Abstract
Rodent models of facial itch and pain provide a valuable tool for distinguishing between behaviors related to each sensation. In rats, pruritogens applied to the face elicit scratching using the hindlimb while algogens elicit wiping using the forelimb. We wished to determine the role of trigeminothalamic tract (VTT) neurons in carrying information regarding facial itch and pain to the forebrain. We have characterized responses to facially applied pruritogens (serotonin, BAM8–22, chloroquine, histamine, capsaicin, and cowhage) and noxious stimuli in 104 VTT neurons recorded from anesthetized rats. Each VTT neuron had a mechanically sensitive cutaneous receptive field on the ipsilateral face. All pruriceptive VTT neurons also responded to noxious mechanical and/or thermal stimulation. Over half of VTT neurons responsive to noxious stimuli also responded to at least one pruritogen. Each tested pruritogen, with the exception of cowhage, produced an increase in discharge rate in a subset of VTT neurons. The response to each pruritogen was characterized, including maximum discharge rate, response duration, and spike timing dynamics. Pruriceptive VTT neurons were recorded from throughout superficial and deep layers of the spinal trigeminal nucleus and were shown to project via antidromic mapping to the ventroposterior medial nucleus or posterior thalamic nuclei. These results indicate that pruriceptive VTT neurons are a subset of polymodal nociceptive VTT neurons and characterize a system conducive to future experiments regarding the similarities and differences between facial itch and pain.
Keywords: itch, pain, trigeminothalamic tract
itch and pain are experienced as distinct sensations. Itch causes the desire to scratch and pain typically results in protective behaviors such as withdrawal from painful stimuli. A substantial body of evidence indicates that neurons that respond to pruritogens comprise a subset of a population of cells that also respond to painful stimuli (Carstens 1997; Davidson et al. 2007; Drzezga et al. 2001; Jinks and Carstens 2002; Johanek et al. 2008; Simone et al. 2004). However, several studies have suggested that itch and pain are processed by separate neurons and may even involve separate types of receptors (Liu et al. 2009; Sun and Chen 2007; Sun et al. 2009; Wilson et al. 2011). Recently, it has been demonstrated that although itch-responsive neurons also respond to painful stimuli, selective activation of these neurons, even by a painful stimulus such as capsaicin, results only in itch-related behaviors (Han et al. 2013), indicating that itch and pain information may indeed be processed by separate populations of neurons.
In rodent studies of the neural mechanisms contributing to itch and pain, pruritogens and algogens that have been used are based on corresponding itch and pain sensations evoked in humans. To study the underlying mechanisms and relationships between itch and pain, it is important to employ experimental models that elicit distinct behavioral responses to each sensation. In many rodent studies pruritogens have been applied to the skin on the nape of the neck, a body region that can only be accessed by the hindlimb (e.g., for scratching) and therefore does not allow study of other potentially relevant behaviors. In the rostral back, algogens and pruritogens each elicit scratching and it is not possible to behaviorally discriminate itchy from painful stimuli. In contrast, application of algogens or pruritogens can elicit distinct behavioral responses when applied to the face in mice (Shimada and LaMotte 2008). In this “cheek model of itch,” algogens applied to the cheek elicited wiping with the forelimb, while pruritogens elicited scratching with the hindlimb. More recently the cheek model has been used to demonstrate that rats also show distinct behavioral responses to stimuli that produce pain or itch in humans. When applied to the face, serotonin and chloroquine elicit scratching with the hindlimb while the algogen mustard oil elicits wiping with the forelimb. Interestingly, facial application of histamine or capsaicin causes a mixture of scratching and wiping in rats, depending on the concentrations used. Histamine induces a greater number of wipes than scratch bouts while capsaicin induces an equivalent number of each type of behavior (Klein et al. 2011). These chemicals can also produce mixed itch and pain sensations in humans when delivered via heat-inactivated cowhage spicule (Sikand et al. 2009), although intradermal injection of capsaicin produces a sensation of burning pain (LaMotte et al. 1991). In the current study, histamine and capsaicin are referred to as “partial pruritogens.”
The differences between the cheek model and the rostral back model of itch in rodents highlight the usefulness of studying itch using the cheek model and examining underlying neural mechanisms within the trigeminal sensory system. However, few studies have focused on this approach (Akiyama et al. 2010; Klein et al. 2011). The neurons in the spinal trigeminal nucleus that convey information to the forebrain about itch occurring on the face have not been identified or characterized. Information about itch in body regions below the head and neck is carried to the brain by cells in the spinothalamic tract (STT), in addition to other possible tracts that have not been examined. In humans, lesions of the anterior lateral quadrant of the spinal cord (including STT axons) abolish both itch and pain sensations (White and Sweet 1969). In nonhuman primates, a subset of STT cells responds with an increase in firing rate to the itchy stimuli histamine or cowhage (Davidson et al. 2007, 2012; Simone et al. 2004). These responses are decreased during scratching of the receptive field (Davidson et al. 2009), suggesting that these neurons are involved in producing the sensation of itch. In the face, information about noxious and pruritic stimuli is carried by the trigeminal nerve (cranial nerve V) to several targets in the brainstem, including the spinal trigeminal nucleus. A subset of neurons in the spinal trigeminal nucleus sends axons to the brain via the trigeminothalamic tract (VTT). We propose that, like the STT for lower body receptive fields, the VTT carries information regarding facial itch to the brain. Here, we have examined the responses of antidromically identified VTT neurons to the application of several pruritogens and partial pruritogens applied to facial receptive fields in anesthetized rats. The pruriceptive and nociceptive responses, receptive fields, recording points, and axon projections of these cells were characterized.
MATERIALS AND METHODS
Animal preparation.
Adult male Sprague-Dawley rats (300–450 g) were used according to protocols approved by the University of Minnesota's Institutional Animal Care and Use Committee. Animals were deeply anesthetized with urethane (1.5 mg/kg ip; Sigma) and tracheotomized. A laminectomy was performed over the first and second cervical segments, and a craniotomy was performed over the right thalamus. A low-impedance stainless steel electrode was positioned at stereotaxic coordinates for the caudal pole of the ventroposterior medial (VPM) nucleus in the thalamus. Pulses of electrical current (300–500 μA, 200 μs, 3 Hz) were delivered through the electrode as an initial search stimulus. A recording electrode (stainless steel, 10 MΩ; FHC for most cases or carbon fiber, 4–10 MΩ for some experiments) was lowered through the contralateral spinal trigeminal nucleus extending from the caudal medulla to the second cervical segment of the spinal cord to search for time-locked single unit responses that met the following standard criteria for antidromically activated VTT neurons: 1) stable antidromic response latency (<0.05 ms variation); 2) ability to follow high-frequency (>300 Hz) antidromic stimulus train; and 3) collision of mechanically evoked orthodromic spike with putative antidromic spike. Any single unit response that met these criteria and had a cutaneous facial receptive field was used for further study, although neurons responsive to noxious stimulation of the receptive field were favored over those maximally responsive to low-threshold mechanical stimuli.
Axon projection mapping.
The stimulating electrode was positioned at 200-μm intervals throughout dorsal-ventral stimulating tracks separated by medial-lateral and rostral-caudal intervals of 300–500 μm in the brain; the amount of current necessary to elicit an antidromic spike was determined at each position. The point at which the threshold for antidromic activation was lowest (≤30 μA) was assumed to be the most accurate indication of the position of the axon terminal (Burstein et al. 1991; Dado et al. 1994). If the antidromic latency remained unchanged by ≤0.05 ms at more rostral positions, it was assumed that the axon did not extend rostrally beyond the identified low threshold point.
Characterization of neurons with mechanical and thermal stimuli.
Innocuous brushing with a soft-bristled brush and the minimum necessary amount of noxious stimuli were used to identify the mechanical receptive field boundaries for each antidromically identified VTT neuron. Pressure was applied using a small clip that produces a distinct subnoxious pressure sensation when applied to the skin in humans; pinching was applied using a small clip that produces a distinct pain sensation when applied to the skin in humans. Brush, pressure, and pinch were used to classify each neuron as low threshold (LT; maximally responsive to innocuous brushing), high threshold (HT; responsive only to noxious pressure and/or pinching), or wide dynamic range (WDR; responsive to innocuous and noxious stimuli, with a higher frequency response to noxious stimuli). Thermal responses were tested by applying a noxious heat stimulus (50°C from a 32°C baseline) to the center of the mechanical receptive field with a feedback controlled Peltier thermode (3 mm2; Yale Instruments).
Pruritic characterization.
After responses to mechanical and thermal stimuli were obtained, sensitivity of each cell to the following chemical stimuli was determined: histamine dihydrochloride (HA; 900 mM; Sigma), serotonin creatinine sulfate complex (5-HT; 47 mM; Sigma), chloroquine diphosphate salt (CQ; 100 mM; Sigma), bovine adrenal medullary 8–22 peptide (BAM8–22; 1 mM; Tocris Bioscience), capsaicin (CAP; 3.3 mM; Sigma), and/or cowhage (COW; ≥10 spicules). Concentrations were chosen within ranges that elicited a significant number of scratch bouts, as reported by Klein et al. (2011). BAM8–22 causes hindlimb scratching upon facial application in mice at an amount comparable to what was used in the current study (Wilson et al. 2011); to our knowledge, the behavioral effects of application of BAM8–22 to the rat face have not been reported. Each drug (except cowhage) was injected intradermally in a 10-μl volume at separate sites within the mechanical receptive field for each cell; for cowhage, ≥10 spicules were inserted within a small area of the receptive field using an applicator consisting of spicules attached to the end of a cotton-tipped swab. Vehicles [pH-matched saline for injected drugs and heat-inactivated spicules for cowhage (IA COW)] were always applied before their respective active stimuli. An attempt was made to test each cell with every drug; however, recording stability did not always allow this. Drugs were applied in random order on each trial, with the exception that capsaicin was always the last drug applied. The ongoing discharge rate of a cell was always allowed to return to baseline before application of a subsequent stimulus, with a minimum of 10 min between drug applications.
Histology.
