were changes in HR, SDNN, and lung function measurements after the morning and afternoon work shifts; and (2) whether those changes were due to exposure to the corresponding PM2.5 exposure. Both 1-h average and peak concentrations of PM2.5 during each work shift were used for examining the effect of PM2.5 exposure on the changes of the health endpoint measurements. To control for the potential effect from PM2.5 exposure before each work shift, the average PM2.5 background concentrations measured 10 min before each work shift were subtracted from the corresponding 1-h average and peak concentrations of PM2.5, that is increases in PM2.5 concentrations were used for analysis. ΔPM2.5-ave-m and ΔPM2.5-peak-m represent for the morning increases in the average and peak PM2.5 concentrations, and ΔPM2.5-ave-a and ΔPM2.5-peak-a represent for the afternoon increases in the average and peak PM2.5 concentrations, respectively. As most crossing guards worked in areas of high traffic, the increase in PM2.5 concentration was primarily due to mobile sources, which allowed for examining the effect from exposure to traffic-related PM2.5 on the cardiac and pulmonary parameters. To control the variability of an individual, the changes in health measures after work shift were used for analysis, that is changes in health outcomes (ΔHR, ΔSDNN, ΔFEV1, ΔFVC, and ΔPEF) were obtained by subtracting baseline health measures before each work shift from those obtained after each work shift. Also, the potential effects from the morning PM2.5 exposure on the afternoon health measures could be controlled. Finally, to avoid potential confounding effects of activities on the recorded signals, the ΔSDNN and ΔHR obtained at the designated resting periods (i.e. 15 min before, 15 min, 2, and 4 h after the morning and afternoon shifts) were used for the analysis of the associations with the ΔPM2.5 exposure. Temperature, relative humidity, and age were included in the model to control potential effects from these parameters. For all analyses, the natural log transformed ΔPM2.5 concentrations were used to normalize the distribution and reduce the influence of a small subset of extremely large values.
A multiple linear regression model was used to examine the associations between the traffic counts (predictor) and the morning increase in PM2.5 concentrations (ΔPM2.5-ave-m) measured during the same time period (dependent variable), controlled by wind speed (another predictor). Both total traffic counts, as well as, the counts of diesel and non-diesel-powered vehicles were examined.
Results
Personal PM2.5 Concentration
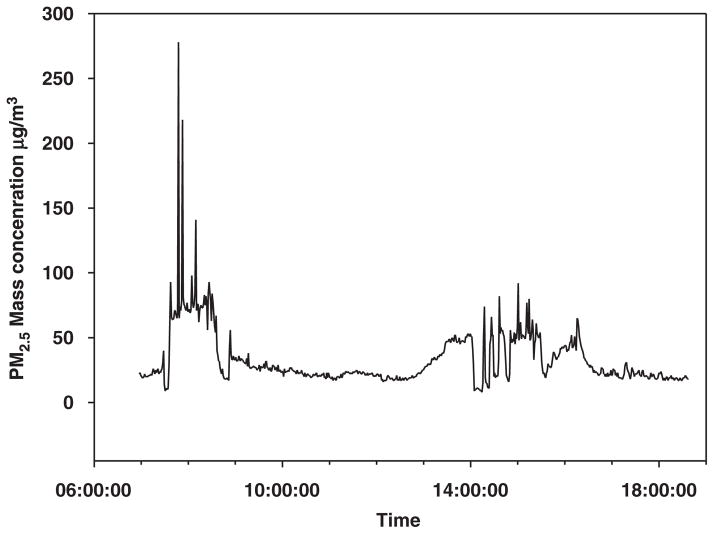
A total of thirty-one 24-h continuous PM2.5 measurements were collected, but three measurements (two from subject 10 and one from subject 11) were excluded due to a malfunction of the PM2.5 monitor. As expected, elevated personal PM2.5 concentrations were observed for most monitoring sessions. The ΔPM2.5-ave-m measured for each subject ranged from 1.1 to 87 μg/m3 and the ΔPM2.5-ave-a ranged from 1.2 to 98 μg/m3 (Table 2). The average increase in PM2.5 concentration for all the subjects in the morning was 35.2±25.9 μg/m3, higher than that in the afternoon (24.1±22.1 μg/m3). Many spikes of PM2.5 concentrations were observed during the work shifts (Figure 1), and the 1-min peak PM2.5 concentrations (ΔPM2.5-peak) were often 10 times higher than the background level (~15 μg/m3), with the maximum ΔPM2.5-peak of 278 μg/m3 during a morning shift.
Table 2.
