Abstract
Scaffoldless engineered 3D skeletal muscle tissue created from satellite cells offers the potential to replace muscle tissue that is lost due to severe trauma or disease. Transforming growth factor-beta 1 (TGF-β1) plays a vital role in mediating migration and differentiation of satellite cells during the early stages of muscle development. Additionally, TGF-β1 promotes collagen type I synthesis in the extracellular matrix (ECM) of skeletal muscle, which provides a passive elastic substrate to support myofibres and facilitate the transmission of force. To determine the role of TGF-β1 in skeletal muscle construct formation and contractile function in vitro, we created tissue-engineered 3D skeletal muscle constructs with varying levels of recombinant TGF-β1 added to the cell culture medium. Prior to the addition of TGF-β1, the primary cell population was composed of 75% Pax7-positive cells. The peak force for twitch, tetanus and spontaneous force were significantly increased in the presence of 2.0 ng/ml TGF-β1 when compared to 0, 0.5 and 1.0 ng/ml TGF-β1. Visualization of the cellular structure with H&E and with immunofluorescence staining for sarcomeric myosin heavy chains and collagen type I showed denser regions of better organized myofibres in the presence of 2.0 ng/ml TGF-β1 versus 0, 0.5 and 1.0 ng/ml. The addition of 2.0 ng/ml TGF-β1 to the culture medium of engineered 3D skeletal muscle constructs enhanced contractility and extracellular matrix organization.
Keywords: tissue engineering, engineered skeletal muscle, scaffoldless, transforming growth factor beta-1, extracellular matrix
1. Introduction
Engineered skeletal muscle has potential uses in muscle repair and replacement after surgery, acute injury and disease (Law et al., 1993; DiEdwardo et al., 1999; Fauza et al., 2001; Rossi et al., 2010). Specifically, conditions such as Duchenne’s muscular dystrophy (Law et al., 1993) and severe burns (Aviss et al., 2010) can cause muscle loss and could be treated with the implantation of engineered muscle. In this study, we (Baker et al., 2003; Larkin et al., 2006) successfully engineered scaffoldless 3D skeletal muscle constructs. Our 3D skeletal muscle constructs were composed of a heterogeneous population of at least two types of cells, which included muscle satellite cells and fibroblasts. Following isolation from soleus muscle, cells proliferated, produced extracellular matrix (ECM) and formed a monolayer. During exposure to differentiation conditions, the monolayer detached and self-assembled into a 3D construct around two constraint points. The transition from a 2D monolayer to a 3D construct provides a novel model for the study of skeletal muscle development in vitro.
Myogenesis involves the precise regulation of numerous developmental events including the migration and proliferation of myoblasts, the alignment of myotubes and the fusion of neighbouring myotubes (Miller et al., 1993; Buckingham, 1994). Though skeletal muscle development is not yet fully understood, a key growth factor involved in skeletal muscle development is transforming growth factor beta 1 (TGF-β1). The physiological effect of TGF-β1 is complex and dependent on the existing intracellular milieu of the cell (Kollias and McDermott, 2008). TGF-β1 activates both Smad2/3 and TAK1 MAPK signal transduction cascades (Macias-Silva et al., 1996; Zhang et al., 1996). During skeletal muscle development, TGF-β 1 inhibits the premature formation of myotubes in the embryonic limb (Kollias and McDermott, 2008) and mediates myogenic cell migration and transmit signals from the neural tube (Olguin et al., 2003; Stern et al., 1997). These studies suggest that inhibiting the premature formation of myotubes prevents overdevelopment of the 2D monolayer and enables a more plastic environment for the 3D development and organization of myotubes. If properly utilized in skeletal muscle tissue engineering applications, TGF-β1 could be a powerful tool for the control of myogenic cell differentiation.
Previous studies have shown that ECM is critical for skeletal muscle development (Buck and Horwitz, 1987), function (Huijing, 1999) and structure (Purslow, 2002). ECM is primarily composed of proteoglycans such as fibronectin and laminin, as well as fibrous proteins such as types I, I, and IV collagens (Beach et al., 1982; Kjaer et al., 2006; Miura et al., 2010). Additionally, ECM facilitates communication between cells and serves as a reservoir for the storage of growth factors (Chen et al., 2007). In skeletal muscle, ECM also provides a passive elastic substrate for muscle fibre contraction and force transmission between neighbouring muscle fibres, then from muscle fibres to the tendons, and eventually to bone (Lawson and Purslow, 2001). Studies indicate that TGF-β 1 enhances the production of collagen type I in the ECM of skeletal muscle both in vitro and in vivo (Roberts et al., 1986; Ignotz et al., 1987). Mechanistically, the activation of Smad3 via TGF-β1 is essential for the synthesis of ECM components and production of collagen type I (Schultze-Mosgau et al., 2004). However, excessive exposure to TGF-β1 can induce fibrosis in skeletal muscle (Leask and Abraham, 2004), which is likely due to overproduction of ECM (Stauber et al., 1996; Duncan et al., 1999). Furthermore, TGF-β1 increases fibroblast proliferation and production of ECM proteins in vivo (Robbins et al., 1997). Future tissue engineering applications involving TGF-β1 in a heterogeneous population of fibroblasts and skeletal muscle cells should carefully balance these effects on each cell type.
Since TGF-β1 is a potent regulator of skeletal muscle development and composition in vivo, an optimized application of TGF-β1 could enhance the function of skeletal muscle tissue engineered in vitro. The purpose of this study was to evaluate the effects of TGF-β1 on the contractile properties and structure of 3D-engineered skeletal muscle constructs. We hypothesized that the addition of recombinant TGF-β1 would increase ECM production and enhance the force-generating capacity of scaffoldless engineered 3D skeletal muscle constructs in vitro. To test our hypothesis, we added 0, 0.5, 1.0 and 2.0 ng/ml of recombinant TGF-β1 to the culture medium during fabrication of 3D skeletal muscle constructs and evaluated each for muscle contractility, muscle differentiation and ECM deposition.
