Abstract
Two studies were performed to 1) characterize changes in local sweat rate (LSR) following fluid ingestion of different temperatures during exercise, and 2) identify the potential location of thermoreceptors along the gastrointestinal tract that independently modify sudomotor activity. In study 1, 12 men cycled at 50% V̇o2peak for 75 min while ingesting 3.2 ml/kg of 1.5°C, 37°C, or 50°C fluid 5 min before exercise; and after 15, 30, and 45-min of exercise. In study 2, 8 men cycled at 50% V̇o2peak for 75 min while 3.2 ml/kg of 1.5°C or 50°C fluid was delivered directly into the stomach via a nasogastric tube (NG trials) or was mouth-swilled only (SW trials) after 15, 30, and 45 min of exercise. Rectal (Tre), aural canal (Tau), and mean skin temperature (Tsk); and LSR on the forehead, upper-back, and forearm were measured. In study 1, Tre, Tau, and Tsk were identical between trials, but after each ingestion, LSR was significantly suppressed at all sites with 1.5°C fluid and was elevated with 50°C fluid compared with 37°C fluid (P < 0.001). The peak difference in mean LSR between 1.5°C and 50°C fluid after ingestion was 0.29 ± 0.06 mg·min−1·cm−2. In study 2, LSR was similar between 1.5°C and 50°C fluids with SW trials (P = 0.738), but lower at all sites with 1.5°C fluid in NG trials (P < 0.001) despite no concurrent differences in Tre, Tau, and Tsk. These data demonstrate that 1) LSR is transiently altered by cold and warm fluid ingestion despite similar core and skin temperatures; and 2) thermoreceptors that independently and acutely modulate sudomotor output during fluid ingestion probably reside within the abdominal area, but not the mouth.
Keywords: body temperatures, exercise, fluid intake, sweating, thermoregulation
to date, several studies (2, 13, 27) have reported that ingestion of cold and warm fluids during exercise leads to large fluid temperature-dependent differences in whole-body sweat loss (WBSL) with no parallel differences in end-exercise core or skin temperatures. However, none of these studies measured the dynamic response of local sweating following the ingestion of fluids of different temperatures during exercise. Therefore, it is not known whether acute changes in local sweat rate (LSR) occur immediately following ingestion, whether the response is localized to specific skin regions, or whether transient changes in core and/or skin temperature occur prior to any changes in LSR. Indeed, given that core temperature is the primary stimulus for changes in sudomotor activity, with additional modifications from skin temperature (3, 17), differences would be expected in advance of any notable divergence in LSR following cold and warm fluid ingestion unless thermoreceptors capable of independently modulating sudomotor activity exist somewhere along the gastrointestinal tract.
Whereas thermoreception in the viscera of humans remains uncertain, one study in sheep identified thermoreceptors in the abdominal wall and small intestine that elicit autonomic thermoeffector responses (21). Several others have studied thermoreception in the mouth (19, 20), esophagus (6), and stomach (9) of birds and mammals by measuring the electrical current produced by thermoafferents, but no attendant thermoeffector responses were assessed in these studies. No study has thus far identified the presence of any thermoreceptors along the gastrointestinal tract of humans that modulate sudomotor activity. An oropharyngeal reflex mechanism modifying sudomotor output that is sensitive to hydration status but without any thermal component has been demonstrated (12, 25). Furthermore, Villanova et al. (26) assessed changes in thermal perception and gastric contractility following ingestion of fluids of different temperatures, but thermoeffector responses were not reported. Indeed, in a recent review on thermoreception and subsequent thermoeffector responses, it was noted that for both humans and animals “how abdominal thermal information contributes to thermoregulatory functions is mostly unknown” (18).
The aim of the present investigation was to explore the mechanism responsible for the different WBSL previously reported following ingestion of fluids of different temperatures during exercise. To this end, two studies were conducted. In the first study, the dynamic local sudomotor response was characterized at three different skin regions (upper back, forehead, and forearm) following serial ingestion of cold (1.5°C), thermoneutral (37°C), and warm (50°C) fluids during exercise. In the second study, the potential location of thermoreceptors along the gastrointestinal tract that independently modulate sudomotor activity was investigated by directing cold (1.5°C) or warm (50°C) fluids either directly into the stomach via a nasogastric (NG) tube or only into the mouth by swilling (SW). It was hypothesized that an immediate but transient change in LSR that is directly dependent upon fluid temperature would be observed at all skin sites with fluid ingestion in the first study despite no differences in core or skin temperatures. In the second study, it was hypothesized that fluid temperature-dependent differences in LSR would be observed in the NG trials, but not the SW trials, indicating that thermoreceptors residing in the abdominal area of humans may independently modify sudomotor output.
METHODS
Ethical Approval
The experimental protocol was approved by the University of Ottawa Research Ethics Board, and was therefore in accordance with the Declaration of Helsinki. Completed Physical Activity Readiness Questionnaire forms and written informed consent were obtained from all the volunteers who participated in the study prior to experimentation.
Participants
For study 1, a power calculation using LSR data from earlier pilot studies was performed using the calculated effect size of 0.80, an α of 0.05, and a β of 0.2, which determined that 12 participants were required for a sufficient level of statistical power to identify whether mean LSR was significantly different between fluid temperatures following fluid ingestion using a Student's t-test. Therefore, 12 non-heat-acclimated men (mean age 23 ± 3 y, body mass 73.9 ± 7.7 kg, V̇o2peak 53.9 ± 5.4 ml·min−1·kg−1) were recruited. For study 2, using the LSR data from the 1.5°C and 50°C trials from study 1, a power calculation was performed using the calculated effect size of 1.25, an α of 0.05, and a β of 0.2, which determined that eight participants were required for a sufficient level of statistical power to identify whether mean LSR was significantly different between fluid temperatures following fluid administration using a Student's t-test. Therefore, eight non-heat-acclimated participants (mean age 22 ± 3 y, body mass 73.4 ± 7.1 kg, V̇o2peak 52.8 ± 5.4 ml·min−1·kg−1) were recruited. Participants did not consume caffeine or alcohol nor partake in any strenuous exercise 24 h prior to testing. Participants were asked to maintain a consistent routine (e.g., sleep schedules) and consume a similar diet during the day before and day of the experimental sessions. To the best of their knowledge, all participants were free from cardiovascular and metabolic health disorders before consenting to the study.
