Abstract
Objectives:
To develop and validate 10-year cumulative incidence functions of intracerebral hemorrhage (ICH) and ischemic stroke (IS).
Methods:
We used data on 27,493 participants from 3 population-based cohort studies: the Atherosclerosis Risk in Communities Study, median age 54 years, 45% male, median follow-up 20.7 years; the Rotterdam Study, median age 68 years, 38% male, median follow-up 14.3 years; and the Cardiovascular Health Study, median age 71 years, 41% male, median follow-up 12.8 years. Among these participants, 325 ICH events, 2,559 IS events, and 9,909 nonstroke deaths occurred. We developed 10-year cumulative incidence functions for ICH and IS using stratified Cox regression and competing risks analysis. Basic models including only established nonlaboratory risk factors were extended with diastolic blood pressure, total cholesterol/high-density lipoprotein cholesterol ratio, body mass index, waist-to-hip ratio, and glomerular filtration rate. The cumulative incidence functions' performances were cross-validated in each cohort separately by Harrell C-statistic and calibration plots.
Results:
High total cholesterol/high-density lipoprotein cholesterol ratio decreased the ICH rates but increased IS rates (p for difference across stroke types <0.001). For both the ICH and IS models, C statistics increased more by model extension in the Atherosclerosis Risk in Communities and Cardiovascular Health Study cohorts. Improvements in C statistics were reproduced by cross-validation. Models were well calibrated in all cohorts. Correlations between 10-year ICH and IS risks were moderate in each cohort.
Conclusions:
We developed and cross-validated cumulative incidence functions for separate prediction of 10-year ICH and IS risk. These functions can be useful to further specify an individual's stroke risk.
Stroke is the second leading cause of death and one of the major causes of disability in most Western countries.1 The incidence of stroke steadily increases from middle age onward. Although most strokes are ischemic strokes (IS), approximately 10% are intracerebral hemorrhages (ICHs), which have a higher case fatality than IS: 41.0% vs 14.3%.2
Multiple risk factors that influence stroke risk are well established and can be used to estimate an individual's stroke risk over a 10-year time period.3–6 Distinguishing the risk of stroke types, i.e., ICH vs IS, could be valuable for various reasons. First, risk factors may vary or may have different or even opposing effects.7 Consequently, the likely effects of modifying these risk factors may vary per stroke type. Second, although antithrombotic therapy has a net preventive effect on stroke by decreasing the occurrence of IS, it also increases the risk of ICH.8 Therefore, decision-making for antithrombotic therapy could be improved by predicting ICH and IS risk separately. Third, the consequences (e.g., the case fatality) of both types differ and a more refined risk communication can be facilitated.
In addition, established stroke risk scores were developed using standard survival analysis with censoring for competing death events. Standard survival analysis will generally overestimate the cumulative incidence, because it fails to consider those who die of nonstroke causes as ineligible for development of stroke events. Methods to adjust for competing risks are now increasingly being used for cardiovascular risk prediction.9 In this study, we aimed to develop and validate separate prediction models for estimation of the 10-year cumulative incidences of ICH and IS.
METHODS
Study design and population.
We performed a combined analysis of individual data from 3 population-based cohort studies: the Atherosclerosis Risk in Communities (ARIC) Study, the Rotterdam Study, and the Cardiovascular Health Study (CHS).
The ARIC Study10 comprises 15,792 individuals aged 45 to 64 years at baseline recruited from 4 different regions in the United States from 1987 to 1989. The Rotterdam Study11 consists of 7,983 inhabitants of Ommoord, Rotterdam, the Netherlands, aged 55 years and older. Baseline examinations were conducted from 1990 to 1993. In the CHS,12 individuals older than 65 years living in 4 US communities were recruited from the Health Care Financing Administration (or Medicare) eligibility lists in 2 phases. First, 5,201 participants were recruited from 1989 to 1990. In a second wave, 687 African Americans were recruited from 1992 to 1993 leading to a cohort of 5,888 participants. For details on baseline measurements of the 3 studies, see appendix e-1 on the Neurology® Web site at Neurology.org.
