Abstract
The liver is a central organ that controls systemic energy homeostasis and nutrient metabolism. Dietary carbohydrates and lipids, and fatty acids derived from adipose tissue are delivered to the liver, and utilized for gluconeogenesis, lipogenesis and ketogenesis, which are tightly regulated by hormonal and neural signals. Hepatic lipogenesis is activated primarily by insulin that is secreted from the pancreas after high carbohydrate meal. SREBP-1c and ChREBP are major transcriptional regulators that induce key lipogenic enzymes to promote lipogenesis in the liver. SREBP-1c is activated by insulin through complex signaling cascades that control SREBP-1c at both transcriptional and post-translational levels. ChREBP is activated by glucose independently of insulin. Here, we attempt to summarize our current understanding of the molecular mechanism for the transcriptional regulation of hepatic lipogenesis, focusing on recent studies that explore the signaling pathways controlling SREBPs and ChREBP.
Introduction
Mammals adapt to the fluctuation of nutrient availability by storing surplus nutrient mainly in adipose tissue in the form of triglyceride (TG). Ingestion of carbohydrates stimulates the conversion of carbohydrate into TG in the liver, which is followed by the mobilization of TG from the liver to adipose tissue for long-term storage. Increased glucose level in circulation after a high-carbohydrate meal activates hepatic lipogenesis through multiple mechanisms. Pancreatic hormones, glucagon and insulin, play central roles in the regulation of both glucose and lipid metabolism. Glucose triggers insulin secretion from pancreatic beta cells, which stimulates glucose uptake and utilization, and promotes glycogen synthesis and lipogenesis in the liver. Insulin also suppresses hepatic glucose production, fat oxidation and ketogenesis, shifting the balance to fat storage. Glucose itself also acts as a signaling molecule to regulate the genes encoding important enzymes in glycolysis and lipogenesis1.
Metabolic and hormonal cues such as glucose, insulin and glucagon regulate gene expression program of glycolysis and lipogenesis via transcription factors. Sterol regulatory element binding protein -1c (SREBP-1c) is considered as the master transcriptional regulator of fatty acid and TG synthesis in response to insulin stimulation. SREBP-1c is expressed at a low level in the liver of fasted animals, but dramatically induced upon feeding, which is mediated by insulin 2,3. SREBP-1c function is also activated by insulin at the post-translational level. Activated SREBP-1c binds to SRE (Sterol Regulatory Element) sequences found on the promoters of its target genes as a homodimer. SREBP-1c induces mRNAs encoding enzymes catalyzing various steps in fatty acid and TG synthesis pathway, such as ATP-citrate lyase (ACL), acetyl-CoA synthetase (ACS), acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), stearoyl-CoA desaturase-1 (SCD1), and glycerol-3-phosphate acyltransferase (GPAT) 2,4,5.
Carbohydrate-responsive element-binding protein (ChREBP) has been recognized as a transcription factor that is activated by high glucose independent of insulin, and plays a key role in glycolysis and lipogenesis 1. ChREBP induces L-Type Pyruvate Kinase (L-PK), ACC, and FAS genes by directly binding to carbohydrate response elements (ChoRE) found in their promoters 6–8. ChREBP is a bZIP transcription factor that forms a heterodimeric complex with another bZIP protein Max-like protein X (MLX) 9.
During recent years, significant advancement has been made in our understanding of the mechanisms by which SREBP and ChREBP are activated in the liver and regulate lipid metabolism. In this review, we will focus on recent studies that provide new insights into the transcriptional regulation of hepatic lipid metabolism.
SREBP transcription factors
SREBPs are major transcription factors that regulate the expression of genes involved in fatty acids, TG and cholesterol metabolism in the liver 10–12. SREBP family consists of SREBP-1a, SREBP-1c and SREBP-2 13,14. SREBP-1a and SREBP-1c are encoded by a single gene, but transcribed by different promoters, producing similar proteins that differ only in the N-terminal region 14. SREBP-1c is the predominant isoform expressed in liver, while SREBP-1a is produced in certain cell types in immune system as well as in cultured cell lines 14,15. Although there is some functional overlap between different isoforms, SREBP-1c is mostly responsible for the expression of genes involved in fatty acid biosynthesis, while SREBP-2 activates cholesterol metabolism genes 10.
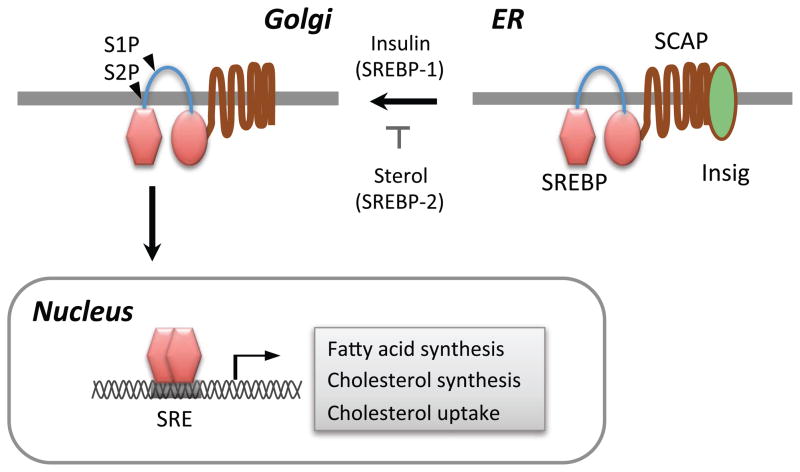
SREBPs are synthesized as precursor forms containing two transmembrane helices that anchor the protein in the ER membrane 16 (Figure 1). SREBPs are associated with the SREBP cleavage activating protein (SCAP) and ER retention protein called Insig 17. In order to be activated, SREBP-SCAP complex should be dissociated from Insig, associate with COPII-coated vesicles and then migrate to Golgi apparatus 18,19. SREBPs are sequentially cleaved by site 1 (S1P) and site 2 (S2P) proteases in the Golgi, which releases the N-terminal cytosolic portion of the protein which enters the nucleus to act as the active transcription factor 10.
Figure 1. Schematic illustration of the proteolytic activation SREBPs.
SREBPs are synthesized as ER-anchored precursor forms. Low cellular sterol concentration triggers the release of SCAP-SREBP-2 complex from Insig. Insulin stimulates the transport of SREBP-1c to Golgi. SREBP is sequentially cleaved by S1P and S2P proteases in the Golgi apparatus. The processed SREBP enter the nucleus to activate the transcription of genes regulating fatty acid and cholesterol metabolism.
Regulation of SREBP activation by proteolytic cleavage
SCAP is a polytopic protein containing eight transmembrane helices and seven loops 20. Transmembrane helices 2–6 are required for the binding of SCAP to Insig 18,21,22. Cholesterol binds to SCAP in loop 1 located in the ER lumen, which triggers a conformational change in the loop 6 facing the cytoplasm 18,21,22. This conformational change of SCAP precludes its interaction with COPII proteins, hence suppresses the mobilization of SCAP-SREBP-2 complex to Golgi. When ER cholesterol level is decreased, SCAP-SREBP-2 complex binds to COPII vesicles to be transported to Golgi for the proteolytic activation. SCAP responds to the changes in ER cholesterol concentration with high precision, such that small changes in the ER cholesterol levels from the threshold level (5%) abruptly turn on/off SCAP-SREBP-2 association with COPII and the consequent SPREP-2 activation, enabling the precise regulation of SREBP-2 by cholesterol abundance 21,22.
