Abstract
Infraorbital nerve (ION) transection in neonatal rats leads to disruption of whisker-specific neural patterns (barrelettes), conversion of functional synapses into silent synapses, and reactive gliosis in the brain stem trigeminal principal nucleus (PrV). Here we tested the hypothesis that neonatal peripheral nerve crush injuries permit better functional recovery of associated central nervous system (CNS) synaptic circuitry compared with nerve transection. We developed an in vitro whisker pad-trigeminal ganglion (TG)-brain stem preparation in neonatal rats and tested functional recovery in the PrV following ION crush. Intracellular recordings revealed that 68% of TG cells innervate the whisker pad. We used the proportion of whisker pad-innervating TG cells as an index of ION function. The ION function was blocked by ∼64%, immediately after mechanical crush, then it recovered beginning after 3 days postinjury and was complete by 7 days. We used this reversible nerve-injury model to study peripheral nerve injury-induced CNS synaptic plasticity. In the PrV, the incidence of silent synapses increased to ∼3.5 times of control value by 2–3 days postinjury and decreased to control levels by 5–7 days postinjury. Peripheral nerve injury-induced reaction of astrocytes and microglia in the PrV was also reversible. Neonatal ION crush disrupted barrelette formation, and functional recovery was not accompanied by de novo barrelette formation, most likely due to occurrence of recovery postcritical period (P3) for pattern formation. Our results suggest that nerve crush is more permissive for successful regeneration and reconnection (collectively referred to as “recovery” here) of the sensory inputs between the periphery and the brain stem.
Keywords: brain stem, nerve damage, rat, silent synapses, trigeminal
peripheral nerve injuries are common in neonates and children, but relatively few studies have focused on long-lasting effects on structural and functional development of the central nervous system (CNS) that normally receive information from the injured nerve. Studies focusing on pain sensitization mechanisms in neonates revealed that neonatal tissue injury can have profound effects later in life on pain sensitivity through altered spinal cord pain circuitry and descending pain modulatory pathways (Beggs et al. 2012; Hathway et al. 2009; Hermann et al. 2006; Ren et al. 2004; Walker et al. 2009; Wollgarten-Hadamek et al. 2009; Zhang et al. 2010). We know very little about how sensory nerve insults in developing mammals affect other modalities of sensory information processing in the CNS.
Whisker-related trigeminal central pathway of rodents is an established model for studying activity-dependent synaptic remodeling during development. The spatial distribution pattern of whiskers is replicated all along the central trigeminal pathway by discrete neural patterns. The development and consolidation of whisker-related patterns depend on the sensory periphery during a critical window after birth. For example, transection of the infraorbital branch (ION) of the trigeminal nerve up to first 3 days after birth leads to disruption or prevention of neural patterning in the brain stem (“barrelettes”), thalamus (“barreloids”), and cerebral cortex (“barrels”) (for review, see Erzurumlu 2010; Erzurumlu and Gaspar 2012).
Previously, we showed that transection of ION in neonatal rats results in substantial synaptic reorganization in the trigeminal principal nucleus (PrV), the first relay station of the trigeminal pathway. Along with disruption of barrelette modules, most of the functional synapses convert to silent synapses by losing functional AMPA receptors (Lo and Erzurumlu 2007). In addition, single barrelette neurons receive substantially more convergent synaptic inputs from trigeminal ganglion (TG) cells through astrocyte-mediated reactive synaptogenesis (Lo et al. 2011). An important question of clinical significance is whether these structural and functional changes are reversible following regeneration of ION fibers. Here we tested the extent of recovery following crush injury of the neonatal ION.
In the nerve transection paradigm, regeneration is a slow process and it is never complete (Jacquin 1989; Jacquin et al. 1989; Jeno and László 2002; Kis et al. 1999; Renehan and Munger 1986; Waite 1984; Waite and Cragg 1982). However, nerve crush leads to a partial blockade of nerve function and relatively rapid functional recovery, evidenced by the appearance of postsynaptic responses to peripheral stimuli (Kis et al. 1999; Renehan and Munger 1986; Waite and Cragg 1982). To further investigate reversibility of nerve injury-induced synaptic changes and potential underlying mechanisms, we developed an in vitro preparation of a cranial cup with the peripheral and central connections of the TG between the whisker pad and the PrV intact. This preparation allowed us to determine responsiveness and action potential (AP) characteristics of TG neurons at varying intervals after ION crush and also enabled us to correlate these changes with synaptic characteristics of the PrV barrelette neurons.
Primary sensory neurons are classified in numerous ways such as expression of specific ion channels, dependence on NGF family of neurotrophins, expression of calcium binding proteins, somatic size, myelination, and conduction velocity, etc. Previous studies showed that neonatal mouse TG neurons can be classified into two groups based on the shape of the AP: S-type neurons that show an inflection in the repolarizing phase of their AP, and F-type neurons that do not show this inflection (Cabanes et al. 2002; Yoshida and Matsuda 1979). We identified these two types of TG cells by using intracellular recordings from the whisker-related TG area (Klein et al. 1986). In normal animals, 69% of the impaled TG cells were responsive to ION stimulation, indicating that they innervate the whisker pad. We took this proportion as an index of normal ION function in the TG. We found that mechanical crush of the ION blocked ∼64% conduction function in the TG. The function started recovering after 3 days postinjury and completely recovered by 7 days postinjury. Both S- and F-type TG cells were affected by the crush equally. We used this reversible nerve-injury model to investigate recovery of normal synaptic function in the PrV. Our results indicate that nerve crush initially leads to conversion of functional synapses to silent synapses and glial reaction, much like more permanent nerve injury (transection). However, rapid recovery of the crushed ION reverses the glial and synaptic responses in the PrV, albeit failure in barrelette formation.
MATERIALS AND METHODS
ION Crush
Sprague-Dawley rat pups of postnatal day 0 (P0) were anesthetized by hypothermia. The ION on the right side was exposed through a microincision of the skin and connective tissue between the eye and the whisker pad, where the nerve exits the infraorbital foramen. The ION was crushed with sterile reverse action forceps (Fine Science Tools 11262-30) for 5 s. Due to its mechanical properties, reverse action forceps produce a relatively constant force on the nerve, and all the crush procedures were done at P0 (the size of the nerve is similar between pups). Thus, we think that there was no significant variability of the extent of crush from case to case. Sham operation without ION crush was performed on the left side as control. The wound was sutured, and the pup was returned to the home cage after recovery from anesthesia. All surgical procedures followed National Institutes of Health guidelines and were approved by the University of Maryland, Baltimore Institutional Animal Care and Use Committee. A total of 45 pups underwent this procedure.
In Vitro Intact Trigeminal Pathway Cranial Cup Preparation
We chose to assess functional recovery of the crushed nerve by recording TG neuron responses following stimulation of the ION distal to the crush site. For this approach, we developed an in vitro TG pathway preparation in postnatal rats (Fig. 1). In this preparation, TG cells innervating the whisker pad are not mechanically damaged and can be kept alive in vitro for more than 8 h. However, it is important to keep in mind that as in other brain slice preparations, many central axonal tracts are axotomized; in our preparation, most notably these are the trigeminothalamic projection cells. While there may be axotomy-related short-term changes in the PrV neurons, we are recording from the ganglion cells with intact axonal projections to both the whisker pad and the PrV. Furthermore, the same preparation was used for both the controls and nerve crush experiments, from the same animal, which obviates possible confounds related to axotomy of the trigeminothalamic projection cells.
Fig. 1.
