Abstract
Objective:
To determine whether the risk of arteriovenous malformation (AVM) rupture is increased during pregnancy and puerperium.
Methods:
Participants included 979 female patients with intracranial AVM admitted to Beijing Tiantan Hospital between 1960 and 2010. Two neurosurgery residents reviewed medical records for each case. Of them, 393 patients with ruptured AVM between 18 and 40 years of age were used for case-crossover analysis. Number of children born and clinical information during pregnancy and puerperium were retrieved to identify whether AVM rupture occurred during this period.
Results:
Of the 979 women, 797 hemorrhages occurred during 25,578 patient-years of follow-up, yielding an annual hemorrhage rate of 3.11%. The annual AVM hemorrhage rate in patients aged 18 to 40 years (n = 579) was 2.78%, lower than the rate in other age groups (odds ratio = 0.75, 95% confidence interval 0.65–0.86, p < 0.05). Of the 393 patients with rupture of AVM aged 18 to 40 years, 12 hemorrhages occurred in 12 patients over 452 pregnancies, yielding a hemorrhage rate of 2.65% per pregnancy or 3.32% per year. Among the remaining 381 patients, 441 hemorrhages occurred during 10,627 patient-years of follow-up, yielding an annual hemorrhage rate of 4.14%. The odds ratio for rupture of AVM during pregnancy and puerperium, compared with the control period, was 0.71 (95% confidence interval 0.61–0.82).
Conclusions:
No increased risk of hemorrhage was found in patients with cerebral AVM during pregnancy and the puerperium. We therefore would not advise against pregnancy in women with intracranial AVM.
Hemorrhagic stroke is a serious complication during pregnancy and puerperium that has a substantial maternal mortality of 35% to 83%, contributing to more than 5% to 12% of all maternal deaths.1 Previous studies suggested that pregnancy and puerperium were associated with an increased risk of stroke.2–4 The rupture of cerebral aneurysm and arteriovenous malformation (AVM) were the main causes.5–7 In a prospective and population-based study, researchers found that pregnancy did not increase AVM bleed rates, but risk of intracranial hemorrhage was increased during the postpartum period.8 Some other studies also found that pregnancy did not increase the risk of hemorrhage from an AVM.9,10 Recently, another study reported the rate of intracranial hemorrhage from AVM was 8.1% per pregnancy, higher than the annual hemorrhage rate of AVM in female patients11; however, this study had a limited sample size. Given the increasing number of reported cases of intracranial hemorrhage from AVM during pregnancy and puerperium, we undertook this study to assess whether the risk of AVM rupture is increased during pregnancy and 6 weeks after delivery.
METHODS
Study population.
Patients were selected from a prospectively collected database of 979 consecutive female patients with a confirmed angiographic or histologic diagnosis of an AVM referred to Beijing Tiantan Hospital from 1960 to 2010. The Beijing Tiantan Hospital served as the referral center for all pregnant women with cerebral AVM rupture in the region of Beijing.
Study design and data analysis.
We conducted a case-crossover design, using cases at previous time points as their own controls. In this design, the risk of cerebral AVM rupture during pregnancy and puerperium (exposure period) was compared with the risk excluding pregnancy and puerperium (the control period). In China, more than 99% of women choose to conceive a child between ages 18 and 40 years12; therefore, only patients with AVM rupture aged 18 to 40 years were used in the case-crossover design (n = 393), and the first 18 years of age were subtracted from the person-time at risk. For each patient, the person-time of exposure period was calculated from the first week of pregnancy to 6 weeks after pregnancy (10 months). The person-time excluding pregnancy and puerperium periods was estimated by subtracting the exposure period from the total person-time. For example, the total person-time at risk for a woman of 28 years who had 2 children was calculated as 28 − 18 years, the time of exposure period was 2 × 10 months, and the control period was (28 − 18) × 12 − 2 × 10 months.
