Abstract
Background
Despite recent developments in preoperative breast cancer imaging, intraoperative localization of tumor tissue can be challenging, resulting in tumor-positive resection margins during breast-conserving surgery. Based on certain physicochemical similarities between Technetium(99mTc)-sestamibi (MIBI), a SPECT radiodiagnostic with a sensitivity of 83–90% to detect breast cancer preoperatively, and the near-infrared (NIR) fluorophore Methylene Blue (MB), we hypothesized that MB might detect breast cancer intraoperatively using NIR fluorescence imaging.
Methods
Twenty-four patients with breast cancer, planned for surgical resection, were included. Patients were divided in 2 administration groups, which differed with respect to the timing of MB administration. N = 12 patients per group were administered 1.0 mg/kg MB intravenously either immediately or 3 h before surgery. The mini-FLARE imaging system was used to identify the NIR fluorescent signal during surgery and on post-resected specimens transferred to the pathology department. Results were confirmed by NIR fluorescence microscopy.
Results
20/24 (83%) of breast tumors (carcinoma in N=21 and ductal carcinoma in situ in N=3) were identified in the resected specimen using NIR fluorescence imaging. Patients with non-detectable tumors were significantly older. No significant relation to receptor status or tumor grade was seen. Overall tumor-to-background ratio (TBR) was 2.4 ± 0.8. There was no significant difference between TBR and background signal between administration groups. In 2/4 patients with positive resection margins, breast cancer tissue identified in the wound bed during surgery would have changed surgical management. Histology confirmed the concordance of fluorescence signal and tumor tissue.
Conclusions
This feasibility study demonstrated an overall breast cancer identification rate using MB of 83%, with real-time intraoperative guidance having the potential to alter patient management.
Keywords: Near-Infrared Fluorescence Imaging, Image-Guided Surgery, Breast Cancer, Methylene Blue
INTRODUCTION
Breast cancer is the most common malignancy in women worldwide and is a leading cause of cancer-related mortality [1]. More than 1.2 million cases are diagnosed every year, affecting 10–12% of the female population and accounting for 500,000 deaths per year worldwide.
In early breast cancer, breast-conserving surgery (BCS) is the preferred standard of care. Despite preoperative imaging modalities such as CT and MRI, intraoperative identification of breast cancer tissue can be challenging. Previous studies reported that the incidence of tumor cells at or near the cut edge of the surgical specimen ranged from 5% to 82%, with the majority of studies indicating positive resection margins in 20% to 40% of patients after resection of the primary tumor [2]. Positive margins may lead to additional surgical procedures, delays in adjuvant treatment, increased morbidity, poor aesthetic results, and increased healthcare costs. Therefore, there is an urgent need for new technology to identify breast cancer tissue intraoperatively.
Technetium(99mTc)-sestamibi (MIBI) is a lipophilic cation used for preoperative, noninvasive identification of malignant tissue via SPECT imaging [3]. Using 99mTc-MIBI, preoperative identification of breast cancer is possible in approximately 83–90% of patients [3–5]. Based on the lipophilic, cationic structure of Methylene Blue (MB), and the fact that like 99mTc-MIBI, MB can function as a perfusion tracer in vivo [6;7], we hypothesized that it too might be able to detect breast tumors. Importantly, MB is a clinically available tracer that can be used at relatively low dose (0.5–1 mg/kg) as a fluorescent tracer during NIR fluorescence imaging. NIR fluorescence imaging is a promising technique to assist in the intraoperative identification of sentinel lymph nodes, tumors, and vital structures [8].
During 99mTc-MIBI SPECT imaging, early (within 30 min after tracer administration) and delayed (3 h post tracer administration) imaging is performed in succession [5;9]. The reason for this is to differentiate more accurately between malignant and benign lesions because it is presumed that tracer uptake in malignant lesions might persist, whereas clearance from benign lesions would be more rapid. Delayed imaging could thereby result in higher tumor-to-background ratios (TBR) from lower background signal.
The aim of this study was to determine the feasibility of using MB as a NIR fluorescent tracer for the identification of breast tumor intraoperatively, and to compare early and delayed imaging protocols.
METHODS
Patients
Breast cancer patients planning to undergo breast surgery were eligible for participation in the trial. Patients planned for either breast conserving surgery (BCS) or modified radical mastectomy (MRM) were included. Consent was performed at the department of Surgery. Exclusion criteria were pregnancy or lactation, and various contraindications to MB including the use of serotonin reuptake inhibitors, serotonin and noradrenalin reuptake inhibitors and/or tricyclic antidepressants, severe renal failure, a G6PD-deficiency or a known allergy to MB. All patients gave informed consent and were anonymized.
Clinical Trial
This clinical trial was approved by the Medical Ethics Committee of the Leiden University Medical Center and was performed in accordance with the ethical standards of the Helsinki Declaration of 1975.