Protocol
Preliminary session.
In both studies, participants attended a preliminary session in which total body mass, height, and peak oxygen consumption were measured. Peak oxygen consumption (V̇o2peak) was measured using an upright cycle ergometer protocol consisting of a 2-min warm-up at 40 W followed by cycling at 100 W for the third minute, with a 20-W increase every minute thereafter until physical exhaustion. This protocol was based on recommendations from the Canadian Society for Exercise Physiology (5).
Experimental sessions.
In both studies, upon arrival to the laboratory, subjects were asked to provide a urine sample, which was analyzed for urine specific gravity (USG) using a spectrometer (Reichert TS 400; Depew, NY) to ensure that all participants were euhydrated prior to each experimental session. Participants were required to have a USG below 1.020 (1) prior to commencing a trial. Mean preexercise USG values were 1.014 ± 0.004. Participants then cycled for 75 min at 50% V̇o2peak. The order in which the trials were performed was determined using an incomplete Latin Square design, and each trial was separated by at least 48 h, but by no more than 1 wk. A mechanical fan placed 1.25 m in front of the participants produced a mean whole-body air velocity of 0.75 m/s, measured using a hot wire anemometer (Omega Engineering, Stamford, CT). Participants were seminude, wearing a standardized clothing ensemble in all experimental trials consisting of only light running shorts, socks, and shoes. All within-subject experimental sessions were completed at the same time of the day to avoid the influence of circadian variation. The ambient conditions were selected to be similar to previous studies (2, 13) reporting differences in WBSL with the ingestion of fluids differing in temperature. As such, air temperature was 23.7 ± 1.3°C, and relative humidity was 32 ± 10%.
In study 1, participants undertook three experimental trials (one trial per fluid temperature) in which they ingested four aliquots of exactly 3.2 ml/kg of 1.5°C, 37°C, or 50°C water at 5 min before exercise and after 15, 30, and 45 min of exercise (equating to a group average of total water consumed per trial of 945 ± 100 ml). This volume of fluid was selected to account for differences in body mass while providing similar volumes to previous studies (2, 13). In study 2, participants completed four experimental trials in which aliquots of exactly 3.2 m/kg of 1.5°C or 50°C water were either swilled in the mouth (SW trials) using 4 equal volume aliquots of water for 15 s at a time for a total swill time of 1 min per administration time point, or delivered directly into the stomach (NG trials) via an NG tube (54–8042; MED-RX, Oakville, ON, Canada) at a rate of ∼12 ml/s after 15, 30, and 45 min of exercise (equating to a group average of total water consumed per trial of 940 ± 90 ml).
Measurements
Water temperature.
In the 1.5°C trials, the water was poured into an insulated thermos with ice, which was then placed in a refrigerator 2 h prior to the experimental trials and left until 2 min before the ingestion of the water. In the 37°C and 50°C trials the water was warmed using a hydrostatic-controlled water bath (DA05A; Polyscience, Niles, IL). The temperature of the water before ingestion was measured using a glass thermometer (Durac Plus, Blue Spirit precision thermometer; Cole-Palmer) that was factory-calibrated with a certified range between −1°C and +51°C with an accuracy of ±0.1°C. Fluid temperatures did not deviate more than 0.5°C from 1.5°C, 37°C, or 50°C for any participant.
Thermometry.
Rectal temperature (Tre) was measured using a pediatric thermocouple probe (Mon-a-therm General Purpose Temperature Probe; Mallinckrodt Medical, St. Louis, MO) inserted to a minimum of 12 cm past the anal sphincter. In study 1, esophageal temperature (Tes) was measured by placing a pediatric thermocouple probe 40 cm past the participant's nostril and into the esophagus. Aural canal temperature (Tau) was measured using a tympanic thermocouple probe (Mon-a-therm Tympanic; Mallinckrodt Medical) placed in the aural canal until resting near the tympanic membrane. The tympanic probe was held in position and isolated from the external environment with large amounts of cotton, which was held in place with surgical tape and an ear defender. Esophageal temperature was used to ensure Tau values were equal to or greater than Tes prior to the start of exercise, thus verifying that the Tau probe had been sufficiently insulated (14). Tes, however, could not be used for the analysis of data during the exercise period due to the influence of fluid ingestion on Tes values.
Skin temperature (Tsk) was measured at eight points over the right side of the body using thermistors integrated into heat flow sensors (2252 Ohms; Concept Engineering, Old Saybrook, CT). The probes were attached using double-sided adhesive discs and surgical tape (Transpore; 3M, London, ON, Canada). Mean Tsk was estimated using a weighted average with the following regional proportions: forehead 7%, chest 17.5%, hand 5%, thigh 19%, scapula 17.5%, calf 20%, shoulder 7%, and triceps 7% (10). All thermometry data were collected using a National Instruments data acquisition module (model NI cDAQ-9172) at a sampling rate of 5 s. Data were simultaneously displayed and recorded in spreadsheet format on a personal computer (Dell Inspirion 545) with LabVIEW 2009 software (National Instruments, Austin, TX). Mean body temperature (Tb) was estimated using a weighting of 0.9 × Tcore (calculated separately both Tau and Tre) and 0.1 × Tsk (8, 15).
Sudomotor measurements.
