Abstract
Objective:
To compare the clinical and imaging characteristics of those PRECEPT (Parkinson Research Examination of CEP-1347 Trial) subjects with a scan without evidence of dopaminergic deficit (SWEDD) to those with dopamine transporter (DAT) deficit scans at study baseline and during a 22-month follow-up.
Methods:
Baseline (n = 799) and 22-month follow-up (n = 701) [123I] β-CIT SPECT scans were acquired. The percent change in [123I] β-CIT striatal binding ratio, the percentage of subjects requiring dopaminergic therapy, the change in Unified Parkinson's Disease Rating Scale (UPDRS) score, and the PRECEPT Study investigators’ diagnosis at study termination were compared between SWEDD and DAT deficit subjects.
Results:
SWEDD subjects (n = 91) compared with DAT deficit subjects (n = 708) showed reduced UPDRS score at baseline (18.7 [SD 8.5] vs 25.5 [SD 10.5], p < 0.05) and minimal change in both [123I] β-CIT striatal binding ratio (−0.2% [SD 12.2] vs −8.5% [SD 11.9], p < 0.0001) and UPDRS score (0.5 [SD 6.9] vs 10.5 [SD 8.9], p < 0.0001) at follow-up assessments. At PRECEPT termination, the diagnosis by study investigators was changed from Parkinson disease (PD) to other disorders not associated with DAT deficit in 44% (95% confidence interval 34.2, 54.7) of SWEDD subjects compared with 3.6% (95% confidence interval 2.3, 5.1) of DAT deficit subjects.
Conclusion:
These results indicate that subjects identified as having a SWEDD, with DAT imaging within the normal range, have minimal evidence of clinical or imaging PD progression. These data strongly suggest that SWEDD subjects are unlikely to have idiopathic PD.
In several recent Parkinson disease (PD) clinical trials, dopaminergic imaging has been used as a tool to assess PD progression. Both dopamine transporter (DAT) SPECT and [18F] fluoro-l-dopa PET imaging identified research subjects having a scan without evidence of dopaminergic deficit (SWEDD) enrolled in PD clinical trials of newly diagnosed untreated patients.1–4 In PD studies enrolling participants within 9 months of PD diagnosis, approximately 10% to 15% of the research subjects have a SWEDD. Longitudinal assessment has demonstrated that those subjects with a SWEDD at baseline continue to have a SWEDD in follow-up scans for at least a 4-year period.5
The Parkinson Research Examination of CEP-1347 Trial (PRECEPT) was a multicenter, randomized, double-masked, placebo-controlled study to examine the efficacy and long-term safety of CEP-1347 as a potential disease-modifying treatment in patients with early PD.3 The primary clinical endpoint for PRECEPT was prespecified as the time to the development of disability requiring dopaminergic therapy. Secondary endpoints included the change from baseline to 22 months in [123I] β-CIT SPECT imaging targeting striatal DATs. Of the 806 subjects enrolled in the PRECEPT Study, 799 underwent [123I] β-CIT imaging at baseline. Approximately 11% of the imaged subjects enrolled in PRECEPT had a SWEDD at baseline, the largest and best clinically characterized group of study subjects with a SWEDD. In this report, we compare the baseline and follow-up clinical and imaging characteristics of those PRECEPT subjects with a SWEDD to those with scans showing a DAT deficit.
METHODS
PRECEPT subjects were enrolled at 65 participating Parkinson Study Group (PSG) research sites (www.parkinson-study-group.org). Eligible patients included those who at the time of PD diagnosis were older than 30 years and who at the screening and baseline evaluations had at least 2 of the cardinal signs of PD (resting tremor, bradykinesia, rigidity) and no current or imminent (in next 3 months) PD disability requiring dopaminergic therapy. Subjects were excluded from participation because of atypical or drug-induced parkinsonism, a diagnosis of PD >5 years' duration, a resting tremor score ≥3 in any limb, Mini-Mental State Examination score ≤26, or a Beck depression score ≥15.
Subjects were randomized equally to CEP-1347 10 mg, 25 mg, or 50 mg, all twice a day, or matching placebo, and evaluated blindly and prospectively. At study baseline, subjects were imaged with [123I] β-CIT at the Institute for Neurodegenerative Disorders in New Haven, CT, in a standardized manner using previously described image acquisition and processing.6,7 Repeat [123I] β-CIT imaging was planned for all subjects after 22 months of follow-up, and the predefined neuroimaging outcome was the percent change from baseline in the [123I] β-CIT striatal binding ratio.
Based on a previously acquired database of 100 healthy subjects, baseline scans were categorized as DAT deficient (≤80% age-expected lowest putamen [123I] β-CIT), or not DAT deficient (>80% age-expected lowest putamen [123I] β-CIT).8,9 Subjects with scans without evidence of DAT deficit were denoted as SWEDD subjects. The neuroimaging personnel were blinded to treatment assignment and to all clinical information. [123I] β-CIT SPECT scan results were transferred to and analyzed by the PSG Biostatistics Center in Rochester, NY.