Summary of the increases in PM2.5 concentrations (1-h average and peak PM2.5 concentrations, μg/m3) during the morning and afternoon shifts.
| Subject ID | ΔPM2.5-ave
|
ΔPM2.5-peak
|
||
|---|---|---|---|---|
| Morning | Afternoon | Morning | Afternoon | |
| 01A | 7.3 | 2.6 | 22 | 39 |
| 01B | 35.1 | 4.4 | 89 | 26 |
| 01C | 25.8 | 11.9 | 39 | 23 |
| 02A | 62.4 | 13.9 | 146 | 120 |
| 02B | 64.4 | 30.5 | 84 | 122 |
| 02C | 86.8 | 47.2 | 148 | 101 |
| 03A | 3.1 | 2.4 | 17 | 18 |
| 03B | 16.1 | 1.2 | 24 | 3 |
| 03C | 17.0 | 11.6 | 41 | 57 |
| 04A | 84.7 | 23.7 | 126 | 45 |
| 04B | 15.2 | 5.8 | 100 | 33 |
| 04C | 1.1 | 2.2 | 4 | 4 |
| 05A | 74.9 | 47.5 | 278 | 92 |
| 05B | 47.9 | 37.6 | 73 | 120 |
| 05C | 20.3 | 26.5 | 32 | 34 |
| 06A | 22.1 | 11.2 | 62 | 67 |
| 06B | 8.0 | 1.6 | 15 | 6 |
| 06C | 65.9 | 63.8 | 77 | 95 |
| 07A | 13.0 | 5.0 | 67 | 18 |
| 07B | 40.8 | 24.9 | 110 | 53 |
| 07C | 21.6 | 13.9 | 45 | 75 |
| 08Aa | 21.2 | 16.8 | 26 | 45 |
| 09A | 32.7 | 21.2 | 50 | 28 |
| 09B | 31.2 | 42.6 | 51 | 150 |
| 09C | 28.8 | 22.0 | 63 | 105 |
| 10Cb | 80.0 | 51.0 | 107 | 72 |
| 11Ac | 15.8 | 25.0 | 46 | 114 |
| 11Cc | 41.6 | 33.3 | 54 | 60 |
| Average±SD | 35.2±25.9 | 24.1±22.1 | 71.3±56.1 | 64.3±43.5 |
| Range | 1.1–87 | 1.2–98 | 4.0–278 | 3.0–150 |
Subject 08 withdrew from the study after one measurement.
One valid exposure measurement for subject 10.
Two valid exposures for subject 11.
Figure 1.
The first 12 h PM2.5 concentration over the 24-h monitoring period monitored by a real-time PM2.5 monitor on 3/11/2005. The PM2.5 was close to baseline between 1800 and 0700 hours in the next morning.
A wide range of the ΔPM2.5-ave and ΔPM2.5-peak was observed among the 3 different working days sampled for most of the subjects (Table 2). Different wind speeds on the sampling day is the primary factor affecting the PM2.5 concentration, as discussed in the following section. The large variations in PM2.5 concentrations among the 3 sampling days allowed the use of each subject as his or her own control in a mixed model to examine the effects of different PM2.5 concentrations on the measured health endpoints.
Traffic Count Information
Different vehicle types and traffic counts were observed at each school location (Table 3). The traffic counts recorded from the 10 locations ranged from 10 to 25 vehicles/min during the morning shift. Although positive relationship were observed, the associations between ΔPM2.5-ave-m and traffic count were not statistically significant (P=0.78, 0.83, and 0.79 for all vehicles, diesel-powered vehicles, or non-diesel-powered vehicles, respectively, Table 4). Only wind speed was inversely associated with ΔPM2.5-ave-m (P=~0.05, Table 4) for all three vehicle categories examined.
Table 3.
Traffic count and type recorded for 15 min during the morning shift at each school location.
| Date | Site | Bus | Car | Large truck | Small truck |
|---|---|---|---|---|---|
| 2/01/05 | 1 | 14 | 212 | 4 | 4 |
| 2/11/05 | 2 | 21 | 180 | 7 | 6 |
| 2/14/05 | 3 | 11 | 154 | 5 | 1 |
| 5/31/05 | 3 | 21 | 397 | 11 | 2 |
| 3/07/05 | 4 | 10 | 324 | 9 | 1 |
| 3/18/05 | 5 | 8 | 196 | 4 | 3 |
| 4/06/05 | 5 | 6 | 209 | 1 | 3 |
| 4/13/05 | 6 | 5 | 233 | 2 | 2 |
| 4/11/05 | 7 | 6 | 162 | 11 | 2 |
| 4/27/05 | 8 | 17 | 137 | 1 | 2 |
| 5/02/05 | 9 | 7 | 76 | 0 | 1 |
| 5/13/05 | 10 | 17 | 395 | 12 | 7 |
Table 4.