It has been well known that insulin transcriptionally activates SREBP-1c in the liver 3,23. But, it has been less clear whether insulin also stimulates proteolytic processing of SREBP-1c, because it is technically difficult to distinguish the contribution by the transcriptional activation and the proteolytic processing to the increased nuclear SREBP-1c level in response to insulin stimulation. To distinguish the effect of insulin on SREBP-1c processing from the transcriptional activation of SREBP1c mRNA, Hegarty et al pretreated rat hepatocytes with LXRα agonist TO-901317 before adding insulin 24. TO-901317 induced SREBP-1c mRNA, which was not further increased by insulin. Under such condition, insulin significantly increased the processed nuclear SREBP-1c protein, indicating that insulin stimulated SREBP-1c processing. Similarly, insulin increased the nuclear processed SREBP-1c level in hepatocytes infected with SREBP-1c adenovirus or in transgenic rat liver that expressed human SREBP-1c under control of apoE promoter 25,26. In contrast, insulin did not increase SREBP-2 processing, highlighting the specific role of insulin in SREBP-1c processing 25. These independent studies clearly demonstrate that insulin not only activates SREBP-1c transcription, but also stimulates SREBP-1c processing.
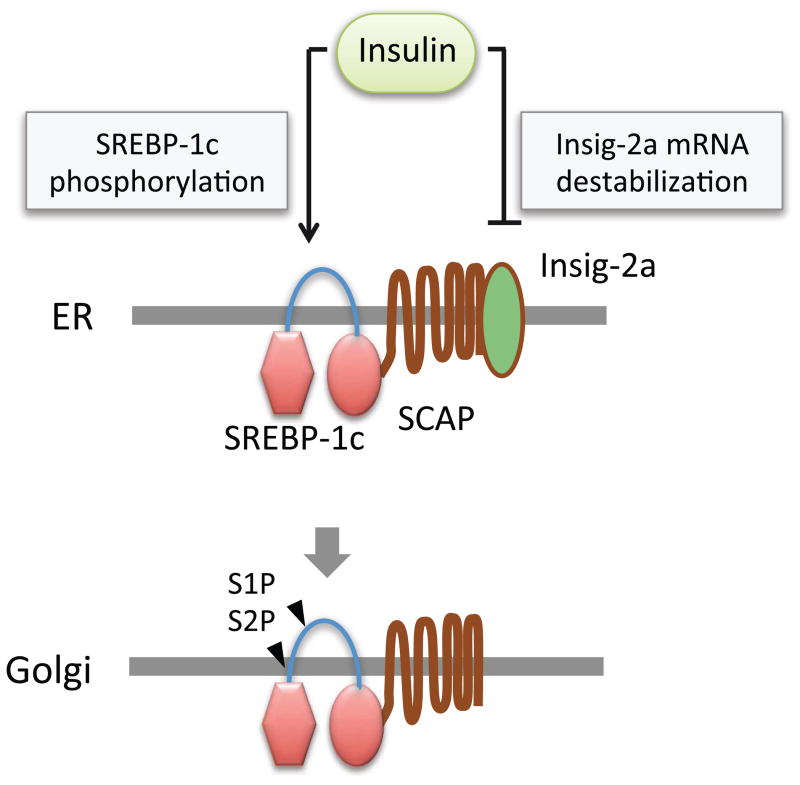
How does insulin stimulate SREBP-1c processing? Stimulation of SREBP-1c processing by insulin was inhibited by small molecule inhibitors of phosphoinositide 3-kinase (PI3K), Akt, and mammalian target of rapamycin (mTOR) complex 1 (mTORC1), and p70 ribosomal S6 kinase (p70S6K), indicating that PI3K/mTOR signaling pathway plays a critical role in SREBP-1c processing 24–27. It has been proposed that Akt directly phosphorylates SREBP-1c, which increases the affinity of SCAP-SREBP-1c complex for Sar1/Sec23/24 proteins of COPII-coated vesicles and facilitates the Golgi transportation of SREBP-1c 25. Interestingly, insulin strongly suppresses the expression of Insig-2a, which is the major Insig isoform expressed in the liver 28. Insig2 gene has two different promoters, from which Insig-2a and Insig-2b mRNAs are transcribed. These two transcripts differ in non-coding exon 1, and hence produce same protein. Suppression of Insig-2a requires Akt activation, and involves mRNA destabilization 27,29. It is notable that Insig-2a preferentially interacts with SCAP-SREBP-1c complex, while Insig-1 binds to SCAP-SREBP-2 29. Hence, it is conceivable that insulin selectively activates SREBP-1c processing through two distinct mechanisms which involve the suppression of Insig-2a, and the induction of SREBP-1c phosphorylation which facilitates the association of SCAP-SREBP-1c complex with COPII-coated vesicles (Figure 2). However, the precise mechanism by which insulin stimulates SREBP-1c processing remains to be further investigated. For example, although SREBP-1c activation correlates well inversely with Insig-2a level, it is not known if the disappearance of Insig-2a protein precedes SREBP-1c activation. Furthermore, Insig-2a is strongly induced by LXRα agonist TO-901317, but does not suppress insulin-mediated SREBP-1c processing, suggesting that Insig-2a down-regulation is not a prerequisite for SREBP-1c processing 24. It is possible that the decreased expression of Insig-2a contributes to SREBP-1c processing upon chronic insulin stimulation, while acute insulin stimulation activates SREBP-1c processing through a distinct mechanism that does not involve Insig-2a down-regulation.
Figure 2. Insulin promotes SREBP-1c processing.
Insulin induces AKT-mediated SREBP-1c phosphorylation, which stimulates the transport of SREBP-1c-SCAP complex to Golgi apparatus. Insulin also induces the degradation of Insig-2a mRNA to promote the Golgi transport and proteolytic processing of SREBP-1c.
Regulation of SREBP activation by nuclear translocation
Although the processed SREBPs contain nuclear localization signal in HLH-Zip domain that mediates spontaneous import of the protein the nucleus 30, a recent study suggests that the nuclear entry of processed SREBP-1 and SREBP-2 could be regulated by mTORC1 31. Lipin 1, a phosphatidic acid phosphatase, is an mTORC1 substrate. Dephosphorylation of lipin 1 by mTOR inhibitor treatment triggers the entry of lipin 1 into the nucleus. Interestingly, dephosphorylated nuclear lipin 1 inhibits nuclear localization of SPREBPs in NIH-3T3 cells. In the presence of lipin 1 in the nucleus, SREBPs appear to localize to the peri-nuclear area in the proximity to the nuclear matrix component lamin A. Lipin 1 construct carrying mutation in mTOC1 phosphorylation site suppressed the nuclear entry of processed SREBP-1c and the expression of lipogenic target genes, indicating that lipin-1 suppresses the transcriptional function of SREBPs by suppressing their nuclear localization. Lipin 1 appears to be critically involved in SREBP regulation by mTORC1 in mouse liver as well, since lipin 1 silencing restored lipogenic gene expression in the liver Raptor knockout mice, where mTORC1 was inactivated. Lipin 1 also regulate fatty acid metabolism through other mechanisms. Lipin 1 dephosphorylates phosphatidic acid to produce diacylglycerol 32,33. It also stimulates fatty acid oxidation in concert with peroxisome proliferator-activated receptor α (PPARα) and its coactivators 34. Further studies will reveal the significance of lipin 1 regulation of SREBP in hepatic lipid metabolism under various pathophysiological conditions.
Transcriptional regulation of SREBP-1c
Hepatic SREBP-1c mRNA level is dynamically regulated by nutritional status. SREBP-1c mRNA expression in liver is suppressed in the fasted animals, and highly induced by ingestion of a high carbohydrate diet 2. SREBP-1c expression was suppressed in diabetic rats induced by streptozotocin treatment, but normalized by insulin injection, indicating that insulin mediates the induction of SREBP-1c mRNA by carbohydrate diet ingestion 23. Insulin strongly induces SREBP-1c mRNA in cultured hepatocytes 14,35. In contrast, glucagon suppresses SREBP-1c mRNA expression via cyclic adenosine 3′,5′-monophosphate/protein kinase A signaling pathway35,36.