2. Materials and Methods
2.1. Animal model and care
Cells for engineered muscle constructs were isolated from female Fischer 344 rats obtained from the Charles River Laboratories, Inc. (Wilmington, MA, USA). The animals were housed under SPF conditions and provided access to chow and water ad libidum. All surgical procedures were performed in an aseptic environment, with animals in a deep plane of anaesthesia induced by intraperitoneal injections of sodium pentobarbital (50 mg/kg). Supplemental doses of pentobarbital were administered as required to maintain adequate depth of anaesthesia. All animal care and surgeries were in accordance with the Guide for Care and Use of Laboratory Animals (Public Health Service, 19965, NIH Publication No. 85–23).
2.2. Preparation of cell culture medium
Growth medium (GM) for engineered muscle culture is composed of Ham’s F-12 (Gibco®, Rockville, MD, USA) supplemented with 20% fetal bovine serum (Gibco®) and 1% antibiotic-antimycotic (Gibco®). The differentiation medium (DM) consists of DMEM (Invitrogen, Carlsbad, CA, USA), 7% Horse Serum (Gibco®), and 1% antibiotic-antimycotic (Gibco®). Excluding the first feeding of DM, concentrations of 0, 0.5, 1.0 or 2.0 ng/ml recombinant human TGF-β1 (PeproTech, Rocky Hill, NJ, USA) and either 67 or 130 μg/ml L-ascorbic acid 2-phosphate (Sigma-Aldrich) were added to the medium. There were no discernable effects of ascorbic acid on the results at the concentrations. Cells cultured with TGF-β1 concentrations greater than 2.0 ng/ml failed to form functional constructs.
2.3. Preparation of culture dishes
3D skeletal muscle constructs were engineered in individual 35 mm plates. Each plate was coated with 2 ml of Sylgard® 184 (Dow Chemical Corporation, Midland, MI, USA) and allowed to cure for 3 weeks prior to use. At least 3 days before the introduction of cells, the plates were sanitized with 70% ethanol and rinsed with 3–4 ml Dulbecco’s Phosphate-Buffered Saline (DPBS) pH 7.2 (Gibco®). The plates were then left to stand for 5 minutes. After aspiration, each plate was covered with 2.0 μg/cm2 natural mouse laminin (Gibco®) in DPBS per plate and left to dry for 24 h. Salt crystals were dissolved and removed by rinsing the plates with 2–3 ml of DPBS. The plates were then filled with 2 ml of previously described GM and decontaminated with UV light (253.7 nm) for 60 min. Afterwards, the plates were stored in a 37°C 5% CO2 incubator for 2 days prior to plating primary muscle cells. Excess media was aspirated prior to use.
2.4. Primary muscle cell isolation
The soleus muscles of the anesthetized rats were surgically removed and placed into transfer medium of 2% antibiotic-antimycotic in DPBS. Using a single edged razor blade and forceps, connective tissue was removed from each muscle. The trimmed muscles were then briefly exposed to a solution of 70% ethanol and rinsed in DPBS. The muscles were further diced into small pieces. For every two muscles, the diced muscle was added to a 100-mm plate with 15 ml Ham’s F-12. The plates then were treated with UV Light (253.7 nm) for 15 min. The UV-treated muscles and HAM’S F-12 were then transferred to 20 ml of dissociation medium consisting of 0.05% Type IV Collagenase (Sigma-Aldrich), 0.4% Dispase (Sigma-Aldrich) and supplemented with Ham’s F-12 (Gibco®). The dissociated tissue was left in a 37°C water bath for 90 min and gently agitated every 15 min. Once large fragments of muscle and connective tissue were no longer visible, the solution was filtered through a 100-μm filter and centrifuged at 100 g and 25°C for 10 min. The pellet was resuspended in GM and incubated in a 100-mm plate for 15 min. A cell count of the suspension was taken using a hemocytometer and plated at an initial density of 20 000 cells/cm2.
2.5. Immunocytochemistry of primary muscle cell population
A subset of 4 separate primary muscle isolations were allowed to attach to 35 mm laminin-coated glass bottom dishes (MatTek, Ashland, MA, USA) for 16 h. These cells were then fixed using 100% acetone for 5 min. Following fixation, the cells were blocked (1% BSA + 1% FBS in PBS) and then labeled with primary IgG antibodies against PAX7 (rabbit monoclonal antibody, 1:1000, AbCam, Cambridge, MA, USA) in binding buffer (0.5% BSA + 0.5% FBS in PBS) for 1 h. For visualization, samples were incubated for 1 h with AlexaFluor-488 (donkey polyclonal antibody, 1:500, Invitrogen) conjugated secondary antibodies, respectively. DAPI was used for nuclear reference staining. Images were taken using a DMIRB inverted fluorescence microscope fitted with an Olympus DP-30 B&W camera and FITC/515-580 fluorescence filters. For each primary isolation, four separate 450 μm2 sections were examined. Image merging and analysis was performed in Photoshop CS3 (Adobe, Mountain View, CA, USA).
2.6. Formation of a 3D muscle construct
The primary cell cultures were incubated for 72 h prior to the first feeding to allow the satellite cells to adhere to the substrate. After 72 h, the non-adherent cells were removed by rinsing with DPBS. Every 2 days thereafter, the media on the plates was removed and subsequently replaced with fresh GM. The cell monolayer was visually inspected to determine when the cells were completely confluent. Monolayers typically became 100% confluent around 2 weeks after plating. At the point of confluence, GM was substituted for DM and was replaced every 2 days. TGF-β1 was added to the culture medium 48 h after the first switch of GM to DM and in every subsequent feeding. After 14 days of total culturing, two pieces of adult rat tail tendon of 5 mm length and 2 mm width were pinned to the plate approximately 12 mm apart using 5 mm steel minutien pins. Care was taken not to damage the monolayer while placing pins. Approximately 1 week later, contraction of the monolayer enabled detachment from the plate and the subsequent formation of a cylindrical construct around two anchor points (see Larkin et al., 2006).