At the end of each experiment, LT antidromic stimulation point(s) in the thalamus and recording point(s) in the spinal trigeminal nucleus were marked with electrolytic lesions. Rats were perfused with 0.9% normal saline followed by 10% formalin with 1% ferrocyanide (for Prussian blue reaction at lesion sites). The brain was removed and cut on a freezing microtome; sections containing thalamus were sectioned by 75 μm and sections containing the spinal trigeminal nucleus were sectioned by 50 μm. Sections were stained with neutral red, and a rat brain atlas (Paxinos and Watson 1982) was used to identify thalamic nuclei for axon projection sites.
Data analysis.
Action potentials were amplified, filtered, and digitized and wave-form discriminated using DAPSYS data acquisition processor system software (www.dapsys.net). A cell was considered responsive to a stimulus if it displayed a poststimulus discharge rate ≥1.5 times the mean discharge rate over 60 s before stimulus application and the increased discharge rate outlasted any response to vehicle. Individual response histograms are reported in 1-s bins. Mean responses are reported in 1-, 5-, or 15-s bins (specified in text) with errors bars representing SE. For statistical analyses, discharge rates were normalized to 60 s of baseline activity preceding stimulus application. To compare variability in spike trains elicited by pruritogens and partial pruritogens, spike timing data was analyzed over a 3-min period beginning 30 s after application of the pruritogen (to avoid activity evoked by insertion of the needle during injection of the pruritogen). To compare variability in spike trains elicited by serotonin and the noxious stimuli heat and pinch, spike timing data were analyzed over a 10-s period beginning 30 s after application of serotonin or during the 5- to 10-s application of heat or pinch. The coefficient of variation (CV) of a spike train was calculated as the SD of interspike intervals (ISIs) divided by the mean duration of ISIs within the spike train. For ISI distribution histograms, the number of ISIs contained in each of 20 log-scaled bins was divided by the total number of ISIs in a given spike train and multiplied by 100 to obtain percentage; mean percent in each bin was calculated for responses to each stimulus. For all statistical tests, effects across stimuli or cell types were compared using Wilcoxon rank sums or Kruskal-Wallis ANOVA analyses with Dunn's posttest, with P < 0.05 considered significant.
RESULTS
One-hundred four VTT neurons from 76 rats were tested for responses to several pruritogens and partial pruritogens, including serotonin, BAM8–22, chloroquine, histamine, capsaicin, and cowhage. Recording points of VTT neurons were located throughout the spinal trigeminal nucleus in the caudal medulla and first and second cervical segments of the spinal cord, and axons terminated in the contralateral thalamus. Every neuron identified using the antidromic stimulation methods employed in this study responded to mechanical stimulation of its receptive field; each time a mechanically sensitive cutaneous receptive field could not be identified, a mechanically sensitive intraoral, intranasal, or corneal receptive field was found to be present. Of the 90 VTT neurons that responded to noxious mechanical stimulation of the facial skin, 54% were classified as WDR and 46% as HT; 31% of the 77 neurons tested with a 50°C stimulus responded to noxious heat. VTT cells that responded to any of the scratch-inducing chemicals tested will be referred to as “pruriceptive” neurons; all pruriceptive VTT neurons also responded to noxious mechanical stimuli. Cells that responded to noxious mechanical or thermal stimulation but did not respond to any tested pruritogen or partial pruritogen will be referred to as “nociceptive-only” neurons. Fourteen VTT neurons responded maximally to subnoxious mechanical stimuli and were classified as LT; none of the LT neurons was pruriceptive.
Pruriceptive VTT neurons.
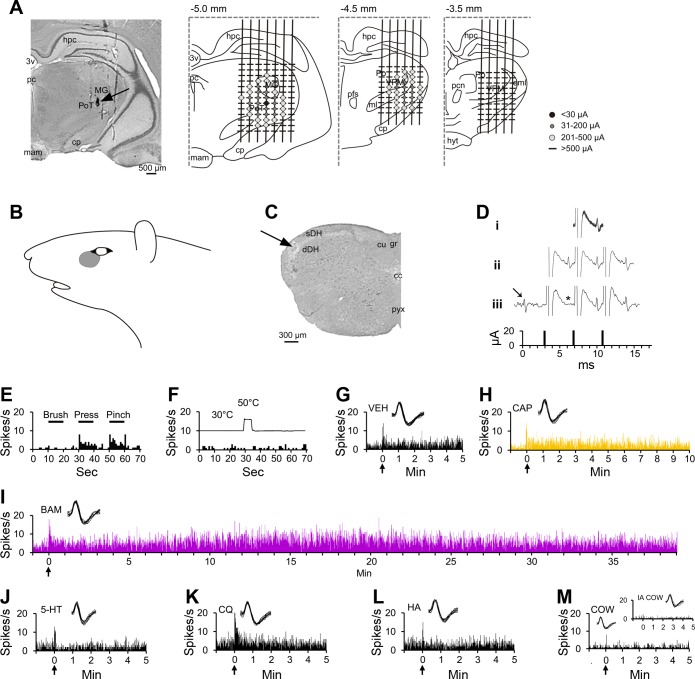
Figure 1 shows an example of a pruriceptive VTT neuron that responded to multiple chemicals injected intradermally within its cutaneous receptive field (Fig. 1B). The recorded unit was located in the superficial layers of the spinal trigeminal nucleus near the level of the pyramidal decussation (Fig. 1C), and its axon projected to the posterior triangular (PoT) nucleus of the contralateral thalamus, as evidenced by antidromic activation of the cell by 20-μA pulses applied to the PoT nucleus but not at levels rostral to the PoT (Fig. 1, A and D). This cell was classified as HT, as it responded selectively to noxious pressure and pinch (Fig. 1E) applied to its receptive field. This cell did not respond to a 50°C heat stimulus (Fig. 1F). Each of the six chemical stimuli employed in this study were consecutively tested on this neuron. The cell responded to intradermal injection of vehicle while the needle was in the skin, but there was no sustained response to vehicle after the needle was removed (Fig. 1G). Likewise, the placement of the needle into the skin during the injection of capsaicin caused a short discharge, but unlike vehicle, capsaicin produced a response that lasted >10 min following removal of the needle (Fig. 1H). Injection of BAM8–22 elicited increased an discharge rate for >40 min (Fig. 1I). The cell did not respond to serotonin, chloroquine, histamine, or cowhage (Fig. 1, J–M).
Fig. 1.
Characterization of a pruriceptive trigeminothalamic tract (VTT) neuron responding to BAM8–22 and capsaicin. A: lesion (arrow) made at the point in the thalamus with the lowest threshold for antidromic activation. Drawings indicate the location of each antidromic test site at 3 rostral-caudal planes within the thalamus; threshold for antidromic activation at each point is indicated by the color/size of the marker at each point (see legend). Numbers above each drawing indicate distance caudal to bregma. B: receptive field. C: lesion (arrow) made at the recording point in the superficial layers of the spinal trigeminal nucleus. D: antidromic spikes have a fixed latency from the antidromic stimulus (i), follow a high-frequency stimulus train (ii), and an orthodromic spike (arrow) collides with an antidromic spike (expected location indicated by *; iii). E: responses to mechanical stimulation of the receptive field (black bar above histogram indicates duration of mechanical stimulus). F: response to thermal stimulation of the receptive field (trace above histogram indicates onset and duration of 50°C thermal stimulus). G: intradermal injection (indicated by arrow) of vehicle into the receptive field produced no sustained response. H–I: the cell responded to the partial pruritogen capsaicin and to the pruritogen BAM8–22, each injected intradermally into the receptive field. Responses are colored differently according to chemical, so that data regarding a single pruritogen or partial pruritogen can be more easily identified throughout the following figures. J–M: the cell did not respond to intradermal injection of serotonin, chloroquine, or histamine, or to application of cowhage spicules (M, inset: response to heat-inactivated cowhage). For this cell, pruritogens and partial pruritogens were applied in the following order: cowhage (COW), BAM8–22 (BAM), chloroquine (CQ), histamine (HA), serotonin (5-HT), and capsaicin (CAP). Insets: 10 randomly selected overlaid spike traces. 3v, third ventricle; cc, central canal; cp, cerebral peduncle; cu, cuneate fasciculus; dDH, deep dorsal horn; eml, external medullary lamina; gr, gracile fasciculus; hpc, hippocampus; hyt, hypothalamus; mam, mammillary n.; MG, medial geniculate n.; ml, medial lemniscus; pc, posterior commissure; pcn, paracentral n.; pfs, parafascicular n.; Po, posterior thalamic n.; PoT, posterior triangular n.; pyx, pyramidal decussation; sDH, superficial dorsal horn; VPM, ventroposterior medial n.
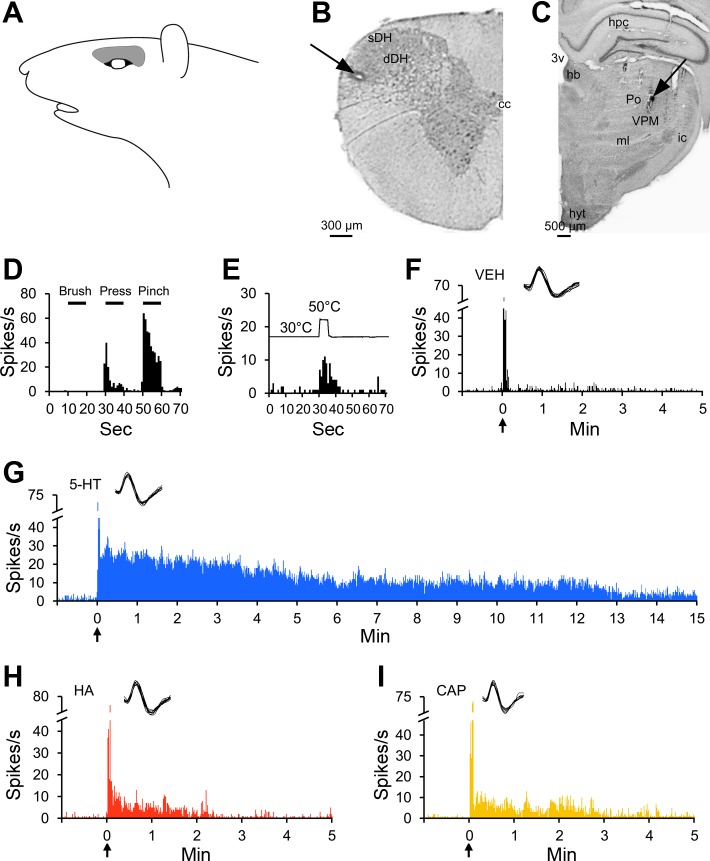
An example of a pruriceptive cell that projected to the VPM nucleus in the contralateral thalamus is illustrated in Fig. 2. This neuron had a mechanical receptive field superior to the eye (Fig. 2A). It was recorded from superficial layers of the spinal trigeminal nucleus at the border between the first and second cervical segments of the spinal cord (Fig. 2B). The location from which its axon was antidromically activated is shown in Fig. 2C. This cell was classified as HT (Fig. 2D) and responded to noxious heat (Fig. 2E). This neuron exhibited a short discharge during needle insertion and injection of vehicle (Fig. 2F); intradermal injection of serotonin, histamine, or capsaicin produced an increase in discharge rate that outlasted the short response to vehicle (Fig. 2, G–I). The response to serotonin lasted >15 min, while responses to histamine and capsaicin returned to baseline within 5 min following injection. None of the other pruritogens elicited a response (data not shown).
