Abstract
To characterize the cerebellar influence on neurons in the abducens (ABD) nucleus, we recorded ABD neurons before and after we inactivated the caudal part of the ipsilateral cerebellar fastigial nucleus (cFN) with muscimol injection. cFN activity influences the horizontal component of saccades. cFN inactivation increased the activity of most ipsilateral ABD neurons (19/22 in 2 monkeys) during ipsiversive (hypermetric) saccades, primarily by increasing burst duration. During contraversive (hypometric) saccades, the off-direction pause of most (10/15) ABD neurons was shorter than normal because of the early resumption of ABD activity. Early ABD firing caused the early contraction of antagonist muscles that reduced eye rotation and made contraversive saccades hypometric. Thus the cerebellum controls ipsilateral ABD activity by truncating on-direction bursts during ipsiversive saccades and extending off-direction pauses during contraversive saccades. We conclude that cFN output keeps saccades accurate by controlling when ABD on-direction bursts and off-direction pauses end.
Keywords: cerebellum, motoneuron, agonist, antagonist, saccade
the brain must coordinate the timing of agonist and antagonist contractions to produce fast and accurate movements. The cerebellum plays a critical role in this coordination. For example, cerebellar patients make dysmetric movements (Holmes 1917; Straube et al. 2001; Waespe and Baumgartner 1992; Waespe and Müller-Meisser 1996), which could be caused by impaired agonist-antagonist coordination. Hore et al. (1991) describe abnormal agonist and antagonist electromyography (EMG) during hypermetric arm movement in cerebellar patients. In the Hore et al. (1991) study, the most salient disorder was the delayed onset of the antagonist EMG. Agonist disorders were not always evident, but, when they were, the agonist EMG grew more slowly and lasted longer than normal.
The objective of this study was to describe how cerebellar output modifies activity in the abducens (ABD) nucleus during horizontal saccades. Saccades are well-suited to evaluating the influence of the cerebellum. The saccade-related parts of the cerebellar cortex, the oculomotor vermis (OMV) and its target nucleus, the caudal fastigial nucleus (cFN, also called the fastigial oculomotor region), have been described in detail. Damage to either area causes saccade dysmetria (OMV: Barash et al. 1999; Kojima et al. 2010b; Takagi et al. 1998; cFN: Goffart et al. 2004; Iwamoto and Yoshida 2002; Robinson et al. 1993). Also, the saccade-related circuits outside of the cerebellum that receive cerebellar input are well-established [Scudder et al. (2002) for review].
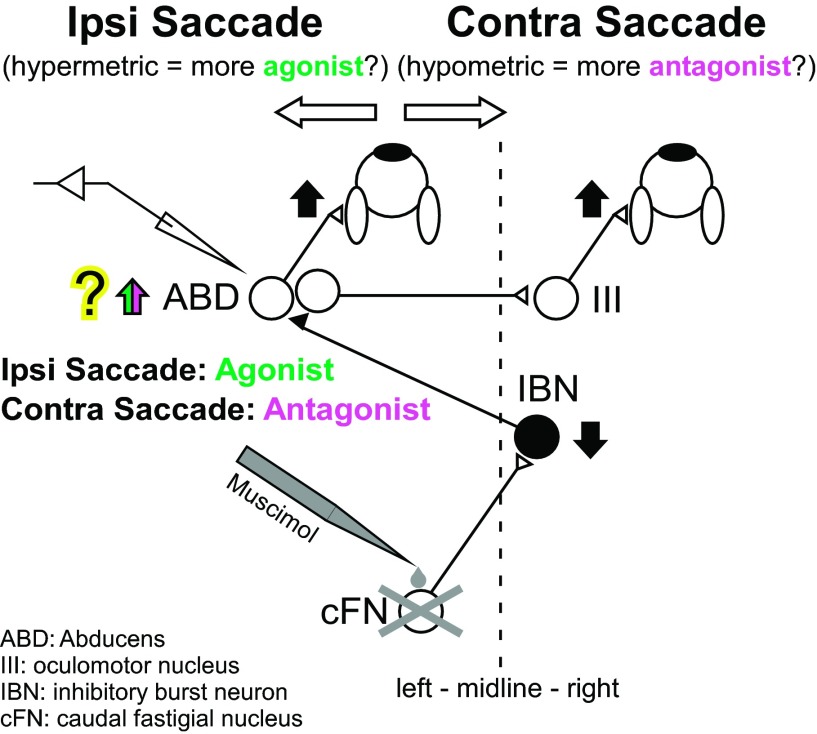
Figure 1 shows the simplified representation of the pathway from the left cFN (Noda et al. 1990) to the left ABD nucleus via inhibitory burst neurons (IBNs) on the right. The left ABD nucleus contains motoneurons for the left lateral rectus muscle and internuclear neurons, which project to motoneurons for the right medial rectus muscle in the right oculomotor nucleus (Büttner-Ennever and Akert 1981; Carpenter and Batton 1980). Thus cFN input to the left ABD affects the muscles for which the contraction rotates both eyes to the left. For a leftward (ipsiversive) saccade, neurons in the left ABD (both motoneurons and internuclear neurons) fire a high-frequency burst of action potentials that causes contraction of the agonist left lateral rectus and right medial rectus muscles (Fuchs and Luschei 1970; Fuchs et al. 1988). For a rightward (contraversive) saccade, ABD neurons pause to relax the same left lateral and right medial rectus muscles, which are the antagonists. Although cFN-IBN-ABD appears to be most significant pathway (Noda et al. 1990), the cFN also projects to other brain-stem structures, e.g., excitatory burst neurons (EBNs; Batton et al. 1977; Noda et al. 1990; Strassman et al. 1986a), omnipause neurons (Batton et al. 1977; Noda et al. 1990), and the superior colliculus (Batton et al. 1977; May et al. 1990; Noda et al. 1990) via which cFN could also influence ABD (see discussion for detail).
Fig. 1.
Simplified schematic of the brain-stem and cerebellar saccade circuitry for leftward (ipsiversive, Ipsi) and rightward (contraversive, Contra) saccades. A signal from the left caudal fastigial nucleus (cFN) reaches right inhibitory burst neurons (IBN), which project to the left abducens (ABD) nucleus, which, in turn, drives the left lateral rectus muscle that is the agonist for leftward (ipsiversive) saccades or the antagonist for rightward (contraversive) saccades. The left ABD nucleus contains motoneurons for the left lateral rectus muscle and internuclear neurons, which project to motoneurons for the right medial rectus muscle in the right oculomotor nucleus (III). Filled neurons are inhibitory. Open neurons are excitatory. Vertical arrows indicate the change in activity that we propose occurs after left cFN inactivation. After left cFN inactivation, leftward (ipsiversive) saccades become hypermetric, and rightward (contraversive) saccades become hypometric. We recorded ABD neurons that act as an agonist and antagonist during ipsi- and contraversive saccade, respectively.
After cFN inactivation, ipsiversive saccades are hypermetric (too large), and contraversive saccades are hypometric (too small; Goffart et al. 2004; Iwamoto and Yoshida 2002; Robinson et al. 1993). The cFN-IBN-ABD pathway (pictured in Fig. 1) is one pathway that can account for these changes. In this view, inactivation of the cFN ipsilateral to saccade direction blocks cFN drive of IBNs and decreases the IBN inhibition of agonist ABD activity. The hypermetria of ipsiversive saccades could result from the increased activity in the agonist ABD. The hypometria of contraversive saccades could result from cFN inactivation increasing antagonist ABD activity. If cFN activity influences saccades, then we expect that inactivating the cFN will increase ipsilateral ABD activity during both ipsiversive and contraversive saccades.
