Abstract
A majority of patients with neuromyelitis optica (NMO) spectrum disorders (NMOSD) have MRI brain abnormalities, some of which are “NMO-typical” with localization in aquaporin 4 (AQP4)–rich circumventricular and periaqueductal regions.1 Although uncommon in adult patients, symptomatic brain involvement occurs in approximately 50% of NMO–immunoglobulin G (IgG) seropositive children. Here we report the clinical characteristics, type, and frequency of hydrocephalus in NMOSD.
A majority of patients with neuromyelitis optica (NMO) spectrum disorders (NMOSD) have MRI brain abnormalities, some of which are “NMO-typical” with localization in aquaporin 4 (AQP4)–rich circumventricular and periaqueductal regions.1 Although uncommon in adult patients, symptomatic brain involvement occurs in approximately 50% of NMO–immunoglobulin G (IgG) seropositive children. Here we report the clinical characteristics, type, and frequency of hydrocephalus in NMOSD.
Methods.
Obstructive hydrocephalus was identified in the index case. Head MRIs from AQP4-IgG-seropositive patients in the Mayo Clinic NMO database (125 NMO; 45 NMOSD) were reviewed.
Standard protocol approvals, registrations, and patient consents.
The study protocol was approved by the Mayo Clinic Institutional Review Board.
Results.
Index case.
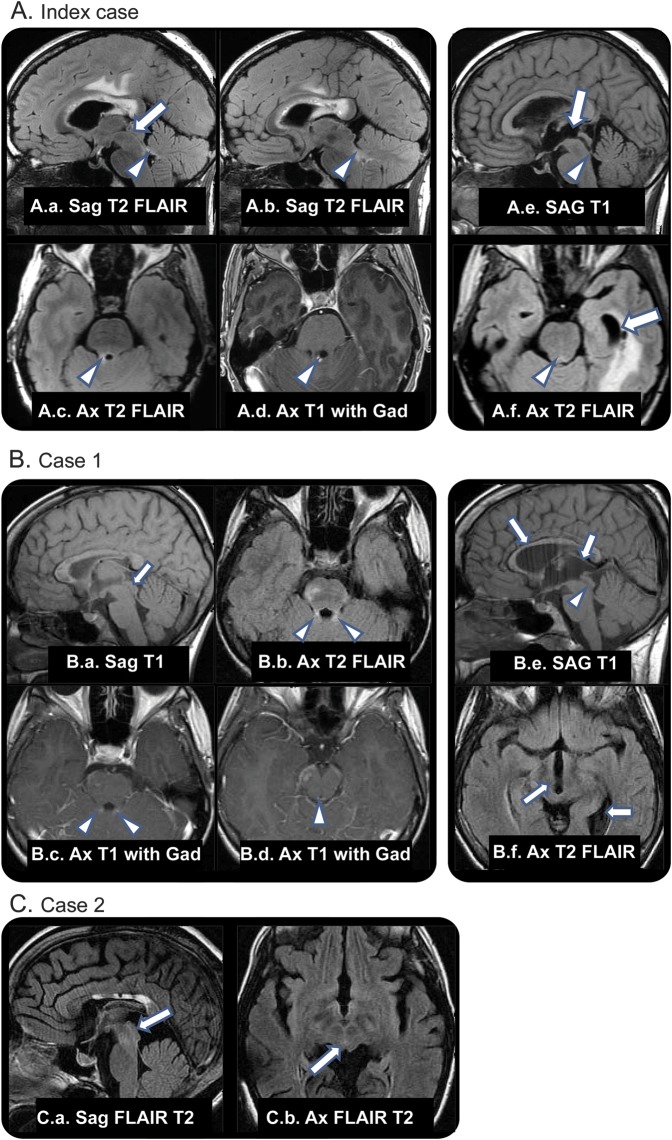
A 40-year-old woman developed right-sided headache, numbness, episodic diplopia, and word-finding difficulties 2 months postpartum. Brain MRI revealed multifocal areas of signal abnormality (figure, index case, A.a–A.d). Parietal lobe biopsy showed demyelination. Eighteen months later, headache, nausea, and vomiting began subacutely. MRI demonstrated obstructive hydrocephalus (figure, index case, A.e and A.f). She required multiple shunt revisions over subsequent years, in the setting of longitudinally extensive transverse myelitis (LETM), encephalopathy/status epilepticus (with multifocal brain and cord enhancement), recurrent sepsis, and ventriculitis. Poor interventricular CSF communication and noncompliant ventricles necessitated bilateral shunt insertion into lateral and temporal horns.
Figure. Cases.