The PRECEPT Study was terminated after an average of 21 months’ follow-up when a planned interim analysis showed that it would be futile to continue study treatment. All subjects who had not yet undergone their planned 22-month [123I] β-CIT SPECT scan were contacted to determine their willingness to undergo repeat imaging. The individuals recruiting PRECEPT subjects for imaging follow-up, the site investigators, and the subjects themselves were unaware of the results of the baseline imaging study at the 22-month scan.
Standard protocol approvals, registrations, and patient consents.
Institutional review board human subject approval was obtained for all study assessments, and written informed consent was obtained before any study activity. The study was registered at clinicaltrials.gov: NCT00605163.
Statistical analysis.
Baseline characteristics for the SWEDD and DAT deficit groups were compared using χ2 tests, Fisher exact tests, or Kruskal-Wallis tests, as appropriate. Kruskal-Wallis tests were also used to compare the 2 groups regarding change in Unified Parkinson's Disease Rating Scale (UPDRS) score from baseline to the final PRECEPT visit and percent change in scan measures from the baseline scan to the 22-month follow-up scan. Analysis of variance was used for the 3-group comparisons of these changes. The method of Kaplan and Meier was used to estimate the probability of reaching the endpoint of disability sufficient to require dopaminergic therapy. All p values are 2-sided.
RESULTS
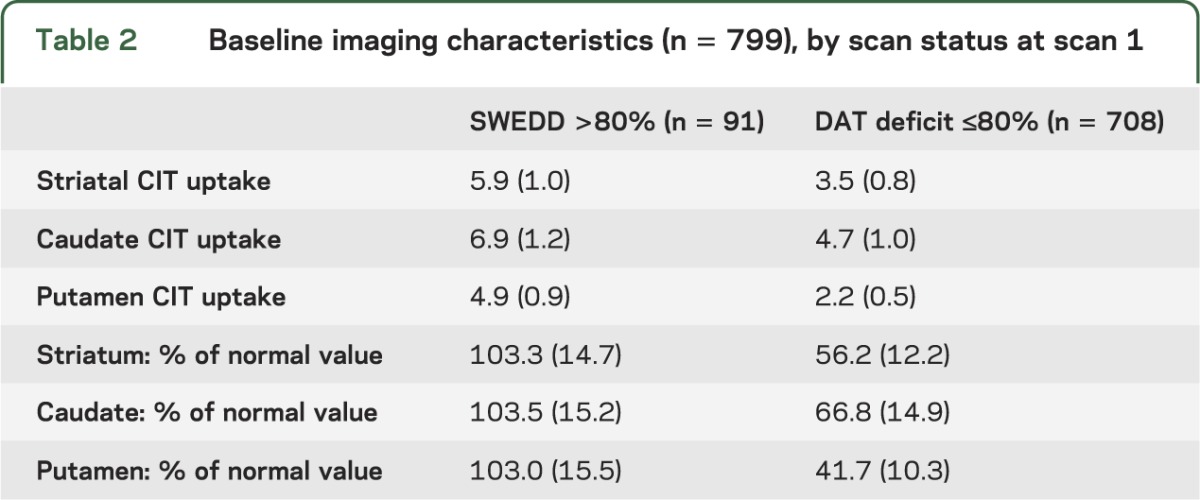
A total of 806 patients diagnosed with early PD were enrolled in the PRECEPT Study at 65 participating PSG research sites (679 subjects at 56 sites in the United States and 127 subjects at 9 sites in Canada). Baseline [123I] β-CIT SPECT scans were acquired in 799 subjects. At baseline, 91 subjects had [123I] β-CIT SPECT scans demonstrating lowest putamen DAT density >80% of age-expected compared with a previously acquired healthy subject database. Comparisons of the baseline characteristics of subjects with a SWEDD to those with scans with DAT deficit (table 1) show a lower proportion of male participants and a lower baseline UPDRS score, including a lower maximal tremor score, among SWEDD subjects. In the 708 DAT deficit subjects imaged at a mean of 0.8 years after diagnosis, [123I] β-CIT putamen uptake was reduced to 41.7% of age-expected normal value (table 2), whereas putamen uptake was essentially normal in SWEDD subjects.
Table 1.
