SUMMARY
Short-term tinnitus develops shortly after the administration of a high dose of salicylate. Since salicylate selectively potentiates N-methyl- D-aspartate (NMDA) currents in spiral ganglion neurons, it may play a vital role in tinnitus by amplifying NMDA-mediated neurotransmission. The aim of this study was to determine whether systemic treatment with a NMDA channel blocker, memantine, could prevent salicylate-induced tinnitus in animals. Additional experiments were performed to evaluate the effect of memantine on the auditory brainstem response (ABR) and distortion product otoacoustic emissions (DPOAE) to test for changes in hearing function. Thirty-six rats were divided into 3 groups and treated daily for four consecutive days. One group (n = 12) was injected with salicylate (300 mg/kg/d, IP), the second (n = 12) was treated with memantine (5 mg/kg/d, IP) and the third group (n = 12) was injected with salicylate and memantine. All rats were tested for tinnitus and hearing loss at 2, 24, 48 and 72 h after the first drug administration and 24 h post treatment; tinnituslike behaviour was assessed with gap prepulse inhibition of acoustic startle (GPIAS), and hearing function was measured with DPOAE, ABR and noise burst prepulse inhibition of acoustic startle (NBPIAS). Rats in the salicylate group showed impaired GPIAS indicative of transient tinnitus-like behaviour near 16 kHz that recovered 24 h after the last salicylate treatment. Memantine did not cause a significant change in GPIAS. Combined injection of salicylate and memantine significantly attenuated GPIAS tinnitus-like behaviour at 48 hours after the first injection. None of the treatments induced permanent threshold shifts in the ABR and DPOAE, which recovered completely within one day post treatment. Animals treated with salicylate plus memantine showed results comparable to animals treated with salicylate alone, confirming that there is no effect of memantine on DPOAE which reflects OHC function. The present study confirms the role of cochlear NMDA receptors in the induction of salicylate-induced tinnitus.
KEY WORDS: Tinnitus, Memantine, Salicylate, Startle reflex, NDMA receptors, Rats
RIASSUNTO
Il sodio salicilato, principio attivo dell'aspirina, è una molecola in grado di indurre un acufene transitorio mediante l'attivazione dei recettori N-metil-D-aspartato (NMDA) a livello periferico e centrale. L'obiettivo primario di questo studio è di valutare la potenzialità della memantina, inibitore selettivo dei recettori NMDA, nel contrastare l'insorgenza e la persistenza dell'acufene indotto da salicilato in un modello animale. Obiettivo secondario è lo studio degli effetti della memantina sulla funzione uditiva e sulle cellule ciliate esterne. Nel nostro studio sono stati utilizzati 36 ratti divisi in tre gruppi: nel primo gruppo (n = 12) gli animali sono stati trattati con salicilato (300 mg/kg/d, IP), nel secondo (n = 12) con memantina (5 mg/kg/d, IP), nel terzo (n = 12) con entrambi. In tutti gli animali è stato studiato l'acufene con la tecnica GPIAS ad intervalli di 2, 24, 48, 72 e 96 ore dalla prima somministrazione e la funzione uditiva mediante i prodotti di distorsione (DPOAE) ed i potenziali evocati uditivi (ABR). Negli animali trattati con salicilato la nostra metodica ha evidenziato la presenza di un acufene con frequenza vicina ai 16 kHz insorto dopo la prima somministrazione e risoltosi spontaneamente 24 ore dopo l'ultima. Negli animali trattati con salicilato e memantina l'acufene, seppur presente, è risultato significativamente attenuato, prevalentemente durante il secondo giorno di trattamento. Né il salicilato né la memantina hanno causato alterazioni permanenti della funzione uditiva; le variazioni registrate mediante i prodotti di distorsione sono regredite al termine del trattamento. Il nostro studio conferma il ruolo dei recettori NMDA nell'acufene da salicilato e le potenzialità della memantina nel contrastarne l'insorgenza e la persistenza. Data la facile reperibilità del farmaco, già utilizzato nel trattamento della malattia di Alzheimer e del morbo di Parkinson, ed i risultati incoraggianti ottenuti nel modello animale, sono auspicabili ulteriori approfondimenti nell'uomo.
Introduction
Subjective tinnitus, defined as the perception of a sound when no external stimulation is present, is a condition that affects a large portion of the world population, with over 16 million subjects in the US reporting frequent tinnitus 1. Tinnitus has been widely studied in humans and animals to better understand the molecular mechanisms that underlie its onset and persistence, and to identify drugs that could be used for treatment.