Regression coefficients for various traffic counts controlled by wind speed (n=12)a.
| Variable | Slope for traffic counts (SE) | P-value | Slope for wind speed (SE) | P-value |
|---|---|---|---|---|
| All vehicles | 0.018 (0.06) | 0.78 | −3.28 (1.52) | 0.06 |
| Diesel-powered vehicles | 0.15 (0.68) | 0.83 | −3.44 (1.38) | 0.04 |
| Non-diesel-powered vehicles | 0.019 (0.07) | 0.78 | −3.28 (1.53) | 0.06 |
Note: a multiple linear regression was used to predict the elevated average PM2.5 (μg/m3) during the recording time. Traffic counts and wind speed are two independent predictors.
Changes in SDNN
The ECG obtained from each subject was reviewed for qualifying beats before analysis. For example, the measurements from subject 2 were excluded from the analysis due to a low proportion of qualified heart beats (<60%) resulting from frequent ectopic heart beats. Overall, 25 measurements were used for analysis.
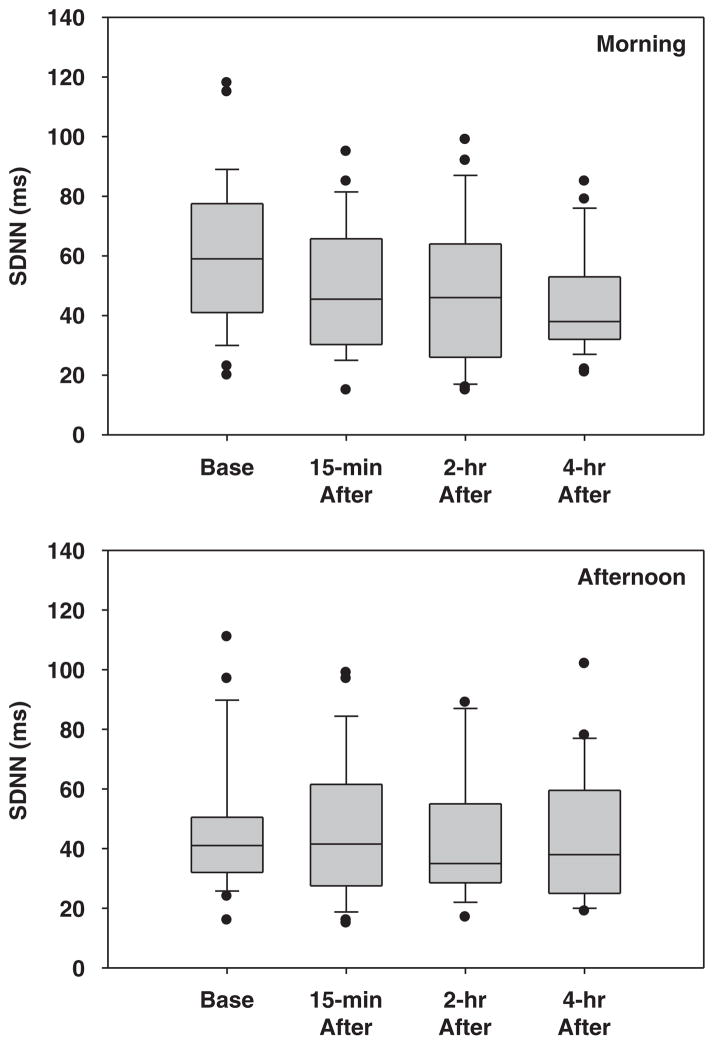
The mean 5-min SDNNs measured at each time point during the morning and afternoon sessions are presented in Figure 2 to examine the change of SDNN with time. The error bar represents the standard deviation of the measurements at each time point. After the morning shift, significant decreases in SDNN were observed, with an average decrease of 18% (P=0.02), 21% (P=0.006), and 26% (P=0.001) at ~15 min, 2 and 4 h, respectively, after the morning shift (Figure 2). To examine whether decrease in SDNN was due to exposure to PM during the morning shift, regression analysis was conducted for the change of SDNN (ΔSDNN) on the increase in PM (ΔPM). The ΔSDNN measured at all three time points were negatively associated with ΔPM2.5-ave-m (Table 5), and the association between ΔSDNN and ΔPM2.5-ave-m was the strongest and most significant (P=0.0002) 2 h after the morning exposure, with 40 ms decrease in HRV for every 10 μg/m3 increase in PM2.5. The association between ΔSDNN and ΔPM2.5-ave-m was only marginal (P=0.06) 15 min after the morning exposure, and insignificant (P=0.78) 4 h after the morning work shift. Relative humidity was found to be a significant covariate for all ΔSDNNs examined (P-values of 0.03–0.05). However, temperature, age, subjects’, and former smoking status were not found to be the significant predictors for the ΔSDNN.
Figure 2.
The variable (SDNN) of heart rate variability measured before and after the morning (top) and afternoon work shift (bottom).
Table 5.
