Abstract
Calmodulin regulation (calmodulation) of the family of voltage-gated CaV1-2 channels comprises a prominent prototype for ion channel regulation, remarkable for its powerful Ca2+ sensing capabilities, deep in elegant mechanistic lessons, and rich in biological and therapeutic implications. This field thereby resides squarely at the epicenter of Ca2+ signaling biology, ion channel biophysics, and therapeutic advance. This review summarizes the historical development of ideas in this field, the scope and richly patterned organization of Ca2+ feedback behaviors encompassed by this system, and the long-standing challenges and recent developments in discerning a molecular basis for calmodulation. We conclude by highlighting the considerable synergy between mechanism, biological insight, and promising therapeutics.
The ancient Ca2+ sensor protein calmodulin (CaM) has emerged as a pervasive modulator of ion channels (Saimi and Kung, 2002), manifesting remarkable Ca2+ sensing capabilities in this context (Dunlap, 2007; Tadross et al., 2008) and furnishing essential Ca2+ feedback in numerous biological settings (Alseikhan et al., 2002; Xu and Wu, 2005; Mahajan et al., 2008; Adams et al., 2010; Morotti et al., 2012). Such modulation was discovered via mutations in CaM that altered the motile behavior of Paramecium, stemming from blunted activation of Ca2+-dependent Na+ current or K+ current (Kink et al., 1990). Since then, numerous ion channels have been found to be modulated by CaM, as extensively reviewed (Budde et al., 2002; Saimi and Kung, 2002; Wei et al., 2003; Halling et al., 2006; Tan et al., 2011; Nyegaard et al., 2012). This paper focuses on the CaM regulation (calmodulation) of voltage-gated Ca2+ channels, which are extensively regulated by intracellular Ca2+ binding to CaM (Ca2+/CaM; Lee et al., 1999; Peterson et al., 1999; Zühlke et al., 1999; Haeseleer et al., 2000; DeMaria et al., 2001; Budde et al., 2002; Halling et al., 2006). Such feedback regulation by Ca2+/CaM demonstrates versatile functional capabilities, represents a prominent prototype for ion-channel modulation, and holds far-reaching biological consequences. These attributes contribute much to the standing of voltage-gated Ca2+ channels as the queen of ion channels, molecules that not only sculpt membrane electrical waveforms but also serve as gatekeepers of the ubiquitous second messenger Ca2+ (Yue, 2004).
Classic history of discovery
The earliest signs of Ca2+-dependent regulation of Ca2+ channels came from data showing that increased Ca2+ could accelerate the inactivation of Ca2+ currents in Paramecium (Brehm and Eckert, 1978), invertebrate neurons (Tillotson, 1979), and insect muscle (Ashcroft and Stanfield, 1981). These results gave birth to the concept of Ca2+-dependent inactivation (CDI), the then counterintuitive notion that entities other than transmembrane voltage could modulate the rapid gating of ion channels (Eckert and Tillotson, 1981).
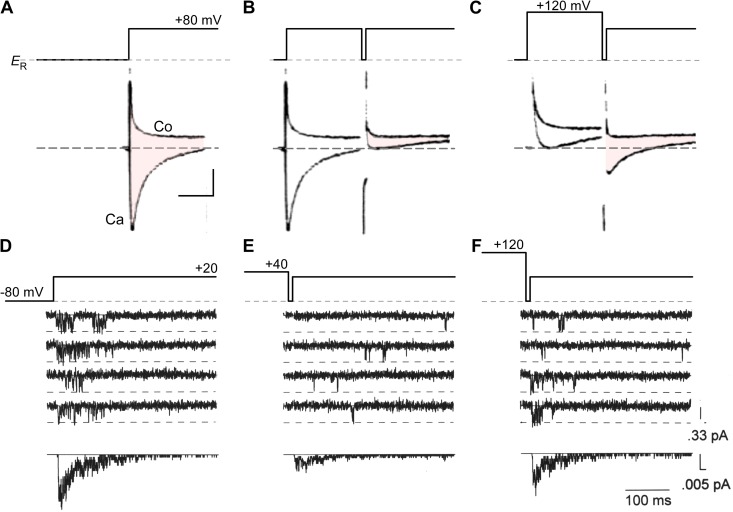
Methodological advances permitting routine isolation and recording of Ca2+ currents within vertebrate preparations clearly revealed the existence of CDI of cardiac L-type Ca2+ currents (carried by CaV1.2 channels) in both multicellular preparations (Kass and Sanguinetti, 1984; Mentrard et al., 1984) and isolated myocytes (Lee et al., 1985). Classic data illustrating CDI of cardiac L-type currents is shown in Fig. 1 (A–C). Fig. 1 A (Ca) shows Ca2+ current from frog cardiocytes, elicited by a test voltage pulse to +80 mV (Mentrard et al., 1984). CDI becomes apparent upon Ca2+ entry during a voltage prepulse to +80 mV (Fig. 1 B), which causes sharply attenuated Ca2+ current in a subsequent test pulse (diminished area of red shading). To exclude voltage depolarization itself as the cause of inactivation, a prepulse to +120 mV (Fig. 1 C) can be demonstrated to produce far less inactivation within a subsequent test-pulse current. Because this prepulse imposed strong depolarization, but diminished Ca2+ entry via decreased driving force, the reemergence of test Ca2+ current suggests that the strong inactivation evident in Fig. 1 B could be attributed to CDI. Data of this type specifies an inactivation mechanism that depends on voltage as a U-shaped function, now considered a hallmark of CDI (Eckert and Tillotson, 1981).
Figure 1.
Classic U-shaped signature of CDI. (A–C) Early voltage-clamp recordings from a multicellular preparation of frog atrial trabeculae cells demonstrates CDI of Ca2+ currents. All listed voltages are relative to resting potential ER (∼−80 mV) Adapted from Mentrard et al. (1984). (A) Ca2+ currents (shaded pink) evoked in response to an +80-mV depolarizing test pulse show robust inactivation. Fast capacitive transient was estimated by blocking these Ca2+ currents with 3 mM Co2+ solution (Co). Vertical bar corresponds to 0.5 µA of current; horizontal bar, 100 ms. (B) The Ca2+ current in response to the test pulse is sharply attenuated when preceded by an +80 mV prepulse. This reduction in current amplitude reflects the inactivation of Ca2+ channels. Format is as in A. (C) Further increase in the prepulse potential to +120 mV restores Ca2+ current amplitude (compare with B). Here, the diminished Ca2+ entry during the prepulse was insufficient to trigger CDI. The format is as in A. (D–F) Single channel recordings of L-type Ca2+ channels from adult rat ventricular myocytes also exhibit U-shaped dependence of Ca2+ channel inactivation. Adapted from Imredy and Yue (1994) with permission from Elsevier. (D) Depolarizing voltage pulse to +20 mV evokes representative elementary Ca2+ currents. Ensemble average currents are shown on the bottom. (E) When depolarizing pulse was preceded by +40 mV prepulse, the elementary Ca2+ currents are sparser and the first opening is delayed. This reduction in open probability parallels the sharp attenuation of macroscopic Ca2+ currents seen in B. The ensemble average is shown on the bottom. (F) Further increase in prepulse voltage to +120 mV led to a reversal of this gating pattern, once again highlighting the U-shaped dependence of CDI. Ensemble average is shown on the bottom.
Still, the actual nature of effects of Ca2+ upon gating remained unclear. Fig. 1 (D–F) reproduces the profile of CDI at the single-molecule level (Yue et al., 1990; Imredy and Yue, 1992, 1994). Resolving data such as these is technically challenging, owing to the diminutive size of unitary Ca2+ currents (∼0.3 pA) and the sub-millisecond gating timescale involved. These data demonstrate the existence of U-shaped inactivation in the gating of a single cardiac L-type Ca2+ channel fluxing Ca2+, as present in an adult rat ventricular myocyte (Imredy and Yue, 1994). These results established that Ca2+ influx of a single channel suffices to trigger CDI, and demonstrated the clear correspondence of single-channel and multicellular behavior (Fig. 1). Thus, CDI emerged as a legitimate molecular-level modulatory process.
Advent of Ca2+ channel calmodulation
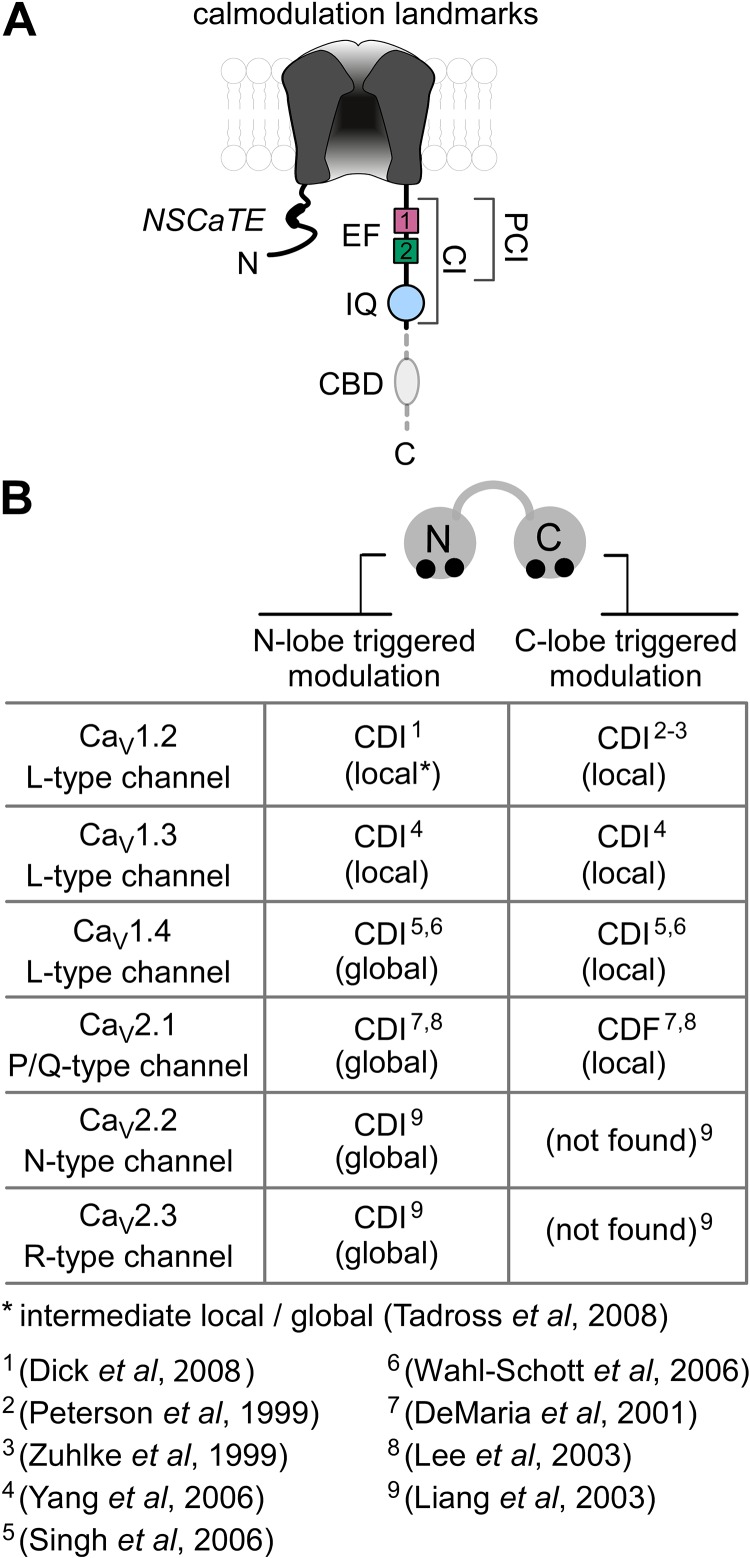
The Ca2+ sensor mediating Ca2+ regulation of Ca2+ channels has long been a matter of debate, with proposals of direct binding of Ca2+ to the channel complex (Standen and Stanfield, 1982; Plant et al., 1983; Eckert and Chad, 1984), and of Ca2+-dependent phosphorylation and/or dephosphorylation of channels (Chad and Eckert, 1986; Armstrong, D., C. Erxleben, D. Kalman, Y. Lai, A. Nairn, and P. Greengard. 1988. Abstracts of papers presented at the forty-second annual meeting of the Society of General Physiologists. Abstr. 20). The road toward identification of the actual Ca2+ sensor began with the cloning and expression of recombinant Ca2+ channels, thereby enabling structure–function studies (Snutch and Reiner, 1992). This phase of discovery was initiated by a bioinformatic observation (Babitch, 1990), pointing out that the carboxy terminus of voltage-gated Ca2+ channels contains a region with weak homology to an EF hand Ca2+-binding motif (Fig. 2 A, EF1). After the recognition of EF1, the existence of a second EF hand–like motif became apparent (Fig. 2 A, EF2). This led to chimeric channel analysis (de Leon et al., 1995), wherein segments of the carboxy termini of L-type (CaV1.2) and R-type (CaV2.3) Ca2+ channels were swapped. These two types of channels exhibit strong and weak CDI, respectively, under conditions of strong intracellular Ca2+ buffering (Liang et al., 2003), making them advantageous for chimeric analysis. Results from these experiments revealed that the proximal third of the carboxy terminus (Fig. 2 A, CI region) was important for CDI, and that EF1 of CaV1.2 channels was essential for the strong CDI in these channels (de Leon et al., 1995). One possible explanation was that EF1 might be the Ca2+ binding site for CDI postulated previously (Standen and Stanfield, 1982; Plant et al., 1983; Eckert and Chad, 1984). However, mutations within the Ca2+ binding loop of EF1 failed to eliminate CDI (Zhou et al., 1997; Peterson et al., 2000), focusing the search for the Ca2+ sensor elsewhere.
