SUMMARY
This draft of the Official Round Table held during the 99th SIO National Congress is an updated review on the diagnostic tools, the importance of polysomnographic recording and a critical analysis of the surgical techniques in obstructive sleep apnoea syndrome (OSAS). The review and analysis of available publications is the premise along with a specific analysis of the relationship between OSAS and metabolic and vascular disorders. In addition, the most recent investigations on sleep disorders and altered glucose metabolism are summarised and discussed together with the results of a study by the authors involving a fairly large number of patients with OSAS and diabetes.
KEY WORDS: OSAS, Diabetes, Polysomnography, Nasal obstruction, Cardiovascular diseases
RIASSUNTO
Questo testo è un estratto della tavola rotonda ufficiale tenutasi durante il 99o Congresso Nazionale SIO. Si tratta di una revisione aggiornata sugli strumenti diagnostici, sull'importanza della polisonnografia e di un'analisi critica delle tecniche chirurgiche dell'OSAS. La revisione e l'analisi di tutti gli studi costituiscono la premessa e il completamento dei capitoli; particolare attenzione è posta sul rapporto tra OSAS e disturbi metabolici e vascolari. Inoltre, i lavori più recenti su disturbi del sonno ed alterazioni del metabolismo glucidico sono riassunti e discussi insieme ai risultati di uno studio portato avanti dagli autori che coinvolge un discreto numero di pazienti con OSAS e diabete.
Introduction
Respiratory disorders play an important role in determining and increasing many metabolic pathologies. Respiration in fact, is a fundamental function of any organism, and it is biologically logical that it should be connected with other critical functions including the control of glucose blood levels.
On the other hand, glucose levels in blood result from the interaction of many factors, one of which is the high overnight activity of the brain.
In patients with diabetes, it has been shown that not only obstructive apnoea, but also simple snoring, has a negative effect on insulin sensitivity, fasting blood glucose and glycated haemoglobin (HbA1c) levels.
As a whole, very recent data point out that sleep disordered breathing (SDB) can be the cause of notable glycaemic variability (GV), and this in turn, is one of the many factors that are associated with complications of diabetes. Both direct and indirect mechanisms can explain the effects of SDB on blood glucose levels. Among these, one of the most important is that anoxia during OSAS is a potent stimulus for catecholamine secretion and glycogenolysis. An indirect effect regards obesity, as it is tightly connected with diabetes (to emphasize this connection the term "diabesity" has been created) 1-3.
Nocturnal awakenings and sleep disruption in OSAS lead to debt in sleep which, in turn, is translated into other activity during diurnal hours, thus promoting obesity.
Relevance of OSAS in metabolic disorders
Some studies have demonstrated a role for OSAS using polysomnographic recording and the oral glucose tolerance test (OGTT) 4. The work of Punjabi 5 in 118 non-diabetic subjects who underwent an insulin sensitivity test, FSIGT (frequently sampled intravenous glucose tolerance) 6, provides evidence that OSAS reduces insulin sensitivity by 27%, 37% and 48% according to the severity of oxygen reduction, defined as modest, moderate or severe, respectively. However, in that report, the criteria used to define these classes is not clear, and it is unknown if the definition was based on the number of apnoea/hypopnoea episodes or on the level of associated oxygen deprivation. Another study on 150 males showed that an increase of the apnoea/hypopnoea index is associated with an increased risk for impaired glucose tolerance (IGT) and development of insulin resistance. Both these conditions are considered as prodromic to type 2 diabetes and an essential part of what is referred to as metabolic syndrome. We recently demonstrated in 60 OSAS subjects that the number of nocturnal awakenings was strictly related with instability of glucose values upon awakening (glycaemic variability) 7. This variability is now deemed to be one of the major risk factors for cardiovascular diseases both in diabetic and non-diabetic individuals. There are many interesting reports on this subject that have been accurately evaluated in a recent review 8. There are also some studies that demonstrate the increase of inflammatory factors in subjects with OSAS: among these, C-reactive protein and cytokines, which are responsible for systemic atherosclerosis and probably have a role in the appearance of neoplasms 9 10. There is also evidence of an increase of atherogenic dyslipidaemia in subjects with OSAS 11.
The metabolic–OSA connection is well studied. It is known that poor sleep/OSA may have a negative effect on body weight. OSAS subjects have substantial difficulty losing weight, and tend to increase it 12. Among the numerous mechanisms hypothesised to explain this effect is a critical that involves the hormone leptin, which regulates appetite 13. OSAS subjects show resistance to leptin and a concomitant increase in appetite drive 14 15. Some years ago, the presence of high but ineffective levels of leptin in obese type 2 diabetic subjects was shown to be a markers of insulin resistance 16. Another interesting connection that is not yet sufficiently explored is the between OSAS and erectile dysfunction 17 18.