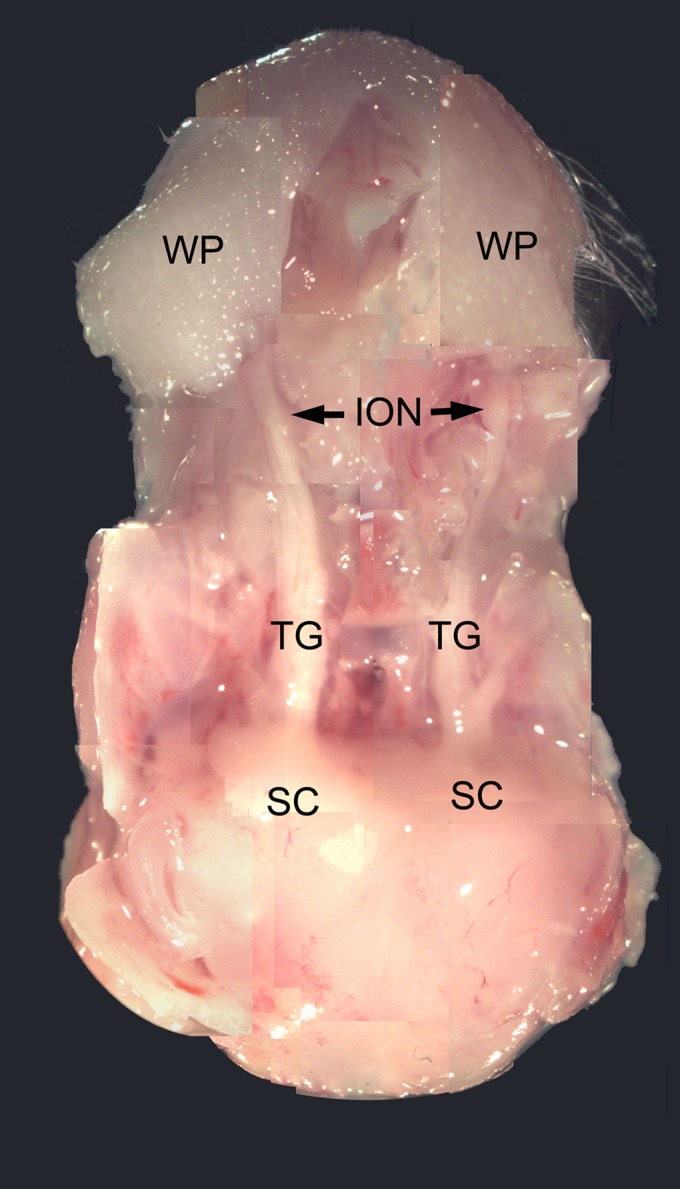
Photograph of an in vitro trigeminal pathway preparation on the third day after right infraorbital nerve (ION) crush. Note that the right TG and ION are thinner than the left control. WP, whisker pad; TG, trigeminal ganglion; SC, superior colliculus.
Neonatal rat pups (P0-P9) were anesthetized with isoflurane and decapitated. The head was immersed in ice-cold artificial cerebrospinal fluid (ACSF) (in mM: 124 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 MgSO4, 26 NaHCO3, 10 glucose, 2 CaCl2, pH 7.4). The skull was opened, and trigeminal ganglia on both sides were exposed by removing the forebrain rostral to the superior colliculus. On each side, the ION branch of the maxillary trigeminal nerve was dissected to the whisker pad, thus allowing direct visualization of the ION between the whisker pad and the TG and leaving the central TG root to the brain stem PrV intact. The cranial cup preparation was placed in a recording chamber perfused (3 ml/min) with oxygenized ACSF at room temperature. A pair of stimulating electrodes was placed on the ION.
Intracellular Recording From TG Cells
We performed intracellular recordings from TG cells in TG pathway preparations from P0 to P9 pups (n = 14) that have undergone P0 unilateral ION crush (and sham operation on the other side) with sharp electrodes filled with 3 M KAc (∼70 MΩ). Because TG cells that innervate whisker pad are widespread in the central part of the TG (Klein et al. 1986), we randomly impaled TG cells in the central TG. Passing biphasic current pulses (±0.7 nA) induced action potentials. Whisker pad-innervating TG cells were identified by the action potentials with fixed latency evoked from ION stimulation (1 ms, <1 mA).
Brain Slice Preparation
Rat pups that have undergone unilateral ION crush on P0 were anesthetized with isoflurane and euthanized by decapitation at various time points between P2 and P7 (n = 26). The brain stem was removed and immersed in cold (4°C) sucrose-based ACSF (in mM: 234 sucrose, 2.5 KCl, 1.25 NaH2PO4, 10 MgSO4, 24 NaHCO3, 11 glucose, 0.5 CaCl2) bubbled with 95% O2 and 5% CO2 (pH 7.4). The brain stem was then embedded in 2% agar and cut into 400-μm-thick transverse sections with a vibratome (Campden 7000smz) in the sucrose-based ACSF at 4°C. Slices containing the PrV were selected under a dissecting microscope; the sham and ION crushed side of each slice was marked. After a 1-h incubation in normal ACSF at 34°C, the slices were stored at room temperature. For recording, each slice was transferred into a submerged-type recording chamber (27L, Warner Instruments) and continuously perfused (>2 ml/min) with normal ACSF at room temperature. During electrophysiological recording, 50 μM picrotoxin was added into the ACSF to block GABAergic responses.
Whole Cell Recording From the PrV
Whole cell patch micropipettes were pulled horizontally in four stages from borosilicate glass with a P-87 puller (Sutter Instrument). The patch electrodes were backfilled with a cesium-based solution (in mM: 115 CsMeSO3, 10 NaCl, 1 KCl, 4 MgCl2, 1 CaCl2, 10 EGTA, 20 HEPES, 3 Na2-ATP, 0.5 Na3-GTP, 0.1 spermine, pH 7.25, >290 mOsm) with a tip resistance of 6–9 MΩ. Inclusion of spermine in the recording pipette prevented loss of inward rectification of AMPAR-mediated excitatory postsynaptic currents (EPSCs) during whole cell recording (Donevan and Rogawski 1995). Neurons in the ventral part of the PrV (barrelette region) were blindly patched (Lo et al. 1999). After “break-in,” the serial resistance was completely compensated with bridge balance of AxoClamp 2B amplifier. We only collected data from cells with resting membrane potential negative to −55 mV and input resistance >200 MΩ with an InstruTECH ITC-16 interface unit and stored data on a Dell DM061 computer with the PULSE (HEKA) software program. A pair of fine-tip stimulating electrodes (0.5 MΩ, WPI, IRM33A05KT, tip diameter 2–3 μm, separated by 300 μm) was inserted into the trigeminal tract (TrV) lateral to the ventral PrV (barrelette region). Electrical pulses (0.2- to 0.3-ms duration) were passed through the electrodes to evoke EPSCs.
Test For Silent Synapses
We first voltage clamped neurons at +60 mV. We stimulated the TrV by 60 pulses at 0.2 Hz. Stimulus intensity was adjusted to around threshold, so that some trials failed to evoke EPSCs. Then we clamped the neurons at −70 mV to investigate changes in failure rate between the two membrane potentials. We measured the amplitude of EPSCs at +60 mV 30 ms after stimulus where NMDA receptor-mediated EPSCs reach their peak. The amplitude of EPSCs at −70 mV was measured at 8 ms after stimulus (the peak of AMPA receptor-mediated EPSCs, Lo and Zhao 2011). The peak amplitude less than two times of noise standard deviation (SD) was determined as the failure of synaptic transmission (Lo and Erzurumlu 2007).
Morphological Studies
P0 ION-crushed rat pups were anesthetized with isoflurane at P3 and P10 (n = 19) and perfused with saline followed by phosphate-buffered 4% paraformaldehyde. The brains were fixed overnight and cytoprotected in 30% sucrose. Coronal sections of 20- to 30-μm thickness were cut in a cryostat and collected in serial order. Alternate sections containing the PrV were processed for cytochrome oxidase (CO) histochemistry and glial fibrillary acidic protein (GFAP; astrocyte marker) and Iba-1 (microglia marker) and vesicular glutamate transporter 1 (VGluT1) (trigeminal afferent terminal marker) immunohistochemistry.
For CO histochemistry, the sections were incubated with PBS containing 0.5 mg/ml cytochrome c, 0.5 mg/ml diaminobenzidine (DAB) (both from Sigma), and 50 mg/ml sucrose for 6–7 h at 37°C in a shaker incubator. For GFAP and Iba-1 immunochemistry, the sections were incubated in 0.6% H2O2 in PBS to block endogenous peroxidase activity. After rinses in PBS, the sections were incubated at 4°C in rabbit anti-GFAP (DAKO 1:20,000) and rabbit anti Iba-1 (Wako 1:2,000) for 48 h, followed by incubation in biotinylated donkey anti-rabbit antibody (Jackson ImmunoResearch). The detection of GFAP and Iba-1 immunoreactivity was performed using the ABC kit (Vector Labs) and visualized with DAB. Iba-1-immunostained sections were counterstained with cresyl violet.
For VGluT1 immunofluorescence, brain stem sections from P3 and P10 rats were incubated in polyclonal anti-vesicular Glutamate Transporter 1 (Millipore 1:3,000) in 0.01 M PBS containing 1% normal donkey serum, 2% BSA, and 0.3% Triton X-100 overnight at 4°C. Next, the sections were rinsed with PBS and then incubated with FITC-conjugated donkey anti-guinea pig (H+L) (Jackson Immunoresearch) at room temperature for 2 h. The sections were rinsed with PBS, mounted onto glass slides, and coverslipped with fluorescence mounting medium.
