Abstract
In the rodent, the parabrachial nucleus of the pons (PbN) receives information about taste directly from the nucleus of the solitary tract (NTS). Here we examined how information about taste quality (sweet, sour, salty, and bitter) is conveyed in the PbN of awake, freely licking rats, with a focus on how this information is transformed from the incoming NTS signals. Awake rats with electrodes in the PbN had free access to a lick spout that delivered taste stimuli (5 consecutive licks; 100 mM NaCl, 10 mM citric acid, 0.01 mM quinine HCl, or 100 mM sucrose and water) or water (as a rinse) on a variable-ratio schedule. To assess temporal coding, a family of metrics that quantifies the similarity of two spike trains in terms of spike count and spike timing was used. PbN neurons (n = 49) were generally broadly tuned across taste qualities with variable response latencies. Some PbN neurons were quiescent during lick bouts, and others, some taste responsive, showed time-locked firing to the lick pattern. Compared with NTS neurons, spike timing played a larger role in signaling taste in the first 2 s of the response, contributing significantly in 78% (38/49) of PbN cells compared with 45% of NTS cells. Also, information from temporal coding increased at a faster rate as the response unfolded over time in PbN compared with NTS. Collectively, these data suggest that taste-related information from NTS converges in the PbN to enable a subset of PbN cells to carry a larger information load.
Keywords: taste, gustatory, brain stem, parabrachial nucleus of the pons, awake recording
in the central pathway for gustation in the rodent, information about taste arriving from peripheral nerves is relayed first to the nucleus of the solitary tract (NTS), where orofacial reflexes may be initiated [through input to the reticular formation (RF); Kinzeler and Travers 2008; Travers et al. 1997], and from there to the parabrachial nucleus of the pons (PbN), where hedonic evaluation is orchestrated (through ventral forebrain projections; Hajnal and Norgren 2005). Although these two functions are interrelated in many ways, there are clear differences between them. One might predict that these differences would be apparent in the response properties in each structure. However, studies of taste responses in these two brain stem nuclei in the anesthetized preparation have revealed only subtle differences in taste response properties (e.g., Di Lorenzo et al. 2009b; Verhagen et al. 2003). Investigations of temporal coding in anesthetized rats have also found little evidence to differentiate taste-responsive cells in the NTS and PbN (Rosen et al. 2011). If there is so little to differentiate taste responses in NTS vs. PbN, the question remains as to how (or even if) information about taste stimuli is transformed as it is passed upstream from NTS to PbN.
The approach to answer this question may require the study of awake, unanesthetized subjects. In fact, there is growing evidence that sensory systems have very different patterns of activity when an organism is awake and actively acquiring information from its environment compared with when it is anesthetized and/or passively presented with stimuli. In the taste system, for example, there are cell types that can only be detected when an animal is actively licking. These are apparent in the gustatory cortex (Stapleton et al. 2006) and in the NTS (Roussin et al. 2012). For example, we found that NTS cells in awake rats (Roussin et al. 2012) are much more broadly tuned than those in anesthetized rats, contrary to previous reports (Nakamura and Norgren 1991, 1993, 1995), and the amount of information conveyed by individual taste-responsive NTS cells, through either temporal coding or rate coding, was far less than what we found in the anesthetized animal (Di Lorenzo et al. 2009a; Di Lorenzo and Victor 2003) and far less than the amount necessary to unambiguously identify a particular taste quality. As a result, we concluded that taste coding in the NTS was supported by ensembles of broadly tuned neurons working in concert, rather than by individual cells each dedicated to a single stimulus, i.e., a labeled line.
In the early 1990s, Norgren's group described taste responses from neurons recorded in the PbN (Nishijo and Norgren 1990, 1991, 1997) in awake rats. As in their parallel work on NTS, they showed that, compared with the anesthetized rat, taste-responsive cells in the PbN of the awake rat were generally more narrowly tuned and showed larger spontaneous firing rates. In fact, in the PbN, 42.4% (25/59) responded specifically to a single taste quality (Nishijo and Norgren 1990), a fraction far greater than the corresponding fraction in anesthetized rats (e.g., Perrotto and Scott 1976). In their study, Nishijo and Norgren (1990) presented taste stimuli passively through an intraoral cannula and did not analyze lick-related activity. In another study, however, they directly compared PbN responses when the rat was licking from a spout vs. receiving taste stimuli passively infused into the mouth (Nishijo and Norgren 1991). Although 37% (17/46) of the cells showed some correlation with EMG activity in that study, their main conclusion was that the responses were nearly identical in both stimulus delivery conditions. This is a stark contrast to the conclusions that we reached in the NTS based on recordings from awake, freely licking rats (Roussin et al. 2012). That is, since we found a wide variety of cells that showed lick-related activity, some of which was also taste-related, we concluded that licking and its neural correlates were an essential aspect of taste coding in the NTS.
The present study was designed to answer the question of whether information about taste is encoded similarly in the NTS and PbN in the awake, freely licking rat, with the goal of gleaning insights into how information about taste quality is transformed as it is transferred from the NTS to the PbN during active sensory acquisition.
MATERIALS AND METHODS
Other than the location of the electrodes, procedures for recording and analysis were similar to those used in Roussin et al. (2012) for recording in NTS of awake, licking rats and are given here for the reader's convenience.
Subjects
Subjects were 31 male Sprague-Dawley rats weighing 300–450 g. Rats were kept on a 12:12-h light-dark cycle (lights on at 0500) and were provided with standard rat chow ad libitum and at least 1 h of access to water daily. All procedures were approved by the Binghamton University Institutional Animal Care and Use Committee.
Electrode Implantation Surgery
Rats were initially anesthetized with a ketamine (100 mg/kg ip)-xylazine (14 mg/kg ip) mixture. The crown of the head was shaved, and the rat was fixed in a stereotaxic instrument with blunt ear bars. The head was angled at a 25° angle downward. Artificial tear gel was applied to the eyes to prevent drying. Body temperature was maintained at 37°C throughout the surgery with a rectal thermometer connected to an autoregulating heating pad. The head was swabbed three times with a Betadine solution and then with alcohol, and an incision was made at the top of the head. The fascia was gently excised with blunt dissection. Six skull screws were implanted in the skull. A small hole was drilled 12.0–12.5 mm posterior to bregma and 1.6–2.0 mm lateral to lambda. The dura was punctured and moved aside for the insertion of the microwire assembly (described below). A stainless steel wire from the microwire assembly was wrapped around a skull screw to provide an electrical ground. The electrode was then lowered slowly to a depth of 5–7 mm below the cerebellar surface. Once the electrode had descended ∼4 mm into the brain, the tongue was periodically bathed with 0.1 M NaCl to test for taste responses, followed by a distilled water rinse. The microwire assembly was lowered until a taste response was observed; at this point, the assembly was fixed to the head with dental acrylic. The animal was then placed in its home cage on top of a warmed surface until it was spontaneously mobile.
After completion of surgery, animals were given 0.05 mg of buprenorphine HCl (sc) and gentamicin (0.05 mg sc) once a day for 3 days. Additionally, topical antibiotic (Neosporin) was applied around the head cap once a day for 5 days to prevent infection. DietGel 76A (ClearH2O, Portland, ME) was placed in the animals' cages to encourage eating after the surgery. Body weight and general well-being (gait, respiration, activity, grooming, etc.) were monitored daily. Testing began 5 days after surgery or when the animal attained 90% of its presurgical body weight.
Apparatus
Taste stimuli and taste stimulus delivery system.
All taste stimuli were reagent-grade chemicals purchased from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA), dissolved in distilled water, and presented at room temperature. Stimuli were chosen to represent the five primary taste qualities: 0.1 M sucrose (S) for sweet, 0.1 M sodium chloride (N) for salty, 0.01 M citric acid (CA) for sour, 0.0001 M quinine HCl (Q) for bitter, and 0.1 M monosodium glutamate (MSG) for umami. Distilled water (W) was also presented as a taste stimulus.
Animals were water deprived 18–20 h before testing, which took place in a clear polypropylene test chamber (Med Associates, St. Albans, VT). Pressurized (∼10 psi) plastic tubes (35 ml) filled with taste stimuli were mounted outside the test chamber. Once inside the chamber, rats were free to move about and had access to a lick spout. The lick spout consisted of a bundle of twelve 20-gauge stainless steel tubes housed within a larger stainless steel tube (the lick spout) 8 mm in diameter. The amount of lick-evoked fluid delivery was calibrated individually for each taste stimulus daily, so that 12 ± 1 μl of fluid was delivered immediately after the animal broke an infrared beam located in front of the recessed lick spout. Fluid delivery was controlled by solenoids (Parker-Hannifin, Fairfield, NY), and stimulus presentations were controlled by MED-PC software (Med Associates). The order of stimulus delivery was as follows: five consecutive reinforced licks (“wet licks”) of a single randomly chosen taste stimulus, followed by a water rinse delivered on a variable ratio 5 (VR5) schedule in which each of five water rinse licks was separated by four to six “dry” licks (no fluid delivered). The next taste stimulus was presented after four to six dry licks following the final water rinse lick.
Microwire electrode assembly.
The microwire assembly consisted of eight tungsten microwires 20 μm in diameter and insulated with formvar. Each wire was soldered to a separate pin on a 10-pin connector (Omnetics, Minneapolis, MN). A stainless steel wire was soldered to the ninth pin of the connector to be wrapped around a bone screw during surgery to serve as an electrical ground. A 10-mm tungsten strut (0.005-in. diameter) was soldered to the 10th pin of the connector. The microwires were gathered into a bundle and cemented to the tungsten strut at the base near the head cap with liquid insulation (GC Electronics, Rockford, IL) so that they extended 1–2 mm past the end of the strut, with the ends staggered over ∼1 mm. The bundle of microwires was dipped into a warmed solution of 50% gelatin-50% sucrose to allow adhesion of the microwires to the tungsten strut. Electrode assemblies were then placed in a refrigerator to cool before implantation. The entire electrode assembly weighed ∼0.7 g prior to implantation.
Recording
Animals were placed into the test box, and the headstage was affixed to the animals' head cap. The beginning of a recording session was marked by illuminating a house-light in the operant chamber; sessions lasted ∼30 min. Neural activity was monitored with Sort Client software (Plexon, Dallas, TX), and the timestamps of neural waveforms and specific stimulus deliveries were recorded (25-μs resolution). Cellular waveforms were imported into Offline Sorter (Plexon). The criteria for isolation included at least a 3-to-1 signal to noise ratio and a refractory period of at least 2 ms.
Data Analysis
General.