The subjects eligible for the current analysis were those without prior stroke (n = 15,297 in the ARIC cohort, n = 7,546 in the Rotterdam Study, n = 5,639 in the CHS), who did not use anticoagulation (n = 15,222 ARIC Study, n = 7,177 Rotterdam Study, n = 5,572 CHS), and who did not have atrial fibrillation (n = 15,217 ARIC cohort, n = 6,910 Rotterdam Study, n = 5,446 CHS) at baseline. The latter 2 exclusion criteria were used because specific guidelines and prediction models already exist for these patients.13 In addition, we excluded participants who were not African American or Caucasian, leaving n = 27,493 subjects (n = 15,170 ARIC Study, n = 6,910 Rotterdam Study, n = 5,413 CHS) for the analysis.
Based on previous literature, we considered age, sex, African American ethnicity, current smoking, systolic blood pressure, antihypertensive medication use, diabetes mellitus, and history of coronary heart disease as candidate predictors in a basic nonlaboratory model for each stroke type.3–5,14–16 Subsequently, we evaluated model extension by the following office-based risk factors: diastolic blood pressure, total cholesterol, high-density lipoprotein cholesterol (HDL-C), body mass index (BMI), waist-to-hip ratio, and estimated glomerular filtration rate.4,5,7,15
Standard protocol approvals, registrations, and patient consents.
All studies received approval from medical ethical committees, and participants gave written informed consent.
Outcome definitions.
Details of outcome ascertainment are described elsewhere17–19 and in appendix e-1. In brief, ARIC outcomes were ascertained through yearly telephone interviews, follow-up examinations, community hospital surveillance, and reported deaths. In the Rotterdam Study, participants were continuously monitored for events through automated linkage of the study database with files from general practitioners and the municipality. Medical records of nursing homes were also evaluated. CHS outcomes were ascertained through 6 monthly telephone interviews, surveillance of Health Care Financing Administration Medicare Utilization files, and reported deaths.
For ICH, we excluded ascertained subarachnoid and traumatic hemorrhages. IS was defined as a combined endpoint of classified ischemic and unspecified stroke as a proxy for true IS events to avoid underestimation.20 Strokes were classified as ischemic if there was no evidence for other diagnoses based on imaging and evident clinical features, or if there was surgical or autopsy evidence of ischemia (also see table e-1). Any stroke was defined as the sum of ICH and IS. Censoring date was December 31, 2009 for the ARIC Study, January 1, 2009 for the Rotterdam Study, and June 30, 2008 for the CHS dataset.
Statistical analysis.
Two separate prediction models for the 10-year cumulative incidence of ICH and IS were developed using Cox regression modeling and considering competing risks (see appendix e-1 for more details). In addition, we developed an “any stroke” model, which can be subdivided into an ICH and IS component. The cause-specific Cox regression models were stratified by study cohort and developed with time since study entry as the time scale. In the basic models, effect modification by sex was evaluated for age, systolic blood pressure, diabetes mellitus, and history of coronary heart disease. An interaction term for systolic blood pressure and antihypertensive medication use was included.3,14 In the extended models, we evaluated replacement of total and HDL-C variables by the total cholesterol/HDL-C ratio and systolic by diastolic blood pressure.21 Finally, we tested heterogeneity of effects across studies by study-predictor interaction terms, and across stroke types by type-predictor interaction terms in full extended models.
Discriminative ability was assessed using Harrell concordance statistic (C statistic) adjusted for competing risks by setting the follow-up time to the maximum value if competing death occurred.22 Model calibration was assessed by calibration plots and χ2 statistics, comparing predicted with observed cumulative incidences using the R “CumInc” function of the R “mstate” library. Equal sized groups per study were made according to age tertiles for ICH and quintiles for IS. To understand the impact of using cumulative incidence functions instead of standard survival analysis, we compared the discriminative ability and model calibration of these 2 statistical methods for ICH and IS prediction.