In addition, because the saccades kinematics are influenced by both agonist and antagonist activity, it is possible that the hypermetria of ipsiversive saccades could result from a combination of increased activity in agonist ABD and decreased activity in antagonist ABD (see discussion for detail). Hypometric contraversive saccades could result from a combination of decreased agonist and increased antagonist activity. To characterize how cFN output affects agonist and antagonist activity in the ABD nucleus, it is necessary to record from ABD neurons in a situation in which agonist and antagonist activity are distinguishable. In this study, we sought to answer the following questions. Does cFN inactivation change ABD activity during ipsi- and contraversive saccades? If so, then when during a saccade does cFN inactivation significantly alter ABD activity? Which attributes of ABD activity (e.g., peak firing rate, burst duration) change after cFN inactivation? The answer will tell us explicitly what cFN activity contributes to ABD firing. To answer these questions, we recorded from single ABD neurons during ipsi- and contraversive saccades before and after we inactivated the ipsilateral cFN.
MATERIALS AND METHODS
Surgery and training.
Two rhesus monkeys (monkeys C and A, Macaca mulatta, male, 6.5 and 8.9 kg) were the subjects of this study. Kojima et al. (2010a) describes our surgical and recording procedures in detail. Briefly, in an aseptic surgery, we implanted each monkey with head-stabilizing fixtures, an eye coil, and two recording chambers attached to the skull. One chamber was aimed at the midline between the left and right ABD nuclei and tilted 15° lateral in the coronal plane. The other chamber was aimed straight down at the midline between the left and right cFNs.
After each monkey recovered from the surgery, we trained it to track a small (0.3°) visual target in a dimly lit, sound-attenuating booth. The monkey sat in a primate chair with its head restrained. We measured eye position with the electromagnetic search coil method (Fuchs and Robinson 1966; Judge et al. 1980). A monkey received a dollop of applesauce via a feeding tube near its mouth when its eyes were within 2° of the horizontal and vertical location of the target spot. The target was a laser spot projected onto a tangent screen via two computer-controlled orthogonal mirror galvanometers and moved within a range of ±20° from straight ahead. The screen was 65 cm from the monkey's eyes.
After a monkey reliably tracked the target spot, we made penetrations with a glass-coated tungsten microelectrode (Alpha Omega, Alpharetta, GA) to locate the ABD and cFN. The electrodes entered the brain via a guide tube that penetrated the tentorium above the cerebellum. We identified the ABD by the characteristic burst tonic activity of its neurons for ipsiversive saccades, the pause for contraversive saccades, and the high tonic firing rate related to eye position (Fuchs and Luschei 1970; Fuchs et al. 1988; Ling et al. 2007; Sylvestre and Cullen 1999). We identified the cFN by their characteristic bursting activity for every saccade and the fact that bursts usually start slightly earlier for contraversive saccades than for ipsiversive saccades (Fuchs et al. 1993; Ohtsuka and Noda 1991; Scudder and McGee 2003).
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (1997) and exceeded the minimal requirements recommended by the Institute of Laboratory Animal Resources and the Association for Assessment and Accreditation of Laboratory Animal Care International. All procedures were evaluated and approved by the local Animal Care and Use Committee of the University of Washington.
Experimental procedures.
In each experiment, we recorded continuously from the same single ABD neuron before and after we inactivated the cFN. At the start of each experiment, we made electrode penetrations to locate the cFN. Once we found it, we withdrew the electrode and replaced it at the same position with an injection pipette (32-gauge stainless steel tube with a pulled glass micropipette tip glued over 1 end). We stopped advancing the pipette when its tip was 5 mm above the cFN to prevent muscimol from leaking into the cFN before we collected preinactivation data from an ABD neuron. We then drove an electrode into the ABD, isolated a neuron, and recorded the preinjection responses of the neuron. After we recorded these data from an isolated ABD neuron, we drove the cFN pipette down to 0.3 mm below the cFN.
We determined the position threshold of the ABD neuron by positioning the target at each of several positions every 5° along the horizontal meridian. The eye position at which the neuron fired at its lowest tonic firing rate without stopping was the position threshold of the neuron. Table 1 shows the threshold of each neuron (column Position Threshold).
Table 1.
Summary of the conditions and results for all 22 experiments
| Ipsiversive Saccade |
Contraversive Saccades |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Summary of Fig. 3, A and B |
Regression Model |
Summary of Fig. 6B |
||||||||||||||||||||
| Side Right/Left |
Saccade |
ABD |
a (Eye Position) |
b (Eye Velocity) |
c (Constant) |
Saccade |
ABD Pause |
|||||||||||||||
| Unit | Monkey | ABD | cFN | Position Threshold | Amp. | Dur. | P-Vel. | n-Spi. | B-Dur. | P-FR | Pre | Post | Pre | Post | Pre | Post | Amp. | Dur. | P-Vel. | Dur. | Start | End |
| 1 | A | L | L | <−25 | * | ns | * | ns | ns | ns | 4.26 ± 0.77 | 4.01 ± 0.73* | 0.67 ± 0.21 | 0.59 ± 0.23* | 32.52 ± 6.69 | 33.73 ± 4.67 ns | * | ns | * | * | ns | * |
| 2 | A | L | L | <−25 | * | * | * | * | * | ns | 1.61 ± 1.23 | 1.81 ± 1.04 ns | 0.37 ± 0.04 | 0.36 ± 0.05* | 78.37 ± 5.27 | 76.90 ± 4.10* | * | * | * | * | ns | * |
| 3 | A | L | L | <−25 | * | * | * | * | ns | ns | 3.74 ± 0.24 | 3.95 ± 0.27* | 0.20 ± 0.03 | 0.19 ± 0.03 ns | 82.14 ± 1.99 | 81.42 ± 2.21* | ns | na | na | na | na | na |
| 4 | A | R | R | <−25 | * | * | * | * | * | * | 2.41 ± 0.72 | 2.58 ± 0.45* | 0.13 ± 0.03 | 0.12 ± 0.03* | 39.80 ± 4.74 | 52.25 ± 4.82* | ns | na | na | na | na | na |
| 5 | C | R | R | <−20 | * | * | * | * | * | ns | 7.15 ± 1.81 | 7.10 ± 1.17 ns | 1.14 ± 0.27 | 0.84 ± 0.21* | 85.00 ± 5.29 | 86.19 ± 4.64 ns | * | * | * | ns | ns | ns |
| 6 | C | R | R | <−20 | * | * | * | * | * | ns | 4.93 ± 0.52 | 4.43 ± 0.53* | 0.25 ± 0.09 | 0.19 ± 0.06* | 40.47 ± 4.15 | 44.27 ± 5.21* | ns | na | na | na | na | na |
| 7 | C | L | L | <−20 | * | ns | * | * | * | * | 5.38 ± 0.37 | 5.40 ± 0.31 ns | 0.16 ± 0.03 | 0.15 ± 0.03* | 60.10 ± 4.22 | 59.58 ± 4.83 ns | * | ns | ns | * | ns | * |
| 8 | C | L | L | <−20 | * | ns | * | * | * | * | 3.76 ± 0.47 | 3.80 ± 0.59 ns | 0.11 ± 0.03 | 0.12 ± 0.03 ns | 85.27 ± 4.40 | 86.61 ± 5.00 ns | * | ns | * | ns | ns | ns |
| 9 | C | R | R | <−20 | * | * | * | * | * | * | 5.44 ± 0.98 | 5.03 ± 1.20 ns | 0.29 ± 0.07 | 0.24 ± 0.09* | 83.74 ± 6.04 | 75.91 ± 8.06* | * | * | * | * | * | * |
| 10 | C | L | L | <−20 | * | ns | * | * | * | * | 4.40 ± 0.45 | 4.21 ± 0.41* | 0.16 ± 0.04 | 0.14 ± 0.04* | 64.38 ± 3.13 | 55.27 ± 5.22* | * | * | * | * | ns | * |
| 11 | C | R | R | <−20 | * | * | * | * | * | * | 6.02 ± 0.63 | 5.58 ± 0.68* | 0.24 ± 0.04 | 0.23 ± 0.03* | 68.11 ± 5.12 | 66.74 ± 4.04 ns | * | * | * | ns | ns | ns |
| 12 | A | L | L | <−20 | * | * | ns | * | * | ns | 5.14 ± 0.50 | 4.77 ± 0.50* | 0.38 ± 0.04 | 0.35 ± 0.03* | 43.70 ± 9.64 | 50.73 ± 13.74* | * | ns | * | * | ns | * |
| 13 | C | R | R | <−15 | * | * | * | * | ns | * | 8.37 ± 2.85 | 9.50 ± 2.68* | 1.02 ± 0.26 | 0.93 ± 0.33 ns | 47.39 ± 15.11 | 47.75 ± 10.40 ns | * | ns | * | * | ns | * |