Fig. 2.
Characterization of a pruriceptive VTT neuron responding to serotonin, histamine, and capsaicin. A: receptive field. B: lesion (arrow) made at the recording point in the superficial layers of the spinal trigeminal nucleus. C: lesion (arrow) made at the point in the thalamus with the lowest threshold for antidromic activation. D: responses to mechanical stimulation of the receptive field. E: response to thermal stimulation of the receptive field. F: the cell did not respond to intradermal injection of vehicle into the receptive field. G–I: the cell responded to the pruritogen serotonin and to the partial pruritogens histamine and capsaicin, each injected intradermally into the receptive field. For this cell, pruritogens and partial pruritogens were applied in the following order: serotonin, cowhage, histamine, chloroquine, BAM8–22, and capsaicin. hb, habenular n.; ic, internal capsule.
An example of a pruriceptive VTT neuron recorded in the deep dorsal horn is provided in Fig. 3. Its cutaneous receptive field was located on the cheek (Fig. 3A). It was recorded in the first cervical segment (Fig. 3B) and projected to the VPM nucleus (Fig. 3C). The cell was classified as WDR (Fig. 3D) and did not respond to noxious heat (Fig. 3E). Histamine, chloroquine, and capsaicin each produced an increase in discharge rate compared with vehicle (Fig. 3, F–I); the response to each of these three chemicals remained elevated above baseline for >5 min. The cell did not respond to serotonin or BAM8–22 (data not shown).
Fig. 3.
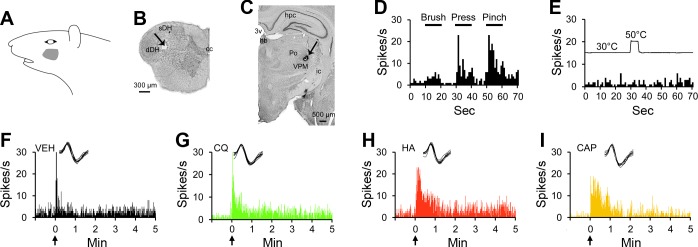
Characterization of a pruriceptive VTT neuron responding to chloroquine, histamine, and capsaicin. A: receptive field. B: lesion (arrow) made at the recording point in the deep layers of the spinal trigeminal nucleus. C: lesion (arrow) made at the point in the thalamus with the lowest threshold for antidromic activation. D: responses to mechanical stimulation of the receptive field. E: response to thermal stimulation of the receptive field. F: the cell did not respond to intradermal injection of vehicle into the receptive field. G–I: the cell responded to the pruritogen chloroquine and to the partial pruritogens histamine and capsaicin, each injected intradermally into the receptive field. For this cell, pruritogens and partial pruritogens were applied in the following order: cowhage, BAM8–22, chloroquine, histamine, serotonin, and capsaicin. sth, subthalamic n.
Nociceptive-only VTT neurons.
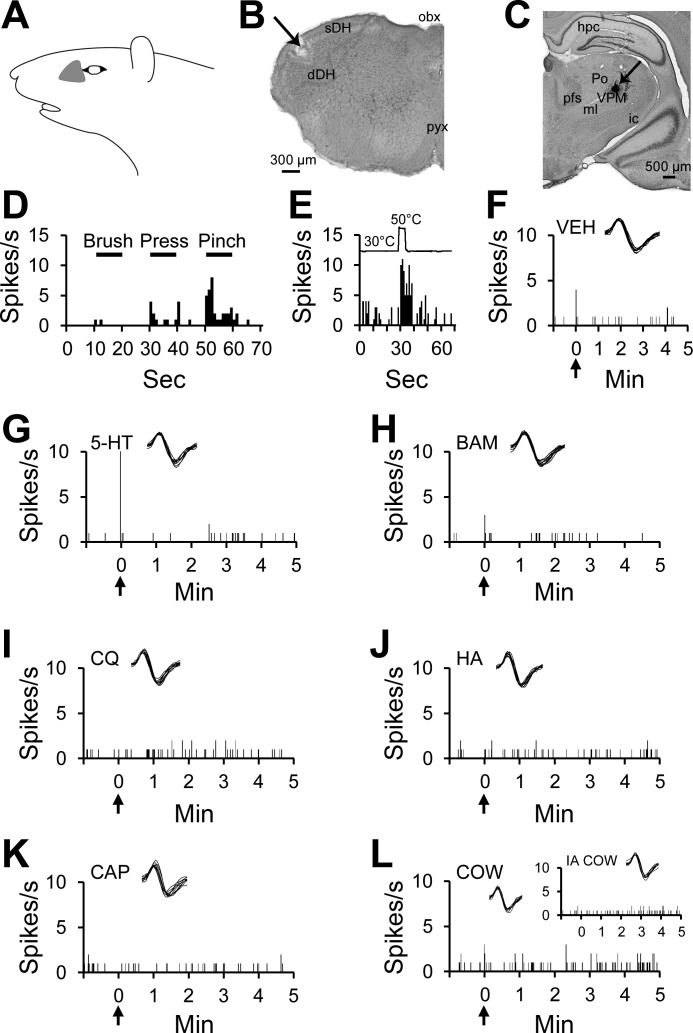
In contrast to pruriceptive VTT neurons, nociceptive-only VTT neurons did not respond to any tested pruritogens or partial pruritogens. An example of a nociceptive-only VTT neuron is shown in Fig. 4. This cell was recorded from superficial layers of the spinal trigeminal nucleus at the level of the pyramidal decussation (Fig. 4B) and projected to the VPM nucleus (Fig. 4C). The neuron responded to noxious mechanical (Fig. 4D) and thermal (Fig. 4E) stimulation of its receptive field (Fig. 4A) and was classified as HT. None of the six tested pruritogens or partial pruritogens produced a response in this cell (Fig. 4, F–L). It was therefore classified as nociceptive only.
Fig. 4.
Characterization of a nociceptive-only neuron responding to noxious mechanical and thermal stimulation, but not to any tested pruritogens or partial pruritogens. A: receptive field. B: lesion (arrow) made at the recording point in the superficial layers of the spinal trigeminal nucleus. C: lesion (arrow) made at the point in the thalamus with the lowest threshold for antidromic activation. D: responses to mechanical stimulation of the receptive field. E: response to thermal stimulation of the receptive field. F–L: the cell did not respond to intradermal injection of vehicle, serotonin, BAM8–22, chloroquine, histamine, or capsaicin or insertion of cowhage spicules into the receptive field [L, inset: response to heat-inactivated (IA) cowhage spicules]. For this cell, pruritogens and partial pruritogens were applied in the following order: serotonin, BAM8–22, chloroquine, histamine, cowhage, and capsaicin. obx, obex.
Characterization of responses to each pruritogen.
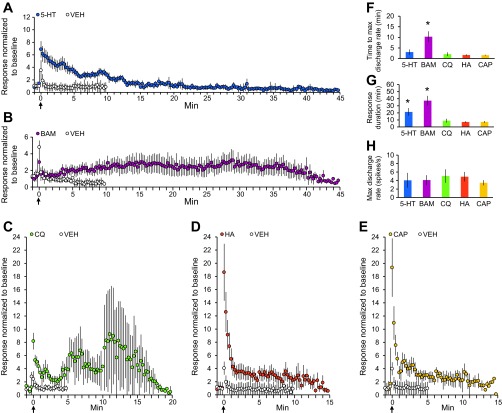
The mean response to each pruritogen for all cells that met the criteria for being responsive to a pruritogen is shown in Fig. 5. The mean response to serotonin (Fig. 5A) reached a maximum discharge rate within 5 min after application (Fig. 5F) and remained elevated above the preserotonin baseline discharge rate for over 20 min (Fig. 5G); the mean duration of the response to serotonin was significantly greater than the mean duration of responses to chloroquine, histamine, or capsaicin. In five neurons, we were able to obtain stable recordings to demonstrate responses to serotonin that remained elevated above baseline for greater than 30 min. The only pruritogen that produced a response that outlasted the response to serotonin was BAM8–22 (Fig. 5 B and G). The mean response to BAM8–22 reached a maximum discharge rate ∼10 min after application (Fig. 5F) and remained elevated above baseline for 35–40 min (Fig. 5G); in four neurons we were able to obtain stable recordings of responses to BAM8–22 lasting at least 40 min. Mean responses to chloroquine, histamine, and capsaicin peaked within the first minute following application (Fig. 5F) and returned to baseline activity levels within 10 min (Fig. 5G). The high SE for the response to chloroquine (Fig. 5C) suggests that responses elicited by chloroquine were more variable across individual cells than those elicited by the other pruritogens or partial pruritogens. The mean maximum discharge rates during the responses to the pruritogens and partial pruritogens did not significantly differ. The mean maximum discharge rate for each chemical ranged from three to five spikes per second (Fig. 5H).
Fig. 5.