Figure 2.
Calmodulation: the ingredients and the flavors. (A) Channel diagram illustrates overall arrangement of structural landmarks critical for CDI. The Ca2+-inactivation (CI) region, spanning ∼160 residues of the channel carboxy terminus, is highly conserved across CaV1/2 channel families and is elemental for CDI. The proximal segment (PCI) of the CI region includes the dual vestigial EF hand (EF) segments (shaded purple and blue-green). The IQ domain, a canonical CaM binding motif critical for CDI, is located just downstream of the PCI segment. The NSCaTE element in the amino terminus of CaV1.2/1.3 channels is an N-lobe Ca2+/CaM effector site. The CBD element (gray) in the carboxy terminus of CaV2 channels is a CaM binding segment thought to be critical for CDI. (B) The table outlines functional bipartition of CaM in CaV channels and the corresponding spatial Ca2+ selectivities. In CaV1.2 and CaV1.3 channels, both the C-lobe and N-lobe of CaM enable fast CDI with local Ca2+ selectivity. Latent CDI of CaV1.4 channels is revealed upon deletion of an autoinhibitory domain in the distal carboxy terminus. Throughout the CaV2 channel family, the N-lobe of CaM evokes slow CDI with global Ca2+ spatial selectivity. In CaV2.1 channels, the C-lobe of CaM supports ultra-fast facilitation (CDF) with local Ca2+ selectivity. No C-lobe triggered modulation has been reported for CaV2.2 and CaV2.3 channels.
Targets of CaM binding themselves often resemble CaM, a bilobed molecule with each lobe composed of two EF hands (Jarrett and Madhavan, 1991). The dual vestigial EF hands in the carboxy terminus of channels (Fig. 2 A) may be seen as resembling a lobe of CaM, which suggests that CaM itself might be the Ca2+ sensor for CDI. Initial studies exploring this notion, however, showed that pharmacological inhibition of CaM did not eliminate CDI of L-type Ca2+ channels (Imredy and Yue, 1994; Zühlke and Reuter, 1998). Nonetheless, deletions within the CaV1.2 channel CI region identified an IQ motif (Fig. 2 A) as a critical element in CDI (Zühlke and Reuter, 1998), and mutagenesis of this IQ domain weakens CDI (Qin et al., 1999). As IQ motifs often bind Ca2+-free CaM (apoCaM; Jurado et al., 1999), the possibility that CaM acts as a CDI sensor reemerged. Definitive evidence came with studies involving a Ca2+-insensitive mutant form of CaM (CaM1234), in which point mutations within all four EF hands eliminate Ca2+ binding (Xia et al., 1998). If “preassociation” of apoCaM with target molecules were a prerequisite for subsequent regulation by Ca2+ binding to this apoCaM, then CaM1234 could act as a dominant negative to silence regulation, as seen for small-conductance Ca2+-activated K channels (Xia et al., 1998). Indeed, when CaV1.2 channels were coexpressed with CaM1234 or its analogues, CDI was ablated or strongly suppressed (Peterson et al., 1999; Zühlke et al., 1999). Additionally, apoCaM was found to bind to the CaV1.2 CI region, with critical dependence upon the IQ segment (Erickson et al., 2001; Pitt et al., 2001; Erickson et al., 2003). In retrospect, apoCaM preassociation with CaV1.2 channels would sterically protect CaM from pharmacological effects (Dasgupta et al., 1989), rationalizing the prior insensitivity of CDI to such small-molecule perturbation. In all, these data firmly substantiated CaM as the sensor for CaV1.2 channel Ca2+ regulation.
Initially, it was believed that only CaV1.2 L-type channels (Fig. 2 B) were subject to CaM-mediated Ca2+ regulation (Zamponi, 2003). However, this system of calmodulation was gradually recognized to pertain to most of the CaV1 and CaV2 (but not CaV3) branches of the CaV channel superfamily (Liang et al., 2003; Fig. 2 B). P-type (CaV2.1) channels were found to be Ca2+ regulated (Lee et al., 1999), and a Ca2+/CaM binding site downstream of the IQ element (CBD; Fig. 2 A) was argued to be important. In this initial study, no role for CaM preassociation was recognized, and no role for the IQ domain was found. Moreover, Ca2+ regulation of CaV2.1 channels manifests as a facilitation of current (Ca2+-dependent facilitation; CDF), followed by a slowly developing CDI. Thus, it appeared possible that the Ca2+ regulation of CaV2.1 channels might diverge mechanistically from that of CaV1.2 channels. However, apoCaM was later found to preassociate with CaV2.1 channels (Erickson et al., 2001), Ca2+-insensitive mutant CaM molecules were found to eliminate Ca2+ regulation in CaV2.1 channels (DeMaria et al., 2001; Lee et al., 2003), and the IQ domain was determined to be structurally essential for this regulation (DeMaria et al., 2001; Lee et al., 2003; Kim et al., 2008; Mori et al., 2008). That said, the role of the CBD segment remains contentious, with some studies still arguing for this segment’s importance in CDI (Lee et al., 2003). In contrast, deletion of the entire carboxy terminus after the IQ domain (including the CBD element) completely spared CDF and CDI of CaV2.1 channels (DeMaria et al., 2001; Chaudhuri et al., 2005). Overall, CaV2.1 and CaV1.2 channels appear to be Ca2+ regulated by a largely conserved scheme.
Soon thereafter, calmodulation was established for the remaining members of the CaV2 class of channels (Fig. 2 B, CaV2.2 and CaV2.3), which indicates that this modulatory system pertains throughout the CaV1-2 channel superfamily (Liang et al., 2003). Strong CaM-mediated CDI was found in CaV1.3 channels (Xu and Lipscombe, 2001; Shen et al., 2006; Yang et al., 2006); a latent capacity for CaM-mediated CDI was observed in CaV1.4 channels (Singh et al., 2006; Wahl-Schott et al., 2006); and potential indications of CaM-mediated regulation of CaV1.1 channels have been described (Stroffekova, 2008, 2011).
A few more nuanced points nonetheless merit attention. First, some types of calmodulation are insensitive to strong intracellular Ca2+ buffering (e.g., CaV1.2 CDI), whereas others are not (CaV2.3 CDI; Fig. 2 B). This contrasting sensitivity enables the use of chimeric channels under increased Ca2+ buffering to identify key structural determinants (de Leon et al., 1995), despite the existence of calmodulation across the channel superfamily. Second, the mechanisms underlying CDF in CaV1.2 channels remain mysterious, as the CDF of native channels of the heart is weak to start, and attenuated by blockade of Ca2+ release from neighboring ryanodine receptor channels (RYRs; Wu et al., 2001). Additionally, CDF of CaV1.2 channels is strongly manifest only in recombinant channels containing point mutations within the IQ domain, and only when they are expressed in frog oocytes (Zühlke et al., 2000). Curiously, CDF is not observed in recombinant CaV1.2 channels expressed in mammalian cell lines (Peterson et al., 1999). In contrast, CDI of CaV1.2 channels is strong and universally observed across experimental platforms. Third, Ca2+/CaM-dependent dephosphorylation by calcineurin was initially suggested as a mechanism for CDI of Ca2+ channels (Chad and Eckert, 1986); however, this proposition has remained controversial as previously reviewed (Budde et al., 2002). Recently, calcineurin was found to preassemble with the CaV1.2 channel complex through the scaffolding protein AKAP79/150 bound to the distal carboxy terminus of the channel (Oliveria et al., 2007). Furthermore, two additional maneuvers were reported to impede CDI of these channels (Oliveria et al., 2012): the overexpression of a mutant AKAP79/150 incapable of binding calcineurin, and inhibition of calcineurin activity. However, others have found that direct inhibition of calcineurin has no effect on the CDI of L-type channels within multiple neuronal preparations (Branchaw et al., 1997; Victor et al., 1997; Zeilhofer et al., 1999). Moreover, deletion of the entire distal carboxy terminus (including the AKAP79/150 binding segment) of CaV1.2 channels preserves strong CDI (de Leon et al., 1995; Erickson et al., 2001; Crump et al., 2013). Similarly, short variants of CaV1.3 channels lacking the AKAP harboring distal carboxy termini (Marshall et al., 2011) also fully support CDI (Xu and Lipscombe, 2001; Shen et al., 2006; Yang et al., 2006; Bock et al., 2011). Altogether, it may be that calcineurin and AKAP79/150 are indirect and context-dependent modulators of CDI, rather than direct effector molecules (Budde et al., 2002).