Baseline clinical characteristics (n = 799), by scan status at scan 1
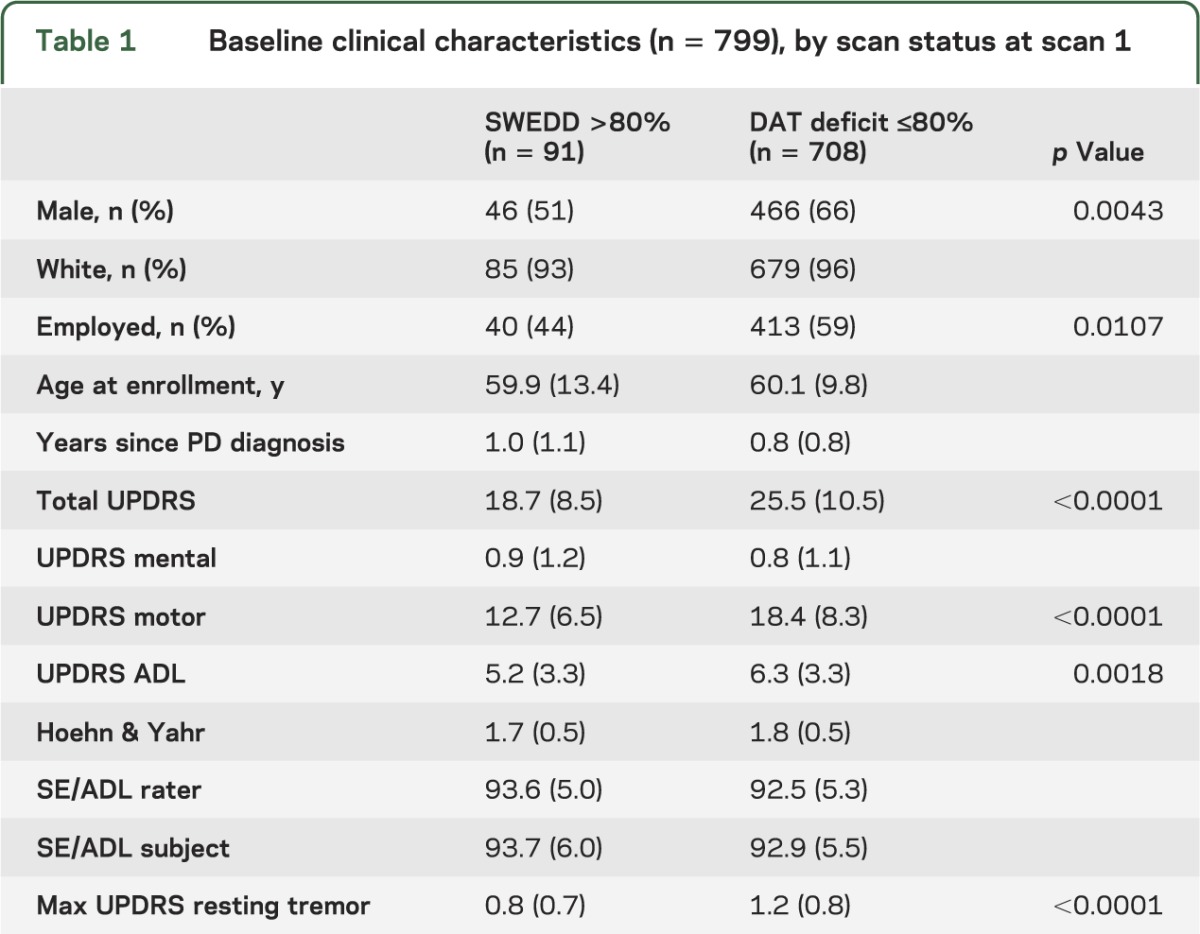
Table 2.
Baseline imaging characteristics (n = 799), by scan status at scan 1
Of the 65 participating clinical sites in PRECEPT, 41 recruited at least one SWEDD subject. Enrollment in these 41 sites showed 14 sites with one SWEDD subject, 16 sites with 2 SWEDD subjects, 4 sites with 3 SWEDD subjects, and 7 sites with 4 or more SWEDD subjects. The mean number of subjects recruited per site was 12.3 (range 4–29). Enrollment of SWEDD subjects occurred throughout the enrollment phase of the study.
Twenty-two–month follow-up.
After an average follow-up of 21.4 months, the independent PRECEPT data monitoring committee reported that a planned interim analysis had reached the prespecified criterion for futility and the PRECEPT Study was terminated. Of the 799 subjects with baseline [123I] β-CIT SPECT, 400 subjects had undergone repeat [123I] β-CIT scans after about 22 months of study medication. After PRECEPT Study termination, an additional 301 subjects underwent repeat [123I] β-CIT scans at about 22 months from baseline, but these subjects had discontinued PRECEPT study medication. The remaining 98 subjects had only a baseline scan.
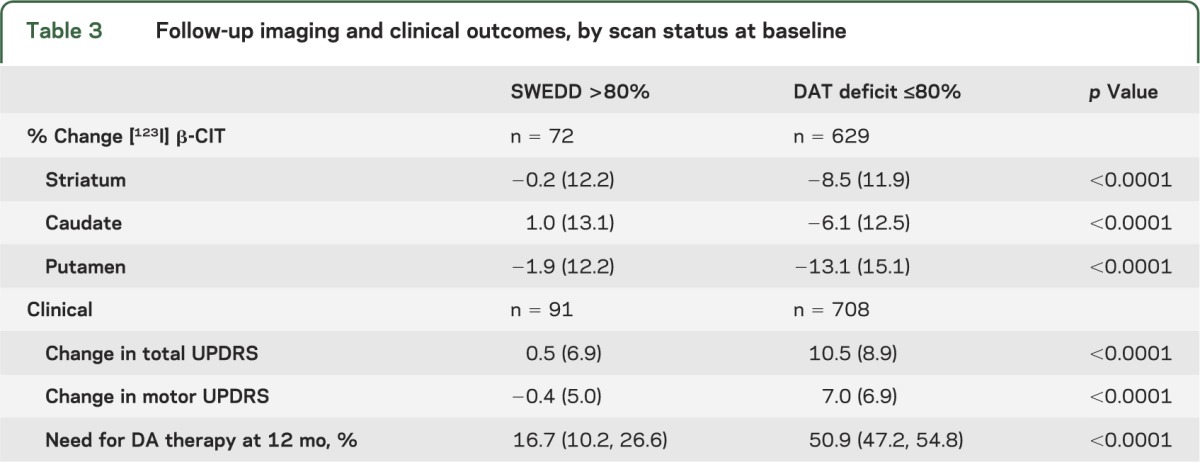
Of these 701 subjects who underwent a baseline and 22-month follow-up [123I] β-CIT SPECT scan, 72 had a baseline SWEDD and 629 a baseline DAT deficit scan. The percent change from baseline in striatal [123I] β-CIT uptake at 22 months was −0.2% (SD 12.2) in subjects with a baseline SWEDD, compared with −8.5% (SD 11.9) in subjects with a baseline DAT deficit scan (p < 0.0001) (table 3). Among the 72 SWEDD subjects, 66 remained SWEDD at 22 months. Of those 6 SWEDD subjects who switched categories, 4 had a 22-month scan in the indeterminate range (65%–80% of age-expected putamen). Among the 629 DAT deficit subjects, 626 remained DAT deficit at 22 months. Of those 3 subjects with DAT deficit scans who switched categories, one had a baseline scan in the indeterminate range (65%–80% of age-expected putamen).