Characterization of the mean response to each pruritogen or partial pruritogen. A: mean response to serotonin (blue); mean response to vehicle in serotonin-responsive cells (white) (n = 22). B: mean response to BAM8–22 (purple); mean response to vehicle in BAM8–22-responsive cells (white) (n = 6). C: mean response to chloroquine (green); mean response to vehicle in chloroquine-responsive cells (white) (n = 7). D: mean response to the partial pruritogen histamine (orange); mean response to vehicle in histamine-responsive cells (white) (n = 23). E: mean response to the partial pruritogen capsaicin (yellow); mean response to vehicle in capsaicin-responsive cells (white) (n = 13). F: mean time to reach maximum discharge rate, calculated by finding the highest discharge rate in a 60-s sliding window following stimulus application for each pruritogen and partial pruritogen. *P = 0.0038, Kruskal-Wallis ANOVA. G: mean time to return to baseline discharge rate, calculated by determining when the mean discharge rate in a 60 s sliding window following stimulus application dropped below 1.5 times the mean discharge rate over 60 s before stimulus application. *P = 0.0057, Kruskal-Wallis ANOVA. H: mean maximum discharge rate for each pruritogen and partial pruritogen. Data only included from neurons for which the receptive field was not manipulated during the response to a chemical. For A–E: data were normalized to 60 s of baseline activity preceding stimulus application and reported in 15-s bins.
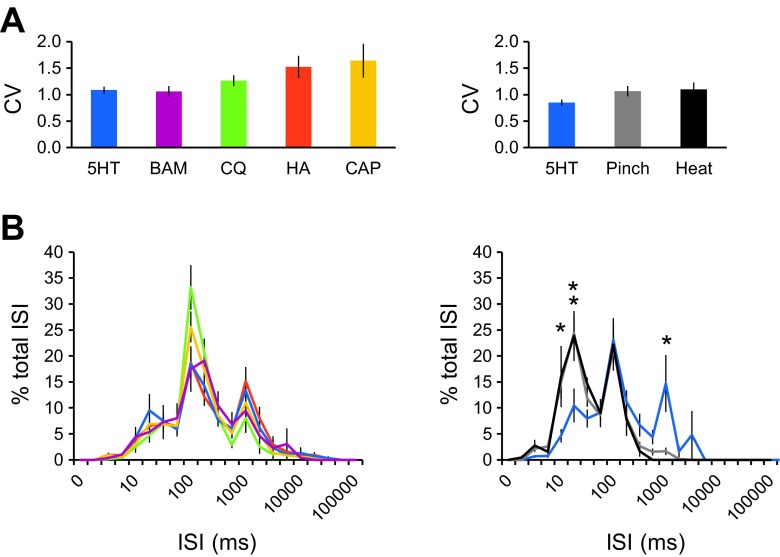
To determine whether VTT cells displayed different spike timing dynamics or spiking patterns in response to different stimuli, the CV was calculated during responses to each pruritogen or partial pruritogen. The CV was also calculated during responses to noxious mechanical (pinch) and thermal stimuli to determine whether there was a difference between pruriceptive and nociceptive responses within pruriceptive VTT neurons responsive to serotonin. The CV is a measure of the variability of ISIs and values >1.0 indicate higher degrees of variability within a spike train. The mean CV did not significantly differ between responses produced by different pruritogens or partial pruritogens. In neurons responsive to serotonin, the mean CV did not significantly differ between responses to the pruritogen vs. the noxious stimuli pinch or heat (Fig. 6A). ISI distribution histograms were compared for responses to each of the pruritogens and partial pruritogens, as well as for responses to pruritic vs. noxious stimuli within serotonin-responsive cells (Fig. 6B). ISIs produced during pruritic responses were highly variable in distribution, but the ISI distributions of the responses to various pruritogens or partial pruritogens did not significantly differ from each other. There was a significant difference in ISI distribution between responses to serotonin vs. responses by the same cells to noxious pinching or heat. Noxious stimuli displayed a bimodal ISI distribution indicative of bursting, with a greater proportion of shorter ISIs (10–50 ms) compared with serotonin that elicited responses with a greater proportion of longer ISIs (1,000–2,500 ms). Because CV and ISI distributions did not differ between pruritogens, analyses of CV and ISI distributions for responses to noxious stimuli are only shown for serotonin-responsive neurons. The same analyses performed with histamine or capsaicin-responsive neurons provided similar results; the numbers of neurons responsive to BAM8–22 or chloroquine were too small to perform reliable statistical testing. Increased numbers of long ISIs were also noted in responses of primate STT neurons to a pruritogen compared with responses to an algogen (Davidson et al. 2012). It is possible, therefore, that low-frequency discharges contribute a distinct signal during pruriceptive processing.
Fig. 6.
Analysis of spike timing dynamics and spike patterns in pruriceptive VTT neurons. A: coefficient of variation (CV) of interspike intervals (ISIs) for responses to each pruritogen or partial pruritogen, and for responses to noxious stimuli within serotonin-responsive neurons. B: ISI distribution histograms for responses to each pruritogen or partial pruritogen, and for responses to noxious stimuli within serotonin-responsive neurons. *P < 0.05, **P < 0.005, Kruskal-Wallis ANOVA. Colors in B correspond to colors labeled for each stimulus in A. Data compiled from same set of neurons used in Fig. 5.
Each pruritogen and partial pruritogen tested elicited a response in a subset of VTT neurons, except cowhage, which did not produce a response in any of the cells in which it was tested. Of the cells in which at least five pruritogens and/or partial pruritogens were tested, 55% responded to at least one pruritogen and/or partial pruritogen (Table 1). Of these pruriceptive cells, 36% responded to multiple pruritogens or partial pruritogens. Twenty-seven percent of neurons responded to serotonin, 22% to BAM8–22, 9% to chloroquine, 28% to histamine, and 27% to capsaicin (Table 2). All cells that responded to a pruritogen or partial pruritogen also responded to noxious mechanical stimulation; roughly one-third of pruriceptive VTT neurons was responsive to noxious heat. For each pruritogen or partial pruritogen, responsive cells consisted of HT and WDR neurons. No LT cells (n = 14) responded to any of the chemical stimuli tested.
Table 1.
Percentage of VTT neurons tested with and responding to a specific number of pruritogens and/or partial pruritogens injected intradermally in the face
| No. of Pruritogens Tested | No. of Pruritogens Eliciting a Response |
|||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| ≥1 (N = 104) | 52% (n = 54) | 31% (n = 32) | 10% (n = 10) | 6% (n = 6) | 2% (n = 2) | 0% (n = 0) |
| ≥2 (N = 92) | 58% (n = 53) | 23% (n = 21) | 11% (n = 10) | 6% (n = 6) | 2% (n = 2) | 0% (n = 0) |
| ≥3 (N = 87) | 60% (n = 52) | 20% (n = 17) | 11% (n = 10) | 7% (n = 6) | 2% (n = 2) | 0% (n = 0) |
| ≥4 (N = 62) | 50% (n = 31) | 21% (n = 13) | 16% (n = 10) | 10% (n = 6) | 3% (n = 2) | 0% (n = 0) |
| ≥5 (N = 47) | 45% (n = 21) | 19% (n = 9) | 21% (n = 10) | 11% (n = 5) | 4% (n = 2) | 0% (n = 0) |
Percentages are calculated by dividing the number of neurons in which a specific number of pruritogens and/or partial pruritogens elicited a response (n) by the total number of neurons in which a specific number of pruritogens and/or partial pruritogens was tested (N). For example, in the 3rd column “23%” refers to the number of neurons in which 1 pruritogen elicited a response (n = 21) divided by the number of neurons in which ≥2 pruritogens were tested (N = 92). Data include neurons tested with the pruritogens serotonin, BAM8-22, chloroquine, cowhage, and/or the partial pruritogens histamine and capsaicin. VTT, trigeminothalamic tract.
Table 2.
Incidence of response of VTT neurons to each pruritogen or partial pruritogen
| Drug | No. Responsive to Drug | Mechanical Classification |
Heat | ||
|---|---|---|---|---|---|
| HT | WDR | LT | |||
| 5-HT (47 mM) | 26 of 95 (27%) | 11 (42%) | 15 (58%) | 0 | 8 of 21 (38%) |
| BAM (1 mM) | 6 of 27 (22%) | 5 (83%) | 1 (17%) | 0 | 2 of 6 (33%) |
| CQ (100 mM) | 7 of 81 (9%) | 2 (29%) | 5 (71%) | 0 | 2 of 7 (29%) |
| HA (900 mM) | 26 of 92 (28%) | 13 (50%) | 13 (50%) | 0 | 9 of 25 (36%) |
| CAP (3.3 mM) | 13 of 49 (27%) | 6 (46%) | 7 (54%) | 0 | 5 of 12 (42%) |
| COW (≥10 spicules) | 0 of 49 | 0 | 0 | 0 | 0 |
For mechanical classification and heat responsiveness, percentages are calculated by dividing the number of neurons responding to noxious stimulus (denoted by column) by the number of neurons responding to the pruritogen or partial pruritogen (denoted by row).
HT, high threshold; WDR, wide dynamic range; LT, low threshold; 5-HT, serotonin; BAM, BAM8-22; CQ, chloroquine; HA, histamine; CAP, capsaicin; COW, cowhage.
VTT neurons were also classified based on their responses to mechanical and thermal stimulation, as well as on the location of their recording points. Of the 90 VTT neurons that responded to noxious mechanical stimulation, 56% were pruriceptive (n = 27 WDR; n = 23 HT) and 44% were nociceptive only (n = 22 WDR; n = 18 HT; Fig. 7A). Thirty-seven percent of pruriceptive neurons and 23% of nociceptive-only neurons responded to a 50°C noxious heat stimulus (Fig. 7B). In regard to recording location, 52% of either pruriceptive or nociceptive-only VTT neurons were located in superficial layers of the spinal trigeminal nucleus (Fig. 7C). The remaining neurons were recorded in the deep dorsal horn. The contribution of each of these classes of VTT neurons (WDR vs. HT; heat responsive vs. nonresponsive; superficial vs. deep dorsal horn recording location) to the mean response to each pruritogen and partial pruritogen was determined by comparing the mean discharge rate during a 5- or 10-min period following pruritogen or partial pruritogen application. Neurons classified as WDR had a significantly greater discharge rate during histamine responses than neurons classified as HT (Fig. 8A); no significant difference was seen between WDR vs. HT neurons for any of the other pruritogens nor was there a significant difference in firing rates during pruritogen application between neurons responsive or nonresponsive to noxious heat (Fig. 8B). Neurons in deep layers exhibited a significantly greater firing rate than those in superficial layers during responses to serotonin or histamine (Fig. 8C).