Patients were divided in 2 administration groups, which differed with respect to the timing of MB administration. 12 patients per group were administered 1.0 mg/kg MB intravenously over 5 minutes either immediately before surgery, or 3 h before surgery. Distribution between groups was based on the logistics of the operating room time on a particular day. Patients scheduled to be first on the day’s surgical program were administered MB immediately before surgery (early imaging). Patients scheduled later in the day were administered MB 3 hours before surgery (delayed imaging).
The mini-FLARE imaging system was used to identify the fluorescent signal during surgery and on post-resected specimens transferred to the pathology department. During surgery images were obtained from the surgical field, resected specimen, and wound bed after resection. When fluorescent signal was observed, the operating surgeon could decide whether to resect the fluorescent tissue or not, based on clinical judgment of the tissue. The resected specimen was sliced at the pathology department, where images from the bisected tumor were obtained. When possible, snap frozen tissue was collected for fluorescence microscopy images.
Intraoperative Near-Infrared Imaging System (Mini-FLARE)
Imaging procedures were performed using the Mini-Fluorescence-Assisted Resection and Exploration (Mini-FLARE) image-guided surgery system, as described earlier [10]. Briefly, the system consists of 2 wavelength isolated light sources: a “white” light source, generating 26,600 l× of 400 to 650 nm light, and a “near-infrared” light source, generating 1.08 mW/came of ≈ 670 nm light. Color video and NIR fluorescence images are simultaneously acquired and displayed in real time using custom optics and software that separate the color video and NIR fluorescence images. A pseudo-colored (lime green) merged image of the color video and NIR fluorescence images is also displayed. The imaging head is attached to a flexible gooseneck arm, which permits positioning of the imaging head at extreme angles virtually anywhere over the surgical field. For intraoperative use, the imaging head and imaging system pole stand are wrapped in a sterile shield and drape (Medical Technique Inc., Tucson, AZ).
Ex vivo Imaging and Fluorescence Microscopy of Resected Lesion
After slicing of the resected lesion at the Pathology department, fluorescent imaging was again performed with the mini-FLARE imaging system. Fluorescence microscopy images were obtained with the Odyssey Infrared Imaging System (LI-COR, USA).
Statistical Analysis
For statistical analysis, SPSS statistical software package (Version 20.0, Chicago, IL) was used. Graphs were generated using GraphPad Prism Software (Version 5.01, La Jolla, CA). TBRs were calculated by dividing the fluorescent signal of the tumor by fluorescent signal of surrounding tissue. Patient age and body mass index (BMI) were reported in median and range and TBR was reported in mean and standard deviation. To compare patient characteristics, independent samples t-test and chi-square tests were used. To compare TBR and background signal between dose groups, independent samples t-test was used. P < 0.05 was considered significant.
RESULTS
A total of 24 patients were included in this study. Patient and tumor characteristics are detailed in Table 1. Mean patient age was 60 years (range 44 – 82 years); 21 patients were planned for BCS, 3 patients received a MRM. Histopathological analysis showed 14 patients with infiltrating ductal adenocarcinoma, 4 patients with infiltrating lobular adenocarcinoma, 3 patients with ductal carcinoma in situ, 1 patient with a primary mucoepidermoid carcinoma, 1 patient with a mucinous adenocarcinoma, and in 1 patient, no tumor was found in the resected specimen even though a preoperative biopsy showed infiltrating adenocarcinoma. Although patients were not assigned to a specific administration group by randomization, groups were comparable as no significant differences in patient or tumor characteristics were found (Table 1). All patients received preoperative ultrasound, and 9 patients also underwent preoperative MRI scan.
TABLE 1.