| 14 | C | R | R | <−15 | * | * | * | * | * | ns | 5.44 ± 0.45 | 5.07 ± 0.31* | 0.23 ± 0.06 | 0.21 ± 0.04* | 64.80 ± 3.34 | 63.15 ± 2.89* | * | * | * | * | ns | * |
| 15 | A | L | L | <−10 | * | * | ns | * | ns | * | 7.76 ± 0.90 | 8.60 ± 0.92* | 0.52 ± 0.11 | 0.44 ± 0.08* | 61.56 ± 10.27 | 67.14 ± 6.65* | ns | na | na | na | na | na |
| 16 | C | R | R | <−5 | * | * | ns | * | * | ns | 7.96 ± 0.51 | 8.25 ± 0.37* | 0.37 ± 0.10 | 0.34 ± 0.07 ns | 54.28 ± 5.57 | 53.09 ± 4.09 ns | ns | na | na | na | na | na |
| 17 | C | L | L | <−5 | * | * | * | * | * | ns | 3.49 ± 0.65 | 3.69 ± 0.49* | 0.11 ± 0.05 | 0.11 ± 0.04 ns | 55.40 ± 5.26 | 52.86 ± 3.70* | ns | na | na | na | na | na |
| 19 | C | R | R | <0 | * | * | * | * | * | * | 9.44 ± 1.24 | 9.20 ± 0.79 ns | 0.48 ± 0.11 | 0.41 ± 0.09* | 42.91 ± 8.65 | 41.64 ± 8.41 ns | * | * | * | * | ns | * |
| 18 | C | R | R | <0 | * | * | * | * | * | * | 9.78 ± 0.74 | 9.39 ± 1.51 ns | 0.38 ± 0.05 | 0.32 ± 0.05* | 46.93 ± 9.66 | 39.95 ± 18.70* | * | ns | ns | ns | ns | ns |
| 20 | C | L | L | <0 | * | ns | * | * | * | * | 3.60 ± 0.97 | 3.97 ± 1.01 ns | 0.11 ± 0.08 | 0.18 ± 0.11* | 59.29 ± 4.99 | 62.94 ± 5.93* | ns | na | na | na | na | na |
| 21 | C | L | L | <+5 | * | ns | * | ns | ns | ns | 3.33 ± 0.78 | 3.65 ± 0.65* | 0.19 ± 0.14 | 0.13 ± 0.06* | 35.84 ± 6.16 | 48.71 ± 9.02* | * | * | * | * | * | * |
| 22 | C | R | R | <+10 | * | ns | * | ns | ns | * | 11.88 ± 0.95 | 11.97 ± 1.04 ns | 0.34 ± 0.07 | 0.33 ± 0.07 ns | 42.86 ± 8.57 | 37.74 ± 7.52* | * | ns | ns | ns | ns | ns |
We ordered these neurons by their position thresholds from most contralateral (−) to most ipsilateral (+).
ABD, abducens; cFN, cerebellar fastigial nucleus; Amp., amplitude; B-Dur., burst duration; Vel., velocity; Spi., spikes; FR, firing rate; L, left; R, right.
Significant; ns, not significant; na, not applicable.
After we determined the position threshold of a neuron, we set the initial fixation target position at 5° ipsilateral to the threshold position to keep the ABD neuron firing consistently during the fixation. The monkey fixated the target for 1.0 or 1.2 s, and then the target jumped pseudorandomly to one of five ipsilateral positions that were 5, 8, 10, 12, or 15° away from the initial fixation position. Saccades to these target steps were ipsiversive to the ABD neuron from which we recorded. After each ipsiversive step, the target then jumped back to the initial fixation location. The monkey tracked these movements with contraversive saccades. This procedure made the starting eye position of all ipsiversive saccades the same for a particular ABD neuron, and it made the end eye position of all contraversive saccades the same. This strategy is necessary because some attributes of the burst for ipsiversive saccades of an ABD neuron depend on initial eye position (Ling et al. 2007). In addition, the pause duration for the contraversive saccades depends on the eye position at the end of the saccade.
Before we inactivated the cFN, we recorded the activity of an ABD neuron during ∼250 targeting saccades. We then inactivated the cFN by using brief pulses of air pressure to inject 1 μl of muscimol (1 μg/μl; dissolved in normal saline solution; MP Biomedicals) into the cFN through the pipette. After this injection, we continued to record the activity of the ABD neuron for as long as the neuron remained isolated. During this postinjection period, the monkey made saccades to targets at the same positions as it did before injection. We injected muscimol only when we recorded saccade-related neurons in cFN so all of our injections produced significant dysmetria (unpaired t-test of saccade amplitude, P < 0.05). Our average recording duration after we injected muscimol into the cFN in the 22 experiments was 16 min (range: 5–32 min). After each cFN inactivation experiment, we waited at least 3 days before the next experiment to be sure that the muscimol injected in the previous experiment had dissipated completely.
In these experiments (Table 1), we recorded ABD neurons that were ipsilateral to the inactivated cFN. In one additional experiment, we recorded a neuron in the ABD contralateral to the inactivated cFN (see discussion).
Data analysis.
We digitized eye and target position signals at 1 kHz and sampled unit activity at 50 kHz using Power1401 data acquisition/controller hardware (Cambridge Electronic Design, Cambridge, United Kingdom). We saved data to a hard disk for later analysis. During the experiment, a custom program running in Spike2 (Cambridge Electronic Design) controlled target movement and the monkey's reward via the Power1401 hardware.
We used a custom program running in Spike2 to analyze the saved data. It detected saccades when eye velocity exceeded 75°/s within 70–800 ms after the target movement. The program marked saccade onset and end when eye vector velocity exceeded or fell below a 20°/s threshold, respectively. The program measured several saccade attributes, e.g., amplitude, peak velocity, and duration, as well as target distance before each saccade.
A voltage threshold trigger detected each ABD action potential, and the program saved its time of occurrence. We verified the marked action potentials by eye and exported saccade attributes, target positions, and action potential times to MATLAB (The MathWorks) to analyze their relationships. We eliminated from analysis saccades for which the initial vertical eye position differed from those of the initial target positions by >5°. To characterize average ABD bursts for ipsiversive saccades, we created a spike density function for the period from 100 ms before saccade start to 100 ms after saccade start by replacing each action potential with a Gaussian function (5-ms SD) centered on the spike time (Kojima et al. 2010a; Sylvestre and Cullen 1999). The duration of every analyzed saccade was <100 ms.
After cFN inactivation, ipsiversive and contraversive saccades were larger and smaller, respectively. We compared ABD activity for saccades of the same target step size before and after the inactivation to compare the hyper- or hypometric saccades of postinactivation with normo-metric saccades before inactivation.
To analyze the ABD burst parameters for ipsiversive saccades, we employed a regression model analysis (Cullen and Guitton 1997; Sylvestre and Cullen 1999). For each saccade, we fit eye position and velocity to ABD spike density with the following equation:
| (1) |
where E is eye position, Ė is eye velocity, a and b are coefficients for the respective components, c is a constant, and d is the burst lead time. The correlation coefficients (r) of the fits for all 22 neurons were >0.86 for pre- and >0.94 for postinactivation.
To analyze the burst duration, we first isolated the saccade-related burst by removing both the position and constant components from the recorded firing rate [Eq. 2; also see Ling et al. (2007)].
| (2) |
ABD(t) is the spike density of the recorded firing rate, and a and c are coefficient and constant determined by the fit of Eq. 1. Next, we detected the peak burst time, as well as the burst onset and end by identifying the times of the firing rate [burst(t)] that was >20 Hz. This threshold was necessary to mark the timings above the baseline noise of the reconstructed burst reliably (Fig. 2, bottom). The burst duration is from burst onset to end. To analyze the number of spikes, we integrated the area of the spike density between burst onset and end.