Fig. 7.
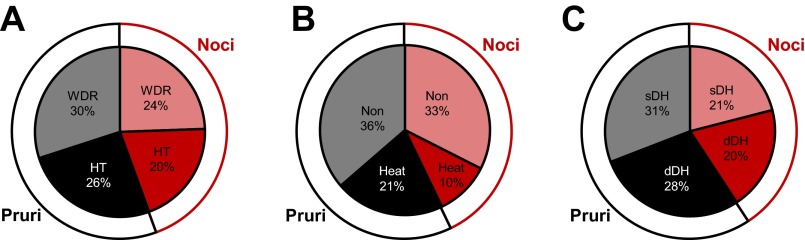
Proportions of pruriceptive (Pruri) and nociceptive-only (Noci) VTT neurons belonging to various subclasses. A: proportions of pruriceptive (black) and nociceptive-only (red) neurons classified as wide dynamic range (WDR) or high threshold (HT) based on responses to mechanical stimulation (n = 90). B: proportions of pruriceptive and nociceptive-only neurons classified as heat responsive or nonresponsive based on responses to thermal (50°C) stimulation (n = 77). C: proportions of pruriceptive and nociceptive-only neurons recorded from superficial or deep dorsal horn (n = 81).
Fig. 8.
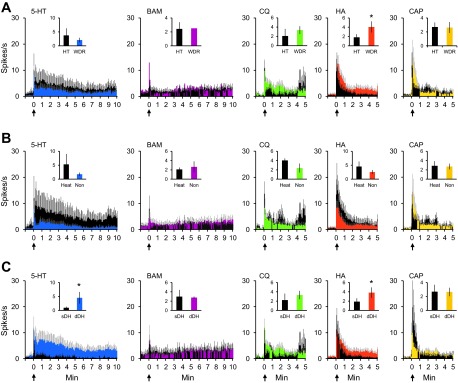
Responses to each pruritogen and partial pruritogen by various subclasses of pruriceptive VTT neurons. A: mean response to each pruritogen and partial pruritogen by WDR vs. HT neurons. *P = 0.014, Wilcoxon rank sums. B: mean response to each pruritogen and partial pruritogen by heat responsive vs. nonresponsive neurons. *P = 0.028 for serotonin; P = 0.030 for histamine, Wilcoxon rank sums. C: mean response to each pruritogen and partial pruritogen by cells recorded from superficial vs. deep dorsal horn. For A–C: histograms represent actual (not normalized) discharge rates and reported in 5-s bins; insets: mean discharge rate across response period shown in histogram (10 min for serotonin and BAM8–22, 5 min for chloroquine, histamine, and capsaicin).
Comparison of responses of pruriceptive and nociceptive-only VTT neurons to mechanical and thermal stimuli.
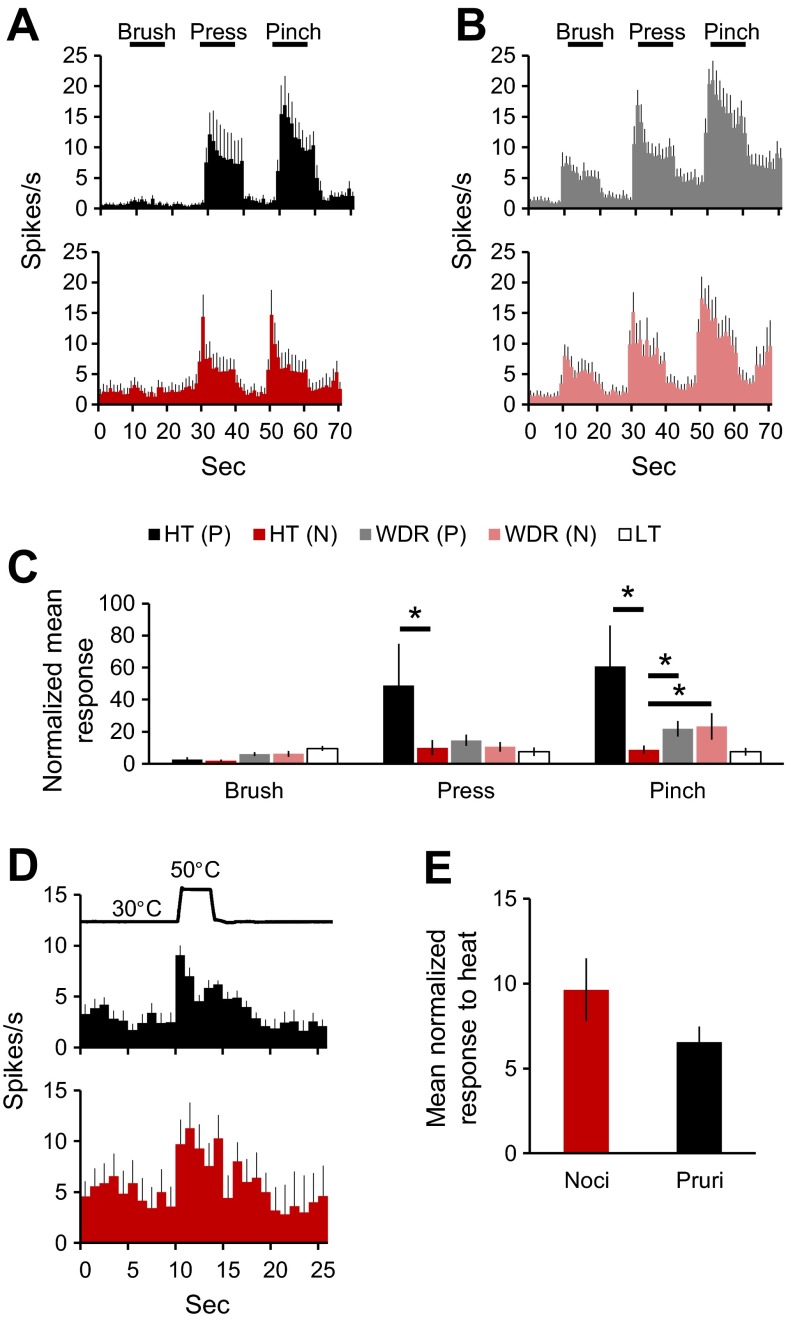
The mean discharge rates of responses of pruriceptive vs. nociceptive-only VTT neurons to mechanical and thermal stimulation were compared. Pruriceptive HT neurons responded with a higher discharge rate during pressure and pinch compared with nociceptive-only HT neurons (Fig. 9A). There was no difference in responses to brush, pressure, or pinch between pruriceptive and nociceptive-only WDR neurons (Fig. 9B). Normalized responses to pressure and pinch by pruriceptive HT neurons were significantly greater than those by nociceptive-only HT neurons (Fig. 9C). Additionally, pinch responses in pruriceptive or nociceptive-only WDR neurons were significantly greater than the response to pinch by nociceptive-only HT neurons but significantly less than the response to pinch by pruriceptive HT neurons. The mean response to noxious heat did not vary between pruriceptive and nociceptive-only VTT neurons (Fig. 9, D and E).
Fig. 9.
Responses to mechanical and thermal stimulation by pruriceptive vs. nociceptive-only VTT neurons. A: mean discharge rate elicited by mechanical stimulation in pruriceptive (black) vs. nociceptive-only (red) HT neurons. B: mean discharge rate elicited by mechanical stimulation in pruriceptive vs. nociceptive-only WDR neurons. C: mean response (normalized to 60 s of baseline activity preceding stimulus application) to brush, pressure, or pinch in pruriceptive, nociceptive-only, or low threshold (LT) neurons. *Statistically significant difference between groups denoted by black bar [P = 0.020 for HT(P) vs. HT(N) press, P = 0.032 for HT(N) vs. WDR(N) pinch, P = 0.010 for HT(P) vs. HT(N) pinch, P = 0.0033 for HT(N) vs. WDR(P) pinch, Kruskal-Wallis ANOVA]. D: mean discharge rate elicited by 50°C heat stimulus in pruriceptive vs. nociceptive-only neurons. E: mean response (normalized to 60 s of baseline activity preceding stimulus application) to 50°C heat stimulus in pruriceptive vs. nociceptive-only neurons.
Recording point and axon projection locations.
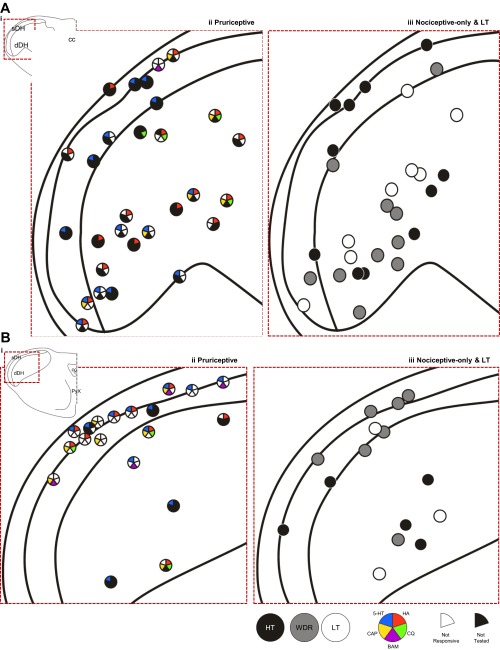
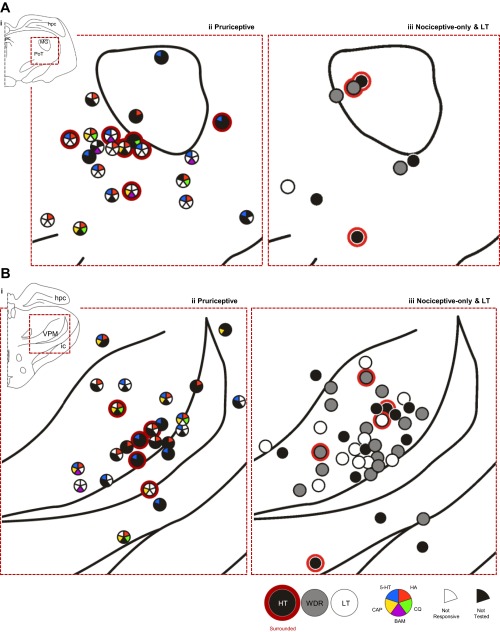
The recovered recording locations for pruriceptive and nociceptive-only VTT neurons are compiled in Fig. 10. Pruriceptive and nociceptive-only VTT neurons were each located throughout the superficial and deep layers of the spinal trigeminal nucleus, with no apparent relationship between location and responsiveness to pruritogens or partial pruritogens (indicated by colored wedges in dots that depict recording location) or mechanical classification. The axon locations for all pruriceptive and nociceptive-only VTT neurons for which the LT stimulation point lesion was recovered are compiled in Fig. 11. Pruriceptive and nociceptive-only VTT axons projected primarily to either the posterior thalamus, often terminating within the PoT, or more rostrally to the VPM nucleus. Axons that were surrounded rostrally by points at which they could not be antidromically activated by ≥300 μA are indicated by a red outline in Fig. 11. The locations to which axons project do not appear to be associated with responsiveness to mechanical stimulation or specific pruritogens.