Regression coefficients of the changes in SDNN (ΔSDNN, in the unit of ms) and heart rate (ΔHR, in the unit of beat/ms) measured after the morning and afternoon shifts relative to increases in the average and peak PM2.5 mass concentration during the 1-h work shift [ln(ΔPM2.5), in the unit of μg/m3] (N=25).
| ΔSDNN–ΔPM2.5
|
ΔHR–ΔPM
|
||||
|---|---|---|---|---|---|
| ln_ΔPM2.5 | Estimate (SE) | P-value | Estimate (SE) | P-value | |
| Morning | |||||
| 15 min | ln_ΔPMave-m | −14.5 (6.9) | 0.06 | 1.2 (3.1) | 0.71 |
| 2 h | ln_ΔPMave-m | −18.9 (4.2) | 0.0002 | −5.5 (2.9) | 0.08 |
| 4 h | ln_ΔPMave-m | −2.5 (8.6) | 0.78 | −3.1 (4.6) | 0.51 |
| 15 min | ln_ΔPMpeak-m | −9.2 (11.2) | 0.43 | 0.8 (4.4) | 0.86 |
| 2 h | ln_ΔPMpeak-m | −5.1 (13.8) | 0.72 | −7.2 (4.2) | 0.11 |
| 4 h | ln_ΔPMpeak-m | 7.4 (12.0) | 0.55 | −7.1 (6.3) | 0.28 |
| Afternoon | |||||
| 15 min | ln_ΔPMave-a | −2.4 (7.6) | 0.77 | −2.0 (4.0) | 0.62 |
| 2 h | ln_ΔPMave-a | −20.2 (10.8) | 0.10 | 0.9 (5.4) | 0.87 |
| 4 h | ln_ΔPMave-a | −0.7 (11.2) | 0.95 | 8.2 (5.2) | 0.14 |
| 15 min | ln_ΔPMpeak-a | 0.6 (8.9) | 0.95 | −5.6 (5.3) | 0.31 |
| 2 h | ln_ΔPMpeak-a | −19.2 (14.6) | 0.23 | 3.1 (8.1) | 0.71 |
| 4 h | ln_ΔPMpeak-a | −6.8 (14.1) | 0.64 | 11.1 (8.1) | 0.20 |
Similar to the morning shift, decreases in SDNN were observed post-afternoon exposure, but the change in SDNN was small and insignificant, with an average decrease of 0.3% (P=0.94), 7% (P=0.55), and 7% (P=0.53) for a lag time of 15 min, 2 and 4 h, respectively (Figure 2). ΔSDNN measured at all three time points were negatively associated with the ΔPM2.5-ave-a (Table 5), but the association between ΔSDNN and ΔPM2.5-ave-a were not significant for the lag time of 15 min (P=0.77) or 4 h (P=0.95) in the afternoon, and only marginal effects were observed (P=0.1) 2 h post-afternoon exposure.
The same analysis was performed to examine the effect of peak PM2.5 exposure (ΔPM2.5-peak) on the change in SDNN (ΔSDNN). Both positive and negative associations between ΔSDNN and ΔPM2.5-peak were observed 15 min or 4 h after the morning and afternoon exposures (Table 5), and none of the associations were found to be significant (P ranged from 0.2 to 0.86). A negative association was observed between the ΔSDNN measured at 2 h after both work shifts, and the corresponding ΔPM2.5-peak, however, was not significant, with P-values of 0.72 and 0.23 for the morning and afternoon exposures, respectively.
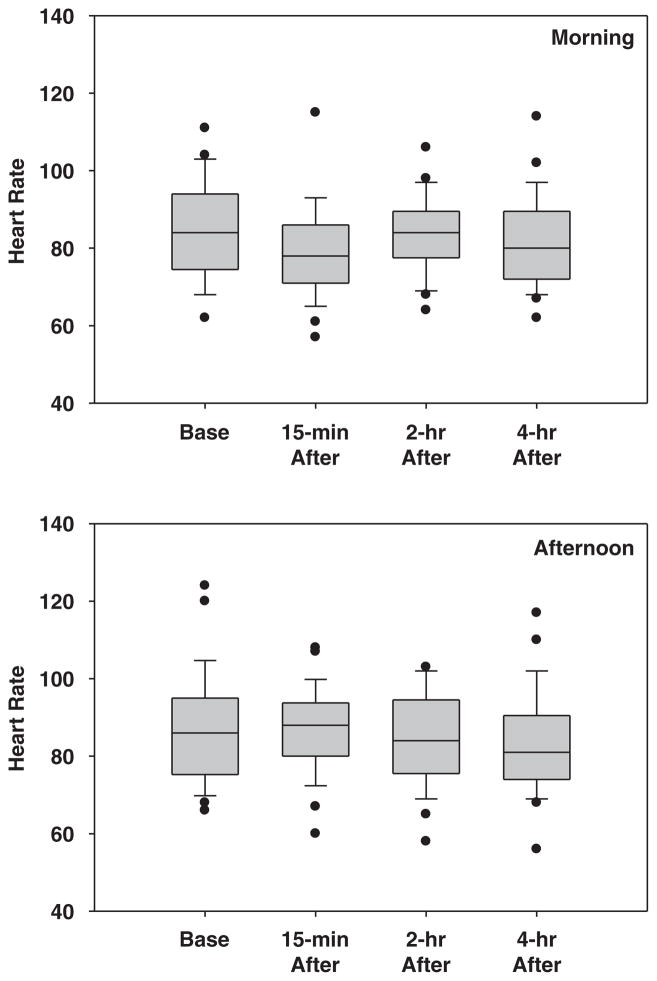
Changes in HR
Both increases and decreases in HR were observed after exposure to PM2.5 during the morning and afternoon shifts (Table 5, Figure 3). The association between ΔHR and ΔPM2.5-ave-m was only marginal for the lag time of 2 h in the morning (P=0.08). It was insignificant for the remaining time points (P-values ranged from 0.14 to 0.87) when we examined the association between either the ΔPM2.5-ave or ΔPM2.5-peak and ΔHR measured during both work shifts (Table 5).