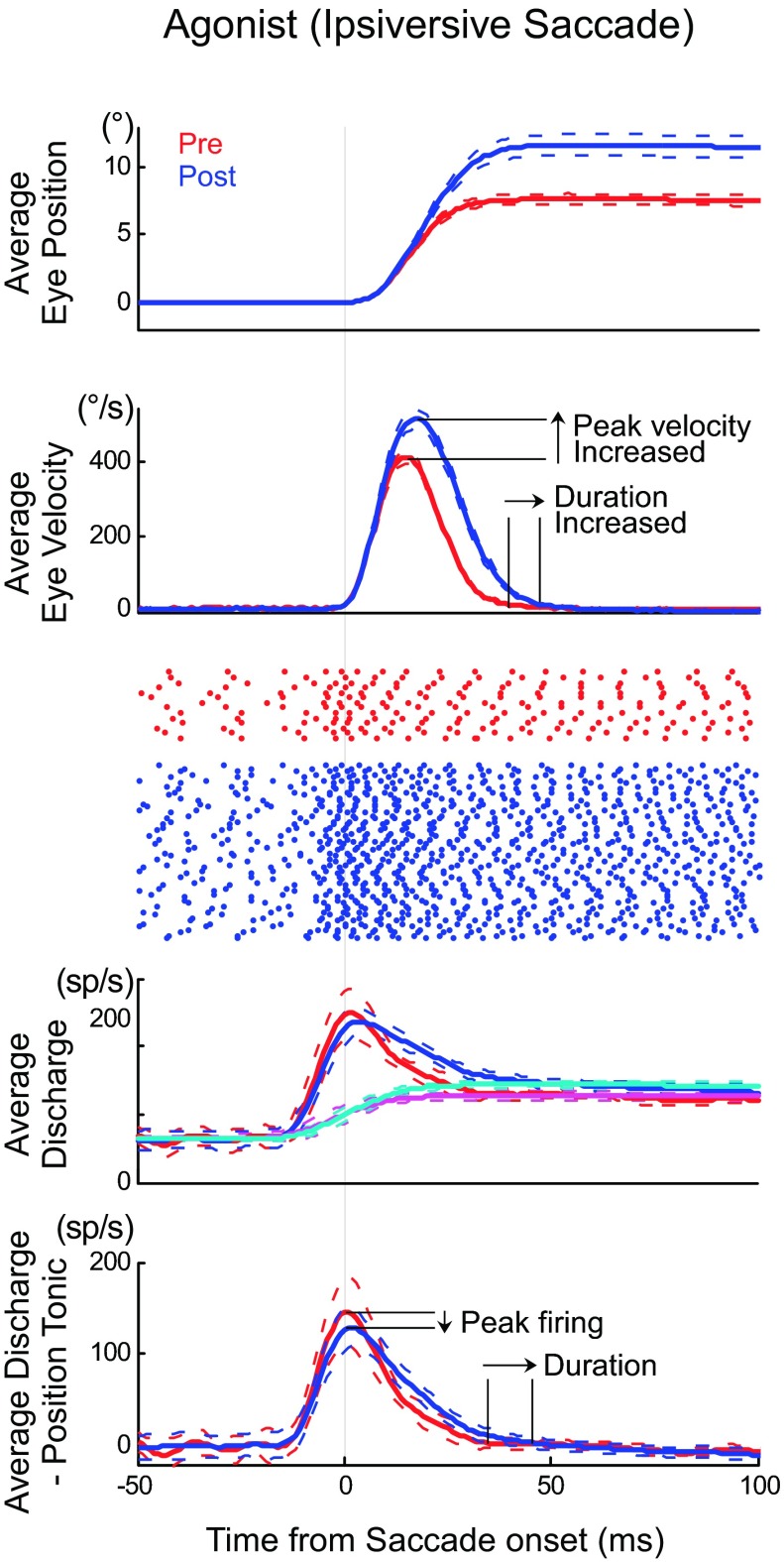
Fig. 2.
Activity in an example ABD neuron (unit 14 in Table 1) during ipsiversive saccades. Top: average pre- (red) and postinactivation (blue) eye position during saccades tracking an 8° target movement. The dashed lines show 1 SD from the mean (number of trials: pre = 14, post = 86). Second panel: average velocity traces of these saccades. Third panel: raster plots of ABD firing during these saccades. Fourth panel: average spike (sp) density function of ABD discharge for these saccades and the tonic activity related to eye position using predicted eye position (pre: magenta, post: cyan). Bottom: burst component of the ABD discharge, i.e., the saccade-related discharge after we subtracted the position-related discharge. All traces aligned on saccade onset.
Across all ipsiversive target movements for a given ABD neuron, we compared the velocity coefficients (b in Eq. 1) of ABD burst pre- and postinactivation. If the postinactivation coefficient was significantly smaller (unpaired t-test, P < 0.05), we concluded that cFN inactivation had decreased the velocity sensitivity of ABD burst, i.e., the same velocity is associated with a lower firing rate.
To analyze the ABD pause for contraversive saccade, we detected the time of the last spike before pause (pause start time) and the first spike after pause (pause end time). We calculated the pause duration as the time between pause start time and pause end time.
Finally, to determine whether a regression line fit to data collected after cFN inactivation was significantly different from a regression line fit to data collected before inactivation, we used an F test (Motulsky and Christopoulos 2004; P < 0.05). To summarize all experiments, we calculated the difference (Δ) between regression lines fit to pre- and postinactivation parameters at a particular target distance with the following equation.
RESULTS
We recorded the activity of 22 burst tonic neurons in the ABD nuclei of 2 monkeys (16 neurons from monkey C, 6 from monkey A) before and after we inactivated the ipsilateral cFN by injecting muscimol. Consistent with previous descriptions of ABD activity (Fuchs and Luschei 1970; Fuchs et al. 1988; Ling et al. 2007; Sylvestre and Cullen 1999), all of the neurons we recorded discharged vigorously for ipsiversive saccades and paused for contraversive saccades. All showed a positive linear relationship between the number of spikes in a burst and saccade size as well as between tonic firing rate and steady eye position. These neurons could have been either motoneurons or internuclear neurons (see discussion).
cFN inactivation produced significant hypermetria of saccades ipsiversive to the inactivated cFN in all 22 experiments. In these experiments, we recorded neurons in the ABD ipsilateral to the inactivated cFN as illustrated in Fig. 1 (Table 1). In 1 experiment, we recorded a neuron in the ABD contralateral to the inactivated cFN (see discussion). Table 1 shows details for each neuron, including the monkey, the recording and injection sides, the position threshold of the ABD neuron (see Experimental procedures), and whether cFN inactivation caused significant hyper- or hypometria.
Agonist activity (during ipsiversive saccades).
Figure 2 shows an example of how cFN inactivation changed ipsiversive saccades and the associated activity of an ipsilateral ABD neuron (unit 14, Table 1) during ipsiversive saccades. cFN inactivation significantly increased the average amplitude of saccades to a 8° target (Fig. 2, top; pre: 7.48 ± 0.75°, n = 14; post: 11.6 ± 2.50°, n = 86; P < 0.05). The average velocity profile of these saccades (Fig. 2, 2nd panel) indicates that the larger saccade amplitude after inactivation is associated with longer saccade duration and higher peak saccade velocity. cFN inactivation increased the ABD discharge (Fig. 2, raster and 3rd panel). We extracted the burst component of ABD discharge by removing the tonic activity related to eye position using predicted eye position (see Experimental procedures for details). After cFN inactivation, the duration of the burst in ABD neurons was longer, but the peak firing rate did not increase and even seems to have slightly decreased (Fig. 2, bottom 2 panels).
