Abstract
Cryptococcus neoformans (Cn) is a fungal pathogen that is a serious health threat to immunocompromised individuals. Upon environmental exposure, infectious fungal propagules are inhaled into the host’s lungs. The anticryptococcal actions of alveolar macrophages (AM), the predominant host phagocyte of the innate immune system in the lungs, are fundamental in determining whether containment and clearance of the pathogen occurs by the development of an adapted immune response or whether infection is established and progresses to disease. However, the fungus is also capable of surviving the antimicrobial actions of AM and exploits these host phagocytes to establish infection and exacerbate disease. In addition, there is evidence suggesting that cryptococcosis may occur following reactivation of latent cryptococcal infection. Currently, the role of AM and the fungal factors contributing to latent cryptococcosis are unknown. This review examines the AM-Cn interaction and how it affects the development of pulmonary disease with a focus on host and pathogen factors enabling latency to occur.
Keywords: Cryptococcus, cryptococcosis, macrophage, lung disease
WORLDWIDE HEALTH BURDEN OF CRYPTOCOCCUS NEOFORMANS
The pathogenic fungus Cryptococcus neoformans (Cn) is a major cause of morbidity and mortality in immunocompromised individuals. Cryptococcosis is one of the most common opportunistic infections in HIV-infected patients, as it is diagnosed in approximately 1,000,000 individuals/year and is responsible for an average of 600,000 deaths/year [1]. The vast majority of these cases occur in regions of the world where the HIV pandemic persists at alarmingly high rates and access to adequate medical care is limited [2].
Since the induction of highly active antiretroviral therapy (HAART) as the gold standard treatment in the management of HIV/AIDS, the incidence of cryptococcosis in medically developed countries has significantly decreased [3]. However, even in countries where HAART has resulted in a decline in opportunistic infections, cryptococcosis remains a serious health concern for non-HIV-infected individuals, such as those with immune deficiencies or patients receiving immunosuppression therapy comprised of corticosteroids, monoclonal antibodies or other immunosuppressive agents. In fact, cryptococcosis is the third-leading invasive fungal infection in solid organ transplant recipients and comprises upwards of 60% of all non-HIV cryptococcal disease cases [4]. The number of non-HIV cryptococcosis cases is suspected to increase proportionally with advances in transplantation medicine and immunity manipulation, along with the broadening application of immunosuppressive therapeutic regimens [3]. Health care facilities have reported mortality rates of 100% within two weeks of clinical presentation when patients are not placed on specific antifungal therapeutic regimens [5]. In addition, acute cryptococcal meningoencephalitis still has a three-month mortality rate of approximately 20% even in patients undergoing advance medical treatment [6].
Unfortunately, with the possible exception of voriconazole in 2002, no new drugs have been approved in the last 10 years useful in the management of cryptococcosis [7]. As emphasized in the 2010 Infectious Diseases Society of America (IDSA) guidelines for the management of cryptococcosis, without ideal anti-Cn agents available, important principals for the management of cryptococcal infections remain in diagnosing cryptococcosis, regulating host immunity and managing toxicity of anti-fungal therapies, [7]. With the dilemmas of using current drug regimens (i.e. drug efficacy versus patient side effects) as well as unacceptably high mortality rates, the discovery of new therapeutic targets and development of new anti-Cn drugs is greatly needed for the treatment and management of cryptococcosis.
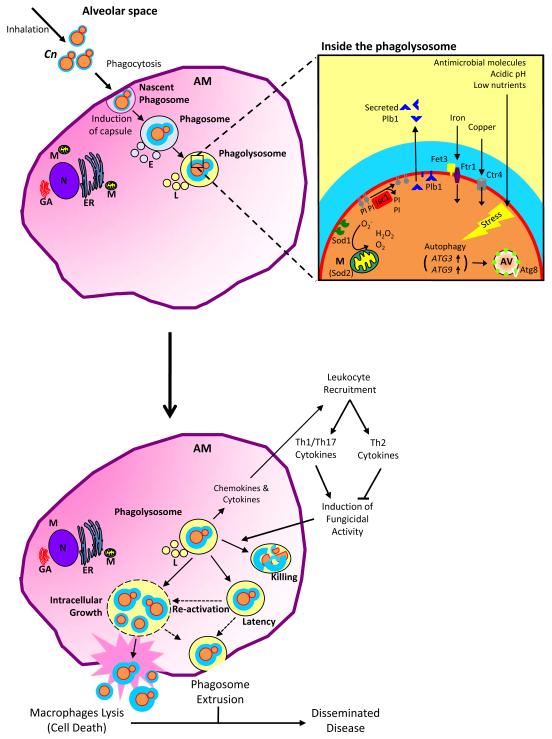
CRYPTOCOCCUS NEOFORMANS EXPOSURE AND THE ESTABLISHMENT OF INFECTION
The basidiomycete Cn is a free-living environmental detritivore found throughout the world. Environmental isolates have been extracted from soil, pigeon droppings, and decomposing/dead wood from certain tree species [8-10]. Cryptococcal infections in humans are believed to occur following inhalation of desiccated yeast or possibly infectious spores. Upon entering the lungs, as a facultative intracellular pathogen, the infectious Cn propagule can reside in the extracellular environment of the alveolar spaces of the lungs or, upon phagocytosis, persist intracellularly within alveolar macrophages (AM). As the predominant resident phagocytes in the lungs, AM possess an essential role in the host immune response to inhaled pathogens. Internalization of Cn via receptor-mediated phagocytosis is a pivotal event in the Cn-AM interaction because it precedes other AM effector actions, which include the secretion of chemokines and cytokines, recruitment of other immune cells, killing of internalized pathogens, and antigen presentation [11-15]. Following internalization there are three possible outcomes in this host-pathogen interaction: 1) the cryptococcal cells are contained and cleared in a concerted effort of the innate and adaptive immune responses; 2) Cn enter a latency stage, allowing for possible reactivation and subsequent symptomatic disease at a later time; or 3) Cn survive and the infection progresses to establish pulmonary cryptococcosis, which can disseminate to cause systematic disease including life-threatening meningoencephalitis. Which of these outcomes occurs is dependent on the balance between Cn fitness and AM anticryptococcal activity (Figure 1).