2.7. 3D muscle construct contractility testing
Contractile properties were initially measured 7–10 days after the formation of a 3D construct as previously described (Larkin et al., 2006). One pin was released from the plate and subsequently attached to the force transducer via wax. The temperature of the construct was maintained at 37 ±1°C using a heated aluminum platform. The diameter of the construct was determined and used to calculate cross-sectional area, assuming a circular cross-section. Platinum wire electrodes were positioned on either side of the construct for field stimulation of the entire construct. Spontaneous force production was recorded in the absence of electrical stimulation. Twitches were elicited using a single 1.2 ms pulse at 2.5, 5, 10, 15 and 20 V. Maximum tetanic force was determined using a train lasting 1 s and consisting of 1.2 ms pulses at 10 V and 10, 20, 40, 60 and 80 Hz. Data files for each peak twitch force and peak tetanic force trace were recorded at 1 000 samples/second and stored for subsequent analysis using LabVIEW (National Instruments, Austin, TX, USA). Peak twitch and tetanic force were normalized using the cross-sectional area to determine maximum specific force.
2.8. Histochemical and immunohistochemical analysis of 3D muscle constructs
Unfixed muscle constructs were placed into TBS medium (Triangle Biological Sciences, Durham, NC, USA), frozen in cold isopentane and stored at −80°C until needed. Samples were sliced to obtain cross- and longitudinal sections with a cryostat at a thickness of approximately 12 μm, adhered to Superfrost Plus microscopy slides, and used for staining. Sections were stained for general morphology observations with hematoxylin and H&E as previously described (Luna, 1968). For immunohisto-chemical analysis, frozen sections were fixed with ice-cold methanol for 10 min and rinsed with Phosphate Buffered Saline (PBS). Sections were blocked for 30 min with PBS with 0.05% Tween-20 (PBST) containing 20% calf serum (PBST-S) at room temperature. Sections were incubated overnight at 4°C, with the primary antibodies diluted in PBST-S. Immunofluorescent staining with specific antibodies was performed to detect the presence of myosin heavy chain (MHC; MF-20 mouse monoclonal antibody, 1:5, obtained from the Developmental Studies Hybridoma Bank, Iowa City, IA, USA), alpha-actinin (mouse monoclonal antibody, 1:200 dilution, Sigma-Aldrich), nebulin (mouse monoclonal antibody, 1:100 dilution, AbCam), and collagen type I (rabbit polyclonal antibody, 5 μg/ml, Chemicon International, Temecula, CA, USA). Following 3 washes in PBST, 1 h room temperature incubation with Cy3-conjugated anti-mouse or anti-rabbit antibodies (Jackson ImmunoResearch Laboratory, West Grove, PA, USA) was used for visualization. Next, nuclei were stained by 5 min incubation with DAPI solution (Sigma-Aldrich) in PBST. The sections were examined and photographed with a Leica microscope.
2.9. Statistical analyses
Statistical analysis was performed using PASW Statistics 18 (IBM, Somers, NY, USA). Values are presented as mean standard error unless otherwise noted. A one-way analysis of variance was conducted to compare differences between constructs, and Fischer’s least significant difference post-hoc test was used for factor analysis. Differences were considered significant at p < 0.05. Contractile data were compiled and analyzed using MATLAB R2010a (Mathworks, Natick, MA, USA).
3. Results
3.1. Composition of initial primary cell culture
Four independent 450 μm2 sections from 4 separate primary cell isolations were immunostained for PAX7 (Figure 1, green). PAX7 was used as an early marker of myogenic satellite cell commitment (Seale et al., 2000). The primary cell isolations were composed of a heterogeneous population. Overall, 74.3% (standard deviation ±7.4%) of the total isolated cell population was positive for PAX7. The remaining cells were predominately fibroblasts.
Figure 1.
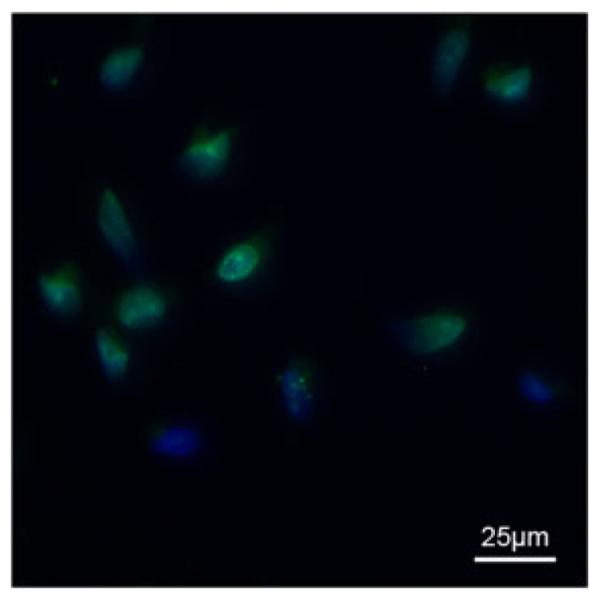
Immunocytochemical analysis of representative primary cell culture. Cells were taken directly from primary cell isolation and antibody labeled for PAX7 (green). DAPI (blue) was used to visualize cell nuclei. Four separate muscle isolations were performed and a representative image of those isolations is shown above
3.2. Culturing of 3D muscle constructs with TGF-β1
Muscle constructs cultured without supplemented TGF-β 1 were more likely to sporadically detach from the substrate prior to construct formation or break apart between the two tendon anchor points once 3D was achieved (data not shown). The addition of TGF-β1 concentrations > 2.0 ng/ml resulted in tendinous constructs that were deficient of contractile myofibres. Additionally, the correct timing of TGF-β1 supplementation was critical for correct formation of 3D-engineered muscle constructs (data not shown). The addition of TGF-β1 with the first application of DM was detrimental to engineered construct formation. For the methods used in this study, the addition of TGF-β1 during the second feeding permitted construct formation.