Amplitude, duration, and peak velocity increased in saccades to every ipsiversive target distance. ABD burst duration also increased for saccades to all ipsiversive targets. Figure 3 shows the characteristics of saccades to ipsiversive targets 5–15° away (A) and of the associated ABD bursts (B) for the same neuron as in Fig. 2. Saccades to all target distances were larger after inactivation (Fig. 3A, top). Inactivation did not change the slope of the linear relationship between saccade and target amplitude but shifted the offset significantly upward (P < 0.05). We attribute the amplitude increase to significantly longer saccade durations and higher peak velocities (Fig. 3A, middle and bottom; P < 0.05). The number of spikes in, and the duration of, ABD bursts increased significantly and by about the same amount for all target amplitudes (Fig. 3B, top 2 panels), but the peak firing rate of these bursts did not change (Fig. 3B, bottom). The change of each neuron was shown in Table 1. The relationship between number of spikes in a burst and saccade amplitude or between burst duration and saccade duration were not significantly changed, but the relationship between peak firing rate and saccade peak velocity was changed (Fig. 3C).
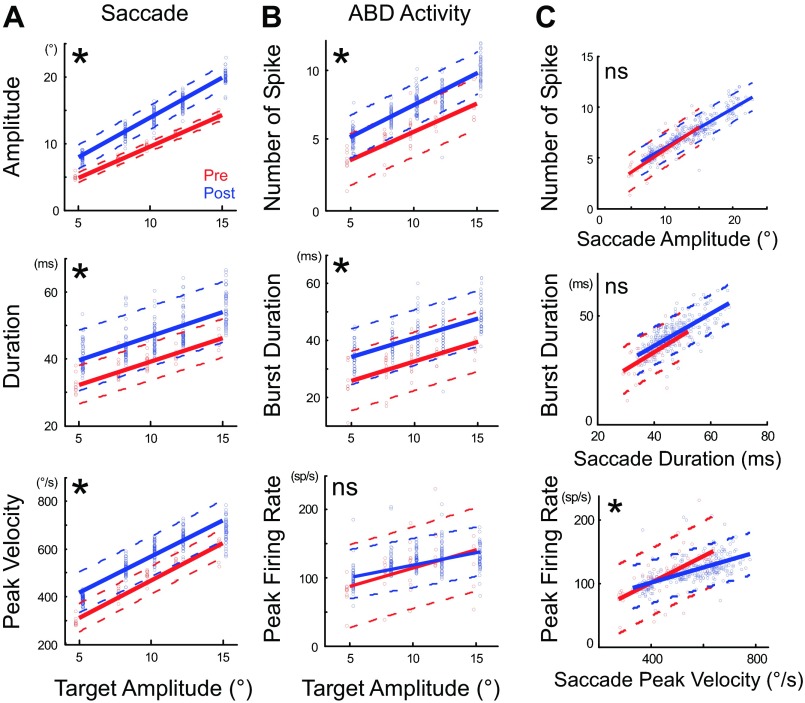
Fig. 3.
Effect of cFN inactivation on the attributes of ipsiversive saccades and the activity of an agonist ABD neuron (unit 14). A: graphs of the saccade amplitude (top), duration (middle), and peak velocity (bottom) as a function of target amplitude. B: graphs of the number of spikes (top), burst duration (middle), and peak firing rate (bottom) in the example ABD neuron as a function of target amplitude. Regression lines of pre- (red) and postinactivation (blue) are significantly different in all except peak firing rate (*P < 0.05; ns, not significant). Each point indicates 1 saccade. We shifted the blue postinactivation points slightly rightward and red preinactivation points slightly leftward to make them distinguishable. C: relationship between the agonist ABD activity and ipsiversive saccade attributes before (red) and after (blue) cFN inactivation. Top shows relationship between the number of spikes and saccade amplitude, middle between burst duration and saccade duration, and bottom between peak firing rate and saccade peak velocity. Regression lines of pre- and postinactivation are not significantly different in both number of spikes vs. amplitude and burst duration vs. saccade duration but differ significantly in peak firing rate vs. peak velocity (P < 0.05).
Figure 4 summarizes the changes caused by cFN inactivation in the burst attributes of all 22 ABD neurons. Because saccades to targets at all of the tested distances increased their amplitude, we concentrated our population analysis to saccades produced by 8° target steps like those in Fig. 2. The increase of amplitude after inactivation was invariably accompanied by increase of the number of spikes as evident from the distribution of all neurons in the right-upper quadrant of the top in Fig. 4 (see also Table 1). The increases in amplitude and the number of spikes were significantly correlated (y = 0.62x + 0.18, P < 0.05, r = 0.71). Inactivation also increased both saccade duration and ABD burst duration (Fig. 4, middle; see also Table 1). These increases were also significantly correlated across neurons (y = 0.45x + 4.86, P < 0.05, r = 0.69). Inactivation consistently increased peak saccade velocity (i.e., points are to the right of 0 in the bottom of Fig. 4; see also Table 1) but did not have a consistent effect on peak ABD firing rate (i.e., neurons distributed above and below the ordinate 0 in the bottom of Fig. 4; see also Table 1). The correlation between Δpeak velocity and Δpeak firing rate was not significant (y = 0.20x − 9.59, P = 0.12, r = 0.35).
Fig. 4.

Summary of all 22 experiments for ipsiversive saccade and agonist ABD activity (units 1–22). Each point indicates average results for 1 experiment. *Regression line (gray line) significantly different from 0 (P < 0.05).
Thus the increases in the number of ABD spikes and burst duration were correlated with the increase in saccade amplitude and duration, respectively. However, we cannot attribute the increased saccade peak velocity after inactivation to an increase in the peak firing rate of ABD bursts. In our discussion, we propose a mechanism through which cFN inactivation could increase saccade peak velocity without increasing ABD peak firing rate.
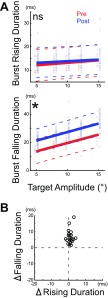
As exemplified in the velocity and burst profiles in Fig. 2, cFN inactivation changed the activity of each ABD neuron more during the falling phase than the rising phase of the burst. To measure this difference, we compared these 2 parts of the burst. Figure 5A shows this comparison in the same example neuron shown in Figs. 2 and 3 (unit 14, Table 1). Inactivation did not change the duration of the rising phase of the burst in this cell (Fig. 5A, top) but significantly increased the duration of the falling phase (Fig. 5A, bottom). Figure 5B shows the summary of all 22 neurons and indicates that inactivation consistently increased the duration of the falling phase (all points are above the ordinate 0 in Fig. 5B) but had little effect on the duration of the rising phase (all cells are near the abscissa 0). Thus the influence of the cFN on ipsilateral ABD neurons during ipsiversive saccades occurred during the falling phase of the burst.
Fig. 5.
Comparison of the change caused in the rising and falling phases of the burst in an ABD neuron by cFN inactivation. A: plot of the burst duration during rising (top) and falling (bottom) phases as a function of target amplitude (unit 14). Regression lines of pre- (red) and postinactivation (blue) are significantly different in falling duration (*P < 0.05). Each point indicates 1 saccade. We shifted the blue postinactivation points slightly rightward and red preinactivation points slightly leftward to make them distinguishable. B: summary of all 22 experiments (units 1–22). Each point indicates average results for 1 experiment.
Antagonist activity (during contraversive saccades).
cFN inactivation caused significant hypometria of contraversive saccades in 15 of our 22 experiments. We analyzed the ABD activity of only these 15 experiments (Table 1, “*” in column Contraversive Saccades, Summary of Fig. 6B, Saccade, Amp.). Figure 6A shows the responses of a representative ABD neuron (unit 12, Table 1) during saccades to a contralateral target 12° away. The top in Fig. 6A shows that inactivation decreased saccade amplitudes significantly (P < 0.05, pre: 12.7 ± 1.32°, post: 9.71 ± 1.29°). The 2nd panel in Fig. 6A shows that inactivation also decreased peak saccade velocity and increased both total saccade duration and deceleration duration. The raster plot at the bottom of Fig. 6A shows that cFN inactivation made the off-direction pause of this ABD neuron end sooner than normal (Fig. 6A, 3rd and 4th panels).