Figure 1.
Strategies for Cryptococcus neoformans survival in host alveolar macrophages.
Following inhalation, infectious Cryptococcus neoformans (Cn) propagules enter the alveolar spaces of the lungs and are confronted by resident alveolar macrophages (AM). Upon phagocytosis, the nascent phagosome surrounds the internalized cryptococcal cell. Phagosome maturation proceeds with the fission and fusion of vesicles including endosomes (E) and then lysosomes (L), which ultimately result in formation of the phagolysosome. Antimicrobial molecules, acidic pH, and low level of nutrients all normally contribute to the killing of internalized pathogens. In addition to its polysaccharide capsule, laccase activity/melanin production, and ability to grow at 37°C, Cn has several other mechanisms enabling it to survive in the phagolysosome. Cn expresses superoxide dismutases 1 (SOD1) and −2 (SOD2) that catalyze the dismutation of antimicrobial superoxide (O2−) to produce hydrogen peroxide (H202) and molecular oxygen (O2). Inositol phosphosphingolipid-phospholipase C1 (Isc1) metabolizes fungal inositol sphingolipids into phytoceramide and is essential for intracellular survival by imparting defense from acidic, oxidative, and nitrosative stresses. Phospholipase B1 (Plb1) is associated with the cell membrane but can also be cleaved from its glycosylphosphatidylinositol anchor and secreted. Plb1 can act as a PLB, a lysopholipase (LPL), and a lysophospholipase transacylase (LPTA) to cleave host lipids. Plb1 contributes to the production of fungal eicosanoids, which are capable of scavenging macrophage-derived arachidonic acid. Ferro-O2-oxidoreductase Fet3 and the iron permease Ftr1, molecular components involved in iron transportation across the plasma membrane, and the Cuf1-dependent copper transporter, Ctr4, are highly expressed during intracellular residency in order to sequester the metal molecules indispensible for survival. Cn autophagy genes ATG3 and ATG9 are stress-induced, resulting in the formation of autophagic vesicles (AV) possessing Atg8. There are three possible outcomes of the AM-Cn interaction: 1) the cryptococcal cells are contained and cleared in a concerted effort of the innate and adaptive immune responses; 2) Cn enter a latency stage, allowing for possible reactivation and subsequent symptomatic disease at a later time; or 3) Cn survive and the infection progresses to establish pulmonary cryptococcosis, which can disseminate to cause systematic disease including life-threatening meningoencephalitis. Which of these outcomes occurs is dependent on the balance between Cn fitness and AM anticryptococcal activity.
ALVEOLAR MACROPHAGES AND THE HOST IMMUNE RESPONSE TO CRYPTOCOCCUS NEOFORMANS
AM have an instrumental role linking innate immunity and adaptive immune responses in the lung. Upon interaction with a pathogen, macrophages secrete cytokines that modulate the development and expression of antigen-experienced CD4+ into Th1, Th2, or Th17 effector cells. In turn, specific cytokine profiles produced by these effector CD4 + Th cells augment macrophage function, which determines their capacity to kill internalized pathogens. A polarization of the host immune response towards CD4+ Th1 immunity is well established as an essential element leading to successful clearance of Cn in the lungs [16-24]. Healthy humans mount predominantly a Th1-mediated response to inhaled Cn cells, resulting in the formation of a granuloma encapsulating the infection site that acts as a physical barrier, which prevents access to vasculature and egress from the lungs to extrapulmonary sites [25, 26]. Th1-associated cytokines, specifically tumor necrosis factor alpha (TNFα) and interferon gamma (IFNγ), evoke classic activation of macrophages, which upregulates NOS2 enzyme to produce fungicidal NO from L-arginine, which kills internalized Cn [27]. The importance of the Th17 immune response to Cn in the host is still being defined, but initial studies suggest it may positively contribute to a host’s ability to prevent cryptococcosis [24, 28-30]. Research into the effect of the Th17-associated cytokines on the Cn-macrophage interactions has shown Il-17 to activate macrophages and limit intracellular proliferation of Cn cells [31]. In contrast, induction of a Th2 response is non-protective to the host, as demonstrated in mouse models and analysis of clinical cases [30, 32, 33]. Th2-associated cytokines, such as IL-4 and IL-13, induce alternative activation of macrophages, which increases phagocytosis and intracellular growth of Cn [27]. Therefore, the fate of Cn cells internalized by AM, and consequently the outcome of the host, is greatly influenced by the CD4+ Th response that is evoked. This has been shown in mouse and rat models of pulmonary cryptococcosis, where differences in susceptibility to cryptococcosis between species were found to correspond to the efficacy of anticryptococcal activities of AM [34]. However, it should also be noted that, as demonstrated by Zhang et al using IL-4/IL-13 knockout mouse model, a robust Th1/Th17 immune response is not sufficient to prevent disease from highly pathogenic Cn strains [30]. Thus, future studies are essential to elucidating host factors affecting susceptibility to cryptococcosis.