LSR was measured using a 4.0-cm2 ventilated capsule placed on the left side of the forehead opposite the thermistor, the right anterior forearm ∼6 cm distal to the antecubital fossa, and the upper left back over the trapezius muscle midway between the neck and the acromion process. Anhydrous compressed air was passed through each capsule over the skin surface at a rate of ∼1.8 l/min. Flow rate for each capsule was measured using a flow rate monitor (FMA-A2307; Omega Engineering, Stamford, CT). Vapor content of the effluent air was measured using a 473 precision dew point mirror (RH Systems, Albuquerque, NM) on the anterior forearm, and two capacitance hygrometers (series HMT333; Vaisala, Helsinki, Finland) for the forehead and upper back. All three hygrometers yielded values accurate to 0.035 mg·min−1·cm−2 and were factory calibrated. Values for LSR were calculated using the exact flow rate, and the difference in water content between effluent and influent air. This value was normalized for the skin surface area under the capsule and expressed in mg·min−1·cm−2.
WBSL was measured using the change in total body mass during the trial to the nearest gram by weighing the participants using a platform scale (Combics 2; Sartorius, Mississauga, ON, Canada) immediately prior to exercise and upon the completion of exercise. Values for WBSL were then corrected for respiratory mass loss, metabolic mass loss, saliva loss, and weight gain through fluid ingestion [see (2) for equations].
Statistical Analysis
All data are expressed as mean ± SD. In studies 1 and 2, to assess the LSR and thermometry data (Tre, Tau, Tsk, and Tb), seven 1-min averages from five time points (minutes 9–15, 17–23, 32–38, 47–53, and 69–75), corresponding with the 7 min prior to the first ingestion during exercise, the 7 min following each fluid ingestion during exercise, and the final 7 min of exercise, were analyzed. Thermometry and LSR data during the first and last time points were analyzed using a one-way repeated measures ANOVA employing the independent variable of fluid temperature. For the three time points directly following ingestion, thermometry data were analyzed using a two-way repeated measures ANOVA employing the independent variables of exercise time and fluid temperature, whereas the LSR data were analyzed using a three-way repeated measures ANOVA employing the independent variables of time point, fluid temperature, and measurement site. In study 2, the NG and SW trials were analyzed separately. As a secondary analysis for the data in study 1, the change in LSR after each ingestion for the next 15 min (from the 1-min average before each ingestion) was calculated separately for the 1.5°C, 37°C, and 50°C fluid trials; and the values from the 37°C trial were subsequently subtracted from the values from the 1.5°C and 50°C trials, thus isolating the independent influence of fluid temperature on changes in LSR. These data were assessed using a three-way repeated measures ANOVA employing the independent variables of postingestion time (0 to 15 min), bolus number (1st, 2nd, 3rd, and 4th), and fluid temperature (warm and cold). In both study 1 and study 2, WBSL was analyzed using a one-way repeated measures ANOVA employing the independent variable of fluid temperature. The effect size of each ANOVA was calculated and reported as partial eta-squared values (η2).
When significant main effects or interactions were found, independent differences were assessed using independent Student's t-tests while maintaining a fixed probability (5%) of making a type I error by using a Holm-Bonferroni correction. The effect size of each t-test was calculated and reported as Cohen's d (d). All analyses were performed using the statistical software package SPSS 21.0 for Windows (SPSS, Chicago, IL).
RESULTS
Study 1
WBSL in the 37°C fluid trial was 767 ± 113 g. In comparison, the WBSL of 671 ± 89 g with 1.5°C fluid ingestion was lower (P = 0.001, d = 0.85), and the WBSL of 815 ± 121 g with 50°C fluid ingestion was greater (P = 0.001, d = 0.42).
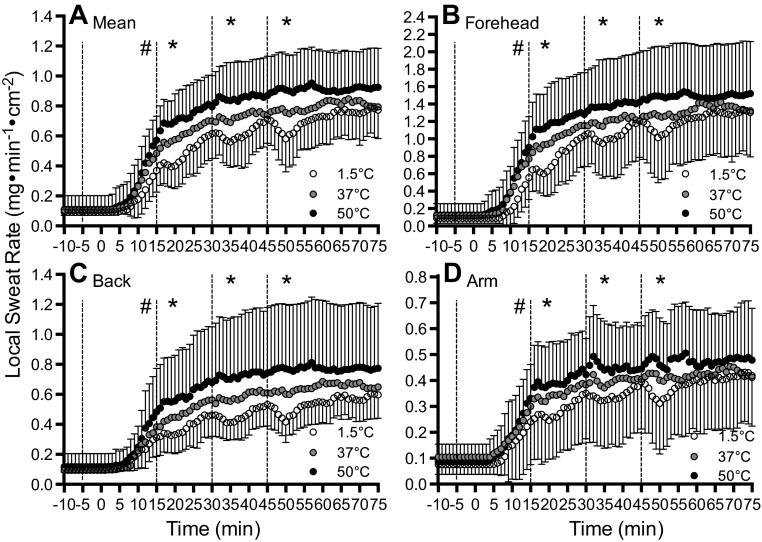
The absolute LSR data with the serial ingestion of 1.5°C, 37°C, and 50°C fluid during exercise are illustrated in Fig. 1. After 15 min of exercise, which followed the preexercise fluid ingestion but preceded the first fluid ingestion during exercise, LSR was lower by 0.15 ± 0.12 mg·min−1·cm−2 with 1.5°C fluid compared with 50°C fluid (P = 0.001, d = 1.07) at all LSR measurement sites. Following the first, second, and third fluid ingestion during exercise (i.e., after 15, 30, and 45 min of exercise, respectively), a fluid temperature-dependent change in LSR was observed (P < 0.001, η2 = 0.235). Specifically, the mean decrease in LSR relative to 37°C fluid ingestion following 1.5°C ingestion was 0.14 ± 0.13 mg·min−1·cm−2 (P = 0.002, d = 0.692) and a mean increase following 50°C fluid ingestion was 0.13 ± 0.18 mg·min−1·cm−2 (P = 0.016, d = 0.576). This influence of fluid temperature was the same for all three fluid ingestions during exercise (P = 0.364, η2 = 0.020) and was not different between LSR measurement sites (P = 0.298, η2 = 0.012). After 75 min of exercise, which was 30 min after the last fluid ingestion, absolute LSR was not different between fluid temperatures (P = 0.251, η2 = 0.014) irrespective of measurement site (P = 0.498, η2 = 0.002).