Functional bipartition of CaM and selectivity for local/global Ca2+ sources
The calmodulatory mechanism for Ca2+ channels supports remarkable forms of Ca2+ decoding. This feature echoes earlier discoveries of “functional bipartition” of CaM in Paramecium (Kink et al., 1990; Saimi and Kung, 2002). Here, “under-excitable” behavioral strains had mutations only in the N-lobe of CaM, whereas “over-excitable” strains had mutations only in the C-lobe. Thus, one lobe of CaM can mediate signaling to one set of functions, whereas the other lobe signals to an alternative set of operations. It was unclear, however, whether this bipartition extended to mammals. The requirement that Ca2+ regulation of mammalian Ca2+ channels requires apoCaM preassociation permitted direct exploration of this question. Coexpressing channels with “half mutant” CaM molecules (CaM12 and CaM34, where only C- or N-terminal lobes, respectively, bind Ca2+) revealed that the individual lobes of CaM can evoke distinct components of channel regulation (Fig. 2 B). In most CaV1 channels, the C-lobe induces a kinetically rapid phase of CDI, whereas the N-lobe yields a slower component (Peterson et al., 1999; Yang et al., 2006; Dick et al., 2008). For CaV1.2 channels, early studies that utilized high intracellular Ca2+ buffering found only minimal N-lobe–mediated CDI (Peterson et al., 1999). However, when interrogated under low intracellular Ca2+ buffering, slow but recognizable N-lobe–mediated CDI of CaV1.2 channels emerges (Dick et al., 2008; Simms et al., 2013). Most strikingly, in CaV2.1 channels, the lobes of CaM produce opposing polarities of regulation: the C-lobe of CaM triggers a kinetically rapid CDF, whereas the N-lobe evokes a slower CDI (DeMaria et al., 2001; Lee et al., 2003). CaV2.2 and CaV2.3 channels manifest CDI triggered mainly by the N-lobe of CaM (Liang et al., 2003). Whether this bipartition orchestrates divergent classes of behavior in higher-order animals remains an intriguing and open question (Wei et al., 2003).
Beyond simply splitting the Ca2+ signal, however, the calmodulation of mammalian Ca2+ channels revealed that the lobes of CaM can selectively decode Ca2+ in different ways. Hints as to this capability came from the differential sensitivity of calmodulation in various channels to Ca2+ buffering. Processes evoked by the C-lobe of CaM are invariably insensitive to introduction of strong Ca2+ buffering, whereas N-lobe–mediated processes in CaV2 channels can be eliminated by the same maneuver. Strong buffering would only spare Ca2+ signals near the cytoplasmic mouth of channels where strong point-source Ca2+ influx would overwhelm buffer capacity (Neher, 1986; Stern, 1992). This argues that local Ca2+ influx through individual channels triggers C-lobe signaling; in other words, the C-lobe of CaM exhibits a “local Ca2+ selectivity.” In contrast, a spatially global elevation of Ca2+ (present in the absence of strong Ca2+ buffering) is required for N-lobe signaling of CaV2 channels (DeMaria et al., 2001; Lee et al., 2003; Liang et al., 2003); thus, the N-lobe in this context exhibits a “global selectivity.” The existence of global selectivity is notable, given that local Ca2+ influx yields far larger Ca2+ increases near a channel than does globally sourced Ca2+ (Tay et al., 2012). One exception to this pattern is the local Ca2+ selectivity of the N-lobe component of CDI in CaV1.2/1.3 channels (see two paragraphs below). Corroboration of spatial Ca2+ selectivity is provided by the ability of single CaV1.2 channels to undergo CDI (Fig. 1, D–F). By definition, only a local Ca2+ source is present in the single-channel configuration; thus, the presence of CDI in this context indicates local Ca2+ selectivity. Additionally, single-channel records of CaV2.1 channels exhibit CDF (driven by C-lobe), but not CDI (triggered by N-lobe; Chaudhuri et al., 2007), which is consistent with the proposed differential selectivities for this channel. Fig. 2 B summarizes the arrangement of spatial Ca2+ selectivities according to the lobes of CaM.
The mechanisms underlying these contrasting spatial Ca2+ selectivities can be interpreted as emergent behaviors of a system in which a lobe of apoCaM must transiently detach from a preassociation site before binding Ca2+, and where a Ca2+-bound lobe of CaM must associate with a channel effector site to mediate regulation (Tadross et al., 2008). When the slow Ca2+-unbinding kinetics of the C-lobe of CaM are imposed on this scenario, local Ca2+ sensitivity invariably results. In contrast, when the rapid Ca2+-unbinding kinetics of the N-lobe are interfaced with this architecture, global Ca2+ selectivity arises if the channel preferentially binds apoCaM versus Ca2+/CaM, as in the case of CaV2 channels (Fig. 2 B). When the channel preferentially interacts with Ca2+/CaM, local Ca2+ selectivity emerges, as in the context of CaV1 channels (Fig. 2 B). The relative roles of the affinities and kinetics of Ca2+ binding to the two lobes of CaM in mediating local and global Ca2+ selectivities have been considered at length elsewhere (Tadross et al., 2008).
As an explicit example of how spatial Ca2+ selectivity of N-lobe regulation is specified, we consider the effects of an N-terminal spatial Ca2+-transforming element (NSCaTE) present only on the amino terminus of CaV1.2/1.3 channels (Fig. 2 A). NSCaTE is a Ca2+/CaM binding site whose presence enhances the aggregate channel affinity for Ca2+/CaM over apoCaM (Ivanina et al., 2000; Dick et al., 2008), endowing the N-lobe component of CDI of these channels with a largely local Ca2+ selectivity of N-lobe CDI in these channels. Elimination of the NSCaTE site in these channels, which tilts channels toward a preference for apoCaM, then switches their N-lobe CDI to a global profile. Conversely, donation of NSCaTE to CaV2 channels, which causes channels to favor Ca2+/CaM binding, then endows their N-lobe CDI with local Ca2+ selectivity (Dick et al., 2008). In this manner, the presence of NSCaTE can tune the spatial Ca2+ selectivity of N-lobe–mediated channel regulation. This overall arrangement of CaM interactions with a target molecule could endow numerous other regulatory systems with spatial Ca2+ selectivity.
Molecular basis of calmodulation
Elucidating the arrangement of apoCaM and Ca2+/CaM on Ca2+ channels is fundamental for the field, given the biological influence of calmodulation, and the importance of this system as an ion-channel regulatory prototype (Dunlap, 2007). This critical task, however, remains an ongoing challenge, given the >2,000 amino acids comprising the main pore-forming α1 subunit alone, the ability of multiple peptide segments of the channel to bind CaM in vitro, and the formidable nature of obtaining atomic structures for these channels.
The stoichiometry of CaM interaction with channels has been debated. Crystal structures of Ca2+/CaM complexed with portions of the carboxy tail CI region of CaV1.2 channels (Fallon et al., 2009; Kim et al., 2010) suggest that multiple CaM molecules may interact to produce the full spectrum of Ca2+ regulatory functions. Moreover, CaM can interact with multiple peptide segments of the channel (Ivanina et al., 2000; Tang et al., 2003; Zhou et al., 2005; Dick et al., 2008; Ben Johny et al., 2013). In contrast, covalent fusion of single CaM molecules to CaV1.2 channels suggests that only one CaM per channel suffices to elicit CDI (Mori et al., 2004). Moreover, live-cell FRET studies of the interactions between CaM and holochannels (CaV1.2) also point to a 1:1 CaM/channel ratio (Ben Johny et al., 2012). In the holochannel, the various CaM binding segments may be arranged in close proximity so as to allow the two lobes of CaM to preferentially interact with the distinct channel segments during Ca2+ regulation (Dick et al., 2008; Ben Johny et al., 2013). Recent biochemical studies of the NSCaTE element show that this segment preferentially interacts with a single lobe of CaM (N-lobe) at a time (Liu and Vogel, 2012). Furthermore, the NSCaTE element can interact also with Ca2+/CaM prebound to an IQ domain peptide (Taiakina et al., 2013). Thus, we favor the interpretation that only one CaM interacts within holochannels, although peptide fragments of Ca2+ channels may bind to multiple CaM molecules.
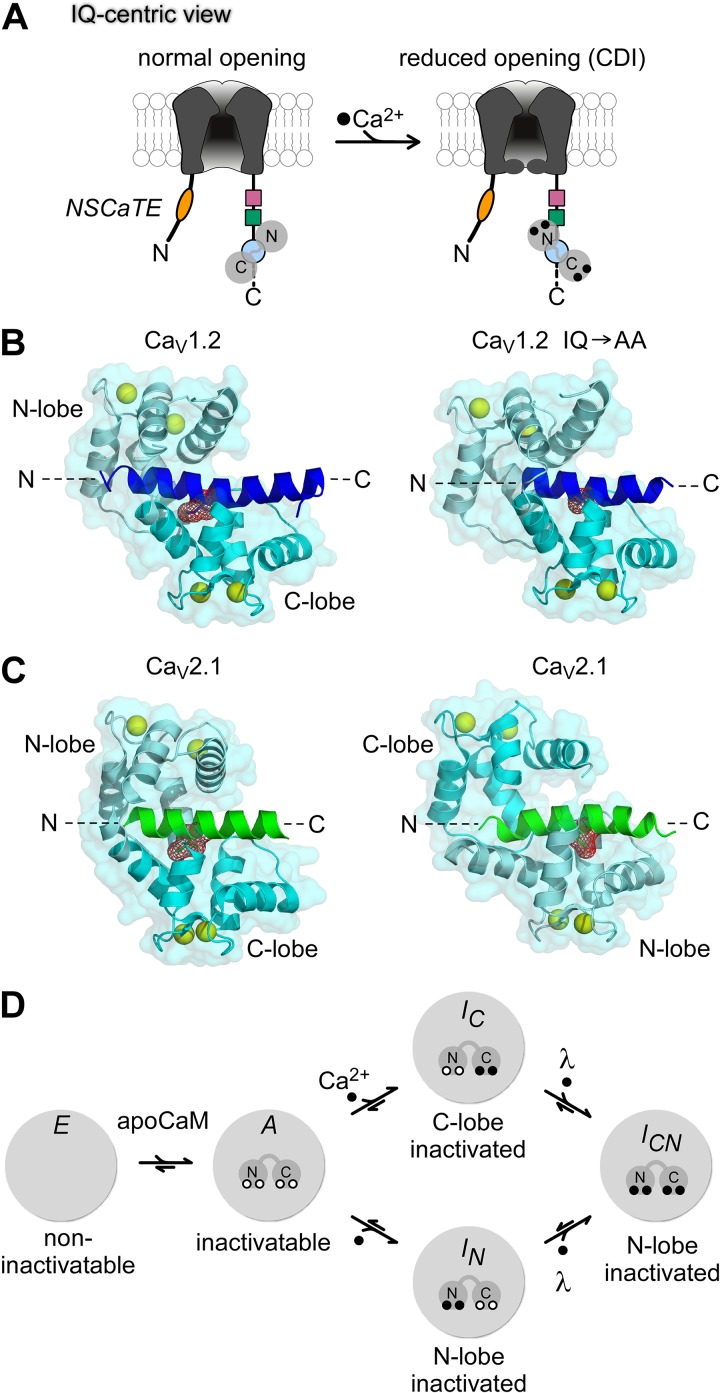
The IQ domain (Fig. 3 A, blue circle) is critical for calmodulation. Mutations within this domain markedly modulate the Ca2+ regulation of CaV1.2 channels (Zühlke and Reuter, 1998; Qin et al., 1999; Zühlke et al., 1999, 2000; Erickson et al., 2003), CaV1.3 channels (Yang et al., 2006; Bazzazi et al., 2013; Ben Johny et al., 2013), CaV2.1 channels (DeMaria et al., 2001; Lee et al., 2003; Kim et al., 2008; Mori et al., 2008), and CaV2.2 and CaV2.3 channels (Liang et al., 2003). Furthermore, apoCaM preassociation with CaV1.2/1.3 channels requires the IQ domain (Erickson et al., 2001; Pitt et al., 2001; Erickson et al., 2003; Bazzazi et al., 2013; Ben Johny et al., 2013). Finally, Ca2+/CaM binds well to IQ-domain peptides of many CaV channels (Peterson et al., 1999; Zühlke et al., 1999; DeMaria et al., 2001; Pitt et al., 2001; Liang et al., 2003; Bazzazi et al., 2013; Ben Johny et al., 2013). All these results gave rise to the IQ-centric hypothesis shown in Fig. 3 A. Here, the IQ domain is important for both apoCaM preassociation (left) and Ca2+/CaM binding (effector configuration shown on the right). This IQ-centric paradigm has motivated efforts to resolve crystal structures of Ca2+/CaM complexed with IQ-domain peptides of various CaV1-2 channels (Fallon et al., 2005; Van Petegem et al., 2005; Kim et al., 2008; Mori et al., 2008), as shown in Fig. 3 (B and C). Throughout, the IQ peptide segment appears as an α-helical entity with N and C termini as labeled.
