Abstract
Background
Hematologic, gastrointestinal, and neurologic complications are common side effects of the platinum/taxane based chemotherapy used in the primary treatment of epithelial ovarian cancer (EOC). While these are well-documented side effects, the effect of the resultant chemotherapy delay or dose reduction on disease free interval has never been studied. The goal of this study was to determine the effect of chemotherapy delays and dose reductions on progression free survival (PFS) and overall survival (OS).
Methods
A retrospective chart review of patients with primary epithelial ovarian, peritoneal, and fallopian tube carcinoma treated between 1/2000-12/2007 was performed. Inclusion criteria were comprehensive surgical staging, advanced stage disease (stage III or IV), and first line chemotherapy with a platinum/taxane-containing regimen. Cox proportional hazard models were used to determine the effect of primary chemotherapy reductions and delays on PFS and OS.
Results
One hundred and fifty eight patients met the inclusion criteria. Patients were divided into four groups: no delays or reductions (48%), delay only (27%), reduction only (8%), and both delay and reduction (18%). The mean number of delays/reductions per patient was 1.1 (range=0-5) and therapy was delayed a mean of 8 days. The most common reasons for delays/reductions were neutropenia (n=53), thrombocytopenia (n=46), and neuropathy (n=20). There were no differences detected in PFS or OS between groups.
Conclusions
There was no difference detected in survival between patients who required dose adjustments and treatment delays and those who did not. The lack of association between survival and chemotherapy alterations suggests that in specific circumstances patients with advanced ovarian cancer should have individualized treatment plans.
Introduction
Though ovarian cancer accounts for only 3% of all cancer cases in women in the United States, it accounts for 5% of all cancer deaths [1,2]. Due to its insidious onset, over 60% of cases will be diagnosed in advanced stages. Over the past several decades, the cornerstone of management for advanced EOC consists of maximal surgical cytoreduction followed by platinum and taxane based chemotherapeutic regimen [3,4]. Advancements in the treatment of epithelial ovarian cancer (EOC), in both surgery and chemotherapy, have resulted in 65% of patients achieving a response to primary treatment [5]. Unfortunately, the majority of patients will develop a recurrence and ultimately, succumb to their disease [2,3,5].
In regards to recurrence and survival, the association between disease related factors such as stage, histology, and grade has been well established [2,8]. Additionally, the amount of residual disease following surgery has been inversely correlated with survival outcomes [6]. Though the selection of chemotherapy agents has a role in regards to progression free and overall survival, the effect of treatment modifications, such as treatment delays and dose reductions has not been evaluated in ovarian cancer patients [7,8]. Therefore, the purpose of this study was to evaluate the effects of treatment modifications on survival outcomes in women with advanced ovarian cancer.
Methods
Approval to conduct this study was obtained from the Ohio State University Medical Center (OSUMC) institutional review board. All patients with pathologically confirmed stage III or IV epithelial ovarian, fallopian tube, and primary peritoneal cancer (hereinafter referred to as EOC) undergoing primary surgery at the OSUMC between January 2000 and December 2007 were retrospectively identified from tumor registries and patient databases. Patients were required to have primary cytoreductive surgery, high-grade carcinoma, and have received first-line chemotherapy with a platinum/taxane-based regimen. Patients were excluded from the study for the following: non-epithelial histology, low-grade or borderline disease, administration of neoadjuvant chemotherapy, synchronous primary tumors, or if they received chemotherapy at an outside institution.
Individual subject data were collected retrospectively from patient medical records. The demographic information collected included the following: age at diagnosis, race, height and weight. Surgical and pathology data obtained included date of surgery, FIGO stage, tumor histology and grade, and debulking status (no residual, optimal—defined as ≤1 cm of residual disease, and suboptimal—defined as >1 cm residual disease). Information related to chemotherapy included the chemotherapy agents administered, recommended schedule and dosages of chemotherapy, number of cycles administered, and route of administration. If applicable, additional data abstracted included the reason for the delay, the number of days delayed, the need for dose reduction, the indication for dose modification, and the use of granulocyte colony stimulating factors. Date of progression (defined as increased disease on physical exam, increased disease on imaging for which Response Evaluation Criteria In Solid Tumors was met, or doubling of Ca-125), platinum response status (platinum refractory-progression during primary therapy, platinum resistant—recurrence within 6 months of completion of primary therapy and platinum sensitive—recurrence ≥ 6 months), and date of last follow-up and date of death were also collected.