As alluded to above, phagocytosis is necessary for containment and clearance of pathogenic microorganisms from the lungs but internalization can also be detrimental and injurious to the host, depending on whether AM are classically activated (Th1-/Th17-associated cytokines) or alternatively activated (Th2-associated cytokines). Unlike many intracellular pathogens, Cn does not actively avoid the potentially microbicidal phagolysosome but, in fact, can readily survive and proliferate within its confinements [35-38]. Research suggests macrophages may serve as a protective microenvironmental niche enabling Cn to avoid the fungicidal actions of other immune cells. Cn cells contained within host phagocytes are capable of egress from the lungs and dissemination to the central nervous system, where they can cause life-threatening meningoencephalitis [39-42]. This dichotomy in the role of phagocytosis in host susceptibility to microbial infection is exemplified in the pathogenesis of Cn where, under conditions when phagocytes are unable to kill internalized Cn, such as during immunosuppressive states, intracellular residency may be beneficial to the fungal cells by enhancing growth and providing refuge [34, 41-43]. Currently, factors influencing whether AM facilitate anticryptococcal actions or act as a protective environmental niche are not fully defined.
ALVEOLAR MACROPHAGES IN LATENT CRYPTOCOCCOSIS
Since Cn causes disease primarily in cases of immune deficiency, one would presuppose that exposure occurs after the development of an immunocompromised condition. However, intriguingly, research suggests latent Cn infection may precede the immunodeficient condition and its reactivation may result in clinical cryptococcosis cases. For example, epidemiology studies of cryptococcal cases from HIV+ immigrant patients showed that the infectious strain genotypes were from the patients’ countries of origin, acquired years before immunosuppression [44, 45]. In addition, seroprevalence studies have shown the majority of healthy children to possess antibodies reactive to the cryptococcal capsule component glucuronoxylomannan (GXM), suggesting Cn exposure occurs during childhood [46-50]. These studies suggest that, for many infections, initial Cn exposure occurs early in life, often during childhood, followed by symptomatic disease during the acquisition of an immunocompromised state.
A model of pathogenesis, where Cn exposure leads to latent infection and, if immune suppression occurs, reactivation of dormant Cn cells results in symptomatic disease, agrees with the concept of the damage-response framework of microbial pathogenesis presented by Casadevall & Pirofski. In this proposition, host damage serves as the essential factor in the host-pathogen interaction facilitating disease by a normally non-pathogenic organism [51]. Recent research has shown immunosuppression of rats previously challenged with Cn induces reactivation of the latent infection, thus mirroring the Cn pathogenesis believed to occur in clinical cases [51]. Yet while conditions affecting cell-mediated immunity could obviously serve as the injurious event to allow reactivation, the host factors facilitating Cn latency in an individual with a seemingly intact immune system are unknown.
Since Cn can survive and proliferate in macrophages, it is intriguing to wonder whether Cn localization (i.e. extracellular versus intracellular within AM) influences its pathogenesis and possible latency. Animal models of pulmonary cryptococcosis have shown that localization of cryptococcal cells in the lungs is not static but, instead, is dynamic over the course of infection and disease progression. Analysis of the lungs of C57BL/6 mice infected with the ATCC Cn strain 24067 revealed that approximately 40% of cryptococcal cells were intracellular 2 hours post infection and the percentage of intracellular Cn peaked 8 hours post-infection (approximately 85%). A dramatic shift towards extracellular localization occurred after 24 hours, with only about 15% of cryptococcal cells observed within host phagocytes at this later time period. Intriguingly, beginning with day 7 after infection and continuing through day 28, intracellular Cn were predominantly observed [52]. It has been shown that Cn strains with mutations affecting their preferential localization within either extracellular spaces or host phagocytes modulate their virulence in murine pulmonary cryptococcosis models [41, 53].
Immunocompetent Fisher rats, whose host immune response and the resulting Cn pathogenesis more closely resemble pulmonary infections in immunocompetent humans than that of mice [54], control and contain the ATCC Cn strain 24067 with granulomatous inflammation without clinical symptoms [36]. Histopathological analysis of the lungs of immunocompetent Fisher rats infected using a pulmonary cryptococcosis model show Cn to have a great propensity for intracellular localization at all time points examined, beginning at 1.5 months post-infection through 18 months post-infection, thereby suggesting that host phagocytes assist in the establishment of latent cryptococcosis. In contrast, the lungs of rats treated with an immunosuppressive regimen of dexamethasone have severely decreased inflammation and granuloma formation, resulting in significantly higher lung fungal burdens and substantially increased extrapulmonary involvement, in comparison to immunocompetent rats. Interestingly, in lungs from dexamethasone-treated rats, the majority of cryptococcal cells are located within the extracellular environment 1.5 months post-infection but are distributed equally between intracellular and extracellular locations 12.5 months after infection [36]. Together, these animal models of pulmonary cryptococcosis demonstrate that Cn localization (extracellular versus intracellular) can be dynamic throughout the course of infection and responsive to the status of the host immune response, thereby affecting the outcome of infection.
The complex host-pathogen interaction between marophages and Cn is further exemplified by studies demonstrating how internalized Cn actively escape from macrophages through a non-lytic mechanism termed expulsion or phagosome extrusion [24, 55, 56]. Voelz and colleagues determined systematically that the frequency of expulsion increases with macrophage-activating Th1 and Th17 cytokines, while the frequency of expulsion is diminished with Th2 cytokines, which inhibits the anti-cryptococcal function of macrophages [24]. Macrophages containing intracellular Cn employ a novel actin-dependent process to preclude expulsion [57]. Although the rate of this mechanism of non-lytic egress from macrophages is observed at relatively low rates in vitro, its occurrence in vivo represents a unique event that may influence Cn pathogenesis by modulating the AM-Cn interaction and possibly affecting reactivation of latent Cn cells. It is possible that when an immunosuppressive state is induced, such as during advanced HIV infection when Th1 cells are severely damaged, macrophages containing intracellular Cn are no longer capable of keeping the fungal cells internalized and/or in a dormant state, at which time Cn cells could employ expulsion [27]. Future research, particularly involving animal models and observations of clinical cases, are required to delineate the role of expulsion or phagosome extrusion in the AM-Cn interaction.