Spontaneous firing rate was calculated from a 10-s sample of a recording session when there were no licks. A taste response was identified as a significant change in firing rate, in spikes per second (sps), compared with the baseline activity. Baseline activity was calculated by taking the 500 ms of activity before the initial stimulus lick (of the 5-stimulus lick block) of which the mean and standard deviation (SD) were calculated in sps. After the first stimulus lick, a window of 100 ms was moved in 20-ms increments until the firing rate was at least 2.58 SD (99% confidence interval) above (excitatory) or below (inhibitory) the average baseline firing rate. The trailing edge of the first time bin that was significantly above baseline was defined as the response latency. Once a significant response was identified, the 100-ms bin was moved in 20-ms increments until the firing rate was no longer significantly different from the baseline firing rate, to determine the duration of the response. Response magnitude was calculated by subtracting the baseline firing rate from the taste-evoked firing rate (i.e., the average firing rate across the response duration). Neurons that showed a significant response to any tastant (including water) were designated as “taste responsive.”
Two-way ANOVAs were calculated with GraphPad Prism software (San Diego, CA). Post hoc analyses used Bonferroni's correction for multiple comparisons. Numerical values are expressed as means ± standard error (SE) unless otherwise stated.
Analysis of temporal coding.
The information content of taste responses was analyzed as previously described (Di Lorenzo and Victor 2003) with an information-theoretic analysis (Victor and Purpura 1996, 1997; for review see Victor 2005). Analyses were performed with MATLAB software (MathWorks, Natick, MA) and the Spike Train Analysis Toolkit. This analysis quantified the amount of information about taste quality conveyed by the timing and number of spikes in a neural response. Briefly, information is measured by determining the extent to which distinct tastants lead to different responses. The difference between a pair of responses is measured by a series of metrics that are sensitive to spike times at a range of levels of precision and to the number of spikes. All analyses were performed for several response durations: the first 200 ms, 500 ms, 1 s, 1.5 s, and 2 s after the initial “wet” lick.
For each metric, the distance between two spike trains is given by the minimum cost required to transform one spike train into the other. For the series of metrics sensitive to spike timing, denoted Dspike[q], the allowed transformations consist of adding a spike (unit cost), deleting a spike (also unit cost), and moving a spike by an amount of time t. The latter transformation has a cost qt, where q is a parameter (in units of 1/s) that sets the level of temporal precision. To examine the temporal characteristics of the response at different levels of temporal precision, we sampled values of the parameter q in half-octave steps from 0 to 512. As the degree of temporal precision q becomes greater, moving a spike in time yields a greater distance in the metric space, and thus the metric is more heavily weighted toward spike timing. When q is set to 0, there is no cost for moving a spike in time; however, the cost of adding or removing a spike still applies. This metric is denoted Dcount. It is simply the difference in spike count between each response, and we use it to measure the information contained in spike count.
Each metric (Dspike[q] or Dcount) provides an estimate of the amount of information (H) that a cell conveyed, based on the extent to which pairs of responses to the same stimulus tended to be more similar to each other than pairs of responses to different stimuli. This estimate was determined in two steps. First, each response spike train (R) is decoded, using the notion of similarity corresponding to the metric. The decoded stimulus, S, is the stimulus for which the average distance in metric space from the spike train R to each of the spike trains elicited by S was shorter than the average distance from R to the set of spike trains elicited by any other stimulus. Information, H, was then calculated from the confusion matrix between the actual stimulus that elicited each response and the decoded stimulus obtained by the above rule.
In our experiment, animals were presented with six stimuli; the amount of information (H) corresponding to perfect discrimination between all taste stimuli is log2(6) = 2.58 bits. If the classification of responses is completely random, then H = 0. The value of information (H) at q = 0 is denoted as Hcount and refers to the amount of information conveyed by spike count alone, which we refer to as rate coding. The value of q at which H is greatest is referred to as qmax, and the maximum value of H is referred to as Hmax. If Hmax is greater than Hcount, then spike timing contributes to the amount of information carried and qmax determines the precision of spike timing that is relevant to carrying this information. Conversely, if Hmax = Hcount (i.e., if qmax = 0), then spike count carries all of the estimated information.
Two additional analyses were performed: shuffle (Hshuffle) and exchange (Hexchange) (Victor and Purpura 1996). First, a shuffle analysis controlled for the well-known upward bias in information estimates (Miller 1955; Treves and Panzeri 1995). For this analysis, information was recalculated for 40 surrogate data sets, created by randomly shuffling the stimulus labels associated with the actual responses. Taste responses were only considered to carry a nonzero amount of information if the information estimated from the original data set (Hcount or Hmax) was larger than the information estimated from the shuffled data sets (Hcount > Hshuffled + 2SD or Hmax > Hshuffled + 2SD). Here Hshuffled was the mean value obtained by applying Dspike[qmax] to the 40 shuffled data sets and 2SD is twice the standard deviation of those values.
Second, to distinguish the contribution of individual spike timing from the contribution of firing rate envelope, we estimated information (Hexchange) from a surrogate data set constructed by randomly exchanging pairs of spikes between recorded responses to the same stimulus. Thus these surrogate data sets preserved the spike count distribution and the rate envelope of the original data but eliminated its patterns of individual spike timing. If Hmax > Hexchange + 2SD (all quantities calculated from Dspike[qmax]), it was concluded that the contribution of spike timing to the cell's ability to discriminate between taste qualities was significant.
All calculations included the Treves-Panzeri-Miller-Carlton bias correction for the limited number of samples (Panzeri et al. 2007). For values of Hcount and Hmax that did not exceed the shuffle correction and were therefore considered insignificant, the final value of these quantities was taken to be 0.
Analysis of relationship of spiking activity to licks.
For all cells with Hmax > Hshuffled, the relationship between licks and neural firing was assessed by calculating the magnitude-squared coherence (Kattla and Lowery 2010), hereafter referred to as “coherence.” The analysis was carried out with NeuroExplorer v.4.109 software (Plexon), applied to spiking data obtained during the entire recording session.
Histology
Animals were deeply anesthetized with a lethal dose of Sleepaway (1 ml/kg; Fort Dodge Animal Health, Fort Dodge, IA), and DC current (1 mA, cathodal; 12 s) was then passed through the microwire from which a taste response was recorded. Animals were perfused transcardially with isotonic saline followed by a 10% formalin solution. Rats were decapitated, and the brain was extracted and placed in a formalin solution for a minimum of 1 wk. Before slicing, brains were rinsed with a phosphate-buffered saline (PBS) three times and then left in a 20% sucrose in PBS solution for at least 24 h. Brains were frozen and sliced in 40-μm sections. Sections were mounted on slides and stained with cresyl violet for reconstruction of the lesion site.
RESULTS
Seventy-seven PbN cells were recorded from 29 awake, freely licking rats. Forty-nine of these (64%, recorded in 20 animals) were taste responsive. The other 28 (36%, recorded in 9 animals) were not taste responsive but showed firing activity that covaried with licking behavior. Additionally, 8 of the 49 taste-responsive cells (16%) showed lick-related activity in addition to taste-related activity. All of these cell types were intermingled within the PbN (see Fig. 12). On average, taste-responsive cells had a spontaneous firing rate of 10.9 ± 2.0 sps (SE). The 28 cells with lick-related activity fell into two categories (see below): lick cells (21 of 28), which had a spontaneous firing rate of 22.6 ± 4.0 sps, and anti-lick cells (7 of 28), which had a spontaneous firing rate of 9.7 ± 4.2 sps. Anti-lick cells became quiescent while the rat licked but were active otherwise.
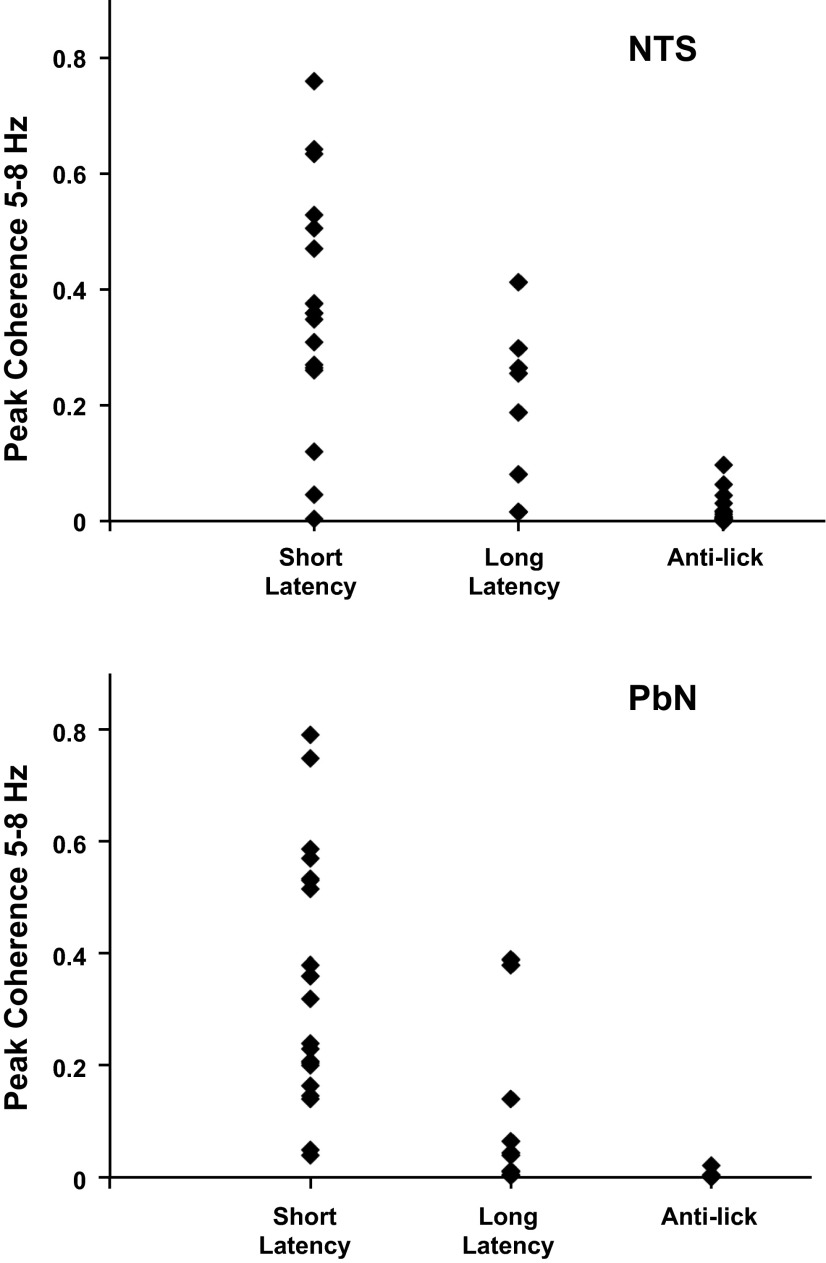
Fig. 12.
Coherence (as magnitude squared) between neuronal firing and lick events in taste-responsive and anti-lick cells in NTS (top) and PbN (bottom). The taste-responsive cells are cells for which Hmax > Hshuffled and are subdivided into those that show only short-latency (<1.0 s) responses and those that show either short-latency and long (>1.0 s)-latency or only long-latency responses. In NTS, n = 17 cells with short-latency responses, n = 8 cells with long-latency responses, n = 21 anti-lick cells; in PbN, n = 21 cells with short-latency responses, n = 10 cells with long-latency responses, n = 8 anti-lick cells.