Statistical analysis
Time to progression was defined as the time from surgery until the date of noted progression. Patients with no recorded progression were censored at the date of their last known follow up. Overall survival was calculated as the time from surgery until the date of death, with patients still alive censored on the date of last follow up. Crude estimates of survival times were calculated using the method of Kaplan and Meier and Cox proportional hazards models were used to estimate hazard ratios and to adjust for confounders [9. 10]. The overall adjusted effect of delays and dose modifications on PFS and OS were made through a likelihood ratio (LR) test, which compared nested models with and without treatment delay and reductions. Model assumptions, including proportional hazards and covariate functional form were reviewed for all final models. Overall comparisons of patient characteristics between dose delay/reductions groups were compared via Fisher’s exact test for categorical variables and by ANOVA for continuous covariates. All p-values presented are two-sided. Analyses were carried out in Stata (version 10.1, StataCorp, College Station, TX).
Results
One hundred and ninety-eight patients met the initial inclusion criteria, of which 193 had information on dose delays and reductions. Of these, 35 patients received intraperitoneal chemotherapy as part of their first line treatment and these patients were excluded from further analysis since toxicities are known to be more severe in this group. Seventy-five patients (48%) experienced no dose delay or reductions, 42 patients (27%) had a delay (schedule modification), 12 patients (8%) had a dose reduction (dose modification), and 29 patients (18%) had both schedule and dose modifications. Demographic factors were similar among the four treatment delay/reduction groups, with the exception of patient’s baseline BMI (post surgery) and age. Patients without delays or reductions had significantly higher average BMIs compared to those with both delays and reductions (3.0 BMI units heavier on average (95% CI: 0.1-5.8). Similarly, patients without delays or reductions were 5.6 years younger on average (95% CI: 0.7-10.4 years) as compared to those with both delays and reductions. (Table 1).
Table 1.
Demographics
| Factor | No Delays or Reductions (N=75) |
Delay Only (N=42) |
Reduction Only (N=12) |
Both Delay and Reduction (N=29) |
|---|---|---|---|---|
| Age, Mean (range) |
57 (27-76) |
58 (22-75) |
60 (42-81) |
62 (40-86) |
|
| ||||
| Race | ||||
| Caucasian (%) | 71(95%) | 37(88%) | 10(91%) | 28(97%) |
| AA (%) | 4(5%) | 5(12%) | 1(9%) | 1(3%) |
|
| ||||
| BMI, Mean (range) |
28 (18-49) |
25 (16-44) |
28 (17-41) |
25 (19-34) |
|
| ||||
| Stage | ||||
| III | 69(92%) | 35(83%) | 10(83%) | 24(83%) |
| IV | 6(8%) | 7(17%) | 2(17%) | 5(17%) |
|
| ||||
| Tumor Grade | ||||
| Grade 1 | 0 (0%) | 1(2%) | 0(0%) | 1(3%) |
| Grade 2 | 6(8%) | 6(14%) | 3(25%) | 2(7%) |
| Grade 3 | 66(88%) | 30 (71%) | 8(67%) | 23(79%) |
| Unknown | 3(4%) | 5(12%) | 1(8%) | 3(7%) |
|
| ||||
| Histology | ||||
| Serous | 66(88%) | 38(90%) | 9(75%) | 28(97%) |
| Mucinous | 0(0%) | 1(2%) | 0(0%) | 0(0%) |
| Endometroid | 3(4%) | 1(2%) | 3(25%) | 0(0%) |
| Clear Cell | 0(0%) | 0(0%) | 0(0%) | 0(0%) |
| Mixed | 6(8%) | 2(5%) | 0(0%) | 1(3%) |
|
| ||||
| Optimal Debulking (%) |
49 (65%) |
23 (55%) |
9 (75%) |
19 (66%) |
|
| ||||
| Platinum Sensitive (%) |
51 (68%) |
22 (52%) |
8 (67%) |
20 (69%) |
In regards to surgical outcomes, optimal cytoreduction was achieved in 100 patients in total and was comparable in each group (Fisher’s exact test p-value=0.56). First line chemotherapy consisted of a platinum/taxane regimen for all patients. Platinum sensitivity was similar between patient subgroups, with rates ranging from 52-69%. In contrast, 65% of patients who underwent suboptimal debulking were classified as platinum refractory or resistant compared to only 32% of patients who underwent optimal debulking and 24% who had no gross residual at completion of their primary cytoreductive surgery (Fisher’s exact test p-value=0.003).