Diagnosis of OSAS
The literature makes it increasingly evident, thanks to increasingly advanced diagnostic techniques, that sleeprelated respiratory disorders are associated not only with cardiovascular pathologies, such as arterial hypertension 19 (as already shown by Coccagna and Lugaresi in the 1970s) 20, but also with systemic endocrine and neurological disorders 3.
It is clear from these premises that otolaryngologists are often the first to encounter such patients. There is, therefore, the challenge to correctly diagnose these patients on the basis of clinical and instrumental investigations while assessing them for therapeutic medical and surgical purposes.
It is especially important to pay attention to diagnostic procedures because of the relative novelty of sleep-related respiratory disorders as a pathology outside the traditional domain of expertise of ENT specialists.
Diagnostic procedures must be aimed at: compiling a thorough clinical history; evaluating, clinically and instrumentally, all alterations of normal anatomy and physiology of the cervical-cephalic regions; locating the obstruction site(s) of the upper airways.
Pursuant to the 2007 Italian Society of Otolaryngology guidelines, it is our view that patients suspected of OSAS should undergo a basic set of tests for gathering clinical data, to be followed by a more advanced clinical-instrumental investigation in case of surgical planning.
Basic Test Set:
thorough anamnestic evaluation;
ENT clinical examination;
Muller's manoeuvre-assisted fiberoptic laryngoscopy.
Advanced clinical-instrumental investigation:
nose function trials;
cephalometric analysis;
sleep endoscopy;
imaging.
Given the large amount of information to be analysed and integrated with the results from nocturnal cardio-respiratory monitoring, it is useful to adopt specially-dedicated medical records for this disorder, as already advanced by several authors 21.
The relationship between nasal obstruction and respiratory disorders has been studied for more than 30 years. A close association has been hypothesised between increased nasal resistance and severity of snoring and sleep apnoea 22. The link between nasal resistance and OSAS severity has been shown by several authors, but there are several contrasting studies which show no association, in clinical trials, between nasal obstruction and apnoea 23-25. On the other hand, perusal of the literature does highlight that many patients affected by sleep-related respiratory disorders show symptoms of nasal obstruction; likewise, it is well known that an integral part of snoring-corrective surgery is nasal surgery. In addition, some authors suggest a decrease in nasal resistance can be of use in CPAP ventilation therapy, due to the lower air pressures required 26. The traditional otolaryngological approach to the patient with nasal obstruction was rendered obsolete by the availability of modern diagnostic tools. Virtually, all instances of nasal ailments can be attributed to their underlying causes and appropriately treated by careful rhinological studies in specialised centres provided with adequate instrumentation. Thus, physical examination needs to be performed with optical endoscopes reaching areas that are traditionally difficult to access, which allows for earlier and more detailed diagnoses; afterwards, tests of nasal function will be necessary for correct diagnostic framing. Basic tests of nose-sinus function, useful for the assessment of suspected OSAS patients, are measurements of airflow and pressure within the nose by active anterior rhinomanometry, acoustic rhinometry and mucociliary transport time–functional assessments of both the nasal fossa and their mucosal lining.
Much has been written for the last 20 years about nasal fiberoptic endoscopy under sedation (sleep endoscopy). This technique was first described in 1991 by Croft and Pringle 24, who started out by observing that snoring and OSAS are dynamic phenomena mainly occurring in sleeping patients, and thought to visualise the sites of obstruction in the upper airways during sleep phases.
In the original study, sleep was induced by short-acting benzodiazepines (midazolam). The fiberoptic endoscope was well-tolerated by 95.8% of study subjects, and the obstructive problem was correctly identified in 79% of cases. Patients are made to lie supine on an operating table with low-intensity lights. Throughout the procedure oximetry and cardiac rhythms are accurately monitored, with supplementary oxygen delivered through a mask if necessary. Propofol is administered at an infusion rate of 50-75 mcg/ kg/min in order to meet the target level of anaesthesia. At drug-induced sleep inception, the flexible endoscope is introduced into the anaesthetised nasal cavity, and the examination (digitally recorded throughout) commences. During the examination, dynamic collapse needs to be evaluated at the level of the retro-palatal and retro-lingual regions, as well as of the laryngeal structures. The severity and type (transverse, circular horizontal pattern) of the collapse needs to be noted for each region. For standardisation purposes, several classification systems have been proposed to report the severity, type and location of obstructions. The most commonly used, at present, are the VOTE classification (Table I), introduced by de Vries 28, and the NOHL classification, introduced and standardised by the Italian School of Vicini 29 (Table II).