It should be noted that in some animals the same recording channel had taste-responsive cells present over multiple days. The number of electrodes that yielded taste-responsive cells that were recorded on 1, 2, 5, 6, and 7 days were 18, 3, 1, 1, and 2, respectively. All recordings from the same wire on multiple days were treated as separate cells, as their response properties differed from day to day and thus represented distinct snapshots of cellular activity in the PbN. However, it is possible that some of these serial recordings represent recordings from a single neuron that remained isolated over multiple daily recording sessions.
General Response Characteristics
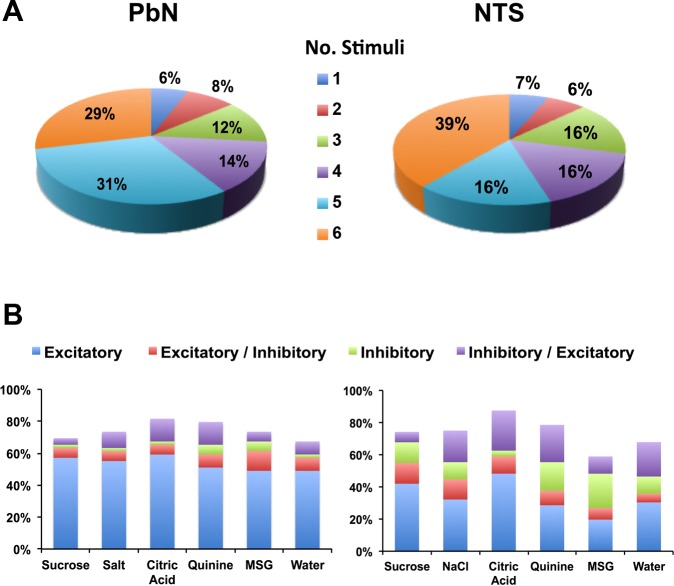
The majority of taste-responsive cells (46/49, 93.8%) were broadly tuned, that is, they responded to more than one taste stimulus. On average, cells responded to 4.4 ± 0.2 of 6 taste stimuli. Figure 1A shows the proportion of cells that responded to one, two, three, four, five, or six of the taste stimuli and compares results from the current PbN recordings with results obtained under similar conditions in NTS (Roussin et al. 2012). The proportion of cells in both PbN and NTS that responded to each stimulus with excitation, inhibition, or mixed excitation-inhibition is shown in Fig. 1B. Taste-responsive cells in the PbN showed a smaller proportion of inhibitory and mixed excitatory-inhibitory responses but a larger proportion of purely excitatory responses (χ2 = 55.1, df = 3, P < 0.001) than cells in the NTS.
Fig. 1.
Taste responses in parabrachial nucleus (PbN) vs. nucleus of the solitary tract (NTS) cells. A: proportion of PbN (left) and NTS (right) cells in awake rats that responded to different numbers of tastants. Most taste-responsive cells in the PbN and NTS in awake rats are broadly tuned across taste qualities. B: proportion of response type (excitatory, inhibitory, or mixed) for each taste stimulus in PbN (left) and NTS (right) cells. There is a preponderance of purely excitatory responses in the PbN compared with the NTS; there are also more inhibitory responses in the NTS compared with the PbN. MSG, monosodium glutamate. NTS data replotted from Roussin et al. (2012).
Each cell was also categorized according to its best stimulus, defined as the taste stimulus that evoked the largest response, either excitatory or inhibitory. There were 9 sucrose best cells (of 49, 18.4%), 9 NaCl best cells (18.4%), 5 citric acid best cells (10%), 12 quinine best cells (25%), 9 MSG best cells (18%), and 5 water best cells (10.2%). Figure 2 shows the average excitatory taste response magnitudes for PbN and NTS cells. Responses to NaCl and MSG were significantly higher in the PbN compared with the NTS [2-way ANOVA, F(5,618) = 2.761, P < 0.02]. In the PbN, the relative efficacy of the mean excitatory responses was as follows: MSG > NaCl > sucrose > citric acid > water > quinine. Response magnitudes evoked by each of the taste stimuli were correlated (Pearson's r) as follows: sucrose to NaCl: 0.84, to MSG: 0.85, to citric acid: 0.87, to quinine: 0.62, to water: 0.70; NaCl to MSG: 0.94, to citric acid: 0.84, to quinine: 0.42, to water: 0.76; MSG to citric acid: 0.85, to quinine: 0.40, to water, 0.73; citric acid to quinine: 0.66, to water: 0.86; quinine to water: 0.73. Only those responses that were significant were included in this analysis.
Fig. 2.
Mean response magnitudes (±SE) for all taste stimuli in PbN and NTS [spikes per second (sps)]. Responses to NaCl and MSG are amplified in the PbN compared with the NTS [2-way ANOVA, F(5,618) = 2.761, *P < 0.02]. NTS data replotted from Roussin et al. (2012).
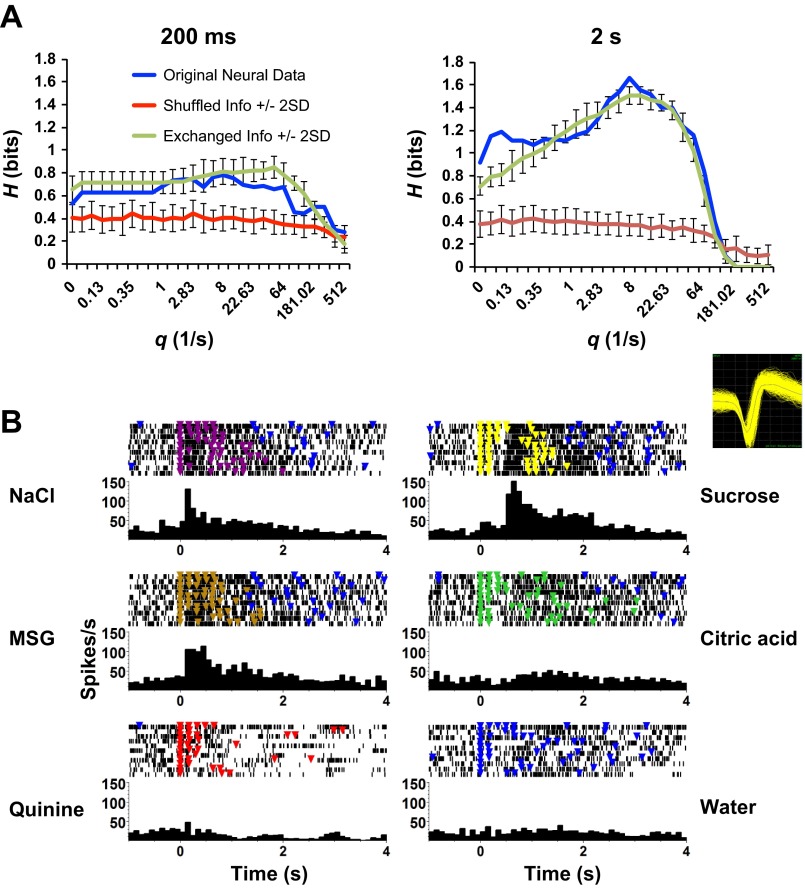
Taste response latencies varied widely across cells. Nearly half (22/49, 45%) of the taste-responsive PbN cells showed responses that began during the five-lick stimulus presentation; an example is shown in Fig. 3. There were an additional six cells (12%) that had no obvious responses when viewed over a several-second timescale (Fig. 4A) but clearly show a response to taste stimuli when the analysis is focused on the first 100 ms of a lick (Fig. 4B). Twenty-one cells (43%) showed “late” responses starting ≥1.5 s after the first stimulus lick, after the stimulus licks ended. Figure 5 shows an example of recordings from such a cell. These long-latency responses were generally associated with more aversive stimuli such as quinine HCl (8 of 21) or citric acid (6 of 21). Some of these responses might be attributable to a response to water following the taste stimulus presentation, as has been commonly reported to occur after acid or quinine (e.g., Rosen et al. 2010); however, other responses clearly occurred before the water rinse licks were presented.
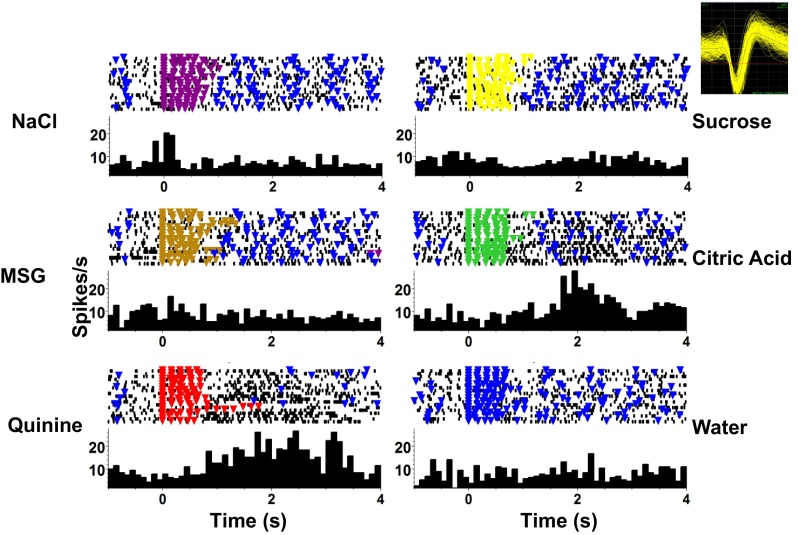
Fig. 3.
An example of a PbN cell with short-latency responses to taste stimuli. This cell showed excitatory responses to NaCl, sucrose, and MSG that began during the stimulus presentation (5 consecutive licks). A significant response to citric acid was also present and increased gradually in magnitude over ∼2.5 s following the first stimulus lick. In each panel, a raster of the stimulus trials is shown at top; each black dot marks the occurrence of a spike, and each colored triangle indicates the occurrence of a reinforced (producing fluid) lick. Taste stimulus presentation consisted of 12 μl of taste stimulus delivered after each of 5 consecutive licks. Taste stimulus delivery was then followed by 5 licks that each produced 12 μl of water separated by 4–6 dry licks where licking was not followed by any fluid delivery. The peristimulus time histogram (PSTH) for each response is shown at bottom of each panel. Inset, top right: extracellular waveform of the recorded cell.
