Abstract
The aim of the present study was to test the hypothesis that the age-associated decrease of tendon stiffness would necessitate greater muscle fascicle strains to produce similar levels of force during isometric contraction. Greater fascicle strains could force sarcomeres to operate in less advantageous regions of their force-length and force-velocity relationships, thus impairing the capacity to generate strong and explosive contractions. To test this hypothesis, sagittal-plane dynamic velocity-encoded phase-contrast magnetic resonance images of the gastrocnemius medialis (GM) muscle and Achilles tendon (AT) were acquired in six young (YW; 26.1 ± 2.3 yr) and six senior (SW; 76.7 ± 8.3 yr) women during submaximal isometric contraction (35% maximum voluntary isometric contraction) of the plantar flexor muscles. Multiple GM fascicle lengths were continuously determined by automatically tracking regions of interest coinciding with the end points of muscle fascicles evenly distributed along the muscle's proximo-distal length. AT stiffness and Young's modulus were measured as the slopes of the tendon's force-elongation and stress-strain curves, respectively. Despite significantly lower AT stiffness at older age (YW: 120.2 ± 52.3 N/mm vs. SW: 53.9 ± 44.4 N/mm, P = 0.040), contraction-induced changes in GM fascicle lengths were similar in both age groups at equal levels of absolute muscular force (4–5% fascicle shortening in both groups), and even significantly larger in YW (YW: 11–12% vs. SW: 6–8% fascicle shortening) at equal percentage of maximum voluntary contraction. These results suggest that factors other than AT stiffness, such as age-associated changes in muscle composition or fascicle slack, might serve as compensatory adaptations, limiting the degree of fascicle strains upon contraction.
Keywords: VE-PC, gastrocnemius muscle, Achilles tendon, stress, strain
it is widely accepted that the aging process is associated with marked losses of skeletal muscle mass and force, with the declines in force significantly exceeding those in muscle mass (5, 6). One factor that has been considered universally to be responsible for this progressive deterioration of muscle function is the age-associated remodeling of muscle architecture, i.e., the structural arrangement of fascicles within a muscle. Numerous reports have proposed that the overall loss of contractile material that is concomitant with aging results in a reduction of muscle fascicle lengths and pennation angles (for a review, see Ref. 30). Moreover, the aging process may influence the mechanical properties of passive intra- and extramuscular tissues, such as tendons and aponeuroses (33, 40). Individually or in concert, these changes may impact the operating range of sarcomeres on their force-tension and force-velocity curves, ultimately impairing a muscle's capacity to generate force under both static and dynamic conditions (24).
One common hypothesis is that the decrease in tendon stiffness that typically accompanies age-associated (33, 40) muscle atrophy would force fascicles, and thus sarcomeres, to shorten more upon isometric contraction than when attached to a younger, less extensible tendon (31, 34). Based on the assumption of a serial arrangement of contractile and elastic components, it is assumed that, at the onset of contraction, fascicles would first have to tauten the tendon, to facilitate force transmission onto the skeleton. Functionally, the postulated greater fascicle shortening has been proposed as one of the factors responsible for the impaired capacity to generate strong and explosive contractions at older age. These considerations, however, may be overly simplistic in that they fail to account for further changes to the muscle-tendon unit (MTU) that may occur with aging. For instance, the excessive accumulation of intramuscular connective tissues that may accompany sarcopenia (45) could lead to a stiffening of the muscle's extracellular matrix, which would reduce fascicle strains, thus countering the effects of decreased tendon stiffness (12). Moreover, there is evidence to suggest that, at rest, gastrocnemius muscle fascicles may be slack over a large range of muscle-tendon lengths (11), which may similarly affect the degree of contraction-induced fascicle shortening. To the best of our knowledge, there is no study to provide direct evidence in support of the postulated greater fascicle shortening upon isometric contraction in elderly subjects.
Presently, ultrasound represents the technique most commonly used to study the effects of aging on either skeletal muscle architecture (28, 32, 41, 42) or the material properties of tendons and aponeuroses (33, 40) in vivo. While ultrasound is generally accepted as a valid and reliable tool for the study of the musculoskeletal system (21), it is limited by its small field of view (FOV) (3), inevitable compression of the tissues of interest due to the necessary fixation of the probe onto the skin, and the relatively strong operator dependency in both image acquisition (17) and analysis. MRI measures of passive and active muscle manipulations offer significant advantages over ultrasound, permitting a large FOV, high soft-tissue contrast, and high pixel resolution of tissue movement, providing the opportunity to simultaneously sample over large regions of muscles, and even multiple muscles, while, at the same time, being able to select small regions of interest within the overall sample and, therefore, study regional variation in musculotendinous tissue behavior. Our group has developed methodologies applying velocity-encoded phase-contrast (VE-PC) MRI to the study of the kinematics of muscular and tendinous tissues in various regions of the triceps surae complex in both healthy muscles (7, 8, 15, 37) and after induction of atrophy via unilateral lower limb suspension (22, 36), based on which we have made some unique observations as well as explanations of skeletal muscle mechanics. For instance, we have been able to demonstrate not only substantial intramuscular, but even intrafascicular, heterogeneity of fascicle strains (37), findings that underline the necessity to study muscle architecture both over large FOVs and with the highest possible soft tissue contrast.
Here we use, for the first time, VE-PC MRI techniques to test the hypothesis that a more compliant Achilles tendon, concomitant with aging, need not a priori necessitate greater fascicle strains to produce similar levels of force. Other factors, such as fascicle slack or changes in muscle composition and stiffness, might serve as compensatory adaptations. To examine this hypothesis, we apply this methodology to the study of the consequences of aging on musculotendinous tissue dynamics and present data on age-associated differences in the mechanical behavior of the plantar flexor MTU, as assessed during submaximal isometric contraction and passive rotation of the ankle joint. For this purpose, sagittal-plane magnetic resonance (MR) images were acquired in six young (∼30 yr) and six senior (∼75 yr) female volunteers to show both the gastrocnemius medialis (GM) muscle and the Achilles tendon from origin to insertion. Specifically, we aimed to assess and compare Achilles tendon material properties and GM fascicle strains between both cohorts.