To identify the occurrence of AVM rupture during pregnancy and puerperium, 2 neurosurgery residents reviewed the history of intracranial bleeding and clinical records for each case. In patients with AVM rupture during pregnancy or puerperium, we collected information on maternal age, parity, gestational age, and Glasgow Outcome Scale scores at admission, modes of delivery, treatment, and maternal and fetal outcome. Maternal outcome was categorized according to the 5-point Glasgow Outcome Scale. Fetal outcome was according to the Apgar score on delivery for 1-5-10 minutes: scores 3 and below are generally regarded as critically low, 4–6 fairly low, and 7–10 generally normal.
Recent studies have reported that AVM is a genetic disease13–16; therefore, patient-years of follow-up were calculated from birth until AVM obliteration. Annual hemorrhage rate was calculated as the ratio of the number of hemorrhages to total number of patient-years of follow-up. Using case-crossover analysis, the ratio of the observed frequency of hemorrhage in the exposure period to the frequency in the control period was used to calculate estimates of the odds ratio (OR). ORs and 95% confidence intervals (CIs) for association between pregnancy and the rupture of AVM were estimated using a Mantel-Haenszel estimator17 (SPSS software, version 16.0; SPSS Inc., Chicago, IL).
Standard protocol approvals, registrations, and patient consents.
This study received approval from the Beijing Tiantan Hospital Research Ethics Committee. Written informed consent was obtained from all participants or their guardians (for each patient, written informed consent was completed after admission).
RESULTS
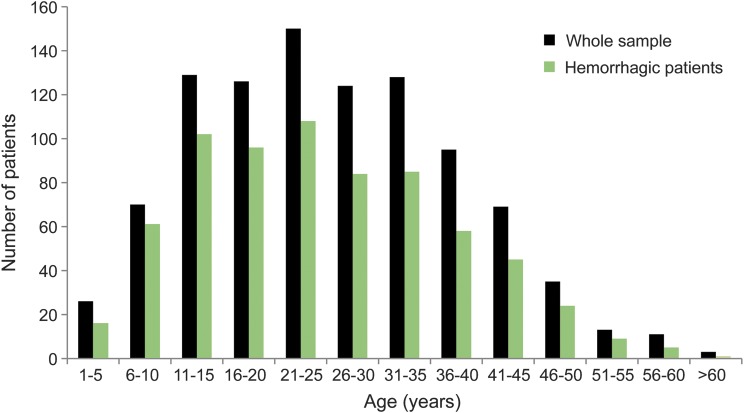
Of a total of 979 patients (mean age 26.1 ± 12.4 years), 797 hemorrhages occurred during 25,578 patient-years of follow-up, yielding an annual hemorrhage rate of 3.11%. Of the 579 patients aged 18 to 40 years, 456 hemorrhages occurred during 16,367 patient-years of follow-up, yielding an annual hemorrhage rate of 2.78%, which is lower than the rate in other age groups (OR = 0.75, 95% CI 0.65–0.86, p < 0.05). Overall, the annual bleeding rate of AVM decreased year by year in the female patients (figure 1). The characteristics of all patients are shown in table 1, and the age distribution of all patients is shown in figure 2.
Figure 1. Changes of annual hemorrhage rate during the past 50 years.
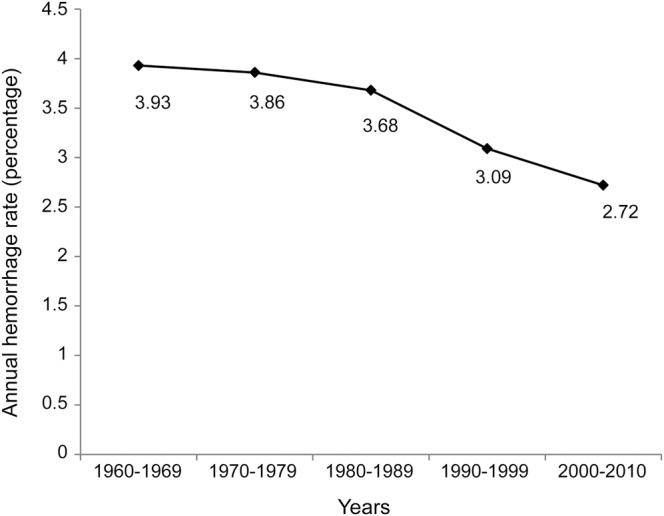
Table 1.