Fig. 1.
Mean local sweat rate (LSR) after the ingestion of 1.5°C (open circles), 37°C (gray circles), and 50°C (black circles) fluid before and during exercise. Dashed lines denote when fluids were ingested. Values given are the grand mean (A) of the following three locations: forehead (B), upper back (C), and forearm (D). *Where 1.5°C < 37°C < 50°C. #Where 1.5°C < 50°C (P < 0.05). Error bars indicate SD.
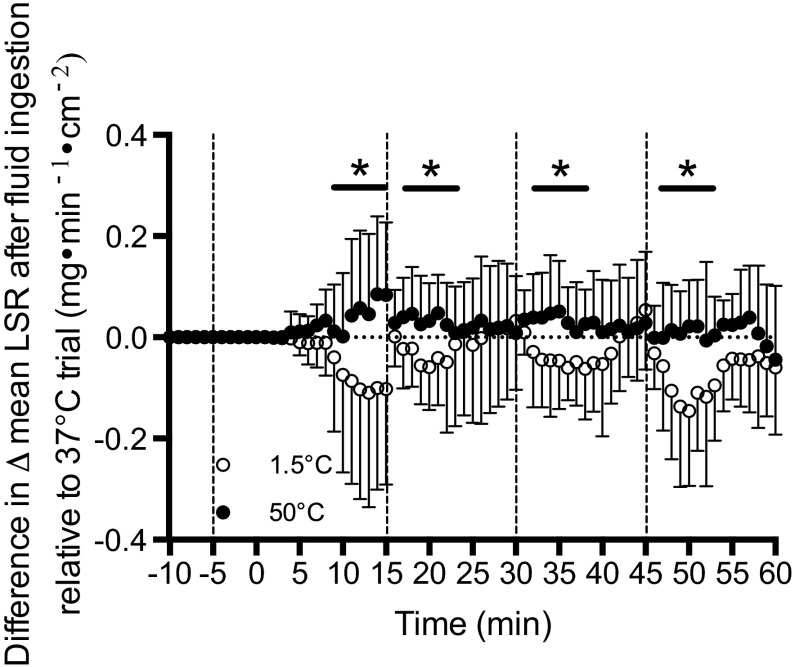
After standardizing LSR in the 1.5°C and 50°C fluids to that of the 37°C fluid trial, a significant main effect of fluid temperature on change in LSR after ingestion was found (P = 0.007, η2 = 0.123). Specifically, LSR in the 1.5°C fluid trial was significantly lower than LSR in the 50°C fluid trial 2 min after ingestion, and remained so for 7 min afterward (Fig. 2).
Fig. 2.
The mean change in LSR following the ingestion of 1.5°C (open circles) and 50°C (black circles) fluid relative to any changes in mean LSR observed during the thermoneutral 37°C fluid control trial. *Time points that 1.5°C < 50°C. Error bars indicate SD. Dashed lines denote when fluids were ingested.
Despite the observed differences in LSR, Tre (P = 0.304, η2 = 0.016), Tau (P = 0.254, η2 = 0.028), and mean Tsk (P = 0.082, η2 = 0.043), were not different between fluid temperatures at any time throughout exercise. Likewise, mean body temperature using Tre (P = 0.879, η2 = 0.002) or Tau (P = 0.773, η2 = 0.028) (Fig. 3) as a representation of the body core, were similar between fluid temperatures throughout exercise.
Fig. 3.
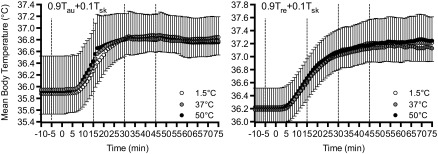
Mean body temperatures using a 0.9/0.1 weighting of core to skin temperatures using aural canal temperature (Tau) (left) and rectal temperature (Tre) (right) as an indication of the body core, following the ingestion of 1.5°C (open circles), 37°C (gray circles), and 50°C (black circles) fluid before and during exercise. Dashed lines denote when fluids were ingested. Error bars indicate SD.
Study 2
WBSL in the SW trials was similar (P = 0.444, d = 0.08) between 1.5°C fluid (693 ± 92 g) and 50°C fluid (685 ± 97 g). In the NG trials, WBSL was greater (P = 0.024, d = 1.18) with 50°C fluid (745 ± 106 g) compared with 1.5°C fluid (630 ± 89 g).