(A.a–A.d) Neuromyelitis optica (NMO) lesions with normal aqueduct. (A.a) Normal caliber aqueduct (arrow). (A.a–A.d) T2 signal and gadolinium enhancement in NMO lesion of right superior cerebellar peduncle (arrowheads). (A.e, A.f) Eighteen months later, dilation of proximal aqueduct, third ventricle, and temporal horns (arrows) with stenosis or occlusion of distal aqueductal orifice (arrowheads). (B) Case 1. Chronic and enhancing NMO lesions, no ventricular obstruction. (B.a) Normal caliber aqueduct (arrow), chronic NMO lesions in corpus callosum. (B.b–B.d) T2 signal and enhancement in superior cerebellar peduncles and across the roof of the aqueduct (arrowheads). Additional enhancing focus in right cerebral peduncle. Thirty-two months later, symptomatic aqueductal stenosis. (B.e) Expansion of third ventricle and elevation of corpus callosum (arrows) and poor definition of aqueduct (arrowhead). (B.f) Dilation of third ventricle and expanded left ventricular atrium (arrows). (C) Case 2. MRI captured 11 years after initial shunt placement (earlier images were discarded). The absence of cerebral aqueduct within a small but otherwise normally developed mesencephalon supports the diagnosis of acquired aqueduct obliteration. (C.a, C.b) Absence of CSF flow void in expected location of aqueduct (arrows). Chronic changes in corpus callosum from NMO lesions. FLAIR = fluid-attenuated inversion recovery.
Two additional patients.
Noncommunicating obstructive hydrocephalus was identified in 1.1% of seropositive NMOSD patients (2 of 177).
Case 1.
A 19-year-old woman with an 11-year history of NMO with brain involvement (figure, case 1, B.a–B.d) had onset of increasingly severe headaches, nausea, and vertigo in 1 week.
MRI was consistent with noncommunicating hydrocephalus (figure, case 1, B.e and B.f). A ventriculoperitoneal shunt was placed. At most recent follow-up (age 23), the patient had undergone successful third ventriculostomy.
Case 2.
A 36-year-old woman had onset of sudden neck and shoulder pain, confusion, bilateral optic neuritis, and quadriparesis. MRI revealed LETM and hydrocephalus (figure, case 2, C.a and C.b). She was treated with steroids and a shunt was placed 5 months after initial symptom onset. LETM recurred in the subsequent 18 years.
Discussion.
The 1% frequency of obstructive hydrocephalus we observed in patients with NMOSD is far greater than in the general adult population. Larger studies will be required to confirm that this observation is not incidental. The incidence of all types of hydrocephalus, annual numbers of new ventricular shunts recorded in the Nationwide Inpatient Sample database and the Californian population, is 2.95 and 5.5 per 100,000 respectively.2,3 Only 16.6% were for obstructive/noncommunicating hydrocephalus.2
We are aware of only one report (abstract) of hydrocephalus (type not specified) related to NMO in a 54-year-old seropositive patient who presented with seizure.4
The cerebral aqueduct, an anatomic bottleneck of the ventricular system, is lined by ependymal cells that express AQP4. We postulate that inflammatory sequelae of IgG binding to AQP4 in this region causes scarring, occlusion, stenosis, or reduced compliance of the aqueductal channel leading to obstruction.
More widespread involvement of AQP4 at ependymal/meningeal surfaces might exacerbate obstructive hydrocephalus through an effect on water transport. A Western blot study comparing human hydrocephalic with control brain tissue showed an increased APQ4 immunoreactivity in hydrocephalic brain.5 Because AQP4 is a bidirectional channel, its lack slows the rate of water entry into the brain in cytotoxic edema but reduces the rate of water outflow from the brain in vasogenic edema. In a rat model of communicating hydrocephalus, the AQP4 profile appeared to adapt to the severity of hydrocephalus, spreading beyond the astrocytic endfeet to the whole astrocytic plasma membrane in rats with the most severe, chronic hydrocephalus.
The sites and mechanisms of CSF resorption are relevant to the pathophysiology of hydrocephalus. It was traditionally held that CSF resorption occurred mainly via arachnoid granulations, but increasing evidence points toward other sites of drainage. Studies by Iliff et al.6 indicate the existence of a brain-wide pathway facilitating exchange of CSF and interstitial fluid via para-arterial CSF influx, para-venous interstitial clearance, and a transparenchymal pathway dependent on water transport through astrocytic AQP4 channels. A recent report that AQP4 protein is significantly elevated in the CSF of infant patients with congenital communicating hydrocephalus compared to controls concluded that AQP4 effects ependymal stability and is involved in CSF production and reabsorption.7 An AQP4-dependent mechanism could facilitate resorption of CSF and clearance from the parenchyma into the microvasculature. In a state of subacutely acquired AQP4 dysfunction, as pertains in NMO, altered CSF resorption could further exacerbate hydrocephalus through a nonobstructive mechanism.