MATERIALS AND METHODS
Subjects
Six young (YW: age: 26.1 ± 2.3 yr, height: 158.6 ± 5.6 cm, mass: 50.8 ± 3.7 kg) and six senior (SW: age: 76.7 ± 8.3 yr, height: 153.0 ± 2.0 cm, mass: 57.4 ± 4.3 kg) Japanese female volunteers were recruited via local Institutional Review Board approved advertisement and instructed about the purpose of, and procedures involved in, the study. Before inclusion, all participants were screened for internal or orthopedic disease. Exclusion criteria were myocardial infarction, unstable angina pectoris, pericarditis, acute infections and fever, as well as severe osteoarthritis and arthroplasty of the hip or knee. Subsequently, participants were requested to give written, informed consent to their participation on a form approved by the above Board. The study was approved by the Institutional Review Board of the University of California at San Diego (project no. 071250) and conducted in agreement with the Ethical Principles for Medical Research Involving Human Subjects outlined in the Declaration of Helsinki, as amended in Fortaleza, Brazil.
Experimental Setup
All MRI scans were performed on a 1.5-T GE (GE Signa version 12, General Electric Medical Systems, Milwaukee, WI) MR scanner, in combination with a custom-made (Millennial MRI), eight-channel, phased-array lower-leg coil system. Given the possibility of selecting different combinations of channels along the superior-inferior direction in this coil, a total FOV of ∼40 cm (sufficient to cover the entire lower leg) could be examined without the need to reposition the coil. Subjects entered the magnet in a supine, feet-first position, with the foot of the dominant leg strapped to a MR-compatible foot pedal device (39), which was placed on top of the coil. Both the knee (∼10° flexion; 0° representing the anatomical position) and ankle (∼10° plantar flexion; 0° representing a right angle between the axis of the foot and the lower leg) were stabilized in a slightly flexed joint position. For this purpose, foam pads and Velcro straps were used to fix the lower leg, ankle, and forefoot to the device and thus minimize inadvertent joint rotation during muscular contraction. For the latter, care was taken to align the center of rotation of the ankle joint with that of the foot pedal. The ball of the foot rested on a carbon-fiber plate, on to which an optical pressure transducer (Luna Innovations, Roanoke, VA) was glued, and which could rotate along with the foot. Pressure against the plate was detected by the transducer, transmitted via a fiber optical cable, and voltage-converted by a spectrometer (Fiberscan, Luna Innovations, Roanoke, VA) stationed in the console room. The voltage output by this device was recorded using an indigenously built LabVIEW module (NI LabVIEW 2011, National Instruments, Austin, TX), stored onto a laptop computer, and, subsequent to a calibration of the system using disk weights, converted into measures of torque (Nm). Images were acquired under two experimental conditions: 1) during submaximal, isometric contraction of the plantar flexor muscles [35% of the individual maximum voluntary isometric contraction (MVIC); see below]; and 2) during passive rotation of the ankle joint over a 30° range of movement (from ∼10° of dorsiflexion to ∼20° of plantar flexion). In VE-PC scans, as in all MRI scans, to generate an image, data has to be acquired over typically 256–128 (in our case reduced to ∼70 using 4 views per segment) phase-encoding repetitions, and all of these data are subsequently Fourier transformed to form an image. Hence, consistency of movement during these ∼70 cycles is crucial for good image quality without any motion artifacts. Therefore, for isometric contractions, the LabVIEW module was also programmed to output a rectified sine wave, which was video projected, along with the actual force generated by the subject, onto the front face of the magnet. Thus the participants were provided with a visual guidance to facilitate the execution of rhythmical contraction-relaxation cycles (24 Hz) at consistent shapes and levels of muscular force. To gate each of the phase-encoding levels of the image acquisition and thus synchronize the image acquisition with the contraction-relaxation cycles, an in-house electronic module differentiated the voltage curve output by the spectrometer, to generate an electrocardiogram-like trigger signal denoting the beginning of a new cycle, which was then fed into the scanner electrocardiogram input. During passive rotation of the ankle joint, this trigger pulse was directly derived from the servomotor driving the foot pedal device, so that the image acquisition always started when the foot was at its most plantar flexed position.
Force Measurements
In the active trials, subjects were instructed to perform cyclical, isometric contractions at a consistent level of force, coinciding with 35% of the individual MVIC. For this purpose, three MVIC trials, interspersed by ∼1 min of passive recovery, were recorded before the acquisition of VE-PC images. The best of these trials was used to set the target torque level. During the subsequent execution of the ∼70 contraction-relaxation cycles, torques were recorded at a sampling frequency of 200 Hz and averaged to produce curves of mean torque. To estimate muscular forces, the so-determined measures of torque were divided by the Achilles tendon moment arm length, which was measured on sagittal-plane MR images of the ankle (Fig. 1B).
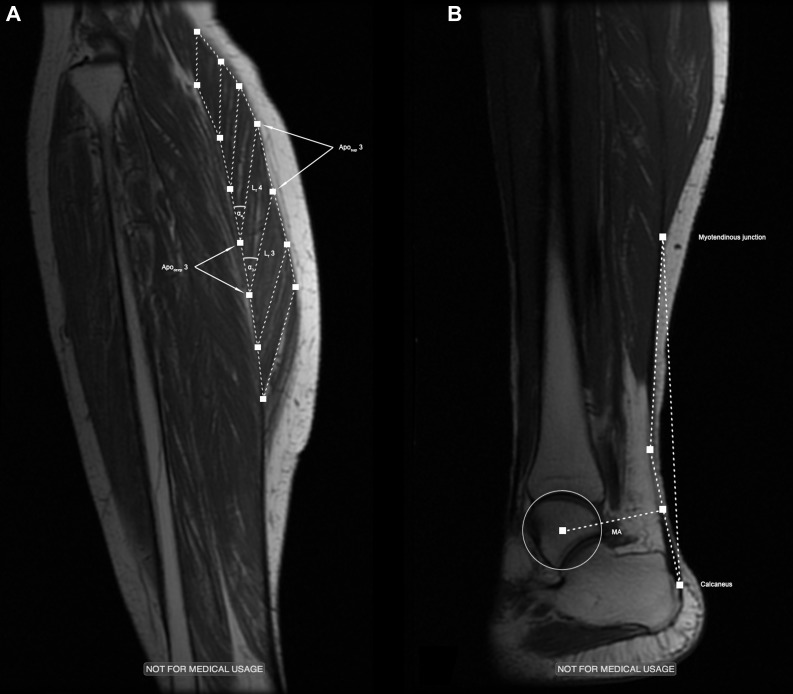
Fig. 1.