Short-term tinnitus has been reported following administration of high-doses of sodium salicylate. The molecular mechanisms through which salicylate induces tinnitus have been explored 2, especially its effects on the cyclooxygenase which blocks the conversion of arachidonic acid to prostaglandin H2 3 4. The increased concentration of arachidonic acid acts on N-methyl-D-aspartic acid (NMDA) receptors, inducing both peripheral and central effects. NMDA receptors are expressed on the synapses between inner hair cells and cochlear spiral ganglion neurons 5. In vitro, salicylate potentiates the NMDA class of glutamatergic currents on cochlear spiral ganglion neurons. Salicylate also impairs outer hair cell (OHC) electromotility 6, although prolonged treatment has been reported to strengthen OHC motility 7 8 and reduces blood flow in the cochlea 9. High doses of salicylate increase the threshold and reduce the amplitude of the compound action potential (CAP), but salicylate paradoxically results in hyperactivity in the central auditory cortex 10.
Memantine, a drug recently approved for the treatment of moderate to severe Alzheimer's disease 11, has been reported to suppress excitatory neurotransmission between the hair cell and auditory nerve afferent fibers by blocking NMDA receptors 12, and is likely to exert effects on the central auditory pathways 13. Memantine has been previously studied for its ability to suppress tinnitus in animals 14 and humans 15 and found to have little or no effect; however, Zheng et al., in 2012 16, reported encouraging effects of memantine in noise-induced tinnitus in the rat.
The animal model used to study tinnitus is the gap prepulse inhibition of the startle reflex (GPIAS); the method was developed by Turner in 2006 17 and later adopted and optimized by others 18-23. Since GPIAS does not require any training and relies on a reflex response, it is a very efficient method of testing for tinnitus compared to time consuming operant conditioning techniques, thereby allowing a larger number of animals to be evaluated 24.
The primary goal of this study was to determine if memantine, a NMDA antagonist, would affect salicylateinduced tinnitus. A secondary goal was to determine what effects memantine or the combination of memantine and salicylate would have on DPOAE and ABR.
Materials and methods
Animals
Thirty-six adult male Sprague Dawley rats (3-5 months, 220-450 g) were used for this study. Rats were divided into three groups: a SAL group (n = 12) injected IP with salicylate (300 mg/kg/d) in bacteriostatic saline, 50 mg/ ml (Sigma); a MEM group (n = 12) treated with memantine at a dose of 5 mg/kg/d diluted in bacteriostatic saline, 50 mg/ml (Sigma); a SAL+MEM group (n = 12) injected with salicylate and memantine combined at the dosage used in the other groups. All animals were treated daily for four consecutive days; drug administration was performed 2 h before testing for tinnitus.
The experimental protocol was approved by the Institutional Animal Care and Use Committee of the Catholic University of the Sacred Heart, Rome Italy. Animals were housed in a colony with a 12 h light-dark cycle; food and water were available ad libitum.
Gap prepulse inhibition of acoustic startle
Tinnitus was assessed using gap prepulse inhibition of acoustic startle as described in our previous investigation 24. Rats were placed in an acoustically transparent wire mesh cage (7.2 cm W, 20 cm L, 6.5 cm H) on a Plexiglas platform; the platform (20 cm × 10 cm) rested on a 50 mm piezoelectric transducer (MCM 28-745). The test apparatus was located in a soundproof chamber equipped with a tweeter (Fostex FT17H) on the chamber's ceiling about 15 cm above the rat's head. The continuous background noise, gap stimulus, prepulse noise burst and startle stimulus were generated with a digital-to-analogue converter at ~100 kHz sampling rate (Tucker Davis Technologies, RP2.1, PA5, SA1). Startle amplitude measured by the piezoelectric transducer was amplified (10-100×), low-pass filtered at 1,000 Hz; WPI, USA) and fed to the analogue-to-digital converter on a separate data acquisition module (TDT, RP2.1) using custom software.