Fig. 10.
Recording locations of each neuron for which a recording point lesion was recovered (n = 92). A: recording points located throughout the first and second cervical segments of the spinal cord. B: recording points located in the medulla, at and rostral to the level of the pyramidal decussation. In A and B, red dashed line indicates area in i, which has been expanded in ii and iii. Cells responsive to each pruritogen or partial pruritogen are indicated by color code; cells tested with a chemical but not responding to that chemical have a white section in the space corresponding to that chemical; cells not tested with a chemical have a black section in the space corresponding to that chemical (see legend).
Fig. 11.
Location of lowest threshold for antidromic activation of each neuron for which a lesion was recovered (n = 96). A: LT points located within the posterior thalamus. B: LT points located within the more rostral thalamus, near VPM. In A and B, red dashed line indicates area in i, which has been expanded in ii and iii. Points surrounded with red indicate points surrounded rostrally by antidromic test sites with a threshold ≥300 μA (see legend).
Receptive fields.
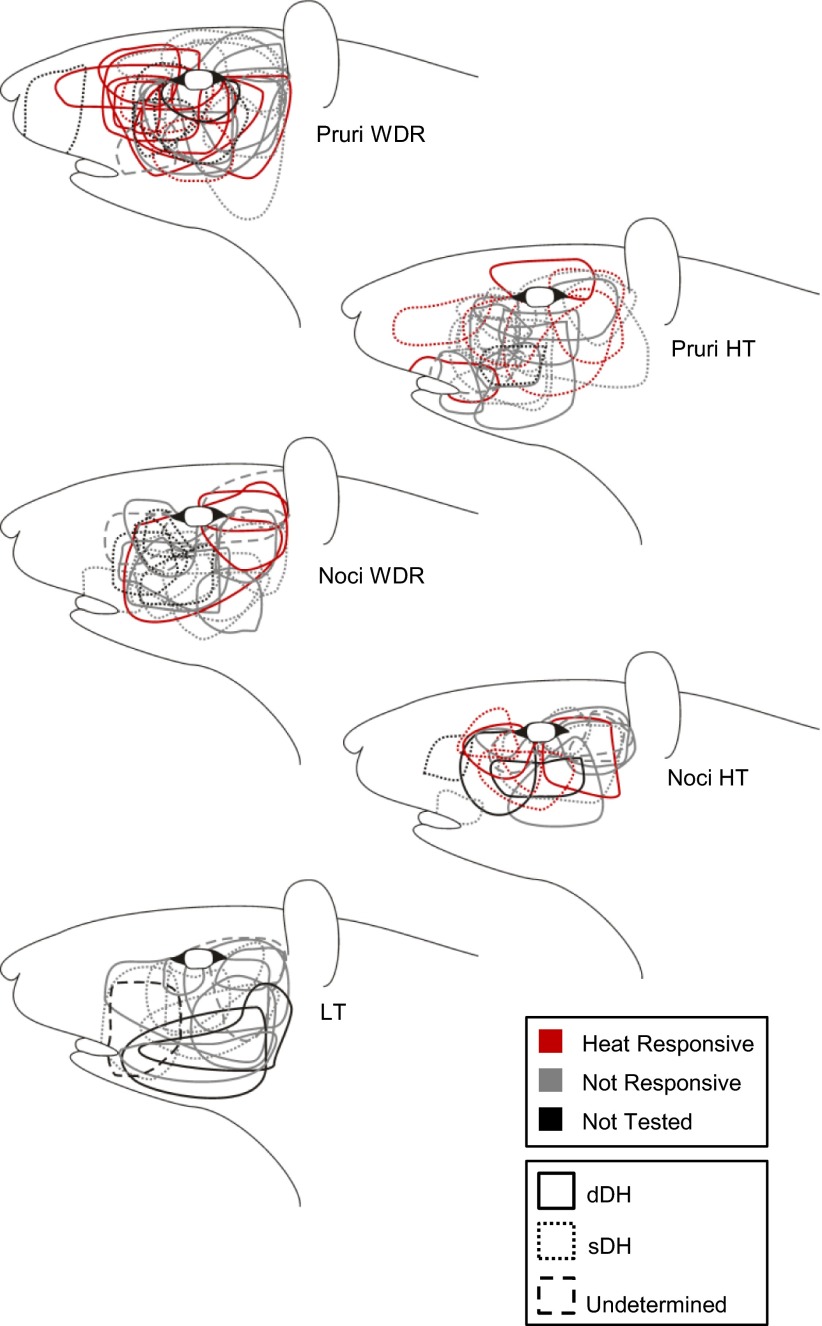
All examined VTT neurons had a cutaneous receptive field on the face ipsilateral to the recording site. The mechanically sensitive receptive field for each neuron is depicted in Fig. 12. The majority of VTT neurons had mechanically sensitive receptive fields located below the eye and caudal to the vibrissal pad, an area of the face corresponding to the area used in studies distinguishing between itch- and pain-related behaviors in rodents. A small number of units had receptive fields located above the eye; to our knowledge, the behavioral effects of pruritogens applied to this area have not been reported.
Fig. 12.
Receptive fields for each neuron included in the current study. Thermal responsiveness and recording location indicated by line color and dash type, respectively (see legend).
DISCUSSION
In this study, we characterized neurons that projected to the thalamus from the spinal trigeminal nucleus and tested their responses to noxious mechanical and thermal stimuli and chemical pruritogens applied to facial cutaneous receptive fields in rats. When tested with all of the six pruritogens and partial pruritogens used in this study, 55% of VTT neurons responded to at least one pruritogen or partial pruritogen. Each pruriceptive VTT cell also responded to noxious mechanical and/or thermal stimulation; no mechanically insensitive cells were identified. The majority of neurons (64%) responded to only one of the chemicals tested, with the remainder responding to multiple pruritogens. Pruriceptive neurons were located in both superficial and deep layers of the spinal trigeminal nucleus extending from the caudal medulla to second cervical segment of the spinal cord and had axons that projected to the VPM, PoT, or other nuclei in the contralateral thalamus.
The current findings describe a population of VTT neurons in which responses to pruritogens paralleled behavioral responses in studies employing the rat cheek model. When injected intradermally in the face of rats, serotonin (47 mM) causes scratching that peaks within 10 min and subsides within an hour (Klein et al. 2011); the mean response to serotonin (47 mM) in VTT neurons has a similar peak timing and duration (Fig. 5). Mean responses to histamine and capsaicin in VTT neurons also match scratching behavior elicited by these chemicals in the cheek, with behavioral and electrophysiological responses to each peaking within 5 min and returning to baseline within 20 min (Fig. 5; Moser HR and Giesler GJ, unpublished observations). Previously, we have shown that pruriceptive VTT neurons respond to intrathecal application of morphine (Moser and Giesler 2013) with a similar response latency and response duration to scratching induced by morphine applied to the central nervous system (Frenk et al. 1984; Koenigstein 1948; Lee et al. 2003; Thomas and Hammond 1995). Intrathecal application of morphine increased responses in pruriceptive VTT neurons to pruritogens and to innocuous mechanical stimuli, possibly contributing to hyperknesis and alloknesis, sensory phenomena that likely involve opioid receptor activation (Fjellner and Hagermark 1982; Heyer et al. 2002; Onigbogi et al. 2000). Together, the present and previously published findings suggest that pruriceptive VTT neurons contribute prominently to producing the sensation of itch located on the face.
Our current data demonstrate that neurons that convey information about itch to the thalamus also respond to noxious mechanical stimuli (pressure and/or pinch). Many pruriceptive VTT cells also responded to noxious heat. These data are in accordance with findings that all nonhuman primate pruriceptive STT cells are also responsive to noxious mechanical, thermal, and/or chemical stimuli (Davidson et al. 2007, 2009, 2012; Simone et al. 2004). In both monkey STT and rat VTT, pruriceptive and nociceptive-only projection neurons are not readily distinguished based on recording point locations in the dorsal horn or on the locations of their axons within the thalamus (Davidson et al. 2012; current data). Instead, pruriceptive vs. nociceptive-only populations can be differentiated by their responsiveness to pruritogens as well as responsiveness to drugs that modulate itch and/or pain, such as morphine (Moser and Giesler 2013). In the periphery, pruriceptive primary afferent neurons can be identified by the presence of cell membrane receptors for various pruritogens. In mice, a population of neurons containing the mas-related gene peptide receptor A3 (MrgprA3) responds to several different pruritogens, including histamine, BAM8–22, and chloroquine. Ablation of this population results in loss of itch-related behaviors while pain-related behavior remains intact; specific activation of MrgprA3-containing neurons, even via a normally painful stimulus, results in scratching (Han et al. 2012). It is possible that, just as was found for primary afferent neurons by Han et al. (2012), selective activation of pruriceptive VTT neurons, without simultaneous activation of nociceptive-only VTT neurons, by any stimulus will result in the sensation of itch. Accordingly, simultaneous activation of both pruriceptive and nociceptive-only VTT populations would likely produce pain. Future studies to test this possibility should provide further insight into how the nervous system codes itch and pain, as well as possibly point out directions for selective treatments of each.