Typical examples of the high-resolution images obtained in the proximal (A) and distal (B) region of the gastrocnemius muscle-tendon unit. Lf, fascicle length; Aposup, superficial aponeurosis; Apodeep, deep aponeurosis; α, pennation angle; MA, Achilles tendon moment arm.
MRI Protocols
The imaging protocol consisted of 1) two sets of oblique sagittal-plane, high-resolution images (obtained at rest); and 2) two sets of spatially registered dynamic VE-PC images for each of the experimental conditions (isometric contraction and passive joint rotation). The first set of each of these sequences was centered to show the proximal part of the lower leg, from the origin of the GM to slightly distal to (depending on the height of the subject) the myotendinous junction (MTJ), i.e., the point where the GM inserts into the gastrocnemius tendon; the second set, showing the distal section of the lower leg, was used to study the free Achilles tendon from the MTJ to its insertion on the calcaneus. 3) In addition, we acquired axial-plane images of the Achilles tendon, to determine its cross-sectional area. Details of these imaging sequences are given below.
High-resolution images in the oblique sagittal plane.
The fast spin echo series parameters are as follows: echo time (TE), 12.9 ms; repetition time (TR), 925 ms; number of excitations (NEX), 4; flip angle (FA), 20°; slice thickness, 3 mm; interslice gap, 0 mm; FOV, 30 × 22.5 cm; 512 × 384 matrix. A quick visual inspection of these images served to decide which of the slices acquired showed best the fascicles within the GM (proximal, Fig. 1A) and the Achilles tendon along its entire superior-inferior length (distal, Fig. 1B). The location and geometry of these slices were noted and used to position the subsequent VE-PC scan slice.
Dynamic VE-PC images.
The VE-PC single slice parameters are as follows: TE, 7.7 ms; TR, 16.4 ms; NEX, 2; FA, 20°; slice thickness, 5 mm; FOV, 30 × 22.5 cm; 256 × 192 matrix; 1 slice; 22 phases; 10 cm/s three-dimensional velocity encoding. Twenty-two phases were collected within each contraction-relaxation cycle of ∼2.5 s (isometric contraction) and plantar flexion-dorsiflexion movement cycle of 3.4 s (passive joint rotation). The resulting VE-PC cine image sets, therefore, consisted of 1 set of 22 (morphological) magnitude images and 3 sets of phase-contrast velocity-encoded images corresponding to each of the X-, Y-, and Z-directions of velocity encoding, with the gray scale of each voxel reflecting the velocity of the tissue in that direction. The VE-PC technique has been exhaustively validated by our group, both against the gold standard flow tube experiments, as well as against tissue movement in prepared muscle specimen tracked by spin-tag experiments (8, 38). These and further experiments determining intrasession variability and day-to-day variability have established the reliability and precision of the VE-PC method of quantitating the trajectory of tissue points in the study of muscle dynamics in humans during different types of contraction of the triceps surae complex (38).
High-resolution images in the axial plane.
The gradient recalled echo image parameters are as follows: TE, 2.7 ms; TR, 225 ms; NEX, 1; FA, 45°; slice thickness, 3 mm; interslice gap, 5 mm; FOV, 18 × 18 cm; 256 × 256 matrix. Tendon cross-sectional areas were measured 3 cm proximal to the tendon's point of insertion on the calcaneus, typically coinciding with the narrowest region of the tendon (27).
MRI Postprocessing and Image Analyses
The paths of the GM muscle fascicles were identified on the proximal set of the fast spin echo images described above. To cover the muscle in its entire length, seven equally spaced pairs of regions of interest (ROIs), representing the end points of muscle fascicles, were placed along the GM's aponeuroses (Fig. 1A). Subsequently, the VE-PC cines were used to track the coordinates of these points across the contraction-relaxation (isometric contractions) or plantar flexion-dorsiflexion (passive joint rotations) cycle. For this purpose, a custom-made MATLAB algorithm (MATLAB R2012b, Mathworks, Natick, MA), which determines the position of each ROI based on its original position and the displacement between phases, calculated as the product of the respective voxel's velocity vector (duly corrected for phase shading and other errors) and the time between subsequent phases (∼78 ms), was used (37). Using the coordinates of the so tracked ROIs as input, further MATLAB routines were applied to automatically calculate fascicle lengths as a straight line between an associated pair of ROIs on the deep and superficial aponeurosis and fascicle pennation angles as the angles enclosed by a fascicle and the respective segment of the deep aponeurosis. For illustrative reasons, the six values (seven in the case of muscle fascicle length) of all measured parameters were grouped into three groups (2 distal, 2–3 middle, 2 proximal) and averaged to represent the typical GM behavior in its distal, middle, and proximal region. Comparisons between age groups were always made for each of these muscular regions. Since, in isometric contractions, the absolute forces produced at equal levels of relative intensity (35% of the individual MVIC) were considerably larger in YW, all parameters are reported at three levels of muscle force: 1) at baseline (values of fascicle lengths and pennation angles as measured at rest); 2) at 199 N, representing the highest force produced by the weakest subject; and 3) at the force level corresponding with 35% of the individual MVIC force.
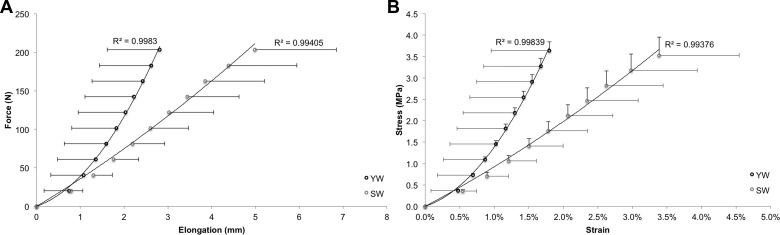
Using the magnitude images from the VE-PC series obtained in the distal region of the lower leg, the positions of the MTJ and the point where the Achilles tendon inserts onto the calcaneus were manually identified on each frame (see Fig. 1B), to measure tendon lengths and elongations. This more time-consuming procedure of data analysis was applied because tendinous tissues appear as signal-void on standard MR images, which complicates the automated tracking of voxels within the tendon and also because the movement of the Y-shaped landmark of the MTJ was easy to track accurately. After plotting tendon elongations against the estimated tendon forces (see below), second-order polynomials were used to obtain smoothed force-elongation curves (R2 > 0.99; see Fig. 2A). Tendon stiffness (N/mm), given by the slope of this curve in its linear region (25), was calculated in the force region coinciding with the highest 20% of force exerted by the weakest subject (162–203 N). Tendon stress (MPa) and strain (%) were determined by normalization of tendon forces to tendon cross-sectional area and tendon elongations to tendon resting length, respectively. Young's modulus, reflecting the tendon's intrinsic material properties, was estimated as the slope of the resulting stress-strain curves (36) (Fig. 2B).