3.3. Contractile testing of 3D muscle constructs
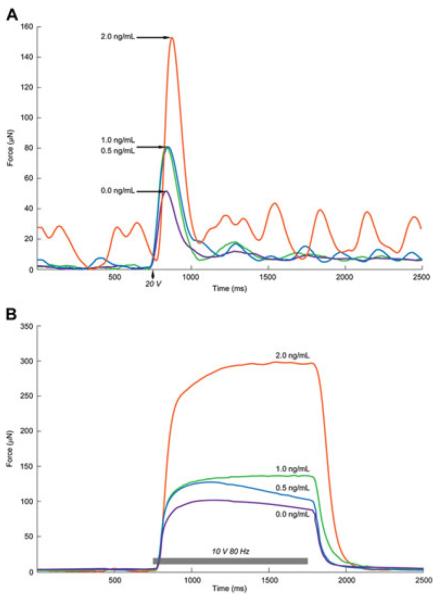
Representative recordings for twitch force (μN) and tetanus (μN) at different TGF-β1 concentrations are shown in Figure 2. At 2.0 ng/ml TGF-β1, the peak force for both twitch and tetanus were significantly increased. When electrical stimulus was not applied, the occurrence of strong spontaneous activity was observed in most functional constructs. There was also a significant increase in magnitude of spontaneous force (Ps) at 2.0 ng/ml TGF-β1 (Table 1).
Figure 2.
Representative twitch and tetanic force traces at different TGF-b1 concentrations. Representative twitch and tetanic force traces from 3D-engineered muscles demonstrate typical muscle mechanics. Constructs were exposed to 20 V twitch stimulus (A) and a 10 V 80 Hz tetanic stimulus (B). Lines represent engineered constructs cultured with TGF-β1 concentrations of 0 (purple line), 0.5 (blue line), 1.0 (green line), and 2.0 (orange line) ng/ml
Table 1.
Contractile properties of engineered constructs at different TGF-β1 concentrations. Values are means±SE; CSA, cross-sectional area; Ps, peak spontaneous force; Pt, peak twitch force; sPt, specific Pt; TTPT, time to peak twitch tension; 1/2RT, half-relaxation time; dP/dt, maximum rise in tension; %P/t, maximum rate of rise in tension; Po, peak tetanic force; sPo, specific Po
| [TGF-β1] (ng/ml) | 0 | 0.5 | 1.0 | 2.0 |
|---|---|---|---|---|
| Sample size, n | 9 | 10 | 12 | 13 |
| CSA, mm 2 | 0.14±0.03 | 0.18±0.03 | 0.12±0.02 | 0.28±0.05* |
| Ps, μN | 3.94±0.47 | 3.20±0.37 | 3.10±0.54 | 18.16±3.68* |
| Pt, μN | 61.70±11.92 | 53.92±8.25 | 74.47±5.53 | 144.82±20.33* |
| sPt, μN/mm 2 | 500±130 | 500±170 | 740±90 | 760±130 |
| TTPT, ms | 68.1±2.7 | 78.1±5.8 | 80.7±3.1 | 92.2±6.6* |
| 1/2RT, ms | 95.2±5.4 | 126.7±10.7* | 120.1±6.4* | 99.4±5.8 |
| dP/dt, μN/ms | 1.67±0.27 | 1.47±0.22 | 1.87±0.13 | 3.52±0.44* |
| %P/t, 1/ms | 2.84±0.11 | 2.78±0.13 | 2.53±0.07* | 2.49±0.08* |
| Po, μN | 111.93±18.82 | 105.09±13.42 | 128.50±9.33 | 290.68±38.20* |
| sPo, mN/mm 2 | 890±200 | 860±230 | 1230±120 | 1520±270* |
Significantly different from 0 ng/ml of TGF-β1 at P<0.05
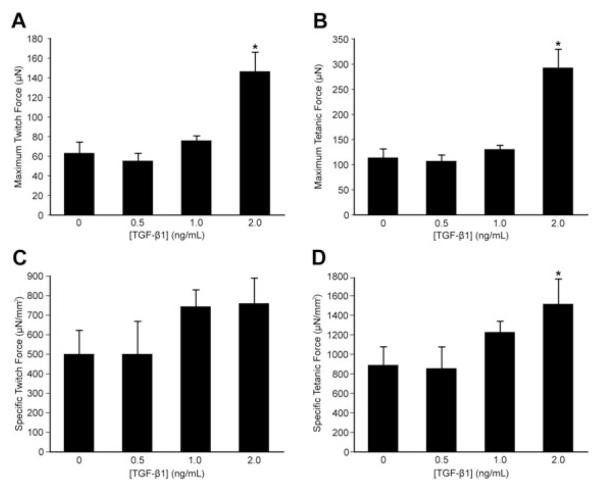
Shown in Table 1 are: the cross-sectional area (CSA), peak twitch force (Pt), specific twitch force (sPt), time to peak twitch tension (TTPT), half-relaxation time (1/2RT), maximum rise in tension (dP/dt), peak tetanic tension (Po), and specific tetanic tension (sPo) for each TGF-β1 concentration tested. A significant increase in CSA was observed at 2.0 ng/ml TGF-β1 (Table 1). Furthermore, a significant increase versus the control for Pt was observed at TGF-β1 concentrations of 2.0 ng/ml (Table 1, Figure 3A), which corresponds to an average increase of 135%. Similarly, Po was significantly greater than the control with the addition of 2.0 ng/ml GF-β1 (Table 1, Figure 3B), resulting in an average 160% increase in peak tetanic tension. Of the measures for specific force, sPo but not sPt was significant with the 2.0 ng/ml TGF-β1 (Table 1, Figures 3C, 3D). A significant increase versus control was observed for 1/2RT with the addition of 0.5 ng/ml and 1.0 ng/ml of TGF-β1 (Table 1). The measures for TTPT and dP/dt significantly increased with 2.0 ng/ml of TGF-β1 (Table 1). When dP/dt was normalized by TTPT, the maximum rate of rise of tension (%Po/t) was significantly decreased from that of the control at 1.0 and 2.0 ng/ml of TGF-β1 (Table 1). The addition of 2.0 ng/ml TGF-β1 significantly affected construct peak force of both twitch and tetanus stimuli, specific tetanic force, and cross-sectional area.
Figure 3.