GPIAS sessions were composed of 80 silent gaps trials (gap) embedded in narrow band noise and 80 no-gap trials (no-gap) in the narrow band noise (Fig. 1A-C). Twenty gap and 20 no-gap trials were made at each of the four narrow band noises centred at 6, 12, 16 or 20 kHz. Gap and no-gap trials were presented in pairs in random order. Trials were separated by a variable period ranging from 7 to 15 sec long. Animals were given a 2-min acclimation period at the beginning of each session during which no gaps or startle sounds occurred. Gap trials were composed of a 60 dB SPL continuous narrow band noise (1,000 Hz wide, centered at 6, 12, 16 and 20 kHz), and a 50 msec startle stimulus (broadband noise burst, 116 dB SPL, 50 msec length, 5 msec rise/fall time) preceded by a 100 msec silent gap that ended 100 msec before the onset of the startle stimulus. In no-gap trials, the background sound was continuous without a silent period preceding the startle stimulus. The amplitude of the startle reflex signal was measured as the root-mean-square (RMS) voltage on gap and no-gap trials. Noise burst prepulse inhibition of the acoustic startle reflex (NBPIAS) was used to determine the audibility of the narrow band noises used for GPIAS assessment (Fig. 1DE). NBPIAS was recorded using the same equipment as GPIAS except that the background noise was removed and the startle stimulus was presented alone (i.e. in quiet) or was preceded by a 60 dB SPL narrow band noise burst (1,000 Hz wide, 100 msec, 5 msec rise/fall time, centred at 6, 12, 16 or 20 kHz.). Twenty noise burst and 20 quiet trials were made at each tested frequency.
Fig. 1.
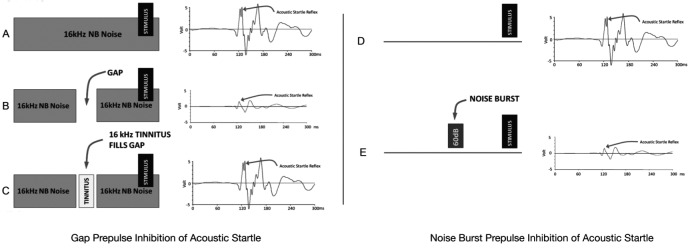
Schematics of the animal model used to highlight the presence and time course of tinnitus in rats. Gap prepulse inhibition of acoustic startle: (A) Following the startle stimulus without a prepulse gap results in a large amplitude startle reflex. (B) When a 50 msec gap prepulse is inserted prior to the startle stimulus, the startle reflex response is significantly reduced. (C) Tinnitus fills in the gap and the animal can no longer hear the silent gap, resulting in a reduction of the startle response similar to the no-gap condition. (D) Noise burst prepulse inhibition of acoustic startle: NBPIAS is used to evaluate whether the animal can hear the background noise used in the GPIAS paradigm. In NBPIAS, the startle stimulus is presented in a silent environment; this results in a large startle response. (E) When a 60 dB noise burst prepulse precedes the startle stimulus, the startle response is reduced.
Gap and noise burst prepulse inhibition of acoustic startle were calculated as a percentage for each frequency using the formulas 1-(gap/no-gap) for GPIAS and 1- (noise burst/quiet) for NBPIAS. For each frequency, a significant reduction of GPIAS was interpreted as behavioural evidence of tinnitus. Conversely, significant inhibition of the startle response in NBPIAS sessions was interpreted as evidence that the animal could hear the narrow band noise used in GPIAS sessions.
Animals were tested daily with GPIAS and NBPIAS before and 2 h after each drug administration; measurements were obtained for five consecutive days (four measurements during treatment; one post treatment).
Auditory brainstem response recordings
Hearing function was evaluated in all animals using the auditory brainstem response (ABR). ABR thresholds were obtained at 6, 12, 16, 24 and 32 kHz in all animals before and 14 days after the end of the drug treatment. Rats were anaesthetized (ketamine 10 mg/kg) and placed in a sound attenuating booth; the non-inverting (+) electrode was inserted at the vertex, the inverting (-) electrode was placed near the pinna of the test ear and the ground electrode was placed near the pinna of the opposite ear. A TDT System 3 (BioSigRP, Tucker–Davis Technologies, Alachua, Florida, USA) data acquisition system was used for stimulus generation and data acquisition. Tone bursts corresponding to tested frequencies were presented monaurally in an open field (Fostex, TD28D, USA) with the speaker pointed towards the test ear at a distance of 1 cm; the contralateral ear was plugged with a silicon plug. Responses were filtered (100-3,000 Hz band pass), digitized (10 kHz sampling rate) and averaged over 1,000 samples at each frequency. Threshold testing began with the stimulus presented at 100 dB SPL in order to generate a clear ABR waveform; then the intensity was reduced in 10 dB steps until the ABR response disappeared. Next, the intensity of the stimulus was increased in 5 dB steps from below threshold until the ABR response reappeared. The ABR threshold was defined as the lowest intensity at which the ABR could be detected and replicated 25 26.