Data Analyses
Physiology.
The duration of spike and afterhyperpolarization (AHP) were measured at their half amplitude. All data are expressed as means ± SE. A Student's t-test and a one-way ANOVA test were used. A linear regression test was used to show developmental changes in the latency.
Morphology.
Glial reaction to nerve injury in the PrV is evident as a conspicuous increase in both astrocyte number and process complexity that can be visualized with GFAP immunocytochemistry. Similar changes in the numbers and morphologies of microglia were observed with Iba-1 immunohistochemistry. We used a stereological approach and counted the number of astrocytes and microglia within a predefined square area (124,238 μm2 or 0.1 mm2) placed over the ventral PrV. This region of interest (ROI) occupied a large sector of the ventral PrV, where barrelettes are located. Both the astrocyte and microglial cell bodies are small, and cell counts from alternate sections from four different animals at each age were made. In addition, ∼20 astrocytes and microglia from representative sections of four pups at P3 and P10 were drawn under ×60 magnification in two-dimensions using a drawing tube attached to a Nikon light microscope. Astrocyte spans in all these cases were around or less than 30 μm, and cell bodies measured around 5–7 μm; thus, the cellular processes can be traced in two dimensions and traced cells were selected from mid-depth of 40-μm-thick sections.
Microscopic observations of the GFAP-immunostained sections gave us the impression that in addition to increased numbers, astrocytes displayed more complex process branching patterns in P3 cases following neonatal ION lesion. We performed Sholl analysis (linear method, Sholl 1953) by placing 10-μm-spaced concentric rings centered on the cell body and counted the number of intersections. We also counted the number of branch points in each drawing. Such measures are routinely used for quantitative analyses of dendritic morphology. Here we used them as measures of astrocyte process complexity.
We also analyzed the intensity of GFAP expression with a Nikon Microphot microscope (Nikon Americas) under bright-field ×10 magnification. Microscope images were captured with a Nikon Digital Sight camera mounted to the microscope, and labeling intensities were obtained using Image J. Two circular ROI of 106,068 μm2 were placed on the dorsal and ventral (barrelette region) PrV separately. Integrated optical density pixel intensities were measured inside each ROI. The intensity of the dorsal ROI was taken as a control. The difference between the dorsal and ventral ROI represented GFAP response to nerve injury in the ventral PrV where the ION central axons terminate.
We did not perform Sholl analysis or count process branch points of microglia because their processes were veil-like with few “spike” protrusions. Instead, we used NIS Elements software and measured the area of individual Iba-1-labeled microglia in representative sections from different pups at P3 and P10.
RESULTS
Classification of TG Cells
We found that biphasic (hyperpolarizing-depolarizing) electrical pulse (±0.7 nA) is more effective than monophasic depolarizing pulse for inducing AP in TG cells. Unlike monophasic depolarizing pulses, with biphasic pulses the membrane is hyperpolarized first and then depolarized, so that the amplitude of the membrane potential change is twofold larger than monophasic depolarization. In addition, some ion channels (for example T-type Ca2+ channels) are inactivated at resting membrane potential. Preceding hyperpolarization releases the inactivation (also called deinactivation), and depolarization activates them.
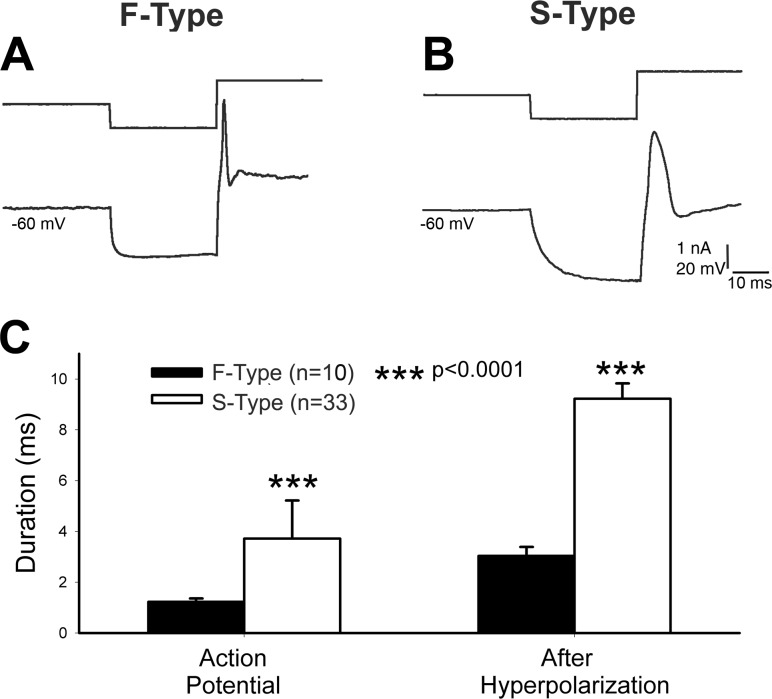
Similar to the mouse TG (Cabanes et al. 2002), the neonatal rat TG cells can be classified as F-type and S-type according to the shape and duration of the AP and AHP. F-type cells showed a narrow AP without a “hump” in the AP repolarization phase and a narrow AHP (Fig. 2A) while S-type cells showed a wide AP and AHP with a marked inflection (hump) in the AP repolarization phase (Fig. 2B). The half-amplitude duration of AP of F-type cells was 1.23 ± 0.13 ms (n = 10) while that of S-type cells was 3.04 ± 0.35 ms (n = 33), which was significantly different (P < 0.0001) from F-type cells (Fig. 2C). The half-amplitude of AHP of F-type cells was 3.72 ± 1.50 ms (n = 10), while that of S-type cells was 9.23 ± 0.60 ms (n = 33), which was significantly (P < 0.0001) longer than F-type cells (Fig. 2C). Next, we calculated the ratio of the two cell types and found that neonatal rat TG contained 23.3% F-type cells and 76.7% S-type cells (n = 43).
Fig. 2.
Classification of TG cells in rat pups. A and B: biphasic electrical pulse (top traces) induces an action potential (AP) with different shape. Note that F-type (A) cell shows a narrow AP and afterhyperpolarization (AHP) and S-type cell, wide AP and AHP. C: comparison of half-duration of AP and AHP between F- and S-type TG cells showing significant difference between the two classes.
Identification of Whisker Pad-Innervating TG Cells
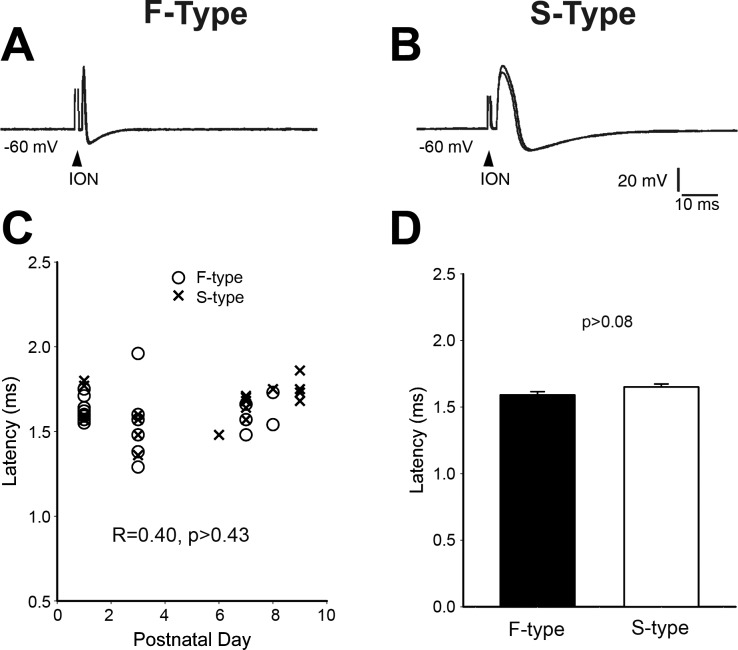
Because TG cells that innervate whisker pad are widespread in the central part of the TG (Klein et al. 1986), we randomly impaled TG cells in the central TG. Then, we identified the whisker pad-innervating TG cells by testing the response to stimulation of the ION. As shown in Fig. 3, A and B, stimulation of the ION induced an AP. Note that each record is made of five superimposed traces. In both F-type and S-type cells, the latency of the AP was fixed. This result indicates that the AP originated from peripheral branch of TG axon (ION) rather than from current spread to the cell body in the TG.