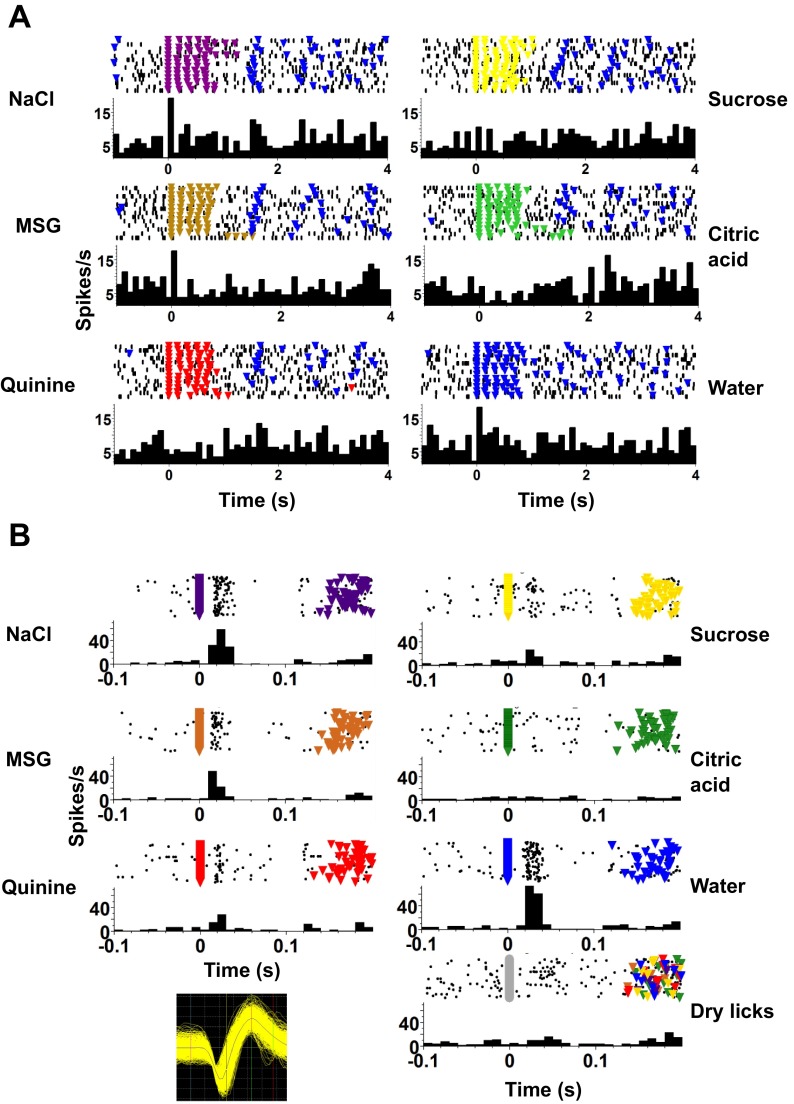
Fig. 4.
An example of a PbN cell that responds to taste stimuli only on a short timescale. A: responses to taste stimuli over several seconds. At this timescale, no taste responses are apparent. B: responses to taste stimuli shown on a shorter timescale. Here it is apparent that the cell responds significantly to all taste stimuli except citric acid. There is also no response to dry licks, indicating that the lick itself is not generating a response. Plotting conventions as in Fig. 3.
Fig. 5.
An example of a PbN cell with long-latency taste responses. This cell shows significant responses to citric acid and quinine that do not begin until after the stimulus presentation has concluded. Format as in Fig. 3.
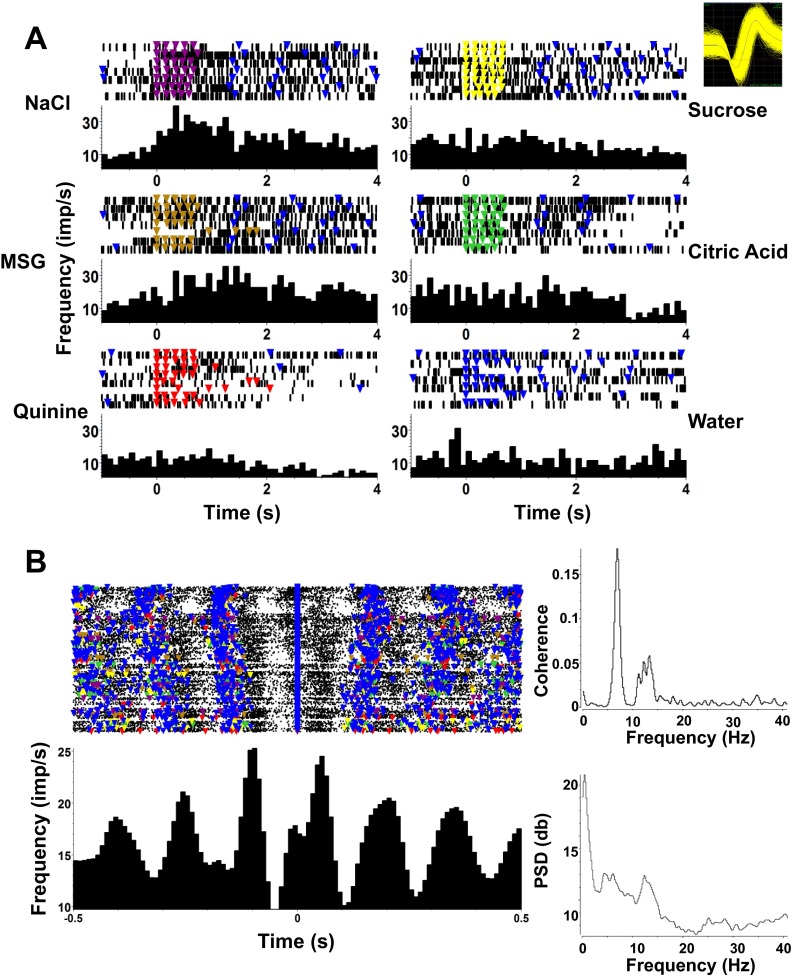
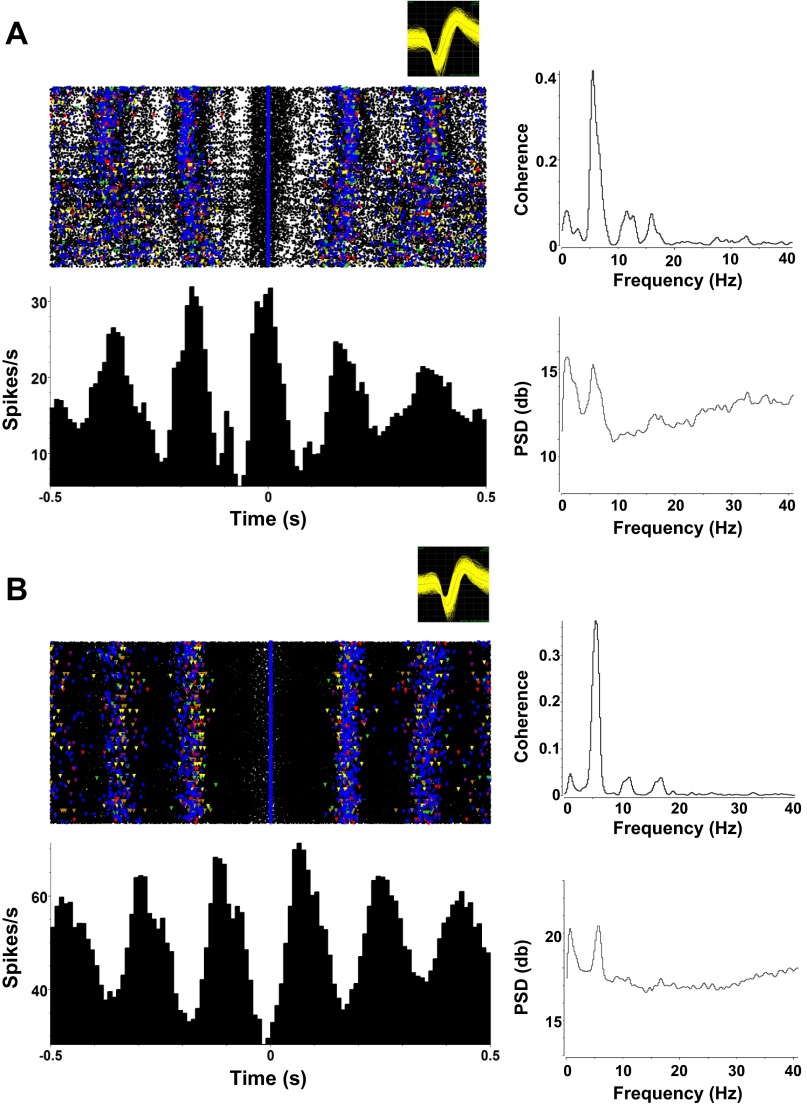
Eight taste-responsive cells (16.3%) showed lick-related activity. These cells fired in phase with licks but also showed taste-specific increases in firing rate. Figure 6A shows an example of such a cell. It was tuned to NaCl and MSG (Fig. 6A) and had a firing rate peak just after a lick occurred (Fig. 6B).
Fig. 6.
Example of a taste-responsive PbN cell that shows lick-related as well as taste-related firing. A: this cell shows significant responses to NaCl and MSG. Format is as in Fig. 3. Inset, top right: waveform of cell recorded in A and B. B, left: raster and PSTH of this cell, centered around the occurrence of a lick, either reinforced with fluid or dry, reveal strongly lick-related firing. Right: spike-lick coherence (top) and spike power spectral density (PSD; bottom) plots.
Temporal Coding of Taste Information in PbN
Metric space analyses (see materials and methods for details) were applied to taste-evoked spike trains for the first 200 ms, 500 ms, 1 s, 1.5 s, and 2 s after the first taste stimulus lick. This allowed examination of how the neural representation of taste quality evolved over time. At each response interval analyses were repeated at varying levels of temporal precision, denoted q (1/s). The value of q at which information is greatest is called qmax; the amount of information at qmax is denoted Hmax. The amount of information at q = 0 is denoted Hcount and is an indication of the amount of information conveyed by spike count (rate coding) alone.
At each time point, the results of the metric space analyses of taste-evoked spike trains were compared against those derived from two surrogates (see materials and methods for further details). First, shuffled data sets were derived from the recorded spike trains with taste stimulus labels randomly shuffled. Second, “exchange” data sets were constructed by randomly exchanging spikes within the responses to each tastant (but keeping their times intact); this yields surrogate responses that retain the rate envelope of the original data but not the temporal patterns of the individual spike trains. For a given cell, if Hmax is larger than Hcount, and larger than Hshuffle + 2SD, then we conclude that temporal aspects of the response (and not just spike count) contribute to the representation of taste quality. If, furthermore, Hmax is greater than Hexchange + 2SD at qmax, then we conclude that individual spike timing conveys information about taste quality, above and beyond spike count and the rate envelope of the response.