Distortion product otoacoustic emissions
DPOAEs were measured unilaterally using an otoacoustic emission system (Intelligent Hearing System, Miami, FL, USA). The f2/f1 ratio of the primary tones was set to 1.2. DPOAE input/output functions were measured at f2 frequencies of 4, 8, 12, 16 and 20 kHz. The f1 intensity, L1, always presented +10 dB above the f2 intensity, L2. Animals were anaesthetized with ketamine as described above and placed on a heating pad in a sound-attenuating booth. The probe assembly was placed in the animal's external ear canal. Input/output functions were obtained by increasing L1 intensity from 25 to 70 dB SPL at f2 frequencies of 4, 8, 12, 16 and 20 kHz (32 sweeps per frequency pair). DPOAEs were recorded before and 2 h after each drug treatment for 5 consecutive days.
Statistical analysis
GPIAS and NBPIAS data were analyzed using a two-way repeated measures analysis of variance (RM-ANOVA, α < 0.05); post-hoc testing was performed using Tukey's test for multiple comparisons. Significant differences between frequency-specific data recorded at each time point and baseline values were analyzed using a one-way ANOVA (p < 0.05). Statistical analysis of ABR and DPOAE measurements were performed using a one-way ANOVA with post-hoc Student-Newman-Keuls analysis. All results were presented as mean ± SEM.
Results
Tinnitus assessment
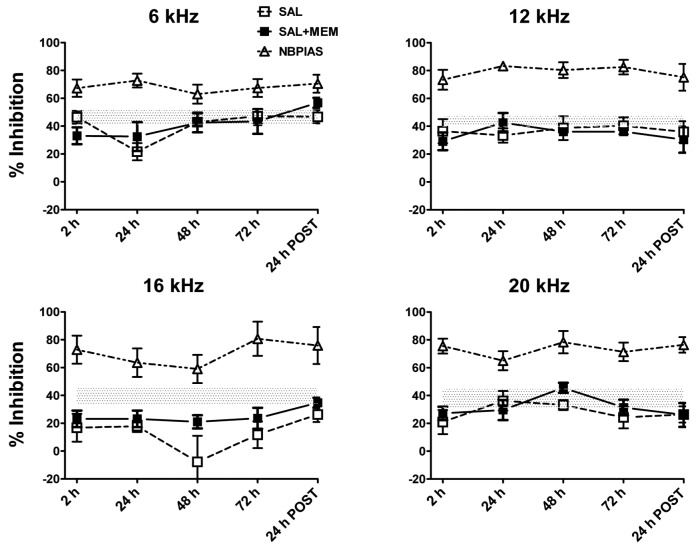
Figure 1A-C is a schematic of the GPIAS paradigm that shows the stimulus conditions and hypothetical results for no-gap, gap and tinnitus conditions. Figure 2 shows the percent GPIAS values at 6, 12, 16 and 20 kHz before and at various time points during the four days of drug treatment. Baseline GPIAS values are represented by the horizontal dotted line (line width equals baseline standard deviation; baseline values were 43.8, 42.3, 37.6 and 38.8% for 6, 12, 16 and 20 kHz respectively.
Fig. 2.
Data obtained in our animal groups showing the time course of tinnitus for different treatments. Percent GPIAS in rats treated with salicylate alone or salicylate and memantine plotted by frequency; NBPIAS data are also shown for comparison. The horizontal dotted line represents baseline data. Rats treated with memantine alone showed no evidence of tinnitus (data not shown). Rats treated with salicylate alone showed transient tinnitus-like behaviour with a pitch near 16 kHz, starting 2 hours after the first injection, lasting for the entire length of treatment (4 days) and resolving spontaneously 24 hours after the last day of drug administration (24 h POST). In rats treated with salicylate and memantine, tinnitus-like behaviour was greatly attenuated, particularly during the first 48 hours of treatment, suggesting that memantine must suppress tinnitus-like behaviour at peripheral or central NMDA receptors.
Rats treated with salicylate alone (SAL) showed a significant reduction in GPIAS at 16 kHz, consistent with tinnituslike behaviour with a pitch near 16 kHz. A significant reduction occurred between 2 and 72 h with a peak at 48 hours; GPIAS returned near to baseline levels 24 h after the last day of drug administration. A statistically significant decrease in mean GPIAS was observed at 16 kHz at 2 h (p = 0.041), 24 h (p = 0.036), 48 h (p = 0.019) and 72 h (p = 0.047) during treatments; 24 h after the end of the treatment mean values returned near to baseline levels (34.95%; p = 0.65). GPIAS values did not show a significant change at 6, 12 and 20 kHz during the salicylate treatment (2 to 72 h).