Fig. 3.
Identification of whisker pad-innervating TG cells. A and B: stimulation of ION induces an AP with fixed latency from both F- and S-type TG cells. Note that each record is formed from 5 superimposed traces. C: plot latencies from both types of TG cells against postnatal days showing that there are no developmental changes in latency. D: averaged latency of F- and S-type TG cells showing no significant difference between them.
We plotted the latencies of both cell types against postnatal days up to P9 (Fig. 3C). There was no developmental change in the latencies (linear regression R = 0.40, P > 0.43) of ION fibers between P2 and P9. We pooled latencies from all time points to get averaged latency for the two types of cells. The averaged latency for F-type cells was 1.59 ± 0.25 ms, while that of S-type cells was 1.65 ± 0.22 (Fig. 3D), which is about the same as F-type cells (P = 0.086). Therefore, the conduction velocity of peripheral branch of both F-type and S-type TG cells is about the same. This conclusion was further supported by the fact that the latencies from both types of cells were overlapped at each time point (Fig. 3C). In the central TG, 29 out of 43 (69%) recorded cells were identified as whisker pad-innervating cells.
Mechanical Crush Affects Both F- and S-Type TG Cells Equally
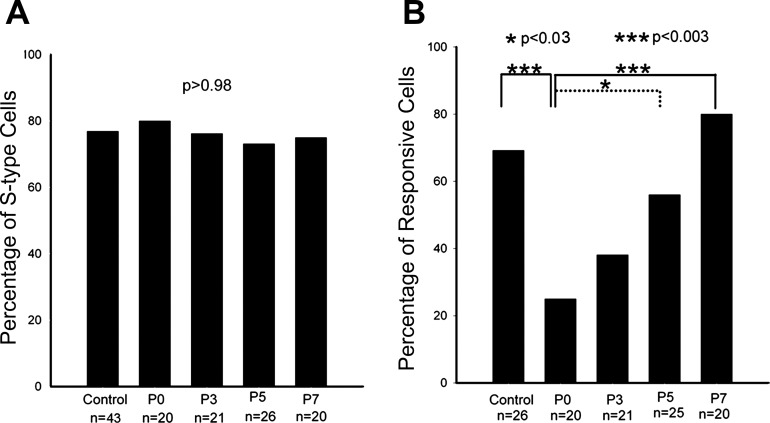
A couple of days after ION crush, the ION and the maxillary branch of the trigeminal nerve appear thinner (Fig. 1). We wanted to determine whether ION crush selectively affected one of the two classes of TG cells. In the normal TG, 76.7% of 43 recorded cells were S-type cells while the rest (23.3%) were F-type cells. Immediately after ION crush, 80.0% of the recorded cells were S-type cells (n = 20), while postinjury 3 days, 76.2% (n = 21), 5 days, 73.1% (n = 26), and 7 days, 75.0% (n = 20) of recorded cells were S-type cells. There was no significant difference (ANOVA test P > 0.98) among them (Fig. 4A). Thus ION crush affects both cell types.
Fig. 4.
Effects of ION crush. A: the percentage of recorded S-type TG cells from normal TG (control) and those at different ages after ION crush. There is no significant difference between them, indicating that mechanical crush affects both types of TG cells. B: the proportion of recorded whisker pad-innervating cells shows the time course of ION functional recovery. Immediately after the crush, about ⅔ ION fibers are blocked, and functional recovery starts after postinjury 3 days and finishes at postinjury day 7.
ION Crush Partially and Temporarily Blocks Conduction Function
As mentioned here earlier, in the normal TG, 69% of 43 recorded cells were identified as whisker pad-innervating cells. Immediately after ION crush, stimulation of ION distal to the crush site induced APs in only 25% of recorded cells (n = 20), which was significantly lower than the normal TG (P < 0.005), suggesting that ∼64% of ION fibers were blocked. The proportion of responded TG cells increased to 38% 3 days after the crush (n = 21), which was not significantly different from immediately after ION crush (P > 0.30). At postinjury day 5, 54% of recorded TG cells (n = 25) innervated whisker pad, which was significantly higher than that of postinjury day 0 (P < 0.03), suggesting that the conduction function of ION recovered to ∼78%. At postinjury day 7, 80% of recorded cells (n = 20) responded to the stimulation of the distal ION, which did not differ from the normal TG (P > 0.40). We interpret this as complete (100%) functional recovery of ION (Fig. 4B). In the next series of experiments, we investigated the effects of nerve crush and recovery on associated synaptic plasticity in the principal sensory nucleus of the trigeminal nerve (PrV) in the brain stem.
Nerve Injury-Induced Silent Synapses Are Reversible After ION Functional Recovery
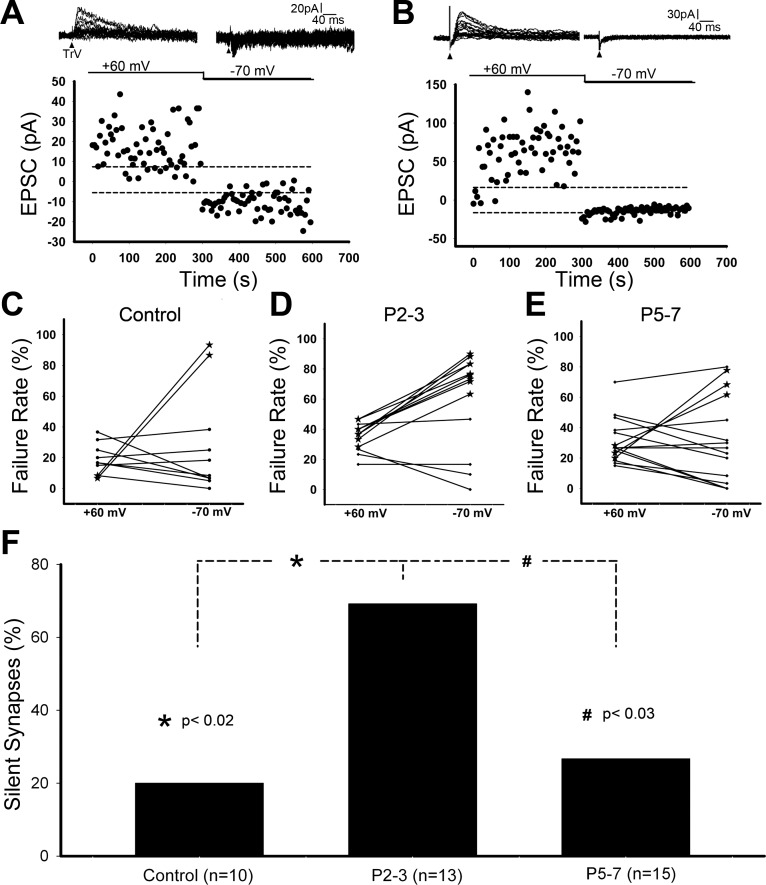
In neonatal rats, most of the synapses in the PrV are functional and show NMDA- and AMPA-mediated EPSCs at holding potentials of +60 mV and −70 mV (Fig. 5A). We previously reported that neonatal ION transection (permanent nerve damage) leads to conversion of functional synapses into silent synapses without AMPA receptor-mediated EPSCs, indicated by high failure rate at −70 mV (Fig. 5B) (Lo and Erzurumlu 2007). Here we tested for the incidence of silent synapses following nerve crush. In normal PrV, only 20% of cells (n = 10) reveal silent synapses with higher failure rate at −70 mV than that at +60 mV (Fig. 5C). The incidence of silent synapses increased to 69% (n = 13, Fig. 5D) 2–3 days after ION crush, which was ∼3.5 times of the control (P < 0.02, Fig. 5F). At postinjury days 5–7, the incidence of silent synapses dropped to 27% (n = 15, Fig. 5, E and F), which was significantly different from postinjury days 2–3 (P < 0.03) but about the same as control (P > 0.71). Thus, the changes in the incidence of silent synapses depend on the conduction function of ION. The activity-dependent AMPA receptor trafficking is reversibly regulated within the first postnatal week.
Fig. 5.