Figure 3.
The heart rate measured before and after the morning (top) and afternoon work shift (bottom).
Changes in Lung Function
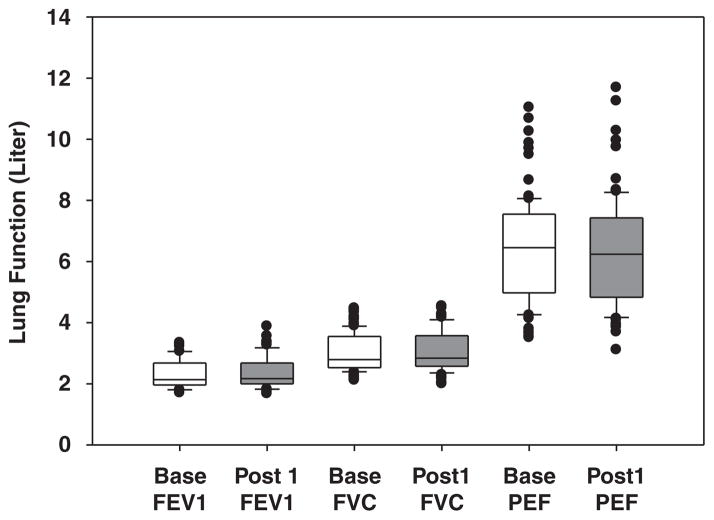
The mean values of lung function parameters (FEV1, FVC, and PEF) measured before and 15 min after the morning shift are presented in Figure 4. Small decreases in FEV1, FVC, and PEF were observed after the morning shift, but the differences in the mean of lung function measures before and after the morning shift were not found to be significant (P>0.23). ΔFEV1, ΔFVC, and ΔPEF were found negatively associated with the ΔPM2.5-ave-m and ΔPM2.5-peak-m (except ΔFEV1 and ln PM2.5-ave-m), however, as shown in Table 6, the association was not significant (P>0.16).
Figure 4.
The lung function (FEV1, FVC, and PEF) measured before and after the morning shift.
Table 6.
Regression coefficients (95% CI) of the changes in lung function (ΔFEV1, ΔFVC, and ΔPEF, in the unit of L) measured after the morning shift relative to increases in the average and peak PM2.5 mass concentration during the 1-h work shift (lnΔPM2.5, in the unit of μg/m3) (N=27).
| Lung function | PM type | Estimate (SE) | P-value |
|---|---|---|---|
| ΔFEV1 | ln_ΔPMave-m | 0.02 (0.04) | 0.68 |
| ΔFVC | ln_ΔPMave-m | −0.10 (0.09) | 0.31 |
| ΔPEF | ln_ΔPMave-m | −0.54 (0.62) | 0.42 |
| ΔFEV1 | ln_ΔPMpeak-m | −0.13 (0.08) | 0.16 |
| ΔFVC | ln_ΔPMpeak-m | −0.12 (0.17) | 0.51 |
| ΔPEF | ln_ΔPMpeak-m | −1.46 (1.12) | 0.24 |
Abbreviations: FVC, forced vital capacity; FEV1, forced expired volume in the first second; PEF, peak expired flow.
Discussion
PM2.5 Exposure
The study showed acute exposure to PM2.5 by being near or in traffic, and the increases in PM2.5 concentrations during the morning shift were found to be higher than during the afternoon shift. This is because the morning shift (7:30–8:30 a.m.) coincides with the rush hour which has an increase in vehicular emissions generated by a variety of vehicles, including diesel-powered school and city buses. In addition, as the morning shift also often coincides with time periods having a low atmospheric mixing height, the concentrations of air pollutants in the intersection during the morning shift are expected to be higher than the afternoon shift.