Rats treated with memantine alone (MEM) showed no significant changes in GPIAS compared to baseline values during the entire length of treatment (data not shown). Rats treated with a combination of salicylate and memantine (SAL+MEM) showed less reduction in GPIAS than rats treated with salicylate alone (SAL), particularly at 16 kHz during the first 48 hours of treatment. There was a statistically significant difference at 48 h between the SAL and the SAL+MEM groups (p = 0.023).
NBPIAS was also tested in the SAL, SAL+MEM and MEM groups; no significant changes were observed over the entire testing period between baseline measures and values obtained during treatments and post-treatment. Data for all groups are compared in Figure 2.
ABR
Baseline ABR threshold values recorded before treatment did not show a statistically significant group difference among the SAL, MEM and SAL+MEM groups (Two-way ANOVA, p = 0.36). No significant difference was seen between baseline and posttreatment thresholds measured 14 days after the end of treatment (p = 0.41). These results indicate that salicylate, memantine or the combination of the two does not produce any long term change in hearing thresholds.
DPOAEs
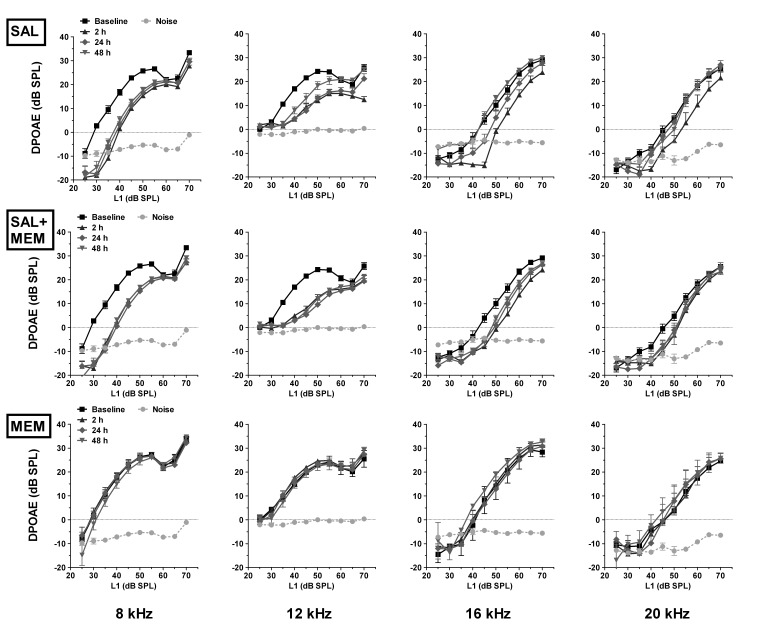
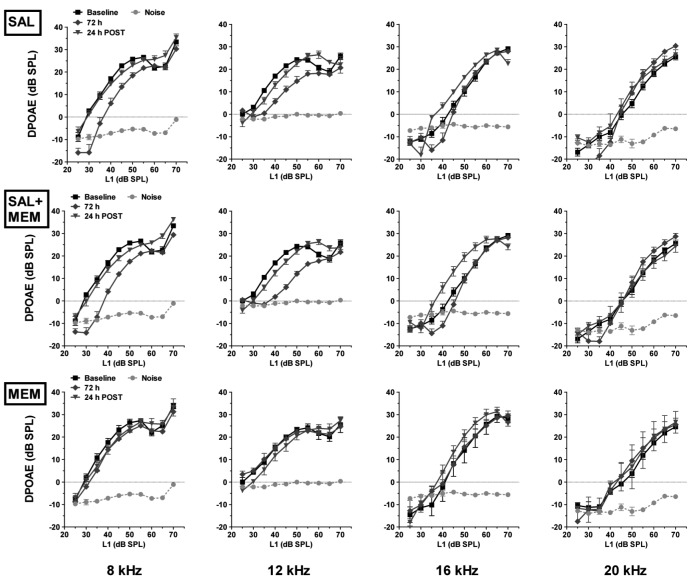
DPOAEs were tested in all animals at 4, 8, 12, 16 and 20 kHz, and were measured before, during (2, 24, 48 and 72 h) and 24 h after SAL, MEM and SAL+MEM treatments. A progressive decrease in amplitude was observed in the salicylate group for low-level DPOAEs at 8, 12 and 16 kHz. High-level DPOAEs were slightly affected; the largest changes occurred 2 h after salicylate injection at 8 and 12 kHz. Amplitudes returned to near baseline values within one day post-treatment. Memantine alone had no effect on DPOAE amplitude. Treatment with SAL+MEM resulted in a decline of DPOAE amplitude comparable to salicylate alone. Data for all groups (SAL, MEM, SAL+MEM) are plotted in Figures 3A and 3B.