Figure 3.
Toward an atomic-level understanding of CDI. (A) IQ-centric view of calmodulation of CaV channels. In this mechanistic scheme, ApoCaM is preassociated with the IQ domain (blue). Upon Ca2+ binding, CaM rebinds the same IQ domain with a higher affinity, and subtle conformational rearrangements are presumed to trigger CaM-mediated channel regulation. (B, left) Crystal structure of CaV1.2 IQ domain peptide in complex with Ca2+/CaM (Protein Data Bank accession no. 2BE6). The IQ domain is colored blue. CaM is show in cyan (N-lobe, pale cyan; C-lobe, cyan). Ca2+ ions are depicted as yellow spheres. The key isoleucine residue (red) serves as a hydrophobic anchor for CaM. Ca2+/CaM adopts a parallel arrangement with the IQ domain in which the N-lobe binds closer to the amino terminus of the IQ domain and the C-lobe binds further downstream. (B, right) Crystal structure of Ca2+/CaM bound to mutant CaV1.2 IQ domain with alanines substituted for the key isoleucine-glutamine residues (accession no. 2F3Z). This double mutation abolishes CDI. Structurally, however, Ca2+/CaM hugs the mutant IQ domain in a similar conformation as to its interaction with the wild-type (left). (C) Crystal structure of Ca2+/CaM bound to CaV2.1 IQ domain (left, accession no. 3BXK; right, accession no. 3DVM). The IQ domain is shaded green, CaM in cyan. Ca2+/CaM was reported to bind to the CaV2.1 IQ domain in both parallel (left) and antiparallel (right) arrangements. The antiparallel arrangement in which the C-lobe of CaM binds upstream of IQ domain has led to speculation that CDF may result from this inverted polarity of Ca2+/CaM association. (D) Simplified configurations of the CaM/channel complex relevant for calmodulation (E, A, IC, IN, and ICN). In configuration E, channels lack preassociated CaM and therefore cannot undergo CDI. Configuration A corresponds to channels bound to apoCaM, and thus capable of undergoing robust CDI. Ca2+ binding to the C-lobe and N-lobe of CaM leads to the inactivated configurations IC and IN, respectively. Ca2+ binding to both lobes of CaM then yields configuration ICN, with both C and N lobes of CaM engaged toward CDI. This final transition may exhibit positive cooperativity as specified by the constant λ >> 1. Under endogenous levels of CaM, channels may reside in any of the five configurations. Strong coexpression of wild-type or mutant CaM restricts accessibility to various states. Reproduced with permission from Ben Johny et al., 2013.
However, this viewpoint remains problematic in three regards. (1) The atomic structures of Ca2+/CaM bound to wild-type and mutant IQ peptides of CaV1.2 (Fig. 3 B) show that a central isoleucine (side chains explicitly shown) in the IQ element is deeply buried within the C-lobe of Ca2+/CaM (Van Petegem et al., 2005), and that alanine substitution at this site hardly changes structure (Fallon et al., 2005). Additionally, Ca2+/CaM dissociation constants for corresponding wild-type and mutant IQ peptides are nearly the same (Zühlke et al., 2000; Bazzazi et al., 2013). Why then would alanine substitution at this well-ensconced site influence the rest of the channel to blunt regulation (Fallon et al., 2005)? (2) For CaV1.2/1.3 channels, the N-lobe of Ca2+/CaM effector site appears to be an NSCaTE element (Fig. 3 A, oval) of the channel amino terminus (Dick et al., 2008; Tadross et al., 2008; Liu and Vogel, 2012), separate from the IQ element. (3) Crystal structures of Ca2+/CaM in complex with the IQ peptide of CaV2.1 channels show that CaM can adopt both a parallel (Mori et al., 2008) and an antiparallel configuration (Kim et al., 2008; Fig. 3 C, left and right, respectively). The apparent inversion in configuration of CaM binding to IQ domain between CaV1.2 and CaV2.1 has been proposed as a mechanism for the opposing polarity of Ca2+ regulation observed in the two channels (Kim et al., 2008; Minor and Findeisen, 2010). However, detailed structural analysis along with functional systematic alanine scanning mutagenesis and chimeric channel analysis argues that the C-lobe effector site resides at a site beyond the IQ module (Mori et al., 2008).
A major concern with older IQ domain analyses is that the regulatory system was not considered conceptually as a whole, as shown in Fig. 3 D (drawn with specific reference to CaV1.3 channels; Ben Johny et al., 2013). Configuration E portrays channels lacking apoCaM. Such channels can open normally, but do not manifest CDI because Ca2+/CaM from bulk solution cannot efficiently access a channel in configuration E to induce CDI (Mori et al., 2004; Yang, P.S., M.X. Mori, E.A. Antony, M.R. Tadross, and D.T. Yue. 2007. Biophysical Journal abstracts issue. 1669-Plat; Liu et al., 2010; Findeisen et al., 2011). ApoCaM binding with configuration E gives rise to configuration A, where opening can also occur normally, but CDI can now take place. For CDI, Ca2+ binding to both lobes of CaM yields configuration ICN, which underlies a fully inactivated channel with strongly reduced opening. For intermediate configurations, Ca2+ binding only to the C-lobe brings about configuration IC, equivalent to a C-lobe inactivated channel; Ca2+ binding only to the N-lobe yields the N-lobe–inactivated arrangement (IN). Of particular importance, ensuing entry into ICN likely involves positively cooperative interactions specified by cooperativity factor λ >> 1.
This scheme emphasizes several challenges for older analyses of the IQ domain, where CDI was mostly assessed with only endogenous CaM present. Results thus obtained would be ambiguous, because IQ-domain mutations could alter calmodulation via changes at multiple steps depicted in Fig. 3 D, whereas other interpretations largely attributed the effects to altered Ca2+/CaM binding with an IQ effector site. In contrast, IQ mutations could well weaken apoCaM preassociation and reduce CDI by favoring configuration E. Moreover, functional deficits caused by mutations that do attenuate interaction with one lobe of Ca2+/CaM may be masked by positively cooperative steps (λ in Fig. 3 D).
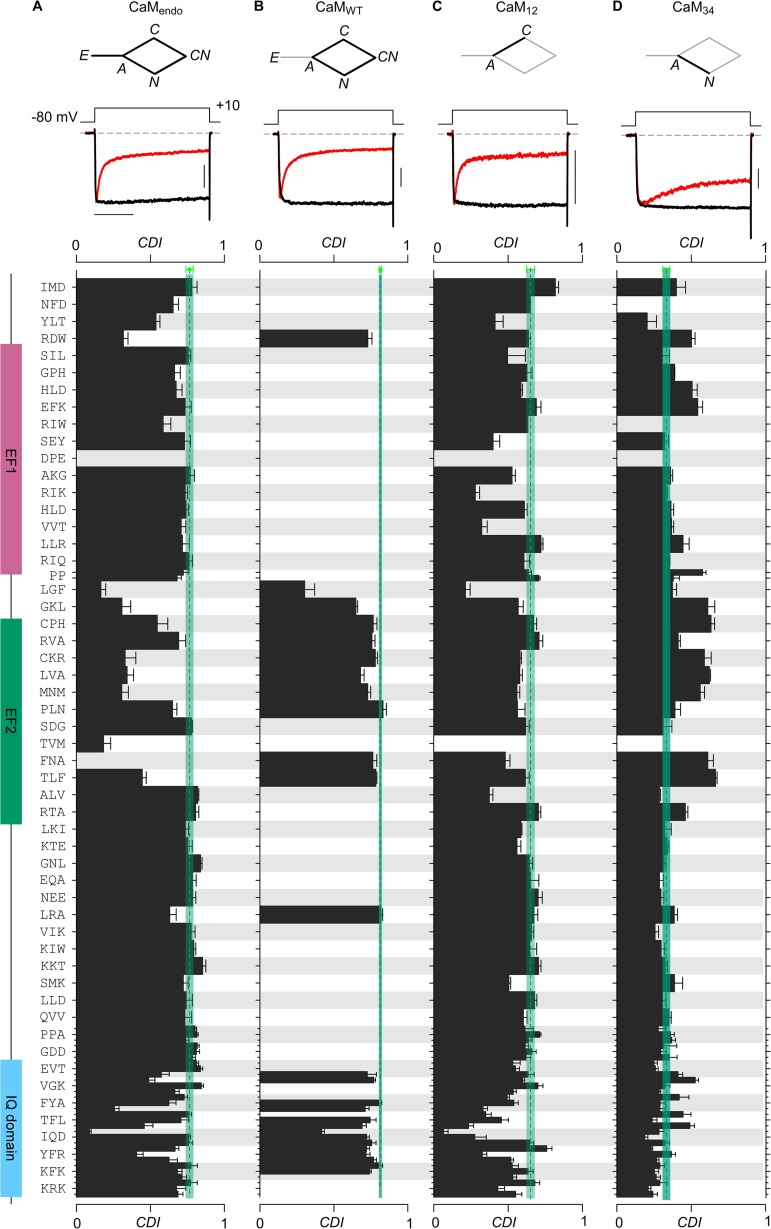
To minimize these issues, CDI of CaV1.3 channels was recently characterized during strong coexpression with various mutant CaM molecules (Bazzazi et al., 2013; Ben Johny et al., 2013) as shown in Fig. 4 (A–C, top). For orientation, CDI of channels expressed with only endogenous CaM present is shown in the upper portion of Fig. 4 A. Strong CDI produces a rapid decay of whole-cell Ca2+ current (red trace), compared with the negligible decline of Ba2+ current (black trace). Because Ba2+ binds CaM poorly (Chao et al., 1984), the decline of Ca2+ versus Ba2+ current after 300 ms of depolarization quantifies CDI (CDI parameter plotted below in bar graphs). One can isolate the diamond-shaped subsystem lacking configuration E (Fig. 4 B, top) by leveraging mass action with strong coexpression of wild-type CaM (CaMWT). Further simplification of CDI was obtained by strongly coexpressing channels with CaM12 (Fig. 4 C, top), which empties configuration E by mass action, and forbids configurations IN and ICN. Thus, the isolated C-lobe component of CDI can be studied, with the signature rapid time course of current decay shown near the top of Fig. 4 C. Critically, this layout avoids interplay with cooperative λ steps in Fig. 3 D. Likewise, strongly coexpressing CaM34 focuses on the slower N-lobe form of CDI, with attendant simplifications.
Figure 4.