The average personal concentrations of PM2.5 measured for crossing guards were found to be higher (about two times) than those reported for patrol officers (Riediker et al., 2004) and other commuters (Sabin et al., 2005; Adar et al., 2007). This is probably because school crossing guards often stand in the middle of traffic or next to school bus exhausts and other vehicles while crossing school children. Moreover, frequent idling of school buses and other vehicles during the morning shift generates higher emissions than moving vehicles, resulting in higher concentrations of PM2.5 at the locations where school crossing guards work and therefore higher personal PM2.5 concentrations. This may also partially explain a lack of association between ΔPM2.5-ave and traffic volume measured in the study. Vehicles continue to emit air pollutants while idling, thus, personal PM2.5 concentrations of crossing guards can be increased without increases in the number of vehicles passing through their work locations, that is the concentrations of air pollutants in the congested areas may not be proportional to the number of vehicles that pass through. These observations suggest that the use of traffic count as a surrogate for acute exposure to vehicular emissions may lead to underestimation of personal exposures to air pollutants in high trafficked areas. For those locations, besides considering the traffic count, the time vehicles are present in the monitoring locations should also be considered when estimating personal exposure to vehicular emissions.
Effects of PM2.5 Exposure on HRV, HR, and Lung Function
For health outcomes, we did not find significant effect of peak PM2.5 exposure (ΔPM2.5-peak) on the heath measures in the study, thus, the following discussion primarily focuses on the impact of the increases in the average PM2.5 (ΔPM2.5-ave) during the 1-h work shifts.
PM2.5 Exposure and SDNN
The present study found a consistent negative association between changes in SDNN and exposure to increases in PM2.5 from vehicular exhaust, suggesting that exposure to elevated PM can result in decrease in SDNN. These observations are in agreement with the findings of previous studies (de Paula Santos et al., 2005; Schwartz et al., 2005; Adar et al., 2007). For example, Adar et al. (2007) reported significant associations between decreases in HRV and particle exposure in the elderly. Also, they reported a stronger association between the decreases in HRV and exposure to PM2.5 experienced during the 2-h bus trip than that from non-bus periods. The findings from this study again suggest that exposure to PM2.5 with traffic origin may alter autonomic nervous system, which may be one of the potential physiological mechanisms linking particle air pollution due to traffic and cardiovascular diseases.
An association between changes in SDNN and increases in PM2.5 (ΔPM2.5-ave-m) was found highest 2 h after morning shift. In addition, the effect of PM2.5 on HRV became insignificant 4 h after the morning exposure. These results suggested that acute exposure to PM2.5 may alter HRV for a short period of time. Our observations are consistent with those reported by Vallejo et al. (2006), who reported the strongest effects on HRV for PM2.5 concentrations that were 1.5 and 2 h before the HRV measurements. Nonetheless, it is worth noting that various lag times for the HRV change associated with exposure to elevated PM2.5 have been reported previously. Gold et al.(2000) reported decreases in HRV in the 21 older adults after exposure to increases in ambient PM2.5 over the previous 4 h; other researchers (Liao et al., 1999; Pope et al., 1999) reported changes in HRV with a lag time of 24 h after exposure to elevated PM2.5; and Park et al.(2005) reported strongest association of HRV with the 48-h moving averages of PM2.5. The mechanisms underlying the effect of PM2.5 on the autonomic system are not well understood and require further investigation.
The decreases in SDNN post-afternoon exposure were very small and insignificantly. The lack of significance in the afternoon sessions may be partially due to the lower afternoon PM2.5 concentrations than in the morning (see Table 2). In addition, we noticed that the SDNN before the afternoon work shift (the afternoon base in Figure 2) was lower than the morning baseline (Figure 2), leading to a small difference in SDNN between post-afternoon and before afternoon exposure. It is not clear whether the lower afternoon SDNN baseline was due to the carry-over effect from the morning exposure on HRV or diurnal pattern of the HRV.
PM2.5 Exposure and HR
Surprisingly, we did not observe any consistent or significant associations between changes in HR and increases in PM2.5 exposure. Lack of such associations should not be attributed to the light activities of the subjects during their work shifts. As described in “Materials and methods” section, the HRs measured at 15 min, 2, and 4 h after work shift were used for analysis. Further, the subjects sat quietly for 10 min, and the ECG from a 5-min period towards the end of the 10 min period was examined. Thus, there should have been sufficient time for the HR to return to the subjects’ resting baselines before the 5-min ECG data period.
Different PM2.5 effects on HR were reported previously. Ibald-Mulli et al. (2004) found a significant decrease in HR associated with PM2.5 exposure in only one of four centers studied, but no significant associations were found for most subjects examined. In contrast, other investigators (Peters et al., 1999; Pope et al., 1999; Adar et al., 2007) reported increases in HR resulted from exposure to PM2.5. The discrepancy observed in different studies are not known. This may be partially due to different exposures and subjects conditions examined in each study.