The pruritogens used in the current study were chosen based on the ability of each to induce scratching with the hindlimb when injected intradermally into the rodent cheek (Akiyama et al. 2010; Klein et al. 2011; Wilson et al. 2011). In addition, the peripheral scratch-inducing actions of each drug have been well characterized and each drug has been implicated in producing itch in humans. It is well-demonstrated that serotonin elicits scratching in rodents via activation of the 5-HT2 receptor (Jinks and Carstens 2002; Nojima and Carstens 2003; Thomsen et al. 2001; Yamaguchi et al. 1999); serotonin produces itch in humans (Hosogi et al. 2006; Rasul et al. 2012; Thomsen et al. 2002; Weisshaar et al. 1997) and is found at increased levels in human patients experiencing itch, including patients with allergic contact dermatitis (Lundeberg et al. 1999) and atopic dermatitis (Soga et al. 2007). Application of BAM8–22 to the face of mice causes scratching with the hindlimb (Wilson et al. 2011) via activation of MrgprC11 (Liu et al. 2009) and downstream opening of TRPA1 channels (Wilson et al. 2011); in humans, heat-inactivated cowhage spicules soaked in BAM8–22 evoke itch accompanied by pricking/stinging and burning sensations (Sikand et al. 2011). Chloroquine produces scratching in rodents via activation of MrgprA3 and downstream opening of TRPA1 channels (Liu et al. 2009; Wilson et al. 2011); in humans, oral administration of chloroquine as a treatment for malaria causes intense itch as a side effect (Mnyika and Kihamia 1991; Sowunmi et al. 2000), although there is little evidence that injection of chloroquine under the skin in humans causes itch (Abila et al. 1994). Unlike these pruritogens, histamine and capsaicin are partial pruritogens that each produce a combination of scratching and pain-related wiping when applied to the face in rats (Klein et al. 2011). In the present study, many cells that responded to histamine and capsaicin also responded to other pruritogens (e.g., serotonin, chloroquine) at concentrations that only cause scratching, suggesting that these cells may be conveying information about the pruritogenic aspects of the stimulus and that the pain responses associated with histamine and capsaicin are mediated by a separate population of neurons. Another possibility is that these cells may be contributing to both itch and pain, depending on the specific spike pattern elicited by a given stimulus, although our spike train analyses did not uncover any obvious differences in spike timing dynamics between the different pruritogens.
The finding that cowhage did not produce a response in any of the VTT cells tested is consistent with findings that cowhage spicules applied to the rat face do not elicit any behavioral response (Klein et al. 2011). In humans, cowhage produces itch via activation of protease-activated receptor (PAR) subtypes 2 and/or 4 by mucunain, the itch-causing protease present in cowhage spicules (Reddy et al. 2008). The short peptide SLIGRL-NH2 has commonly been used as a PAR2 agonist to induce scratching in mice (Akiyama et al. 2009b) and study itch responses in spinal neurons (Akiyama et al. 2009a,b, 2011). However, it has been shown that in mice, SLIGRL-induced scratching does not require activation of PAR2 but instead requires activation of MrgprC11, the same receptor underlying itch induced by BAM8–22 (Liu et al. 2011). Neither the PAR2/MrgprC11 agonist SLIGRL-NH2 nor the PAR4 agonist AYPGKF-NH2 induces scratching when applied to the rat face (Klein et al. 2011); SLIGRL-NH2 does cause pain-related wiping, an effect not seen with cowhage spicules or the PAR4 agonist. Accordingly, in future studies it will be important to maintain a distinction between the target receptors for cowhage spicules vs. injections of SLIGRL-NH2.
In the central nervous system, less is known about the specific receptors involved in conveying information about pruritogens to the brain. The gastrin-releasing peptide receptor (GRPR) is implicated in itch processing in the spinal cord, as both the receptor and spinal neurons containing the receptor are necessary for scratching responses induced by a variety of histaminergic and nonhistaminergic pruritogens (Sun and Chen 2007; Sun et al. 2009). Primary afferent neurons within dorsal root ganglia and trigeminal ganglia contain the GRPR agonist gastrin-releasing peptide (GRP) (Takanami et al. 2013). However, GRP is likely released from spinal neurons receiving primary afferent input, rather than from primary afferent terminals themselves, to produce itch (Mishra and Hoon 2013). GRPR is likely located on tertiary spinal neurons receiving input from GRP-containing secondary spinal neurons. Therefore, it is possible that GRPR is located on spinal projection neurons such as pruriceptive VTT neurons, although this has not yet been directly demonstrated via immunohistochemistry or other anatomical methods. Axons of pruriceptive VTT neurons appear to terminate primarily in either the posterior thalamus or more rostrally in the VPM nucleus (Fig. 11). The posterior thalamus, including PoT and medial geniculate nuclei, also receives input from a large portion of rat STT neurons in the cervical enlargement (Zhang and Giesler 2005). This region of the thalamus sends somatosensory input, both directly (Bordi and LeDoux 1994; LeDoux et al. 1985; Ottersen and Ben-Ari 1979) and indirectly via cortical loops (Gauriau and Bernard 2004; Kurokawa et al. 1990; Ledoux et al. 1985; Linke 1999; Linke and Schwegler 2000), to the amygdala and has been implicated in fear conditioning (LeDoux et al. 1986a,b; Shi and Davis 1999). It is possible that affective responses to itch are mediated via these projections.
Our study highlights several interesting differences between itch responses in spinal projection neurons in rats vs. other species. Previously, a small subset of STT neurons in cats was found to be mechanically insensitive yet respond to histamine. It was suggested that these cells may be part of a labeled line for itch information, although the number of histamine-responsive neurons that did not also respond to the algogen mustard oil, when tested, was low (n = 2). In addition, other algogens, such as capsaicin, which activates a large portion of STT neurons, were not tested (Andrew and Craig 2001). In contrast, in monkey STT neurons as well as in rat VTT neurons, all pruriceptive cells were responsive to noxious mechanical stimulation (Davidson et al. 2007, 2009, 2012; Moser and Giesler 2013; Simone et al. 2004). Pruriceptive monkey STT neurons were found to respond either to histamine or to cowhage (Davidson et al. 2007), suggesting a separation between histaminergic and nonhistaminergic forms of itch. Our current data suggest that this separation of histaminergic and nonhistaminergic responses in spinal projection neurons does not exist in the rat trigeminal system. In fact, the only predictive relationship identified in rat VTT neurons is that all neurons that responded to chloroquine (a pruritogen generally referred to as nonhistaminergic) also, when tested, responded to histamine. This is in accordance with the finding that MrgprA3-containing primary afferent fibers also respond to both histamine and chloroquine (Han et al. 2013). It is possible that, as more pruritogens are tested in monkey STT neurons, the separation of populations responding to histamingeric and nonhistaminergic stimuli will become less distinct. In addition, it is likely that the previous finding that approximately one-third of monkey STT neurons is pruriceptive (vs. approximately half of rat VTT neurons) will change when monkey STT neurons are characterized with a greater number of pruritogens. Another major difference between species was found for the contribution of superficial vs. deep dorsal horn units to itch responses. Monkey STT neurons located within the superficial dorsal horn exhibited a greater discharge rate than cells located within the deep dorsal horn in response to histamine, cowhage, or capsaicin (Davidson et al. 2012). In contrast, in rat VTT neurons, deep dorsal horn units exhibited a greater discharge rate compared with superficial dorsal horn units in response to the pruritogens histamine and serotonin, highlighting the importance of including both superficial and deep dorsal horn neurons in studies of itch. It should also be noted that observed differences between previous studies of STT neurons in monkeys and the current studies of VTT neurons in rats may be due in part, or in full, to differences between the characteristics of skin and/or underlying nervous system of the body vs. face.
Application of pruritogens and algogens to the rodent cheek provides a reliable approach to distinguish between itch-evoked and pain-evoked behaviors and thus is valuable for examining how the nervous system distinguishes between these distinct sensations. VTT neurons in rats respond to a variety of pruritogens that evoke scratching with the hindlimb when applied to the face in rats. The time course of pruritic responses in VTT neurons is similar to the time course of facial scratching for corresponding pruritogens. Therefore, the VTT appears to a highly promising system for future studies of processing and transmission of pruriceptive and nociceptive information within the central nervous system.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants P01-NS-047399 (to G. J. Giesler), F31-NS-077554 (to H. R. Moser), T32-NS-048944, and NS-062158 (core funding).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.R.M. and G.J.G. conception and design of research; H.R.M. performed experiments; H.R.M. analyzed data; H.R.M. and G.J.G. interpreted results of experiments; H.R.M. prepared figures; H.R.M. drafted manuscript; H.R.M. and G.J.G. edited and revised manuscript; H.R.M. and G.J.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank H. Truong for valuable technical assistance and B. Lipshetz for help in some experiments. We also thank Dr. D. Simone for critically reading this manuscript.