Residue-level roadmap for calmodulation of CaV1.3 channels. Systematic alanine scanning mutagenesis of the entire CI domain of CaV1.3 channels. (A) CDI of wild-type and mutant CaV1.3 channels recombinantly expressed in HEK293 cells was measured with only endogenous CaM present. CDI observed here reflects properties of the entire system (stick figure) diagrammed in Fig. 3 D. Exemplar whole-cell current shows robust CDI for wild-type (WT) CaV1.3, reflecting their high affinity for apoCaM. Here and throughout, the vertical bar pertains to 0.2 nA of Ca2+ current (black); the Ba2+ current (gray) has been scaled ∼3-fold downward to aid comparison of decay kinetics. Horizontal bar, 100 ms. CDI is measured as the fractional reduction of Ca2+ current in comparison to Ba2+ at 300 ms (Ben Johny et al., 2013). Bottom, bar graph summary of CDI for alanine substitutions for indicated residues. CDI for WT channels, blue-green vertical line. Alanine substitutions in both EF hand regions and IQ domain resulted in diminished CDI. (B) Overexpression of CaMWT isolates the behavior of the diamond-shaped subsystem. Such overexpression of CaMWT should rescue CDI for mutations that weakened apoCaM preassociation. For WT CaV1.3, exemplar current shows that CDI is unaltered, as expected for a channel with high affinity for apoCaM. Bottom, bar graph summary of CDI for various mutant channels with CaMWT overexpressed. Format is as in A. CDI is rescued for several mutations in the EF hand region (EF2) and the IQ domain, reflecting the preassociation of N-lobe and C-lobe of apoCaM. (C) Overexpression of CaM12 isolates C-lobe CDI. Exemplar currents for WT channels show the fast C-lobe form of CDI. Bottom, bar graph summary shows the footprint of C-lobe CDI. Mutants in both the first EF hand and the IQ domain disrupted the C-lobe CDI (see the LGF loci in the EF hand regions and TFL and IQD loci in the IQ domain). The format is as in A. (D) Overexpression of CaM34 isolates N-lobe CDI. N-lobe CDI was largely preserved by mutations in the CI region. Mutations that weakened N-lobe apoCaM preassociation exhibited enhanced N-lobe CDI (see EF2 segment). Reassuringly, alanine scanning mutagenesis of NSCaTE element resulted in specific disruption of N-lobe CDI of CaV1.3 channels (Tadross et al., 2008). Adapted with permission from Ben Johny et al. (2013) and Bazzazi et al. (2013).
Accordingly, a new framework of CaM/channel configurations that underlie calmodulation could be deduced, as diagrammed in Fig. 5 (Ben Johny et al., 2013). For convenience, Fig. 4 compiles results from a systematic alanine scan covering the entire CI domain of the carboxyl tail of CaV1.3 channels (Bazzazi et al., 2013; Ben Johny et al., 2013), where substitution positions are denoted to the left of the bar graph in Fig. 4 A. The N-terminal end of the CI segment corresponds to the top of the graph, purple and blue-green regions indicate EF1 and EF2, and the lavender zone denotes the IQ domain. Electrophysiological characterization for each of the substitutions was performed for all the various subsystems (Fig. 4, A–D, top), and bar graphs plot the strength of CDI for the corresponding subsystems in each of the panels. The blue-green vertical lines reference the profile for wild-type channels. The results thus obtained were combined with CaM binding assays (not depicted) to identify meaningful structure–function correlations.
Figure 5.
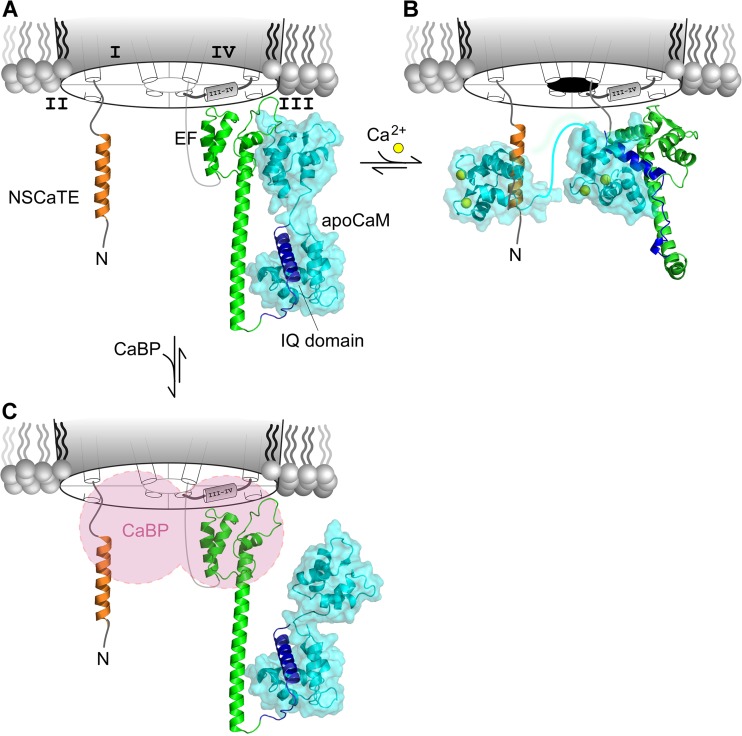
Next-generation blueprint for CaM/CaBP regulation of CaV channels. (A) De novo molecular model of CaV1.3 CI region docked to apoCaM. The N-lobe of apoCaM is thought to preassociate with the PCI region (green), whereas the C-lobe binds to the IQ domain (blue). The NSCaTE segment (tan) is unoccupied. This model corresponds to configuration A in Fig. 3 D, where the channels are charged with an apoCaM and ready to undergo CDI. (B) Proposed model for the Ca2+ inactivated state of the channel. The N-lobe of Ca2+/CaM is shown binding to the NSCaTE segment based on a recent NMR structure (Protein Data Bank accession no. 2LQC). This configuration is believed to result in N-lobe CDI. The de novo molecular model shows C-lobe of Ca2+/CaM, the EF hand segment, and the IQ domain forming a tripartite complex resulting in C-lobe CDI. Overall, this model corresponds to the inactive configuration ICN in Fig. 3 D. (C) Proposed allosteric mechanism of CaBP action. CaBP and apoCaM may dually bind the CaV1 channel. However, CaBP may allosterically inhibit CDI of CaV1 channels by interacting with the NT, III-IV loop, or PCI segment, thereby preventing Ca2+/CaM from reaching its effector configuration. (A–C) Adapted with permission from Yang et al. (2014).
Under the regimen in Fig. 4 A, where all configurations are potentially accessible, diminished CDI by alanine substitutions occurred not only in the IQ domain, but upstream in the EF hand regions (between LGF and TLF residues). The latter effects strongly suggested that something outside the IQ domain was essential. Some of these deficits in CDI could be attributed to weakening of apoCaM preassociation with channels, because overexpressing wild-type CaM to depopulate configuration E (in Fig. 4 B) substantially recovered many of these CDI deficits. Binding assays of apoCaM with various CI segments confirmed this interpretation (Bazzazi et al., 2013; Ben Johny et al., 2013).
Turning to potential deficits relating to diminished Ca2+/CaM action through channel effector sites, Fig. 4 (C and D) separately interrogates the C-lobe and N-lobe components of CDI. Importantly, isolating the C- and N-lobe components minimizes masking of mutational effects by positive cooperativity, allowing CDI deficits to be more sensitively observed in this regimen. That said, Fig. 4 C displays C-lobe CDI deficits in the EF hand region (strongest for LGF substitution), as well as in the IQ domain (with the strongest effect upon substitution at the central isoleucine). These outcomes support a hypothesis where the Ca2+/CaM effector configuration for the C-lobe component of CDI involves both of these functional hotspots, as developed two paragraphs below. For the N-lobe component of CDI (Fig. 4 D), no appreciable deficits were observed, as would be expected if interaction with the channel NSCaTE element (in the channel amino terminus) serves as the effector element.
Fig. 5 A displays a proposed configuration A for apoCaM interaction with the channel. This includes a homology model of the apoCaM C-lobe complexed with the IQ domain (blue), based on analogous NaV structures (Chagot and Chazin, 2011; Feldkamp et al., 2011). The portrayal of the apoCaM N-lobe incorporates ab initio structural prediction of the CI domain, with two vestigial EF hands (EF), and a protruding helix (preIQ subelement). The EF hand module (EF) resembles the structure of a homologous NaV segment (Chagot et al., 2009; Wang et al., 2012), and the preIQ helix resembles a helical segment observed in crystal structures of analogous CaV1.2 peptides (Fallon et al., 2009; Kim et al., 2010). The atomic structure of the apoCaM N-lobe (1CFD) was interfaced with shape-complementarity docking algorithms. Regarding the proposed interaction interface of the N-lobe with the channel EF domain, we note that alanine substitution therein produced a curious enhancement of N-lobe CDI seen with certain alanine substitutions, especially in the EF region (Fig. 4 D). This feature is interesting because weakening of channel interaction with the N-lobe of apoCaM would be predicted to strengthen CDI produced by the N-lobe of Ca2+/CaM (Tadross et al., 2008).
Fig. 5 B displays a proposed arrangement for the Ca2+/CaM-bound configuration ICN. The N-lobe complex with NSCaTE is an NMR structure (Liu and Vogel, 2012). An alternative ab initio model of the PCI is computationally docked with the C-lobe of Ca2+/CaM (Protein Data Bank accession no. 3BXL) and IQ module, which together form a ternary complex. This ternary arrangement is consistent with the importance of both IQ and EF domains for C-lobe CDI (Fig. 4 C). Intriguingly the C-lobe configuration resembles a rather canonical CaM–peptide complex, where the channel EF module contributes a surrogate lobe of CaM. Importantly, assays of Ca2+/CaM binding to the IQ segment alone would correlate poorly with functional effects relating to the ternary complex, as experimental studies observe (Bazzazi et al., 2013; Ben Johny et al., 2013). Overall, the proposed framework in Fig. 5 (A and B) may aid future structural biology and structure–function work. In addition, this scheme of CaM exchange within its target molecule may generalize beyond the Ca2+ channel family.
Biological consequences and prospects for new disease therapies
The biological consequences of Ca2+ regulation by CaM promise to be wide ranging and immense. In the heart, elimination of CaV1.2 CDI by means of dominant-negative CaM elicits marked prolongation of ventricular action potential duration (APD), implicating CDI as a dominant control factor in specifying APD (Alseikhan et al., 2002; Mahajan et al., 2008). As APD is one of the main determinants of electrical stability and arrhythmias in the heart, pharmacological manipulation of such regulation looms as a future antiarrhythmic strategy (Mahajan et al., 2008; Anderson and Mohler, 2009). Most recently, genome-wide linkage analysis in humans has uncovered heritable and de novo CaM mutations as the probable cause of several cases of catecholaminergic polymorphic ventricular tachycardia (CPVT), with altered CaM-ryanodine receptor function implicated as a major contributing factor (Nyegaard et al., 2012). Whole-exome sequencing has revealed an association between three de novo missense CaM mutations and severe long-QT (LQT) syndrome with recurrent cardiac arrest (Crotti et al., 2013), quite plausibly acting via disruption of CaV1.2/1.3 channel CDI (Limpitikul et al., 2014).
In brain, state-of-the-art analysis of genome-wide single-nucleotide polymorphisms (SNPs) has identified CaV channels as a major risk factor for several psychiatric disorders (Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013). More specifically to calmodulation, CaV1.3 channels constitute a prominent Ca2+ entry portal into pacemaking and oscillatory neurons (Bean, 2007), owing to the more negative voltages required to open these ion channels. These channels are subject to extensive alternative splicing (Hui et al., 1991; Xu and Lipscombe, 2001; Shen et al., 2006; Bock et al., 2011; Tan et al., 2011) and RNA editing (Huang et al., 2012) in the carboxy tail, in ways that strongly modulate the strength of CDI (Shen et al., 2006; Liu et al., 2010; Huang et al., 2012). This fine tuning of CDI appears to be important for circadian rhythms (Huang et al., 2012). From the specific perspective of disease, CaV1.3 channels contribute a substantial portion of Ca2+ entry into substantia nigral neurons (Bean, 2007; Chan et al., 2007; Puopolo et al., 2007; Guzman et al., 2009), which exhibit high-frequency pacemaking (Chan et al., 2007) that drives dopamine release important for movement control. Notably, loss of these neurons is intimately related to Parkinson’s disease, and Ca2+ disturbances and overload are critical to this neurodegeneration (Bezprozvanny, 2009; Surmeier and Sulzer, 2013). Accordingly, an attractive therapeutic possibility for Parkinson’s disease involves discovery of small molecules that selectively down-regulate the opening of CaV1.3 versus other closely related Ca2+ channels (Kang et al., 2012). Thus, understanding the mechanisms underlying the modulation of CaV1.3 CDI is crucial, particularly to furnish specific molecular interfaces as targets of rational screens for small-molecule modulators.