Table I.
VOTE Classification according de Vries et al. 28 (shaded boxes reflect the fact that a specific structure-configuration cannot be seen). Degree of obstruction:
0 No obstruction/vibration < del 50%
1 Partial obstruction/vibration >del 50%< del 75%
2 Complete collapse
X Not visualised
| STRUCTURE | DEGREE OF OBSTRUCTION | CONFIGURATION | ||
|---|---|---|---|---|
| A-P | Lateral | Concentric | ||
| Velum | ||||
| Oropharynx lateral walls | ||||
| Tongue base | ||||
| Epiglottis | ||||
Table II.
NOHL Classification according Vicini et al. (This is associated with the pattern of collapse and tonsil size) Example: N3O4cTS3H2tLn.
| Site | Nose | Oropharynx | Hypopharynx | Larynx Supraglottic Glottic |
|---|---|---|---|---|
| Grade of Obstruction/Collapse | Grade 1: 0-25% | Grade 1: 0-25% | Grade 1: 0-25% | Collapse |
| Grade 2: 25-50% | Grade 2: 25-50% | Grade 2: 25-50% | present/absent | |
| Grade 3: 50-75% | Grade 3: 50-75% | Grade 3: 50-75% | ||
| Grade 4: 75-100% | Grade 4: 75-100% | Grade 4: 75-100% |
To date, several modifications of the technique have been published 30 31, with varying results. A recent review of the literature by Ravesloot and de Vries 32 of patients who underwent sleep endoscopy showed that different regions of the upper airways collapse, such as the velopharynx, oropharynx, base of the tongue and epiglottis, but it also underscored that the collapse is oftentimes multi-level. Furthermore, according to Rodriguez 33-35, sleep endoscopy has demonstrated high test-retest reliability compared with other diagnostic techniques for OSAS.
The role of polysomnography
Among sleep disorders, those with the greatest impact on health and highest healthcare-related costs are respiratory disorders in sleep (mainly night apnoeas-OSAS).
Their incidence in the Italian population is estimated at about 180,000 people, 95% of which is probably undiagnosed.
There has been barely any increase in newly-diagnosed patients over the last 20 years, a greatly concerning figure from a public health and social point of view. Unfortunately, this problem is still inadequately addressed, in all its complexity, at the educational (university), preventative or patient-care level.
Daytime sleepiness, a paramount symptom of OSAS, results from non-restful sleep at night and can lead to very serious consequences (traffic accidents, workplace accidents, etc.). Furthermore, traffic accidents by excessive daytime sleepiness carry high costs, both public and private, are generally more severe and result in a mortality rate almost twice that of accidents due to other causes.
Different, but equally relevant for public health, is the impact of sleep apnoea syndromes on cardiovascular risk: few disorders such as sleep apnoea display such a strong and important association with hypertension, cardiac arrhythmias and heart failure.
Several studies have reported that 45-50% of all hypertensive subjects are actually suffering from a hidden, undiagnosed sleep apnoea, and the same applies to 25-50% of patients with heart failure.
The most impressive datum concerns patients who have had a transient ischaemic attack (TIA) or stroke: a sleep apnoea syndrome may be present in 60% of these cases, and this figure increases consistently once the presence of a relatively common heart defect, such as a patent "foramen ovalis", is factored in.