In the PbN, spike timing conveyed a significant amount of information about taste quality beyond what was conveyed by spike count alone in 38 cells (of 49, 78%) for the 2-s response interval. An example cell that uses temporal coding to convey information about taste quality is shown in Fig. 7. Two response intervals are analyzed in Fig. 7A, and the corresponding rasters and peristimulus time histogram (PSTHs) are shown in Fig. 7B. In the first 200 ms of the response (Fig. 7A, left), the maximum value of information (Hmax) was 0.78 bits and occurs at qmax = 8; the information estimate from spike count alone (q = 0) is Hcount = 0.53. In this interval, the rate envelope conveys more information about taste quality than spike count alone (since Hmax > Hcount and Hmax > Hshuffle + 2SD). However, there was no identifiable contribution to the information carried by the pattern of spike times (Hmax < Hexchange + 2SD during the first 200 ms). When a longer portion of the response is analyzed, the amount of information increases, and the way that it is conveyed changes as well (Fig. 7A, right). Specifically, Hmax = 1.66 for the first 2 s of the response, which is not only greater than Hcount (0.92 bits) and Hshuffle + 2SD but also greater than Hexchange + 2SD. Thus, as this neuron's response unfolds, temporal coding (either as rate envelope or spike timing) plays a progressively greater role, a trend that was typical of the full data set.
Fig. 7.
Information-theoretic analysis of a single taste-responsive PbN cell. A: information (H) is shown as a function of temporal precision (q), for the first 200 ms (left) and first 2 s (right) of the response. At 200 ms, the information in the recorded responses (Hmax = 0.78 bits) exceeded the information in the shuffled (Hshuffle + 2SD = 0.63 bits) but not the exchange-resampled (Hexchange + 2SD = 1.05 bits) responses, indicating that the rate envelope conveys more information than spike count, but spike timing does not make a detectable contribution. At 2 s, the information in the recorded responses (Hmax = 1.66 bits) at qmax (q = 8) exceeds the information conveyed by both the shuffled (Hshuffle + 2SD = 0.54 bits) and exchange-resampled (Hexchange + 2SD = 1.50 bits) responses, indicating that spike timing contributes to the information carried about taste quality, in addition to the information carried by spike count and rate envelope. Blue, recorded responses; red, shuffled responses; green, exchange-resampled responses; error bars indicate ±2SD. B: rasters and PSTHs of the cell shown in A; format as in Fig. 3. This cell responded significantly to NaCl, sucrose, and MSG. Inset: extracellular waveform of the recorded cell.
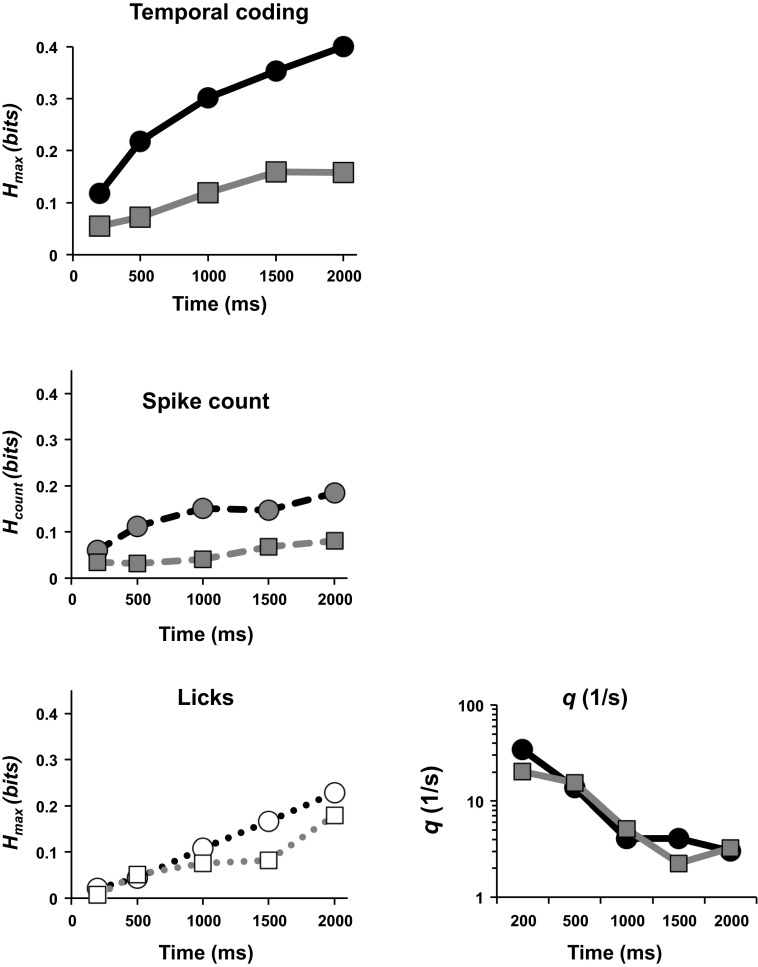
Figure 8 shows the amount of information conveyed by temporal coding (Hmax: spike timing or rate envelope) vs. spike count alone (Hcount) across cells as a function of response interval for the PbN. For both PbN and NTS, as the response unfolds over time, significantly more information per cell is conveyed by temporal coding (Student's t-test, P < 0.02) or by spike count alone (Student's t-test, P < 0.01; Fig. 8). Moreover, as the response evolves, the contribution of temporal coding (the gap between Hmax and Hcount) increases. Notably, the gap is wider in PbN than in NTS, indicating a relatively greater role for temporal coding in PbN.
Fig. 8.
Information in neural responses (average per cell) for PbN and NTS cells conveyed by spike timing (top left), by spike count alone (middle left), and in the pattern of licks (bottom left; see text for further details). Circular symbols denote results from PbN; square symbols denote results from NTS. Each point was calculated as the sum of Hmax for the neural response (top left), Hcount (middle left), or Hmax for licks (bottom left) values for all cells that showed a significant amount of information about taste quality, divided by the total number of cells recorded. As longer response intervals are considered, the amount of information carried by temporal coding is larger in PbN cells compared with NTS cells and exceeds the information in the lick responses. Bottom right: geometric mean for temporal precision of all cells with significant information from temporal coding in both PbN and NTS. NTS data replotted from Roussin et al. (2012).
In addition, more cells in PbN and NTS contribute information through the temporal aspects of their response as longer response intervals are considered. For PbN, the number of cells that made use of temporal coding grew from 16 cells (36%) at 200 ms to 38 cells (78%) at 2 s. For NTS, the corresponding quantities were 6 cells (15%) at 200 ms increasing to 18 cells (45%) at 2 s. Figure 8 also compares the information carried by the neural responses with the information contained in the licks themselves (determined by applying the same metric-space analysis to the lick events). In both PbN and NTS, the information in the neural data is greater (Student's t-test, P < 0.01 for PbN; P < 0.05 for NTS when the 2-s point is excluded). This means that the information contained in the spike trains is not simply a reflection of a dependence of the lick pattern on the tastant and must contain a substantial contribution due to the tastant itself. Reassuringly, the information contained in licking activity is very similar in the present data set and in the comparison recordings from the NTS (Student's t-test, P < 0.6; Roussin et al. 2012).
Figure 8, right, is a plot of the temporal precision, qmax, across response intervals between 200 ms and 2 s for both PbN and NTS. For both structures, temporal precision in the early portions of the response is high, but as longer response intervals are considered (and information about taste quality increases) temporal precision becomes more coarse. In the PbN, the temporal precision is greater than that in the NTS in the earliest response interval (200 ms), likely reflecting convergence and refinement of the temporal pattern of the taste-evoked spike trains.
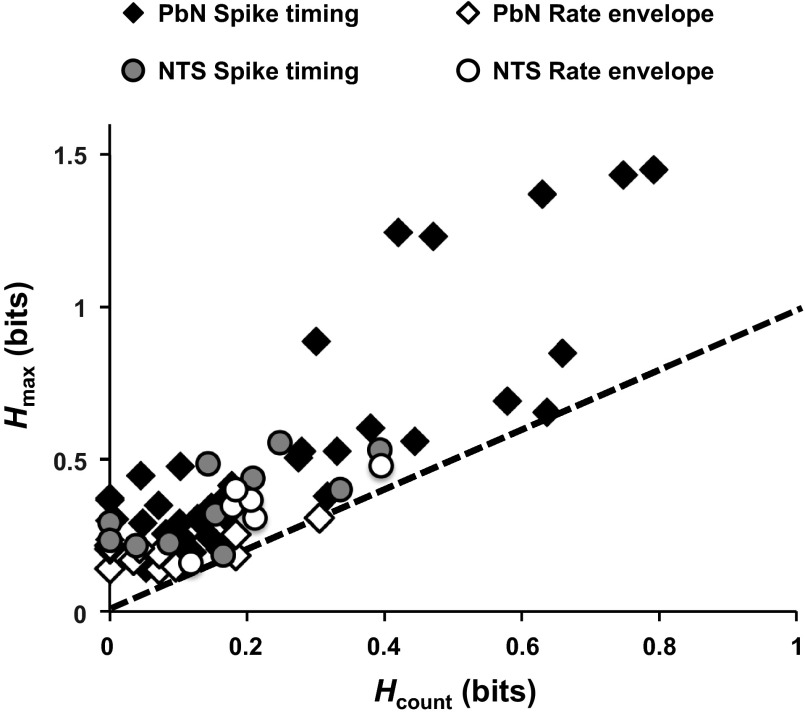
The greater role of temporal coding in PbN is further illustrated in Fig. 9, which compares information conveyed by spike count (Hcount) to information conveyed by temporal coding in PbN and NTS cells that showed evidence of temporal coding at the 2-s response interval. Filled symbols in Fig. 9 indicate cells in which individual spike timing contributed to the representation of taste quality (Hmax > Hshuffle + 2SD and Hmax > Hexchange + 2SD at qmax), and the distances of these symbols from the line of identity indicate the magnitude of this contribution. As is seen, there is a subset of PbN cells in which this contribution is >0.5 bits, a level not encountered in the comparison sample from the NTS.
Fig. 9.
Information conveyed about taste quality (average per cell) at 2 s in PbN and NTS by rate and spike timing. Dashed diagonal line indicates equality between Hmax and Hcount. Cells above the diagonal use the rate envelope (n = 27 for PbN, n = 11 for NTS) or spike timing (n = 11 for PbN, n = 7 for NTS) in addition to spike count to discriminate among taste quality at 2 s. NTS data replotted from Roussin et al. (2012).
Lick-Related Activity
Twenty-eight PbN cells (of 77, 36%) exhibited firing activity that covaried with licks but were not taste responsive. Seven (of 77, 9%) of these cells were “anti-lick” cells (see Roussin et al. 2012). That is, they were relatively quiescent during the lick bout but fired rapidly between lick bouts. Two of these cells showed a burst of activity immediately before and immediately after a lick bout, as shown by the example in Fig. 10A. The five other “anti-lick” cells did not show a peribout burst of activity but were nevertheless relatively silent during the lick bout, as shown by the example in Fig. 10B. In addition to the anti-lick cells, 21 cells showed phase-locked activity with the lick cycle. Most of these cells (n = 15; Figure 11A) had a peak firing rate close to the time of individual licks. Firing rate in the remaining lick-related cells (n = 6; Fig. 11B) peaked between individual licks (but still had a higher overall firing rate during the lick bout than during a period in which the animal was not licking).