Deafferentation-induced silent synapses are reversible after ION functional recovery. A: example record of functional synapses from a trigeminal principal nucleus (PrV) neuron. B: example record of silent synapses from a PrV neuron. C: plot of failure rate at holding potential of +60 mV and −70 mV for normal PrV showing silent synapses from two cells (denoted by asterisks) with high failure rate at −70 mV (lacking AMPA receptor-mediated EPSCs). D: at postinjury days 2–3, more PrV cells reveal silent synapses. E: at postinjury days 5–7, just a few PrV cells show silent synapses. F: the incidence of recorded silent synapses demonstrates that deafferentation-induced silent synapses are reversible after ION functional recovery.
Disruption of Barrelettes After ION Crush Is Not Reversible After ION Functional Recovery
The central axons of TG cells that contribute to the ION in the periphery develop discrete clusters of terminals and together with small diameter thalamocortical projection cells of the PrV form a neural pattern (barrelettes) that replicates the punctate distribution of the whiskers on the snout (Bates et al. 1982; Erzurumlu et al. 1980; Ma and Woolsey 1984). The PrV is a peanut-shaped nucleus laterally capped by the trigeminal tract. The barrelette region, or the ION central projection zone of the nucleus, is confined to the ventral half of the nucleus, whereas the dorsal portion receives inputs from the mandibular division of the trigeminal nerve. To delineate the effects of ION crush and functional recovery on barrelette patterns, we used immunocytochemistry for VGluT1 to label sensory afferent terminal patterns and CO histochemistry, a generic barrelette and barrel marker. On the sham side, whisker-related sensory axon terminal patches and CO-positive barrelettes could be seen at P3 and more distinctly at P10 (Fig. 6, A, C, E, G). After ION crush, TG terminals failed to form whisker-specific patches, and CO-positive barrelettes were absent both at P3 and P10 (Fig. 6, B, D, F, H). These observations confirm previous reports that damage to the ION during a sensitive period up to P3 irreversibly abolishes barrelette patterns (Belford and Killackey 1980; Durham and Woolsey 1984) and even functional recovery of the crushed ION does not rescue whisker-related neural patterning in the PrV.
Fig. 6.
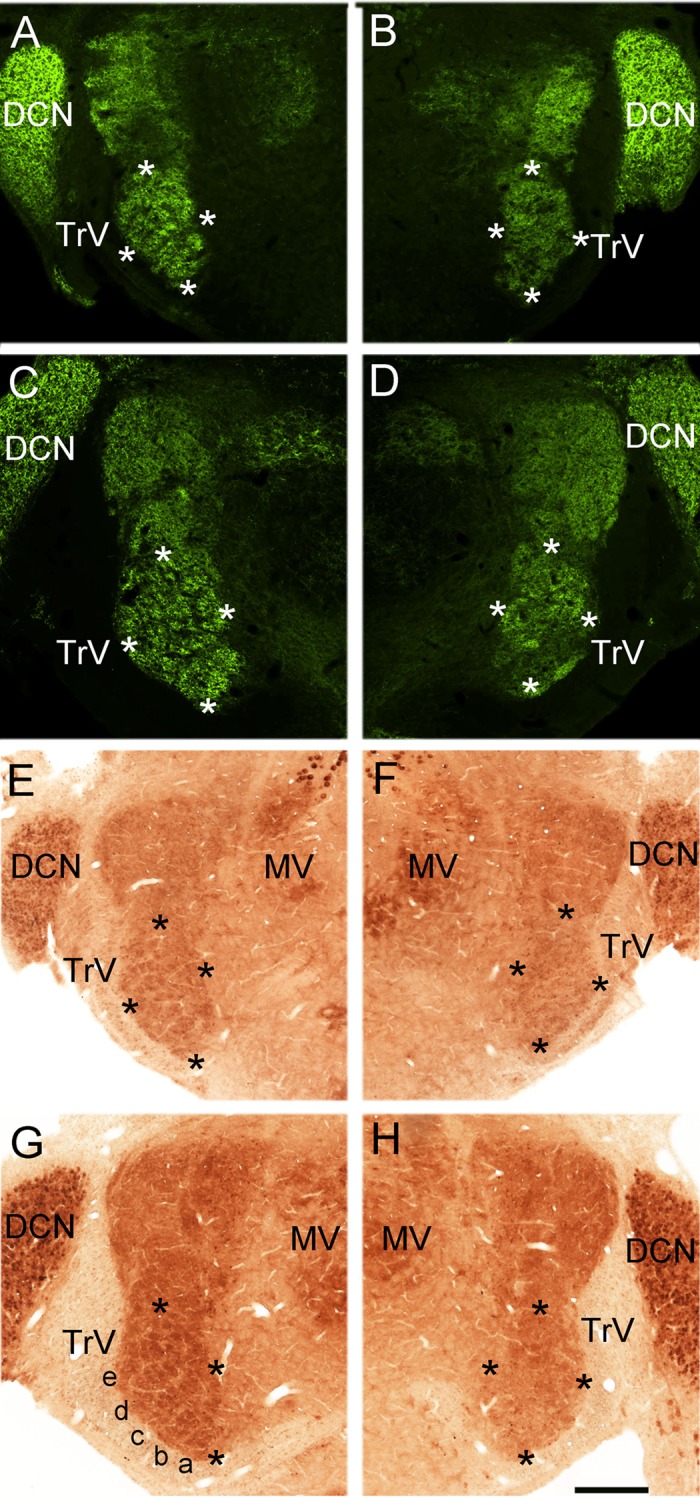
ION crush-induced disruption of barrelettes in the PrV is not reversible. The PrV is a peanut-shaped nucleus, and the barrelettes are confined to the ventral half of the nucleus. In the brain stem, most sensory afferent terminals are positive for vesicular glutamate transporter 1 (VGluT1). VGluT1-labeled TG fiber terminals form whisker-related patches (barrelettes) in the sham side PrV at both postnatal day 3 (A) and 10 (C). Three (B) and ten (D) days after ION crush, the patchy terminal distribution is disrupted. Cytochrome oxidase staining reveals barrelette patterns in P3 (E) and P10 (G) sham-operated side better. The barrelettes are absent in nerve crushed side at P3 (F) and P10 (H). DCN, dorsal cochlear nucleus; TrV, trigeminal tract; MV, motor trigeminal nucleus. Asterisks outline the ventral, barrelette region of the PrV. In G, whisker barrelette rows a-e are marked. Scale bar = 250 μm.
Reaction of Astrocytes and Microglia to ION Crush and Functional Recovery
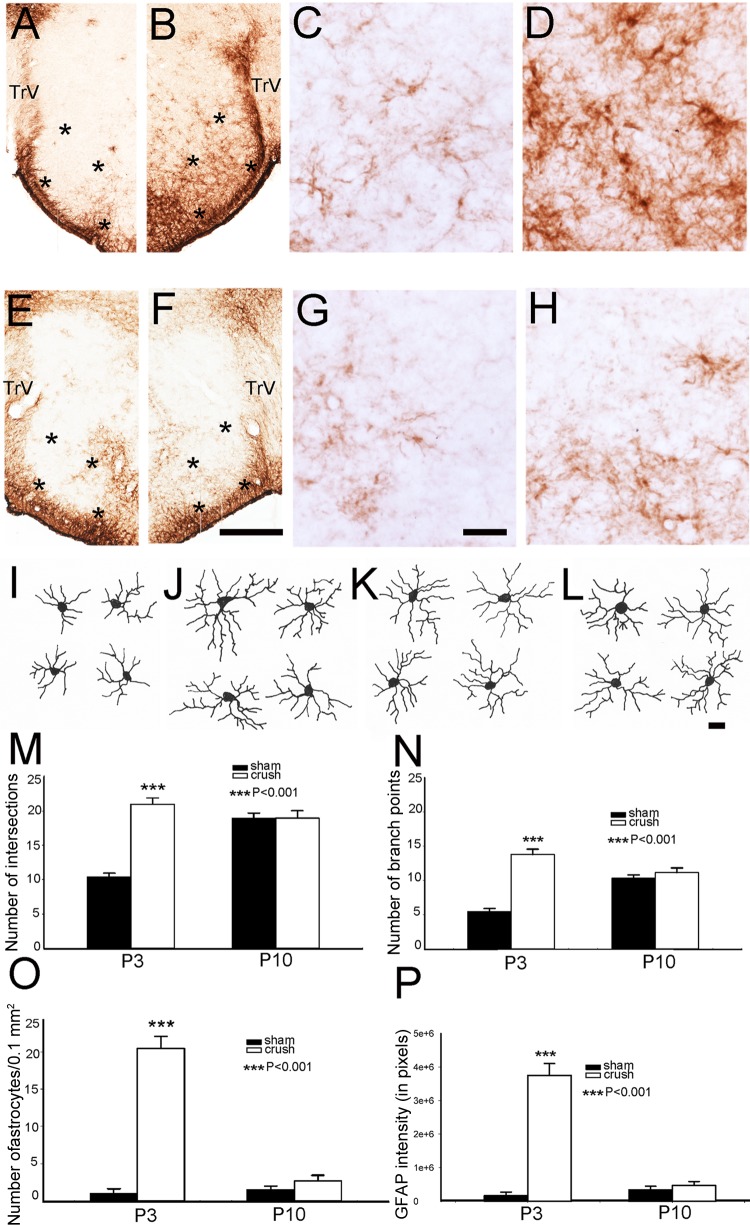
Previously, we showed that neonatal ION transection leads to significant gliosis in the PrV, and ensuing synaptic plasticity is mediated by astrocytes (Lo et al. 2011). Neonatal ION crush also resulted in upregulation of GFAP (an astrocyte marker) expression in the barrelette region of the PrV at P3 (Fig. 7, A–H). A dramatic increase in astrocyte numbers, length, and thickness of their processes was evident in the ION-recipient zone of the PrV under light microscopic observation. After ION functional recovery at P10, the expression of GFAP returned to normal levels (Fig. 7, E–H). We used stereological quantification to compare the numbers of GFAP-positive astrocytes, Sholl analysis for astrocytic process complexity, and quantitative analysis of the intensity of GFAP labeling (Fig. 7, M–P). In sham-operated side, astrocyte processes showed increased complexity from P3 to P10 (Fig. 7, I, K), and this was confirmed with quantitative analyses (Fig. 7, M, N). At P3, nerve-crushed side showed highly arborized astrocyte processes (Fig. 7J), and quantitative analyses showed significant increase in astrocyte process complexity (Fig. 7, M, N). However, this difference was no longer evident at P10 when functional recovery has taken place (Fig. 7, M, N). At P3, the PrV on the nerve-crushed side had dramatically increased GFAP-positive astrocyte numbers and immunostaining intensity compared with controls and also compared with P10 (Fig. 7, O, P).