Patient characteristics
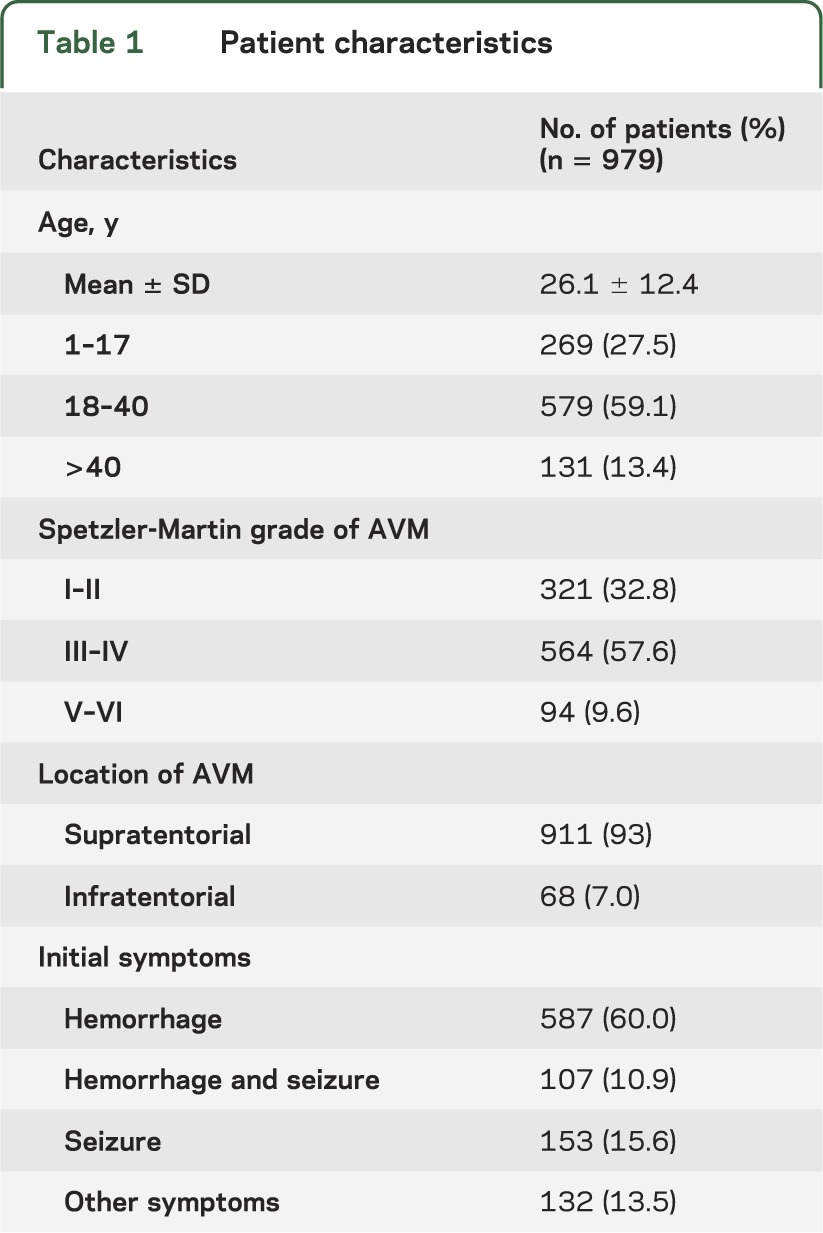
Figure 2. Age distribution of the patients.