CRYPTOCOCCUS NEOFORMANS VIRULENCE FACTORS AND MACROPHAGES
Cn possesses a unique array of virulence factors enabling survival in the lung environment, both as an extracellular and intracellular pathogen, and for the establishment of pulmonary infection. Unlike other intracellular pathogens such as Mycobacterium species, Histoplasma capsulatum, Legionella pneumophila and Toxoplasma gondii, Cn does not actively avoid phagolysosome acidification [38]. In fact, in vitro assays using primary and macrophage-like cells lines shows Cn proliferates better in an acidic environment than under alkaline conditions [35, 38, 58]. Thus, as a free-living organism, Cn has evolved mechanisms to survive in its natural environmental niche that translate to the intracellular microenvironment of mammalian phagocytes.
Cn has three preeminent virulence factors: a polysaccharide capsule, laccase activity/melanin production, and the ability to grow at 37°C. These main virulence traits are pertinent to Cn survival in the mammalian host at different stages of infection and are particularly crucial for Cn to subsist as an intracellular pathogen following internalization by host phagocytes. The Cn capsule has been observed to undergo alterations in both murine models of pulmonary cryptococcosis and clinical histology showing that it is a dynamic entity whose composition and size changes according to various stimuli within its microenvironment in the host [44, 59, 60]. Following phagocytosis, the polysaccharide capsule enlarges and, thereby, imparts greater resistance to microbicidal agents within the lumen of the phagolysosome such as reactive oxygen species (ROS), and antimicrobial peptides [61]. Laccase activity, specifically Lac1, protects intracellular Cn from the harsh microenvironment within the host phagocyte as well. Lac1 is a cell wall-associated enzyme that oxidizes exogenous iron and catecholamines to produce the pigment melanin, a molecule linked to antioxidative properties in macrophages [62]. While in the phagolysosome, the iron oxidase activity of Lac1 protects internalized Cn by competing with AM for phagosomal iron and preventing the formation of Fe (III), thus inhibiting macrophage ability to produce hydroxyl radicals [62-64].
Other intracellular pathogens, such as pathogenic Mycobacterium species [65, 66] and Francisella tularensis [67], also modulate host iron homeostasis, thereby lessening macrophage antimicrobial actions. Laccase also produces the immunosuppressing prostaglandin, PGE2, which reduces the ability to recruit and activate macrophages [68-70]. The ability of Cn to tolerate the high body temperature of the mammalian host is connected to the gene product of CNA1, calcineurin A, which is a Ca2+-calmodulin-regulated protein phosphatase [71]. In addition to modulating temperature sensitivity, calcineurin A-linked signaling pathways control induction of genes required to survive under metabolic and oxidative stress, such as those characterizing various environments of the host, including that within host phagocytes [72].
CRYPTOCOCCUS NEOFORMANS INTRACELLULAR SURVIVAL FACTORS AND MACROPHAGES
The capacity of a pathogen to survive and propagate in the host is often thought of as its pathogenic fitness [73]. As cryptococcosis may occur following latency and reactivation, a key to understanding Cn virulence and pathogenesis may be to elucidate genes and pathways providing cryptococcal cells the pathogenic fitness to subsist for extended periods of time within the host. Fan and colleagues provided an important look into Cn factors affecting intracellular survival by examining the transcriptional response of Cn following phagocytosis by macrophages. In this work, transcription profiles of the clinical isolate the H99 Cn strain were examined at 2 hours and 24 hours post-internalization by the murine macrophage-like J774A.1 cells. After deducing the magnitude of change in phagocytic-specific gene expression and using a threshold of two-fold or greater compared to internal controls, there were 157 down-regulated genes and 123 up-regulated genes. Importantly, the gene expression profile of internalized Cn differed at 2 hours post-internalization compared to 24 hours post-internalization [74]. Several of these genes, which bestow the ability to tolerate harsh conditions during the intracellular parasitism of Cn, have garnered significant attention. Of particular interest are the factors contributing to the aptitude of Cn cells to persist in the phagolysosome, as host phagocytes may serve as a protective microenvironment niche under specific conditions (Figure 1).
Several Cn genes and pathways that affect intracellular survival independent of the three classical virulence factors have been identified. Cryptococcus species express superoxide dismutases 1 (SOD1) and −2 (SOD2) that produce hydrogen peroxide and molecular oxygen from antimicrobial molecule superoxide released by phagocytes [75]. Both SOD1 and SOD2 protect from intracellular antimicrobial actions, albeit by different roles as they differ in their biochemical requirements and cellular localization [75-77]. Through a Pma1-dependent mechanism(s), inositol phosphosphingolipid-phospholipase C1 (Isc1) imparts defense from acidic, oxidative, and nitrosative stresses of the phagolysosome [53, 78]. Isc1 is an enzyme that metabolizes fungal inositol sphingolipids into phytoceramide, and is essential for intracellular survival by imparting defense from acidic, oxidative, and nitrosative stresses of the phagolysosome [53, 78]. In murine models of pulmonary cryptococcosis, the Δisc1 mutant strain is almost exclusively localized in the extracellular environment of the lung. Phospholipase B1 (Plb1) is a cell membrane-associated enzyme that can be cleaved from its glycosylphosphatidylinositol (GPI) anchor and secreted from the cryptococcal cell [79, 80]. Plb1 has several enzymatic activities: it can function as a PLB, a lysophospholipase (LPL), and a lysophospholipase transacylase (LPTA) to cleave host lipids. The Δplb1 mutant strain has an intracellular growth defect attributed to the production of fungal eicosanoids, which are capable of scavenging macrophage-derived arachidonic acid [81]. Cn metabolism of host arachidonic acid diminishes the host cellular pool, which is required for critical macrophage functions, while producing fungal eicosanoids that may protect Cn from the host response.