For cross-validation, models were fit in 2 cohorts and evaluated in the other. Reclassification by extending basic models was assessed by the continuous net reclassification improvement.23 Ninety-five percent confidence intervals (CIs) were estimated by bootstrapping datasets with recalculation of the observed cumulative incidences within each bootstrap sample. Scatter plots showing the relationship between the ICH and IS components within any stroke risk were made for each dataset using extended models. Finally, we constructed an Excel risk calculator for the risk assessment of 10-year ICH, IS, and any stroke (as a sum of ICH and IS) risks (appendix e-2). Total cholesterol/HDL-C ratios are automatically calculated from total cholesterol and HDL-C. To individualize absolute risk differences and numbers needed to treat for each stroke type and any stroke events, estimated treatment effects expressed as hazard ratios (HRs) can be added.9
Missing covariable values were imputed for each study separately using single imputation with the R “aregImpute” function of the R “Hmisc” library. Imputation models included all potential predictors and the log cumulative hazard for each outcome. Hypothesis tests were 2-sided and decisions on selection of predictor main effects were made based on an improvement of the Akaike Information Criterion after exclusion from a full model. Interactions and nonlinear effects were included using a p value <0.05. The effect of excluding predictors with highly significant heterogeneous HRs (p < 0.01 for ICH, p < 0.001 for IS and competing death) on cross-validated model performance was evaluated in sensitivity analyses (see tables e-2 to e-6). We used R version 2.14.2 for all statistical analyses.
RESULTS
Study population.
The baseline characteristics of included ARIC (median age 54, 45% male), Rotterdam Study (median age 68, 38% male), and CHS (median age 71, 41% male) participants are given in table 1. In total, 325 participants experienced an ICH, 2,559 experienced an IS event, and 9,909 died from a competing death cause. The total number of individuals with ICH was low, especially in the ARIC Study, in which only 103 experienced an ICH. The 10-year cumulative incidence for ICH was approximately one-ninth of the 10-year cumulative incidence of IS in all studies (table 2).
Table 1.
Baseline characteristics

Table 2.
Incident event data
Hazard ratios.
Sex, diabetes, prior coronary heart disease, waist-to-hip ratio, and estimated glomerular filtration rate were not found to be statistically significant and were excluded from ICH models, whereas these were included in IS models. Table 3 shows the multivariable-adjusted HRs and 95% CIs for incident ICH and IS events (see table e-7 for competing death). For both ICH and IS, replacement of total and HDL-C by total cholesterol/HDL-C ratio and the simultaneous inclusion of systolic and diastolic blood pressure (despite correlations of 0.69, 0.59, and 0.51 in ARIC, Rotterdam, and CHS cohorts) improved the Akaike Information Criterion (figures e-1 through e-4 show the multivariable adjusted relations with ICH and IS event rates). The extended ICH model is reported without BMI, although BMI statistically significantly decreased the ICH hazard: 0.97 (95% CI 0.94–0.99) per unit increase. However, the BMI association varied significantly across the 3 studies (see table e-2) and exclusion improved the cross-validated model performance (see table e-5). BMI was removed from the extended IS model because it was not statistically significant (p = 0.27).
Table 3.
Included predictors and hazard ratios (95% confidence intervals)
Midrange total cholesterol/HDL-C ratio values as compared with low and high values decreased ICH risk, whereas high total cholesterol/HDL-C ratio monotonically increased IS risk (see figures e-2 and e-4). The association of the total cholesterol/HDL-C ratio statistically differed across stroke types (p < 0.001). The HRs for ICH additionally censored for IS and vice versa are given in tables e-8 and e-9; these did not largely differ from those shown in table 3.
Model performance.
For ICH prediction, C statistics of cumulative incidence functions were higher than C statistics of standard survival models (see tables 4 and e-10). For IS prediction, C statistics of both statistical methods were comparable. Extending the basic cumulative incidence functions generally led to small improvements in C statistics, ranging from 0.001 to 0.020 for ICH, and 0.001 to 0.009 for IS. The continuous total net reclassification improvements were positive, with more pronounced changes in the ARIC cohort. Improvements in C statistics were reproduced by cross-validation except for IS predictions in Rotterdam Study data (table 4). C statistics for any stroke predictions were similar to IS predictions, and did not improve with model extension (table e-11).
Table 4.
Prognostic performance
As compared with standard survival models, calibration generally improved using cumulative incidence functions, especially within the elderly, except for ICH prediction below the second age tertile in the CHS cohort (see table e-10 and figures e-5 to e-8). Overall, model calibration did not differ to a relevant extent between basic and extended models for both ICH and IS prediction (figures e-5 to e-8). Results on calibration by the any-stroke cumulative incidence functions were similar to those for IS prediction (table e-11).