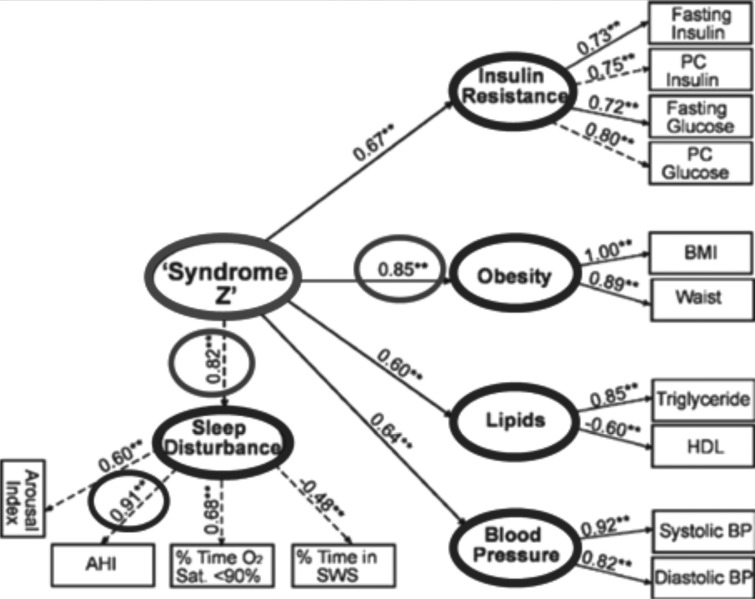
The majority of OSAS patients also display a constellation of metabolic and non-metabolic cardiovascular risk factors typical of metabolic syndrome. Indeed, the suggestion has been put forth that OSAS may be a manifestation of metabolic syndrome ("syndrome Z") 36 (Fig. 1).
Fig. 1.
"Hierarchical" hypothesis of the prevalence of metabolic and non-metabolic risk factors of sleep disorders. Adapted from: NL Nock et al.: Empirical evidence for "syndrome Z": a hierarchical 5-factor model of metabolic syndrome incorporating measures of sleep disturbance. Sleep 2009, 32:615-622.
Polysomnography permits detection and classification of a number of respiratory (apnoeas, O2 desaturations) and neurological phenomena (e.g. arousals, or periodic movements of the lower limbs), as well as blood pressure and cardiac activity; it is therefore essential, for the interpretation of such data, to have some basic knowledge of specific technical standards.
In order to establish adequate treatment, OSAS medical specialists need to make the best use of the available severity criteria provided by international guidelines. However, it is often the case that the indexes resulting from the scoring parameters of sleep-related respiratory events (RDI, AHI, ODI) are inadequate to classify and confidently deal with all clinical situations.
Indeed, patterns of sleep-related events frequently occur that hardly fit a patient on the exclusive basis of strict taxonomic criteria, providing an interesting interpretative and therapeutic challenge. This consideration, together with the high incidence of OSAS in the general population, and the vast array of disease-associated cardiovascular and neurological complications, further highlights the need to assess OSAS patients in a clinical and multidisciplinary framework, reserving instrumentation exclusively to the task of confirming the physician's diagnostic hypotheses, developed on the basis of the patient's signs and symptoms.
It is now necessary to address the question: who should be tested by polysomnography? In Italy, are the current AIMS–AIPO guidelines the most appropriate to deal with the problem? Who are the best specialists for expanding diagnostic opportunities? The otolaryngologist has always been closely linked to diagnosis and surgical treatment of OSAS. Is it possible to broaden his role in the diagnosis and treatment of OSAS patients?
There is some rationale in this regard:
higher frequency of OSAS patients' first visit;
early identification of obstruction sites;
widespread availability of ENT specialists;
and some challenges to be met:
management of diagnostic polysomnography;
management of the multidisciplinary team;
carrying out prospective studies to evaluate the efficacy of a surgical approach.
The role of severe OSAS in atherosclerotic disease: pharmacological treatment with statins.
The recently published meta-analysis in The Lancet by CTT (Cholesterol Treatment Trialists Collaborators) provides further evidence that statins are a safe and effective way to reduce the risk of heart attack and stroke, even among people at very low risk for major cardiovascular events. A recent review 37, on the usefulness of longterm statins in primary prevention of cardiovascular disease concluded by inviting clinicians to exercise caution in prescribing statins for primary prevention in patients at low cardiovascular risk. On the basis of these results, patients older than 50 years of age with severe OSAS at high risk for coronary heart disease and stroke should take statins to reduce carotid and coronary early vascular risk. One study 38 showed that 20% of severe OSAS patients developed early carotid atherosclerosis.
On the basis of the CTT study, the suggestion can be made that it may be appropriate to prescribe statins to subjects aged > 50 years, since 83% of men over 50 have a 10-year cardiovascular risk of 10%. In fact, the benefits to be gained by giving statins to anyone older than 50 years of age would probably result in a net saving for the public health service by reducing health care costs resulting from heart attacks and strokes, and prevented by statins. OSAS is, among cardiac-metabolic disorders, still underestimated from an epidemiological point of view; it is associated in 67% of cases with an asymptomatic coronary syndrome, and in 51.2% with metabolic syndrome. In the future, substantial public health resources will be needed for diagnosis and early treatment.