Fig. 7.
Astrocytosis in response to ION crush. A–D show low- and high-power micrographs of GFAP labeling in the sham side PrV (A, C) and ION-crushed side PrV (B, D) on P3. E–H: similar series of micrographs showing GFAP labeling at P10. Note that increased glial response is diminished on the ION crush side (F, H) compared with that seen at P3 (B, D). I–L show representative camera lucida drawings of individual astrocytes from control (I, K) and nerve-crushed (J, L) PrV at P3 (I, J) and P10 (K, L). Asterisks outline the barrelette region of the PrV. In F, scale bar = 250 μm for all low-power micrographs, and in G, scale bar = 20 μm for all high-power micrographs; in L, scale bar = 10 μm for all camera lucida drawings. Sholl analysis of number of intersections is shown in M, and number of astrocyte process branch points is shown in N. Bar graphs in O and P show comparisons of astrocyte numbers and GFAP staining intensity, respectively.
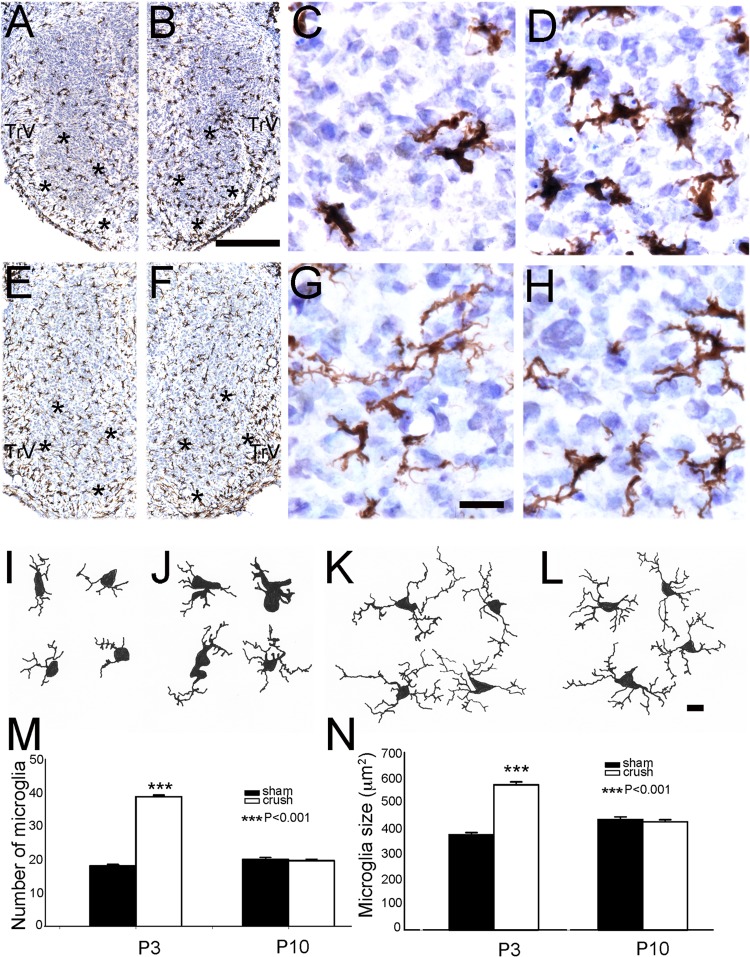
Figure 8 illustrates our findings with Iba-1 immunostaining of microglia. As with astrocytes, we saw a pronounced increase in the numbers of microglia in the PrV on the nerve-crushed side compared with the sham-operated side (Fig. 8, A–D). This was notable at P3 but no longer evident by P10 (Fig. 8, E–H). There is also a marked change in the morphology of microglia between P3 and P10. Early on, microglia have rotund cell bodies and few lamellar processes (Fig. 8I). At P10, characteristic sentient appearance of microglia is evident with ramified processes (Fig. 8L). On the nerve-crushed side, microglia in the ventral PrV had larger cell bodies in addition to increased numbers (Fig. 8, J, M, N) compared with sham-operated side at P3. No difference was observed in these parameters between the two sides on P10 (Fig. 8, K–N).
Fig. 8.
Microglial response to ION crush. A–D show low- and high-power micrographs of Iba-1 immunolabeling in the sham-operated side PrV (A, C) and ION-crushed side PrV (B, D) on P3. E–H: similar series of micrographs showing Iba-1 immunolabeling at P10. Note that increased glial response is diminished on the ION crush side (F, H) compared with that seen at P3 (B, D). I–L show representative camera lucida drawings of individual astrocytes from control (I, K) and nerve-crushed (J, L) PrV at P3 (I, J) and P10 (K, L). Asterisks outline the barrelette region of the PrV. In B, scale bar = 250 μm for all low-power micrographs, and in G, scale bar = 20 μm for all high-power micrographs; in L, scale bar = 10 μm for all camera lucida drawings. Bar graphs in M and N show comparisons of microglia numbers and sampled microglia size (including the processes), respectively.
DISCUSSION
How does the CNS respond to peripheral nerve injuries during the normal developmental period of synaptic wiring of somatosensory pathways? In the mature CNS, deafferentation results in a rapid synaptic loss followed by a prolonged period of new synapse formation. This form of neural plasticity has been referred to as “reactive synaptogenesis,” implying that it is a reaction to deafferentation (Cotman et al. 1981; Hamori 1990; Matthews et al. 1976). In the mature state, the time course of reactive synaptogenesis varies among different central pathways. For example, in the entorhinal cortex-dentate gyrus pathway, reactive synaptogenesis starts on postlesion day 6 and persists for more than a month (Cotman et al. 1981; Marrone et al. 2004). In the dorsal column lemniscal pathway, reactive synaptogenesis begins in the thalamus about a month after lesion and continues for several weeks (Wells and Tripp 1987a,b).
Not much is known about reactive synaptogenesis during development of the somatosensory pathways, particularly following peripheral nerve injuries. A number of studies have noted that experimentally induced inflammation of the skin and abnormal activation of “pain” fibers in the dorsal root ganglia in newborn animals cause long-lasting, and often irreversible, expansion of dorsal root afferent terminal zones in the spinal cord with significant behavioral and physiological consequences in the adult (Peng et al. 2003; Ruda et al. 2000; Walker et al. 2003). Obstetric injuries to the brachial plexus during birth and orofacial injuries and fractures in young children are common occurrences, which can lead to permanent brachial plexus palsy or trigeminal nerve pathologies (see reviews by Alcalá-Galiano et al. 2008; Eggensperger Wymann et al. 2008; Sandmire et al. 2008; Vyas et al. 2008). While these neurological cases are extensively studied at the peripheral nerve level, CNS consequences are unknown.