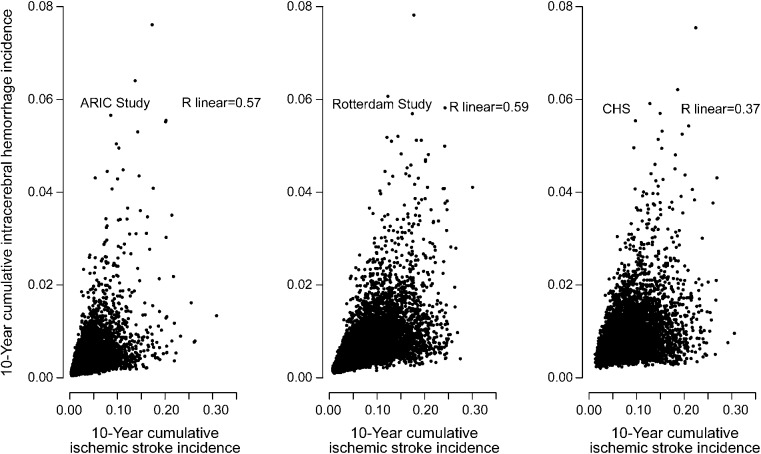
Predicted ICH risk tended to increase with IS risk for each study, but the correlation between both predicted risks was moderate in ARIC, Rotterdam, and CHS cohorts (r = 0.57, 0.59, 0.37, respectively; figure 1). The Excel risk calculator for predicting the 10-year cumulative incidence of any stroke subdivided into ICH and IS components can be accessed online (appendix e-2). HRs used in the calculator are provided in tables e-7 to e-9.
Figure 1. Contribution of intracerebral hemorrhage and ischemic stroke to 10-year any stroke incidence.
ARIC = Atherosclerosis Risk in Communities; CHS = Cardiovascular Health Study.
DISCUSSION
In this study, we developed and cross-validated 10-year ICH and IS risk models within middle-aged and elderly individuals. As compared with standard survival analysis, the cumulative incidence functions showed improved discriminative ability for ICH prediction and better model calibration, especially in the elderly. Extending basic nonlaboratory models led to limited improvement of discriminative ability. By using our cumulative incidence functions, individuals can be identified with low 10-year IS risk but high ICH risk, and vice versa.
Studies on hemorrhagic stroke prediction are scarce. By performing a systematic literature search (see appendix e-1), we found only 2 studies, both conducted in Chinese populations. One study24 was performed in a cohort of 4,400 steelworkers free of stroke at baseline with an average age of 45 years. The number of hemorrhagic strokes was low: 33 in the development set and 15 in the validation set. Multivariable-adjusted HRs of age (1.89 per 10 years) and systolic blood pressure (1.22 per 10 mm Hg) were similar to ours. For diastolic blood pressure (1.49 per 10 mm Hg) and total cholesterol (1.00 per mmol/L), nonlinearity was not explored, and therefore associations are not comparable with ours. In addition, the model was not validated in the general population and the elderly. In the other study,25 major bleeding risk scoring schemes designed for patients with atrial fibrillation were validated in 3,602 individuals without atrial fibrillation. C statistics of the various risk scores ranged from 0.59 to 0.72. Individuals with previous stroke were, however, not excluded, and ICH event ascertainment was registry-based. Other prognostic studies focused on either assessment of any stroke risk3,4,14,16,26–32 or IS risk5,6,20,33,34 usually within a time horizon of 5 to 10 years.
In contrast, we developed models for the separate 10-year risk assessment of ICH and IS while considering competing risks. By combining data from 3 large population-based cohorts, we acquired a sufficient number of ICH events for multivariable prediction modeling. The number of ICH events was low in the ARIC Study, probably explained by the younger age of this cohort. Furthermore, we included elderly individuals older than 75 years. Especially in the elderly, competing risks become relevant, because the competing death rate rapidly increases. We demonstrated that also in the elderly, predictions were well calibrated as opposed to predictions using standard survival analysis.