Table 3.
Follow-up imaging and clinical outcomes, by scan status at baseline
Clinical outcomes.
The primary clinical endpoint for PRECEPT was the time to the development of disability requiring dopaminergic therapy. The figure plots the probability of reaching the endpoint during the PRECEPT study period. The probability of reaching the endpoint after 12 months’ follow-up for those subjects with a baseline SWEDD was 16.7% (95% confidence interval [CI] 10.2, 26.6), compared with 50.9% (95% CI 47.2, 54.8) in subjects with a baseline DAT deficit scan.
Figure. Need for dopaminergic therapy in SWEDD and DAT deficit subjects.
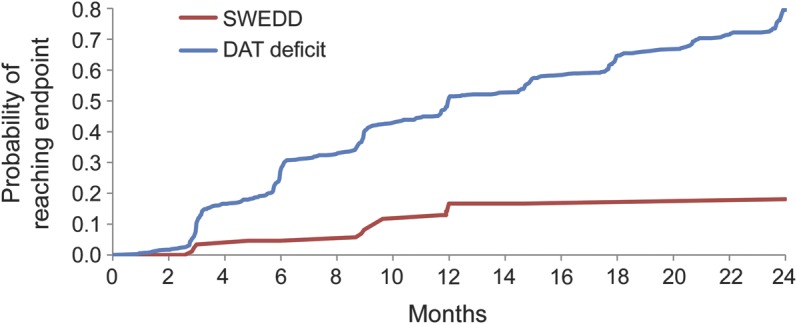
Kaplan-Meier curve showing the probability of disability requiring dopaminergic therapy in SWEDD and DAT deficit subjects during the PRECEPT Study. DAT = dopamine transporter; PRECEPT = Parkinson Research Examination of CEP-1347 Trial; SWEDD = scan without evidence of dopaminergic deficit.
The mean change in total UPDRS score (parts I, II, and III at 12 hours defined off) from baseline to either primary clinical endpoint or study completion or termination (in non-endpointers) was 0.5 (SD 6.9) in those subjects with a baseline SWEDD, while the mean change in UPDRS score in subjects with a baseline DAT deficit scan was 10.5 (SD 8.9) (table 3). The change in motor UPDRS score was also reduced in SWEDD subjects compared with DAT deficit subjects.
Subjects were further compared at their 22-month scans by whether they were treated with dopaminergic medications. Based on scan status at their 22-month scans, 13% of subjects (9/69) with a SWEDD were undergoing dopaminergic treatment, compared with 68% (428/632) of DAT deficit subjects.
Diagnosis.
At study termination, the PRECEPT investigators at the clinical sites were asked to complete a questionnaire identifying the likely diagnosis of the subjects enrolled at their site (table 4). The investigators were asked to provide their best diagnosis based on all available clinical data but had no access to the imaging data. The possible diagnoses listed at study termination can be divided into 2 groups: (1) degenerative parkinsonian syndrome with loss of neurons in the substantia nigra compacta expected to result in a striatal DAT deficit, termed here DAT deficit parkinsonism (DDP) (PD, progressive supranuclear palsy, multiple system atrophy, corticobasal ganglionic degeneration, Lewy body disease, hemiparkinson syndrome, and juvenile parkinsonism); and (2) pseudo-parkinsonian (PP) syndrome unlikely to have a DAT deficit (essential tremor, dystonic tremor, vascular parkinsonism, psychogenic illness, Alzheimer disease, dopa-responsive dystonia, other unspecified neurologic illness, or no neurologic disorder).10–13 Comparison of diagnoses in those subjects with a baseline [123I] β-CIT SPECT SWEDD to subjects with a baseline DAT deficit scan demonstrated that 44.4% (40/90) (95% CI 34.2, 54.7) of SWEDD subjects and 3.7% (26/707) (95% CI 2.3, 5.1) of subjects with DAT deficit were given clinical diagnoses at study termination consistent with PP.
Table 4.