There were 393 patients with AVM rupture between the ages of 18 and 40 years. Of them, 54 patients had previous bleeding, and 24 patients (6.1%) received AVM treatment before the first pregnancy, including surgical resection (n = 18), gamma knife (n = 4), and interventional therapy (n = 2). Twelve hemorrhages occurred in 12 patients over 452 pregnancies, yielding a hemorrhage rate of 2.65% per pregnancy or 3.32% per year. Among the remaining 381 patients, 441 hemorrhages occurred during 10,627 patient-years of follow-up, yielding an annual hemorrhage rate of 4.14%. The OR for rupture of AVM during pregnancy and puerperium in patients with AVM, compared with the period of time excluding pregnancy and puerperium, was 0.71 (95% CI 0.61–0.82).
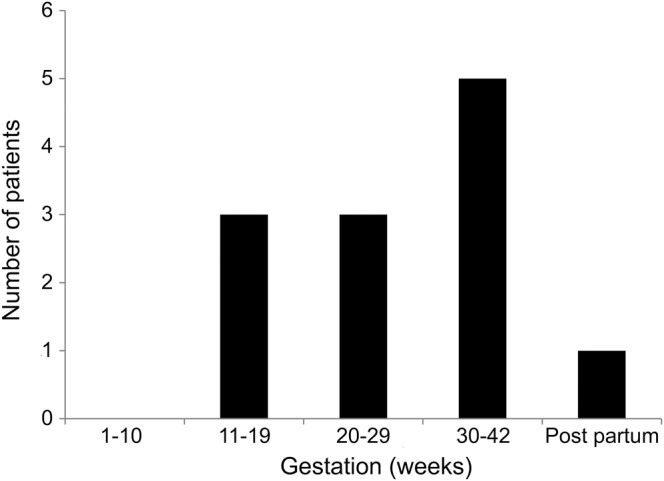
Of the 12 patients with AVM rupture during pregnancy and puerperium, headache, vomiting, limb dysfunction, and altered consciousness were the main clinical manifestations. Eleven patients were in antepartum, including 6 in the second trimester and 5 in the third trimester. The mean gestation time was 26.3 ± 7.6 weeks (range 15 to 38 weeks). Only one patient had rupture of AVM in postpartum. There was no case in the first trimester or during delivery (figure 3).
Figure 3. Distribution of gestation weeks of the 12 patients with arteriovenous malformation rupture during pregnancy.
All patients received emergency CT brain scans and 9 patients underwent emergency digital subtraction angiography (DSA) examinations to confirm the cerebral AVMs. Two patients were confirmed by pathology after operation and one patient was previously diagnosed with a cerebral AVM by DSA. Eight patients had a gestation age of less than 35 weeks. Of them, 4 patients chose to continue pregnancy, 3 of them delivered a healthy baby by cesarean section weeks later, and one delivered a premature baby at 31 weeks; the other 4 patients chose abortion considering the potential biological effects of radiation exposure after extensive counseling.
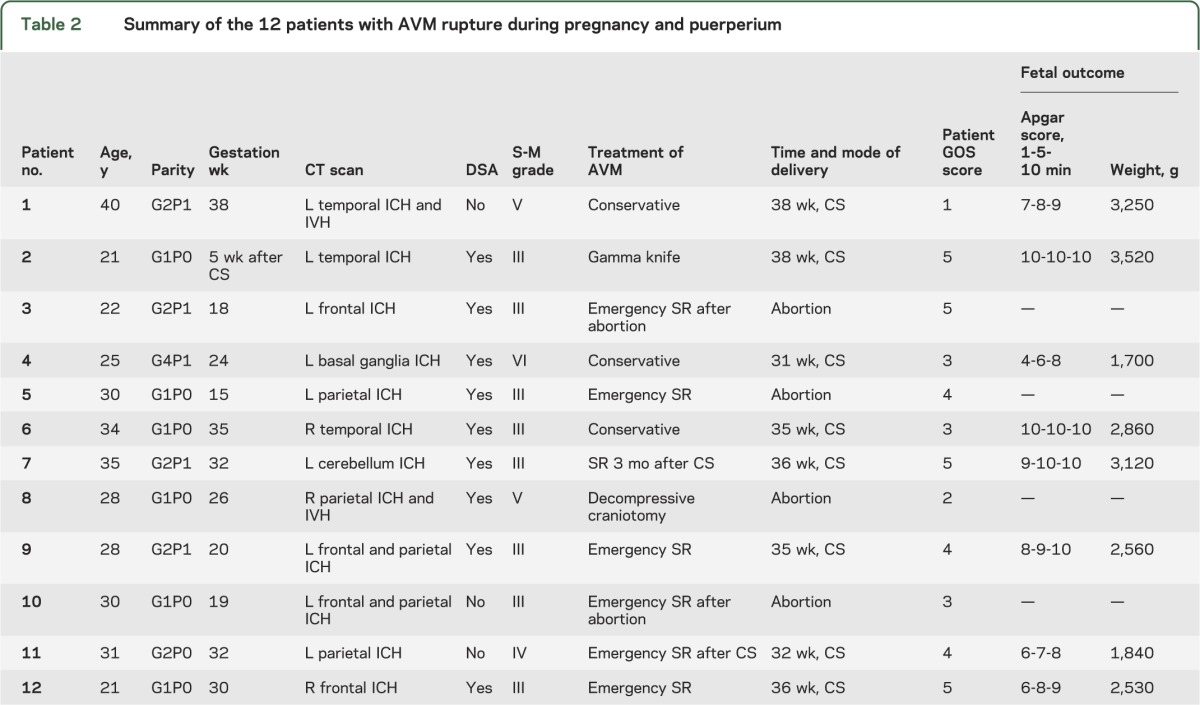
All patients were managed by multidisciplinary care teams (neurosurgery, neurology, obstetrics and gynecology, pediatrics, intensive care). Nine patients received surgical interventions, including 6 (50%) emergency surgical clot evacuation and resections of AVM, one elective surgical resection of AVM 3 months after cesarean delivery, one decompressive craniotomy, and one gamma knife. There were 4 fetal deaths (4 abortions) and no maternal deaths in the surgical treatment group. Three patients were managed conservatively, 2 with Spetzler-Martin grade V or VI AVM and one with a grade III AVM with a small stable intracranial hemorrhage. Of those managed conservatively, one maternal death occurred. The characteristics of the 12 patients, location of the AVM, mode of delivery, treatment, and maternal and fetal outcome are summarized in table 2.