Fig. 2.
Achilles tendon force-elongation (A) and stress-strain (B) curves as obtained in six young (YW) and six senior women (SW). Values are means ± SD.
Statistical Analyses
After verifying normal distribution of data (Shapiro-Wilk test, P > 0.05), independent sample t-tests were applied to assess differences between age groups. One-way ANOVAs and Bonferroni adjusted post hoc t-tests were used to test differences in fascicle lengths and pennation angles between the proximal, middle, and distal muscle region. For correlational analyses, Pearson's coefficients were used. The statistical level of significance was set at α = 0.05, and data are reported as means ± SD. All statistical tests were performed using SPSS for Mac OSX (SPSS 21.0, SPSS, Chicago, IL).
RESULTS
Baseline Measurements
The results of baseline measurements of muscle architecture and tendon dimensions, as obtained at rest, are summarized in Table 1. Across age groups, both fascicle lengths and pennation angles were subject to considerable intramuscular variability, with the average differences between the shortest and longest single fascicle, as well as the smallest and largest single pennation angle, amounting to 9.5 ± 6.1 mm and 15.5 ± 4.8°, respectively. One-way ANOVAs revealed that fascicle lengths [F(2,33) = 1.070, P = 0.355] did not differ statistically between muscle regions, whereas the regional differences in pennation angles were found to be significant [F(2,33) = 7.600, P = 0.002]. Post hoc t-tests performed to follow up this finding showed that pennation angles in the distal muscle region were significantly smaller than those in the middle and proximal region, with no statistical differences between the latter two. With regard to differences between age groups, we found GM fascicles to be longer in YW in all muscle regions examined (differences: proximal, 20.0%; middle, 13.3%; distal, 9.8%), reaching statistical significance, however, only in the GM's most proximal region. On average, the intramuscular heterogeneity of fascicle lengths was larger in YW (∼11.5 vs. ∼6.0%). For fascicle pennation angles, no significant between-group differences were found.
Table 1.
Baseline measurements of gastrocnemius medialis muscle architecture and Achilles tendon dimensions
| Young Women |
Senior Women |
||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P Value | |
| Lf distal, cm | 3.4 | 0.5 | 3.1 | 0.2 | 0.211 |
| Lf middle, cm | 3.8 | 0.4 | 3.3 | 0.5 | 0.088 |
| Lf proximal, cm | 3.9 | 0.5 | 3.1 | 0.2 | 0.022 |
| PA distal, ° | 23.5 | 4.4 | 23.2 | 5.7 | 0.937 |
| PA middle, ° | 30.4 | 5.3 | 28.4 | 4.2 | 0.484 |
| PA proximal, ° | 31.0 | 7.6 | 31.5 | 4.4 | 0.881 |
| Tendon length, cm | 15.9 | 1.6 | 14.6 | 1.5 | 0.180 |
| Tendon CSA, mm2 | 56.1 | 3.1 | 58.3 | 7.2 | 0.499 |
Values represent the average of 2 (3 in the midregion) neighboring fascicles.
Lf, fascicle length; PA, pennation angle; CSA, cross-sectional area.
Submaximal Contraction
In submaximal contractions at an intensity level coinciding with 35% of the individual isometric MVIC, the force developed by YW (344.8 ± 72.2 N) was higher by ∼30% compared with the force measured in SW (253.8 ± 88.9 N). Although these differences were found to be nonsignificant (P = 0.080), all parameters of interest are compared at both identical relative intensities (35% MVIC) and equal levels of absolute force (199 N), dictated by the maximum force developed by the weakest (senior) subject.
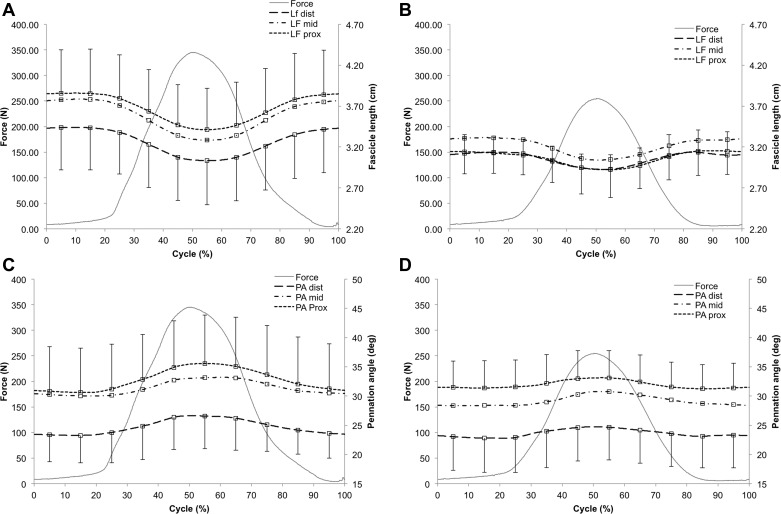
During active, submaximal contractions of the triceps surae muscle group, GM fascicle length decreased in all muscle regions and both age groups (Fig. 3, A and B). At 199 N, the degree of fascicle shortening ranged, on average, between 1 and 2 mm, coinciding with fascicle strains of −4–5%, with no significant differences observed between age groups (distal: YW −2 ± 1 mm vs. SW −1 ± 1 mm, P = 0.504; middle: YW −2 ± 1 mm vs. SW −2 ± 1 mm, P = 0.592; proximal: YW −2 ± 1 mm vs. SW −1 ± 1 mm, P = 0.488). At greater force (35% MVIC), fascicle shortening was generally more pronounced and significantly greater in YW in the distal (YW −4 ± 1 mm vs. SW −2 ± 1 mm, P = 0.032), middle (YW −5 ± 1 mm vs. SW −3 ± 1 mm, P = 0.012), and proximal (YW −4 ± 2 mm vs. SW −2 ± 1 mm, P = 0.021) GM region (see Fig. 3). This greater fascicle shortening was also reflected in a trend toward greater fascicle strains in YW (distal: YW −12 ± 5% vs. SW −6 ± 5%, P = 0.065; middle: YW −12 ± 3% vs. SW −8 ± 4%, P = 0.050; proximal: YW −11 ± 4% vs. SW −7 ± 3%, P = 0.059).