REFERENCES
- Abila B, Ezeamuzie IC, Igbigbi PS, Ambakederemo AW, Asomugha L. Effects of two antihistamines on chloroquine and histamine induced weal and flare in healthy African volunteers. Afr J Med Med Sci 23: 139–142, 1994 [PubMed] [Google Scholar]
- Akiyama T, Iodi Carstens M, Carstens E. Excitation of mouse superficial dorsal horn neurons by histamine and/or PAR-2 agonist: potential role itch. J Neurophysiol 102: 2176–2183, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Merrill AW, Iodi Carstens M, Carstens E. Activation of superficial dorsal horn neurons in the mouse by a PAR-2 agonist and 5-HT: potential role for itch. J Neurosci 29: 6691–6699, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Iodi Carstens M, Carstens E. Facial injections of pruritogens and algogens excite partly overlapping populations of primary and second-order trigeminal neurons in mice. J Neurophysiol 104: 2442–2450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Iodi Carstens M, Carstens E. Enhanced responses of lumbar superficial dorsal horn neurons to intradermal PAR-2 agonist but not histamine in a mouse hindpaw dry skin itch model. J Neurophysiol 105: 2811–2817, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D, Craig AD. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci 4: 72–77, 2001 [DOI] [PubMed] [Google Scholar]
- Bordi F, LeDoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. II. Cells receiving convergent auditory and somatosensory inputs and cells antidromically activated by amygdala stimulation. Exp Brain Res 98: 275–286, 1994 [DOI] [PubMed] [Google Scholar]
- Burstein R, Dado RJ, Cliffer KD, Giesler GJ., Jr Physiological characterization of spinohypothalamic tract neurons in the lumbar enlargement of rats. J Neurophysiol 66: 261–284, 1991 [DOI] [PubMed] [Google Scholar]
- Carstens E. Responses of rat spinal dorsal horn neurons to intracutaneous microinjection of histamine, capsaicin, and other irritants. J Neurophysiol 77: 2499–2514, 1997 [DOI] [PubMed] [Google Scholar]
- Dado RJ, Katter JT, Giesler GJ., Jr Spinothalamic and spinohypothalamic tract neurons in the cervical enlargement of rats. I. Locations of antidromically identified axons in the thalamus, and hypothalamus. J Neurophysiol 71: 959–980, 1994 [DOI] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci 27: 10007–10014, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat Neurosci 12: 544–546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Khasabov SG, Moser HR, Honda CN, Simone DA, Giesler GJ., Jr Pruriceptive spinothalamic tract neurons: physiological properties and projection targets in the primate. J Neurophysiol 108: 1711–1723, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzezga A, Darsow U, Treede RD, Siebner H, Frisch M, Munz F, Weilke F, Ring J, Schwaiger M, Bartenstein P. Central activation by histamine-induced itch: analogies to pain processing: a correlational analysis of O-15 H2O positron emission tomography studies. Pain 92: 295–305, 2001 [DOI] [PubMed] [Google Scholar]
- Fjellner B, Hägermark Ö. Potentiation of histamine-induced itch and flare responses in human skin by the enkephalin analog FK 33–824, β-endorphin and morphine. Arch Dermatol Res 274: 29–37, 1982 [DOI] [PubMed] [Google Scholar]
- Frenk H, Watkins LR, Mayer DJ. Differential behavioral effects induced by intrathecal microinjection of opiates: comparison of convulsive and cataleptic effects produced by morphine, methadone, and d-Ala2-methionine-enkephalinamide. Brain Res 299: 31–42, 1984 [DOI] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF. Posterior triangular thalamic neurons convey nociceptive messages to the secondary somatosensory and insular cortices in the rat. J Neurosci 24: 752–761, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Ma C, Liu Q, Weng HJ, Cui Y, Tang Z, Kim Y, Nie H, Qu L, Patel KN, Li Z, McNeil B, He S, Guan Y, Xiao B, LaMotte RH, Dong X. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci 16: 174–182, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer G, Groene D, Martus P. Efficacy of naltrexone on acetylcholine-induced alloknesis in atopic eczema. Exp Dermatol 11: 448–455, 2002 [DOI] [PubMed] [Google Scholar]
- Hosogi M, Schmelz M, Miyachi Y, Ikoma A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain 126: 16–23, 2006 [DOI] [PubMed] [Google Scholar]
- Jinks SL, Carstens E. Responses of superficial dorsal horn neurons to intradermal serotonin and other irritants: comparison with scratching behavior. J Neurophysiol 87: 1280–1289, 2002 [DOI] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci 28: 7659–7669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Carstens MI, Carstens E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J Neurophysiol 106: 1078–1088, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigstein H. Experimental study of itch stimuli in animals. Arch Derm Syphilol 57: 828–849, 1948 [DOI] [PubMed] [Google Scholar]
- Kurokawa T, Yoshida K, Yamamoto T, Oka H. Frontal cortical projections from the suprageniculate nucleus in the rat, as demonstrated with the PHA-L method. Neurosci Lett 120: 259–262, 1990 [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Shain CN, Simone DA, Tsai E. Neurogenic hyperalgesia: psychophysical studies of underlying mechanisms. J Neurophysiol 66: 190–211, 1991 [DOI] [PubMed] [Google Scholar]
- Lee H, Naughton NN, Woods JH, Ko MC. Characterization of scratching responses in rats following centrally administered morphine or bombesin. Behav Pharmacol 14: 501–508, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Ruggiero DA, Reis DJ. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J Comp Neurol 242: 182–213, 1985 [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Pearl D, Reis DJ. Disruption of auditory but not visual learning by destruction of intrinsic neurons in the rat medial geniculate body. Brain Res 371: 395–399, 1986a [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Sakaguchi A, Iwata J, Reis DJ. Interruption of projections from the medial geniculate body to an archi-neostriatal field disrupts the classical conditioning of emotional responses to acoustic stimuli. Neuroscience 17: 615–627, 1986b [DOI] [PubMed] [Google Scholar]
- Linke R. Organization of projections to temporal cortex originating in the thalamic posterior intralaminar nucleus of the rat. Exp Brain Res 127: 314–320, 1999 [DOI] [PubMed] [Google Scholar]
- Linke R, Schwegler H. Convergent and complementary projections of the caudal paralaminar thalamic nuclei to rat temporal and insular cortex. Cereb Cortex 10: 753–771, 2000 [DOI] [PubMed] [Google Scholar]
- Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139: 1353–1365, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Weng HJ, Patel KN, Tang Z, Bai H, Steinhoff M, Dong X. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci Signal 4: 1–6, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundeberg L, Sundstrom E, Nordlund K, Verhofstad A, Johansson O. Serotonin in human allergic contact dermatitis. Ann NY Acad Sci 885: 422–426, 1999 [DOI] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science 340: 968–971, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnyika KS, Kihamia CM. Chloroquine-induced pruritus: its impact on chloroquine utilization in malaria control in Dar es Salaam. J Trop Med Hyg 94: 27–31, 1991 [PubMed] [Google Scholar]
- Moser HR, Giesler GJ., Jr Itch and analgesia resulting from intrathecal application of morphine: contrasting effects on different populations of trigeminothalamic tract neurons. J Neurosci 33: 6093–6101, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima H, Carstens E. Quantitative assessment of directed hind limb scratching behavior as a rodent itch model. J Neurosci Meth 126: 137–143, 2003 [DOI] [PubMed] [Google Scholar]
- Onigbogi O, Ajayi AA, Ukponmwan E. Mechanisms of chloroquine-induced body-scratching behavior in rats: evidence of involvement of endogenous opioid peptides. Pharmacol Biochem Behav 65: 333–337, 2000 [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Ben-Ari Y. Afferent connections to the amygdaloid complex of the rat and cat. I. Projections from the thalamus. J Comp Neurol 187: 401–424, 1979 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 1982 [DOI] [PubMed] [Google Scholar]
- Rasul A, Nordlund K, Wahlgren CF. Pruritic and vascular responses induced by serotonin in patients with atopic dermatitis and in healthy controls. Acta Derm Venereol 93: 277–280, 2013 [DOI] [PubMed] [Google Scholar]
- Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci 28: 4331–4335, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Davis M. Pain pathways involved in fear conditioning measured with fear-potentiated startle: lesion studies. J Neurosci 19: 420–430, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain 139: 681–687, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikand P, Dong X, LaMotte RH. BAM8–22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J Neurosci 31: 7563–7567, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine, and cowhage. Pain 144: 66–75, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone DA, Zhang X, Li J, Zhang JM, Honda CN, LaMotte RH, Giesler GJ., Jr Comparison of responses of primate spinothalamic tract neurons to pruritic and algogenic stimuli. J Neurophysiol 91: 213–222, 2004 [DOI] [PubMed] [Google Scholar]
- Soga F, Katoh N, Inoue T, Kishimoto S. Serotonin activates human monocytes and prevents apoptosis. J Invest Dermatol 127: 1947–1955, 2007 [DOI] [PubMed] [Google Scholar]
- Sowunmi A, Fehintola FA, Adedeji AA, Falade AG, Falade CO, Akinyinka OO, Oduola AM. Comparative efficacy of chloroquine plus chlorpheniramine alone and in a sequential combination with sulfadoxine-pyrimethamine, for the treatment of acute, uncomplicated, falciparum malaria in children. Ann Trop Med Parasitol 94: 209–217, 2000 [DOI] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448: 700–703, 2007 [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science 325: 1531–1534, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanami K, Sakamoto H, Ken-Ichi M, Keita S, Takashi T, Yamada S, Inoue K, Oti T, Sakamoto T, Kawata M. Distribution of gastrin-releasing peptide in the rat trigeminal and spinal somatosensory systems. J Comp Neurol 2013. November 20 [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- Thomas DA, Hammond DL. Microinjection of morphine into the rat medullary dorsal horn produces a dose-dependent increase in facial scratching. Brain Res 695: 267–270, 1995 [DOI] [PubMed] [Google Scholar]
- Thomsen JS, Petersen MB, Benfeldt E, Jensen SB, Serup J. Scratch induction in the rat by intradermal serotonin: a model for pruritus. Acta Derm Venereol 81: 250–254, 2001 [DOI] [PubMed] [Google Scholar]
- Thomsen JS, Sonne M, Benfeldt E, Jensen SB, Serup J, Menné T. Experimental itch in sodium lauryl sulphate-inflamed and normal skin in humans: a randomized, double-blind, placebo-controlled study of histamine and other inducers of itch. Br J Dermatol 146: 792–800, 2002 [DOI] [PubMed] [Google Scholar]
- Weisshaar E, Ziethen B, Gollnick H. Can a serotonin type 3 (5-HT3) receptor antagonist reduce experimentally-induced itch? Inflamm Res 46: 412–416, 1997 [DOI] [PubMed] [Google Scholar]
- White JC, Sweet WH. Pain and the Neurosurgeon. Springfield, IL: Charles C. Thomas, 1969 [Google Scholar]
- Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Autista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci 14: 595–602, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Nagasawa T, Satoh M, Kuraishi Y. Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci Res 35: 77–83, 1999 [DOI] [PubMed] [Google Scholar]
- Zhang X, Giesler GJ., Jr Response characteristics of spinothalamic tract neurons that project to the posterior thalamus in rats. J Neurophysiol 93: 2552–2564, 2005 [DOI] [PubMed] [Google Scholar]