The engagement of insulin with its cell surface receptor induces the phosphorylation of the scaffolding protein family insulin-receptor substrates (IRS), which then initiates a signaling cascade that culminates with the transcriptional suppression of gluconeogenesis and the activation of lipogenesis 37,38 (Figure 3). Tyrosine phosphorylation of IRS by insulin receptor recruits phosphoinositide-3-kinase (PI3K), which then phosphorylates phosphatidylinositol (4,5) bisphosphate (PtdIns(4,5)P2) to produce Ptd(3,4,5)P3 (PIP3). As a phospholipid second messenger, PIP3 recruits the Ser/Thr kinase AKT to the plasma membrane, where it is phosphorylated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) to be activated. Consequently, active AKT phosphorylates a wide range of downstream targets involved in cell metabolism, such as forkhead box protein O1 (Foxo1), glycogen synthase kinase 3 (GSK3), and tuberous sclerosis 2 (TSC2) within TSC1-TSC2 complex 39,40. TSC2 is a critical regulator of the mammalian TOR (mTOR) complex 1 (mTORC1), which plays a central role in cell growth and metabolism 41,42. Phosphorylation of TSC2 by AKT results in the activation of mTORC1, as the phosphorylated TSC2 no longer inhibits the Ras homolog enriched in brain (Rheb) protein that is critically required for mTORC1 functions 40.
Figure 3. Regulation of SREBP by insulin signaling pathway.
Insulin activates SREBP-1 through multiple mechanisms. Insulin stimulates SREBP-1c transcription, promotes proteolytic processing, facilitates the nuclear import of the the processed protein, and suppresses the proteosomal degradation of SREBP-1.
Recently, mTORC1 has emerged as an important regulator of SREBP-1c that activates both SREBP-1c transcription 26,43,44 and the proteolytic processing in response to insulin stimulation 24–27. Suppression of mTORC1 activity by rapamycin inhibited the expression of SREBP-1c and lipogenic genes regulated by SREBP-1c in the livers of rodents subjected to fasting followed by refeeding with a high carbohydrate diet, uncovering a critical role of mTORC1 pathway in insulin-induced lipogenesis program 43. Similarly, insulin-induced SREBP-1c mRNA expression was abolished by rapamycin or small molecule inhibitors of PI3K or AKT that blocks mTORC1 activation in cultured rat hepatocytes 43. mTORC1 protein kinase directly phosphorylates two major downstream targets, initiation factor 4E-binding protein (4E-BP) and p70 ribosomal S6 kinase (p70S6K), increasing mRNA translation 41,45,46. p70S6K has growing number of downstream targets in addition to the ribosomal protein S6 47,48. Notably, a p70S6K inhibitor had no effect on insulin-induce SREBP-1c mRNA expression 43, but inhibited the proteplytic processing of SREBP-1c protein 26, indicating that mTORC1 regulates SREBP-c mRNA expression and protein processing through distinct mechanisms.
Although the acute inhibition of mTORC1 activity suppresses insulin-induced SREBP-1c mRNA expression and lipogenic gene expression, constitutive activation of mTORC1 ironically suppresses SREBP-1c activation 27. Genetic ablation of TSC1 in the liver caused constitutive activation of mTORC1, but suppressed age- and diet-induced hepatic steatosis, possibly due to defective SREBP-1c expression. This unexpected phenotype reflects the complex feedback regulation of insulin signaling pathway leading to SREBP-1c activation 12,40. It has been well established that mTORC1 negatively regulates insulin signaling at multiple steps of the signaling cascade as feedback mechanisms 48–50. Indeed, AKT was markedly suppressed in TSC1 deficient hepatocytes, which might contribute to the decreased expression of SREBP-1c and lipogenic genes. An important question that remains to be answered is what ultimately increases SREBP-1c transcription in response to insulin stimulation. So far, liver X receptors (LXRs) and SREBP-1c itself are known to activate SREBP-1c promoter 51–55. The relative contribution of LXR and SREBP-1c in the insulin-induced transcriptional activation of SREBP-1c remains to be further investigated.
LXRs are members of the nuclear hormone receptor superfamily that play a critical role in cholesterol efflux, excretion and absorption 56. LXRα also plays an important role in fatty acid and triglyceride synthesis, as it induces SREBP-1c expression via an LXR response element on its promoter 51,53,57. The lipogenic activity of LXRα was abrogated in SREBP-1c deficient mice, indicating that LXR promotes lipogenesis through SREBP-1c 4. LXRα deficient mice exhibit reduced expression of SREBP-1c and lipogeneic genes such as SCD1 and FAS in the liver 53,54,58,59. In contrast, high cholesterol diet or LXRα agonist TO-901317 increases SREBP-1c and stimulates lipogenesis in the liver 53,54,58,59. Importantly, disruption of LXR binding sites on SREBP-1c promoter abolished the induction of the promoter activity by insulin or TO-901317, suggesting that LXRα is responsible for SREBP-1c induction in response to insulin 51. However, it is not known how insulin activates LXRα. The role of LXRα in insulin signaling cascade appears to be specific to SREBP-1c, since insulin does not induce other LXRα target genes 60,61. It has been reported that insulin modestly increases LXRα mRNA in cultured rat hepatocytes 62. It is also possible that insulin stimulates the production of LXRα ligands to activate LXRα.
Regulation of SREBP protein stability
The nuclear form of SREBP protein is highly unstable, as it is degraded via ubiquitin-dependent proteasomal degradation pathway 63,64. Treatment of proteasome inhibitors increases the amount of nuclear SREBP, but not the precursor form, indicating that only the processed nuclear forms of SREBPs are subjected to proteasomal degradation. Ubiquitination and degradation of SREBPs are closely associated with their transcriptional activities 65. Inhibition of transcriptional activity of SREBPs by mutating critical functional domains, or by treating with RNA polymerase inhibitor prevented the degradation of SREBP proteins. It is conceivable that the proteosomal degradation of SREBPs portrays a feedback regulation of SREBP activity to fine tune transcriptional response of lipogenesis. Ubiquitination of SREBP could be suppressed by SREBP coactivators, CREB-binding protein (CBP) and p300 that competitively acetylate the lysine residue that is also targeted by ubiquitination, leading to stabilization of SREBP and the induction of SREBP target genes (LDLR, HMG-CoA reductase) and sterol synthesis 66.
Phosphorylation of SREBP is critically required for its ubiquitination. F-box and WD repeat domain-containing 7 (Fbw7) is a cullin-RING type E3 ubiquitin ligase that has emerged as the major ubiquitin ligase for SREBPs. Phosphorylation of SREBP induces its interaction with Fbw7, and thus facilitates its ubiquitination and degradation 67,68. GSK3 phosphorylates SREBP-1a at T426 and S430 residues, which resemble Cdc4 phosphodegron (CPD) motif, a recognition site for Fbw7. SREBP-1c and SREBP-2 are also similarly ubiquitinated by Fbw7. DNA binding of SREBP facilitates the recruitment of GSK3 to the promoter, and the subsequent interaction between SREBP1 and GSK3 69. Insulin regulates the stability of SREBP by controlling its phosphorylation by GSK3 and interaction with Fbw7 70. Insulin-mediated AKT activation induces Ser-9 phosphorylation of GSK3, leading to the suppression of its kinase activity 71. Consequently, insulin suppresses SREBP phosphorylation and the following Fbw7-dependent degradation. Cyclin-dependent kinase 8 (CDK8) can also phosphorylate SREBP-1c, and thus trigger its ubiquitination by Fbw7 and proteasomal degradation 72. As CDK8 expression is suppressed by insulin, CDK8-triggered SREBP-1c ubiquitination/degradation would constitute a regulatory mechanism of lipogenesis program. Indeed, knockdown of CDK8 in mouse liver increased SREBP-1c target genes (FAS, ACS, and SCD1) and hepatic triglyceride level 72.
Fbw7 deficiency stabilizes nuclear SREBPs and enhances the expression of their target genes, leading to increased synthesis of fatty acids, TG, and cholesterol, and increased receptor-mediated uptake of Low-density lipoprotein (LDL) 73. Liver-specific deletion of Fbw7 in vivo increased the expression of hepatic SREBP-1c and lipogenic genes, which was accompanied by massive lipid deposition and the occurrence of nonalcoholic steatohepatitis (NASH) in the mutant mice 73. These findings establish Fbw7 as an important regulator of SREBP protein stability and lipid metabolism.