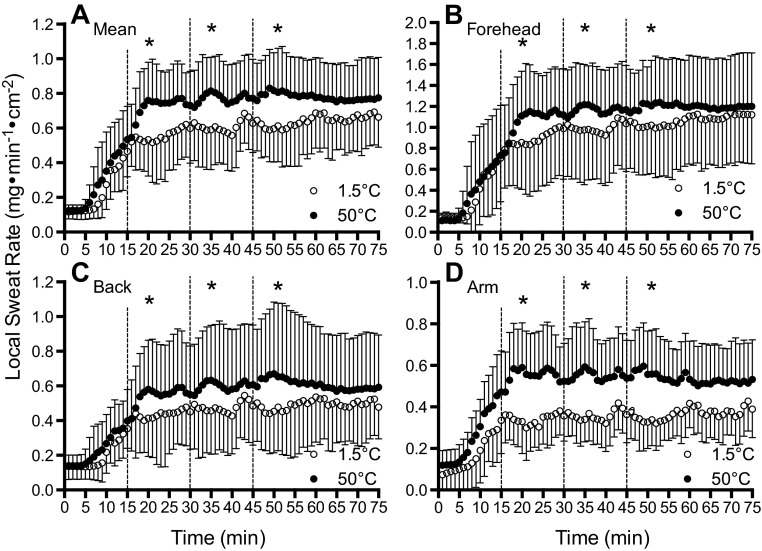
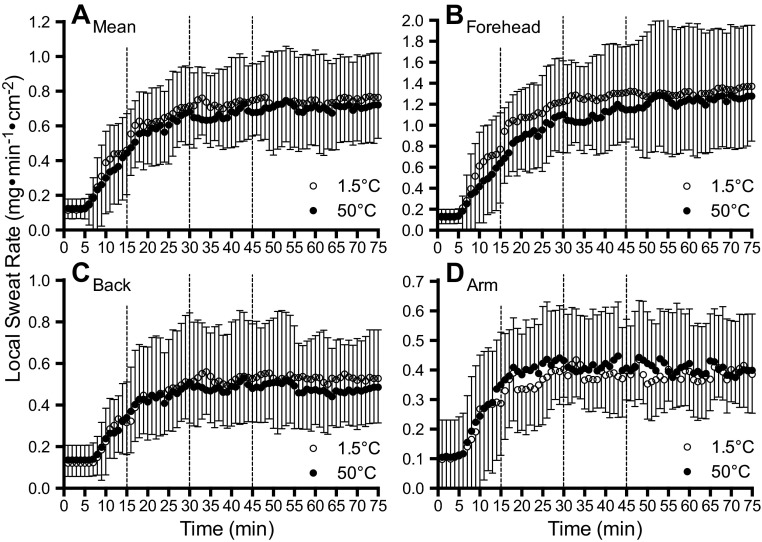
A comparison of LSR data between 1.5°C and 50°C fluid during NG and SW are illustrated in Figs. 4 and 5, respectively. In the SW trials, no differences were observed in LSR between 1.5°C and 50°C fluids at any point throughout exercise (P = 0.738, η2 = 0.003) irrespective of when the fluids were swilled (P = 0.668, η2 < 0.001) or LSR measurement site (P = 0.630, η2 < 0.001). On the other hand, when fluid was delivered directly into the stomach via nasogastric tube, lower LSR values were observed at all sites with 1.5°C fluid after the first, second, and third ingestion (P < 0.001, η2 = 0.131), which occurred after 15, 30, and 45 min of exercise, respectively. Specifically, the mean difference in LSR with 1.5°C and 50°C fluid ingestion was 0.20 ± 0.20 mg·min−1·cm−2 (P = 0.012, d = 0.264). There was no interaction between fluid temperature and fluid ingestion time (P = 0.573, η2 < 0.001) during exercise (i.e., after 15, 30, and 45 min of exercise), or an interaction between fluid temperature and LSR measurement site (i.e., forearm, upper back, or forehead) (P = 0.650, η2 < 0.001). After 75 min of exercise, which was 30 min after the last fluid ingestion, LSR was not different between 1.5°C and 50°C fluids at all measurement sites (P = 0.118, d = 0.138).
Fig. 4.
Absolute LSR after the ingestion of 1.5°C (open circles) and 50°C (black circles) fluid through a nasogastric tube (NG trials) during exercise. Dashed lines denote when fluids were ingested. Values given are the mean (A) of the following three locations: forehead (B), upper back (C), and forearm (D). #Where 1.5°C < 50°C. Error bars indicate SD.
Fig. 5.
Mean LSR after mouth-swilling (SW trials) 1.5°C (open circles) and 50°C (black circles) fluid during exercise. Dashed lines denote when mouth-swills were administered. Values given are the grand mean (A) of the following three locations: forehead (B), upper back (C), and forearm (D). Error bars indicate SD.
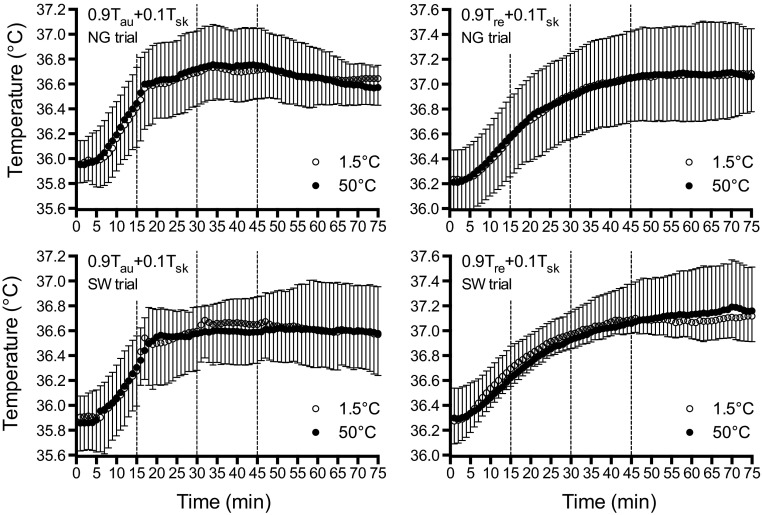
Rectal temperature (SW, P = 0.444, η2 = 0.005; NG, P = 0.561, η2 = 0.008), Tau (SW, P = 0.844, η2 = 0.018; NG, P = 0.737, η2 < 0.001), and Tsk (SW, P = 0.430, η2 = 0.015; NG, P = 0.598, η2 = 0.013) were similar between 1.5°C and 50°C fluids throughout exercise both during the SW and NG trials. Similarly, Tb using either Tre (SW, P = 0.471, η2 = 0.012; NG, P = 0.485, η2 = 0.013) or Tau (SW, P = 0.681, η2 = 0.020; NG, P = 0.612, η2 = 0.001) as a representation of the body core yielded similar values throughout exercise during both the NG and SW trials (Fig. 6).
Fig. 6.
Mean body temperatures using a 0.9/0.1 weighting of core to skin temperatures using Tau (left) and Tre (right) as an indication of the body core, with 1.5°C (open circles) and 50°C (black circles) fluid during the NG trials (top) and SW trials (bottom). Dashed lines denote when fluids were ingested/administered. Error bars indicate SD.