Fig. 6.
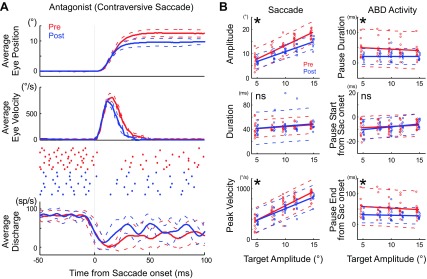
Antagonist activity in an example ABD neuron (unit 12) during contraversive saccades. A: illustrated with the same formats as Fig. 2. Number of trials: pre = 11, post = 7. B: effect of cFN inactivation on the attributes of contraversive saccades and the antagonist ABD pause. Regression lines of pre- (red) and postinactivation (blue) are significantly different in all except pause start time (*P < 0.05). Each point indicates 1 saccade. We shifted the blue postinactivation points slightly rightward and red preinactivation points slightly leftward to make them distinguishable.
Figure 6B summarizes the changes caused by cFN inactivation in saccades (left column) and ABD pauses (right column) in the same experiment for which the data appears in Fig. 6A during saccades to targets 5–15° away. Inactivation made saccades to all target distances significantly smaller. The linear relationship between saccade and target amplitude was significantly shallower after inactivation (P < 0.05; Fig. 6B, left top). Inactivation did not change the duration of the saccades, but it decreased the peak velocity (Fig. 6B, left middle and bottom). The intercept of linear relationship between ABD pause duration and target amplitude was significantly lower after inactivation (Fig. 6B, right top). Thus inactivation decreased the durations of pauses.
We measured when the pause in ABD activity started and ended relative to saccade onset. Inactivation did not change the time when the pause started (Fig. 6B, right middle). In contrast, the intercept of linear relationship between the pause ended and target amplitude was significantly lower after inactivation (Fig. 6B, right bottom).
Similar to the neuron in Fig. 6, 3 other neurons also showed significant decrease in intercept of the regression lines for pause duration (Fig. 6B, right top) and pause end (Fig. 6B, right bottom). In addition, 6 other neurons showed shallower slopes after inactivation, and remaining 5 neurons showed no significant difference in either slope or intercept (1-tailed t-test).
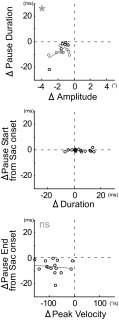
Figure 7 summarizes the pause attributes of ABD neurons recorded in the 15 experiments in which contraversive saccades were significantly hypometric. Larger contraversive saccades showed clearer changes; therefore, we show data from saccades to targets 12° away. In all 15 neurons, a decrease in pause duration accompanied the decrease in saccade amplitude after cFN inactivation (Fig. 7, top, all points are in the lower left quadrant; see also Table 1). Pause duration and the decrease in saccade amplitude were positively correlated (y = 4.20x + 0.03, P < 0.05, r = 0.54). cFN inactivation increased saccade duration in 8 experiments (Table 1; mean duration increase for all 15 experiments = 4.40 ± 6.73 ms, P < 0.05, 1-sample t-test) but did not change pause start time (points near ordinate 0 in the middle of Fig. 7; see also Table 1). Inactivation decreased saccade peak velocity and the pause end time (points are below 0 in the bottom of Fig. 7; see also Table 1). Changes in pause end time and peak velocity are not significantly correlated. We interpret this to mean that, after cFN inactivation, the hypometria of contraversive saccades is caused by a decrease in the duration of off-direction pauses in ABD neurons.
Fig. 7.
Summary of the change in each attribute of all 15 experiments that showed a significant hypometria for contraversive saccade (Table 1). Each point indicates the average results of 1 experiment. *Regression line (gray line) significantly different from 0 (P < 0.05).
DISCUSSION
We characterized how temporarily blocking output from the saccade-related part of the cerebellum (the cFN) changes the activity of neurons in the ipsilateral ABD nucleus. Inactivating the cFN for a brief period avoids confusing the immediate cerebellar influence on ABD neurons with potential compensation that may occur after a permanent lesion. Unilateral cFN inactivation allowed us to assess cFN effects on the ipsiversive and contraversive saccades. Our findings are consistent with a previous description of EMG abnormalities in cerebellar patients (Hore et al. 1991).
cFN influences on the ipsilateral ABD during ipsiversive saccades.
Inactivating the cFN on one side of the cerebellum increased the duration of, and number of spikes in, the saccade-related bursts of most of the ABD neurons that we tested (19/22; Table 1). These changes in ABD activity increased both saccade duration and saccade amplitude (Figs. 2–4).
Human cerebellar patients making arm movements often exhibit a similar increase in the duration of the agonist EMG activity (Hallett et al. 1975; Hore et al. 1991) as do monkeys making arm movements during cerebellar nuclear cooling (Flament and Hore 1986). Thus, like previous findings, our current evidence indicates that cerebellar output normally shortens agonist contraction.
How does cFN activity shorten the agonist burst in ABD neurons? As noted in the previous studies (Fuchs et al. 1993; Goffart et al. 2004; Iwamoto and Yoshida 2002; Kojima et al. 2008, 2010a; Ohtsuka and Noda 1991; Robinson et al. 1993; Scudder and McGee 2003), ABD neurons receive two saccade-related inputs. One is a high-frequency burst signal from the saccade burst generator (SBG). The other is a signal from the cerebellum. As Fig. 1 emphasizes, much of the cerebellar signal reaches ABD neurons via contralateral IBNs. Inhibitory input to the ABD from contralateral IBNs suppresses a part of the burst signal from the SBG. The bursts of most cFN neurons begin after the onset of ipsiversive saccades (Fuchs et al. 1993; Ohtsuka and Noda 1991; Scudder and McGee 2003). These late cFN bursts drive the contralateral IBNs (Noda et al. 1990) for which activity also begins after the onset of these saccades (“off-direction activity”; Kojima et al. 2008; Scudder et al. 1988; Strassman et al. 1986b). Consistent with late bursts in the cFN and IBN, cFN inactivation increased ABD activity late during the burst, i.e., during the falling phase of the burst in all ABD neurons (Fig. 5). The late influence of cFN inactivation on ipsiversive saccades is consistent with the previous finding cFN inactivation makes ipsiversive saccades hypermetric largely by increasing the distance that the eye rotates during saccade deceleration. cFN inactivation increases eye rotation the most during the last part of saccade deceleration (Buzunov et al. 2013).
There are at least three other possible pathways from the cFN to the ipsilateral ABD in addition to the one through IBNs. First, the cFN projects to the superior colliculus that sends a signal to the brain-stem SBG (Batton et al. 1977; May et al. 1990; Noda et al. 1990). The cFN might adjust the duration of the burst of a collicular neuron to produce an appropriate ipsiversive saccade. Second, the cFN projects to the omnipause neurons in the midline pontine region (Batton et al. 1977; Noda et al. 1990). These neurons control the duration of the burst of SBG neurons so they could affect the duration of ABD bursts. Finally, the cFN projects to contralateral EBNs (Batton et al. 1977; Noda et al. 1990; Strassman et al. 1986a; Fig. 8A). EBNs project to ABD neurons contralateral to the inactivated cFN. Thus, during saccades ipsiversive to the cFN on one side, the pathway from that cFN to contralateral EBNs and then to the contralateral ABD could increase the activity in the ABD contralateral to saccade direction, thus increasing antagonist resistance to the saccade (Fig. 8A).
Fig. 8.