Patient and Tumor Characteristics
| Total N=24 |
Early Imaging N=12 |
Delayed Imaging N=12 |
|||||
|---|---|---|---|---|---|---|---|
| Characteristic | Mean | Range | Mean | Range | Mean | Range | P |
| Age | 60 | (44–82) | 59 | (44–82) | 60 | (46–71) | 0.97 |
| Body mass index | 24 | (19–37) | 24 | (19–37) | 27 | (22–37) | 0.16 |
| Pathological tumor size (mm) | 15 | (6–33) | 14 | (7–27) | 16 | (6–33) | 0.58 |
| N | % | N | % | N | % | ||
| Clinical Stage | 0.22 | ||||||
| Stage 0 | 3 | 13 | 3 | 25 | 0 | 0 | |
| Stage 1A | 13 | 54 | 7 | 58 | 6 | 50 | |
| Stage 1B | 1 | 4 | 0 | 0 | 1 | 9 | |
| Stage 2A | 4 | 17 | 2 | 17 | 2 | 17 | |
| Stage 2B | 2 | 8 | 0 | 0 | 2 | 17 | |
| Stage 3C | 1 | 4 | 0 | 0 | 1 | 9 | |
| Type of operation | 0.54 | ||||||
| Mastectomy | 3 | 13 | 1 | 9 | 2 | 17 | |
| Wide local excision | 21 | 87 | 11 | 92 | 10 | 84 | |
| Histological type | 0.22 | ||||||
| Infiltrating ductal type adenocarcinoma | 15 | 63 | 6 | 50 | 9 | 75 | |
| Infiltrating lobular type adenocarcinoma | 4 | 17 | 2 | 17 | 2 | 17 | |
| Mucinous adenocarcinoma | 1 | 4 | 1 | 9 | |||
| Primary mucoepidermoid carcinoma | 1 | 4 | 1 | 4 | |||
| Ductal carcinoma in situ | 3 | 12 | 3 | 25 | |||
| Receptor status | |||||||
| ER positive | 19 | 79 | 8 | 67 | 11 | 92 | 0.83 |
| PR positive | 11 | 46 | 6 | 50 | 5 | 42 | 0.26 |
| HER2/NEU positive | 1 | 4 | 0 | 0 | 1 | 9 | 0.38 |
| Triple Negative | 2 | 8 | 1 | 9 | 1 | 9 | 0.95 |
| Histological grade (Bloom-Richardon) | 0.34 | ||||||
| I | 5 | 21 | 1 | 9 | 4 | 34 | |
| II | 9 | 38 | 5 | 42 | 4 | 34 | |
| III | 4 | 16 | 2 | 17 | 2 | 17 | |
| No grading possible | 6 | 25 | 4 | 34 | 2 | 17 | |
In 20 out of 24 patients (83%), breast tumors (carcinoma in N=21 and ductal carcinoma in situ in N=3) were identified in the resection specimen with NIR fluorescence imaging after bisection in the Pathology department. Tumors were identified as a bright fluorescent spot in the sliced specimen (Figure 1). Table 2 shows patient and tumor characteristics related to detectability of the tumor. Patients with non-detectable tumors were significantly older (mean age 68 years old versus 58 years old; P=0.03). Both infiltrating ductal type adenocarcinoma and infiltrating lobular type adenocarcinoma were detectable. In patients with mucinous adenocarcinoma or primary mucoepidermoid carcinoma, no fluorescent tumor was found. No significant relation was found regarding receptor status or tumor grade.
Figure 1. NIR fluorescence imaging of a tumor resection with negative margins.
A. Resected specimen after wide local excision. No fluorescent signal was seen at resection margins. B. Inspection of wound bed after resection. No fluorescent signal was seen at resection margins. C. Sliced resection specimen at Pathology department. A clear fluorescent spot (arrow) was seen at the location of the tumor. Tumor was an infiltrating ductal adenocarcinoma, grade 2, ER+ PR+ Her2/neu−.
TABLE 2.
Patient and Tumor Characteristics Related to Detectability
| Detectable N=20 |
Not Detectable N=4 |
|||
|---|---|---|---|---|
| Characteristic | Mean | Range | Mean | Range |
| Age | 58 | (44–71) | 68 | (56–82) |
| Body mass index | 26 | (19–37) | 24 | (22–26) |
| Pathological tumor size (mm) | 16 | (6–33) | 10 | (7–12) |
| N | % | N | % | |
| Clinical Stage | ||||
| Stage 0 | 2 | 8 | 1 | 4 |
| Stage 1A | 10 | 42 | 3 | 13 |
| Stage 1B | 1 | 4 | 0 | 0 |
| Stage 2A | 4 | 17 | 0 | 0 |
| Stage 2B | 2 | 8 | 0 | 0 |
| Stage 3C | 1 | 4 | 0 | 0 |
| Histological type | ||||
| Infiltrating ductal type adenocarcinoma | 14 | 59 | 1 | 4 |
| Infiltrating lobular type adenocarcinoma | 4 | 17 | 0 | 0 |