In total, 83 (53%) patients experienced a schedule modification, dose modification, or both during primary treatment. The mean number of modifications (either schedule or dose) per patient was 1.1 (standard deviation (sd): 1.2, range: 0-5). Of the 71 patients who experienced a schedule modification, 40 patients (56%) had a delay between 3 to 14 days and 31 patients (42%) had a delay greater than 14 days. In this group of patients, the mean number of days delayed was 8.2 (sd =13.6, range: 0-98). The most common reasons for delays or reductions from most to least frequent were neutropenia (n=53), thrombocytopenia (n=46), neuropathy (n=20), gastrointestinal symptoms (n=11), patient request (n=11), fatigue (n=10), anemia (n=7), infection (n=5), performance status (n=4), venous thromboembolic events (n=2), decreased and unanticipated surgery (n=2)(Figure 2). Nineteen patients received granulocyte colony stimulating factor as treatment for their neutropenia.
Figure 2.
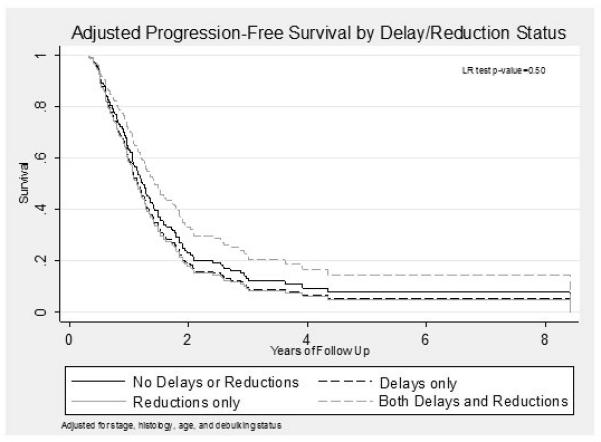
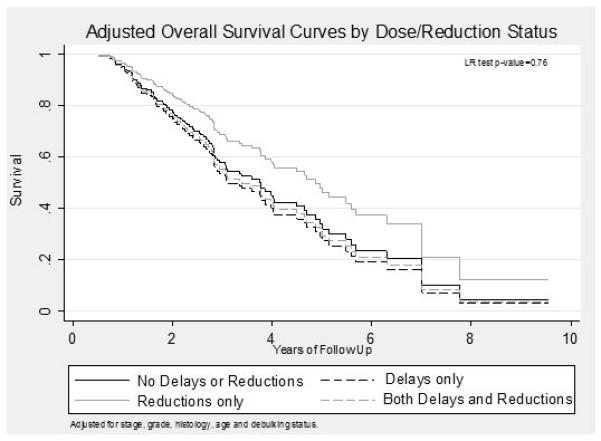
Crude and adjusted progression free and overall survival times across all groups were comparable (Table 2). Adjusted Kaplan-Meier survival curves for both progression-free and overall survival are shown in Figures 2 and 3, respectively. There were no statistically significant differences between the progression free and overall survival between the four groups (p=0.50 and p=0.76 respectively).
Table 2.