Peak and specific forces for twitch and tetanic stimulations. (A) Maximum twitch force (Pt) and (B) maximum tetanic force (Po) for 0, 0.5, 1.0, and 2.0 ng/ml of TGF-β1. (C) Specific twitch force (sPt) and (D) specific tetanic force (sPo) for 0, 0.5, 1.0, and 2.0 ng/ml of TGF-β1. Values are means ± standard errors. *Significantly different from 0 ng/ml of TGF-b1 (p < 0.05)
3.4. Morphology of 3D muscle constructs
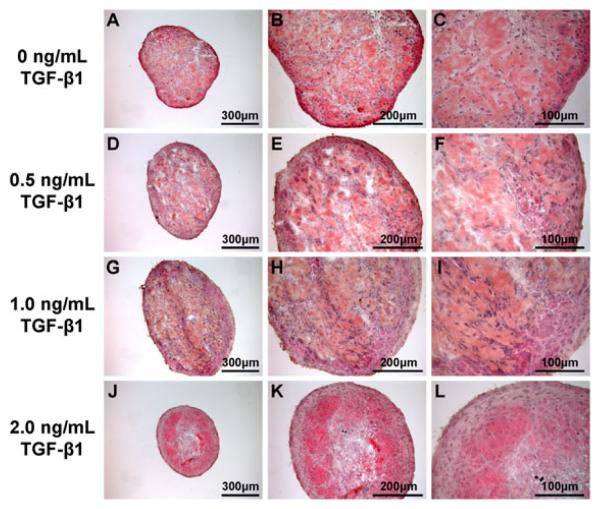
The cross-sections of engineered constructs were stained with H&E to examine the effects of TGF-β1 on general construct morphology (Figures 4A-L). In the sections of constructs formed in the presence of 0, 0.5 and 1.0 ng/ml TGF-β1 (Figures 4A-I), connective tissue and unorganized myofibres appeared throughout the construct. In the presence of 2.0 ng/ml TGF-β1 (Figures 4J-K), denser regions of better organized myofibres appeared closer to the periphery of the construct.
Figure 4.
Hematoxylin and eosin stained cross-sections of muscle constructs. Cross-sections of 3D-engineered skeletal muscle constructs formed in the presence of 0 (A-C), 0.5 (D-F), 1.0 (G-I), and 2.0 (J-K) ng/ml of TGF-β1 were stained with H&E
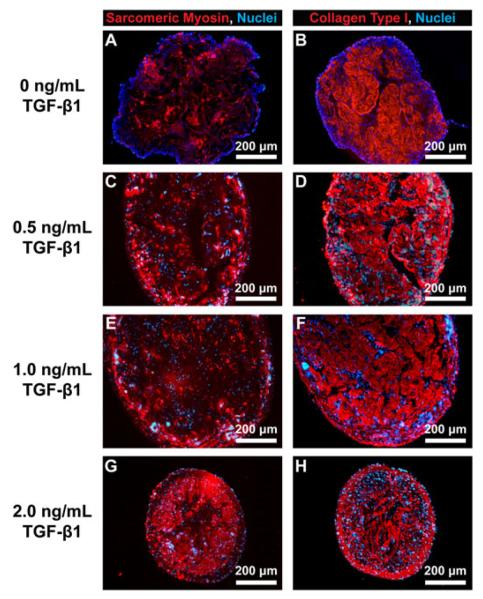
Immunostaining of the cross-sections of 3D-engineered muscle constructs was used to visualize sarcomeric myosin heavy chains (red, Figures 5A, C, E, G) and collagen type I (red, Figures 5B, D, F, H) in the constructs cultured with varying concentrations of TGF-β1. Cell nuclei (blue) were visible throughout the cross-sections at each of the concentrations of TGF-β1 used. Sarcomeric myosin was present at the higher level and was located closer to the periphery of the constructs in the presence of 2.0 ng/ml TGF-β1 (Figure 5G). Collagen type I immunostaining showed that at 2.0 ng/ml TGF-β1, a distinct epimysium-like outer layer of collagen was formed at the periphery of the constructs (Figure 5H).
Figure 5.
Immunofluorescence staining of cross-sections of 3D muscle constructs Immunostaining of the cross-sections of 3D-engineered skeletal muscle constructs formed in the presence of 0 (A and B), 0.5 (C and D), 1.0 (E and F), and 2.0 (G and H) ng/ml of TGF-b1. Antibodies for sarcomeric myosin heavy chains (red in A, C, E, G) and collagen 1 (red in B, D, F, H) were used to visualize myofibres and ECM, respectively. DAPI was used to visualize cell nuclei (blue in A-H)
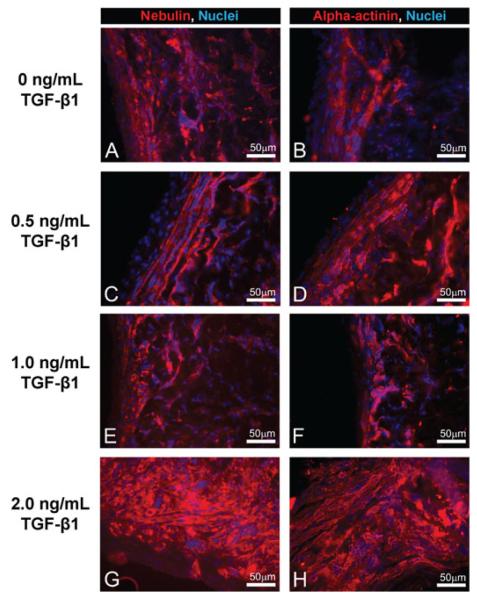
The longitudinal sections of 3D-engineered constructs were immunostained to visualize striations with antibodies to nebulin (red, Figures 6A, C, E, G) and alpha-actinin (red, Figures 6B, D, F, H). Alpha-actinin can cross-link actin and titan filaments at the Z disk, and nebulin associates with and helps organize thin filaments in the sarcomeres of skeletal muscle (Kontrogianni-Konstantopoulos et al., 2009). Cell nuclei (blue) were visible throughout the constructs at all concentrations of TGF-β1. At 2.0 ng/ml TGF-β1, the stainings for nebulin (Figure 6G) and alpha-actinin (Figure 6H) were both more prevalent in comparison to stainings at lower TGF-β1 concentrations (Figures 6A-F). However, very few multinucleated muscle fibres and no visible striations were observed in the constructs at all concentrations of TGF-β1.
Figure 6.