Table 2.
Summary of the 12 patients with AVM rupture during pregnancy and puerperium
DISCUSSION
In the present study, we did not find an increased risk of AVM rupture in patients aged 18 to 40 years, which were the prime childbearing ages in the Chinese population. The case-crossover analysis using the time of pregnancy and the puerperium as a trigger also did not reveal an increased risk of AVM rupture in patients aged 18 to 40 years. The annual rate of hemorrhage in these patients was similar to the rate reported by natural-history studies of untreated AVM.18–20 These findings show that pregnancy is not associated with an increased risk of intracranial AVM rupture. Therefore, based on our data, we would not advise against pregnancy in women with intracranial AVMs. However, the issues facing patients with a ruptured AVM in pregnancy remained challenging.
Several predictors of hemorrhage in patients with an untreated cerebral AVM have been established, including patient age, AVM location, and deep venous drainage.21 Recently, there is an increasing number of reported cases of AVM rupture during pregnancy and puerperium,1,9–11,22–24 and in some studies, it was assumed that pregnancy was a risk factor for AVM rupture. In an earlier study, researchers reviewed 113 young patients with intracranial stroke aged 15 to 45 years and found that pregnancy posed a risk for AVM rupture.23 However, another study did not find that the natural history of AVM was altered by pregnancy.24 A recent study reviewed 54 women with cerebral AVM and found 4 patients with hemorrhages that occurred over 62 pregnancies.11 The results revealed that the rate of AVM rupture during pregnancy was higher than the annual hemorrhage rate. However, an important limitation of these studies was the small sample size. In this study, we included a larger sample size and used a case-crossover design to analyze the risk of AVM rupture during pregnancy and puerperium. To our knowledge, cases act as their own controls in a case-crossover study design. Only patients having an event of interest were enrolled and their exposures were intermittent. Pregnancy and the puerperium are transient and the rupture of AVM is abrupt. Therefore, the exposure during pregnancy and the puerperium is a valid trigger that can be used in a case-crossover study.25
In this study, most patients presented with intracranial hemorrhages in the second and third gestational trimester, accounting for 91.6% of all the patients (figure 3). No case of AVM rupture occurred in the first trimester or during delivery. Although we did not find an increased risk of AVM rupture during pregnancy and the puerperium, we found that the AVM rupture occurred more frequently in late gestational age. This may be correlated with the more extensive changes of hemodynamic parameters, coagulative function, and vessel wall in the third trimester of pregnancy.