| Mucinous adenocarcinoma | 0 | 0 | 1 | 4 |
| Primary mucoepidermoid carcinoma | 0 | 0 | 1 | 4 |
| Ductal carcinoma in situ | 2 | 8 | 1 | 4 |
| Receptor status | ||||
| ER positive | 17 | 71 | 2 | 8 |
| PR positive | 9 | 38 | 2 | 8 |
| HER2/NEU positive | 1 | 4 | 0 | 0 |
| Triple Negative | 2 | 4 | 0 | 0 |
| Histological grade (Bloom-Richardon) | ||||
| I | 4 | 17 | 1 | 4 |
| II | 9 | 37 | 0 | 0 |
| III | 3 | 13 | 1 | 4 |
| No grading possible | 4 | 17 | 2 | 8 |
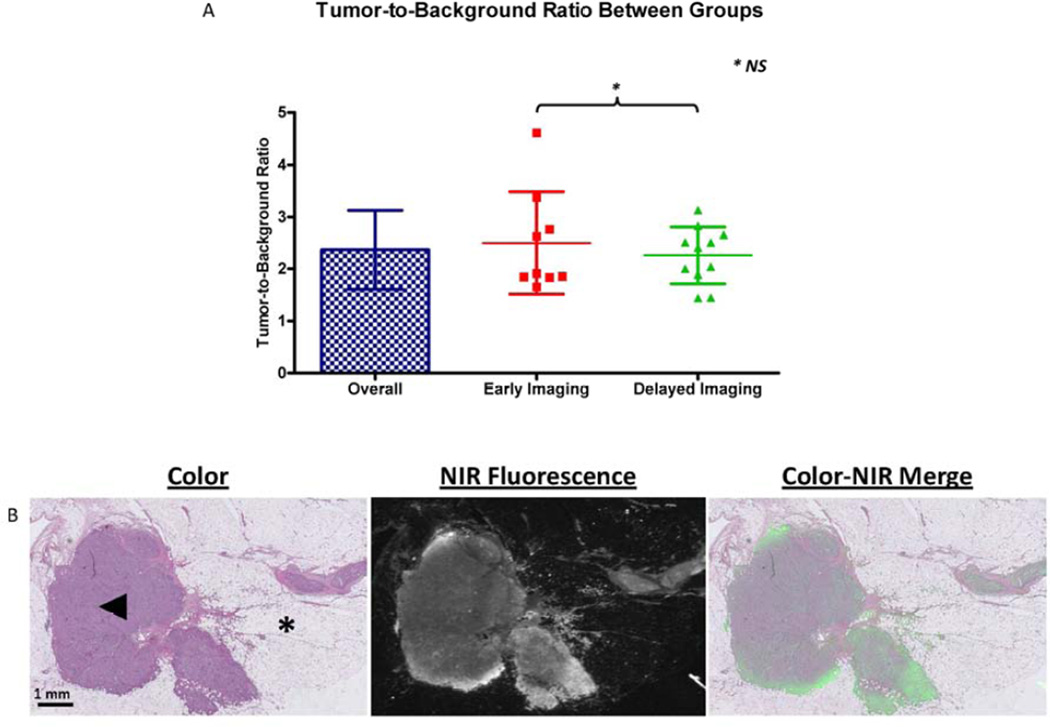
The overall TBR was 2.4 ± 0.8 (Figure 2A). There was no significant difference between administration groups in TBR (2.5 ± 0.9 vs. 2.3 ± 0.5; P=0.50) or background signal in arbitrary units (333 ± 215 vs. 262 ± 134; P = 0.37, data not shown) in the sliced specimens.
Figure 2. Tumor-to-Background Ratio and microscopic images of resected lesion.
A. Overall Tumor-to-Background Ratio (TBR) and TBR per administration group are shown. No differences in TBR between administration groups were observed (P = 0.50; 95% CI −0,49 – 0,96). B. Microscopic images of Hematoxylin and Eosin staining of the resected lesion and fluorescent signal (Odyssey Infrared Imaging System, LI-COR). At the NIR fluorescent image, fluorescent signal is seen as white, where surrounding breast tissue remains black. A clear overlay between fluorescent signal and tumor tissue was seen. Normal breast tissue was indicated by an asterisk (*). Tumor (indicated by arrowhead) was an infiltrating ductal adenocarcinoma, grade 2, ER + PR + Her2/neu −.
Four patients (17%) were found to have positive resection margins. In case 1, tumor tissue was identified both on the surface of the resected specimen (Figure 3A) and intraoperatively in the wound bed using NIR fluorescence imaging (Figure 3B). Direct re-resection was performed, and histopathological analysis confirmed that the fluorescent resected tissue was tumor (data not shown). Both primary resected and re-resected tumor tissue was an infiltrating lobular adenocarcinoma, grade 2, ER+ PR− Her2/neu −. In case 2, no fluorescent tumor signal was seen on the surface of the resected specimen. In the bisected specimen, no fluorescent signal was seen at location of the tumor. This could have been because of lack of uptake or intracellular conversion of MB to its leuco [11], non-fluorescing form, but either way explains the lack of fluorescent signal in the tumor-positive resection margin. Tumor was a DCIS grade 3. In case 3, only images of the sliced specimen were available due to logistics, so no intraoperative fluorescent images were available. In case 4, clear fluorescent spots were identified in the wound bed intraoperatively, however, the operating surgeon believed that they were a false positive and did not resect them. Histopathology, though, confirmed positive margins at the location of the fluorescent spots. Afterwards, patient underwent a mastectomy in which the residual lobular infiltrating adenocarcinoma was found near the lumpectomy cavity.
Figure 3. NIR fluorescence imaging of a tumor resection with positive margins.
A. Resected specimen after wide local excision. Fluorescent signal was seen at the deep margin of the resection specimen, indicated by arrows. B. Inspection of wound bed after resection. Fluorescent signal was seen at resection margins indicated by arrows. Direct re-resection of the fluorescent tissue was performed, which contained malignant tumor tissue. Tumor was an infiltrating lobular adenocarcinoma, grade 2, ER+ PR− Her2/neu −.
In the patients with negative resection margins, median minimal distance of tumor tissue to resection margin was 3 (range 1–20).