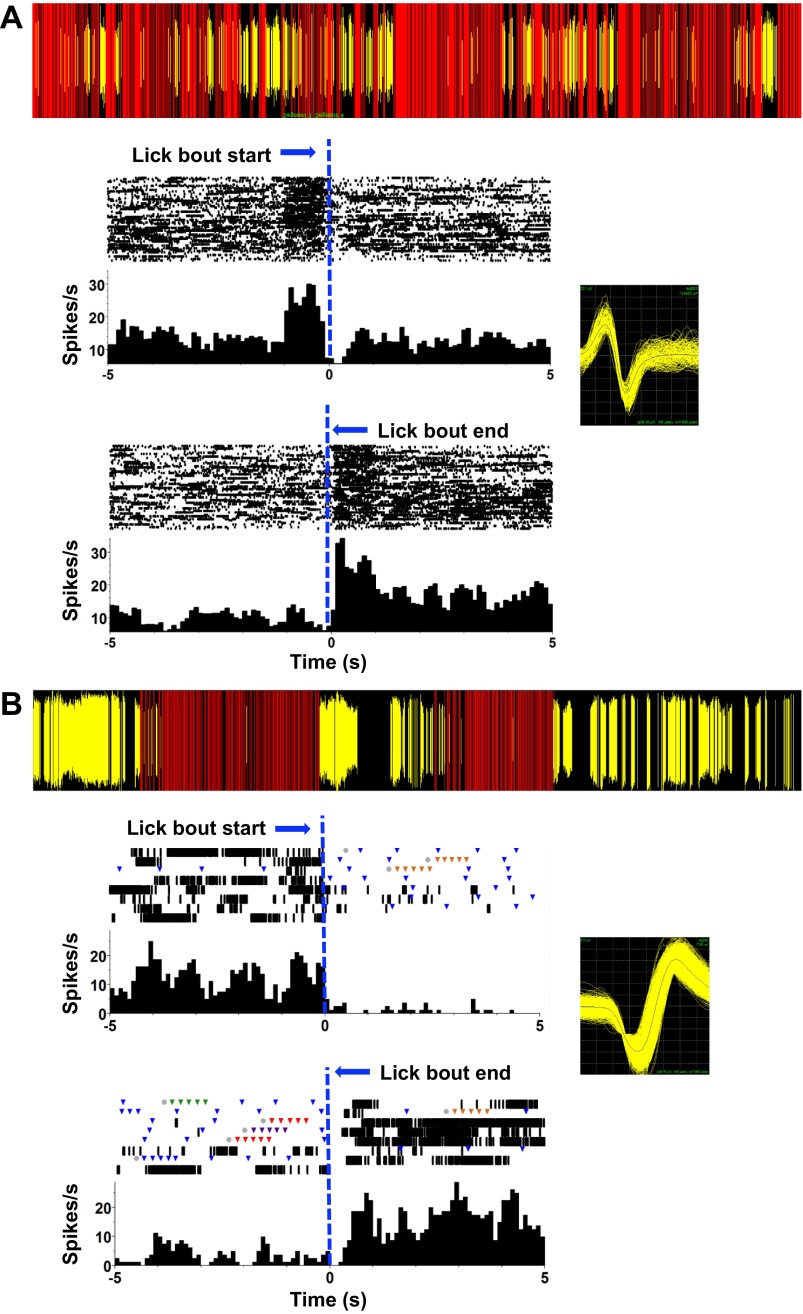
Fig. 10.
Examples of firing patterns of anti-lick cells in PbN. The lick events and firing patterns of the cells over a short time period during the recording session are shown at top of each panel. Red lines indicate the occurrence of a lick; yellow lines indicate the occurrence of a spike. Rasters and PSTHs below in each panel show activity 5 s before the lick bout begins (top) and 5 s after the lick bout ends (bottom). Lick bout beginning or end is indicated by a vertical dashed blue line positioned at the transition time. Bin size = 100 ms. Insets: waveforms of the cells recorded in A and B. A: example of an anti-lick PbN cell that shows a surge in firing rate before a lick bout and again at the end of a lick bout and a moderate decrease in firing rate while the animal is licking. B: example of an anti-lick PbN cell that fires before and after a lick bout and is completely silent during the lick bout.
Fig. 11.
Lick-related activity not associated with taste responsivity. Left: rasters and associated PSTHs of spiking activity referenced to the occurrence of a lick (zero point). Right: spike-lick coherence (top) and spike PSD (bottom) plots. A: example of a cell with a peak firing rate at the time of a lick. B: example of a cell with a peak firing rate between licks.
Relationship of Spiking Activity and Licks
To quantify the relationship of spiking activity with lick events, we calculated the coherence between these time series, both for the present taste-responsive PbN recordings and the previously recorded taste-responsive NTS neurons (Roussin et al. 2012). Results are shown in Fig. 12. Lick coherence values for taste-responsive cells with only short-latency taste responses are plotted separately from those with either both short- and long (>1.0 s)-latency or only long-latency responses. Parallel results for anti-lick cells in both structures are also plotted. These data show that most taste-responsive cells in both PbN and NTS have a detectable coherence with licks. This coherence tends to be strong among taste-responsive cells with short-latency responses and relatively weaker in those with long-latency taste responses. These findings are consistent across PbN and NTS; there is a statistically significant difference between the lick coherence values in cells with short-latency responses and those with long-latency responses for both NTS and PbN (Student's t-test, P < 0.05). Average coherence value for cells with short-latency responses was 0.37 ± 0.05 in NTS and 0.35 ± 0.05 in PbN. Average coherence value for cells with long-latency responses was 0.19 ± 0.05 in NTS and 0.11 ± 0.05 in PbN. Not surprisingly, lick coherence in anti-lick cells is negligible, as these cells fire most of their spikes when there is no licking at all.
Histology
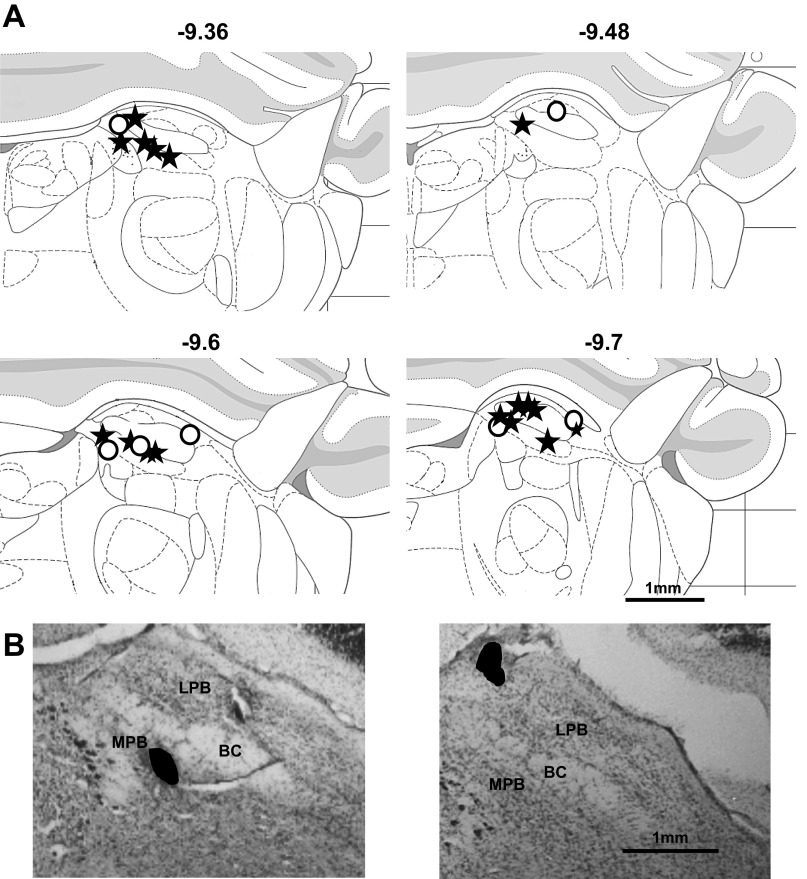
Taste-responsive cells, as well as lick-related cells, were histologically identified within the brachium conjunctivum, or in either lateral or medial PbN, as shown in Fig. 13. Fifteen lesions were located within the medial PbN, eight were located within the lateral PbN, and one was located in the brachium. Histological data were lost for five animals. There was no apparent difference in the distribution of sites where long-latency responses were recorded and those where short-latency responses were recorded. Additionally, the location of sites where lick-related activity, but not taste-responsive activity, was recorded was not different from that where taste-responsive activity was recorded.
Fig. 13.
Results of histological analyses of recording sites in the PbN. A: schematic of coronal sections through the PbN showing the location of lesions [reproduced with permission from Paxinos and Watson (2007)]. Numbers at top indicate distance from bregma (in mm). Stars are locations of lesions from animals that had taste-responsive cells; open circles are locations of lesions from animals that had lick-related activity. B: representative photos of lesions in the PbN of 2 rats. Black-filled areas indicate the location and shape of the lesion in each photomicrograph. BC, brachium conjunctivum; LPB, lateral parabrachial nucleus; MPB, medial parabrachial nucleus.
DISCUSSION
Responses to representatives of the five basic taste qualities plus water were recorded from 77 single PbN neurons in the awake, freely licking rat. While aspects of the recorded activity were qualitatively similar to what was found in the NTS under similar conditions (Roussin et al. 2012), a quantitative analysis of PbN taste responses revealed systematic differences in the way that taste quality was represented. Most importantly, temporal coding of taste quality information was significantly more prominent in the PbN compared with the NTS of the awake, freely licking rat. The convergence of taste-related information from the NTS to the PbN resulted in a significantly larger amount of information about taste quality over the first 2 s of response in the PbN compared with the NTS and a greater information load carried by individual PbN cells. These results likely reflect the convergence of NTS cells onto PbN cells.
In addition to differences in the quantity of information conveyed per cell, PbN and NTS recordings differed in the way that taste quality information is formatted (Fig. 8 and Fig. 9): the proportion of PbN cells conveying information about taste quality through the temporal properties of their responses was much greater in the PbN than in the NTS within the first 2 s of response (78% in PbN, 45% in NTS; Roussin et al. 2012). Although the collective information conveyed by the temporal characteristics of the responses grew over the first 2 s of taste responses in both NTS and PbN, the proportion and amount of information conveyed in this fashion were greater in PbN than in NTS. The information conveyed by the lick pattern was nearly identical in both structures at all response intervals considered (as expected since the experiments were carried out under similar conditions) and was less than the amount of information in the neural responses, indicating that the information about taste quality in NTS and PbN responses cannot simply reflect motor activity.