The fundamental mechanism of action of a natural modulator of CaV1.3 CDI may be especially relevant. In particular, recent discoveries identify Ca2+-binding proteins (CaBPs), a family of CaM-like brain molecules (Haeseleer et al., 2000) that may also bind CaV channels and other targets (Yang et al., 2002; Kasri et al., 2004). Like CaM, CaBPs are bilobed, with each lobe containing two EF hand Ca2+ binding motifs (Haeseleer et al., 2000), and a recent crystal structure shows overall similarity to CaM (Findeisen and Minor, 2010). However, whereas all four EF hands bind Ca2+ in CaM, one lobe is nonfunctional in CaBPs. Coexpressing CaBPs with CaV1 channels eliminates their CDI, thereby potentially influencing diverse biological processes (Zhou et al., 2004; Yang et al., 2006). Some have argued that CaBPs may simply compete for apoCaM on channels (Lee et al., 2002; Findeisen et al., 2013; Oz et al., 2013). More recent data argue for a different mechanism (Fig. 5 C), in which CaBP and apoCaM can both preassociate with the channel (Yang et al., 2014). In particular, by binding to channel regions that overlap Ca2+/CaM effector loci, CaBPs may preemptively retard the ability of Ca2+/CaM to reach its effector configuration (Fig. 5 B), allowing low concentrations of CaBP molecules in the CNS to still exert functional effects in the presence of much higher CaM levels (Yang et al., 2014). If this scheme is correct, the basic mechanisms of CaBP action may have implications for drug discovery, in that small molecules that target channel interaction surfaces for CaBP and/or Ca2+/CaM may exert potent modulatory actions.
In conclusion, this overview of the calmodulation of voltage-gated Ca2+ channels highlights an impressive synergy among elegant molecular regulatory mechanisms, vital biological functions, and pathogenesis and potential therapy. As such, this field promises considerable mystery, enrichment, and enlightenment in the years ahead.
Acknowledgments
We thank Dr. Philemon Yang and members of the Ca2+ signals laboratory for valuable insights.
This work was supported by grants from the National Institutes of Health National Heart, Lung, and Blood Institute (R37HL076795), National Institute of Mental Health (R01MH065531), and National Institute of Neurological Disorders and Stroke (R01NS073874) to D.T. Yue; and the National Institute of Mental Health (F31MH088109) to M. Ben-Johny.
The authors declare no competing financial interests.
Elizabeth M. Adler served as editor.
Footnotes
Abbreviations used in this paper:
- CaM
- calmodulin
- CaM regulation
- calmodulation
- CaBP
- Ca2+-binding protein
- CDF
- Ca2+-dependent facilitation
- CDI
- Ca2+-dependent inactivation
- NSCaTE
- N-terminal spatial Ca2+-transforming element
References
- Adams P.J., Rungta R.L., Garcia E., van den Maagdenberg A.M., MacVicar B.A., Snutch T.P. 2010. Contribution of calcium-dependent facilitation to synaptic plasticity revealed by migraine mutations in the P/Q-type calcium channel. Proc. Natl. Acad. Sci. USA. 107:18694–18699 10.1073/pnas.1009500107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alseikhan B.A., DeMaria C.D., Colecraft H.M., Yue D.T. 2002. Engineered calmodulins reveal the unexpected eminence of Ca2+ channel inactivation in controlling heart excitation. Proc. Natl. Acad. Sci. USA. 99:17185–17190 10.1073/pnas.262372999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M.E., Mohler P.J. 2009. Rescuing a failing heart: think globally, treat locally. Nat. Med. 15:25–26 10.1038/nm0109-25 [DOI] [PubMed] [Google Scholar]
- Ashcroft F.M., Stanfield P.R. 1981. Calcium dependence of the inactivation of calcium currents in skeletal muscle fibers of an insect. Science. 213:224–226 10.1126/science.213.4504.224 [DOI] [PubMed] [Google Scholar]
- Babitch J. 1990. Channel hands. Nature. 346:321–322 10.1038/346321b0 [DOI] [PubMed] [Google Scholar]
- Bazzazi H., Ben Johny M., Adams P.J., Soong T.W., Yue D.T. 2013. Continuously tunable Ca2+ regulation of RNA-edited CaV1.3 channels. Cell Rep. 5:367–377 10.1016/j.celrep.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B.P. 2007. Neurophysiology: stressful pacemaking. Nature. 447:1059–1060 10.1038/4471059a [DOI] [PubMed] [Google Scholar]
- Ben Johny M., Yue D.N., Yue D.T. 2012. A Novel FRET-Based Assay Reveals 1:1 Stoichiometry of Apocalmodulin Binding Across Voltage-Gated Ca and Na ion channels. Biophys. J. 102:125a–126a 10.1016/j.bpj.2011.11.700 [DOI] [Google Scholar]
- Ben Johny M., Yang P.S., Bazzazi H.X., Yue D.T. 2013. Dynamic switching of calmodulin interactions underlies Ca2+ regulation of CaV1.3 channels. Nat Commun. 4:1717 10.1038/ncomms2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I. 2009. Calcium signaling and neurodegenerative diseases. Trends Mol. Med. 15:89–100 10.1016/j.molmed.2009.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock G., Gebhart M., Scharinger A., Jangsangthong W., Busquet P., Poggiani C., Sartori S., Mangoni M.E., Sinnegger-Brauns M.J., Herzig S., et al. 2011. Functional Properties of a Newly Identified C-terminal Splice Variant of Cav1.3 L-type Ca2+ channels. J. Biol. Chem. 286:42736–42748 10.1074/jbc.M111.269951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchaw J.L., Banks M.I., Jackson M.B. 1997. Ca2+- and voltage-dependent inactivation of Ca2+ channels in nerve terminals of the neurohypophysis. J. Neurosci. 17:5772–5781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Eckert R. 1978. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 202:1203–1206 10.1126/science.103199 [DOI] [PubMed] [Google Scholar]
- Budde T., Meuth S., Pape H.C. 2002. Calcium-dependent inactivation of neuronal calcium channels. Nat. Rev. Neurosci. 3:873–883 10.1038/nrn959 [DOI] [PubMed] [Google Scholar]
- Chad J.E., Eckert R. 1986. An enzymatic mechanism for calcium current inactivation in dialysed Helix neurones. J. Physiol. 378:31–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagot B., Chazin W.J. 2011. Solution NMR structure of Apo-calmodulin in complex with the IQ motif of human cardiac sodium channel NaV1.5. J. Mol. Biol. 406:106–119 10.1016/j.jmb.2010.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagot B., Potet F., Balser J.R., Chazin W.J. 2009. Solution NMR structure of the C-terminal EF-hand domain of human cardiac sodium channel NaV1.5. J. Biol. Chem. 284:6436–6445 10.1074/jbc.M807747200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C.S., Guzman J.N., Ilijic E., Mercer J.N., Rick C., Tkatch T., Meredith G.E., Surmeier D.J. 2007. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature. 447:1081–1086 10.1038/nature05865 [DOI] [PubMed] [Google Scholar]
- Chao S.H., Suzuki Y., Zysk J.R., Cheung W.Y. 1984. Activation of calmodulin by various metal cations as a function of ionic radius. Mol. Pharmacol. 26:75–82 [PubMed] [Google Scholar]
- Chaudhuri D., Alseikhan B.A., Chang S.Y., Soong T.W., Yue D.T. 2005. Developmental activation of calmodulin-dependent facilitation of cerebellar P-type Ca2+ current. J. Neurosci. 25:8282–8294 10.1523/JNEUROSCI.2253-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri D., Issa J.B., Yue D.T. 2007. Elementary Mechanisms Producing Facilitation of Cav2.1 (P/Q-type) channels. J. Gen. Physiol. 129:385–401 10.1085/jgp.200709749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium. 2013. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 381:1371–1379 10.1016/S0140-6736(12)62129-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotti L., Johnson C.N., Graf E., De Ferrari G.M., Cuneo B.F., Ovadia M., Papagiannis J., Feldkamp M.D., Rathi S.G., Kunic J.D., et al. 2013. Calmodulin mutations associated with recurrent cardiac arrest in infants. Circulation. 127:1009–1017 10.1161/CIRCULATIONAHA.112.001216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump S.M., Andres D.A., Sievert G., Satin J. 2013. The cardiac L-type calcium channel distal carboxy terminus autoinhibition is regulated by calcium. Am. J. Physiol. Heart Circ. Physiol. 304:H455–H464 10.1152/ajpheart.00396.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta M., Honeycutt T., Blumenthal D.K. 1989. The γ-Subunit of Skeletal Muscle Phosphorylase Kinase Contains Two Noncontiguous Domains That Act in Concert to Bind Calmodulin. J. Biol. Chem. 264:17156–17163 [PubMed] [Google Scholar]
- de Leon M., Wang Y., Jones L., Perez-Reyes E., Wei X., Soong T.W., Snutch T.P., Yue D.T. 1995. Essential Ca2+-binding motif for Ca2+-sensitive inactivation of L-type Ca2+ channels. Science. 270:1502–1506 10.1126/science.270.5241.1502 [DOI] [PubMed] [Google Scholar]
- DeMaria C.D., Soong T.W., Alseikhan B.A., Alvania R.S., Yue D.T. 2001. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 411:484–489 10.1038/35078091 [DOI] [PubMed] [Google Scholar]
- Dick I.E., Tadross M.R., Liang H., Tay L.H., Yang W., Yue D.T. 2008. A modular switch for spatial Ca2+ selectivity in the calmodulin regulation of CaV channels. Nature. 451:830–834 10.1038/nature06529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. 2007. Calcium channels are models of self-control. J. Gen. Physiol. 129:379–383 10.1085/jgp.200709786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Chad J.E. 1984. Inactivation of Ca channels. Prog. Biophys. Mol. Biol. 44:215–267 10.1016/0079-6107(84)90009-9 [DOI] [PubMed] [Google Scholar]
- Eckert R., Tillotson D.L. 1981. Calcium-mediated inactivation of the calcium conductance in caesium-loaded giant neurones of Aplysia californica. J. Physiol. 314:265–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson M.G., Alseikhan B.A., Peterson B.Z., Yue D.T. 2001. Preassociation of Calmodulin with Voltage-Gated Ca2+ Channels Revealed by FRET in Single Living Cells. Neuron. 31:973–985 10.1016/S0896-6273(01)00438-X [DOI] [PubMed] [Google Scholar]
- Erickson M.G., Liang H., Mori M.X., Yue D.T. 2003. FRET two-hybrid mapping reveals function and location of L-type Ca2+ channel CaM preassociation. Neuron. 39:97–107 10.1016/S0896-6273(03)00395-7 [DOI] [PubMed] [Google Scholar]
- Fallon J.L., Halling D.B., Hamilton S.L., Quiocho F.A. 2005. Structure of Calmodulin Bound to the Hydrophobic IQ Domain of the Cardiac Cav1.2 Calcium Channel. Structure. 13:1881–1886 10.1016/j.str.2005.09.021 [DOI] [PubMed] [Google Scholar]