Unadjusted Median Recurrence and Survival times (95% CI)
| Group | N | PFS (months) | OS (months) |
|---|---|---|---|
|
No Delays or
Reduction |
95 | 16.0 (12.7-19.0) | 45.3 (34.2-59.7) |
| Delay Only | 42 | 12.7 (11.0-14.5) | 33.9 (25.2-56.0) |
|
Reduction
Only |
12 | 13.8 (5.3-22.2) | 60.3 (11.3 - ) |
|
Both Delay
and Reduction |
29 | 18.9 (11.6-29.0) | 43.1 (30.4-59.3) |
Figure 3.
Conclusions
The optimal chemotherapeutic agents and route of administration of chemotherapy for the treatment of EOC has been the subject of many retrospective and prospective studies over the last decade. While the standard of care continues to be surgical cytoreduction followed by platinum/taxane-based chemotherapy, few studies have looked at the impact of time to completion of chemotherapy after surgical staging in ovarian cancer [2-8, 11-12]. To our knowledge, this is the first study analyzing the association between adjustments in the dose or schedule of primary chemotherapy as related to PFS and OS in advanced stage EOC. Although there was an increased risk of disease progression in patients with delays only (without reductions) compared to those patients without delays or reductions in the unadjusted analysis (HR=1.4, 95% CI: 0.9-2.1), this finding was not significant after correction for stage, grade, histology, age and debulking status (HR=1.1, 95% CI: 0.7-1.8). Ultimately, we did not find a difference in PFS or OS between groups who had chemotherapy delays, reductions in dosage, or both delays and dose reductions when compared to those who had none.
Time to completion of therapy has been shown to have an impact on survival in patients undergoing radiation therapy, yet this relationship is less well-defined in patients receiving chemotherapy (13,14). Though no studies have been performed in patients with EOC, decreased survival has been documented in breast cancer patients who experience delays and dose reductions in chemotherapeutic regimens [15-18]. In 1994, the Cancer and Leukemia Group B (CALGB) conducted a randomized trial in women with locally advanced breast cancer, which showed that patients who received lower dosages and had a longer interval between cycles had a significantly worse outcome [15]. Another study in breast cancer patients showed that patients who were unable to complete 85% of their initial calculated dose did not appear to gain any benefit from adjuvant therapy [16]. Thus, the importance of adhering to recommended dosages and scheduling is apparent.
One of the most common reasons for chemotherapy dose reduction or delay is neutropenia. In a study of adjuvant chemotherapy in breast cancer, the overall rate of neutropenia was 29%, most of which resulted in a treatment delay [19]. Furthermore, patients who experienced one neutropenic event were also more likely to experience another and 17% of patients were unable to receive 85% of their scheduled chemotherapy dose intensity [19]. Our study showed similar results in that neutropenia was the most common reason for a dose reduction or delay (58%) in our patient population followed closely by thrombocytopenia and then gastrointestinal complications. Despite this similarity, our study demonstrates that even with these delays and reductions, there was no observed impact on progression free and overall survival in our patient population.
As a retrospective study we are limited by potential selection bias. While modeling attempts to adjust imbalances in characteristics between comparison groups on observed covariates, we do not know the impact of unobserved/measured variables. In addition, chemotherapy regimens, particularly after recurrence, varied and the impact of specific regimens on overall survival were not measurable. However, to our knowledge, the current study was the first study to evaluate dose modifications on survival in patients with advanced epithelial ovarian cancer. Further work is needed on a larger cohort to confirm these observational results.
In conclusion, our study did not demonstrate an association between progression free and overall survival related to modifications in primary chemotherapy regimens in patients with advanced stage EOC. Though treatment plans should be individualized, prospective studies are warranted to evaluate the impact of dose modifications and treatment delays on survival in ovarian cancer patients.
Figure 1.