Diagnosis at study termination, by scan status at scan 1
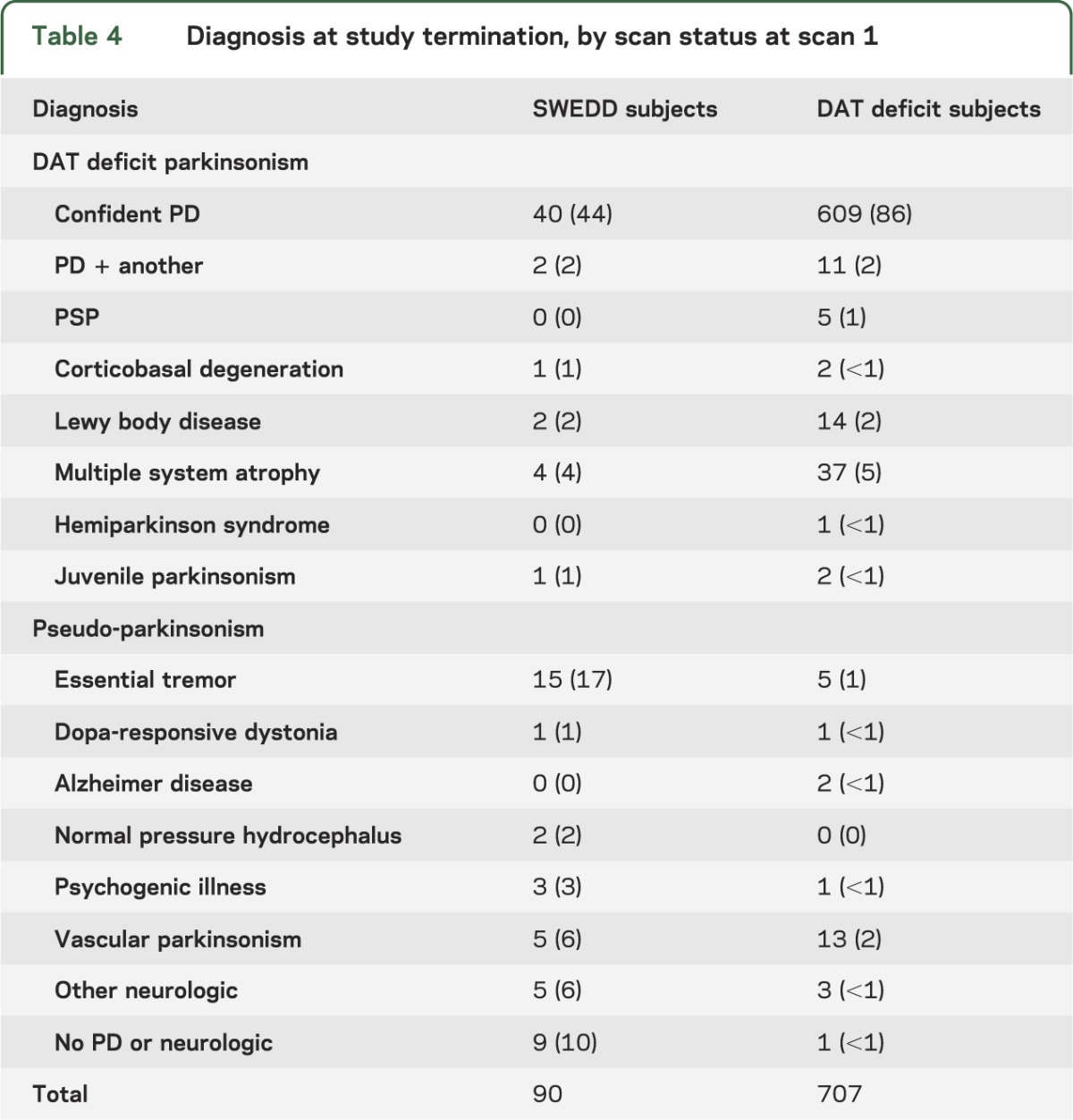
There were no differences in baseline clinical or imaging characteristics among the SWEDD subjects with PP at PRECEPT Study termination (n = 40) compared with those with a DDP diagnosis at PRECEPT Study termination (n = 50) except for an increase in resting tremor among those with a DDP diagnosis compared with a PP diagnosis (mean maximum UPDRS score resting tremor 1.0 [SD 0.7] vs 0.6 [SD 0.6], p < 0.005). Among SWEDD subjects with follow-up scans, while 6 of 40 DDP subjects changed to either indeterminate (n = 4) or DAT deficit (n = 2), none of 32 PP subjects changed status. Comparison of follow-up striatal imaging outcomes showed no significant differences (change from baseline of −1.3 [SD 13.1] vs 1 [SD 11.1]), but the change in total UPDRS score was significantly greater in the DDP group (2.2 [SD 6.4] vs −1.7 [SD 6.9], p < 0.05). The proportion of subjects needing dopaminergic therapy was higher in the DDP group, but this comparison did not reach statistical significance (p = 0.11). Despite the minor differences between the DDP and PP groups, the change in striatal [123I] β-CIT uptake, the change in UPDRS score, and the probability of development of disability requiring dopaminergic therapy were significantly different for both the DDP and PP SWEDD groups compared with the DAT deficit group (p < 0.001 for each of the 6 comparisons).