Footnotes
Author contributions: Study design and conceptualization: S.L.C., C.F.L., S.J.P. Drafting of manuscript: S.L.C., S.J.P. Acquisition, analysis, and interpretation of data: S.L.C., O.O., B.G.W., C.F.L., K.K., C.D.B., S.K., C.M., S.J.P. Critical revision of the manuscript: C.F.L., K.N.K., V.A.L., S.J.P. Obtained funding: S.J.P.
Study funding: Supported by the NIH (R01 NS065829) and the Guthy Jackson Foundation.
Disclosure: S. Clardy reports no disclosures relevant to the manuscript. C. Lucchinetti shares in royalties from marketing of kits for detecting AQP4 autoantibody and from the sale of Blue Books of Neurology: Multiple Sclerosis 3 (Saunders Elsevier, 2010); and receives research support from the NIH (RO1-NS49577), the Guthy-Jackson Charitable Foundation, and the National Multiple Sclerosis Society (RG 3185-B-3). K. Krecke reports no disclosures relevant to the manuscript. V. Lennon is a named inventor on two patent applications filed by Mayo Foundation for Medical Education and Research that relate to aquaporin-4 (AQP4) autoantibody and its application to cancer and functional assays for its detection. She shares in royalties from marketing of kits for detecting AQP4-IgG. Royalties received to date by Dr. Lennon and Mayo Clinic exceed the federal threshold for significant financial interest. Serologic testing for neural autoantibodies is offered on a service basis by Mayo Collaborative Service, Inc., an agency of Mayo Foundation. Neither Dr. Lennon nor her laboratory benefits financially from this testing. Dr. Lennon has received research support from the Guthy-Jackson Charitable Foundation and the NIH (R01-DK71209, P01-DK068055, and R01-NS065829). O. O'Toole reports no disclosures relevant to the manuscript. B. Weinshenker serves on data safety monitoring boards for Novartis, Biogen Idec, and Mitsubishi Pharmaceuticals; serves on the editorial boards of the Canadian Journal of Neurological Sciences and the Turkish Journal of Neurology; and receives license royalties (<$5,000 to date) from RSR Ltd. for marketing of kits for the detection of AQP4 antibodies as a diagnostic aid for neuromyelitis optica. He has received consulting fees from Asahi Kasei Medical Company, GlaxoSmithKline, Ono Pharmaceuticals, CHORD Pharmaceuticals, and Elan Pharmaceuticals. C. Boyd, S. Krieger, C. McGraw, and Y. Guo report no disclosures relevant to the manuscript. S. Pittock has received no royalties to date but may accrue revenue for patents relating to AQP4 antibodies for diagnosis of neuromyelitis optica and AQP4 autoantibody as a cancer marker. He has received research support from the Guthy-Jackson Charitable Foundation, Alexion Pharmaceuticals, Inc., and the National Institutes of Health (R01-NS065829). Dr. Pittock has provided consultation to Alexion Pharmaceuticals, Medimmune, and Chugai Pharma USA but has received no personal fees or personal compensation for these consulting activities. All compensation for consulting activities is paid directly to Mayo Clinic. Go to Neurology.org for full disclosures.
References
- 1.Pittock SJ, Weinshenker BG, Lucchinetti CF, Wingerchuk DM, Corboy JR, Lennon VA. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol 2006;63:964–968 [DOI] [PubMed] [Google Scholar]
- 2.Patwardhan RV, Nanda A. Implanted ventricular shunts in the United States: the billion-dollar-a-year cost of hydrocephalus treatment. Neurosurgery 2005;56:139–144; discussion 144–135 [DOI] [PubMed] [Google Scholar]
- 3.Wu Y, Green NL, Wrensch MR, Zhao S, Gupta N. Ventriculoperitoneal shunt complications in California: 1990 to 2000. Neurosurgery 2007;61:557–562; discussion 562–553 [DOI] [PubMed] [Google Scholar]
- 4.Gratton S, Mora C. Unexplained hydrocephalus in a patient with neuromyelitis optica. In: North American Neuro-Ophthalmology Society annual meeting, San Antonio, 2013 [Google Scholar]
- 5.Skjolding AD, Holst AV, Broholm H, Laursen H, Juhler M. Differences in distribution and regulation of astrocytic aquaporin-4 in human and rat hydrocephalic brain. Neuropathol Appl Neurobiol 2013;39:179–191 [DOI] [PubMed] [Google Scholar]
- 6.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 2012;4:147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castaneyra-Ruiz L, Gonzalez-Marrero I, Gonzales-Toledo JM, et al. Aquaporin-4 expression in the cerebrospinal fluid in congenital human hydrocephalus. Fluids Barriers CNS 2013;18:10–18 [DOI] [PMC free article] [PubMed] [Google Scholar]