Our results must be interpreted in the light of some limitations. First, we did not consider biomarkers, genetic risk factors, and imaging tests. For example, studies have demonstrated an independent association of C-reactive protein with IS but not ICH risk,35 and carotid intima-media thickness measurement and APOE genotype with both ICH and IS risk.36,37 However, carotid intima-media thickness and APOE genotype are generally difficult to assess during an office-based risk assessment, which would limit the translation to clinical practice, and C-reactive protein was not available as a baseline variable in the ARIC Study. Second, neuroimaging was not performed in all participants with stroke symptoms. Consequently, a proportion of strokes were not further specified. We included these as IS, which could have led to a small overestimation of the IS risk and underestimation of ICH risk. Third, the quality of the individual studies may have influenced the overall validity of the results. For example, the Rotterdam Study had more missing values for the included predictors than the other 2 cohorts. Because of the computational demand of constructing the cumulative incidence function, it was infeasible to perform multiple imputation. Therefore, the precision of our models is subject to a small overestimation. In addition, validity of our results is hampered if the missingness was actually nonrandom. Fourth, the baseline age ranges of the ARIC, Rotterdam, and CHS cohorts did not entirely overlap. As a consequence, the age association was not fully determined by the 3 datasets combined. Finally, we excluded individuals with stroke, atrial fibrillation, or anticoagulation use at baseline, and individuals who were not Caucasian and not African American, such as Asian and Hispanic participants. Also, we did not consider use of antiplatelet and statin therapy in the analysis. The effect of these preventive drugs should have been captured in the baseline survival function or within the associations of included predictors. The validity of our predictions can thus be affected if the use of these drugs differs by population or varies over time because of changing medical practice. Consequently, our predictions should be validated in other and more recent populations. For patients with atrial fibrillation, clinicians should use the specific stroke and bleeding risk models validated for this population.
We evaluated extension of a basic nonlaboratory model using C statistic as a criterion. The C statistic would improve if a predictor is added that increases risk in early cases and/or decreases risk in late cases and controls. The impact on the C statistic depends both on the HR of the added predictor and its distribution in the context of included predictors.38 Because we evaluated the heterogeneity of associations within sensitivity analyses, the varying distribution of predictor values across the 3 cohorts must have led to the differences in (changes of) the C statistic. However, because discriminative ability generally improved for all 3 cohorts, and the added predictors are expected to incur limited extra costs and potential harms, we recommend the use of the extended models as the preferred choice.
Specifying whether a first stroke is ICH or IS is clinically valuable. More refined estimates of the expected benefits and harms can be made about preventive interventions with different effects on ICH and IS risk. For example, aspirin use is recommended when the potential benefit of reduction in IS outweighs the bleeding risks.39 This trade-off is especially relevant in the elderly,40 making well-calibrated predictions at older ages more important. Our cumulative incidence functions may therefore be useful to refine communication of the expected benefit (by number of IS events avoided) and harm (by number of induced ICH events in addition to gastrointestinal bleedings) to support shared decision-making. However, differences in case-fatality rate of ICH and IS should be considered as well. In this context, we anticipate that our prediction models for 10-year ICH and IS risk are useful to further specify an individual's stroke risk.
Supplementary Material
ACKNOWLEDGMENT
The authors thank David van Klaveren and Yvonne Vergouwe (Department of Public Health, Erasmus MC) for their valuable methodologic advice, and Irene Doherty (ARIC Study) and Tony Wilsdon (CHS) for preparing the ARIC and CHS datasets. The contributions of inhabitants, general practitioners, and pharmacists of the Ommoord district to the Rotterdam Study are gratefully acknowledged.
GLOSSARY
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- CHS
Cardiovascular Health Study
- CI
confidence interval
- HDL-C
high-density lipoprotein cholesterol
- HR
hazard ratio
- ICH
intracerebral hemorrhage
- IS
ischemic stroke
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Ferket: drafting the manuscript for content, study concept and design, analysis and interpretation of data. Dr. van Kempen: revising the manuscript for content, interpretation of data. Dr. Wieberdink: revising the manuscript for content. Dr. Steyerberg: study concept and design, revising the manuscript for content, interpretation of data. Dr. Koudstaal, Dr. Hofman, Dr. Shahar, Dr. Gottesman, Dr. Rosamond, Dr. Kizer, Dr. Kronmal, and Dr. Psaty: revising the manuscript for content. Dr. Longstreth, Jr.: study concept and design, revising the manuscript for content. Dr. Mosley: revising the manuscript for content. Dr. Folsom, Dr. Hunink, and Dr. Ikram: study concept and design, revising the manuscript for content.