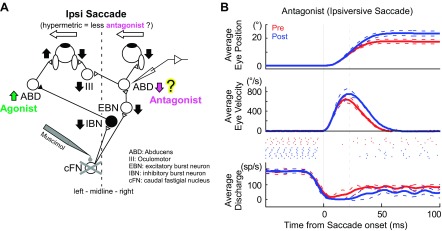
Activity of an antagonist right ABD neuron during hypermetric leftward saccades after left cFN inactivation. A: simplified schematic of the brain-stem and cerebellar saccade circuitry showing the relationship between the inactivated cFN and the recorded ABD neuron. A signal from the left cFN reaches right excitatory burst neurons (EBN), which project to right ABD nuclei, which, in turn, drive muscle that acts as antagonist for leftward saccades. Filled neurons are inhibitory, and open neurons are excitatory. Vertical arrows indicate the change in activity that might occur after left cFN inactivation. Inactivation of the left cFN removed off-direction EBN bursts (Strassman et al. 1986a), and right antagonist ABD activity decreased, so leftward saccades become hypermetric. B: antagonist ABD activity during hypermetric saccades ipsiversive to the inactivated cFN. Illustrated with the same formats as Fig. 2. Number of trials: pre = 5, post = 8. The cFN inactivation made the pause of this ABD neuron end later.
The increase in the peak velocity of an ipsiversive saccade after cFN inactivation was not consistently correlated with the peak firing of ABD burst. Peak saccade velocity could increase without an increase in peak firing rate if antagonist muscles exert less resistance during the saccade after cFN inactivation. Our regression analysis (see Data analysis, Eq. 1) supports this explanation by demonstrating a significantly lower velocity sensitivity (parameter b) in most (16/22, 73%; Table 1) of our neurons after cFN inactivation. This means that, after cFN inactivation, these neurons fired fewer action potentials for a given velocity. The response of the 1 ABD neuron that we recorded contralateral to an inactivated cFN is consistent with this explanation. During hypermetric saccades ipsiversive to the inactivated cFN, this contralateral ABD neuron fired less than normal (Fig. 8). Data from this 1 neuron cannot confirm that cFN inactivation reduces activity in many antagonist ABD neurons. Still, until we have more data, we have no reason to reject the proposal that unilateral cFN inactivation makes ipsiversive saccades hypermetric partly by reducing the contraction of antagonist muscles. If future data support this proposal, then we would conclude that cFN output normally increases the activity of antagonist muscles for ipsiversive saccades.
cFN influences on the ABD during contraversive saccades.
After cFN inactivation, contraversive saccades were hypometric and had lower peak velocities (Fig. 7; Goffart et al. 2004; Iwamoto and Yoshida 2002; Robinson et al. 1993). In addition, saccade duration was longer in the majority of the experiments (Fig. 7; Buzunov et al. 2013; Goffart et al. 2004). How does cFN inactivation cause these changes in contraversive saccades? After cFN inactivation, the off-direction pauses of ABD neurons driving antagonist muscles ended earlier than normal (Fig. 6A). This early pause end will cause antagonist muscles to contract earlier than normal. In turn, earlier antagonist resistance makes saccades shorter and decreases their peak velocity.
In the current study, we showed an earlier onset of antagonist activity during hypometric movement. The antagonist EMG of human cerebellar patients during hypermetric arm movements showed a delayed onset of the activity (Hore et al. 1991). We also recorded one antagonist ABD neuron during hypermetric movement and saw a result similar to the human study, i.e., impairing the cerebellum delayed the resumption of the tonic activity and presumably delayed the antagonist contraction (Fig. 8). The results of our current study and the Hore et al. (1991) report both indicate that the cerebellum sets the timing of antagonist muscle contraction appropriately for accurate movement. Without cerebellar output, delayed antagonist contraction makes movements hypermetric, whereas advanced antagonist contraction makes movements hypometric.
How does normal cFN activity extend the pause of antagonist ABD neurons during contraversive saccades? Bursts in IBNs contralateral to the ABD inhibit ABD neurons during contraversive saccades. The main source of IBN bursts is the superior colliculus, and the cFN adds activity late in the IBN burst. The input from the superior colliculus may help start the saccade by initiating a pause of antagonist ABD, and our result suggests that the cFN helps set the duration of this pause, probably by driving IBNs.
Different magnitude of cFN influences on ipsiversive and contraversive saccades.
The effect of inactivating the cFN is weaker on contraversive than on ipsiversive saccades (Goffart et al. 2004; Iwamoto and Yoshida 2002; Robinson et al. 1993). Even considering this, the effect of cFN inactivation on contraversive saccade in this study was weaker and less consistent than in previous studies. This might be the consequence of our starting to collect postinjection data as soon as ipsiversive saccades became hypermetric. This always occurred sooner than the hypometria of contraversive saccades. Previous studies did not collect data until at least 5 min after cFN inactivation. We did not wait for 5 min because we wanted to collect as much postinjection data as possible before we lost unit isolation. Injected muscimol may not have spread throughout the cFN by the time we began collecting postinjection data. Our collecting data immediately after we saw ipsiversive hypermetria may also explain why we did not see the fixation offset toward the side of the injection that previous work describes (Goffart et al. 2004; Iwamoto and Yoshida 2002; Robinson et al. 1993). Indeed, the longest recording experiment in monkey A (31 min, 40 s, unit 1 in Table 1) showed ∼2° fixation offset toward the injection side at the end of the experiment, and monkey C (31 min, 5 s, unit 10 in Table 1) showed ∼1° offset.
Similar to the difference of the ipsi- and contraversive saccades, the effect of cFN inactivation on ABD neurons during contraversive saccades was weaker than its effect on ABD activity during ipsiversive saccades. cFN inactivation significantly increased the firing of every neuron in the ABD for ipsiversive saccades but only 10/15 ABD neurons for contraversive saccades. cFN bursts for ipsi- and contralateral saccades are about the same duration and rate (Fuchs et al. 1993; Ohtsuka and Noda 1991; Scudder and McGee 2003). Why do cFN bursts of the same size affect ipsiversive saccades more than contraversive saccades and affect the same ABD neurons more during ipsiversive saccades than during contraversive saccades?
The difference in IBN activity for on- (ipsiversive) and off- (contraversive) saccades may explain this asymmetry. Note that an on-direction, ipsiversive saccade for IBNs is a contraversive saccade for the cFN that influences those IBNs and for the ABD that receives input from those IBNs (Fig. 1). For saccades in the on-direction for IBNs, these neurons exhibit a strong burst beginning just before saccade onset. For off-direction saccades, some of IBNs fire a weak burst that begins after saccade onset (Kojima et al. 2008; Scudder et al. 1988; Strassman et al. 1986b). The strong on-direction burst of IBNs causes a pause in the activity of contralateral, antagonist ABD neurons (Fig. 1). The weak off-direction activity of IBNs suppresses spikes near the end of bursts in the same ABD for ipsiversive saccades (Fig. 1). If the cFN adds the same number of spikes to both off- and on-direction IBN activity, then the spikes added by the cFN are a larger proportion of the off-direction activity than of on-direction activity because on-direction bursts contain more spikes. Thus inactivating the left cFN removes a larger proportion of off-direction IBN bursts than of on-direction bursts, thereby impairing ipsiversive saccades more than contraversive saccades.
Are the ABD neurons motoneurons?
The neurons that we recorded in the ABD could have been motoneurons that terminate in the ipsilateral lateral rectus muscle or internuclear neurons that terminate in the contralateral oculomotor nucleus on the motoneurons for the contralateral medial rectus muscle. We did not identify our neurons with action-potential-triggered EMG average of lateral rectus muscles (Fuchs et al. 1988; Gamlin et al. 1989), so we cannot say conclusively whether an ABD neuron was a motoneuron or an internuclear neuron. Although Gamlin et al. (1989) indicated that the position thresholds for identified ABD internuclear neurons were similar to those for overall population of ABD neurons, Fuchs et al. (1988) indicated that the position threshold of the motoneurons was widely distributed from −40 to +20° (negative means contra-, and positive means ipsilateral), whereas that of the internuclear neurons was from −50 to −20°. If we use the Fuchs criteria, about half of our neurons (units 13–22, Table 1) are most likely motoneurons because their thresholds are more ipsilateral than >−20°. The remaining half (units 1–12) could be motoneurons or internuclear neurons. Neurons in these two groups (units 1–12 vs. 13–22) exhibit very similar effects after cFN inactivation. If some of our ABD neurons are interneurons, then cFN activity does not affect them differently from motoneurons.