Fig. 3.
The effect of salicylate and memantine on outer hair cells. DPOAE measurements in animals treated with memantine, salicylate or a combination of both. Data from baseline and 2, 24 and 48 h time points are shown in Fig 3A; data from baseline and 72, 24 h post time points are shown in Fig 3B. Memantine alone had no effect on DPOAE amplitude whereas salicylate alone caused a progressive decrease in DPOAE over the 4-day treatment. The combination of both drugs resulted in DPOAE amplitudes similar to those obtained in animals treated with salicylate alone.
Discussion
The effect of memantine on salicylate-induced tinnitus
Memantine, a non-competitive NMDA antagonist that affects neurotransmission between inner hair cells and afferent auditory nerve fibers, has been hypothesized to suppress tinnitus. Memantine is currently approved for the treatment of moderate to severe cases of Alzheimer's disease, and has been proposed for the treatment of Parkinson's disease and certain forms of dementia 11. Given its availability in clinical settings, memantine and its analogues have been considered as potential treatments for tinnitus 27. To test this hypothesis, Lobarinas and colleagues treated rats with a moderate dose (150 mg/kg) of salicylate and obtained behavioural evidence of tinnitus using an operant lick suppression technique 14. Rats were then treated with 1.5 or 3 mg/kg of memantine combined with 150 mg/kg of salicylate to determine if memantine would suppress tinnitus-like behaviour. While tinnitus was not completely suppressed by 1.5 or 3 mg/kg memantine, the molecule tended to reduce the tinnitus-like behaviour, which was more pronounced at the higher dose. Therefore, while tinnitus was not completely abolished by memantine, the evidence suggested that tinnitus severity might be somewhat reduced. Lobarinas attempted to use a higher dose of memantine, but found that 10 mg/ kg disrupted the operant behaviour; consequently, higher doses of memantine were not evaluated for suppression of tinnitus.
Figueredo et al. published a randomized, double-blind placebo-controlled trial in which tinnitus was studied in 60 patients, and its severity was monitored using the Tinnitus Handicap Inventory (THI). In this study, memantine was administered at a dose of 20 mg per day for 90 days. They reported no statistically significant differences between patients in the memantine group and those in the placebo group 15. Taken together, these two studies suggest that memantine may have little or no effect on tinnitus.
In the current study, using GPIAS as our behavioural metric for tinnitus, we observed evidence of tinnitus at 16 kHz with 300 mg/kg of salicylate and found that the 5 mg/kg dose of memantine significantly reversed tinnitus- like GPIAS behaviour. Thus, our results suggest that a 5 mg/kg dose of memantine, nearly twice that used by Lobarinas, can suppress tinnitus. Importantly, animals treated with memantine alone did not show significant changes in GPIAS.
A recent paper from Zheng et al. 16 investigated the efficacy of a 5 mg/kg dose of memantine in rats with behavioural evidence of tinnitus assessed with an operant lick-suppression technique. They found that a 1 h, 110 dB, 16 kHz traumatizing tone induced tinnitus having a pitch of 32 kHz in five of eight exposed rats. After treatment with 5 mg/kg memantine, only 2 of the 5 rats continued to show evidence of noise-induced tinnitus, i.e., memantine eliminated signs of tinnitus in 3 of 5 rats.
Our results are consistent with those of Zheng suggesting that higher doses of memantine may suppress tinnitus. However, it should be noted that while the 5 mg/kg dose of memantine caused a statistically significant improvement in tinnitus, our GPIAS values during SAL+MEM were still slightly below their baseline GPIAS values. In other words, while 5 mg/kg memantine significantly improved tinnitus-like behaviour, it may not have completely suppressed tinnitus. Likewise, memantine only led to a reduction in noise-induced tinnitus in 60% of subjects in the Zheng study. It is conceivable that higher doses of memantine may be more effective in suppressing tinnitus; however, the behavioural side effects, as noted by Lobarinas 28 may outweigh the benefits.