Because of small samples and sparse data about the treatment of AVM rupture during pregnancy and puerperium, the management of these patients is very difficult and controversial. Timely and accurate diagnosis of intracranial AVM is of utmost importance. At present, DSA is the gold standard in diagnosing AVM and CT scan is the preferred method in diagnosing intracranial hemorrhage. However, fetal radiation exposure is the major concern when using these examinations. In our group, there were no cases of radiation-induced fetal malformation in patients who chose to continue pregnancy after CT and DSA examinations. Several studies have indicated that the effective radiation dose to the fetus during DSA was very small.26–28 Recent studies about radiation exposure and pregnancy also demonstrated that a brain CT can be performed with lead shielding of the abdomen and pelvis.29 Although the additional radiation dose to the fetus from CT and DSA under lead shielding might be small, especially during middle to late pregnancy, further studies with a high level of evidence are needed to confirm this.
At present, there is no Class I or II evidence to guide the treatment of intracranial AVM in women during pregnancy. The timing of surgical intervention is controversial.30 Dias and Sekhar1 reported that early surgical resection of AVM did not have a better outcome during pregnancy compared with conservative treatment. In this study, one maternal death was encountered in the 12 patients with AVM rupture during pregnancy (8.3% maternal mortality). This seemingly high maternal mortality was in keeping with other large AVM series that quoted approximately 10% risk of death with initial AVM rupture.24 No maternal death occurred in the 6 patients who received emergent surgical resections of AVM (table 2). However, emergency surgical resection of AVM is not the first choice for all patients. For patients with Spetzler-Martin grade V or VI,24 conservative treatment was preferred because of very high morbidity and mortality associated with operating on these lesions. According to our experience, for patients with grades I to IV AVM, urgent surgical evacuation of the intracranial hematoma and resection of AVM should be performed if signs of brain herniation occur. For patients of later pregnancy, surgical intervention can be performed after cesarean delivery. Furthermore, close collaboration among multidisciplinary teams including the departments of neurology, neurosurgery, obstetrics and anesthesiology, pediatrics, and intensive care has been important for the management of this group of patients.
A strength of this study is that it included a large sample size of female patients with intracranial AVM. We used a case-crossover design to compare the risk of rupture of AVM during pregnancy and puerperium with the risk excluding this period for the cohort of women aged 18 to 40 years. However, we are aware that the single-center retrospective design of this study is one of its limitations, and the study includes exclusively Chinese patients. Second, there might be some patients who had a bleed but were admitted to a different hospital or died before reaching our hospital; therefore, a case ascertainment bias may exist. Third, we did not include patients who might have received AVM resection before 18 years of age in the case-crossover analysis, and these patients would have lower risk of a bleed during pregnancy. In addition, we did not follow up the young patients who presented with hemorrhage before pregnancy to determine whether hemorrhage occurred again during a subsequent pregnancy, so the study cannot answer the question of whether one or more pregnancies increase the risk of AVM rupture. However, we believe that this would be a very small possibility because most patients would have surgical resection of the AVM before their next pregnancy.
Our data suggest that the risk of cerebral AVM rupture is not increased during pregnancy and the puerperium. Therefore, we would not advise against pregnancy in patients with intracranial AVM. However, we found that AVM rupture usually occurred in late gestational age; therefore, more attention should be provided to maintain relative hemodynamic stability in these patients during mid to late pregnancy. The issues facing patients with ruptured AVM in pregnancy remained challenging and required multidisciplinary management including neurosurgeons, obstetricians, pediatricians, and intensivists.