STUDY FUNDING
The Atherosclerosis Risk in Communities Study is performed as a collaborative study supported by National Heart, Lung, and Blood Institute (contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC Study for their important contributions. The Rotterdam Study is supported by the Erasmus Medical Center Rotterdam, the Erasmus University Rotterdam, the Netherlands Organization for Scientific Research (NWO), The Netherlands Organization for Health Research and Development (ZonMW), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. B.S.F. was funded through the Netherlands Organization for Scientific Research (NWO) training grant 022.002.023. The Cardiovascular Health Study was supported by contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by AG023629 from the National Institute on Aging. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/PI.htm.
DISCLOSURE
B. Ferket, B. van Kempen, R. Wieberdink, E. Steyerberg, P. Koudstaal, A. Hofman, E. Shahar, R. Gottesman, and W. Rosamond report no disclosures relevant to the manuscript. J. Kizer has provided expert witness consultation to Pfizer relating to Prempro and stroke. R. Kronmal reports no disclosures relevant to the manuscript. B. Psaty serves on the DSMB of a clinical trial of a device funded by the manufacturer (Zoll LifeCor) and on the Steering Committee of the Yale Open Data Access Project funded by Johnson & Johnson. W. Longstreth, Jr., T. Mosley, A. Folsom, M. Hunink, and M. Ikram report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
DISCLAIMER
The funding sources had no role in the design, conduct, or analysis of this study or in the decision to submit the article for publication.
REFERENCES
- 1.OECD.StatExtracts. OECD health data 2011 [online]. Available at: http://stats.oecd.org/index.aspx?DataSetCode=HEALTH_STAT. Accessed August 5, 2013
- 2.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009;8:355–369 [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke 1991;22:312–318 [DOI] [PubMed] [Google Scholar]
- 4.Lumley T, Kronmal RA, Cushman M, Manolio TA, Goldstein S. A stroke prediction score in the elderly: validation and Web-based application. J Clin Epidemiol 2002;55:129–136 [DOI] [PubMed] [Google Scholar]
- 5.Chambless LE, Heiss G, Shahar E, Earp MJ, Toole J. Prediction of ischemic stroke risk in the Atherosclerosis Risk in Communities Study. Am J Epidemiol 2004;160:259–269 [DOI] [PubMed] [Google Scholar]
- 6.Assmann G, Schulte H, Cullen P, Seedorf U. Assessing risk of myocardial infarction and stroke: new data from the Prospective Cardiovascular Munster (PROCAM) Study. Eur J Clin Invest 2007;37:925–932 [DOI] [PubMed] [Google Scholar]
- 7.Leppala JM, Virtamo J, Fogelholm R, Albanes D, Heinonen OP. Different risk factors for different stroke subtypes: association of blood pressure, cholesterol, and antioxidants. Stroke 1999;30:2535–2540 [DOI] [PubMed] [Google Scholar]
- 8.Baigent C, Blackwell L, Collins R, et al. ; Antithrombotic Trialists’ (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koller MT, Raatz H, Steyerberg EW, Wolbers M. Competing risks and the clinical community: irrelevance or ignorance? Stat Med 2012;31:1089–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702 [PubMed] [Google Scholar]
- 11.Hofman A, van Duijn CM, Franco OH, et al. The Rotterdam Study: 2012 objectives and design update. Eur J Epidemiol 2011;26:657–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–276 [DOI] [PubMed] [Google Scholar]
- 13.Goldstein LB, Bushnell CD, Adams RJ, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:517–584 [DOI] [PubMed] [Google Scholar]
- 14.D'Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke 1994;25:40–43 [DOI] [PubMed] [Google Scholar]
- 15.Sturgeon JD, Folsom AR, Longstreth WT, Jr, Shahar E, Rosamond WD, Cushman M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke 2007;38:2718–2725 [DOI] [PubMed] [Google Scholar]
- 16.Voko Z, Hollander M, Koudstaal PJ, Hofman A, Breteler MM. How do American stroke risk functions perform in a Western European population? Neuroepidemiology 2004;23:247–253 [DOI] [PubMed] [Google Scholar]
- 17.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1999;30:736–743 [DOI] [PubMed] [Google Scholar]
- 18.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol 1995;5:278–285 [DOI] [PubMed] [Google Scholar]