Immunofluorescence staining for striations in longitudinal sections of 3D muscle constructs. Immunostaining of the longitudinal sections of 3D-engineered skeletal muscle constructs formed in the presence of 0 (A and B), 0.5 (C and D), 1.0 (E and F), and 2.0 (G and H) ng/ml of TGF-β1 with antibodies against nebulin (red in A, C, E and G) and alpha-actinin (red in B, D, F and H). Nuclei were stained with DAPI (blue in A-H)
4. Discussion
We successfully engineered 3D skeletal muscle constructs with improved peak force production using an optimized TGF-β1 concentration in the culture medium during construct formation. The addition of 2.0 ng/ml TGF-β1 after 2 days of myotube differentiation improved maximum force production and increased construct diameter in our experimental procedure. The utilization of TGF-β 1 during skeletal muscle tissue engineering requires a balance of timing, concentration, thickness, and the correct formation of muscle fibers.
3D skeletal muscle constructs engineered with the addition of 2.0 ng/ml TGF-β1 showed greater contractile properties than at lower concentrations. The addition of 2.0 ng/ml TGF-β1 to the culture medium of 3D skeletal muscle constructs increased the magnitude of both twitch and tetanic force. The control group was cultured in similar conditions and was consistent with previously reported data (Larkin et al., 2006). Additionally, spontaneous activity was more notable in constructs grown in 2.0 ng/ml of TGF-β1. Cross-sectional area and specific force also significantly increased in constructs cultured in the presence of 2.0 ng/ml of TGF-β1. For an engineered construct, maximum force production is directly proportional to the functional myofibre cross-sectional area. An increase in similarly functional muscle cross-sectional area would lead to a proportional increase in maximum force production but no increase in specific force. This suggests that the increase in force production at 2.0 ng/ml TGF-β1 was not solely due to an increase in size but could be due to an enhanced density and contractile activity of myofibres.
Immunohistochemistry of the muscle constructs supported this finding, as immunostaining for the myosin heavy chains was more prominent at the periphery of the constructs than at lower TGF-β1 concentrations. Increases in other sarcomeric markers, alpha-actinin and nebulin, were also found at the periphery of the constructs. Nebulin is important for proper organization and contraction of skeletal muscle sarcomeres (Kontrogianni-Konstantopoulos et al., 2009). The presence of alpha-actinin, a major component of the Z disk, further supports the development of sarcomeres. However, few multinucleated myofibres and no striations were detected in constructs at any of the TGF-β1 concentrations used, which indicates an undeveloped phenotype. Our latest experiments showed that striations only began to appear in some areas of the 3D-engineered muscle constructs cultured in vitro with the same method and implanted in vivo for 1 week (Williams et al., 2011). We conclude that implantation in vivo is required for improved development of sarcomeres in 3D-engineered muscle constructs.
The correct timing and amount of TGF-β1 need to be considered for any skeletal muscle tissue engineering application. TGF-β1 can either promote or inhibit the normal function of muscle based on the maturity and developmental age of the tissue. While an excess of TGF-β1 leads to atrophy and fibrosis in adult muscle (Li et al., 2004), inhibition of TGF-β1 during embryonic development severely disrupts the formation of connective tissue in limbs (Pryce et al., 2009). We found that the addition of TGF-β1 immediately after the application of differentiation medium was detrimental to engineered construct formation, presumably due to inhibition of myotube fusion as previously reported (Vaidya et al., 1989). The ideal application of TGF-β1 was during and after the transition from 2D mono-layer to 3D-engineered construct. The controlled inhibition of skeletal muscle differentiation during this delamination and reorganization could protect against tearing due to self-contraction of the fibroblasts in the ECM, the myotubes, or both. Supplementation with TGF-β1 could also effectively produce a beneficial increase in ECM content, which could subsequently improve intracellular structural support during the 2D to 3D transition and lateral transmission of force between myotubes. An increased density of myotubes in the 3D constructs may have also stimulated the increase in ECM content (Rao et al., 1985). Furthermore, mechanical loading, which has been shown to improve engineered muscle thickness and force production in vitro (Powell et al., 2002), is associated with an up-regulation of both TGF-β1 and collagen type I (Heinemeier et al., 2009). Hence, the application of TGF-β1 during the 3D phase of engineered construct formation could biochemically emulate part of the response to mechanical loading for maintenance and improvement of skeletal muscle construct formation in vitro.
The primary muscle cell population used in this study was a heterogeneous population of at least 75% satellite cells and approximately 25% fibroblasts. In addition to its effects on skeletal muscle, TGF-β1 can also stimulate fibroblast growth and collagen type I production (Robbins et al., 1997). Many engineered constructs deficient in TGF-β1 lacked sufficiently stiff ECM, erratically detached from the substrate, and frequently tore apart. Excessive TGF-β1 supplementation resulted in tendon-like constructs that did not detach from the substrate during 3D construct formation or were often not contractile. Mathematical models for ECM and myofibre interactions in adult skeletal muscle predict that an increase in stiffness and thickness of ECM will improve the capacity for force transmission (Purslow, 2010). Exposure of skeletal muscle to collagenase type I ex vivo led to decreased passive elastic stiffness (Rowe et al., 2010), indicating a role of collagen in the elastic properties of ECM. The ECM of constructs that did not properly form may not have been sufficiently stiff or thick enough to provide ideal structural support and force transmission. Conversely, the inherent disadvantage of increased thickness in vitro is cell death due to lack of nutrient diffusion in the centre of the constructs. A balance of cell populations and the effects of TGF-β1 between satellite cells and fibroblasts is important for improving muscle construct performance. Remarkably, a recent study indicated that signaling between satellite cells and fibroblasts is critical in the regulation of myogenesis (Mathew et al., 2011). When using a heterogeneous population of satellite cells and fibroblasts, an optimal dose of TGF-β1 will depend on the context of the application as well as the composition and behaviour of the cell source.