GLOSSARY
- AVM
arteriovenous malformation
- CI
confidence interval
- DSA
digital subtraction angiography
- OR
odds ratio
AUTHOR CONTRIBUTIONS
Dr. Xing-ju Liu: study design, acquisition, data collection, analysis, and writing the manuscript. Professor Shuo Wang: study design and critical revision of the manuscript. Dr. Yuan-li Zhao: data interpretation and manuscript revision. Dr. Mario Teo: data interpretation, critical revision of the manuscript for important intellectual content. Drs. Peng Guo and Dong Zhang: data collection, analysis, and interpretation. Dr. Rong Wang: data collection and analysis. Drs. Yong Cao, Xun Ye, and Shuai Kang: data analysis and interpretation. Professor Ji-Zong Zhao: study concept, design, supervision, and critical revision of the manuscript.
STUDY FUNDING
Supported by the “12th Five-Year Plan” National Science and Technology supporting plan (2011BAI08B00), and the Beijing Municipal Science and Technology Commission (D101107049310001).
DISCLOSURE
X. Liu reports no disclosures relevant to the manuscript. S. Wang serves as vice chairman of the Neurosurgical Society of the Chinese Medical Association, and received institutional support from the National Science and Technology supporting plan. Y. Zhao, M. Teo, P. Guo, D. Zhang, R. Wang, Y. Cao, Y. Xun, and S. Kang report no disclosures relevant to the manuscript. J. Zhao serves as former chairman of the Neurosurgical Society of the Chinese Medical Association and president of the National Research Center of Neurological Diseases, and received institutional support from the National Science and Technology supporting plan and National 973 Plan Project. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Dias MS, Sekhar LN. Intracranial hemorrhage from aneurysms and arteriovenous malformations during pregnancy and the puerperium. Neurosurgery 1990;27:855–865 [DOI] [PubMed] [Google Scholar]
- 2.Kittner SJ, Stern BJ, Feeser BR, et al. Pregnancy and the risk of stroke. N Engl J Med 1996;335:768–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ros HS, Lichtenstein P, Bellocco R, et al. Increased risks of circulatory diseases in late pregnancy and puerperium. Epidemiology 2001;12:456–460 [DOI] [PubMed] [Google Scholar]
- 4.Sharshar T, Lamy C, Mas J. Incidence and causes of strokes associated with pregnancy and puerperium: a study in public hospitals of Ile de France. Stroke 1995;26:930–936 [DOI] [PubMed] [Google Scholar]
- 5.Davie CA, O'Brien P. Stroke and pregnancy. J Neurol Neurosurg Psychiatry 2008;79:240–245 [DOI] [PubMed] [Google Scholar]
- 6.Tiel GA, Rinkel GJ, vander Bom JG, Algra A, Klijn CJ. The risk of aneurysmal subarachnoid hemorrhage during pregnancy, delivery, and the puerperium in the Utrecht population: case-crossover study and standardized incidence ratio estimation. Stroke 2009;40:1148–1151 [DOI] [PubMed] [Google Scholar]
- 7.James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol 2005;106:509–516 [DOI] [PubMed] [Google Scholar]
- 8.Bateman BT, Schumacher HC, Bushnell CD, et al. Intracerebral hemorrhage in pregnancy: frequency, risk factors, and outcome. Neurology 2006;67:424–429 [DOI] [PubMed] [Google Scholar]
- 9.Horton JC, Chambers WA, Lyons SL, et al. Pregnancy and the risk of hemorrhage from cerebral arteriovenous malformations. Neurosurgery 1990;27:867–871 [DOI] [PubMed] [Google Scholar]
- 10.Uchide K, Terada S, Akasofu K, et al. Cerebral arteriovenous malformations in a pregnancy with twins: case report. Neurosurgery 1992;31:780–782 [DOI] [PubMed] [Google Scholar]