Salicylate, memantine and auditory function
Our results show that memantine had no effect on DPOAE amplitudes during treatment. These results are consistent with the view that memantine acts on neuronal NMDA receptors, which are not expressed in adult OHC 29. In addition, our results show that memantine had no effect on OHC function, as reflected in DPOAE, when administered with salicylate; results are consistent with the view that memantine is not acting on the OHC. Short-term treatment with salicylate, memantine or the combination of the two had no permanent effects on ABR; thresholds recorded 14 days after drug treatments showed no differences compared to baseline. Serial DPOAE measurements from 2 to 72 h of salicylate treatment revealed a cumulative dose-dependent effect mainly on low-level DPOAEs at 8, 12 and 16 kHz; i.e. DPOAE amplitudes tended to decrease over the 3 day treatment. These results are consistent with previous results showing a gradual decline in DPOAE amplitude over several days of salicylate treatment 30. However, DPOAE amplitudes recovered to within normal limits within 1 day after the end of treatment. Since animals treated with salicylate and memantine showed DPOAE alteration comparable to salicylate alone, the effects of memantine on GPIAS are unlikely to be due to changes in OHC function.
Conclusions
The present study confirms that salicylate can induce transient, reversible tinnitus when administered at high doses. More importantly, our behavioural assessment of tinnitus using GPIAS suggests that a 5 mg/kg dose of memantine, which was nearly two fold greater than that used by Lobarinas, can significantly reduce tinnitus-like behaviour. Since higher doses (10 mg/kg) of memantine can disrupt behaviour, effective treatment of tinnitus using NMDA antagonists may require careful titration of the dose to obtain clinical efficacy without inducing deleterious side effects. The suppression of tinnitus-like behaviour in our study was achieved with systemic memantine treatment, it is therefore unclear if the therapeutic effect of memantine is occurring at the IHC-auditory nerve fibre synapse, as proposed by Puel et al. 5, or if it is occurring at multiple sites within the central auditory pathway. Given the encouraging results and clinical availability of memantine, it would be interesting to further explore its efficacy in humans with tinnitus. Since the effective therapeutic window for drug dosing appears to be relatively narrow, effective tinnitus therapy may require careful escalation of the dose of memantine to achieve optimal therapy with minimal side effects.
Acknowledgements
Research supported in part by grants from Fondi di Ateneo, UCSC Rome Italy and Office of Naval Research (N000141210731), NIH (R01DC009091; R01DC009219).
References
- 1.Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123:711–718. doi: 10.1016/j.amjmed.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Cazals Y. Auditory sensorineural alterations induced by salicylate. Prog Neurobiol. 2000;62:583–631. doi: 10.1016/s0301-0082(00)00027-7. [DOI] [PubMed] [Google Scholar]
- 3.Vane JR, Botting RM. Mechanism of action of anti-inflammatory drugs. Int J Tissue React. 1998;20:3–15. [PubMed] [Google Scholar]
- 4.Mitchell JA, Akarasereenont P, Thiemermann C, et al. Selectivity of nonsteroidal anti-inflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci USA. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruel J, Chabbert C, Nouvian R, et al. Salicylate enables cochlear arachidonic-acid-sensitive NMDA receptor responses. J Neurosci. 2008;28:7313–7323. doi: 10.1523/JNEUROSCI.5335-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ermilov SA, Murdock DR, El-Daye D, et al. Effects of salicylate on plasma membrane mechanics. J Neurophysiol. 2005;94:2105–2110. doi: 10.1152/jn.00414.2005. [DOI] [PubMed] [Google Scholar]
- 7.Chen GD, Kermany MH, Ralli M, et al. Too much of a good thing: long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hear Res. 2010;265:63–69. doi: 10.1016/j.heares.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang K, Huang ZW, Liu ZQ, et al. Long-term administration of salicylate enhances prestin expression in rat cochlea. Int J Audiol. 2009;48:18–23. doi: 10.1080/14992020802327998. [DOI] [PubMed] [Google Scholar]
- 9.Didier A, Miller JM, Nuttall AL. The vascular component of sodium salicylate ototoxicity in the guinea pig. Hear Res. 1993;69:199–206. doi: 10.1016/0378-5955(93)90108-d. [DOI] [PubMed] [Google Scholar]