PM2.5 Exposure and Lung Function
No significant changes in lung function measures were observed for the crossing guards after their acute exposures to elevated PM2.5 concentrations during their work shifts. The findings are consistent with previous studies of effects on lung function with controlled PM exposure (Salvi et al., 1999; Devlin et al., 2003). For example, Salvi et al. (1999) observed increases in systemic and pulmonary inflammatory markers in healthy human volunteers after their 1-h exposure to diesel particles (300 μg/m3 of PM10), but found no changes in standard lung function measurements.
It is recognized that some limitations exist in the study design. First, the small size of the study may have resulted in insufficient power to detect some associations between health outcomes and elevated personal PM2.5 levels. Second, personal exposures to other traffic-related air pollutants, such as CO and NO2 that may affect the cardiopulmonary endpoints, were not measured. Third, we did not control other exposure-related factors that might have confounded the association between PM2.5 exposure and HRV, such as stress and noise during the work shift. Thus, we cannot determine whether the co-pollutants and/or other factors may have contributed to the effects observed. Nonetheless, the findings from the study suggest that common acute exposure to mobile source emissions may have important cardiovascular health consequences. Further studies are needed to investigate how the acute decline in HRV due to exposure to vehicular emission may be related to the increased risks of adverse cardiovascular events. Moreover, this study suggests that research is needed to examine the correlation between personal exposure levels of traffic generated air pollutants and traffic count in traffic congested areas to define the proper surrogates for future exposure and health studies associated with traffic air pollution.
Acknowledgments
This research was supported in part by the NIEHS sponsored UMDNJ Center for Environmental Exposures and Disease (NIEHS P30ES005022), Q. Meng is a US EPA/NCEA-DOE/ORISE research fellow. We thank Dr. Linda Bonanno from the New Jersey Department of Environmental Protection for providing contact information for the Paterson Police Department in New Jersey. We also thank the subjects for their participation and the support from the Paterson Police Department. The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the funding agencies and US EPA.
References
- Adar SD, Gold DR, Coull BA, Schwartz J, Stone PH, Suh H. Focused exposures to airborne traffic particles and heart rate variability in the elderly. Epidemiology. 2007;18(1):95–103. doi: 10.1097/01.ede.0000249409.81050.46. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society. Standardization of Spirometry 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- Brauer M, Hoek G, Van Vliet P, Meliefste K, Fischer PH, Wijga A, et al. Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. Am J Respir Crit Care Med. 2002;166(8):1092–1098. doi: 10.1164/rccm.200108-007OC. [DOI] [PubMed] [Google Scholar]
- Buckeridge DL, Glazier R, Harvey BJ, Escobar M, Amrhein C, Frank J. Effect of motor vehicle emissions on respiratory health in an urban area. Environ Health Perspect. 2002;110(3):293–300. doi: 10.1289/ehp.02110293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula Santos U, Braga AL, Giorgi DM, Pereira LA, Grupi CJ, Lin CA, et al. Effects of air pollution on blood pressure and heart rate variability: a panel study of vehicular traffic controllers in the city of Sao Paulo, Brazil. Eur Heart J. 2005;26(2):193–200. doi: 10.1093/eurheartj/ehi035. [DOI] [PubMed] [Google Scholar]
- Devlin RB, Ghio AJ, Kehrl H, Sanders G, Cascio W. Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. Eur Respir J. 2003;21:76s–80s. doi: 10.1183/09031936.03.00402403. [DOI] [PubMed] [Google Scholar]
- Finkelstein MM, Jerrett M, Sears MR. Traffic air pollution and mortality rate advancement periods. Am J Epidemiol. 2004;160(2):173–177. doi: 10.1093/aje/kwh181. [DOI] [PubMed] [Google Scholar]
- Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, et al. Ambient pollution and heart rate variability. Circulation. 2000;101(11):1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- Gold DR, Litonjua AA, Zanobetti A, Coull BA, Schwartz J, MacCallum G, et al. Air pollution and ST-segment depression in elderly subjects. Environ Health Perspect. 2005;113(7):883–887. doi: 10.1289/ehp.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek G, Brunekreef B, Goldbohm S, Fischer P, van den Brandt PA. Association between mortality and indicators of traffic-related air pollution in the Netherlands: a cohort study. Lancet. 2002;360(9341):1203–1209. doi: 10.1016/S0140-6736(02)11280-3. [DOI] [PubMed] [Google Scholar]