Regeneration of the transected ION fibers is a slow process, and it is never complete in neonates. Central effects of neonatal ION transection have been reported and include cell death, expansion of representation for noninfraorbital vibrissae, and sprouting of axon terminals in laminae I and II in the medullary trigeminal dorsal horn (Golden et al. 1997; Jacquin et al. 1993). Previously, we found that ION transection in newborn rat pups induces rapid reactive synaptogenesis within the PrV as early as 2 days after injury, then reaches a peak by P5, and remains at that level for 2 wk (Lo et al. 2011). Furthermore, ION lesions during the critical period for barrelette formation initially produce “silent” synapses. High-frequency stimulation, which mimics sensory afferent activity, rapidly switches silent synapses into functional synapses (Lo and Erzurumlu 2007) via activity-dependent AMPAR exocytosis. Our experiments were designed to test the hypothesis that neonatal peripheral nerve crush injuries permit better functional recovery of associated central synaptic circuitry compared with nerve transection. We found that nerve crush is more permissive for successful regeneration and reconnection (recovery) of the synaptic communication between the periphery and the brain stem. We sought to determine whether regenerative peripheral nerve injury (nerve crush) in neonates would arrest reactive synaptogenesis, permit de novo synaptic refinement, and resume synaptic function. Unfortunately, in the present study we could not use the MII assay because the time course of recovery after mechanical crush injury is short and overlaps with the normal course of synaptogenesis. Our results show that impulse conduction along the ION is blocked by ∼64% immediately after mechanical crush but it began recovering 3 days postinjury and was complete by 7 days. Similar to ION transection effects in the PrV, initial response to ION crush was conversion of functional synapses to silent synapses (∼3.5 times of control value by 2–3 days postinjury), but these silent synapses did not persist. They became functional synapses paralleling ION conduction recovery. Thus, activity-dependent AMPAR trafficking in response to peripheral nerve injury in neonates is reversible depending on the recovery of function along the injured nerve.
Earlier studies on neonatal ION crush in rats recorded whisker-evoked responses in the cortex 2–3 days postinjury (Waite and Cragg 1982); in adult rats, evoked responses could be evoked in the PrV only 7–9 days postinjury (Kis et al. 1999). Our in vitro preparation allows for quantitative measurement of ION functional recovery at the level of the TG. In this preparation, we can perform the nerve crush and immediately assess the effects in the TG or several hours thereafter or use this preparation from rat pups that have undergone ION crush on P0 at different postnatal days. In assessing the CNS effects of peripheral nerve crush, we took the incidence of silent synapses as an index of synaptic plasticity in the PrV as a function of changes in ION conduction. This index provides a quantitative way to show synaptic plasticity after ION crush, especially before ION functional recovery. We found recovery of conduction function along the crushed ION and regain of functional synapses (from silent synapses) in the PrV following nerve recovery. However, CNS effects of neonatal peripheral nerve crush injuries are not fully compensated after nerve recovery. For example, barrelette patterning in the PrV is not restored even though ION function returns and functional synapses between the trigeminal afferents and barrelette neurons are established. Earlier studies on the effects of neonatal ION crush in rat pups also found that somatotopic representation is modified both in the brain stem and cortical levels, and ION input recipient cells show larger, more complex receptive fields, altered latencies and following frequencies (Waite 1984). Most likely, upon recovery from crush injury, central axons of ION-contributing TG cells converge onto multiple barrelette neurons leading to poor spatial resolution of whisker inputs along the central pathways leading to the neocortex.
Our morphological results provide hints on the mechanisms underlying CNS synaptic plasticity after peripheral nerve damage. Both astrocytes and microglia reacted to ION crush during blockade of ION conduction and returned to quiescent status (at the morphological level) after recovery of ION function, suggesting that astrocytes and microglia are involved. Astrocytes have long been considered as the main player in the inhibition of CNS repair via formation of glial scar, but now it is accepted that astrocytes play an important role in CNS repair via promoting maturation of oligodendrocyte progenitors and removing damaged myelin debris (Barnett and Linington 2012; Baumgartner and Stangel 2013; Moore et al. 2011; Skripuletz et al. 2013). Previously, we demonstrated that neonatal ION transection induces reactive astrocytes that promote synaptogenesis in the PrV. Pharmacological blockade of astrocyte function and related synaptogenesis events prevents the formation of new synapses (Lo et al. 2011). Similarly, microglial activation has been considered harmful for neurons, but also has neuroprotective effects, such as phagocytosis of dead neurons and clearance of debris. Recent data support the protective function of microglia through the release of neuroprotective molecules (Aldskogius 2011; Doring and Yong 2011; Polazzi and Monti 2010; Walter and Neumann 2009). While our present morphological results are correlative, they suggest a role for astrocytes and microglia in CNS response to peripheral nerve injury in neonates.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-037070 (to R. S. Erzurumlu).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
F.-S.L. and R.S.E. conception and design of research; F.-S.L. and S.Z. performed experiments; F.-S.L. and S.Z. analyzed data; F.-S.L. and R.S.E. interpreted results of experiments; F.-S.L., S.Z., and R.S.E. prepared figures; F.-S.L. drafted manuscript; R.S.E. edited and revised manuscript; R.S.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. A. Püche for help with stereological analyses.
REFERENCES
- Alcalá-Galiano A, Arribas-García IJ, Martín-Pérez MA, Romance A, Montalvo-Moreno JJ, Juncos JM. Pediatric facial fractures: children are not just small adults. Radiographics 28: 441–461, 2008 [DOI] [PubMed] [Google Scholar]
- Aldskogius H. Mechanisms and consequences of microglial responses to peripheral axotomy. Front Biosci 3: 857–868, 2011 [DOI] [PubMed] [Google Scholar]
- Barnett SC, Linington C. Myelination: do astrocytes play a role? Neuroscientist 10.1177/1073858412465655, 2012 [DOI] [PubMed] [Google Scholar]
- Baumgartner W, Stangel M. Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination. Brain 136: 147–167, 2013 [DOI] [PubMed] [Google Scholar]
- Bates CA, Erzurumlu RS, Killackey HP. Central correlates of peripheral pattern alterations in the trigeminal system of the rat. III. Neurons of the principal sensory nucleus. Brain Res 281: 108–113, 1982 [DOI] [PubMed] [Google Scholar]
- Beggs S, Currie G, Salter MW, Fitzgerald M, Walker SM. Priming of adult pain responses by neonatal pain experience: maintenance by central neuroimmune activity. Brain 135: 404–417, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belford GR, Killackey HP. The sensitive period in the development of the trigeminal system of the neonatal rat. J Comp Neurol 193: 335–350, 1980 [DOI] [PubMed] [Google Scholar]
- Cabanes C, De Armentia ML, Viana AF, Belmonte C. Postnatal changes in membrane properties of mice trigeminal ganglion neurons. J Neurophysiol 87: 2398–2407, 2002 [DOI] [PubMed] [Google Scholar]
- Cotman CW, Nieto-Sampedro M, Harris EW. Synapse replacement in the nervous system of adult vertebrates. Physiol Rev 61: 684–784, 1981 [DOI] [PubMed] [Google Scholar]
- Donevan SD, Rogawski MA. Intracellular polyamines mediate inward rectification of Ca2+-permeable α-amino-3-hydroxy-5-methyo-4-isoxazolepionic acid receptors. Proc Natl Acad Sci USA 92: 9298–9302, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doring A, Yong VW. The good, the bad and the ugly. Macrophages/microglia with a focus on myelin repair. Front Biosci 3: 846–856, 2011 [DOI] [PubMed] [Google Scholar]
- Durham D, Woolsey TA. Effects of neonatal whisker lesions on mouse central trigeminal pathways. J Comp Neurol 223: 424–447, 1984 [DOI] [PubMed] [Google Scholar]