The larger amount of information about taste quality conveyed by the temporal characteristics of PbN taste responses compared with those in the NTS likely reflects the selective convergence of input from NTS cells with similar response patterns. Evidence from functionally connected pairs of simultaneously recorded taste-responsive cells in the NTS and PbN in anesthetized rats (Di Lorenzo and Monroe 1997) shows that NTS cells with taste response profiles (relative response rates across taste stimuli) similar to their PbN targets are more effective at driving PbN taste responses than NTS cells with dissimilar response profiles. In the context of rate coding, these results suggest a bias toward connections between NTS and PbN cells that respond preferentially to the same tastant. In the context of temporal coding, these data suggest that the temporal characteristics of NTS responses are transferred to PbN cells from NTS cells with some fidelity. The convergence of many such temporally informative spike trains from NTS cells onto PbN cells may reinforce the essential informative spikes in the spike train but enable filtering of noise.
One important caveat must be considered when comparing coding strategies in NTS vs. PbN. That is, there may be functional differences among the cells that were recorded in each area. For example, different groups of cells may use different neurotransmitters and/or modulators. Some may be inhibitory interneurons, while others may be projection neurons. Some may receive centrifugal input from one or more sources. Simple electrophysiological recording of taste responses as we report here cannot differentiate these possibilities. We are, however, comparing only taste-responsive cells, with the tacit assumption that their function is similar in both structures. The present PbN data and those from the NTS (Roussin et al. 2012) have shown that both structures contain a variety of cell types in addition to taste-responsive cells, including lick-only cells and anti-lick cells. These latter cell types may have a special role in taste coding that we have yet to discern.
Anesthesia Effects on Coding of Taste Information in PbN
There were major differences between the characteristics of the activity recorded during awake, spontaneous licking and during passive presentation under anesthesia in the PbN (Rosen et al. 2011) or the NTS (Di Lorenzo and Victor 2003). In both structures, the information about taste quality contained in individual neuronal responses was far less under awake conditions than under anesthesia. For example, during the first 2 s of response the average amount of information about taste quality contributed by spike timing across cells in the PbN of the awake animal was 0.4 bits compared with 0.8 bits in PbN cells in the anesthetized animal (Rosen et al. 2011). The temporal precision at which information about taste quality is maximal (qmax) in the awake animal (3.2; 313 ms) was also considerably lower than that of the anesthetized animal (7.9; 127 ms) for 2 s of response.
These differences likely represent the effects of anesthesia on the signal-to-noise ratio of taste responses. That is, anesthesia may preferentially eliminate spikes that are not due to taste stimuli (e.g., related to motor activity or “noise”) and be relatively sparing of taste-driven responses. Consistent with this notion is the observation that spontaneous firing rates in the PbN are threefold greater in unanesthetized vs. anesthetized rats. Specifically, spontaneous firing rates are ∼10–13 sps in the PbN of unanesthetized rats (e.g., Di Lorenzo 1988; Nishijo and Norgren 1991; present study) vs. ∼2–4 sps in the anesthetized rat (e.g., Geran and Travers 2009; Rosen et al. 2011). However, despite these enhanced spontaneous firing rates, response magnitudes reported for most taste stimuli are similar between anesthetized and unanesthetized rats. Since response magnitudes are normally calculated by subtracting a measure of spontaneous firing, the signal-to-noise ratio of taste responses in the brain stem would obviously be smaller in the awake rat. Results of analyses of temporal coding may reflect this degraded signal-to-noise ratio.
Water Responses
The existence of responses to water in taste-responsive cells seems to be a common feature in studies of taste-responsive areas in awake animals. Schwartzbaum (1983) noted them in the PbN of rabbits, as did Nishijo and Norgren (1991) in rat PbN. Water responses were also common in the NTS of awake rats (Nakamura and Norgren 1991; Roussin et al. 2012) and the gustatory cortex (Stapleton et al. 2006). Water-specific neurons have been identified as well in the gustatory cortex in awake rats (MacDonald et al. 2012) and in the brain stem of anesthetized rats (Rosen et al. 2010). These data are consistent with results showing that water may have its own representation in the cortex of humans (de Araujo et al. 2003) and rats (Acolla et al. 2007). Collectively, evidence is converging on the idea that water may be perceived as a tastant, in addition to other more traditional taste qualities.
Late Responses
Long-latency (“late”) responses, observed in PbN cells in the present study, were also observed in the PbN of awake rats by Nishijo and Norgren (1991) and in the PbN of awake rabbits by Schwartzbaum (1983). This type of response was also seen in the NTS of awake rats by Roussin et al. (2012). Nishijo and Norgren (1991) found that of the 46 PbN cells recorded 9 (20%) exhibited long-latency responses; 7 of those 9 were to citric acid, 1 was to quinine, and the last was to NaCl. Similarly, in the present study, late responses were observed in PbN cells only after the presentation of quinine or citric acid. This contrasts with data recorded from NTS cells in the awake rat, where some late responses were observed for all taste qualities (Roussin et al. 2012). Nishijo and Norgren (1991) suggested that these late responses could be conditional water responses following taste stimulus presentation (see Rosen et al. 2010) or responses to tastants originating from the posterior lingual taste receptors. However, it is also possible that these late responses originate from extraoral chemoreceptors in the gut, i.e., the vagal visceral system. Cells in the gut are known to express taste receptor proteins, similar to those on the tongue (Höfer et al. 1996). Moreover, gastric projections have been shown to terminate in the medial as well as lateral subnuclei of the PbN (Baird et al. 2001a, 2001b; Hajnal et al. 1999; Karimnamazi et al. 2002). The fact that both PbN and NTS cells with these late responses show very little coherence with licks (in comparison to the lick coherence seen in cells with short-latency responses) is consistent with the idea that these responses do not originate in the oropharyngeal area.
Lick-Related Activity
The observation of firing patterns that covaried with the phase of licks in 36% of PbN cells reflects the rhythmic nature in which the sensory input is acquired in freely licking rats. These findings are remarkably consistent with other studies of the PbN in awake animals. For example. Nishijo and Norgren (1991) showed that 37% (17 of 46) of taste-responsive cells showed neural activity that was correlated with tongue EMG activity in the awake rat. Moreover, Schwartzbaum (1983) also found that 37% (35 of 94) of PbN cells in the awake rabbit showed activity correlated with the lick pattern but not related to taste. In that study, Schwartzbaum noted that that 15% of PbN cells showed both lick- and taste-related activity. Interestingly, we found a similar proportion of PbN cells with such activity (8 of 49, 16%). Furthermore, coherence with licks was a common feature of taste-responsive cells, even in those cells where lick-related activity was not altogether obvious by visual inspection.
The issue of the origin of lick-related activity in both PbN and NTS remains unresolved. It is possible that lick-related activity in one structure, derived from either motor or somatosensory input, could be driving such activity in the other structure. Motor input to the PbN associated with licking may originate in the RF or the motor trigeminal nucleus (Herbert et al. 1990; Li et al. 1996; Tokita et al. 2009) and may drive lick-related activity in the PbN directly and in the NTS indirectly through projections from the PbN to the NTS (Krukoff et al. 1993). Alternatively, the NTS may be driving the phase-locked activity with licks seen in the PbN. Although there is no direct motor input to the NTS, tactile stimulation of the oral cavity, specifically the posterior tongue, has been shown to produce firing rate changes in NTS cells of the anesthetized animal (Halsell et al. 1993). In a study by Ogawa et al. (1984), 22 of 36 NTS-PbN projection neurons displayed firing rate changes in response to pinching of the tongue. Furthermore, Ogawa et al. (1982) showed that most taste-responsive cells in the PbN showed some type of receptive field for mechanical stimulation of the palate.
Another finding of the present study is the presence of anti-lick cells, i.e., cells that were silent during lick bouts. This type of cells was also observed in the NTS of the awake, freely licking rat (Roussin et al. 2012). However, there were proportionally fewer anti-lick cells in the PbN than in the NTS (7 of 74, 9% in PbN; 28 of 107, 26% in NTS; Roussin et al. 2012) and there were some subtle differences between anti-lick cells in these structures. For example, anti-lick cells in the NTS showed a sharp increase in firing rate just before and just after a lick bout occurred, but only two of seven anti-lick cells in PbN showed this effect. In Roussin et al. (2012) we hypothesized that these cells might be driven by cells with similar response patterns in the nucleus accumbens (NAc) shell (Krause et al. 2010) mediated by the lateral hypothalamus. That is, anti-lick cells in the NAc may be indirectly driving anti-lick cells in the NTS through their action on the lateral hypothalamus. Anti-lick cells in the PbN may then be driven by input from anti-lick cells in the NTS. Alternatively, or perhaps in addition, direct projections from the NAc to the PbN may regulate firing in anti-lick cells in the PbN. Consistent with this notion is evidence showing that electrical stimulation of the NAc shell inhibited the great majority of taste-responsive cells in the hamster PbN (Li et al. 2012). Balancing excitatory (e.g., from NTS) and inhibitory (e.g., from NAc) input is known to be an important function of the PbN in its role as a modulator of ingestive behavior (Wu et al. 2012).
Although the function of anti-lick cells is unknown, their pattern of activity suggests that they may suppress licking when active and thereby regulate the central pattern generator responsible for the lick response. In addition, since they are intermingled with taste-responsive cells in both NTS and PbN, they may also enhance encoding of taste stimuli by increasing the signal-to-noise ratio of the taste message while quiescent. Although anti-lick cells were far fewer in the PbN than in the NTS, their purpose might be similar.
Conclusions—Information Transfer from NTS to PbN
Comparison of the present results with those recorded under identical conditions in the NTS (Roussin et al. 2012) suggests some principles that characterize how information is transformed in its passage from the NTS to the PbN. Most importantly, evidence suggests that information about taste quality conveyed by the temporal characteristics of the responses in individual PbN cells is greater on average than that in individual cells in the NTS. This was evident in the average amount of information about taste quality conveyed by spike timing (see Fig. 8) and in the proportion of cells that use temporal coding to convey taste quality information across the first 2 s of response (see Fig. 9). Both of these measures were greater in the PbN than in the NTS, and both measures exceeded the corresponding values for spike count and lick pattern. In the initial ∼500 ms of response, during which taste quality discrimination is complete (Perez et al. 2013; Weiss and Di Lorenzo 2012), a relatively small proportion of both NTS (Roussin et al. 2012) and PbN cells convey information about taste quality through either rate or temporal coding, suggesting that in both structures taste identification is accomplished by ensembles. The apparent increase in the amount of taste-related information conveyed by PbN cells compared with NTS cells may be the result of extensive, tastant-specific convergence of input from the NTS onto PbN cells (Geran and Travers 2006, 2009; Halsell et al. 1996; Tokita et al. 2009). As a consequence, at the level of the NTS information about taste quality may require the participation of more cells acting cooperatively than in the PbN, where individual cells may carry more of the “information load.” This idea is consistent with the hypothesis that one of the functions of the PbN is to parse information about hedonics and taste quality. That is, information about taste quality might be focused on PbN-thalamic relay cells while information about hedonics might be routed to other PbN-ventral forebrain cells.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grant RO1-DC-006914 to P. M. Di Lorenzo.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.S.W. performed experiments; M.S.W. analyzed data; M.S.W., J.D.V., and P.M.D.L. interpreted results of experiments; M.S.W. and P.M.D.L. prepared figures; M.S.W. drafted manuscript; M.S.W., J.D.V., and P.M.D.L. approved final version of manuscript; J.D.V. and P.M.D.L. edited and revised manuscript; P.M.D.L. conception and design of research.