DISCUSSION
To the best of our knowledge, the present study is the first to demonstrate that transient fluid temperature-dependent changes in local sudomotor activity across the body surface occur immediately following the ingestion of fluids of different temperatures during exercise (Figs. 1 and 2). In parallel, no differences in core or skin temperatures were observed between the three fluid temperatures (Fig. 3), suggesting that thermoreceptors residing somewhere along the gastrointestinal tract were likely responsible for independently modifying sudomotor activity. In study 2, the potential location of these thermoreceptors was investigated by administering warm (50°C) and cold (1.5°C) fluid either into the mouth area only (SW trials), or directly into the stomach area bypassing the mouth and esophagus using an NG tube (NG trials). Fluid temperature-dependent differences in LSR were observed in the NG trials (Fig. 4), but similar sweat rates were found between 1.5°C and 50°C fluids in the SW trials (Fig. 5), again despite no differences in core and skin temperatures between 1.5°C and 50°C fluid trials in the NG or SW trials (Fig. 6). The findings of the second study suggest that thermoreceptors residing in the abdominal area, but not the mouth, can independently alter sudomotor output immediately following fluid ingestion.
The different LSR values observed between fluid temperatures in both studies are clear evidence of a thermally mediated response. In study 1, temperature-dependent deviations in local sweating were evident in that changes in LSR were minimal with the ingestion of a thermoneutral (37°C) fluid, moderate with ingestion of warm (50°C) fluids, and large with the ingestion of cold (1.5°C) fluids, even when accounting for any changes in LSR in the thermoneutral trial (Fig. 2). Although ingesting cold fluids resulted in an immediate and pronounced change in LSR with each aliquot, the bulk of the difference in LSR with warm fluid ingestion occurred early on, and subsequent aliquots moderately increased and sustained the elevated sweating rate. Nonthermal modifiers of the central drive for sweating have been previously shown with fluid ingestion. The act of drinking temporarily inhibits the osmoregulatory inhibition of sudomotor activity in dehydrated subjects, with the ingestion of a small (∼4 ml/kg of body wt) aliquot of 38°C fluid eliciting an immediate rise in sweating (12, 22, 25). However, when subjects are euhydrated or isoosmotic, this nonthermal reflex sweating response is abolished (25). In the present study, euhydration was verified prior to the commencement of all trials, and large differences in LSR were observed between 1.5°C and 50°C fluids when the fluid bypassed the mouth altogether in the NG trials.
The sweating response was similar on the torso (upper back), a peripheral limb (forearm), and the head (forehead), indicating that the modification of sudomotor activity with fluid temperature appears to be a systemic rather than a localized response. Additionally, large differences in WBSL were observed between fluid temperatures. From study 1, a residual effect of fluid temperature on absolute LSR values was evident due to the preexercise ingestion of 1.5°C and 50°C fluid, and successive ingestions during exercise separated by 15 min. Moreover, when accounting for any differences in absolute LSR prior to each ingestion, significant suppressions and elevations in sudomotor activity were observed with 1.5°C and 50°C fluids, respectively, relative to 37°C fluid (Fig. 2), thereby demonstrating an observed response to each individual ingestion. To remove any potential confounding effect of the preexercise fluid ingestion in study 1, there was no fluid ingestion before exercise in study 2. LSR values were almost identical prior to the first fluid ingestion during exercise in both the SW and NG trials in study 2; however, following the first ingestion, significant fluid temperature-dependent differences in LSR were observed in the NG trials, similar to those observed in study 1.
In both study 1 and study 2, the observed differences in sudomotor activity between 1.5°C and 50°C fluids occurred without any parallel differences in core temperature measured in the rectum and aural canal, or mean Tsk measured at eight different sites. Both Tc measurement sites have purported limitations. Tre exhibits a temporal lag relative to pulmonary artery, esophageal, and aural canal temperature measurements (7, 23), however, no differences in Tre between fluid temperatures were observed following fluid ingestion at any time point. Additionally, Tau measurements have been criticized in the past for being overly influenced by ambient temperatures (28); however, these errors can be minimized if the probe is properly insulated (4, 7). Moreover, Tau measurements were validated in the present study prior to exercise by ensuring that values were equal to or greater than Tes (14).
Given the difficulty of directly measuring brain temperature in awake, exercising humans, we cannot be certain that undetected changes in hypothalamic temperature did not occur with the ingestion of cold and warm fluids in both study 1 and study 2. A particular concern may be that local heating or cooling of the hypothalamic region may occur when fluid is either held in the mouth or as it passes down the esophagus (via direct heat exchange with blood in the carotid arteries) (24). This notion, however, is not supported by the data in study 2. There were no fluid temperature-related differences in LSR in the SW trials, and when fluid was delivered directly to the stomach (thereby bypassing the mouth and esophagus) in the NG trials, large differences in LSR were observed between 1.5°C and 50°C fluids. It may be possible that if the water remained in the mouth for a longer duration, previously unstimulated oral thermoreceptors would have become active or sufficient heat transfer would have occurred with the surrounding tissues to affect hypothalamic temperature and, subsequently, alter LSR. However, these possibilities seem unlikely given that during pilot testing when 50°C and 1.5°C water remained in the mouth for as long as 2 min, no changes in LSR were observed.
Whereas it can be conclusively stated that the thermoreceptors responsible for the present sudomotor response are not located in the mouth, the precise location, or combined locations, is less certain. The NG tube employed in the NG trials was not perfectly insulated, and some minor heat transfer with the nasopharynx and esophagus probably occurred. The role of any thermoreceptors residing in the nasopharynx on the observed LSR response seems unlikely because similar changes in sweating were observed between the NG trials and study 1 when no stimulation of any nasopharyngeal thermoreceptors could have possibly occurred. Moreover, because the NG tube delivered fluids directly to the bottom of the esophagus, cooling/heating of any potential esophageal thermoreceptors would have been much less in the NG trials than with standard drinking in study 1. Therefore, the similar LSR responses between trials also do not seem to support the role of thermoreceptors in the esophagus. It follows that thermoreceptors residing in or around the stomach seem most likely responsible for the fluid temperature-dependent changes in sudomotor output.