The results of this study further indicate that 3D-engineered muscle constructs can be utilized as a unique in vitro model for the study of skeletal muscle development and function. Optimization of these carefully mediated processes could improve force production and organization as well as reduce variability between engineered muscle constructs. Early in monolayer formation, the effects of growth factors could be tested and utilized to study the effects on cell proliferation and activation of satellite cells. Hepatocyte growth factor (HGF) is an essential activator of satellite cells (Tatsumi et al., 1998), and fibroblast growth factors (FGFs) are known to affect the rates of proliferation and differentiation of satellite cells (Allen and Boxhorn, 1989). During delamination, HGF is also critical and could be utilized to improve the homogeny of detachment of the monolayer (Heymann et al., 1996). Other TGF-β superfamily members such as myostatin and connective tissue growth factor (CTGF) have similar roles in the control of muscle growth and differentiation (Whittemore et al., 2003; Croci et al., 2004), and could be potential alternatives to TGF-β1 in controlling the function of myogenic cells in vitro. A previous study indicated that TGF-β2 and TGF-β3 were involved in the recruitment of hematopoietic stem cells and myotube fusion events during muscle regeneration (McLennan and Koishi, 1997). In the current study, TGF-β1 was chosen for its known effects in both ECM production and muscle development. A large hurdle in the future will be combating necrosis due to poor nutrient diffusion in the centre of engineered 3D skeletal muscle constructs. For example, vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF-B) have been previously used in scaffold tissue engineering to induce vascularization (Levenberg et al., 2005). Future studies are planned to investigate the effects of these additional growth factors on the advancement of the structure and function of engineered skeletal muscle constructs.
In the current study, the correct use of 2.0 ng/ml TGF-β1 in the culture medium improved the size and force production of 3D-engineered skeletal muscle constructs. The success of TGF-β1 concentration optimization opens the door for the study of the effects of other growth factors on construct formation. Utilization of TGF-β1 during monolayer detachment of 3D-engineered constructs significantly expands the potential to control the phenotype of the muscle tissue in culture and increases the usefulness of engineered muscle for many different applications.
Acknowledgements
This research was supported by a NIH, NIAMS, NIBIB funded grant R01 AR054778-02.
References
- Allen RE, Boxhorn LK. Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol. 1989;138:311–315. doi: 10.1002/jcp.1041380213. [DOI] [PubMed] [Google Scholar]
- Aviss KJ, Gough JE, Downes S. Aligned electrospun polymer fibres for skeletal muscle regeneration. Eur Cell Mater. 2010;19:193–204. doi: 10.22203/ecm.v019a19. [DOI] [PubMed] [Google Scholar]
- Baker EL, Dennis RG, Larkin LM. Glucose transporter content and glucose uptake in skeletal muscle constructs engineered in vitro. In Vitro Cell Dev Biol Anim. 2003;39:434–439. doi: 10.1290/1543-706X(2003)039<0434:GTCAGU>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Beach RL, Burton WV, Hendricks WJ, et al. Extracellular matrix synthesis by skeletal muscle in culture. Proteins and effect of enzyme degradation. J Biol Chem. 1982;257:11437–11442. [PubMed] [Google Scholar]
- Buck CA, Horwitz AF. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- Buckingham M. Muscle differentiation. Which myogenic factors make muscle? Curr Biol. 1994;4:61–63. doi: 10.1016/s0960-9822(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Chen Q, Sivakumar P, Barley C, et al. Potential role for heparan sulfate proteoglycans in regulation of transforming growth factor-beta (TGF-beta) by modulating assembly of latent TGF-beta-binding protein-1. J Biol Chem. 2007;282:26418–26430. doi: 10.1074/jbc.M703341200. [DOI] [PubMed] [Google Scholar]
- Croci S, Landuzzi L, Astolfi A, et al. Inhibition of connective tissue growth factor (CTGF/CCN2) expression decreases the survival and myogenic differentiation of human rhabdomyosarcoma cells. Cancer Res. 2004;64:1730–1736. doi: 10.1158/0008-5472.can-3502-02. [DOI] [PubMed] [Google Scholar]
- DiEdwardo CA, Petrosko P, Acarturk TO, et al. Muscle tissue engineering. Clin Plast Surg. 1999;26:647–656. [PubMed] [Google Scholar]
- Duncan MR, Frazier KS, Abramson S, et al. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. FASEB J. 1999;13:1774–1786. [PubMed] [Google Scholar]
- Fauza DO, Marler JJ, Koka R, et al. Fetal tissue engineering: diaphragmatic replacement. J Pediatr Surg. 2001;36:146–151. doi: 10.1053/jpsu.2001.20034. [DOI] [PubMed] [Google Scholar]
- Heinemeier KM, Olesen JL, Haddad F, et al. Effect of unloading followed by reloading on expression of collagen and related growth factors in rat tendon and muscle. J Appl Physiol. 2009;106:178–186. doi: 10.1152/japplphysiol.91092.2008. [DOI] [PubMed] [Google Scholar]
- Heymann S, Koudrova M, Arnold H, et al. Regulation and function of SF/HGF during migration of limb muscle precursor cells in chicken. Dev Biol. 1996;180:566–578. doi: 10.1006/dbio.1996.0329. [DOI] [PubMed] [Google Scholar]
- Huijing PA. Muscle as a collagen fiber reinforced composite: a review of force transmission in muscle and whole limb. J Biomech. 1999;32:329–345. doi: 10.1016/s0021-9290(98)00186-9. [DOI] [PubMed] [Google Scholar]
- Ignotz RA, Endo T, Massague J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987;262:6443–6446. [PubMed] [Google Scholar]
- Kjaer M, Magnusson P, Krogsgaard M, et al. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat. 2006;208:445–450. doi: 10.1111/j.1469-7580.2006.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollias HD, McDermott JC. Transforming growth factor-beta and myostatin signaling in skeletal muscle. J Appl Physiol. 2008;104:579–587. doi: 10.1152/japplphysiol.01091.2007. [DOI] [PubMed] [Google Scholar]