MicroRNA-SREBP connection in lipid metabolism
MicoRNAs (miRNAs) are small non-protein-coding RNAs of ~23nt in length that are produced from longer primary miRNA transcripts via sequential processing by DROSHA and DICER ribonucleases 74. miRNAs bind to the 3′ untranslated regions of target mRNAs, and thereby either promote the degradation or suppress the translation of target mRNAs 74. Given that a single miRNA can control the expression of multiple target genes in the same pathway, miRNAs have emerged as critical regulators of a variety of biological processes, including nutrient metabolism 75,76.
Interestingly, recent reports revealed that SREBP genes (SREBF1 and SREBF2) harbor miRNAs within introns that are consequently cotranscribed with the respective SREBP genes. In human, miR-33a is located in intron 16 of SREBF2 gene (encoding SREBP-2), and miR-33b is within intron 17 of SREBF1 gene (encoding SREBP-1a and -1c) 77–81. Mature miR-33a and miR-33b have similar nucleotide sequences and hence expected to regulate overlapping target mRNAs. While miR-33a is evolutionary conserved in multiple animal species, miR-33b exists in human, but absent in rodent genome 79,81. Reminiscing the critical role of SREBP proteins in lipid metabolism, miR-33a and miR-33b also regulate cholesterol and fatty acid homeostasis 77–81. miRNA target sequence analysis predicted that miR-33a and miR-33b target adenosine triphosphate–binding cassette A1 (ABCA1) mRNA, which encodes a cholesterol transporter that plays a crucial role in cholesterol efflux. Indeed, silencing or genetic ablation of miR-33 markedly increased ABCA1 expression both in cultured hepatocytes and macrophages, and increased plasma high-density lipoprotein (HDL) levels 77–82.
Given the beneficial effects of miR-33 antagonism in increasing plasma HDL levels, miR-33 inhibition arose great interests as a potential therapeutic approach to treat cardiovascular diseases 83. Indeed, Rayner et al demonstrated that anti-miR-33 treatment promoted reverse cholesterol transport and reduced atherosclerotic plaques in LDL receptor knockout (Ldlr−/−) mice 84. Similarly, genetic loss of miR-33 in ApoE null mice increased circulating HDL-cholesterol levels and reduced plaque size 85. A recent study in nonhuman primates also reported the increase of HDL cholesterol by anti-miR-33 therapy, highlighting strong potential of anti-miR-33 as a new therapy for coronary heart disease 86. However, subsequent independent studies using anti-miR-33 anti-sense oligonucleotides or locked nucleic acids reported somewhat inconsistent effects of miR-33 inhibition on HDL cholesterol levels and atherosclerotic lesion development in Ldlr−/− mice 87,88. For example, both studies found that anti-miR-33 had no effect on HDL cholesterol levels in Ldlr−/− mice fed western diet, although animals on chow diet exhibited increased HDL cholesterol by anti-miR-33 treatment. Nonetheless, Rotllan et al demonstrated that anti–miR-33 therapy significantly reduced atherosclerotic lesion and macrophage infiltration 88. In contrast, Marquart et al failed to detect any significant changes in the size or composition of atherosclerotic plaques in anti-miR-33 treated mice, while plasma TG levels were significantly increased 87. Further studies should address the effectiveness of anti-miR-33 treatment as a therapeutic approach, and identify the full spectrum of miR-33 target mRNAs.
A recent elegant study identified a pair of microRNAs that are transcriptionally induced by SREBPs, and in turn suppress SREBPs, constituting a negative feedback loop 89. SREBPs directly activate the transcription of a primary miRNA transcript that is processed to three miRNAs; miR-96, -182, and -183. Interestingly, miR-96 and -182 suppressed the expression of the processed SREBPs and the synthesis of fatty acids and cholesterol, suggesting that these miRNAs regulates the processing or stability of SREBPs. Target sequence analysis predicted that Insig-2 and Fbw7, which regulate the processing and proteasomal degradation of SREBPs, might be regulated by miR-96 and miR-182, respectively. Indeed, miR-96 and miR-182 suppressed the synthesis of Insig-2 and Fbw7, and increased the processed SREBP1 and SREBP2 protein levels. This study reveals a new layer of regulatory mechanism in lipid metabolism.
ChREBP
Carbohydrate response element binding protein (ChREBP) was first identified as a glucose responsive transcription factor, which regulates glycolytic, gluconeogenic and lipogenic gene expression 6,7. Transcriptional targets of ChREBP encodes important enzymes in these pathways including L-pyruvate kinase (L-PK) for glycolysis, Glucose 6 phosphatase catalytic subunit (G6PC) for gluconeogenesis, Fatty acid synthase (FAS), Acetyl coA carboxylase 1 (ACC1) and Stearyl coA desaturase 1 (SCD 1) for lipogenesis 6. Carbohydrate-response elements (ChoREs) have been identified in promoters of these genes, which are composed of two E-box (CACGTG) or E-box-like sequences separated by 5 nucleotides 90,91. ChREBP and its interaction partner Max-like protein X (MLX) form heterodimmers and bind to the ChoREs to induce the expression of its target genes 92,93 (Figure 4).
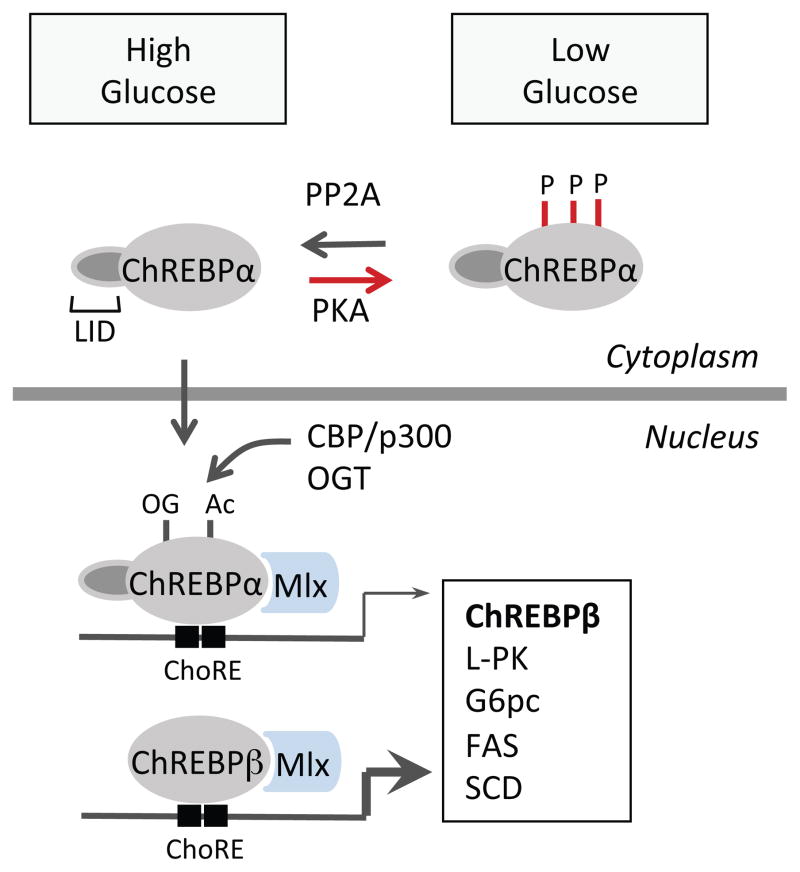
Figure 4. Regulation of ChREBP activity.
The phosphorylation/dephosphorylation of ChREBPα by PKA/protein phosphatase 2A (PP2A) is involved in ChREBPα nuclear translocation and activation. Acetylation by coactivator CBP/P300 and O-GlcNAcylation by O-GlcNAc transferase (OGT) also contribute to ChREBPα transcriptional activities. ChREBPα forms heterodimer with Max-like protein X (MLX) and binds to the Carbohydrate-response elements (ChoREs) in the nucleus to induce its target genes involved in glycolytic and lipogenic pathways. In the adipose tissue, active ChREBPα induces expression of ChREBPβ, a new ChREBP isoform which lacks the low glucose inhibitory domain (LID), and hence constitutively active regardless of glucose concentration.