DISCUSSION
The PRECEPT cohort was utilized in this study to compare baseline and 22-month follow-up of clinical and imaging outcomes in subjects with [123I] β-CIT SPECT scans without evidence of dopamine deficit (SWEDD subjects) to those subjects with scans showing a dopamine deficit. These data demonstrate that both at baseline and with 22-month follow-up, the PRECEPT SWEDD subjects were markedly different from the DAT deficit group regarding both clinical and imaging characteristics. SWEDD subjects showed minimal change in [123I] β-CIT uptake during the 22-month follow-up, consistent with the rate of change previously seen among healthy subjects.14–16 UPDRS scores at baseline were considerably lower in SWEDD subjects compared with DAT deficit subjects, and there was no change in UPDRS scores during the 22-month follow-up in SWEDD subjects, compared with an increase in DAT deficit subjects consistent with other clinical studies of newly diagnosed PD.2,17–19 Subjects with a baseline SWEDD showed an equal sex distribution, rather than the expected male predominance in PD evident in most PD clinical studies. The percentage of subjects reaching the PRECEPT Study endpoint of disability requiring dopaminergic therapy was reduced in those subjects with a SWEDD compared with DAT deficit subjects. Finally, the proportion of SWEDD subjects treated with dopaminergic medication at their 22-month scan was markedly lower than for DAT deficit subjects. In summary, these data indicate that subjects identified as SWEDD, with DAT imaging within the normal range, have minimal evidence of clinical or imaging PD progression. While these data are not definitive, they strongly suggest that SWEDD subjects are unlikely to have idiopathic PD.
If SWEDD subjects do not have PD, what is their diagnosis? This question was partially answered by the site investigators in PRECEPT, who were asked to select their best clinical diagnosis at study termination from a list including PD and common alternatives. All subjects were required to have a clinical diagnosis of PD at enrollment. The possible diagnoses at study termination can be divided into DDP (those expected to have a striatal DAT deficit) and PP (those unlikely to have a DAT deficit). Among the SWEDD subjects, 40 of 90 subjects received revised diagnoses that would be consistent with PP. Essential tremor (n = 15) and no neurologic illness (n = 9) were the most common diagnoses among the SWEDD subjects with revised diagnoses at termination, but no single diagnosis was predominant. Among the SWEDD subjects with DDP, 6 of 50 subjects changed from SWEDD to either indeterminate (n = 4) or DAT deficit (n = 2), suggesting that these subjects might be on a trajectory of reduced DAT.
Comparison of SWEDD subjects diagnosed at PRECEPT termination with DDP and PP may provide some insight into the diagnostic rationale of the investigators. Regardless of final diagnosis, the SWEDD subjects had similar baseline clinical and imaging characteristics except for an increase in resting tremor in those with a DDP diagnosis. For SWEDD subjects with follow-up scans, the clinical and imaging characteristics were also similar but showed an increased change in UPDRS score in the SWEDD subjects with DDP compared with PP diagnoses. The presence of resting tremor may have contributed to the persistent DDP diagnosis and may have resulted in the slight increase in UPDRS scores among the DDP compared with PP SWEDD subjects. It has been suggested that SWEDD subjects may have a dystonic tremor masquerading as PD, but it is uncertain whether the tremor in any of the PRECEPT subjects had those characteristics.20,21
The change in diagnosis from idiopathic PD at baseline to a PP alternative at study termination in 44% of SWEDD subjects compared with 3.7% of those with DAT deficit [123I] β-CIT scans at baseline again demonstrates that the SWEDD group differs from those with baseline DAT deficit scans. Based on investigator report, the baseline SWEDD subjects with a final diagnosis of PP were less likely to manifest tremor, bradykinesia, and rigidity than those with a DDP diagnosis. However, SWEDD subjects with either a revised PP or a persistent DDP diagnosis at study termination showed minimal change in clinical and imaging outcomes compared with subjects with baseline [123I] β-CIT DAT deficit scans. This suggests that while SWEDD subjects may have variable clinical presentations, the baseline [123I] β-CIT imaging is highly predictive of subsequent clinical and imaging PD progression. The persistent diagnosis of DDP in 56% of SWEDD subjects at PRECEPT Study termination likely reflects the difficulty in revising diagnoses in a clinically variable and slowly progressive illness such as PD.