A gastric tension thermoreflex caused by stimulation of hot and cold receptors in the stomach and small intestine has been demonstrated in humans (26). Similarly to the present study, the changes in gastric tension occurred within 2 min of thermal stimulation. Additionally, electrophysiological and immunohistochemical studies in cats and mice have demonstrated the existence of temperature-sensitive neurons in the stomach (6, 29). Whether the reflexes observed in the present study were due solely to stimulation of thermoreceptors in the stomach is unclear because thermoreceptors in the small intestine and abdominal wall but not the liver are known to elicit thermoeffector responses (i.e., panting and shivering) in sheep (21). Because the sudomotor response in the present study and the gastric tension reflex in the study by Villanova (26) appeared 2 min poststimulation, it is possible this was the time required for a sufficient transfer of heat from the stomach to influence thermoreceptors present in the abdominal wall and small intestine.
Perspectives
In a previous study by Nadel and colleagues (16), the ingestion of hot pudding and ice cream caused changes in LSR similar to those of the present study; however, changes in Tc occurred in most but not all conditions. The different observations between that study and the present study are likely due to the different magnitudes of heat load or debt imposed, which with 50°C fluid, was approximately one-seventh of the hot pudding trials of Nadel et al. (+13 vs. +88 kJ), and with 1.5°C fluid was about one-fifth of the ice cream trials (−38 vs. −195 kJ). Additionally, during warm pudding ingestion in a hot environment (i.e., 44°C) (16), a much greater increase in evaporative heat loss occurred relative to the internal heat load but with minimal changes in core temperature. This result is similar to an earlier study from our laboratory demonstrating a lower body heat storage with a 50°C fluid than with a 1.5°C fluid despite similar changes in Tc (2). These different changes in total body heat content between conditions despite similar core temperatures suggest modified levels of peripheral heat storage, and further highlight the limitations of using thermometry to estimate body heat storage during exercise (11).
From a practical standpoint, hydration status for a given ingested volume of fluid could potentially be better maintained with colder fluid temperatures. On an absolute scale, the difference in WBSL between 1.5°C and 37°C fluid ingestion was not large (∼100 ml). However, it is possible that under conditions that elicit greater WBSL along with low levels of evaporative efficiency (e.g., hot/humid conditions, higher intensity exercise, and/or high clothing insulation), a greater difference in WBSL between fluid temperature could be achieved because inefficient sweat losses (i.e., dripping sweat) may be reduced without altering whole-body evaporation. Additionally, if reductions in WBSL are proportional to the heat sink of the ingested fluid (2), greater absolute reductions in WBSL could potentially be accomplished through the ingestion of a fluid mixed with ice, because this would greatly expand the internal heat sink due to the enthalpy of fusion of ice (334 J/g). Future research however, is required to assess this notion. From a mechanistic point of view, further evidence of abdominal thermoreceptors independently modulating thermoeffector responses could be obtained by assessing physiological thermoregulatory responses following the ingestion of warm and cool fluids during exposure to the cold. Additionally, it is unknown whether this fluid temperature-dependent sudomotor reflex is altered following heat acclimation.
Limitations
Because participants were exercising, skin temperatures could not be fixed in either study. Although there were no differences in Tsk between trials in either study 1 or study 2, future studies should consider a passive heating protocol to clamp core and skin temperatures at different levels while fluids are delivered to the stomach at different rates. Furthermore, skin blood flow was not measured in the present study, so the potential influence of abdominal thermoreceptors upon vasomotor control also needs to be investigated.
An additional consideration is that fluids were ejected from the mouth after 1 min in the SW trials in study 2, whereas they remained in the stomach in study 1 and in the NG trials in study 2, thereby allowing for a larger net amount of heat transfer to occur. However, peak temperature stimulus/heat transfer would occur immediately upon fluid administration, and LSR changes in the in the NG trials were observed 1 min postingestion. Therefore, whereas leaving water in the stomach could have contributed to differences in LSR observed several minutes following fluid ingestion, it is unlikely that this contributed to the acute changes in LSR observed 1–2 min after ingestion. Nonetheless, future studies should use a protocol isolating the acute from the residual effect of fluid temperatures, by fixing the amount of time the water remains in the mouth and stomach.
Conclusion
The serial ingestion of 1.5°C and 50°C fluid elicited significant suppressions and elevations, respectively, in local sweat rate at all sites (i.e., forehead, forearm, and upper back) relative to a thermoneutral trial (37°C fluid ingestion) despite no differences in core and skin temperatures between fluid temperatures throughout. In a second study, these LSR responses following 1.5°C and 50°C fluid ingestion during exercise without any differences in Tc and Tsk were replicated when fluid was delivered directly to the stomach, bypassing the mouth and esophagus, using an NG tube. However, almost identical LSR responses were observed when 1.5°C and 50°C fluid was swilled in the mouth only. Collectively, these data suggest that thermoreceptors modulating sudomotor output during fluid ingestion independently of core and skin temperatures, probably reside in the abdominal area, but not the mouth. Such a mechanism may possibly protect against differences in thermal load arising from fluid ingestion that are not detected by hypothalamic or cutaneous thermoreceptors and additionally could help to preserve body-water stores when possible.
GRANTS
This research was supported by Natural Sciences and Engineering Research Council (NSERC) of Canada Discovery Grant 386143-2010 to O. Jay. N.B. Morris is supported by a University of Ottawa Master's Scholarship. A.R. Bain and M.N. Cramer are supported by an NSERC PhD Scholarship. M.N. Cramer is also supported by a University of Ottawa Excellence Scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.B.M., A.R.B., M.N.C., and O.J. conception and design of research; N.B.M., A.R.B., and M.N.C. performed experiments; N.B.M. and O.J. analyzed data; N.B.M., A.R.B., M.N.C., and O.J. interpreted results of experiments; N.B.M. and O.J. prepared figures; N.B.M. and O.J. drafted manuscript; N.B.M., A.R.B., M.N.C., and O.J. edited and revised manuscript; N.B.M., A.R.B., M.N.C., and O.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the participants who volunteered for the study. We also thank N. Lespérance, Z. Novak, and N. Ravanelli for their assistance during various stages of data collection.