Strengthening prevention strategies against primary atherosclerosis will need to be one of the focal aims of future healthcare programs. Furthermore, as increased protection from risk factors hinges on an increased level of education, this means that education towards healthy lifestyles needs to be greatly enhanced and to become an integral part of public healthcare policies. This is a public health service yielding slower effects, but certainly more efficacious considering the economy of the entire public healthcare system. In another paper Ye et al., found increased cell-free DNA in the serum of patients with OSAS. The authors interpret these results by suggesting that chronic hypoxia-induced apnoea makes cancer cells more resistant and accelerates their growth. This study also provides important evidence that OSAS is associated with increased tumour mortality as well as general and cardiovascular mortality 39.
OSAS surgical treatment
While most patients undergo surgery because of CPAP intolerance, it is imperative that they use their CPAP for at least two weeks prior to and after surgery in order to not accumulate sleep debt. Hypertension should be treated aggressively in the perioperative period. After surgery, admission with pulse oximetry and pain management with narcotics is required. Patients need to demonstrate the ability to tolerate a liquid diet, have adequate pain control and have a safe airway prior to discharge.
Nasal surgery: various observational and cross-sectional studies have documented a relationship between chronic nasal obstruction and OSA 40. Rhinitis constitutes a pathologic condition characterised by an increase in nasal airway resistance due to mucosal swelling, and therefore it may represent a risk factor for OSA. Several studies have correlated nasal congestion from allergic and non-allergic rhinitis as a risk factor for OSAS with differing results. Epidemiological data have shown that chronic rhinitis symptoms and increased nasal resistance measured by rhinomanometry are associated with habitual snoring, but a similar association is not documented for OSAS.
Kohler 40 reviewed the literature about the role of the nose in the pathogenesis of OSA. From the currently available data, it was concluded that nasal congestion may contribute to the pathogenesis of OSA. Architecture and quality of sleep could be improved by treating nasal congestion, but the clinical relevance remains to be demonstrated. Nasal surgery may be helpful in patients who are unable to tolerate CPAP because of nasal obstruction, but this has never been shown in randomised controlled trials. Although there is no role for nasal surgery as a single treatment for OSA, it is quite useful in improving symptoms in simple snorers and potentially useful as part of multilevel surgery in many patients with SRBD. The ERS task force 41 concludes that nasal surgery as a single intervention is not recommended for treatment of OSAS (grade of recommendation C), but is recommended for reducing high therapeutic CPAP pressure due to nasal obstruction.
Velo-palatal and pharyngeal surgery: the tonsils and retropalatal areas clearly represent common sites of obstruction in OSA.
Palatal stiffening with the pillar implant technique may be useful only for patients with mild to moderate OSA who refused other conservative approaches. Palatal implants are expensive and a partial extrusion occurs in 10.3% of patients 37. The overall success rate is limited.
Laser-assisted uvulopalatoplasty (LAUP) is an officebased surgical procedure that progressively shortens and tightens the uvula and palate through a series of carbon dioxide laser incisions and vaporisations. This technique is associated with moderate to severe pain immediately after the procedure, and is weighed against the risk of scar contracture which can reduce the effectiveness of surgery and lead to complications. In mild OSAS, LAUP is not recommended (recommendation B) 41.
Radiofrequency (RF) surgery of the soft palate is applied by inserting electrodes submucosally usually into five sites of the soft palate. This procedure represents a good treatment option in habitual snorers, but it is not recommended as a single-stage approach in mild OSAS and is not superior to placebo 42.
Other procedures for soft palate stabilisation have been proposed such as using nasal septum cartilage or concha cartilage 43.
Uvulopalatopharyngoplasty 44 45 (UP3) is the single most common surgical performed procedure for the correction of retropalatal obstruction causing or contributing to OSAS. This basic procedure only corrects obstruction of the palate and tonsils. Firstly, Fujita himself recognised that half of patients submitted to UP3 were non-responders. For those with a component of hypopharyngeal obstruction (Types II and III in Fujita and Simmons classification 46, the response rate is only 5.3% 47. Actually, for patients with morbid obesity and airway involvement, UP3 treatment is unsuccessful.
Anterior palatoplasty (modified cautery assisted palatoplasty) was proposed by Pang 48 in management of patients with mild-moderate OSA. This procedure is very simple and safe, and if associated with UP3 gives excellent results in patients type I Fujita.
Tucker Woodson 49 described a method of reconstructing the upper pharynx by performing a posterior maxillary osteotomy and advancing the soft palate anteriorly (posterior palatal osteotomy and palatal advancement flap). A 67% successful response rate was observed in patients who underwent transpalatal advancement. This procedure enlarges the upper oropharyngeal airway, and can be indicated in UP3 failures.