ChREBP protein contains two nuclear export signals and one nuclear localization signal near the N terminal, prolin-rich domains, a basic loop-helix-leucine-zipper (b/HLH/Zip), and a leucine-zipper-like (Zip-like) domain 7,94.
Posttranslational modifications of ChREBP is required for its activation. The phosphorylation/dephosphorylation of ChREBP has been proposed to be important for ChREBP nuclear translocation and activation. Under basal conditions, like starvation or low glucose concentrations, ChREBP is phosphorylated on Ser-196, Ser626 and Thr66 by cAMP-dependent protein kinase (PKA), on Ser568 by AMP-activated protein kinase (AMPK) and localized in the cytosol 8,95. Upon high glucose stimulation, xylulose 5-phosphate (X5P), an intermediate of the pentose phosphate pathway, activates protein phosphatase 2A (PP2A) and dephosphorylates ChREBP, allowing its translocation into nucleus and activation 96. However, some studies shows that mutation of one or several PKA phosphorylation sites did not affect the responsiveness of ChREBP to high glucose levels, suggesting a more complex mechanism could be involved 97. Transactivity of ChREBP can also be modulated through acetylation on Lys672 by histone acetyl-transfrase (HAT) and its co-activator p300 98 and by O-linked-β-N-actetylation 99,100.
Alternatively, intramolecular interaction has been proposed to be another way to modulate ChREBP activity. ChERBP contains a glucose-sensing module near the N-terminus, which consists of a low glucose inhibitory domain (LID) and a glucose response activation conserved element (GRACE). Due to the inhibition of LID domain on GRACE, ChREBP is restrained in of LID domain on GRACE confers ChREBP a unfavorable conformation for DNA binding and activation, which is reversed by high glucose 94,101–103. In line with this model, deletion of LID domain produced a constitutively active ChREBP even under low glucose conditions 102. The involvement of glucose-sensing module and conformational modulation has been implicated in the regulation of ChREBP activity by glucose metabolites, such as glucose 6 phosphate (G6P) 104,105.
The mechanism of carbohydrate mediated ChREBP activation may involve feedforward regulation, since changes of ChREBP activity can also be reflected on ChREBP mRNA levels 106,107. Recently, the self-regulation of ChREBP in adipose tissue has been revealed with the discovery of a novel ChREBP isoform, ChREBPβ 108. ChREBPβ is transcribed from an alternative promoter, differing from the previously identified ChREBP, or ChREBPα. ChREBPβ protein does not contain LID and nucleus export signals, therefore exhibits constitutively higher transactivation ability than ChREBPα with increased nuclear localization 102, regardless of the glucose concentration 108. ChREBPβ expression was markedly increased by co-transfection of ChREBPα and LMX in a glucose dose dependent manner. The ChoREs are also identified in the promoter region of ChREBPβ and the deletion of these elements completely abolished the responsiveness of ChREBPβ promoter to ChREBPα/MLX 108. Therefore, ChREBPα may be activated by high glucose concentrations as previously reported, and induces ChREBPβ expression as a feedforward regulation. It remains to be determined if this feedforward regulation of ChREBP also occurs in the liver and other tissues.
As ChREBP directly regulates genes involved in both glucose and lipid metabolism which indirectly influence each other, genetic manipulation of ChREBP expression in vivo results in rather complex metabolic changes. ChREBP−/− mice have impaired glycolytic and lipogenic pathways in the liver and show moderate glucose intolerance 6. Global or liver-specific deletion of ChREBP greatly ameliorated fatty liver diseases, and improved overall glucose tolerance and insulin sensitivity in ob/ob mice, possibly through decreasing de novo lipogenesis 109,110. Overexpression of ChREBP in the liver increased hepatic stestosis associated with the increased expression of genes regulating fatty acid and TG synthesis in the liver. Interestingly, ChREBP transgenic mice exhibited elevated monounsaturated fatty acids in the liver, which conferred improved glucose tolerance and insulin sensitivity upon high fat diet feeding despite greater hepatic steatosis 111.
ChREBP expression is induced during adipocyte differentiation, and by refeeding with a high-carbohydrate diet in adipose tissue, suggesting it might have a metabolic function in adipocytes 112. Indeed, a recent study demonstrated that ChREBP regulates de novo lipogenesis program in response to glucose flux in adipocytes, and adipose tissue ChREBP level correlates well with glucose tolerance and insulin sensitivity in humans 108. ChREBP is also expressed in pancreatic β-cells. Glucose stimulated the expression of ChREBP target genes in β-cells 107,113, and activation of ChREBP promoted glucose stimulated β-cell proliferation 114.
Concluding remarks
In this review, we summarized the complex signaling network that controls hepatic lipogenesis transcriptional program activated directly or indirectly by carbohydrate ingestion. SREBPs and ChREBP are major transcriptional regulators that are activated by carbohydrate signal, and stimulate de novo hepatic lipogenesis. Recent studies revealed that AKT/mTORC1 signaling pathway are critically involved not only in the transcriptional activation, but also in the post-translational processing of SREBP-1c.
Uncontrolled de novo lipogenesis causes hepatic steatosis, which is closely associated with the onset of obesity, insulin resistance and type 2 diabetes. Excessive lipogenesis induced by transgenic overexpression of SREBP-1, ingestion of high fructose diet or leptin deficiency causes hepatic steatosis. Under insulin resistant state, hyperinsulinemia can also activate SREBP-1 to induce hepatic steatosis, with the loss of insulin-mediated suppression of gluconeogenesis 115. On the other hand, inhibition of SREBP-1 could be a potential therapeutic approach to treat dyslipidemia and metabolic syndrome. A better understanding of the signaling pathway controlling lipogenesis may lead to the identification of novel targets for metabolic diseases.
The role of ChREBP in sensing glucose and regulating nutrient homeostasis, especially lipid synthesis is of great interest, considering its therapeutic potentials on diabetes and metabolic syndrome. With the discovery of ChREBPα and β isoforms, tissue specific distributions of ChREBP should be more carefully considered when studying its regulation on lipid metabolism and systemic glucose homeostasis.
Acknowledgments
This work was supported by NIH grant DK089211 to A.-H.L.