REFERENCES
- 1.American College of Sports Medicine Sawka MN, Burke LM, Eichner ER, Maughan RJ, Montain SJ, Stachenfeld NS. American College of Sports Medicine position stand. Exercise and fluid replacement. Med Sci Sports Exerc 39: 377–390, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bain AR, Lesperance NC, Jay O. Body heat storage during physical activity is lower with hot fluid ingestion under conditions that permit full evaporation. Acta Physiol (Oxf) 206: 98–108, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Benzinger TH. On physical heat regulation and the sense of temperature in man. Proc Natl Acad Sci USA 45: 645–659, 1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinnel H, Cabanac M. Tympanic temperature is a core temperature in humans. J Therm Biol 14: 47–53, 1989 [Google Scholar]
- 5.Canadian Society for Exercise Physiology. Canadian Society for Exercise Physiology: Certified Fitness Appraiser Resource Manual. Canadian Society for Exercise Physiology, Ottawa, 1986 [Google Scholar]
- 6.El Ouazzani T, Mei N. Electrophysiologic properties and role of the vagal thermoreceptors of lower esophagus and stomach of cat. Gastroenterology 83: 995–1001, 1982 [PubMed] [Google Scholar]
- 7.Gagnon D, Lemire BB, Jay O, Kenny GP. Aural canal, esophageal, and rectal temperatures during exertional heat stress and the subsequent recovery period. J Athl Train 45: 157–163, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gisolfi CV, Wenger CB. Temperature regulation during exercise: old concepts, new ideas. Exerc Sport Sci Rev 12: 339–372, 1984 [PubMed] [Google Scholar]
- 9.Gupta BN, Nier K, Hensel H. Cold-sensitive afferents from the abdomen. Pflugers Arch 380: 203–204, 1979 [DOI] [PubMed] [Google Scholar]
- 10.International Organization for Standardization. ISO 9886:1992. Evaluation of thermal strain by physiological measurements. Geneva, International Organization for Standardization, 1992 [Google Scholar]
- 11.Jay O, Reardon FD, Webb P, Ducharme MB, Ramsay T, Nettlefold L, Kenny GP. Estimating changes in mean body temperature for humans during exercise using core and skin temperatures is inaccurate even with a correction factor. J Appl Physiol 103: 443–451, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Kamijo YI, Okumoto T, Takeno Y, Okazaki K, Inaki M, Masuki S, Nose H. Transient cutaneous vasodilatation and hypotension after drinking in dehydrated and exercising men. J Physiol (Lond) 568: 689–698, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JK, Maughan RJ, Shirreffs SM. The influence of serial feeding of drinks at different temperatures on thermoregulatory responses during cycling. J Sports Sci 26: 583–590, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Mariak Z, Lewko J, Luczaj J, Polocki B, White MD. The relationship between directly measured human cerebral and tympanic temperatures during changes in brain temperatures. Eur J Appl Physiol 69: 545–549, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Nadel ER, Bullard RW, Stolwijk JA. Importance of skin temperature in the regulation of sweating. J Appl Physiol 31: 80–87, 1971 [DOI] [PubMed] [Google Scholar]
- 16.Nadel ER, Horvath SM, Dawson CA, Tucker A. Sensitivity to central and peripheral thermal stimulation in man. J Appl Physiol 29: 603–609, 1970 [DOI] [PubMed] [Google Scholar]
- 17.Nadel ER, Mitchell JW, Saltin B, Stolwijk JA. Peripheral modifications to the central drive for sweating. J Appl Physiol 31: 828–833, 1971 [DOI] [PubMed] [Google Scholar]
- 18.Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol 301: R1207–R1228, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Poulos DA, Lende RA. Response of trigeminal ganglion neurons to thermal stimulation of oral-facial regions. I. Steady-state response. J Neurophysiol 33: 508–517, 1970 [DOI] [PubMed] [Google Scholar]
- 20.Poulos DA, Lende RA. Response of trigeminal ganglion neurons to thermal stimulation of oral-facial regions. II. Temperature change response. J Neurophysiol 33: 518–526, 1970 [DOI] [PubMed] [Google Scholar]
- 21.Rawson RO, Quick KP. Localization of intra-abdominal thermoreceptors in the ewe. J Physiol (Lond) 222: 665–667, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senay LC, Jr, Christensen ML. Cardiovascular and sweating responses to water ingestion during dehydration. J Appl Physiol 20: 975–979, 1965 [DOI] [PubMed] [Google Scholar]
- 23.Shiraki K, Konda N, Sagawa S. Esophageal and tympanic temperature responses to core blood temperature changes during hyperthermia. J Appl Physiol 61: 98–102, 1986 [DOI] [PubMed] [Google Scholar]
- 24.Siegel R, Laursen PB. Keeping your cool: possible mechanisms for enhanced exercise performance in the heat with internal cooling methods. Sports Med 42: 89–98, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Takamata A, Mack GW, Gillen CM, Jozsi AC, Nadel ER. Osmoregulatory modulation of thermal sweating in humans: reflex effects of drinking. Am J Physiol Regul Integr Comp Physiol 268: R414–R422, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Villanova N, Azpiroz F, Malagelada JR. Perception and gut reflexes induced by stimulation of gastrointestinal thermoreceptors in humans. J Physiol (Lond) 502, Pt 1: 215–222, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wimer GS, Lamb DR, Sherman WM, Swanson SC. Temperature of ingested water and thermoregulation during moderate-intensity exercise. Can J Appl Physiol 22: 479–493, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Zehner WJ, Terndrup TE. The impact of moderate ambient temperature variance on the relationship between oral, rectal, and tympanic membrane temperatures. Clin Pediatr 30: 61–64, 1991 [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Jones S, Brody K, Costa M, Brookes SJ. Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse. Am J Physiol Gastrointest Liver Physiol 286: G983–G991, 2004 [DOI] [PubMed] [Google Scholar]