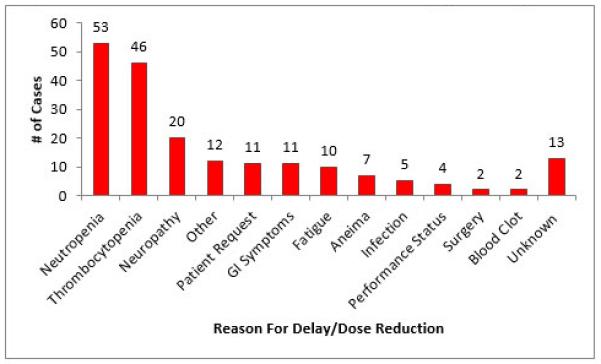
Reasons for Chemotherapy Delays and Dose
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Gerestein C, Eijkemans M, de Jong D, et al. The prediction of progression-free and overall survival in women with an advanced stage of epithelial ovarian carcinoma. BJOG. 2009;116(3):372–380. doi: 10.1111/j.1471-0528.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 3.Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review, 1975- 2006. National Cancer Institute; [Accessed August 10, 2009]. http://seer.cancer.gov/faststats/selections.php?series=cancer. [Google Scholar]
- 4.McGuire WP, Markman M. Primary ovarian cancer chemotherapy: current standards of care. British Journal of Cancer. 2003;89(suppl 3):S3–S8. doi: 10.1038/sj.bjc.6601494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–59. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 6.Omura GA, Brady MF, Homesly HD, et al. Long-term follow-up and prognostic factor analysis in advanced ovarian carcinoma: the Gynecologic Oncology group experience. J Clin Oncol. 1991;9(7):1138–50. doi: 10.1200/JCO.1991.9.7.1138. [DOI] [PubMed] [Google Scholar]
- 7.Fung-Kee-Fung M, Provencher D, Rosen B, et al. Intraperitoneal chemotherapy for patients with advanced ovarian cancer: A review of the evidence and standards for the delivery of care. Gynecol Oncol. 2007;105(3):747–756. doi: 10.1016/j.ygyno.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2003;21(17):3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53(282):457–481. [Google Scholar]
- 10.Cox DR. Regression Models and Life Tables. Journal of the Royal Statistical Society B. 1972;34(2):187–220. [Google Scholar]
- 11.Chi DS, Liao J, Leon LF, et al. Identification of prognostic factors in advanced epithelial ovarian carcinoma. Gynecol Oncol. 2001;82(3):532–7. doi: 10.1006/gyno.2001.6328. [DOI] [PubMed] [Google Scholar]
- 12.Muggia F, Braly P, Brady MF, et al. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2000;18(1):106–115. doi: 10.1200/JCO.2000.18.1.106. [DOI] [PubMed] [Google Scholar]
- 13.Perez CA, Grigsby PW, Castro-Vita H, Lockett MA. Carcinoma of the uterine cervix. I. Impact of prolongation of overall treatment time and timing of brachytherapy on outcome of radiation therapy. Int J Radiation Oncology Biol Phys. 1995;32(5):1275–1288. doi: 10.1016/0360-3016(95)00220-S. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Josef E, Moughan J, Ajani J, et al. Impact of overall treatment time on survival and local control in patients with anal cancer: A pooled data analysis of Radiation Therapy Oncology Group Trials. J Clin Oncol. 2010;28(34):5061–5066. doi: 10.1200/JCO.2010.29.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood WC, Budman DR, Korzun AH, et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med. 1994;330(18):1253–1259. doi: 10.1056/NEJM199405053301801. [DOI] [PubMed] [Google Scholar]
- 16.Bonadonna G, Valagussa B, Moliterni A, et al. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer. N Engl J Med. 1995;332(14):901–906. doi: 10.1056/NEJM199504063321401. [DOI] [PubMed] [Google Scholar]
- 17.Chang J. Chemotherapy dose reduction and delay in clinical practice: evaluating the risk to patient outcome in adjuvant chemotherapy for breast cancer. Eur J Cancer. 2000;36(S1):11–14. doi: 10.1016/s0959-8049(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 18.Leonard RC, Miles D, Thomas R, Nussey F. Impact of neutropenia on delivering planned adjuvant chemotherapy: UK audit of primary breast cancer patients. Br J Cancer. 2003;89(11):2062–8. doi: 10.1038/sj.bjc.6601279. [DOI] [PMC free article] [PubMed] [Google Scholar]