Fig. 3.
Dynamic changes in gastrocnemius medialis fascicle length (LF; A and B) and pennation angles (PA; C and D) during submaximal isometric contraction as measured in YW (n = 6) and SW (n = 6). Note, subplots A and C reflect the data measured in YW (n = 6), B and D represent the results obtained in the SW cohort (n = 6). Values are means ± SD.
Correlational analyses revealed that, when the average behavior of all identified fascicles (7 fascicles per subject, 12 subjects) was considered, the degree of fascicle shortening was strongly linearly related to plantar flexion force (YW: r = 0.97, P < 0.001; SW: r = 0.99, P < 0.001). As opposed to the decrease in fascicle length, pennation angles increased during active contraction of the plantar flexor muscles in both age groups (Fig. 3, C and D). At 199 N, these changes were generally small, yet significantly larger in YW in the proximal muscle region (distal: YW +1.2 ± 1.0° vs. SW +1.1 ± 1.0°, P = 0.867; middle: YW +0.6 ± 0.7° vs. SW +0.9 ± 0.7°, P = 0.450; proximal: YW + 1.8 ± 0.7° vs. SW + 0.9 ± 0.7°, P = 0.044). With increasing force (35% MVIC), the age-related differences in the changes of pennation angles increased, to reach significance in both the distal (YW +3.2 ± 1.4° vs. SW +1.5 ± 0.9°, P = 0.033) and proximal (YW +4.5 ± 1.0° vs. SW +1.6 ± 0.9°, P < 0.001) muscle region (no significant differences found for the middle muscle region: YW +2.6 ± 1.2° vs. SW +2.3 ± 1.5°, P = 0.707).
Achilles tendon stiffness (YW 120.2 ± 52.3 N/mm vs. SW 53.9 ± 44.4 N/mm, P = 0.040) and Young's modulus (YW 340.1 ± 163.3 MPa vs. SW 139.7 ± 130.5 MPa), derived as the slopes of the tendon's force-elongation and stress-strain relationships (see Fig. 2), were significantly larger in YW. By contrast, tendon resting length and cross-sectional area were not statistically different between groups (Table 1).
Passive Joint Rotation
With the foot held in a plantar flexed joint position, very low levels of passive tension (YW 4.1 ± 5.5 N vs. SW 5.2 ± 1.8 N, P = 0.132) were recorded for both age groups. During passive rotation of the foot, a rise in passive tension was observed in both cohorts, but this was more pronounced in YW. At a fully dorsiflexed joint, passive tension was greater by ∼17% in the YW cohort, although these differences in passive tension were not statistically significant (YW 209.7 ± 68.3 N vs. SW 177.6 ± 48.2 N, P = 0.369).
With respect to muscle architecture, passive rotation of the ankle from a plantar- to a dorsiflexed joint position was found to coincide with an increase in fascicle length in all muscle regions and both age groups. In absolute terms, the fascicle length changes were larger in YW in the distal (YW +7 ± 1 mm vs. SW +5 ± 2 mm; P = 0.278), middle (YW +8 ± 1 mm vs. SW +6 ± 3 mm; P = 0.221), and proximal (YW +7 ± 1 mm vs. SW +6 ± 3 mm; P = 0.583) section of the GM, although these differences failed to reach significance. As a consequence of the shorter resting fascicle length in SW, very similar fascicle strains were recorded for both age groups (distal: YW 23 ± 5% vs. SW 25 ± 12%, P = 0.817; middle: YW 23 ± 2% vs. SW 26 ± 13% mm, P = 1.000; proximal: YW 18 ± 3% vs. SW 21 ± 11% mm, P = 0.937). Passive rotation of the ankle joint from a plantar- to a dorsiflexed joint position was also found to coincide with a decrease in fascicle pennation angles. However, comparisons between age groups revealed that these changes were of the same dimension in the distal (YW −4.9 ± 1.4° vs. SW −4.9 ± 1.6°, P = 0.998) and only insignificantly larger in SW in the middle (YW −5.3 ± 2.1° vs. SW −6.9 ± 1.5°, P = 0.148) and proximal region (YW −5.9 ± 1.5° vs. SW −7.8 ± 2.4°, P = 0.138) of the GM muscle.
DISCUSSION
In the present study, we applied the elegant VE-PC MR imaging technique to the study of soft tissue dynamics within the GM MTU in YW and SW. Specifically, using our indigenous analysis software, we aimed to assess age-associated differences in Achilles tendon material properties and the mechanical behavior of GM muscle fascicles in response to submaximal, isometric contraction and passive rotations of the ankle joint. Confirming our hypothesis, our data demonstrate that, despite significantly greater tendon compliance in SW, contraction-induced changes in muscle architecture (fascicle lengths and pennation angles) are similar in both age groups when equal levels of absolute muscular force are compared, and even significantly larger in YW at an equal percentage of maximum voluntary contraction. These findings contradict the notion that a more compliant tendon inevitably entails greater fascicle shortening on isometric contraction (31, 34). Fascicle strains and changes in pennation angles induced by passive rotation of the ankle joint did not differ significantly between groups.
In agreement with our expectation of a deterioration of tendon mechanical properties at older age, we found the stiffness of the Achilles to be significantly lower in the SW cohort. While the direct comparison of our results and those obtained in previous studies is complicated by the limited sample size studied here and the associated large variance of measurement results observed in the SW cohort, a decline of tendon stiffness has also been found in studies using ultrasound (33, 40). However, the age-associated differences found here were larger than those previously reported [for instance, Onambele et al. (33) reported differences in tendon stiffness of ∼49% (33), compared with ∼76% in the present study]. In part, these larger differences may be attributed to the significantly older age of our SW cohort, but it could be argued that the different methodologies applied here and in former studies may also account for these differences in age-associated changes. In ultrasound-based examinations, tendon stiffness is usually measured at near maximal tendon force, whereas the necessity to acquire multiple contraction-relaxation cycles limits VE-PC MRI to the study of submaximal contractions. While it is possible that, at higher forces, the force-elongation curves used to determine Achilles tendon stiffness would have altered their shape, the region at which tendon stiffness was estimated here (∼200 N) was found to be linear, not only in our study, but also in previously performed, ultrasound-based experiments (33, 40). However, if bias related to the relatively low tendon forces was indeed present, it is plausible to assume that it would have affected the results obtained in SW more strongly, since their tendon compliance and the associated “toe region” in the force-elongation curve are presumably greater. At the same time, it should be noted that the execution of everyday tasks, such as walking, does not require the development of maximal muscular forces, and that the cyclical, submaximal contraction-relaxation cycles studied here may better represent real-life demands.