ACKNOWLEDGMENTS
The authors acknowledge the contribution of Andrew Fooden, who helped with data analyses. This project served as partial fulfillment of the Master's degree for M. S. Weiss.
REFERENCES
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron 44: 865–876, 2004 [DOI] [PubMed] [Google Scholar]
- Accolla R, Bathellier B, Petersen CC, Carleton A. Differential spatial representation of taste modalities in the rat gustatory cortex. J Neurosci 27: 1396–1404, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JP, Travers JB, Travers SP. Parametric analysis of gastric distension responses in the parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 281: R1568–R1580, 2001a [DOI] [PubMed] [Google Scholar]
- Baird JP, Travers SP, Travers JB. Integration of gastric distension and gustatory responses in the parabrachial nucleus. Am J Physiol Regul Integr Comp Physiol 281: R1581–R1593, 2001b [DOI] [PubMed] [Google Scholar]
- Bathellier B, Buhl DL, Accolla R, Carleton A. Dynamic ensemble odor coding in the mammalian olfactory bulb: sensory information at different timescales. Neuron 57: 586–598, 2008 [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Kringelbach ML, Rolls ET, McGlone F. Human cortical responses to water in the mouth, and the effects of thirst. J Neurophysiol 90: 1865–1876, 2003 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM. Taste responses in the parabrachial pons of decerebrate rats. J Neurophysiol 59: 1871–1887, 1988 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Chen JY, Victor JD. Quality time: representation of a multidimensional sensory domain through temporal coding. J Neurosci 29: 9227–9238, 2009a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo PM, Monroe S. Transfer of information about taste from the nucleus of the solitary tract to the parabrachial nucleus of the pons. Brain Res 763: 167–181, 1997 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Platt D, Victor JD. Information processing in the parabrachial nucleus of the pons: Temporal relationships of input and output. Ann NY Acad Sci 1170: 365–371, 2009b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo PM, Victor JD. Taste response variability and temporal coding in the nucleus of the solitary tract of the rat. J Neurophysiol 90: 1418–1431, 2003 [DOI] [PubMed] [Google Scholar]
- Geran LC, Travers SP. Single neurons in the nucleus of the solitary tract respond selectively to bitter taste stimuli. J Neurophysiol 96: 2513–2527, 2006 [DOI] [PubMed] [Google Scholar]
- Geran LC, Travers SP. Bitter-responsive gustatory neurons in the rat parabrachial nucleus. J Neurophysiol 101: 1598–1612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnal A, Norgren R. Taste pathways that mediate accumbens dopamine release by sapid sucrose. Physiol Behav 84: 363–369, 2005 [DOI] [PubMed] [Google Scholar]
- Hajnal A, Takenouchi K, Norgren R. Effect of intraduodenal lipid on parabrachial gustatory coding in awake rats. J Neurosci 19: 7182–7190, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsell CB, Travers JB, Travers SP. Gustatory and tactile stimulation of the posterior tongue activate overlapping but distinctive regions within the nucleus of the solitary tract. Brain Res 632: 161–173, 1993 [DOI] [PubMed] [Google Scholar]
- Halsell CB, Travers SP, Travers JB. Ascending and descending projections from the rostral nucleus of the solitary tract originate from separate neuronal populations. Neuroscience 72: 185–197, 1996 [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293: 540–580, 1990 [DOI] [PubMed] [Google Scholar]
- Höfer D, Püschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci USA 93: 6631–6634, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimnamazi H, Travers SP, Travers JB. Oral and gastric input to the parabrachial nucleus of the rat. Brain Res 957: 193–206, 2002 [DOI] [PubMed] [Google Scholar]
- Kattla S, Lowery MM. Fatigue related changes in electromyographic coherence between synergistic hand muscles. Exp Brain Res 202: 89–99, 2010 [DOI] [PubMed] [Google Scholar]
- Kinzeler NR, Travers SP. Licking and gaping elicited by microstimulation of the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 295: R436–R448, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, German PW, Taha SA, Fields HL. A pause in nucleus accumbens neuron firing is required to initiate and maintain feeding. J Neurosci 30: 4746–4756, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukoff TL, Harris KH, Jhamandas JH. Efferent projections from the parabrachial nucleus demonstrated with the anterograde tracer Phaseolus vulgaris leucoagglutinin. Brain Res Bull 30: 163–72, 1993 [DOI] [PubMed] [Google Scholar]
- Lei Q, Yan J, Huang T, Shi J, Yang X, Lv B, Li Q. Role of the lateral hypothalamus in modulating responses of parabrachial gustatory neurons in the rat. Brain Res Bull 77: 165–171, 2008 [DOI] [PubMed] [Google Scholar]
- Li CS, Chung S, Lu DP, Cho YK. Descending projections from the nucleus accumbens shell suppress activity of taste-responsive neurons in the hamster parabrachial nuclei. J Neurophysiol 108: 1288–1298, 2012 [DOI] [PubMed] [Google Scholar]
- Li YQ, Takada M, Kaneko T, Mizuno N. GABAergic and glycinergic neurons projecting to the trigeminal motor nucleus: a double labeling study in the rat. J Comp Neurol 373: 498–510, 1996 [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Meck WH, Simon SA. Distinct neural ensembles in the rat gustatory cortex encode salt and water tastes. J Physiol 590: 3169–3184, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA. Note on the bias on information estimates. In: Information Theory in Psychology: Problems and Methods. Glencoe, IL: Free Press, 1955, II-B, p. 95–100 [Google Scholar]
- Nakamura K, Norgren R. Gustatory responses of neurons in the nucleus of the solitary tract of behaving rats. J Neurophysiol 66: 1232–1248, 1991 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Norgren R. Taste responses of neurons in the nucleus of the solitary tract of awake rats: an extended stimulus array. J Neurophysiol 70: 879–891, 1993 [DOI] [PubMed] [Google Scholar]
- Nakamura K, Norgren R. Sodium-deficient diet reduces gustatory activity in the nucleus of the solitary tract of behaving rats. Am J Physiol Regul Integr Comp Physiol 269: R647–R661, 1995 [DOI] [PubMed] [Google Scholar]
- Nishijo H, Norgren R. Responses from parabrachial gustatory neurons in behaving rats. J Neurophysiol 63: 707–724, 1990 [DOI] [PubMed] [Google Scholar]
- Nishijo H, Norgren R. Parabrachial gustatory neural activity during licking by rats. J Neurophysiol 66: 974–985, 1991 [DOI] [PubMed] [Google Scholar]
- Nishijo H, Norgren R. Parabrachial neural coding of taste stimuli in awake rats. J Neurophysiol 78: 2254–2268, 1997 [DOI] [PubMed] [Google Scholar]
- Ogawa H, Hayama T, Ito S. Convergence of input from tongue and palate to the parabrachial nucleus neurons of rats. Neurosci Lett 28: 9–14, 1982 [DOI] [PubMed] [Google Scholar]
- Ogawa H, Imoto T, Hayama T. Responsiveness of solitario-parabrachial relay neurons to taste and mechanical stimulation applied to the oral cavity in rats. Exp Brain Res 54: 349–358, 1984 [DOI] [PubMed] [Google Scholar]
- Panzeri S, Senatore R, Montemurro MA, Petersen RS. Correcting for the sampling bias problem in spike train information measures. J Neurophysiol 98: 1064–1072, 2007 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (6th ed.) San Diego, CA: Academic, 2007 [Google Scholar]
- Perez IO, Villavicencio M, Simon SA, Gutierrez R. Speed and accuracy of taste identification and palatability: impact of learning, reward expectancy, and consummatory licking. Am J Physiol Regul Integr Comp Physiol 305: R252–R270, 2013 [DOI] [PubMed] [Google Scholar]
- Perrotto RS, Scott TR. Gustatory neural coding in the pons. Brain Res 110: 283–300, 1976 [DOI] [PubMed] [Google Scholar]
- Rosen AM, Roussin AT, Di Lorenzo PM. Water as an independent taste modality. Front Neurosci 4: 175, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AM, Victor JD, Di Lorenzo PM. Temporal coding of taste in the parabrachial nucleus of the pons of the rat. J Neurophysiol 105: 1889–1896, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussin AT, D'Agostino AE, Fooden AM, Victor JD, Di Lorenzo PM. Taste coding in the nucleus of the solitary tract of the awake, freely licking rat. J Neurosci 32: 10494–10506, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzbaum JS. Electrophysiology of taste-mediated functions in parabrachial nuclei of behaving rabbit. Brain Res Bull 11: 61–89, 1983 [DOI] [PubMed] [Google Scholar]
- Stapleton JR, Lavine ML, Wolpert RL, Nicolelis MA, Simon SA. Rapid taste responses in the gustatory cortex during licking. J Neurosci 26: 4126–4138, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokita K, Inoue T, Boughter JD., Jr Afferent connections of the parabrachial nucleus in C57BL/6J mice. Neuroscience 161: 475–488, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JB, DiNardo LA, Karimnamazi H. Motor and premotor mechanisms of licking. Neurosci Biobehav Rev 21: 631–647, 1997 [DOI] [PubMed] [Google Scholar]
- Treves A, Panzeri S. The upward bias in measures of information derived from limited data samples. Neural Comput 7: 399–407, 1995 [Google Scholar]
- Verhagen JV, Giza BK, Scott TR. Responses to taste stimulation in the ventroposteromedial nucleus of the thalamus in rats. J Neurophysiol 89: 265–275, 2003 [DOI] [PubMed] [Google Scholar]
- Victor JD. Spike train metrics. Curr Opin Neurobiol 15: 585–592, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor JD, Purpura KP. Nature and precision of temporal coding in visual cortex: a metric-space analysis. J Neurophysiol 76: 1310–1326, 1996 [DOI] [PubMed] [Google Scholar]
- Victor JD, Purpura KP. Metric-space analysis of spike trains: theory, algorithms and application. Network 8: 127–164, 1997 [Google Scholar]
- Weiss MS, Di Lorenzo PM. Not so fast: taste stimulus coding time in the rat revisited. Front Integr Neurosci 6: 27, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI. Neural and behavioral mechanisms of olfactory perception. Curr Opin Neurobiol 18: 408–412, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Clark MS, Palmiter RD. Deciphering a neuronal circuit that mediates appetite. Nature 483: 594–597, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]