- Eggensperger Wymann NM, Hölzle A, Zachariou Z, Iizuka T. Pediatric craniofacial trauma. J Oral Maxillofac Surg 66: 58–64, 2008 [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS. Critical period for the whisker-barrel system. Exp Neurol 222: 10–12, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Bates CA, Killackey HP. Differential organization of thalamic projection cells in the brain stem trigeminal complex of the rat. Brain Res 198: 427–433, 1980 [DOI] [PubMed] [Google Scholar]
- Erzurumlu RS, Gaspar P. Development and critical period plasticity of the barrel cortex. Eur J Neurosci 35: 1540–1553, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden JP, Demaro JA, Robinson PL, Jacquin MF. Development of terminals and synapses in laminae I and II of the rat medullary dorsal horn after infraorbital nervetransection at birth. J Comp Neurol 383: 339–348, 1997 [PubMed] [Google Scholar]
- Hamori J. Morphological plasticity of postsynaptic neurons in reactive synaptogenesis. J Exp Biol 153: 251–260, 1990 [DOI] [PubMed] [Google Scholar]
- Hathway GJ, Koch S, Low L, Fitzgerald M. The changing balance of brainstem-spinal cord modulation of pain processing over the first weeks of rat postnatal life. J Physiol 587: 110–118, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann C, Hohmeister J, Demirakca S, Zohsel K, Flor H. Long-term alteration of pain sensitivity in school aged children with early pain experiences. Pain 125: 278–285, 2006 [DOI] [PubMed] [Google Scholar]
- Jacquin MF. Structure-function relationships in rat brainstem subnucleus interpolaris: V. Functional consequences of neonatal infraorbital nerve section. J Comp Neurol 282: 63–79, 1989 [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Chiaia NL, Klein BG, Rhoades RW. Structure-function relationships in the rat brainstem subnucleus interpolaris: VI. Cervical convergence in cells deafferented at birth and a potential primary afferent substrate. J Comp Neurol 283: 513–525, 1989 [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Zahm DS, Henderson TA, Golden JP, Johnson EM, Renehan WE, Klein BG. Structure-function relationships in rat brainstem subnucleus interpolaris. X. Mechanisms underlying enlarged spared whisker projections after infraorbital nerve injury at birth. J Neurosci 13: 2946–2964, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeno P, László N. Reinnervation of a single vibrissa after nerve excision in the adult rat. Neuroreport 13: 1743–1746, 2002 [DOI] [PubMed] [Google Scholar]
- Kis Z, Farkas T, Rábl K, Kis E, Kóródi K, Simon L, Marusin I, Rojik I, Toldi J. Comparative study of the neuronal plasticity along the neuraxis of the vibrissal sensory system of adult rat following unilateral infraorbital nerve damage and subsequent regeneration. Exp Brain Res 126: 259–269, 1999 [DOI] [PubMed] [Google Scholar]
- Klein BG, MacDonald GJ, Szczepanik AM, Rhoades RW. Topographic organization of peripheral trigeminal ganglionic projections in newborn rats. Dev Brain Res 27: 257–262, 1986 [DOI] [PubMed] [Google Scholar]
- Lo FS, Erzurumlu RS. Conversion of functional synapses into silent synapses in the trigeminal brainstem after neonatal peripheral nerve transection. J Neurosci 27: 4929–4934, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo FS, Guido W, Erzurumlu RS. Electrophysiological properties and synaptic responses of cells in the trigeminal principal sensory nucleus of postnatal rats. J Neurophysiol 82: 2765–2775, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo FS, Zhao S. NMDA receptor subunit composition in the rat trigeminal principal nucleus remains constant during postnatal development and following neonatal denervation. Neuroscience 178: 240–249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo FS, Zhao S, Erzurumlu RS. Astrocytes promote peripheral nerve injury induced reactive synaptogenesis in the neonatal CNS. J Neurophysiol 106: 2876–2887, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma PM, Woolsey TA. Cytoarchitectonic correlates of the vibrissae in the medullary trigeminal complex of the mouse. Brain Res 306: 374–379, 1984 [DOI] [PubMed] [Google Scholar]
- Marrone DF, LeBoutillier JC, Petit TL. Comparative analyses of synaptic densities during reactive synaptogenesis in the rat dentate gyrus. Brain Res 996: 19–30, 2004 [DOI] [PubMed] [Google Scholar]
- Matthews DA, Cotman C, Lynch G. An electron microscopic study of lesion-induced synaptogenesis in the dentate gyrus of the adult rat. II. Reappearance of morphologically normal synaptic contacts. Brain Res 115: 23–41, 1976 [DOI] [PubMed] [Google Scholar]
- Moore CS, Abdullah SL, Brown A, Arulpragasam A, Crocker SJ. How factors secreted from astrocytes impact myelin repair. J Neurosci Res 89: 13–21, 2011 [DOI] [PubMed] [Google Scholar]
- Peng YB, Ling QD, Ruda MA, Kenshalo DR. Electrophysiological changes in adult rat dorsal horn neurons after neonatal peripheral inflammation. J Neurophysiol 90: 73–80, 2003 [DOI] [PubMed] [Google Scholar]
- Polazzi E, Monti B. Microglia and neuroprotection: from in vitro studies to therapeutic applications. Prog Neurobiol 92: 293–315, 2010 [DOI] [PubMed] [Google Scholar]
- Ren K, Anseloni V, Zou SP, Wade EB, Novikova SI, Ennis M, Traub RJ, Gold MS, Dubner R, Lidow MS. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain 110: 588–596, 2004 [DOI] [PubMed] [Google Scholar]
- Renehan WE, Munger BL. Degeneration and regeneration of peripheral nerve in the rat trigeminal system. II. Response to nerve lesions. J Comp Neurol 249: 429–459, 1986 [DOI] [PubMed] [Google Scholar]
- Ruda MA, Ling QD, Hohmann AG, Peng YB, Tachibana T. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science 289: 628–631, 2000 [DOI] [PubMed] [Google Scholar]
- Sandmire H, Morrison J, Racinet C, Hankins G, Pecorari D, Gherman R. Newborn brachial plexus injuries: the twisting and extension of the fetal head as contributing causes. J Obstet Gynaecol 28: 170–172, 2008 [DOI] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87: 387–406, 1953 [PMC free article] [PubMed] [Google Scholar]
- Skripuletz T, Hackstette D, Bauer K, Gudi V, Pul R, Voss E, Berger K, Kipp M, Baumgartner W, Stangel M. Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination. Brain 136: 147–167, 2013 [DOI] [PubMed] [Google Scholar]
- Vyas RM, Dickinson BP, Wasson KL, Roostaeian J, Bradley JP. Pediatric facial fractures: current national incidence, distribution, and health care resource use. J Craniofac Surg 19: 339–349, 2008 [DOI] [PubMed] [Google Scholar]
- Waite PM. Rearrangement of neuronal responses in the trigeminal system of the rat following peripheral nerve section. J Physiol 352: 425–445, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite PM, Cragg BG. The peripheral and central changes resulting from cutting or crushing the afferent nerve supply to the whiskers. Proc R Soc Lond B Biol Sci 214: 191–211, 1982 [DOI] [PubMed] [Google Scholar]
- Walker SM, Meredith-Middleton J, Cooke-Yarborough C, Fitzgerald M. Neonatal inflammation and primary afferent terminal plasticity in the rat dorsal horn. Pain 105: 185–195, 2003 [DOI] [PubMed] [Google Scholar]
- Walker SM, Tochiki KK, Fitzgerald M. Hindpaw incision in early life increases the hyperalgesic response to repeat surgical injury: critical period and dependence on initial afferent activity. Pain 147: 99–106, 2009 [DOI] [PubMed] [Google Scholar]
- Walter L, Neumann H. Role of microglia in neuronal degeneration and regeneration. Semin Immunopathol 31: 513–525, 2009 [DOI] [PubMed] [Google Scholar]
- Wells J, Tripp LN. Time course of reactive synaptogenesis in the subcortical somatosensory system. J Comp Neurol 255: 466–475, 1987a [DOI] [PubMed] [Google Scholar]
- Wells J, Tripp LN. Time course of the reaction of glia fibers in the somatosensory thalamus after lesions in the dorsal column nuclei. J Comp Neurol 255: 476–482, 1987b [DOI] [PubMed] [Google Scholar]
- Wollgarten-Hadamek I, Hohmeister J, Demirakca S, Zohsel K, Flor H, Hermann C. Do burn injuries during infancy affect pain and sensory sensitivity in later childhood? Pain 141: 165–172, 2009 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Matsuda Y. Study on sensory neurons of the mouse with intracellular-recording and horseradish peroxidase-injection techniques. J Neurophysiol 42: 1134–1145, 1979 [DOI] [PubMed] [Google Scholar]
- Zhang YH, Wang XM, Ennis M. Effects of neonatal inflammation on descending modulation from the rostroventromedial medulla. Brain Res Bull 83: 16–22, 2010 [DOI] [PubMed] [Google Scholar]