Another interesting comparison is between the Achilles tendon material properties measured here, where age-associated effects can be loosely termed chronic, vs. in a former study investigating the effects of acute atrophy, induced by unilateral lower limb suspension (36). While the differences between the Young's moduli measured in YW and SW were significant (∼83%), the respective changes were comparably small following unilateral suspension of the lower limb (∼17%; Ref. 36). It has been speculated that the deterioration of tendon material properties from various pathological conditions, including atrophy, may be associated with a reduction in ground substances (a mixture of proteoglycans and glycoproteins surrounding the collagen fibers) and the number of longitudinally oriented collagen fibers, as well as a decrease in fibril diameter, possibly related to the secretion of cytokines, which lead to an increased activation of matrix metalloproteinases, enzymes catalyzing the degradation of collagens (16). While a deterioration of tendon material properties may be triggered by both disuse and aging, our data suggest that the long-term chronic process of aging may lead to a significantly greater remodeling of collagenous structures than short-term inactivity. In support of this observation, recent experiments in rodents suggest that the upregulation of matrix metalloproteinase activity may be associated with aging (43), but not artificially induced, and acute, hindlimb disuse (44).
Our data further show age-associated differences in muscle architecture. Fascicle lengths within the GM were shorter by ∼10–20% (statistically significant in the proximal muscle region), and fascicle pennation angles were lower by 1–7% in SW (although failing to reach statistical significance). Prior findings in the literature of these variations are not consistent. Our findings agree approximately with that of Narici et al. (32) and Morse et al. (28), who reported that overall age-associated losses of muscle mass were accompanied by decreases of both fascicle length (−10–17%) and pennation angles (−14%). However, no such differences between age groups were found by Kubo et al. (19) and Karamanidis and Arampatzis (13), although the SW subjects examined in the latter study were younger (60–69 yr) than those included in our and the aforementioned papers.
During submaximal isometric contraction of the triceps surae complex, fascicle shortening and increases in pennation angles were observed in both age groups. While this behavior was expected, because fascicles are known to rotate about their origin during contraction, thereby increasing the angle of pennation (14, 29, 37), we aimed to assess whether the dynamic changes seen in GM architecture at different proximo-distal locations would differ between YW and SW participants. In the most simplistic model of muscle mechanics, assuming a serial arrangement of muscle fascicles and tendon, homogeneous development of force during fascicle shortening and a perfectly linear force-elongation relationship of the tendon, the fascicle shortening s required for the generation of a given force F would be given by s = F·k−1, where k is tendon stiffness. If the degree of fascicle shortening during isometric contraction was indeed solely determined by the stiffness of the free tendon, conventional understanding of muscle mechanics would therefore dictate larger fascicle strains in SW, in whom tendon compliance was substantially larger (31, 34). This is because, with a serial arrangement of contractile and elastic components, fascicles would have to shorten more to tauten a more compliant tendon and thereby facilitate force transmission onto the skeleton (31, 34). More specifically, in our study, the ∼76% difference in tendon stiffness would have to result in ∼2.2 times greater fascicle shortening in SW. Such a less direct coupling of muscles and bones through a more compliant tendon would impair a subject's capacity to explosively generate force (2), which may be of considerable clinical relevance in the avoidance of falls. Contrary to our expectation of greater fascicle strains, however, the degree of fascicle shortening was generally small (1–2 mm), not significantly different between the cohorts of YW and SW participants at low levels of force (∼200 N), and thus substantially smaller in SW than would have to be expected if fascicle shortening were determined by tendon mechanical properties alone. While a lack of statistical differences does not prove the null hypothesis to be true, particularly when sample sizes are small, our results strongly suggest that factors other than tendon stiffness strongly affect the degree of fascicle shortening on isometric contraction and act to offset the consequences of a more compliant tendon in SW. In addition, it is noteworthy that, while comparisons made at different force levels may be of limited significance, fascicle shortening was even significantly larger in YW at equal levels of relative intensity (35% MVIC).
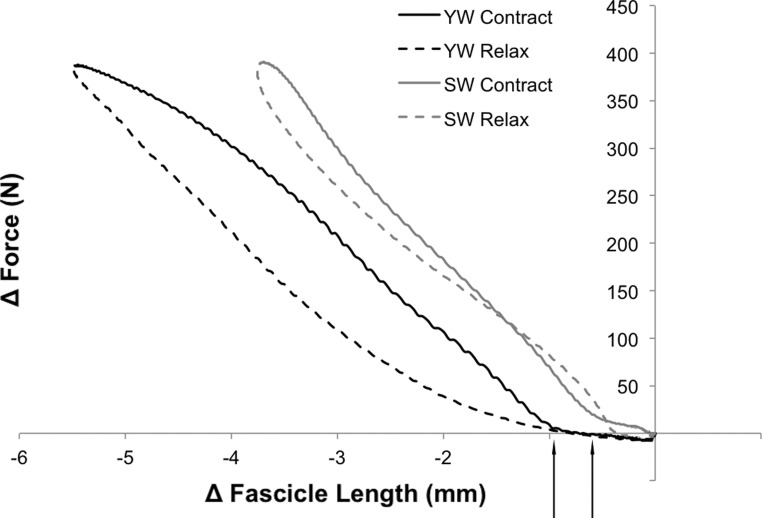
One possible explanation for this unexpected result may lie in fascicle slack. Studying the GM fascicle behavior during passive rotation of the ankle joint, Herbert et al. (11) recently reported that fascicle lengthening may be delayed with respect to the lengthening of the total GM MTU, indicating fascicle slack at short MTU length. It may be speculated that fascicle slack increases with fascicle length, which, in our study, was found to be significantly larger in YW. Qualitative support for this hypothesis is provided by subject-specific plots relating the length changes of single fascicles to the rise in muscle force. These plots revealed that, on contraction, fascicles shortened by a small amount while no or only a minimal rise of force was seen. This “toe” region was typically followed by a marked bend in the curves, reflecting the changes in fascicle lengths and muscular forces. The representative examples shown in Fig. 4 reflect the typical shortening of one fascicle located in the middle of the muscle belly (fascicle 3, see Fig. 1A) in one YW and one SW participant (subjects chosen for their similar maximal force output), respectively. As indicated in these illustrative figures, the toe region typically appeared to be longer in YW, which lends support to the hypothesis that fascicle slack might be greater in YW. However, it should be noted that these effects were generally very small in dimension (∼1 mm), which makes these data liable to measurement inaccuracies. If fascicle slack were indeed present, the effects of larger fascicle slack and greater tendon stiffness would tend to cancel each other, resulting in the similar degree of fascicle shortening observed at equal levels of absolute force.