- Fallon J.L., Baker M.R., Xiong L., Loy R.E., Yang G., Dirksen R.T., Hamilton S.L., Quiocho F.A. 2009. Crystal structure of dimeric cardiac L-type calcium channel regulatory domains bridged by Ca2·calmodulins. Proc. Natl. Acad. Sci. USA. 106:5135–5140 10.1073/pnas.0807487106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldkamp M.D., Yu L., Shea M.A. 2011. Structural and energetic determinants of apo calmodulin binding to the IQ motif of the NaV1.2 voltage-dependent sodium channel. Structure. 19:733–747 10.1016/j.str.2011.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findeisen F., Minor D.L., Jr 2010. Structural basis for the differential effects of CaBP1 and calmodulin on CaV1.2 calcium-dependent inactivation. Structure. 18:1617–1631 10.1016/j.str.2010.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findeisen F., Tolia A., Arant R., Kim E.Y., Isacoff E., Minor D.L., Jr 2011. Calmodulin overexpression does not alter Cav1.2 function or oligomerization state. Channels (Austin). 5:320–324 10.4161/chan.5.4.16821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findeisen F., Rumpf C.H., Minor D.L., Jr 2013. Apo states of calmodulin and CaBP1 control CaV1 voltage-gated calcium channel function through direct competition for the IQ domain. J. Mol. Biol. 425:3217–3234 10.1016/j.jmb.2013.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman J.N., Sánchez-Padilla J., Chan C.S., Surmeier D.J. 2009. Robust pacemaking in substantia nigra dopaminergic neurons. J. Neurosci. 29:11011–11019 10.1523/JNEUROSCI.2519-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F., Sokal I., Verlinde C.L., Erdjument-Bromage H., Tempst P., Pronin A.N., Benovic J.L., Fariss R.N., Palczewski K. 2000. Five members of a novel Ca2+-binding protein (CABP) subfamily with similarity to calmodulin. J. Biol. Chem. 275:1247–1260 10.1074/jbc.275.2.1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling D.B., Aracena-Parks P., Hamilton S.L. 2006. Regulation of voltage-gated Ca2+ channels by calmodulin. Sci. STKE. 2006:er1. [DOI] [PubMed] [Google Scholar]
- Huang H., Tan B.Z., Shen Y., Tao J., Jiang F., Sung Y.Y., Ng C.K., Raida M., Köhr G., Higuchi M., et al. 2012. RNA editing of the IQ domain in Cav1.3 channels modulates their Ca2+-dependent inactivation. Neuron. 73:304–316 10.1016/j.neuron.2011.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui A., Ellinor P.T., Krizanova O., Wang J.J., Diebold R.J., Schwartz A. 1991. Molecular cloning of multiple subtypes of a novel rat brain isoform of the α1 subunit of the voltage-dependent calcium channel. Neuron. 7:35–44 10.1016/0896-6273(91)90072-8 [DOI] [PubMed] [Google Scholar]
- Imredy J.P., Yue D.T. 1992. Submicroscopic Ca2+ diffusion mediates inhibitory coupling between individual Ca2+ channels. Neuron. 9:197–207 10.1016/0896-6273(92)90159-B [DOI] [PubMed] [Google Scholar]
- Imredy J.P., Yue D.T. 1994. Mechanism of Ca2+-sensitive inactivation of L-type Ca2+ channels. Neuron. 12:1301–1318 10.1016/0896-6273(94)90446-4 [DOI] [PubMed] [Google Scholar]
- Ivanina T., Blumenstein Y., Shistik E., Barzilai R., Dascal N. 2000. Modulation of L-type Ca2+ channels by Gβγ and calmodulin via interactions with N and C termini of α1C. J. Biol. Chem. 275:39846–39854 10.1074/jbc.M005881200 [DOI] [PubMed] [Google Scholar]
- Jarrett H.W., Madhavan R. 1991. Calmodulin-binding proteins also have a calmodulin-like binding site within their structure. The flip-flop model. J. Biol. Chem. 266:362–371 [PubMed] [Google Scholar]
- Jurado L.A., Chockalingam P.S., Jarrett H.W. 1999. Apocalmodulin. Physiol. Rev. 79:661–682 [DOI] [PubMed] [Google Scholar]
- Kang S., Cooper G., Dunne S.F., Dusel B., Luan C.H., Surmeier D.J., Silverman R.B. 2012. CaV1.3-selective L-type calcium channel antagonists as potential new therapeutics for Parkinson’s disease. Nat Commun. 3:1146 10.1038/ncomms2149 [DOI] [PubMed] [Google Scholar]
- Kasri N.N., Holmes A.M., Bultynck G., Parys J.B., Bootman M.D., Rietdorf K., Missiaen L., McDonald F., De Smedt H., Conway S.J., et al. 2004. Regulation of InsP3 receptor activity by neuronal Ca2+-binding proteins. EMBO J. 23:312–321 10.1038/sj.emboj.7600037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R.S., Sanguinetti M.C. 1984. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J. Gen. Physiol. 84:705–726 10.1085/jgp.84.5.705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.Y., Rumpf C.H., Fujiwara Y., Cooley E.S., Van Petegem F., Minor D.L., Jr 2008. Structures of CaV2 Ca2+/CaM-IQ Domain Complexes Reveal Binding Modes that Underlie Calcium-Dependent Inactivation and Facilitation. Structure. 16:1455–1467 10.1016/j.str.2008.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.Y., Rumpf C.H., Van Petegem F., Arant R.J., Findeisen F., Cooley E.S., Isacoff E.Y., Minor D.L., Jr 2010. Multiple C-terminal tail Ca2+/CaMs regulate CaV1.2 function but do not mediate channel dimerization. EMBO J. 29:3924–3938 10.1038/emboj.2010.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kink J.A., Maley M.E., Preston R.R., Ling K.Y., Wallen-Friedman M.A., Saimi Y., Kung C. 1990. Mutations in Paramecium calmodulin indicate functional differences between the C-terminal and N-terminal lobes in vivo. Cell. 62:165–174 10.1016/0092-8674(90)90250-I [DOI] [PubMed] [Google Scholar]
- Lee K.S., Marban E., Tsien R.W. 1985. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J. Physiol. 364:395–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Wong S.T., Gallagher D., Li B., Storm D.R., Scheuer T., Catterall W.A. 1999. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature. 399:155–159 10.1038/20194 [DOI] [PubMed] [Google Scholar]
- Lee A., Westenbroek R.E., Haeseleer F., Palczewski K., Scheuer T., Catterall W.A. 2002. Differential modulation of Cav2.1 channels by calmodulin and Ca2+-binding protein 1. Nat. Neurosci. 5:210–217 10.1038/nn805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Zhou H., Scheuer T., Catterall W.A. 2003. Molecular determinants of Ca2+/calmodulin-dependent regulation of Cav2.1 channels. Proc. Natl. Acad. Sci. USA. 100:16059–16064 10.1073/pnas.2237000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H., DeMaria C.D., Erickson M.G., Mori M.X., Alseikhan B.A., Yue D.T. 2003. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron. 39:951–960 10.1016/S0896-6273(03)00560-9 [DOI] [PubMed] [Google Scholar]
- Limpitikul W.B., Dick I.E., Joshi-Mukherjee R., Overgaard M.T., George A.L., Jr, Yue D.T. 2014. Calmodulin mutations associated with long QT syndrome prevent inactivation of cardiac L-type Ca2+ currents and promote proarrhythmic behavior in ventricular myocytes. J. Mol. Cell. Cardiol. In press 10.1016/j.yjmcc.2014.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Vogel H.J. 2012. Structural basis for the regulation of L-type voltage-gated calcium channels: interactions between the N-terminal cytoplasmic domain and Ca2+-calmodulin. Front Mol Neurosci. 5:38 10.3389/fnmol.2012.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Yang P.S., Yang W., Yue D.T. 2010. Enzyme-inhibitor-like tuning of Ca2+ channel connectivity with calmodulin. Nature. 463:968–972 10.1038/nature08766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan A., Sato D., Shiferaw Y., Baher A., Xie L.H., Peralta R., Olcese R., Garfinkel A., Qu Z., Weiss J.N. 2008. Modifying L-type calcium current kinetics: consequences for cardiac excitation and arrhythmia dynamics. Biophys. J. 94:411–423 10.1529/biophysj.106.98590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M.R., Clark J.P., III, Westenbroek R., Yu F.H., Scheuer T., Catterall W.A. 2011. Functional roles of a C-terminal signaling complex of CaV1 channels and A-kinase anchoring protein 15 in brain neurons. J. Biol. Chem. 286:12627–12639 10.1074/jbc.M110.175257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentrard D., Vassort G., Fischmeister R. 1984. Calcium-mediated inactivation of the calcium conductance in cesium-loaded frog heart cells. J. Gen. Physiol. 83:105–131 10.1085/jgp.83.1.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor D.L., Jr, Findeisen F. 2010. Progress in the structural understanding of voltage-gated calcium channel (CaV) function and modulation. Channels (Austin). 4:459–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M.X., Erickson M.G., Yue D.T. 2004. Functional stoichiometry and local enrichment of calmodulin interacting with Ca2+ channels. Science. 304:432–435 10.1126/science.1093490 [DOI] [PubMed] [Google Scholar]
- Mori M.X., Vander Kooi C.W., Leahy D.J., Yue D.T. 2008. Crystal structure of the CaV2 IQ domain in complex with Ca2+/calmodulin: high-resolution mechanistic implications for channel regulation by Ca2+. Structure. 16:607–620 10.1016/j.str.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morotti S., Grandi E., Summa A., Ginsburg K.S., Bers D.M. 2012. Theoretical study of L-type Ca2+ current inactivation kinetics during action potential repolarization and early afterdepolarizations. J. Physiol. 590:4465–4481 10.1113/jphysiol.2012.231886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. 1986. Concentration profiles of intracellular calcium in the presence of a diffusible chelator. Exp. Brain Res. 14:80–96 [Google Scholar]
- Nyegaard M., Overgaard M.T., Søndergaard M.T., Vranas M., Behr E.R., Hildebrandt L.L., Lund J., Hedley P.L., Camm A.J., Wettrell G., et al. 2012. Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. Am. J. Hum. Genet. 91:703–712 10.1016/j.ajhg.2012.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria S.F., Dell’Acqua M.L., Sather W.A. 2007. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 55:261–275 10.1016/j.neuron.2007.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria S.F., Dittmer P.J., Youn D.H., Dell’Acqua M.L., Sather W.A. 2012. Localized calcineurin confers Ca2+-dependent inactivation on neuronal L-type Ca2+ channels. J. Neurosci. 32:15328–15337 10.1523/JNEUROSCI.2302-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz S., Benmocha A., Sasson Y., Sachyani D., Almagor L., Lee A., Hirsch J.A., Dascal N. 2013. Competitive and non-competitive regulation of calcium-dependent inactivation in CaV1.2 L-type Ca2+ channels by calmodulin and Ca2+-binding protein 1. J. Biol. Chem. 288:12680–12691 10.1074/jbc.M113.460949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B.Z., DeMaria C.D., Adelman J.P., Yue D.T. 1999. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron. 22:549–558 10.1016/S0896-6273(00)80709-6 [DOI] [PubMed] [Google Scholar]