GRANTS
This study was supported by National Eye Institute (NEI) Grants EY-019258 (R. Soetedjo), EY-023277 (Y. Kojima), EY-018585 (F. R. Robinson), and EY-07031 and Grant RR-00166 from the National Center for Research Resources (NCRR), components of the National Institutes of Health (NIH). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.K. and F.R.R. conception and design of research; Y.K. and R.S. performed experiments; Y.K. analyzed data; Y.K. interpreted results of experiments; Y.K. prepared figures; Y.K. drafted manuscript; Y.K., F.R.R., and R.S. edited and revised manuscript; Y.K., F.R.R., and R.S. approved final version of manuscript.
REFERENCES
- Barash S, Melikyan A, Sivakov A, Zhang M, Glickstein M, Their P. Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. J Neurosci 19: 10931–10939, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batton RR, 3rd, Jayaraman A, Ruggiero D, Carpenter MB. Fastigial efferent projections in the monkey: an autoradiographic study. J Comp Neurol 174: 281–305, 1977 [DOI] [PubMed] [Google Scholar]
- Büttner-Ennever JA, Akert K. Medial rectus subgroups of the oculomotor nucleus ant their abducens internuclear input in the monkey. J Comp Neurol 197: 17–27, 1981 [DOI] [PubMed] [Google Scholar]
- Buzunov E, Mueller A, Straube A, Robinson FR. When during horizontal saccades in monkey does cerebellar output affect movement? Brain Res 1503: 33–42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MB, Batton RR., 3rd Abducens internuclear neurons and their role in conjugate horizontal gaze. J Comp Neurol 189: 191–209, 1980 [DOI] [PubMed] [Google Scholar]
- Cullen KE, Guitton D. Analysis of primate IBN spike trains using system identification techniques. I. Relationship to eye movement dynamics during head-fixed saccades. J Neurophysiol 78: 3259–3282, 1997 [DOI] [PubMed] [Google Scholar]
- Flament D, Hore J. Movement and electromyographic disorders associated with cerebellar dysmetria. J Neurophysiol 55: 1221–1233, 1986 [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Luschei ES. Firing patterns of abducens neurons of alert monkeys in relationship to horizontal eye movement. J Neurophysiol 33: 382–392, 1970 [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson FR, Straube A. Role of the caudal fastigial nucleus in saccade generation. I. Neuronal discharge patterns. J Neurophysiol 70: 1723–1740, 1993 [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol 21: 1068–1070, 1966 [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Scudder CA, Kaneko CR. Discharge patterns and recruitment order of identified motoneurons and internuclear neurons in the monkey abducens nucleus. J Neurophysiol 60: 1874–1895, 1988 [DOI] [PubMed] [Google Scholar]
- Gamlin PD, Gnadt JW, Mays LE. Abducens internuclear neurons carry an inappropriate signal for ocular convergence. J Neurophysiol 62: 70–81, 1989 [DOI] [PubMed] [Google Scholar]
- Goffart L, Chen LL, Sparks DL. Deficits in saccades and fixation during muscimol inactivation of the caudal fastigial nucleus in the rhesus monkey. J Neurophysiol 92: 3351–3367, 2004 [DOI] [PubMed] [Google Scholar]
- Hallett M, Shahani BT, Young RR. EMG analysis of patients with cerebellar deficits. J Neurol Neurosurg Psychiatry 38: 1163–1169, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G. The symptoms of acute cerebellar injuries due to gunshot injuries. Brain 40: 461–535, 1917 [Google Scholar]
- Hore J, Wild B, Diener HC. Cerebellar dysmetria at the elbow, wrist, and fingers. J Neurophysiol 65: 563–571, 1991 [DOI] [PubMed] [Google Scholar]
- Iwamoto Y, Yoshida K. Saccadic dysmetria following inactivation of the primate fastigial oculomotor region. Neurosci Lett 325: 211–215, 2002 [DOI] [PubMed] [Google Scholar]
- Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980 [DOI] [PubMed] [Google Scholar]
- Kojima Y, Iwamoto Y, Robinson FR, Noto CT, Yoshida K. Premotor inhibitory neurons carry signals related to saccade adaptation in the monkey. J Neurophysiol 99: 220–230, 2008 [DOI] [PubMed] [Google Scholar]
- Kojima Y, Soetedjo R, Fuchs AF. Changes in simple spike activity of some Purkinje cells in the oculomotor vermis during saccade adaptation are appropriate to participate in motor learning. J Neurosci 30: 3715–3727, 2010a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Soetedjo R, Fuchs AF. Effects of GABA agonist and antagonist injections into the oculomotor vermis on horizontal saccades. Brain Res 1366: 93–100, 2010b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling L, Fuchs A, Siebold C, Dean P. Effects of initial eye position on saccade-related behavior of abducens nucleus neurons in the primate. J Neurophysiol 98: 3581–3599, 2007 [DOI] [PubMed] [Google Scholar]
- May PJ, Hartwich-Young R, Nelson J, Sparks DL, Porter JD. Cerebellotectal pathways in the macaque: implications for collicular generation of saccades. Neuroscience 36: 305–324, 1990 [DOI] [PubMed] [Google Scholar]
- Motulsky H, Christopoulos A. Using global fitting to test a treatment effect in one experiment. In: Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting (1st ed.) New York: Oxford Univ. Press, 2004, p. 163–165. [Google Scholar]
- Noda H, Sugita S, Ikeda Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol 302: 330–348, 1990 [DOI] [PubMed] [Google Scholar]
- Ohtsuka K, Noda H. Saccadic burst neurons in the oculomotor region of the fastigial nucleus of macaque monkeys. J Neurophysiol 65: 1422–1434, 1991 [DOI] [PubMed] [Google Scholar]
- Robinson FR, Straube A, Fuchs AF. Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J Neurophysiol 70: 1741–1758, 1993 [DOI] [PubMed] [Google Scholar]
- Scudder CA, Fuchs AF, Langer TP. Characteristics and functional identification of saccadic inhibitory burst neurons in the alert monkey. J Neurophysiol 59: 1430–1454, 1988 [DOI] [PubMed] [Google Scholar]
- Scudder CA, Kaneko CS, Fuchs AF. The brainstem burst generator for saccadic eye movements: a modern synthesis. Exp Brain Res 142: 439–462, 2002 [DOI] [PubMed] [Google Scholar]
- Scudder CA, McGee DM. Adaptive modification of saccade size produces correlated changes in the discharges of fastigial nucleus neurons. J Neurophysiol 90: 1011–1026, 2003 [DOI] [PubMed] [Google Scholar]
- Strassman A, Highstein SM, McCrea RA. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. I. Excitatory burst neurons. J Comp Neurol 249: 337–357, 1986a [DOI] [PubMed] [Google Scholar]
- Strassman A, Highstein SM, McCrea RA. Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. II. Inhibitory burst neurons. J Comp Neurol 249: 358–380, 1986b [DOI] [PubMed] [Google Scholar]
- Straube A, Deubel H, Ditterich J, Eggert T. Cerebellar lesions impair rapid saccade amplitude adaptation. Neurology 57: 2105–2108, 2001 [DOI] [PubMed] [Google Scholar]
- Sylvestre PA, Cullen KE. Quantitative analysis of abducens neuron discharge dynamics during saccadic and slow eye movements. J Neurophysiol 82: 2612–2632, 1999 [DOI] [PubMed] [Google Scholar]
- Takagi J, Zee D, Tamargo R. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol 80: 1911–1931, 1998 [DOI] [PubMed] [Google Scholar]
- Waespe W, Baumgartner R. Enduring dysmetria and impaired gain adaptivity of saccadic eye movements in Wallenberg's lateral medullary syndrome. Brain 115: 1123–1146, 1992 [PubMed] [Google Scholar]
- Waespe W, Müller-Meisser E. Directional reversal of saccadic dysmetria and gain adaptivity in a patient with a superior cerebellar artery infarction. Neuroophthalmology 16: 65–74, 1996 [Google Scholar]