Fig. 4.
Representative curves illustrating the typical relationship between fascicle shortening and rise of force in YW and SW. Note: the data presented were obtained in one YW and one SW, who were selected due to their similar muscular force and baseline LF. Solid and dashed lines represent the phase of contraction and relaxation, respectively. The arrows below the x-axis highlight the “toe” regions, typically seen at the onset of contraction. Δ, Change.
Moreover, muscle stiffness may have differed between YW and SW. The results of several animal studies suggest that the stiffness of skeletal muscles increases throughout life (for a review, see Ref. 18), which may be associated with an increase in the total amount of intramuscular connective tissues (1, 35, 45). In a recent study performed in our laboratory (unpublished observations), we aimed to quantify the extracellular matrix within the gastrocnemius muscles by means of a specific (ultra-short echo time) MR sequence and estimated the total amount of intramuscular connective tissue to be greater by ∼49% in SW, compared with YW. The accumulation of excess extracellular matrix, often referred to as fibrosis, affects both the endo- and perimysium (10) and may strongly affect muscle mechanics. Computational models comparing compliant and stiffer intramuscular passive matrix suggest that a stiffer passive matrix reduces both fascicle strain and total muscle force output of an isometrically contracting muscle (12). Similar to the proposed smaller fascicle slack, greater muscle stiffness would act to counter the effects of lower tendon stiffness. Importantly, in support of this argument, we also found the age-associated differences in passive tension at a fully dorsiflexed joint (greater in YW by 17%) and the degree of fascicle length changes during passive joint rotation (across all fascicles greater in YW by ∼25%) to be substantially smaller than those measured for tendon stiffness. Another factor that may affect the degree of fascicle shortening on isometric contraction, unaccounted for in our study, is the proportion of plantar flexion moment generated by different heads of the triceps surae complex. Since aging is known to be associated with changes in fiber type and diameter, which are indicative of a preferential atrophy of fast-twitching type II fibers (23), it could be argued that young and senior subjects might adopt different motor unit recruitment strategies, with the elderly candidates relying more strongly on the predominantly slow-twitch soleus muscle. In this report, we are able to elucidate only one aspect, namely the influence of tendon compliance on fascicle shortening.
Another interesting observation was made regarding the fascicle behavior during the force-relaxation cycle. From Fig. 4 it is apparent that, at any given level of muscular force and in both age groups, fascicle length was longer in the phase of muscle contraction (solid lines) and shorter during the phase of relaxation (dashed lines), although these differences were small in magnitude. This result may reflect the effects of tendon hysteresis. During contraction-relaxation cycles, the force-elongation curves of tendons have been found to describe a hysteresis loop that is mirror inverted to the fascicle force-elongation curves shown here; i.e., Achilles tendon length is shorter during contraction and longer during relaxation (20, 26). Since, in isometric contractions, the overall length of the MTU must remain constant, fascicles might be forced to alter their length to adjust for changes in Achilles tendon length that result from previous muscular contractions. However, it should be noted that measurement inaccuracies, possibly related to inadvertent joint rotations during isometric contraction, cannot be ruled out and might have affected our results. Moreover, as pointed out by Finni and colleagues (9), observations of hysteresis can also stem from artifacts related to imperfect synchronization of force and length recordings. Such bias is more likely when sampling rates are low, as is unavoidable with the VE-PC technique applied in the present study.
In conclusion, we successfully applied VE-PC MRI to the study of the GM MTU tissue dynamics during submaximal contraction of the plantar flexor muscles and passive rotation of the ankle joint. Our comparison of data obtained in cohorts of YW and SW revealed that, despite significantly greater tendon compliance in SW, the contraction-induced changes in fascicle lengths and pennation angles were similar between groups at equal levels of muscle force and even larger in YW when equal relative intensities (35% MVIC) were compared. While the generalizability of our results is complicated by the small size and specific nature (only Japanese women were studied) of our cohort, these data suggest that factors other than tendon compliance, such as muscle stiffness or fascicle slack, may counter the effects of age-associated decreases in tendon stiffness, thus limiting the degree of fascicle strains.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant 5RO1-AR-053343-07 (S. Sinha)
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.C. and S.S. conception and design of research; R.C., V.M., and S.S. performed experiments; R.C. and V.M. analyzed data; R.C., J.A.H., and S.S. interpreted results of experiments; R.C. prepared figures; R.C. drafted manuscript; R.C., V.M., J.A.H., and S.S. approved final version of manuscript; J.A.H. and S.S. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. Ryuta Kinugasa for help with subject recruitment.