- Peterson B.Z., Lee J.S., Mulle J.G., Wang Y., de Leon M., Yue D.T. 2000. Critical determinants of Ca2+-dependent inactivation within an EF-hand motif of L-type Ca2+ channels. Biophys. J. 78:1906–1920 10.1016/S0006-3495(00)76739-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt G.S., Zühlke R.D., Hudmon A., Schulman H., Reuter H., Tsien R.W. 2001. Molecular basis of calmodulin tethering and Ca2+-dependent inactivation of L-type Ca2+ channels. J. Biol. Chem. 276:30794–30802 10.1074/jbc.M104959200 [DOI] [PubMed] [Google Scholar]
- Plant T.D., Standen N.B., Ward T.A. 1983. The effects of injection of calcium ions and calcium chelators on calcium channel inactivation in Helix neurones. J. Physiol. 334:189–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puopolo M., Raviola E., Bean B.P. 2007. Roles of subthreshold calcium current and sodium current in spontaneous firing of mouse midbrain dopamine neurons. J. Neurosci. 27:645–656 10.1523/JNEUROSCI.4341-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N., Olcese R., Bransby M., Lin T., Birnbaumer L. 1999. Ca2+-induced inhibition of the cardiac Ca2+ channel depends on calmodulin. Proc. Natl. Acad. Sci. USA. 96:2435–2438 10.1073/pnas.96.5.2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saimi Y., Kung C. 2002. Calmodulin as an ion channel subunit. Annu. Rev. Physiol. 64:289–311 10.1146/annurev.physiol.64.100301.111649 [DOI] [PubMed] [Google Scholar]
- Shen Y., Yu D., Hiel H., Liao P., Yue D.T., Fuchs P.A., Soong T.W. 2006. Alternative splicing of the Cav1.3 channel IQ domain, a molecular switch for Ca2+-dependent inactivation within auditory hair cells. J. Neurosci. 26:10690–10699 10.1523/JNEUROSCI.2093-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms B.A., Souza I.A., Zamponi G.W. 2013. A novel calmodulin site in the Cav1.2 N-terminus regulates calcium-dependent inactivation. Pflugers Arch. In press 10.1007/s00424-013-1423-9 [DOI] [PubMed] [Google Scholar]
- Singh A., Hamedinger D., Hoda J.C., Gebhart M., Koschak A., Romanin C., Striessnig J. 2006. C-terminal modulator controls Ca2+-dependent gating of Cav1.4 L-type Ca2+ channels. Nat. Neurosci. 9:1108–1116 10.1038/nn1751 [DOI] [PubMed] [Google Scholar]
- Snutch T.P., Reiner P.B. 1992. Ca2+ channels: diversity of form and function. Curr. Opin. Neurobiol. 2:247–253 10.1016/0959-4388(92)90111-W [DOI] [PubMed] [Google Scholar]
- Standen N.B., Stanfield P.R. 1982. A binding-site model for calcium channel inactivation that depends on calcium entry. Proc. R. Soc. Lond. B Biol. Sci. 217:101–110 10.1098/rspb.1982.0097 [DOI] [PubMed] [Google Scholar]
- Stern M.D. 1992. Buffering of calcium in the vicinity of a channel pore. Cell Calcium. 13:183–192 10.1016/0143-4160(92)90046-U [DOI] [PubMed] [Google Scholar]
- Stroffekova K. 2008. Ca2+/CaM-dependent inactivation of the skeletal muscle L-type Ca2+ channel (Cav1.1). Pflugers Arch. 455:873–884 10.1007/s00424-007-0344-x [DOI] [PubMed] [Google Scholar]
- Stroffekova K. 2011. The IQ motif is crucial for Cav1.1 function. J. Biomed. Biotechnol. 2011:504649 10.1155/2011/504649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier D.J., Sulzer D. 2013. The pathology roadmap in Parkinson disease. Prion. 7:85–91 10.4161/pri.23582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadross M.R., Dick I.E., Yue D.T. 2008. Mechanism of local and global Ca2+ sensing by calmodulin in complex with a Ca2+ channel. Cell. 133:1228–1240 10.1016/j.cell.2008.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiakina V., Boone A.N., Fux J., Senatore A., Weber-Adrian D., Guillemette J.G., Spafford J.D. 2013. The Calmodulin-Binding, Short Linear Motif, NSCaTE is Conserved in L-Type Channel Ancestors of Vertebrate Cav1.2 and Cav1.3 channels. PLoS ONE. 8:e61765 10.1371/journal.pone.0061765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B.Z., Jiang F., Tan M.Y., Yu D., Huang H., Shen Y., Soong T.W. 2011. Functional characterization of alternative splicing in the C terminus of L-type CaV1.3 channels. J. Biol. Chem. 286:42725–42735 10.1074/jbc.M111.265207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Halling D.B., Black D.J., Pate P., Zhang J.Z., Pedersen S., Altschuld R.A., Hamilton S.L. 2003. Apocalmodulin and Ca2+ calmodulin-binding sites on the CaV1.2 channel. Biophys. J. 85:1538–1547 10.1016/S0006-3495(03)74586-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay L.H., Dick I.E., Yang W., Mank M., Griesbeck O., Yue D.T. 2012. Nanodomain Ca2+ of Ca2+ channels detected by a tethered genetically encoded Ca2+ sensor. Nat Commun. 3:778 10.1038/ncomms1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson D. 1979. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc. Natl. Acad. Sci. USA. 76:1497–1500 10.1073/pnas.76.3.1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Petegem F., Chatelain F.C., Minor D.L., Jr 2005. Insights into voltage-gated calcium channel regulation from the structure of the CaV1.2 IQ domain-Ca2+/calmodulin complex. Nat. Struct. Mol. Biol. 12:1108–1115 10.1038/nsmb1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor R.G., Rusnak F., Sikkink R., Marban E., O’Rourke B. 1997. Mechanism of Ca2+-dependent inactivation of L-type Ca2+ channels in GH3 cells: direct evidence against dephosphorylation by calcineurin. J. Membr. Biol. 156:53–61 10.1007/s002329900187 [DOI] [PubMed] [Google Scholar]
- Wahl-Schott C., Baumann L., Cuny H., Eckert C., Griessmeier K., Biel M. 2006. Switching off calcium-dependent inactivation in L-type calcium channels by an autoinhibitory domain. Proc. Natl. Acad. Sci. USA. 103:15657–15662 10.1073/pnas.0604621103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Chung B.C., Yan H., Lee S.Y., Pitt G.S. 2012. Crystal structure of the ternary complex of a NaV C-terminal domain, a fibroblast growth factor homologous factor, and calmodulin. Structure. 20:1167–1176 10.1016/j.str.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F., Xia X.M., Tang J., Ao H., Ko S., Liauw J., Qiu C.S., Zhuo M. 2003. Calmodulin regulates synaptic plasticity in the anterior cingulate cortex and behavioral responses: a microelectroporation study in adult rodents. J. Neurosci. 23:8402–8409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Dzhura I., Colbran R.J., Anderson M.E. 2001. Calmodulin kinase and a calmodulin-binding ‘IQ’ domain facilitate L-type Ca2+ current in rabbit ventricular myocytes by a common mechanism. J. Physiol. 535:679–687 10.1111/j.1469-7793.2001.t01-1-00679.x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Xia X.M., Fakler B., Rivard A., Wayman G., Johnson-Pais T., Keen J.E., Ishii T., Hirschberg B., Bond C.T., Lutsenko S., et al. 1998. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 395:503–507 10.1038/26758 [DOI] [PubMed] [Google Scholar]
- Xu W., Lipscombe D. 2001. Neuronal CaV1.3α1 L-Type Channels Activate at Relatively Hyperpolarized Membrane Potentials and Are Incompletely Inhibited by Dihydropyridines. J. Neurosci. 21:5944–5951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Wu L.G. 2005. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron. 46:633–645 10.1016/j.neuron.2005.03.024 [DOI] [PubMed] [Google Scholar]
- Yang J., McBride S., Mak D.O., Vardi N., Palczewski K., Haeseleer F., Foskett J.K. 2002. Identification of a family of calcium sensors as protein ligands of inositol trisphosphate receptor Ca2+ release channels. Proc. Natl. Acad. Sci. USA. 99:7711–7716 10.1073/pnas.102006299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P.S., Alseikhan B.A., Hiel H., Grant L., Mori M.X., Yang W., Fuchs P.A., Yue D.T. 2006. Switching of Ca2+-dependent inactivation of Cav1.3 channels by calcium binding proteins of auditory hair cells. J. Neurosci. 26:10677–10689 10.1523/JNEUROSCI.3236-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P.S., Johny M.B., Yue D.T. 2014. Allostery in Ca2+ channel modulation by calcium-binding proteins. Nat. Chem. Biol. 10:231–238 10.1038/nchembio.1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D.T. 2004. The dawn of high-resolution structure for the queen of ion channels. Neuron. 42:357–359 10.1016/S0896-6273(04)00259-4 [DOI] [PubMed] [Google Scholar]
- Yue D.T., Backx P.H., Imredy J.P. 1990. Calcium-sensitive inactivation in the gating of single calcium channels. Science. 250:1735–1738 10.1126/science.2176745 [DOI] [PubMed] [Google Scholar]
- Zamponi G.W. 2003. Calmodulin Lobotomized: Novel Insights into Calcium Regulation of Voltage-Gated Calcium Channels. Neuron. 39:879–881 10.1016/S0896-6273(03)00564-6 [DOI] [PubMed] [Google Scholar]
- Zeilhofer H.U., Blank N.M., Neuhuber W.L., Swandulla D. 1999. Calcium-dependent inactivation of neuronal calcium channel currents is independent of calcineurin. Neuroscience. 95:235–241 10.1016/S0306-4522(99)00434-0 [DOI] [PubMed] [Google Scholar]
- Zhou J., Olcese R., Qin N., Noceti F., Birnbaumer L., Stefani E. 1997. Feedback inhibition of Ca2+ channels by Ca2+ depends on a short sequence of the C terminus that does not include the Ca2+-binding function of a motif with similarity to Ca2+-binding domains. Proc. Natl. Acad. Sci. USA. 94:2301–2305 10.1073/pnas.94.6.2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Kim S.A., Kirk E.A., Tippens A.L., Sun H., Haeseleer F., Lee A. 2004. Ca2+-binding protein-1 facilitates and forms a postsynaptic complex with Cav1.2 (L-type) Ca2+ channels. J. Neurosci. 24:4698–4708 10.1523/JNEUROSCI.5523-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Yu K., McCoy K.L., Lee A. 2005. Molecular mechanism for divergent regulation of Cav1.2 Ca2+ channels by calmodulin and Ca2+-binding protein-1. J. Biol. Chem. 280:29612–29619 10.1074/jbc.M504167200 [DOI] [PubMed] [Google Scholar]
- Zühlke R.D., Reuter H. 1998. Ca2+-sensitive inactivation of L-type Ca2+ channels depends on multiple cytoplasmic amino acid sequences of the α1C subunit. Proc. Natl. Acad. Sci. USA. 95:3287–3294 10.1073/pnas.95.6.3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zühlke R.D., Pitt G.S., Deisseroth K., Tsien R.W., Reuter H. 1999. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 399:159–162 10.1038/20200 [DOI] [PubMed] [Google Scholar]
- Zühlke R.D., Pitt G.S., Tsien R.W., Reuter H. 2000. Ca2+-sensitive inactivation and facilitation of L-type Ca2+ channels both depend on specific amino acid residues in a consensus calmodulin-binding motif in the α1C subunit. J. Biol. Chem. 275:21121–21129 10.1074/jbc.M002986200 [DOI] [PubMed] [Google Scholar]