SWEDD subjects have been identified in several PD clinical trials using dopaminergic imaging. Because of the difficulties of making PD diagnoses, the percentage of SWEDD subjects among those enrolled depends on the duration from diagnosis at baseline of the study subjects. In both the ELLDOPA and REAL PET studies, the duration from PD diagnosis was 9 months and the percentage of SWEDD subjects was 15% (21/142) and 11% (21/186).2,21 In the CALM-PD study, the duration from PD diagnosis was 18 months and the percentage of SWEDD subjects was only 5% (4/82), while in the GPI-1485 study, duration from PD diagnosis was 24 months and the percentage of SWEDD subjects was only 1% (2/201).1,22 The SWEDD group in the PRECEPT Study is consistent with previous studies enrolling subjects with short duration from diagnosis. These data strongly suggest that the probability of enrolling SWEDD subjects increases as subjects with earlier diagnoses are included in the study. The longitudinal follow-up in the PRECEPT Study showing largely unchanging imaging diagnoses among SWEDD and DAT deficit subjects between baseline and 22 months further makes it very unlikely that SWEDD occurs due to lack of sensitivity of imaging outcomes in early PD. Evidence from other cross-sectional studies demonstrates that even at the threshold of PD symptoms, DAT imaging uptake is reduced by 50% to 60%.8,23
Finally, identification of the SWEDD subjects in the PRECEPT Study raises the practical question of whether dopaminergic imaging should be used as an inclusion criterion for early PD clinical trials. Because the study sample size required is inversely proportional to the square of the hypothesized average treatment effect, assuming a drug had no effect on SWEDD subjects, for a given study, eliminating SWEDD subjects would reduce sample size by 19% (if 10% SWEDD) and 28% (if 15% SWEDD). While imaging would likely enrich the study sample for idiopathic PD, it also adds cost, complexity, and patient burden to the trial. If there is substantial experience with the study drug demonstrating a good safety profile, it may be more desirable to conduct the study without imaging but with the understanding that some subjects who do not have PD will likely be recruited, resulting in increased sample size and time of study recruitment compared with studies with baseline imaging eligibility criterion.
As studies focus on early diagnosis of PD or even premotor diagnosis, the use of imaging in PD clinical studies becomes both more crucial and more complicated. The PRECEPT imaging data indicate that DAT imaging at study baseline is a sensitive predictor of follow-up measures of clinical and imaging progression and that those subjects with baseline scans without evidence of dopaminergic deficit are very unlikely to have PD.
Supplementary Material
GLOSSARY
- CI
confidence interval
- CIT
carbomethoxy-3-β-(4-iodophenyl)tropane
- DAT
dopamine transporter
- DDP
dopamine transporter deficit parkinsonism
- PD
Parkinson disease
- PP
pseudo-parkinsonian
- PRECEPT
Parkinson Research Examination of CEP-1347 Trial
- PSG
Parkinson Study Group
- SWEDD
scan without evidence of dopaminergic deficit
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Kenneth Marek: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, study supervision, obtaining funding. John Seibyl: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data, study supervision. Shirley Eberly: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, statistical analysis. David Oakes: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, statistical analysis. Ira Shoulson: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, obtaining funding. Anthony E. Lang: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and will give final approval, acquisition of data. Chris Hyson: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and will give final approval, acquisition of data, study supervision. Danna Jennings: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and will give final approval, acquisition of data, study supervision.
STUDY FUNDING
Supported by Cephalon, Inc. (Frazer, PA) and H. Lundbeck A/S (Copenhagen and Valby, Denmark). Supported in part by DOD W81XWH-05-1-0603 at the Institute for Neurodegenerative Disorders.
DISCLOSURE
K. Marek holds ownership interest in Molecular NeuroImaging, LLC; has served as a paid consultant for GE Healthcare, Bayer Healthcare, Eli Lilly, Sanofi, Merck Serono, AstraZeneca, Prothena, nLife, BMS, Pfizer, and Merck; and holds grants with the Department of Defense and The Michael J. Fox Foundation for Parkinson's Research. J. Seibyl reports that he holds equity interest in Molecular NeuroImaging, LLC; has served as a consultant for GE Healthcare; holds grants from the Department of Defense; and has received payment for the development of educational presentations from Bayer Healthcare. S. Eberly and D. Oakes report no disclosures relevant to the manuscript. I. Shoulson reports that he received support for travel to study meetings from Cephalon, Inc.; serves on the board as associate editor for Archives of Neurology; is a paid consultant for the Archives of Neurology, the RJG Foundation, Partners Health Care, Link Medicine, Merck Serono SA, NIH Treasury, Medtronic, Lundbeck, Inc., Salamandra, LLC, Washington University, Adolor Corporation, The Michael J. Fox Foundation for Parkinson's Research, Montefiore Medical Center, Goodwin Proctor, CSL Ltd., Prana Biotechnology, New York University, University of Tennessee, Alkermes, Duke University, Ovation, Cornell University, the American Academy of Neurology, MSI Methylation Sciences, NuPathe, Inc., Auspex Pharma, Seneb BioSciences, Inc., Isis Pharmaceuticals, Edison