The dynamic nature of Cn as an intracellular pathogen responding to a host microenvironment has also been shown when examining membrane-associated transporters regulating nutrient uptake from the external environment, such as carbohydrate transporters and nitrogen starvation-associated transporters located on the plasma membrane. The expression levels of the ferro-O2-oxidoreductase Fet3 and the iron permease Ftr1, both of which are molecular components involved in iron transportation across the plasma membrane, were significantly upregulated over time during intracellular residency [74], showing that sequestering metal molecules from the host is an aspect of nutritional acquisition important to gene regulatory networks of Cn. The iron-responsive transcription factor Cir1 has been identified as a regulator of capsule formation, melanin formation in the cell wall, and temperature sensitivity through its control of genes modulating iron acquisition [82]. In addition, the ferroxidase Cfo1, which is essential for the reductive uptake system that acquires iron from host transferrin during infection, is upregulated when Cn resides in an iron-limited environment [83].
Similarly, the acquisition of copper is also vital to Cn to survival within the host. The cryptococcal copper dependent transcription factor 1 (Cuf1) is required for growth and virulence factor expression in the presence of low copper concentrations, such as the phagolysosome [84]. The importance of copper on the intracellular parasitism of Cn is further exemplified by the fact that the Cuf1-dependent copper transporter, Ctr4, is highly expressed during intracellular residence [84]. Mutant strains with impaired ability to acquire both iron and copper from the host have either attenuated virulence or are avirulent, and clinical strains having intrinsically reduced CTR4 expression were less likely to cause meningitis rather than more localized pulmonary disease in a cohort of organ transplant patients [83, 84]. These transcription factors, which respond to metal molecules in the host environment, make intriguing therapeutic targets to complement the antifungal drugs amphotericin and fluconazole [83].
Research has found internalization by macrophages induces autophagy in Cn [74, 85]. Autophagy is a pro-survival adaption of eukaryotes initiated in response to both extracellular stresses, such as nutritional deprivation, and intracellular stresses, including damaged proteins and organelles. To survive within a host phagocyte, internalized microbial pathogens must scavenge nutrients from the vesicles of the phagocytic pathway in which they reside and undergo necessary metabolic alterations. As carbon sources and micronutrients are limited in pathogen-containing vesicles, induction of autophagy provides a mechanism to prolong intracellular survival by recycling organelles and cytoplasmic proteins to provide essential elements for survival. Cn autophagy gene ATG3 is induced at 2 hours after phagocytosis while ATG9 is induced after 24 hours of internalization within murine macrophage-like J774A.1 cells [74]. Autophagy has been demonstrated directly to occur in Cn following phagocytosis by J774A.1 cells through microscopic analysis of the localization of Atg8, a microtubule-associated protein that is induced and associated with vesicles during autophagy [85]. A Cn mutant strain lacking the Vps34 phosphatidylinositol 3-kinase (vps34Δ), which is known to be involved in autophagy in ascomycete yeast, was defective in the formation of Atg8-labeled vesicles and was rapidly killed within hours by macrophages following phagocytosis in vitro, despite normal growth in fungal media at 37°C [85]. Importantly, vps34Δ is avirulent in a murine model pulmonary cryptococcosis, where the cryptococcal cells are amazingly killed in the lungs within hours of challenge [85].
CONCLUSIONS
AM serve as a vital link between innate immunity and the development of an adaptive immune response. The AM-Cn interaction is part of an intricate host-pathogen relationship that greatly influences whether cryptococcal cells are contained and cleared from the lungs or whether cryptococcal cells persist to establish infection and disease. Under conditions not fully defined, Cn can survive within AM to seemingly exploit the intracellular residency within these host phagocytes. The fact that cryptococcal cells adapt to survive and persist in the host for extended periods of time supports the view that the ability to establish infection, whether it leads to acute or latent disease, has a strong component of pathogenic fitness.
Therefore, future therapeutic regimens should take into account Cn pathogenic fitness as it relates to the status of the host immune system. Research also suggests reactivation of latent cryptococcal cells residing within lungs may cause a significant proportion of cryptococcosis cases. Therefore, to identify factors modulating the pathogenic fitness of Cn, development of a consensus animal model of latent pulmonary cryptococcosis is required and greater explorations into clinical cases are necessary. Lastly, the Cn factors enabling the fungus to survive for an extended period of time and/or reactivate within the host make intriguing targets for drug development. For example, in the case of organ transplantation, drugs can be administered prior to manipulation of host immunity to preclude reactivation and prevent cryptococcosis. The advancements in knowledge thus obtained from these future studies defining host factors, particularly involving macrophages, and distinguishing Cn factors contributing to latent infection and reactivation hold a great degree of potential to prevent cryptococcosis in high-risk patient groups, such as HIV-infected individuals and patients beginning immunosuppressive therapy.
Acknowledgment
This work was supported by the Intramural Research Program of the NIH, NIAID. We would like also to acknowledge manuscript editorial review by J. Abbott.