Histological validation of MB positive tumors with fluorescence microscopy showed a clear overlay between fluorescent signal and tumor tissue (Figure 2B).
Three patients in the early administration group experienced transient mild pain of the lower arm during administration of MB, which disappeared after flushing the intravenous cannula with saline. No other adverse reactions associated with the use of MB or the Mini- FLARE™ intraoperative NIR fluorescence camera were observed.
DISCUSSION
The current study demonstrated feasibility of real-time identification of breast cancer using NIR fluorescence imaging and MB. In 83% of patients, tumor demarcation as identified by NIR fluorescence imaging corresponded to histological presence of tumor. In addition, in one case surgical management was changed based on intraoperative NIR fluorescence findings, which avoided the need for re-resection.
During breast cancer surgery, the distinction between healthy and malignant tissue is often not evident, resulting in positive resection margins in up to 40% of patients undergoing breast conserving surgery [12;13]. Despite major improvements in preoperative imaging, real-time intraoperative imaging modalities are lacking [2]. Therefore, breast cancer surgeons often still have to rely on palpation and previously obtained mammography or MRI to determine the extent of resection. Optical imaging using exogenous contrast agents could usher in a new era in surgical oncology. However, to successfully use this modality both a clinical-grade intraoperative fluorescence imaging system and a tumor-specific NIR probe are obligatory. To date multiple camera systems have become clinically available, however FDA/EMA approved tumor-specific probes are still lacking. Therefore, it is important to exploit clinically available contrast agents, such as indocyanine green and MB [14] whenever possible.
Several papers have reported the intraoperative use of MB for identification of various tumors. As early as 1971, MB was used at high concentration as a visible blue dye to stain parathyroid adenomas after systemic administration [15]. Thereafter, it was shown that there was a high uptake of the tracer in various kinds of neuroendocrine tumors [16–18]. One of the disadvantages of using MB is that the high dose (7.5 mg/kg) used in these prior studies, which was needed for visualization of the blue colored dye the human eye, brings substantial risk of serious adverse events, such as toxic metabolic encephalopathy [19].
NIR fluorescence imaging permits visualization of MB at low concentration because MB fluoresces at 700 nm. In transgenic mice, Winer et al [20] showed intraoperative identification of insulinoma, a pancreatic neuroendocrine tumor, using NIR fluorescence imaging and MB. In a clinical setting, this technique has been used for the intraoperative identification of parathyroid adenomas and a solitary fibrous tumor of the pancreas [21;22]. In these prior studies, a dose of only 0.5 – 0.1 mg/kg MB was used, which resulted in clear fluorescent signal in the identified lesions. Although we did not explore dose in this feasibility study, our results suggest that a formal study of dose, which includes higher doses than 1.0 mg/kg, is warranted. Such a study could answer definitively if the 17% of tumors that were not identified using MB were because the dose was too low.
The exact mechanism of tracer uptake in these tumors is unclear. The physicochemical similarities of MB and 99mTc-MIBI, both lipophilic and cationic, suggest a possible common mechanism. Lipophilic captions were originally developed as myocardial perfusion agents, and subsequently used as tumor-seeking agents in a variety of tumors [23]. They emerged as suitable tools to explore specific cellular processes and functions in malignant tumors. In several studies, it was shown that lipophilic cations show passive influx into cells with large negative plasma membrane and mitochondrial membrane potentials [23;24], with influx being reversible. Cellular processes that are associated with uptake of these tracers are apoptosis, proliferation, P-glycoprotein expression, and neoangiogenesis. However, it has to be further explored whether any of these mechanisms result in MB accumulation in breast cancer tissue.
Using 99mTc-MIBI, a breast tumor identification rate of 83–90% has been reported. Although our present study was small (N = 24 subjects), our identification rate is in agreement with the sensitivity of 99mTc-MIBI described in the literature. Based on clinical experience with 99mTc-MIBI in breast cancer, early and delayed imaging is used to discriminate between malignant and benign lesions, as tracer accumulation is prolonged in malignant lesions. This is in contrary to MR imaging of breast malignancies, where malignant lesions tends to enhance but also washout quicker than benign lesions [25]. This is caused by endothelial fenestration in microvasculature, which leads to increased capillary leakage, and connections between the arteriolar and venular systems (arteriovenous shunting), which leads to less perfusion of the capillary bed. Complicating the understanding of mechanism even further is the fact that MB can be converted to a non-fluorescent leuco form under certain intracellular redox and pH conditions [11]. Because the exact mechanism of 99mTc-MIBI and MB uptake in breast tumors is still unclear, future studies have to be done regarding this effect. In the present study, though, no difference was seen between different administration groups in TBR and background signal, suggesting that the more convenient early imaging protocol could be used in future studies.