Pharmaceuticals, Johns Hopkins University, Johnson & Johnson, Omeros Corporation, Knopp Biosciences, LLC, Clarion Healthcare, and Polaris Venture Partners; and holds current or pending grants with the Food and Drug Administration, the Parkinson Disease Foundation, and Johns Hopkins University. A. Lang reports no disclosures relevant to the manuscript. C. Hyson reports no disclosures relevant to the manuscript. D. Jennings is an employee of Molecular NeuroImaging, LLC; has received money for a consultancy with Teva; holds and has pending grants with The Michael J. Fox Foundation for Parkinson's Research; and has received payments for lectures from the American Pharmacists Association and the Movement Disorders Society. Authors of this report from the Parkinson Study Group (PSG) received grant support from the sponsors through their academic institutions (coinvestigator appendix on the Neurology® Web site at Neurology.org), but they neither had equity interests in nor received any personal remuneration from the sponsoring companies since initiation of the study. Some authors of the report are employees of the sponsor and are so designated in the coinvestigator appendix. The PSG maintained the PRECEPT database and performed independent analysis of the data. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Parkinson Study Group. Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA 2002;287:1653–1661 [DOI] [PubMed] [Google Scholar]
- 2.Parkinson Study Group. Levodopa and the progression of Parkinson's disease. N Engl J Med 2004;351:2498–2508 [DOI] [PubMed] [Google Scholar]
- 3.Parkinson Study Group. Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology 2007;69:1480–1490 [DOI] [PubMed] [Google Scholar]
- 4.Whone AL, Watts RL, Stoessl AJ, et al. Slower progression of Parkinson's disease with ropinirole versus levodopa: the REAL-PET Study. Ann Neurol 2003;54:93–101 [DOI] [PubMed] [Google Scholar]
- 5.Marek K, Seibyl J; Parkinson Study Group. β-CIT scans without evidence of dopaminergic deficit (SWEDD) in the ELLDOPA-CIT and CALM-CIT Study: long-term imaging assessment. Neurology 2003;60(suppl 1):A298 [Google Scholar]
- 6.Seibyl JP, Marek K, Quinlan D, et al. Decreased single-photon emission computed tomographic [123I]beta-CIT striatal uptake correlates with symptom severity in Parkinson's disease. Ann Neurol 1995;38:589–598 [DOI] [PubMed] [Google Scholar]
- 7.Seibyl JP. Single-photon emission computed tomography of the dopamine transporter in parkinsonism. J Neuroimaging 1999;9:223–228 [DOI] [PubMed] [Google Scholar]
- 8.Jennings D, Seibyl JP, Oakes D, et al. [123I]β-CIT and SPECT imaging versus clinical evaluation in parkinsonian syndrome: unmasking an early diagnosis. Arch Neurol 2004;61:1219–1228 [DOI] [PubMed] [Google Scholar]
- 9.van Dyck CH, Seibyl JP, Malison RT, et al. Age-related decline in dopamine transporters: analysis of striatal subregions, nonlinear effects, and hemispheric asymmetries. Am J Geriatr Psychiatry 2002;10:36–43 [PubMed] [Google Scholar]
- 10.Marshall V, Grosset D. Role of dopamine transporter imaging in routine clinical practice. Mov Disord 2003;18:1415–1423 [DOI] [PubMed] [Google Scholar]
- 11.Parkinson Study Group. A multicenter assessment of dopamine transporter imaging with DOPASCAN/SPECT in parkinsonism. Neurology 2000;55:1540–1547 [DOI] [PubMed] [Google Scholar]
- 12.Scherfler C, Schwarz J, Antonini A, et al. Role of DAT-SPECT in the diagnostic work up of parkinsonism. Mov Disord 2007;22:1229–1238 [DOI] [PubMed] [Google Scholar]
- 13.Varrone A, Marek KL, Jennings D, et al. [(123)I]beta-CIT SPECT imaging demonstrates reduced density of striatal dopamine transporters in Parkinson's disease and multiple system atrophy. Mov Disord 2001;16:1023–1032 [DOI] [PubMed] [Google Scholar]
- 14.Marek K, Innis R, van Dyck C, et al. [123I]beta-CIT SPECT imaging assessment of the rate of Parkinson's disease progression. Neurology 2001;57:2089–2094 [DOI] [PubMed] [Google Scholar]
- 15.Morrish PK, Sawle GV, Brooks DJ. An [18F]dopa-PET and clinical study of the rate of progression in Parkinson's disease. Brain 1996;119:585–591 [DOI] [PubMed] [Google Scholar]
- 16.Nurmi E, Ruottinen HM, Kaasinen V, et al. Progression in Parkinson's disease: a positron emission tomography study with a dopamine transporter ligand [18F]CFT. Ann Neurol 2000;47:804–808 [PubMed] [Google Scholar]
- 17.Parkinson Study Group. A controlled trial of rasagiline in early Parkinson disease: the TEMPO Study. Arch Neurol 2002;59:1937–1943 [DOI] [PubMed] [Google Scholar]
- 18.Shults CW, Oakes D, Kieburtz K, et al. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol 2002;59:1541–1550 [DOI] [PubMed] [Google Scholar]
- 19.The NINDS NET-PD Investigators. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology 2006;66:664–671 [DOI] [PubMed] [Google Scholar]
- 20.Schneider SA, Edwards MJ, Mir P, et al. Patients with adult-onset dystonic tremor resembling parkinsonian tremor have scans without evidence of dopaminergic deficit (SWEDDs). Mov Disord 2007;22:2210–2215 [DOI] [PubMed] [Google Scholar]
- 21.Bajaj NP, Gontu V, Birchall J, et al. Accuracy of clinical diagnosis in tremulous parkinsonian patients: a blinded video study. J Neurol Neurosurg Psychiatry 2010;81:1223–1228 [DOI] [PubMed] [Google Scholar]
- 22.The GPI 1485 Investigators. GPI 1485, a neuroimmunophilin ligand, fails to alter disease progression in mild to moderate Parkinson's disease. Mov Disord 2006;21:S607 [Google Scholar]
- 23.Booij J, Bergmans P, Winogrodzka A, et al. Imaging of dopamine transporters with [123I]FP-CIT SPECT does not suggest a significant effect of age on the symptomatic threshold of disease in Parkinson's disease. Synapse 2001;39:101–108 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