Abbreviations
- ABCA1
Adenosine triphosphate–binding cassette A1
- ACC
Acetyl-CoA carboxylase
- ACL
ATP-citrate lyase
- ACS
Acetyl-CoA synthetase
- AMPK
AMP-activated protein kinase
- CBP
CREB-binding protein
- CDK8
Cyclin-dependent kinase 8
- ChoREs
Carbohydrate-response elements
- ChREBP
Carbohydrate-responsive element-binding protein
- FAS
Fatty acid synthase
- Fbw7
F-box and WD repeat domain-containing 7
- Foxo1
Forkhead box protein O1
- G6PC
Glucose 6 phosphatase catalytic subunit
- GPAT
Glycerol-3-phosphate acyltransferase
- GRACE
Glucose response activation conserved element
- GSK3
Glycogen synthase kinase 3
- HAT
Histone acetyl-transfrase
- HDL
High-density lipoprotein
- L-PK
L-Type Pyruvate Kinase
- LDL
Low-density lipoprotein
- LDLR
Low-density lipoprotein receptor
- LID
Low glucose inhibitory domain
- LXR
Liver X receptor
- MLX
Max-like protein X
- mTORC
Mammalian target of rapamycin (mTOR) complex
- p70S6K
p70 ribosomal S6 kinase
- PDK1
3-phosphoinositide-dependent protein kinase-1
- PI3K
Phosphoinositide 3-kinase
- PKA
cAMP-dependent protein kinase
- PP2A
Protein phosphatase 2A
- PPARα
Peroxisome proliferator-activated receptor a
- SCAP
SREBP cleavage activating protein
- SCD1
Stearoyl-CoA desaturase-1
- SREBP-1c
Sterol regulatory element binding protein -1c
- TSC2
Tuberous sclerosis 2
- X5P
Xylulose 5-phosphate
References
- 1.Towle HC. Glucose as a regulator of eukaryotic gene transcription. Trends in endocrinology and metabolism: TEM. 2005;16(10):489–494. doi: 10.1016/j.tem.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci U S A. 1998;95(11):5987–5992. doi: 10.1073/pnas.95.11.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JB, Sarraf P, Wright M, et al. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J Clin Invest. 1998;101(1):1–9. doi: 10.1172/JCI1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang G, Yang J, Horton JD, et al. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem. 2002;277(11):9520–9528. doi: 10.1074/jbc.M111421200. [DOI] [PubMed] [Google Scholar]
- 5.Horton JD, Shah NA, Warrington JA, et al. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci U S A. 2003;100(21):12027–12032. doi: 10.1073/pnas.1534923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci U S A. 2004;101(19):7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamashita H, Takenoshita M, Sakurai M, et al. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci U S A. 2001;98(16):9116–9121. doi: 10.1073/pnas.161284298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawaguchi T, Takenoshita M, Kabashima T, Uyeda K. Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein. Proc Natl Acad Sci U S A. 2001;98(24):13710–13715. doi: 10.1073/pnas.231370798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma L, Robinson LN, Towle HC. ChREBP*Mlx is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem. 2006;281(39):28721–28730. doi: 10.1074/jbc.M601576200. [DOI] [PubMed] [Google Scholar]
- 10.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferre P, Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes, obesity & metabolism. 2010;12 (Suppl 2):83–92. doi: 10.1111/j.1463-1326.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- 12.Jeon TI, Osborne TF. SREBPs: metabolic integrators in physiology and metabolism. Trends in endocrinology and metabolism: TEM. 2012;23(2):65–72. doi: 10.1016/j.tem.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hua X, Wu J, Goldstein JL, Brown MS, Hobbs HH. Structure of the human gene encoding sterol regulatory element binding protein-1 (SREBF1) and localization of SREBF1 and SREBF2 to chromosomes 17p11. 2 and 22q13. Genomics. 1995;25(3):667–673. doi: 10.1016/0888-7543(95)80009-b. [DOI] [PubMed] [Google Scholar]
- 14.Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest. 1997;99(5):838–845. doi: 10.1172/JCI119247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Im SS, Yousef L, Blaschitz C, et al. Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab. 2011;13(5):540–549. doi: 10.1016/j.cmet.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89(3):331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein JL, Rawson RB, Brown MS. Mutant mammalian cells as tools to delineate the sterol regulatory element-binding protein pathway for feedback regulation of lipid synthesis. Arch Biochem Biophys. 2002;397(2):139–148. doi: 10.1006/abbi.2001.2615. [DOI] [PubMed] [Google Scholar]
- 18.Sun LP, Li L, Goldstein JL, Brown MS. Insig required for sterol-mediated inhibition of Scap/SREBP binding to COPII proteins in vitro. J Biol Chem. 2005;280(28):26483–26490. doi: 10.1074/jbc.M504041200. [DOI] [PubMed] [Google Scholar]
- 19.Adams CM, Goldstein JL, Brown MS. Cholesterol-induced conformational change in SCAP enhanced by Insig proteins and mimicked by cationic amphiphiles. Proc Natl Acad Sci U S A. 2003;100(19):10647–10652. doi: 10.1073/pnas.1534833100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hua X, Nohturfft A, Goldstein JL, Brown MS. Sterol resistance in CHO cells traced to point mutation in SREBP cleavage-activating protein. Cell. 1996;87(3):415–426. doi: 10.1016/s0092-8674(00)81362-8. [DOI] [PubMed] [Google Scholar]
- 21.Sun LP, Seemann J, Goldstein JL, Brown MS. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Insig renders sorting signal in Scap inaccessible to COPII proteins. Proc Natl Acad Sci U S A. 2007;104(16):6519–6526. doi: 10.1073/pnas.0700907104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS. Switch-like control of SREBP-2 transport triggered by small changes in ER cholesterol: a delicate balance. Cell Metab. 2008;8(6):512–521. doi: 10.1016/j.cmet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimomura I, Bashmakov Y, Ikemoto S, et al. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci U S A. 1999;96(24):13656–13661. doi: 10.1073/pnas.96.24.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hegarty BD, Bobard A, Hainault I, et al. Distinct roles of insulin and liver X receptor in the induction and cleavage of sterol regulatory element-binding protein-1c. Proc Natl Acad Sci U S A. 2005;102(3):791–796. doi: 10.1073/pnas.0405067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yellaturu CR, Deng X, Cagen LM, et al. Insulin enhances post-translational processing of nascent SREBP-1c by promoting its phosphorylation and association with COPII vesicles. J Biol Chem. 2009;284(12):7518–7532. doi: 10.1074/jbc.M805746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owen JL, Zhang Y, Bae SH, et al. Insulin stimulation of SREBP-1c processing in transgenic rat hepatocytes requires p70 S6-kinase. Proc Natl Acad Sci U S A. 2012;109(40):16184–16189. doi: 10.1073/pnas.1213343109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yecies JL, Zhang HH, Menon S, et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011;14(1):21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yabe D, Komuro R, Liang G, Goldstein JL, Brown MS. Liver-specific mRNA for Insig-2 down-regulated by insulin: implications for fatty acid synthesis. Proc Natl Acad Sci U S A. 2003;100(6):3155–3160. doi: 10.1073/pnas.0130116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yellaturu CR, Deng X, Park EA, Raghow R, Elam MB. Insulin enhances the biogenesis of nuclear sterol regulatory element-binding protein (SREBP)-1c by posttranscriptional down-regulation of Insig-2A and its dissociation from SREBP cleavage-activating protein (SCAP) SREBP-1c complex J Biol Chem. 2009;284(46):31726–31734. doi: 10.1074/jbc.M109.050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagoshi E, Imamoto N, Sato R, Yoneda Y. Nuclear import of sterol regulatory element-binding protein-2, a basic helix-loop-helix-leucine zipper (bHLH-Zip)-containing transcription factor, occurs through the direct interaction of importin beta with HLH-Zip. Molecular biology of the cell. 1999;10(7):2221–2233. doi: 10.1091/mbc.10.7.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson TR, Sengupta SS, Harris TE, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146(3):408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donkor J, Sariahmetoglu M, Dewald J, Brindley DN, Reue K. Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J Biol Chem. 2007;282(6):3450–3457. doi: 10.1074/jbc.M610745200. [DOI] [PubMed] [Google Scholar]
- 33.Harris TE, Huffman TA, Chi A, et al. Insulin controls subcellular localization and multisite phosphorylation of the phosphatidic acid phosphatase, lipin 1. J Biol Chem. 2007;282(1):277–286. doi: 10.1074/jbc.M609537200. [DOI] [PubMed] [Google Scholar]