References
- 1.Park BJ, et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–30. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Warnock DW. Fungal diseases: an evolving public health challenge. Med Mycol. 2006;44(8):697–705. doi: 10.1080/13693780601009493. [DOI] [PubMed] [Google Scholar]
- 3.Chayakulkeeree M, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2006;20(3):507–44. v–vi. doi: 10.1016/j.idc.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Singh N, et al. Cryptococcosis in solid organ transplant recipients: current state of the science. Clin Infect Dis. 2008;47(10):1321–7. doi: 10.1086/592690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.French N, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002;16(7):1031–8. doi: 10.1097/00002030-200205030-00009. [DOI] [PubMed] [Google Scholar]
- 6.Dromer F, et al. Determinants of disease presentation and outcome during cryptococcosis: the CryptoA/D study. PLoS Med. 2007;4(2):e21. doi: 10.1371/journal.pmed.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perfect JR, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010;50(3):291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett JE, Kwon-Chung KJ, Howard DH. Epidemiologic differences among serotypes of Cryptococcus neoformans. Am J Epidemiol. 1977;105(6):582–6. doi: 10.1093/oxfordjournals.aje.a112423. [DOI] [PubMed] [Google Scholar]
- 9.Nishikawa MM, et al. Serotyping of 467 Cryptococcus neoformans isolates from clinical and environmental sources in Brazil: analysis of host and regional patterns. J Clin Microbiol. 2003;41(1):73–7. doi: 10.1128/JCM.41.1.73-77.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiremath SS, et al. Long-distance dispersal and recombination in environmental populations of Cryptococcus neoformans var. grubii from India. Microbiology. 2008;154(Pt 5):1513–24. doi: 10.1099/mic.0.2007/015594-0. [DOI] [PubMed] [Google Scholar]
- 11.Guillot L, et al. Enhanced innate immune responsiveness to pulmonary Cryptococcus neoformans infection is associated with resistance to progressive infection. Infect Immun. 2008;76(10):4745–56. doi: 10.1128/IAI.00341-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez-Mejorada G, Rosales C. Signal transduction by immunoglobulin Fc receptors. J Leukoc Biol. 1998;63(5):521–33. doi: 10.1002/jlb.63.5.521. [DOI] [PubMed] [Google Scholar]
- 13.Vecchiarelli A, et al. Role of human alveolar macrophages as antigen-presenting cells in Cryptococcus neoformans infection. Am J Respir Cell Mol Biol. 1994;11(2):130–7. doi: 10.1165/ajrcmb.11.2.8049074. [DOI] [PubMed] [Google Scholar]
- 14.Vecchiarelli A, et al. Encapsulation of Cryptococcus neoformans regulates fungicidal activity and the antigen presentation process in human alveolar macrophages. Clin Exp Immunol. 1994;98(2):217–23. doi: 10.1111/j.1365-2249.1994.tb06128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vecchiarelli A, et al. Purified capsular polysaccharide of Cryptococcus neoformans induces interleukin-10 secretion by human monocytes. Infect Immun. 1996;64(7):2846–9. doi: 10.1128/iai.64.7.2846-2849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abe K, et al. Th1-Th2 cytokine kinetics in the bronchoalveolar lavage fluid of mice infected with Cryptococcus neoformans of different virulences. Microbiol Immunol. 2000;44(10):849–55. doi: 10.1111/j.1348-0421.2000.tb02573.x. [DOI] [PubMed] [Google Scholar]
- 17.Arora S, et al. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J Immunol. 2005;174(10):6346–56. doi: 10.4049/jimmunol.174.10.6346. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez Y, et al. Distinct roles for IL-4 and IL-10 in regulating T2 immunity during allergic bronchopulmonary mycosis. J Immunol. 2005;174(2):1027–36. doi: 10.4049/jimmunol.174.2.1027. [DOI] [PubMed] [Google Scholar]
- 19.Herring AC, et al. Induction of interleukin-12 and gamma interferon requires tumor necrosis factor alpha for protective T1-cell-mediated immunity to pulmonary Cryptococcus neoformans infection. Infect Immun. 2002;70(6):2959–64. doi: 10.1128/IAI.70.6.2959-2964.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huffnagle GB, et al. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J Leukoc Biol. 1994;55(1):35–42. doi: 10.1002/jlb.55.1.35. [DOI] [PubMed] [Google Scholar]
- 21.Okano M, et al. Interleukin-4-independent production of Th2 cytokines by nasal lymphocytes and nasal eosinophilia in murine allergic rhinitis. Allergy. 2000;55(8):723–31. doi: 10.1034/j.1398-9995.2000.00429.x. [DOI] [PubMed] [Google Scholar]
- 22.Olszewski MA, et al. Regulatory effects of macrophage inflammatory protein 1alpha/CCL3 on the development of immunity to Cryptococcus neoformans depend on expression of early inflammatory cytokines. Infect Immun. 2001;69(10):6256–63. doi: 10.1128/IAI.69.10.6256-6263.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snelgrove RJ, et al. In the absence of reactive oxygen species, T cells default to a Th1 phenotype and mediate protection against pulmonary Cryptococcus neoformans infection. J Immunol. 2006;177(8):5509–16. doi: 10.4049/jimmunol.177.8.5509. [DOI] [PubMed] [Google Scholar]
- 24.Voelz K, Lammas DA, May RC. Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect Immun. 2009;77(8):3450–7. doi: 10.1128/IAI.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill JO. CD4+ T cells cause multinucleated giant cells to form around Cryptococcus neoformans and confine the yeast within the primary site of infection in the respiratory tract. J Exp Med. 1992;175(6):1685–95. doi: 10.1084/jem.175.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibuya K, et al. Granuloma and cryptococcosis. J Infect Chemother. 2005;11(3):115–22. doi: 10.1007/s10156-005-0387-x. [DOI] [PubMed] [Google Scholar]
- 27.Olszewski MA, Zhang Y, Huffnagle GB. Mechanisms of cryptococcal virulence and persistence. Future Microbiol. 2010;5(8):1269–88. doi: 10.2217/fmb.10.93. [DOI] [PubMed] [Google Scholar]