- 19.Wieberdink RG, Ikram MA, Hofman A, Koudstaal PJ, Breteler MM. Trends in stroke incidence rates and stroke risk factors in Rotterdam, the Netherlands from 1990 to 2008. Eur J Epidemiol 2012;27:287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hippisley-Cox J, Coupland C, Brindle P. Derivation and validation of QStroke score for predicting risk of ischaemic stroke in primary care and comparison with other risk scores: a prospective open cohort study. BMJ 2013;346:f2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franklin SS, Lopez VA, Wong ND, et al. Single versus combined blood pressure components and risk for cardiovascular disease: the Framingham Heart Study. Circulation 2009;119:243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolbers M, Koller MT, Witteman JC, Steyerberg EW. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology 2009;20:555–561 [DOI] [PubMed] [Google Scholar]
- 23.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 2011;30:11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang XF, Attia J, D'Este C, Yu XH, Wu XG. A risk score predicted coronary heart disease and stroke in a Chinese cohort. J Clin Epidemiol 2005;58:951–958 [DOI] [PubMed] [Google Scholar]
- 25.Lip GY, Lin HJ, Hsu HC, et al. Comparative assessment of the HAS-BLED score with other published bleeding risk scoring schemes, for intracranial haemorrhage risk in a non-atrial fibrillation population: the Chin-Shan Community Cohort Study. Int J Cardiol 2013;168:1832–1836 [DOI] [PubMed] [Google Scholar]
- 26.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J 1991;121(1 pt 2):293–298 [DOI] [PubMed] [Google Scholar]
- 27.Bineau S, Dufouil C, Helmer C, et al. Framingham stroke risk function in a large population-based cohort of elderly people: the 3C Study. Stroke 2009;40:1564–1570 [DOI] [PubMed] [Google Scholar]
- 28.Borglykke A, Andreasen AH, Kuulasmaa K, et al. Stroke risk estimation across nine European countries in the MORGAM project. Heart 2010;96:1997–2004 [DOI] [PubMed] [Google Scholar]
- 29.Jee SH, Park JW, Lee SY, et al. Stroke risk prediction model: a risk profile from the Korean study. Atherosclerosis 2008;197:318–325 [DOI] [PubMed] [Google Scholar]
- 30.Moons KG, Bots ML, Salonen JT, et al. Prediction of stroke in the general population in Europe (EUROSTROKE): is there a role for fibrinogen and electrocardiography? J Epidemiol Community Health 2002;(56 suppl 1):i30–i36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Truelsen T, Lindenstrom E, Boysen G. Comparison of probability of stroke between the Copenhagen City Heart Study and the Framingham Study. Stroke 1994;25:802–807 [DOI] [PubMed] [Google Scholar]
- 32.Chien KL, Su TC, Hsu HC, et al. Constructing the prediction model for the risk of stroke in a Chinese population: report from a cohort study in Taiwan. Stroke 2010;41:1858–1864 [DOI] [PubMed] [Google Scholar]
- 33.Simons LA, McCallum J, Friedlander Y, Simons J. Risk factors for ischemic stroke: Dubbo Study of the Elderly. Stroke 1998;29:1341–1346 [DOI] [PubMed] [Google Scholar]
- 34.Qiao Q, Gao W, Laatikainen T, Vartiainen E. Layperson-oriented vs. clinical-based models for prediction of incidence of ischemic stroke: National FINRISK Study. Int J Stroke 2012;7:662–668 [DOI] [PubMed] [Google Scholar]
- 35.Kaptoge S, Di Angelantonio E, Lowe G, et al. ; Emerging Risk Factors Collaboration. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375:132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohira T, Shahar E, Iso H, et al. Carotid artery wall thickness and risk of stroke subtypes: the Atherosclerosis Risk in Communities Study. Stroke 2011;42:397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sudlow C, Martinez Gonzalez NA, Kim J, Clark C. Does apolipoprotein E genotype influence the risk of ischemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage? Systematic review and meta-analyses of 31 studies among 5961 cases and 17,965 controls. Stroke 2006;37:364–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demler OV, Pencina MJ, D'Agostino RB., Sr Impact of correlation on predictive ability of biomarkers. Stat Med Epub May 3 2013 [DOI] [PMC free article] [PubMed]
- 39.US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2009;150:396–404 [DOI] [PubMed] [Google Scholar]
- 40.Nelson MR, Liew D, Bertram M, Vos T. Epidemiological modelling of routine use of low dose aspirin for the primary prevention of coronary heart disease and stroke in those aged ≥70. BMJ 2005;330:1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.