- 11.Gross BA, Du R. Hemorrhage from arteriovenous malformations during pregnancy. Neurosurgery 2012;71:349–355 [DOI] [PubMed] [Google Scholar]
- 12.Qiu X, He J, Qiu L, Larson CP, Xia H, Lam KB. Willingness of pregnant women to participate in a birth cohort study in China. Int J Gynaecol Obstet 2013;122:216–218 [DOI] [PubMed] [Google Scholar]
- 13.Sturiale CL, Gatto I, Puca A, et al. Association between the rs1333040 polymorphism on the chromosomal 9p21 locus and sporadic brain arteriovenous malformations. J Neurol Neurosurg Psychiatry 2013;84:1059–1062 [DOI] [PubMed] [Google Scholar]
- 14.Young WL, Yang GY. Are there genetic influences on sporadic brain arteriovenous malformations? Stroke 2004;35:2740–2745 [DOI] [PubMed] [Google Scholar]
- 15.Hademenos GJ, Alberts MJ, Awad I, et al. Advances in the genetics of cerebrovascular disease and stroke. Neurology 2001;56:997–1008 [DOI] [PubMed] [Google Scholar]
- 16.Nishida T, Faughnan ME, Krings T, et al. Brain arteriovenous malformations associated with hereditary hemorrhagic telangiectasia: gene-phenotype correlations. Am J Med Genet 2012;158:2829–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology, 3rd ed Philadelphia: Lippincott Williams & Wilkins; 2008 [Google Scholar]
- 18.Brown RD, Wiebers DO, Forbes G, et al. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg 1988;68:352–357 [DOI] [PubMed] [Google Scholar]
- 19.Ondra SL, Troupp H, George ED, Schwab K. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg 1990;73:387–391 [DOI] [PubMed] [Google Scholar]
- 20.da Costa L, Wallace MC, Ter Brugge KG, O'Kelly C, Willinsky RA, Tymianski M. The natural history and predictive features of hemorrhage from brain arteriovenous malformations. Stroke 2009;40:100–105 [DOI] [PubMed] [Google Scholar]
- 21.Stapf C, Mast H, Sciacca RR, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology 2006;66:1350–1355 [DOI] [PubMed] [Google Scholar]
- 22.Liu XJ, Wang S, Zhao YL, Zhang D, Zhao JZ. A single-center study of hemorrhagic stroke caused by cerebrovascular disease during pregnancy and puerperium in China. Int J Gynaecol Obstet 2011;113:82–83 [DOI] [PubMed] [Google Scholar]
- 23.Bevan H, Sharma K, Bradley W. Stroke in young adults. Stroke 1990;21:382–386 [DOI] [PubMed] [Google Scholar]
- 24.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg 1986;65:476–483 [DOI] [PubMed] [Google Scholar]
- 25.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol 1991;133:144–153 [DOI] [PubMed] [Google Scholar]
- 26.Marshman LA, Rai MS, Aspoas AR. Comment to “Endovascular treatment of ruptured intracranial aneurysms during pregnancy: report of three cases”. Arch Gynecol Obstet 2005;272:93. [DOI] [PubMed] [Google Scholar]
- 27.Le TT, Haskal ZJ, Holland GA, et al. Endovascular stent placement and magnetic resonance angiography for management of hypertension and renal artery occlusion during pregnancy. Obstet Gynecol 1995;85:822–825 [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Ramos L, Chami YG, Bass TA, et al. Myocardial infarction during pregnancy: management with transluminal coronary angioplasty and metallic intracoronary stents. Am J Obstet Gynecol 1994;171:1392–1393 [DOI] [PubMed] [Google Scholar]
- 29.Cynthia H, McCollough CH, Schueler BA, et al. Radiation exposure and pregnancy: when should we be concerned? Radiographics 2007;27:909–917 [DOI] [PubMed] [Google Scholar]
- 30.Trivedi RA, Kirkpatrick PJ. Arteriovenous malformations of the cerebral circulation that rupture in pregnancy. J Obstet Gynaecol 2003;23:484–489 [DOI] [PubMed] [Google Scholar]