As NIR fluorescence imaging is a surface technology (≈ 5 mm penetration depth), it is important to understand its capabilities and limitations. To date, research and development of new imaging modalities for oncology mainly focus on the detection of small tumor deposits in the human body. In breast cancer, these small tumor deposits can change surgical decision making, but do not provide such prognostic relevance in breast conserving surgery, as postoperative radiation eliminates microscopic tumor deposits in most cases [26]. For example, the rate of small tumor deposits is 2 to 3 times higher than the incidence of local recurrence using preoperative MR imaging, resulting in mastectomies that may not be beneficial to patient survival [27]. On the other hand, adequate visualization of tumor margins intraoperatively would be beneficial to lower the number of R1 resections and to avoid the need for re-resections and radiotherapeutic boost therapy [28]. Using intraoperative NIR fluorescence imaging, it is not possible to image the whole breast for small tumor deposits due to the limited penetration depth. However, the optical properties of NIR fluorescence are very well suited for the visualization of possible residual tumor cells at the resection margin. Additionally, NIR fluorescence has an exceptionally high spatial resolution compared to conventional imaging techniques.
To benefit from the full potential for NIR fluorescence imaging several factors are of paramount importance and need to be optimized: 1) the concentration of the NIR fluorophore in the target tissue, 2) minimizing photon absorption and scattering in the tissue, 3) maximizing excitation power of NIR excitation without inducing photobleaching or photo damage to tissue, and 4) the sensitivity of the CCD chip on the detector. Methylene blue becomes a moderate-strength fluorophore when used at low concentrations, with an excitation maximum of 670 nm. Contrast agents with an emission peak of ≈700 nm have several limitations compared to 800 nm fluorophores with respect to quantum yield, penetration depth, and autofluorescence. It is hoped that new tumor-specific “800 nm” contrast agents will become widely available during the next few years, although MB could fill the gap in the meantime. Improved laser or LED light sources and cameras are under constant development to overcome the imaging system associated factors.
Another important consideration is extraneous NIR fluorescence generated from other drugs in the surgical field. During this study we found that Patent Blue, used for sentinel lymph node mapping, exhibited a weak NIR fluorescence at 700 nm that could confound the MB results (data not shown). Because we have previously demonstrated that blue dye can be omitted from sentinel lymph node mapping when indocyanine green (ICG) is used [29], and the FLARE imaging system is capable of imaging 2 independent channels of NIR fluorescence, e.g., NIR Channel 1 for MB-guided breast cancer resection and NIR Channel 2 for ICG-guided sentinel lymph node mapping, it should be possible to eliminate Patent Blue from future protocols and thus eliminate this potential confounder.
The primary endpoint of this study was the identification ratio of breast cancer using NIR fluorescence and MB and optimization of injection timing. As it was the first feasibility study with MB in breast cancer, no outcomes data were collected to correlate with intraoperative NIR fluorescence findings. Therefore, is not possible to draw conclusions on the prognostic relevance of the technology, although one might ponder that true negative MB uptake selected for tumors of low perfusion and relatively low metastatic potential. Thereby only one patient directly benefitted from this technique with respect to direct resection of residual tumor tissue due to the reasons mentioned above. Future studies will need to address this and other remaining questions.
In conclusion, this is the first study to demonstrate the use of low dose MB for the real-time identification of breast cancer using NIR fluorescence and MB. No difference was seen between different administration groups in TBR and background signal, suggesting that the more convenient early imaging protocol could be used in future studies. Although larger studies are necessary to determine patient benefit, results with MB are promising and improved contrast agents will likely become available in the future.
ACKNOWLEDGEMENTS
Q.R.J.G. Tummers and F.P.R. Verbeek share first authorship. We thank David Burrington jr. for editing. This work was supported in part by the Dutch Cancer Society grant UL2010- 4732 and National Institutes of Health grant R01-CA-115296. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported in part by the Center of Translational Molecular Medicine (MUSIS project, grant 03O-202-04).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
Quirijn R.J.G. Tummers, M.D.: Nothing to declare
Floris P.R. Verbeek, MSc: Nothing to declare
Boudewijn E. Schaafsma, M.D.: Nothing to declare
Martin C. Boonstra, BSc.: Nothing to declare
Joost R. van der Vorst, M.D.: Nothing to declare
Gerrit-Jan Liefers, M.D., Ph.D.: Nothing to declare
Cornelis J.H. van de Velde, M.D., Ph.D.: Nothing to declare
John V. Frangioni, M.D., Ph.D.: FLARE™ technology is owned by Beth Israel Deaconess Medical Center, a teaching hospital of Harvard Medical School. Dr. Frangioni has started three for-profit companies, Curadel, Curadel ResVet Imaging, and Curadel Surgical Innovations, which has optioned FLARE™ technology for potential licensing from Beth Israel Deaconess Medical Center.