- 34.Finck BN, Gropler MC, Chen Z, et al. Lipin 1 is an inducible amplifier of the hepatic PGC-1alpha/PPARalpha regulatory pathway. Cell Metab. 2006;4(3):199–210. doi: 10.1016/j.cmet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Foretz M, Pacot C, Dugail I, et al. ADD1/SREBP-1c is required in the activation of hepatic lipogenic gene expression by glucose. Molecular and cellular biology. 1999;19(5):3760–3768. doi: 10.1128/mcb.19.5.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang X, Ma H, Shen Z, et al. Dehydroepiandrosterone activates cyclic adenosine 3′,5′-monophosphate/protein kinase A signalling and suppresses sterol regulatory element-binding protein-1 expression in cultured primary chicken hepatocytes. The British journal of nutrition. 2009;102(5):680–686. doi: 10.1017/S0007114509289021. [DOI] [PubMed] [Google Scholar]
- 37.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lizcano JM, Alessi DR. The insulin signalling pathway. Current biology: CB. 2002;12(7):R236–238. doi: 10.1016/s0960-9822(02)00777-7. [DOI] [PubMed] [Google Scholar]
- 39.Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annual review of physiology. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- 40.Howell JJ, Manning BD. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends in endocrinology and metabolism: TEM. 2011;22(3):94–102. doi: 10.1016/j.tem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer cell. 2007;12(1):9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Laplante M, Sabatini DM. An emerging role of mTOR in lipid biosynthesis. Current biology: CB. 2009;19(22):R1046–1052. doi: 10.1016/j.cub.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107(8):3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porstmann T, Santos CR, Griffiths B, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8(3):224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown EJ, Beal PA, Keith CT, et al. Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature. 1995;377(6548):441–446. doi: 10.1038/377441a0. [DOI] [PubMed] [Google Scholar]
- 46.Brunn GJ, Hudson CC, Sekulic A, et al. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997;277(5322):99–101. doi: 10.1126/science.277.5322.99. [DOI] [PubMed] [Google Scholar]
- 47.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nature reviews Molecular cell biology. 2011;12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Um SH, Frigerio F, Watanabe M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431(7005):200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 49.Yu Y, Yoon SO, Poulogiannis G, et al. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science. 2011;332(6035):1322–1326. doi: 10.1126/science.1199484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hsu PP, Kang SA, Rameseder J, et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332(6035):1317–1322. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci U S A. 2004;101(31):11245–11250. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen W, Chen G, Head DL, Mangelsdorf DJ, Russell DW. Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell Metab. 2007;5(1):73–79. doi: 10.1016/j.cmet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Repa JJ, Liang G, Ou J, et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14(22):2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Breevoort SR, Angdisen J, et al. Liver LXRalpha expression is crucial for whole body cholesterol homeostasis and reverse cholesterol transport in mice. J Clin Invest. 2012;122(5):1688–1699. doi: 10.1172/JCI59817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amemiya-Kudo M, Shimano H, Yoshikawa T, et al. Promoter analysis of the mouse sterol regulatory element-binding protein-1c gene. J Biol Chem. 2000;275(40):31078–31085. doi: 10.1074/jbc.M005353200. [DOI] [PubMed] [Google Scholar]
- 56.Calkin AC, Tontonoz P. Liver x receptor signaling pathways and atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2010;30(8):1513–1518. doi: 10.1161/ATVBAHA.109.191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshikawa T, Shimano H, Amemiya-Kudo M, et al. Identification of liver X receptor-retinoid X receptor as an activator of the sterol regulatory element-binding protein 1c gene promoter. Molecular and cellular biology. 2001;21(9):2991–3000. doi: 10.1128/MCB.21.9.2991-3000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peet DJ, Turley SD, Ma W, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93(5):693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 59.Kalaany NY, Gauthier KC, Zavacki AM, et al. LXRs regulate the balance between fat storage and oxidation. Cell Metab. 2005;1(4):231–244. doi: 10.1016/j.cmet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Kok T, Wolters H, Bloks VW, et al. Induction of hepatic ABC transporter expression is part of the PPARalpha-mediated fasting response in the mouse. Gastroenterology. 2003;124(1):160–171. doi: 10.1053/gast.2003.50007. [DOI] [PubMed] [Google Scholar]
- 61.Bloks VW, Bakker-Van Waarde WM, Verkade HJ, et al. Down-regulation of hepatic and intestinal Abcg5 and Abcg8 expression associated with altered sterol fluxes in rats with streptozotocin-induced diabetes. Diabetologia. 2004;47(1):104–112. doi: 10.1007/s00125-003-1261-y. [DOI] [PubMed] [Google Scholar]
- 62.Tobin KA, Ulven SM, Schuster GU, et al. Liver X receptors as insulin-mediating factors in fatty acid and cholesterol biosynthesis. J Biol Chem. 2002;277(12):10691–10697. doi: 10.1074/jbc.M109771200. [DOI] [PubMed] [Google Scholar]
- 63.Wang X, Sato R, Brown MS, Hua X, Goldstein JL. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell. 1994;77(1):53–62. doi: 10.1016/0092-8674(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 64.Hirano Y, Yoshida M, Shimizu M, Sato R. Direct Demonstration of Rapid Degradation of Nuclear Sterol Regulatory Element-binding Proteins by the Ubiquitin-Proteasome Pathway. Journal of Biological Chemistry. 2001;276(39):36431–36437. doi: 10.1074/jbc.M105200200. [DOI] [PubMed] [Google Scholar]
- 65.Sundqvist A, Ericsson J. Transcription-dependent degradation controls the stability of the SREBP family of transcription factors. Proceedings of the National Academy of Sciences. 2003;100(24):13833–13838. doi: 10.1073/pnas.2335135100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ericsson J, Edwards PA. CBP Is Required for Sterol-regulated and Sterol Regulatory Element-binding Protein-regulated Transcription. Journal of Biological Chemistry. 1998;273(28):17865–17870. doi: 10.1074/jbc.273.28.17865. [DOI] [PubMed] [Google Scholar]
- 67.Kim KH, Song MJ, Yoo EJ, et al. Regulatory Role of Glycogen Synthase Kinase 3 for Transcriptional Activity of ADD1/SREBP1c. Journal of Biological Chemistry. 2004;279(50):51999–52006. doi: 10.1074/jbc.M405522200. [DOI] [PubMed] [Google Scholar]
- 68.Sundqvist A, Bengoechea-Alonso MT, Ye X, et al. Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCFFbw7. Cell Metabolism. 2005;1(6):379–391. doi: 10.1016/j.cmet.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 69.Punga T, Bengoechea-Alonso MT, Ericsson J. Phosphorylation and Ubiquitination of the Transcription Factor Sterol Regulatory Element-binding Protein-1 in Response to DNA Binding. Journal of Biological Chemistry. 2006;281(35):25278–25286. doi: 10.1074/jbc.M604983200. [DOI] [PubMed] [Google Scholar]
- 70.Krycer JR, Sharpe LJ, Luu W, Brown AJ. The Akt-SREBP nexus: cell signaling meets lipid metabolism. Trends in endocrinology and metabolism: TEM. 2010;21(5):268–276. doi: 10.1016/j.tem.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Cross DAE, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 72.Zhao X, Feng D, Wang Q, et al. Regulation of lipogenesis by cyclin-dependent kinase 8-mediated control of SREBP-1. The Journal of Clinical Investigation. 2012;122(7):2417–2427. doi: 10.1172/JCI61462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Onoyama I, Suzuki A, Matsumoto A, et al. Fbxw7 regulates lipid metabolism and cell fate decisions in the mouse liver. The Journal of Clinical Investigation. 2011;121(1):342–354. doi: 10.1172/JCI40725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fernandez-Hernando C, Ramirez CM, Goedeke L, Suarez Y. MicroRNAs in metabolic disease. Arteriosclerosis, thrombosis, and vascular biology. 2013;33(2):178–185. doi: 10.1161/ATVBAHA.112.300144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rottiers V, Naar AM. MicroRNAs in metabolism and metabolic disorders. Nature reviews Molecular cell biology. 2012;13(4):239–250. doi: 10.1038/nrm3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davalos A, Goedeke L, Smibert P, et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci U S A. 2011;108(22):9232–9237. doi: 10.1073/pnas.1102281108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gerin I, Clerbaux LA, Haumont O, et al. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J Biol Chem. 2010;285(44):33652–33661. doi: 10.1074/jbc.M110.152090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marquart TJ, Allen RM, Ory DS, Baldan A. miR-33 links SREBP-2 induction to repression of sterol transporters. Proc Natl Acad Sci U S A. 2010;107(27):12228–12232. doi: 10.1073/pnas.1005191107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Najafi-Shoushtari SH, Kristo F, Li Y, et al. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;328(5985):1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rayner KJ, Suarez Y, Davalos A, et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328(5985):1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Horie T, Ono K, Horiguchi M, et al. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc Natl Acad Sci U S A. 2010;107(40):17321–17326. doi: 10.1073/pnas.1008499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Naar AM. Anti-atherosclerosis or No Anti-atherosclerosis: That is the miR-33 question. Arteriosclerosis, thrombosis, and vascular biology. 2013;33(3):447–448. doi: 10.1161/ATVBAHA.112.301021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rayner KJ, Sheedy FJ, Esau CC, et al. Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest. 2011;121(7):2921–2931. doi: 10.1172/JCI57275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Horie T, Baba O, Kuwabara Y, et al. MicroRNA-33 deficiency reduces the progression of atherosclerotic plaque in ApoE−/− mice. Journal of the American Heart Association. 2012;1(6):e003376. doi: 10.1161/JAHA.112.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rayner KJ, Esau CC, Hussain FN, et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;478(7369):404–407. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marquart TJ, Wu J, Lusis AJ, Baldan A. Anti-miR-33 therapy does not alter the progression of atherosclerosis in low-density lipoprotein receptor-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2013;33(3):455–458. doi: 10.1161/ATVBAHA.112.300639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rotllan N, Ramirez CM, Aryal B, Esau CC, Fernandez-Hernando C. Therapeutic Silencing of MicroRNA-33 Inhibits the Progression of Atherosclerosis in Ldlr−/− Mice. Arteriosclerosis, thrombosis, and vascular biology. 2013 doi: 10.1161/ATVBAHA.113.301732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jeon TI, Esquejo RM, Roqueta-Rivera M, et al. An SREBP-Responsive microRNA Operon Contributes to a Regulatory Loop for Intracellular Lipid Homeostasis. Cell Metab. 2013;18(1):51–61. doi: 10.1016/j.cmet.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rufo C, Teran-Garcia M, Nakamura MT, et al. Involvement of a unique carbohydrate-responsive factor in the glucose regulation of rat liver fatty-acid synthase gene transcription. J Biol Chem. 2001;276(24):21969–21975. doi: 10.1074/jbc.M100461200. [DOI] [PubMed] [Google Scholar]
- 91.Shih HM, Liu Z, Towle HC. Two CACGTG motifs with proper spacing dictate the carbohydrate regulation of hepatic gene transcription. J Biol Chem. 1995;270(37):21991–21997. doi: 10.1074/jbc.270.37.21991. [DOI] [PubMed] [Google Scholar]
- 92.Ma L, Tsatsos NG, Towle HC. Direct role of ChREBP. Mlx in regulating hepatic glucose-responsive genes. J Biol Chem. 2005;280(12):12019–12027. doi: 10.1074/jbc.M413063200. [DOI] [PubMed] [Google Scholar]
- 93.Stoeckman AK, Ma L, Towle HC. Mlx is the functional heteromeric partner of the carbohydrate response element-binding protein in glucose regulation of lipogenic enzyme genes. J Biol Chem. 2004;279(15):15662–15669. doi: 10.1074/jbc.M311301200. [DOI] [PubMed] [Google Scholar]
- 94.Sakiyama H, Wynn RM, Lee WR, et al. Regulation of nuclear import/export of carbohydrate response element-binding protein (ChREBP): interaction of an alpha-helix of ChREBP with the 14-3-3 proteins and regulation by phosphorylation. J Biol Chem. 2008;283(36):24899–24908. doi: 10.1074/jbc.M804308200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K. Mechanism for fatty acid “sparing” effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem. 2002;277(6):3829–3835. doi: 10.1074/jbc.M107895200. [DOI] [PubMed] [Google Scholar]
- 96.Kabashima T, Kawaguchi T, Wadzinski BE, Uyeda K. Xylulose 5-phosphate mediates glucose-induced lipogenesis by xylulose 5-phosphate-activated protein phosphatase in rat liver. Proc Natl Acad Sci U S A. 2003;100(9):5107–5112. doi: 10.1073/pnas.0730817100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsatsos NG, Towle HC. Glucose activation of ChREBP in hepatocytes occurs via a two-step mechanism. Biochem Biophys Res Commun. 2006;340(2):449–456. doi: 10.1016/j.bbrc.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 98.Bricambert J, Miranda J, Benhamed F, et al. Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. J Clin Invest. 2010;120(12):4316–4331. doi: 10.1172/JCI41624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guinez C, Filhoulaud G, Rayah-Benhamed F, et al. O-GlcNAcylation increases ChREBP protein content and transcriptional activity in the liver. Diabetes. 2011;60(5):1399–1413. doi: 10.2337/db10-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sakiyama H, Fujiwara N, Noguchi T, et al. The role of O-linked GlcNAc modification on the glucose response of ChREBP. Biochem Biophys Res Commun. 2010;402(4):784–789. doi: 10.1016/j.bbrc.2010.10.113. [DOI] [PubMed] [Google Scholar]
- 101.Davies MN, O’Callaghan BL, Towle HC. Activation and repression of glucose-stimulated ChREBP requires the concerted action of multiple domains within the MondoA conserved region. Am J Physiol Endocrinol Metab. 2010;299(4):E665–674. doi: 10.1152/ajpendo.00349.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li MV, Chang B, Imamura M, Poungvarin N, Chan L. Glucose-dependent transcriptional regulation by an evolutionarily conserved glucose-sensing module. Diabetes. 2006;55(5):1179–1189. doi: 10.2337/db05-0822. [DOI] [PubMed] [Google Scholar]
- 103.Li MV, Chen W, Poungvarin N, Imamura M, Chan L. Glucose-mediated transactivation of carbohydrate response element-binding protein requires cooperative actions from Mondo conserved regions and essential trans-acting factor 14-3-3. Molecular endocrinology. 2008;22(7):1658–1672. doi: 10.1210/me.2007-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McFerrin LG, Atchley WR. A novel N-terminal domain may dictate the glucose response of Mondo proteins. PloS one. 2012;7(4):e34803. doi: 10.1371/journal.pone.0034803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dentin R, Tomas-Cobos L, Foufelle F, et al. Glucose 6-phosphate, rather than xylulose 5-phosphate, is required for the activation of ChREBP in response to glucose in the liver. J Hepatol. 2012;56(1):199–209. doi: 10.1016/j.jhep.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 106.Iizuka K, Takeda J, Horikawa Y. Hepatic overexpression of dominant negative Mlx improves metabolic profile in diabetes-prone C57BL/6J mice. Biochem Biophys Res Commun. 2009;379(2):499–504. doi: 10.1016/j.bbrc.2008.12.100. [DOI] [PubMed] [Google Scholar]
- 107.Iizuka K, Wu W, Horikawa Y, Takeda J. Role of glucose-6-phosphate and xylulose-5-phosphate in the regulation of glucose-stimulated gene expression in the pancreatic beta cell line, INS-1E. Endocrine journal. 2013;60(4):473–482. [PubMed] [Google Scholar]
- 108.Herman MA, Peroni OD, Villoria J, et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 2012;484(7394):333–338. doi: 10.1038/nature10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dentin R, Benhamed F, Hainault I, et al. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes. 2006;55(8):2159–2170. doi: 10.2337/db06-0200. [DOI] [PubMed] [Google Scholar]
- 110.Iizuka K, Miller B, Uyeda K. Deficiency of carbohydrate-activated transcription factor ChREBP prevents obesity and improves plasma glucose control in leptin-deficient (ob/ob) mice. Am J Physiol Endocrinol Metab. 2006;291(2):E358–364. doi: 10.1152/ajpendo.00027.2006. [DOI] [PubMed] [Google Scholar]
- 111.Benhamed F, Denechaud PD, Lemoine M, et al. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J Clin Invest. 2012;122(6):2176–2194. doi: 10.1172/JCI41636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.He Z, Jiang T, Wang Z, Levi M, Li J. Modulation of carbohydrate response element-binding protein gene expression in 3T3-L1 adipocytes and rat adipose tissue. Am J Physiol Endocrinol Metab. 2004;287(3):E424–430. doi: 10.1152/ajpendo.00568.2003. [DOI] [PubMed] [Google Scholar]
- 113.Wang H, Kouri G, Wollheim CB. ER stress and SREBP-1 activation are implicated in beta-cell glucolipotoxicity. Journal of cell science. 2005;118(Pt 17):3905–3915. doi: 10.1242/jcs.02513. [DOI] [PubMed] [Google Scholar]
- 114.Metukuri MR, Zhang P, Basantani MK, et al. ChREBP mediates glucose-stimulated pancreatic beta-cell proliferation. Diabetes. 2012;61(8):2004–2015. doi: 10.2337/db11-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7(2):95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]