- Ibald-Mulli A, Timonen KL, Peters A, Heinrich J, Wolke G, Lanki T, et al. Effects of particulate air pollution on blood pressure and heart rate in subjects with cardiovascular disease: a multicenter approach. Environ Health Perspect. 2004;112(3):369–377. doi: 10.1289/ehp.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Smorodinsky S, Lipsett M, Singer BC, Hodgson AT, Ostro B. Traffic-related air pollution near busy roads: the East Bay Children’s Respiratory Health Study. Am J Respir Crit Care Med. 2004;170(5):520–526. doi: 10.1164/rccm.200403-281OC. [DOI] [PubMed] [Google Scholar]
- Laden F, Neas LM, Dockery DW, Schwartz J. Association of fine particulate matter from different sources with daily mortality in six US cities. Environ Health Perspect. 2000;108(10):941–947. doi: 10.1289/ehp.00108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao DP, Creason J, Shy C, Williams R, Watts R, Zweidinger R. Daily variation of particulate air pollution and poor cardiac autonomic control in the elderly. Environ Health Perspect. 1999;107(7):521–525. doi: 10.1289/ehp.99107521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Munsie JP, Hwang SA, Fitzgerald E, Cayo MR. Childhood asthma hospitalization and residential exposure to state route traffic. Environ Res. 2002;88(2):73–81. doi: 10.1006/enrs.2001.4303. [DOI] [PubMed] [Google Scholar]
- Marr LC, Grogan LA, Wohrnschimmel H, Molina LT, Molina MJ, Smith TJ, et al. Vehicle traffic as a source of particulate polycyclic aromatic hydrocarbon exposure in the Mexico City metropolitan area. Environ Sci Technol. 2004;38(9):2584–2592. doi: 10.1021/es034962s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, O’Neill MS, Vokonas PS, Sparrow D, Schwartz J. Effects of air pollution on heart rate variability: the VA normative aging study. Environ Health Perspect. 2005;113(3):304–309. doi: 10.1289/ehp.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Perz S, Doring A, Stieber J, Koenig W, Wichmann HE. Increases in heart rate during an air pollution episode. Am J Epidemiol. 1999;150(10):1094–1098. doi: 10.1093/oxfordjournals.aje.a009934. [DOI] [PubMed] [Google Scholar]
- Pope CA, III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109(1):71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- Pope CA, Verrier RL, Lovett EG, Larson AC, Raizenne ME, Kanner RE, et al. Heart rate variability associated with particulate air pollution. Am Heart J. 1999;138(5):890–899. doi: 10.1016/s0002-8703(99)70014-1. [DOI] [PubMed] [Google Scholar]
- Riediker M, Cascio WE, Griggs TR, Herbst MC, Bromberg PA, Neas L, et al. Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med. 2004;169(8):934–940. doi: 10.1164/rccm.200310-1463OC. [DOI] [PubMed] [Google Scholar]
- Rowan WH, III, Campen MJ, Wichers LB, Watkinson WP. Heart rate variability in rodents: uses and caveats in toxicological studies. Cardiovasc Toxicol. 2007;7(1):28–51. doi: 10.1007/s12012-007-0004-6. [DOI] [PubMed] [Google Scholar]
- Sabin LD, Behrentz E, Winer AM, Jeong S, Fitz DR, Pankratz DV, et al. Characterizing the range of children’s air pollutant exposure during school bus commutes. J Expo Anal Environ Epidemiol. 2005;15(5):377–387. doi: 10.1038/sj.jea.7500414. [DOI] [PubMed] [Google Scholar]
- Salvi S, Blomberg A, Rudell B, Kelly F, Sandstrom T, Holgate ST, et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med. 1999;159(3):702–709. doi: 10.1164/ajrccm.159.3.9709083. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Litonjua A, Suh H, Verrier M, Zanobetti A, Syring M, et al. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax. 2005;60(6):455–461. doi: 10.1136/thx.2004.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter JC, Kim E, Sheppard L, Sullivan JH, Larson TV, Claiborn C. Association between particulate matter and emergency room visits, hospital admissions and mortality in Spokane, Washington. J Expo Anal Environ Epidemiol. 2005;15(2):153–159. doi: 10.1038/sj.jea.7500382. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Venditti FJ, Jr, Manders ES, Evans JC, Larson MG, Feldman CL, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90(2):878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- Vallejo M, Ruiz S, Hermosillo AG, Borja-Aburto VH, Cardenas M. Ambient fine particles modify heart rate variability in young healthy adults. J Expo Sci Environ Epidemiol. 2006;16(2):125–130. doi: 10.1038/sj.jea.7500447. [DOI] [PubMed] [Google Scholar]
- Wellenius GA, Bateson TF, Mittleman MA, Schwartz J. Particulate air pollution and the rate of hospitalization for congestive heart failure among medicare beneficiaries in Pittsburgh, Pennsylvania. Am J Epidemiol. 2005;161(11):1030–1036. doi: 10.1093/aje/kwi135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc. 2002;52(9):1032–1042. doi: 10.1080/10473289.2002.10470842. [DOI] [PubMed] [Google Scholar]