- Kontrogianni-Konstantopoulos A, Ackermann MA, Bowman AL, et al. Muscle giants: molecular scaffolds in sarcomero-genesis. Physiol Rev. 2009;89:1217–1267. doi: 10.1152/physrev.00017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin LM, Calve S, Kostrominova TY, et al. Structure and functional evaluation of tendon-skeletal muscle constructs engineered in vitro. Tissue Eng. 2006;12:3149–3158. doi: 10.1089/ten.2006.12.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PK, Goodwin TG, Fang Q, et al. Cell transplantation as an experimental treatment for Duchenne muscular dystrophy. Cell Transplant. 1993;2:485–505. doi: 10.1177/096368979300200607. [DOI] [PubMed] [Google Scholar]
- Lawson MA, Purslow PP. Development of components of the extracellular matrix, basal lamina and sarcomere in chick quadriceps and pectoralis muscles. Br Poult Sci. 2001;42:315–320. doi: 10.1080/00071660120055269. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- Levenberg S, Rouwkema J, Macdonald M, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- Li Y, Foster W, Deasy BM, Chan Y, et al. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol. 2004;164:1007–1019. doi: 10.1016/s0002-9440(10)63188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna A, Meister HP, Szanto PB. Esophageal varices in the absence of cirrhosis, Incidence and characteristics in congestive heart failure and neoplasm of the liver. Am J Clin Pathol. 1968;49:710–717. doi: 10.1093/ajcp/49.5.710. [DOI] [PubMed] [Google Scholar]
- Macias-Silva M, Abdollah S, Hoodless PA, et al. MADR2 is a substrate of the TGF-beta receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell. 1996;87:1215–1224. doi: 10.1016/s0092-8674(00)81817-6. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Hansen JM, Merrell AJ, et al. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development. 2011;138:371–384. doi: 10.1242/dev.057463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan IS, Koishi K. Cellular localisation of transforming growth factor -beta 2 and -beta 3 (TGF-beta2, TGF-beta3) in damaged and regenerating skeletal muscles. Dev Dyn. 1997;208:278–289. doi: 10.1002/(SICI)1097-0177(199702)208:2<278::AID-AJA14>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Miller JB, Everitt EA, Smith TH, et al. Cellular and molecular diversity in skeletal muscle development: news from in vitro and in vivo. Bioessays. 1993;15:191–196. doi: 10.1002/bies.950150308. [DOI] [PubMed] [Google Scholar]
- Miura T, Kishioka Y, Wakamatsu J, et al. Interaction between myostatin and extra-cellular matrix components. Anim Sci J. 2010;81:102–107. doi: 10.1111/j.1740-0929.2009.00700.x. [DOI] [PubMed] [Google Scholar]
- Olguin HC, Santander C, Brandan E. Inhibition of myoblast migration via decorin expression is critical for normal skeletal muscle differentiation. Dev Biol. 2003;259:209–224. doi: 10.1016/s0012-1606(03)00180-5. [DOI] [PubMed] [Google Scholar]
- Powell CA, Smiley BL, Mills J, et al. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am J Physiol Cell Physiol. 2002;283:C1557–C1565. doi: 10.1152/ajpcell.00595.2001. [DOI] [PubMed] [Google Scholar]
- Pryce BA, Watson SS, Murchison ND, et al. Recruitment and maintenance of tendon progenitors by TGF-beta signaling are essential for tendon formation. Development. 2009;136:1351–1361. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purslow PP. The structure and functional significance of variations in the connective tissue within muscle. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:947–966. doi: 10.1016/s1095-6433(02)00141-1. [DOI] [PubMed] [Google Scholar]
- Purslow PP. Muscle fascia and force transmission. J Bodyw Mov Ther. 2010;14:411–417. doi: 10.1016/j.jbmt.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Rao JS, Beach RL, Festoff BW. Extra-cellular matrix (ECM) synthesis in muscle cell cultures: quantitative and qualitative studies during myogenesis. Biochem Biophys Res Commun. 1985;130:440–446. doi: 10.1016/0006-291x(85)90436-x. [DOI] [PubMed] [Google Scholar]
- Robbins JR, Evanko SP, Vogel KG. Mechanical loading and TGF-beta regulate proteoglycan synthesis in tendon. Arch Biochem Biophys. 1997;342:203–211. doi: 10.1006/abbi.1997.0102. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB, Assoian RK, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi CA, Pozzobon M, De Coppi P. Advances in musculoskeletal tissue engineering: moving towards therapy. Organo-genesis. 2010;6:167–172. doi: 10.4161/org.6.3.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe J, Chen Q, Domire ZJ, et al. Effect of collagen digestion on the passive elastic properties of diaphragm muscle in rat. Med Eng Phys. 2010;32:90–94. doi: 10.1016/j.medengphy.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze-Mosgau S, Blaese MA, Grabenbauer G, et al. Smad-3 and Smad-7 expression following anti-transforming growth factor beta 1 (TGF-beta-1)-treatment in irradiated rat tissue. Radiother Oncol. 2004;70:249–259. doi: 10.1016/j.radonc.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, et al. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Stauber WT, Knack KK, Miller GR, et al. Fibrosis and intercellular collagen connections from four weeks of muscle strains. Muscle Nerve. 1996;19:423–430. doi: 10.1002/mus.880190402. [DOI] [PubMed] [Google Scholar]
- Stern HM, Lin-Jones J, Hauschka SD. Synergistic interactions between bFGF and a TGF-beta family member may mediate myogenic signals from the neural tube. Development. 1997;124:3511–3523. doi: 10.1242/dev.124.18.3511. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Anderson JE, Nevoret CJ, et al. HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol. 1998;194:114–128. doi: 10.1006/dbio.1997.8803. [DOI] [PubMed] [Google Scholar]
- Vaidya TB, Rhodes SJ, Taparowsky EJ, et al. Fibroblast growth factor and transforming growth factor beta repress transcription of the myogenic regulatory gene MyoD1. Mol Cell Biol. 1989;9:3576–3579. doi: 10.1128/mcb.9.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittemore LA, Song K, Li X, Aghajanian J, et al. Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun. 2003;300:965–971. doi: 10.1016/s0006-291x(02)02953-4. [DOI] [PubMed] [Google Scholar]
- Williams ML, Kostrominova TY, Arruda EM, et al. Effect of Implantation on engineered skeletal muscle constructs. Revision Submitted for Publication in Tissue Engineering and Regenerative Medicine, September. 2011;12 doi: 10.1002/term.537. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Feng X, We R, et al. Receptor-associated Mad homologues synergize as effectors of the TGF-beta response. Nature. 1996;383:168–172. doi: 10.1038/383168a0. [DOI] [PubMed] [Google Scholar]