REFERENCES
- 1.Alnaqeeb MA, Al Zaid NS, Goldspink G. Connective tissue changes and physical properties of developing and ageing skeletal muscle. J Anat 139: 677–689, 1984 [PMC free article] [PubMed] [Google Scholar]
- 2.Bojsen-Moller J, Magnusson SP, Rasmussen LR, Kjaer M, Aagaard P. Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structures. J Appl Physiol 99: 986–994, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Cronin NJ, Lichtwark G. The use of ultrasound to study muscle-tendon function in human posture and locomotion. Gait Posture 37: 305–312, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Dalton BH, Harwood B, Davidson AW, Rice CL. Triceps surae contractile properties and firing rates in the soleus of young and old men. J Appl Physiol 107: 1781–1788, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr 90: 1579–1585, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finni T, Hodgson JA, Lai AM, Edgerton VR, Sinha S. Mapping of movement in the isometrically contracting human soleus muscle reveals details of its structural and functional complexity. J Appl Physiol 95: 2128–2133, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Finni T, Hodgson JA, Lai AM, Edgerton VR, Sinha S. Nonuniform strain of human soleus aponeurosis-tendon complex during submaximal voluntary contractions in vivo. J Appl Physiol 95: 829–837, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Finni T, Peltonen J, Stenroth L, Cronin NJ. Viewpoint: On the hysteresis in the human Achilles tendon. J Appl Physiol 114: 515–517, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 44: 318–331, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbert RD, Clarke J, Kwah LK, Diong J, Martin J, Clarke EC, Bilston LE, Gandevia SC. In vivo passive mechanical behaviour of muscle fascicles and tendons in human gastrocnemius muscle-tendon units. J Physiol 589: 5257–5267, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodgson JA, Chi SW, Yang JP, Chen JS, Edgerton VR, Sinha S. Finite element modeling of passive material influence on the deformation and force output of skeletal muscle. J Mech Behav Biomed Mater 9: 163–183, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karamanidis K, Arampatzis A. Mechanical and morphological properties of human quadriceps femoris and triceps surae muscle-tendon unit in relation to aging and running. J Biomech 39: 406–417, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Kawakami Y, Ichinose Y, Fukunaga T. Architectural and functional features of human triceps surae muscles during contraction. J Appl Physiol 85: 398–404, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Kinugasa R, Shin D, Yamauchi J, Mishra C, Hodgson JA, Edgerton VR, Sinha S. Phase-contrast MRI reveals mechanical behavior of superficial and deep aponeuroses in human medial gastrocnemius during isometric contraction. J Appl Physiol 105: 1312–1320, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84: 649–698, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Klimstra M, Dowling J, Durkin JL, MacDonald M. The effect of ultrasound probe orientation on muscle architecture measurement. J Electromyogr Kinesiol 17: 504–514, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Kragstrup TW, Kjaer M, Mackey AL. Structural, biochemical, cellular, and functional changes in skeletal muscle extracellular matrix with aging. Scand J Med Sci Sports 21: 749–757, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Kubo K, Kanehisa H, Azuma K, Ishizu M, Kuno SY, Okada M, Fukunaga T. Muscle architectural characteristics in women aged 20–79 years. Med Sci Sports Exerc 35: 39–44, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Kubo K, Kanehisa H, Fukunaga T. Effects of transient muscle contractions and stretching on the tendon structures in vivo. Acta Physiol Scand 175: 157–164, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Kwah LK, Pinto RZ, Diong J, Herbert RD. Reliability and validity of ultrasound measurements of muscle fascicle length and pennation in humans: a systematic review. J Appl Physiol 114: 761–769, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Lee HD, Finni T, Hodgson JA, Lai AM, Edgerton VR, Sinha S. Soleus aponeurosis strain distribution following chronic unloading in humans: an in vivo MR phase-contrast study. J Appl Physiol 100: 2004–2011, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50: Spec No. 11–16, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Lieber RL, Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve 23: 1647–1666, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Maganaris CN. Tensile properties of in vivo human tendinous tissue. J Biomech 35: 1019–1027, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Maganaris CN, Paul JP. Hysteresis measurements in intact human tendon. J Biomech 33: 1723–1727, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Magnusson SP, Beyer N, Abrahamsen H, Aagaard P, Neergaard K, Kjaer M. Increased cross-sectional area and reduced tensile stress of the Achilles tendon in elderly compared with young women. J Gerontol A Biol Sci Med Sci 58: 123–127, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Morse CI, Thom JM, Birch KM, Narici MV. Changes in triceps surae muscle architecture with sarcopenia. Acta Physiol Scand 183: 291–298, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Narici MV, Binzoni T, Hiltbrand E, Fasel J, Terrier F, Cerretelli P. In vivo human gastrocnemius architecture with changing joint angle at rest and during graded isometric contraction. J Physiol 496: 287–297, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull 95: 139–159, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Narici MV, Maffulli N, Maganaris CN. Ageing of human muscles and tendons. Disabil Rehabil 30: 1548–1554, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Narici MV, Maganaris CN, Reeves ND, Capodaglio P. Effect of aging on human muscle architecture. J Appl Physiol 95: 2229–2234, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Onambele GL, Narici MV, Maganaris CN. Calf muscle-tendon properties and postural balance in old age. J Appl Physiol 100: 2048–2056, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Reeves ND, Narici MV, Maganaris CN. In vivo human muscle structure and function: adaptations to resistance training in old age. Exp Physiol 89: 675–689, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Serrano AL, Munoz-Canoves P. Regulation and dysregulation of fibrosis in skeletal muscle. Exp Cell Res 316: 3050–3058, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Shin D, Finni T, Ahn S, Hodgson JA, Lee HD, Edgerton VR, Sinha S. Effect of chronic unloading and rehabilitation on human Achilles tendon properties: a velocity-encoded phase-contrast MRI study. J Appl Physiol 105: 1179–1186, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin D, Hodgson JA, Edgerton VR, Sinha S. In vivo intramuscular fascicle-aponeuroses dynamics of the human medial gastrocnemius during plantar flexion and dorsiflexion of the foot. J Appl Physiol 107: 1276–1284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sinha S, Hodgson JA, Finni T, Lai AM, Grinstead J, Edgerton VR. Muscle kinematics during isometric contraction: development of phase contrast and spin tag techniques to study healthy and atrophied muscles. J Magn Reson Imaging 20: 1008–1019, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Sinha S, Shin DD, Hodgson JA, Kinugasa R, Edgerton VR. Computer-controlled, MR-compatible foot-pedal device to study dynamics of the muscle tendon complex under isometric, concentric, and eccentric contractions. J Magn Reson Imaging 36: 498–504, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stenroth L, Peltonen J, Cronin NJ, Sipila S, Finni T. Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J Appl Physiol 113: 1537–1544, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Strasser EM, Draskovits T, Praschak M, Quittan M, Graf A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age (Dordr) 35: 2377–2388, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thom JM, Morse CI, Birch KM, Narici MV. Influence of muscle architecture on the torque and power-velocity characteristics of young and elderly men. Eur J Appl Physiol 100: 613–619, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Yu TY, Pang JH, Wu KP, Chen MJ, Chen CH, Tsai WC. Aging is associated with increased activities of matrix metalloproteinase-2 and -9 in tenocytes. BMC Musculoskelet Disord 14: 2, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Q, Joshi SK, Manzano G, Lovett DH, Kim HT, Liu X. Original article Muscle extracellular matrix degradation and contractibility following tendon rupture and disuse. Muscles Ligaments Tendons J 3: 35–41, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zoico E, Corzato F, Bambace C, Rossi AP, Micciolo R, Cinti S, Harris TB, Zamboni M. Myosteatosis and myofibrosis: Relationship with aging, inflammation and insulin resistance. Arch Gerontol Geriatr 57: 411–416, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]