- 10.Chen GD, Stolzberg D, Lobarinas E, et al. Salicylate-induced cochlear impairments, cortical hyperactivity and re-tuning, and tinnitus. Hear Res. 2013;295:100–113. doi: 10.1016/j.heares.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olivares D, Deshpande VK, Shi Y, et al. N-methyl D-aspartate (NMDA) receptor antagonists and memantine treatment for Alzheimer's disease, vascular dementia and Parkinson's disease. Curr Alzheimer Res. 2012;9:746–758. doi: 10.2174/156720512801322564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oestreicher E, Arnold W, Ehrenberger K, et al. Memantine suppresses the glutamatergic neurotransmission of mammalian inner hair cells. ORL J Otorhinolaryngol Relat Spec. 1998;60:18–21. doi: 10.1159/000027556. [DOI] [PubMed] [Google Scholar]
- 13.Tikhonravov D, Neuvonen T, Pertovaara A, et al. Doserelated effects of memantine on a mismatch negativity- like response in anesthetized rats. Neuroscience. 2010;167:1175–1182. doi: 10.1016/j.neuroscience.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Lobarinas E, Yang G, Sun W, et al. Salicylate- and quinineinduced tinnitus and effects of memantine. Acta Otolaryngol Suppl. 2006;(556):13–19. doi: 10.1080/03655230600895408. [DOI] [PubMed] [Google Scholar]
- 15.Figueiredo RR, Langguth B, Mello de Oliveira P, et al. Tinnitus treatment with memantine. Otolaryngol Head Neck Surg. 2008;138:492–496. doi: 10.1016/j.otohns.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 16.Zheng Y, McNamara E, Stiles L, et al. Evidence that memantine reduces chronic tinnitus caused by acoustic trauma in rats. Front Neurol. 2012;3:127–127. doi: 10.3389/fneur.2012.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turner JG, Brozoski TJ, Bauer CA, et al. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci. 2006;120:188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- 18.Yang G, Lobarinas E, Zhang L, et al. Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res. 2007;226:244–253. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Brozoski TJ, Turner JG, et al. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009;164:747–759. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longenecker RJ, Galazyuk AV. Development of tinnitus in CBA/CaJ mice following sound exposure. J Assoc Res Otolaryngol. 2011;12:647–658. doi: 10.1007/s10162-011-0276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dehmel S, Eisinger D, Shore SE. Gap prepulse inhibition and auditory brainstem-evoked potentials as objective measures for tinnitus in guinea pigs. Front Syst Neurosci. 2012;6:42–42. doi: 10.3389/fnsys.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longenecker RJ, Galazyuk AV. Methodological optimization of tinnitus assessment using prepulse inhibition of the acoustic startle reflex. Brain Res. 2012;1485:54–62. doi: 10.1016/j.brainres.2012.02.067. [DOI] [PubMed] [Google Scholar]
- 23.Chen G, Lee C, Sandridge SA, et al. Behavioral evidence for possible simultaneous induction of hyperacusis and tinnitus following intense sound exposure. J Assoc Res Otolaryngol. 2013;14:413–424. doi: 10.1007/s10162-013-0375-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ralli M, Lobarinas E, Fetoni AR, et al. Comparison of salicylate- and quinine-induced tinnitus in rats: development, time course, and evaluation of audiologic correlates. Otol Neurotol. 2010;31:823–831. doi: 10.1097/MAO.0b013e3181de4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fetoni AR, Mancuso C, Eramo SL, et al. In vivo protective effect of ferulic acid against noise-induced hearing loss in the guinea-pig. Neuroscience. 2010;169:1575–1588. doi: 10.1016/j.neuroscience.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Fetoni AR, Ralli M, Sergi B, et al. Protective effects of Nacetylcysteine on noise-induced hearing loss in guinea pigs. Acta Otorhinolaryngol Ital. 2009;29:70–75. [PMC free article] [PubMed] [Google Scholar]
- 27.Suckfull M, Althaus M, Ellers-Lenz B, et al. A randomized, double-blind, placebo-controlled clinical trial to evaluate the efficacy and safety of neramexane in patients with moderate to severe subjective tinnitus. BMC Ear Nose Throat Disord. 2011;11:1–1. doi: 10.1186/1472-6815-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobarinas E, Sun W, Stolzberg D, et al. Human brain imaging of tinnitus and animal models. Semin Hear. 2008;29:333–349. doi: 10.1055/s-0028-1095893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knipper M, Kopschall I, Rohbock K, et al. Transient expression of NMDA receptors during rearrangement of AMPAreceptor- expressing fibers in the developing inner ear. Cell Tissue Res. 1997;287:23–41. doi: 10.1007/s004410050729. [DOI] [PubMed] [Google Scholar]
- 30.Puel JL. Cochlear NMDA receptor blockade prevents salicylate- induced tinnitus. B-Ent 3 Suppl. 2007;7:19–22. [PubMed] [Google Scholar]