Alexander L. Vahrmeijer, M.D., Ph.D.: Nothing to declare
REFERENCES
- 1.Hortobagyi GN, de la Garza SJ, Pritchard K, Amadori D, Haidinger R, Hudis CA, Khaled H, Liu MC, Martin M, Namer M, O'Shaughnessy JA, Shen ZZ, Albain KS. The Global Breast Cancer Burden: Variations in Epidemiology and Survival. Clin Breast Cancer. 2005;6(5):391–401. doi: 10.3816/cbc.2005.n.043. [DOI] [PubMed] [Google Scholar]
- 2.Pleijhuis RG, Graafland M, de VJ, Bart J, de jong JS, van Dam GM. Obtaining Adequate Surgical Margins in Breast-Conserving Therapy for Patients With Early-Stage Breast Cancer: Current Modalities and Future Directions. Ann Surg Oncol. 2009;16(10):2717–2730. doi: 10.1245/s10434-009-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu HB, Li L, Xu Q. Tc-99m Sestamibi Scintimammography for the Diagnosis of Breast Cancer: Meta-Analysis and Meta-Regression. Nucl Med Commun. 2011;32(11):980–988. doi: 10.1097/MNM.0b013e32834b43a9. [DOI] [PubMed] [Google Scholar]
- 4.O'Connor M, Rhodes D, Hruska C. Molecular Breast Imaging. Expert Rev Anticancer Ther. 2009;9(8):1073–1080. doi: 10.1586/era.09.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SJ, Kim IJ, Bae YT, Kim YK, Kim DS. Comparison of Early and Delayed Quantified Indices of Double-Phase (99m)Tc MIBI Scintimammography in the Detection of Primary Breast Cancer. Acta Radiol. 2005;46(2):148–154. doi: 10.1080/02841850510020752. [DOI] [PubMed] [Google Scholar]
- 6.Nakayama A, Bianco AC, Zhang CY, Lowell BB, Frangioni JV. Quantitation of Brown Adipose Tissue Perfusion in Transgenic Mice Using Near-Infrared Fluorescence Imaging. Mol Imaging. 2003;2(1):37–49. doi: 10.1162/15353500200303103. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka E, Chen FY, Flaumenhaft R, Graham GJ, Laurence RG, Frangioni JV. Real-Time Assessment of Cardiac Perfusion, Coronary Angiography, and Acute Intravascular Thrombi Using Dual-Channel Near-Infrared Fluorescence Imaging. J Thorac Cardiovasc Surg. 2009;138(1):133–140. doi: 10.1016/j.jtcvs.2008.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image-Guided Cancer Surgery Using Near-Infrared Fluorescence. Nat Rev Clin Oncol. 2013 doi: 10.1038/nrclinonc.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu G, Shih WJ, Huang HY, Long MQ, Sun Q, Liu YH, Chou C. 99Tcm-MIBI Mammoscintigraphy of Breast Masses: Early and Delayed Imaging. Nucl Med Commun. 1995;16(3):150–156. [PubMed] [Google Scholar]
- 10.Mieog JS, Troyan SL, Hutteman M, Donohue KJ, van der Vorst JR, Stockdale A, Liefers GJ, Choi HS, Gibbs-Strauss SL, Putter H, Gioux S, Kuppen PJ, Ashitate Y, Löwik CW, Smit VT, Oketokoun R, Ngo LH, van de Velde CJ, Frangioni JV, Vahrmeijer AL. Towards Optimization of Imaging System and Lymphatic Tracer for Near-Infrared Fluorescent Sentinel Lymph Node Mapping in Breast Cancer. Ann Surg Oncol. 2011;18:2483–2491. doi: 10.1245/s10434-011-1566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsui A, Tanaka E, Choi HS, Kianzad V, Gioux S, Lomnes SJ, Frangioni JV. Real-Time, Near-Infrared, Fluorescence-Guided Identification of the Ureters Using Methylene Blue. Surgery. 2010;148(1):78–86. doi: 10.1016/j.surg.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park CC, Mitsumori M, Nixon A, Recht A, Connolly J, Gelman R, Silver B, Hetelekidis S, Abner A, Harris JR, Schnitt SJ. Outcome at 8 Years After Breast-Conserving Surgery and Radiation Therapy for Invasive Breast Cancer: Influence of Margin Status and Systemic Therapy on Local Recurrence. J Clin Oncol. 2000;18(8):1668–1675. doi: 10.1200/JCO.2000.18.8.1668. [DOI] [PubMed] [Google Scholar]
- 13.Miller AR, Brandao G, Prihoda TJ, Hill C, Cruz AB, Jr, Yeh IT. Positive Margins Following Surgical Resection of Breast Carcinoma: Analysis of Pathologic Correlates. J Surg Oncol. 2004;86(3):134–140. doi: 10.1002/jso.20059. [DOI] [PubMed] [Google Scholar]