- 28.Guerrero A, et al. Cryptococcus neoformans variants generated by phenotypic switching differ in virulence through effects on macrophage activation. Infect Immun. 2010;78(3):1049–57. doi: 10.1128/IAI.01049-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muller U, et al. A gene-dosage effect for interleukin-4 receptor alpha-chain expression has an impact on Th2-mediated allergic inflammation during bronchopulmonary mycosis. J Infect Dis. 2008;198(11):1714–21. doi: 10.1086/593068. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, et al. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. Am J Pathol. 2009;175(6):2489–500. doi: 10.2353/ajpath.2009.090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller U, et al. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. J Immunol. 2007;179(8):5367–77. doi: 10.4049/jimmunol.179.8.5367. [DOI] [PubMed] [Google Scholar]
- 32.Hoag KA, et al. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am J Respir Cell Mol Biol. 1995;13(4):487–95. doi: 10.1165/ajrcmb.13.4.7546779. [DOI] [PubMed] [Google Scholar]
- 33.Wormley FL, Jr., et al. Protection against cryptococcosis by using a murine gamma interferon-producing Cryptococcus neoformans strain. Infect Immun. 2007;75(3):1453–62. doi: 10.1128/IAI.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shao X, et al. An innate immune system cell is a major determinant of species-related susceptibility differences to fungal pneumonia. J Immunol. 2005;175(5):3244–51. doi: 10.4049/jimmunol.175.5.3244. [DOI] [PubMed] [Google Scholar]
- 35.Diamond RD, Bennett JE. Growth of Cryptococcus neoformans within human macrophages in vitro. Infect Immun. 1973;7(2):231–6. doi: 10.1128/iai.7.2.231-236.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldman DL, et al. Persistent Cryptococcus neoformans pulmonary infection in the rat is associated with intracellular parasitism, decreased inducible nitric oxide synthase expression, and altered antibody responsiveness to cryptococcal polysaccharide. Infect Immun. 2000;68(2):832–8. doi: 10.1128/iai.68.2.832-838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldmesser M, Tucker S, Casadevall A. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol. 2001;9(6):273–8. doi: 10.1016/s0966-842x(01)02035-2. [DOI] [PubMed] [Google Scholar]
- 38.Levitz SM, et al. Cryptococcus neoformans resides in an acidic phagolysosome of human macrophages. Infect Immun. 1999;67(2):885–90. doi: 10.1128/iai.67.2.885-890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Charlier C, et al. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun. 2009;77(1):120–7. doi: 10.1128/IAI.01065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chretien F, et al. Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J Infect Dis. 2002;186(4):522–30. doi: 10.1086/341564. [DOI] [PubMed] [Google Scholar]
- 41.Kechichian TB, Shea J, Del Poeta M. Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect Immun. 2007;75(10):4792–8. doi: 10.1128/IAI.00587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luberto C, et al. Identification of App1 as a regulator of phagocytosis and virulence of Cryptococcus neoformans. J Clin Invest. 2003;112(7):1080–94. doi: 10.1172/JCI18309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McQuiston T, Luberto C, Del Poeta M. Role of host sphingosine kinase 1 in the lung response against Cryptococcosis. Infect Immun. 2010;78(5):2342–52. doi: 10.1128/IAI.01140-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Hermoso D, Janbon G, Dromer F. Epidemiological evidence for dormant Cryptococcus neoformans infection. J Clin Microbiol. 1999;37(10):3204–9. doi: 10.1128/jcm.37.10.3204-3209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dromer F, Ronin O, Dupont B. Isolation of Cryptococcus neoformans var. gattii from an Asian patient in France: evidence for dormant infection in healthy subjects. J Med Vet Mycol. 1992;30(5):395–7. [PubMed] [Google Scholar]
- 46.Abadi J, Pirofski L. Antibodies reactive with the cryptococcal capsular polysaccharide glucuronoxylomannan are present in sera from children with and without human immunodeficiency virus infection. J Infect Dis. 1999;180(3):915–9. doi: 10.1086/314953. [DOI] [PubMed] [Google Scholar]
- 47.Abadi J, et al. Cryptococcosis in children with AIDS. Clin Infect Dis. 1999;28(2):309–13. doi: 10.1086/515130. [DOI] [PubMed] [Google Scholar]
- 48.Davis J, et al. Serologic evidence for regional differences in pediatric cryptococcal infection. Pediatr Infect Dis J. 2007;26(6):549–51. doi: 10.1097/INF.0b013e318047e073. [DOI] [PubMed] [Google Scholar]
- 49.Goldman DL, et al. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics. 2001;107(5):E66. doi: 10.1542/peds.107.5.e66. [DOI] [PubMed] [Google Scholar]
- 50.Houpt DC, et al. Occurrences, immunoglobulin classes, and biological activities of antibodies in normal human serum that are reactive with Cryptococcus neoformans glucuronoxylomannan. Infect Immun. 1994;62(7):2857–64. doi: 10.1128/iai.62.7.2857-2864.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casadevall A, Pirofski L. Host-pathogen interactions: the attributes of virulence. J Infect Dis. 2001;184(3):337–44. doi: 10.1086/322044. [DOI] [PubMed] [Google Scholar]
- 52.Feldmesser M, et al. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun. 2000;68(7):4225–37. doi: 10.1128/iai.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shea JM, et al. The cryptococcal enzyme inositol phosphosphingolipid-phospholipase C confers resistance to the antifungal effects of macrophages and promotes fungal dissemination to the central nervous system. Infect Immun. 2006;74(10):5977–88. doi: 10.1128/IAI.00768-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldman D, Lee SC, Casadevall A. Pathogenesis of pulmonary Cryptococcus neoformans infection in the rat. Infect Immun. 1994;62(11):4755–61. doi: 10.1128/iai.62.11.4755-4761.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma H, et al. Expulsion of live pathogenic yeast by macrophages. Curr Biol. 2006;16(21):2156–60. doi: 10.1016/j.cub.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 56.Alvarez M, Casadevall A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr Biol. 2006;16(21):2161–5. doi: 10.1016/j.cub.2006.09.061. [DOI] [PubMed] [Google Scholar]