- 14.Schaafsma BE, Mieog JS, Hutteman M, van der Vorst JR, Kuppen PJ, Lowik CW, Frangioni JV, van de Velde CJ, Vahrmeijer AL. The Clinical Use of Indocyanine Green As a Near-Infrared Fluorescent Contrast Agent for Image-Guided Oncologic Surgery. J Surg Oncol. 2011;104(3):323–332. doi: 10.1002/jso.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudley NE. Methylene Blue for Rapid Identification of the Parathyroids. Br Med J. 1971;3(5776):680–681. doi: 10.1136/bmj.3.5776.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon DL, Airan MC, Thomas W, Seidman LH. Parathyroid Identification by Methylene Blue Infusion. Br J Surg. 1975;62(9):747–749. doi: 10.1002/bjs.1800620919. [DOI] [PubMed] [Google Scholar]
- 17.Keaveny TV, Fitzgerald PA, McMullin JP. Selective Parathyroid and Pancreatic Staining. Br J Surg. 1969;56(8):595–597. doi: 10.1002/bjs.1800560812. [DOI] [PubMed] [Google Scholar]
- 18.Keaveny TV, Tawes R, Belzer FO. A New Method for Intra-Operative Identification of Insulinomas. Br J Surg. 1971;58(3):233–234. doi: 10.1002/bjs.1800580319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kartha SS, Chacko CE, Bumpous JM, Fleming M, Lentsch EJ, Flynn MB. Toxic Metabolic Encephalopathy After Parathyroidectomy With Methylene Blue Localization. Otolaryngol Head Neck Surg. 2006;135(5):765–768. doi: 10.1016/j.otohns.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 20.Winer JH, Choi HS, Gibbs-Strauss SL, Ashitate Y, Colson YL, Frangioni JV. Intraoperative Localization of Insulinoma and Normal Pancreas Using Invisible Near-Infrared Fluorescent Light. Ann Surg Oncol. 2009 doi: 10.1245/s10434-009-0868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Vorst JR, Vahrmeijer AL, Hutteman M, Bosse T, Smit VT, van de Velde CJ, Frangioni JV, Bonsing BA. Near-Infrared Fluorescence Imaging of a Solitary Fibrous Tumor of the Pancreas Using Methylene Blue. World J Gastrointest Surg. 2012;4(7):180–184. doi: 10.4240/wjgs.v4.i7.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Vorst JR, Schaafsma BE, Verbeek FP, Swijnenburg RJ, Tummers Q, Hutteman M, Hamming JF, Kievit J, Frangioni JV, van de Velde CJ, Vahrmeijer AL. Intraoperative Near-Infrared Fluorescence Imaging of Parathyroid Adenomas Using Low-Dose Methylene Blue. Head Neck. 2013 doi: 10.1002/hed.23384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Vecchio S, Salvatore M. 99mTc-MIBI in the Evaluation of Breast Cancer Biology. Eur J Nucl Med Mol Imaging. 2004;31(Suppl 1):S88–S96. doi: 10.1007/s00259-004-1530-0. [DOI] [PubMed] [Google Scholar]
- 24.Murphy MP. Targeting Lipophilic Cations to Mitochondria. Biochim Biophys Acta. 2008;1777(7–8):1028–1031. doi: 10.1016/j.bbabio.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 25.Millet I, Curros-Doyon F, Molinari N, Bouic-Pages E, Prat X, Alili C, Taourel P. Invasive Breast Carcinoma: Influence of Prognosis and Patient-Related Factors on Kinetic MR Imaging Characteristics. Radiology. 2014;270(1):57–66. doi: 10.1148/radiol.13122758. [DOI] [PubMed] [Google Scholar]
- 26.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y. Effects of Radiotherapy and of Differences in the Extent of Surgery for Early Breast Cancer on Local Recurrence and 15-Year Survival: an Overview of the Randomised Trials. Lancet. 2005;366(9503):2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 27.Bloom S, Morrow M. A Clinical Oncologic Perspective on Breast Magnetic Resonance Imaging. Magn Reson Imaging Clin N Am. 2010;18(2):277–294. doi: 10.1016/j.mric.2010.02.007. ix. [DOI] [PubMed] [Google Scholar]
- 28.Poortmans PM, Collette L, Horiot JC, Van den Bogaert WF, Fourquet A, Kuten A, Noordijk EM, Hoogenraad W, Mirimanoff RO, Pierart M, Van LE, Bartelink H. Impact of the Boost Dose of 10 Gy Versus 26 Gy in Patients With Early Stage Breast Cancer After a Microscopically Incomplete Lumpectomy: 10-Year Results of the Randomised EORTC Boost Trial. Radiother Oncol. 2009;90(1):80–85. doi: 10.1016/j.radonc.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 29.van der Vorst JR, Schaafsma BE, Verbeek FP, Hutteman M, Mieog JS, Lowik CW, Liefers GJ, Frangioni JV, van de Velde CJ, Vahrmeijer AL. Randomized Comparison of Near-Infrared Fluorescence Imaging Using Indocyanine Green and 99(m) Technetium With or Without Patent Blue for the Sentinel Lymph Node Procedure in Breast Cancer Patients. Ann Surg Oncol. 2012;19(13):4104–4111. doi: 10.1245/s10434-012-2466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]