- 57.Johnston SA, May RC. The human fungal pathogen Cryptococcus neoformans escapes macrophages by a phagosome emptying mechanism that is inhibited by Arp2/3 complex-mediated actin polymerisation. PLoS Pathog. 2010;6(8) doi: 10.1371/journal.ppat.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levitz SM, et al. Chloroquine induces human mononuclear phagocytes to inhibit and kill Cryptococcus neoformans by a mechanism independent of iron deprivation. J Clin Invest. 1997;100(6):1640–6. doi: 10.1172/JCI119688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cherniak R, et al. Variation in the structure of glucuronoxylomannan in isolates from patients with recurrent cryptococcal meningitis. Infect Immun. 1995;63(5):1899–905. doi: 10.1128/iai.63.5.1899-1905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zaragoza O, et al. The capsule of the fungal pathogen Cryptococcus neoformans. Adv Appl Microbiol. 2009;68:133–216. doi: 10.1016/S0065-2164(09)01204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zaragoza O, et al. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol. 2008;10(10):2043–57. doi: 10.1111/j.1462-5822.2008.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu L, Tewari RP, Williamson PR. Laccase protects Cryptococcus neoformans from antifungal activity of alveolar macrophages. Infect Immun. 1999;67(11):6034–9. doi: 10.1128/iai.67.11.6034-6039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu X, et al. Laccase of Cryptococcus neoformans is a cell wall-associated virulence factor. Infect Immun. 2001;69(9):5589–96. doi: 10.1128/IAI.69.9.5589-5596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu X, et al. Copper-mediated reversal of defective laccase in a Deltavph1 avirulent mutant of Cryptococcus neoformans. Mol Microbiol. 2003;47(4):1007–14. doi: 10.1046/j.1365-2958.2003.03340.x. [DOI] [PubMed] [Google Scholar]
- 65.Wagner D, et al. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell’s endosomal system. J Immunol. 2005;174(3):1491–500. doi: 10.4049/jimmunol.174.3.1491. [DOI] [PubMed] [Google Scholar]
- 66.Serafin-Lopez J, et al. The effect of iron on the expression of cytokines in macrophages infected with Mycobacterium tuberculosis. Scand J Immunol. 2004;60(4):329–37. doi: 10.1111/j.0300-9475.2004.01482.x. [DOI] [PubMed] [Google Scholar]
- 67.Pan X, et al. Modulation of iron homeostasis in macrophages by bacterial intracellular pathogens. BMC Microbiol. 2010;10:64. doi: 10.1186/1471-2180-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erb-Downward JR, Huffnagle GB. Cryptococcus neoformans produces authentic prostaglandin E2 without a cyclooxygenase. Eukaryot Cell. 2007;6(2):346–50. doi: 10.1128/EC.00336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Noverr MC, et al. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect Immun. 2003;71(3):1538–47. doi: 10.1128/IAI.71.3.1538-1547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noverr MC, et al. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect Immun. 2001;69(5):2957–63. doi: 10.1128/IAI.69.5.2957-2963.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Odom A, et al. The immunosuppressant FK506 and its nonimmunosuppressive analog L-685,818 are toxic to Cryptococcus neoformans by inhibition of a common target protein. Antimicrob Agents Chemother. 1997;41(1):156–61. doi: 10.1128/aac.41.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kozubowski L, Lee SC, Heitman J. Signalling pathways in the pathogenesis of Cryptococcus. Cell Microbiol. 2009;11(3):370–80. doi: 10.1111/j.1462-5822.2008.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Panepinto JC, Williamson PR. Intersection of fungal fitness and virulence in Cryptococcus neoformans. FEMS Yeast Res. 2006;6(4):489–98. doi: 10.1111/j.1567-1364.2006.00078.x. [DOI] [PubMed] [Google Scholar]
- 74.Fan W, et al. Cryptococcus neoformans gene expression during murine macrophage infection. Eukaryot Cell. 2005;4(8):1420–33. doi: 10.1128/EC.4.8.1420-1433.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 76.Cox GM, et al. Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect Immun. 2003;71(1):173–80. doi: 10.1128/IAI.71.1.173-180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Narasipura SD, Chaturvedi V, Chaturvedi S. Characterization of Cryptococcus neoformans variety gattii SOD2 reveals distinct roles of the two superoxide dismutases in fungal biology and virulence. Mol Microbiol. 2005;55(6):1782–800. doi: 10.1111/j.1365-2958.2005.04503.x. [DOI] [PubMed] [Google Scholar]
- 78.Garcia J, et al. Mathematical modeling of pathogenicity of Cryptococcus neoformans. Mol Syst Biol. 2008;4:183. doi: 10.1038/msb.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Siafakas AR, et al. Cell wall-linked cryptococcal phospholipase B1 is a source of secreted enzyme and a determinant of cell wall integrity. J Biol Chem. 2007;282(52):37508–14. doi: 10.1074/jbc.M707913200. [DOI] [PubMed] [Google Scholar]
- 80.Siafakas AR, et al. Lipid rafts in Cryptococcus neoformans concentrate the virulence determinants phospholipase B1 and Cu/Zn superoxide dismutase. Eukaryot Cell. 2006;5(3):488–98. doi: 10.1128/EC.5.3.488-498.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wright LC, et al. Cryptococcal lipid metabolism: phospholipase B1 is implicated in transcellular metabolism of macrophage-derived lipids. Eukaryot Cell. 2007;6(1):37–47. doi: 10.1128/EC.00262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jung WH, et al. Iron regulation of the major virulence factors in the AIDS-associated pathogen Cryptococcus neoformans. PLoS Biol. 2006;4(12):e410. doi: 10.1371/journal.pbio.0040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jung WH, et al. Role of ferroxidases in iron uptake and virulence of Cryptococcus neoformans. Eukaryot Cell. 2009;8(10):1511–20. doi: 10.1128/EC.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Waterman SR, et al. Role of a CUF1/CTR4 copper regulatory axis in the virulence of Cryptococcus neoformans. J Clin Invest. 2007;117(3):794–802. doi: 10.1172/JCI30006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu G, et al. PI3K signaling of autophagy is required for starvation tolerance and virulenceof Cryptococcus neoformans. J Clin Invest. 2008;118(3):1186–97. doi: 10.1172/JCI32053. [DOI] [PMC free article] [PubMed] [Google Scholar]