Lateral pharyngoplasty was proposed by Cahali 50 in 2003 for patients with moderate to severe OSA to enlarge the collapsed lateral pharyngeal wall: the results seemed initially promising, but many patients had postoperative dysphagia. Expansion sphincter pharyngoplasty consists of a tonsillectomy, expansion pharyngoplasty with or without superolateral incision on the soft palate, horizontal section and superolateral rotation of the palatopharyngeus muscle, partial uvulectomy and a closure of the anterior and posterior tonsillar pillars. The key to this procedure is to not completely isolate the muscle and rotate it. This procedure is simple to perform, but significant pain and swallowing problems may occur.
Tongue surgery: a variety of approaches have been described for lingual tonsillectomy and advancement of the tongue base. Exposure of the tongue base is optimised via suspension laryngoscopy with endoscopy. Isolated retrolingual obstruction is present in only 25% or less of patients with OSAS.
Radiofrequency tongue base ablation (RFA): Success rates are higher according to the subjects' posture with a rate of 87.5% for the supine position and 56.6% in nonsupine positions 51.
Lingual tonsillectomy can be done with laser, diathermy, cryotherapy, ultrasonic coagulating dissector, or microdebrider. The surgical technique should be always performed under visualisation with telescope, and care should be taken to avoid damage to the lingual neurovascular bundle 52. Laser midline glossectomy 53 enlarges the retrolingual airway by reducing the base of tongue by approximately 2.5 x 5 cm through an intraoral approach. Lingual tonsillectomy, epiglottectomy and aryepiglottic fold reduction may be performed at the same setting. More aggressive resection is associated with more frequent complications such as lingual and airway oedema necessitating tracheotomy.
Submucosal minimally invasive lingual excision (SMILE) 54 is a modified version of the description by Robinson et al. of tongue base reduction using coblation through a suprahyoid neck approach. It is a mucosal sparing approach, while allowing aggressive tissue removal using a plasma-mediated radiofrequency device under ultrasonic and endoscopic guidance.
In 1999, Chabolle 55 proposed tongue base reduction with hyoepiglottoplasty (TBRHE) through a cervical approach. The lingual neurovascular bundle must be carefully identified and a subtotal tongue base resection is performed. Next, the hyoid bone is suspended at the lower border of the mandible and finally a temporary tracheotomy is carried out.
The tongue-base suspension can also be obtained by means of REPOSE system 56. In our opinion, this procedure belongs to the past and should be forgotten.
Recently some reports about the partial glossectomy using transoral robotic surgery (TORS) have been reported 57. This procedure can be performed without the need for tracheotomy, but has an increased morbidity compared with the other techniques as RFA or SMILE 56.
Hyoid advancement: Riley et al. described hyoid suspension in 1986, although with osteotomy of the mandible and fixation of the hyoid bone to the mandible 58. The modified hyoid suspension according to Hörmann and Baish 59 may provide good benefits for successful surgical therapy of OSA. Lewis 60 proposed a modified technique of inferior hyoid advancement using four sutures between just below the superior border of the thyroid cartilage and the hyoid bone pulled completely over the anterior surface of the thyroid cartilage.
Maxillo-mandibular osteotomy: clearly a very valid option for OSAS failures. This procedure is usually performed after the above surgeries have failed to improve obstructive sleep apnoea and is considered a phase II surgery. Most of these patients have already undergone a staged surgery, often with UP3 and genioglossal advancement as a part of a multilevel surgical program. The success rate of this group varies from 65.2% to 97.5% 61-63. This surgery is perceived as an unattractive treatment modality because of the possibility of a significant change in the facial profile. MMO is particularly indicated in presence of craniofacial abnormalities.
Laryngeal surgery: the larynx can also be a possible site of obstruction in OSA. It is documented that an increase in the concavity of the posterior surface of the epiglottis can be correlated with an increase in BMI 64. Epiglottis reshaping with CO2 laser irradiation gives a significant improvement even in children with laryngomalacia and obstructive sleep apnoea. The data suggest that this form of airway distress characterised by prolapse of supraglottic structures into the glottic airway during inspiration is an important contributor to OSA, and that its correction can significantly improve sleep in children.
Skin-lined tracheostomy: this procedure bypasses the laryngeal airway and is reserved for use in patients with severe OSA who have failed to improve with other medical and surgical treatments and in special cases in which these modalities are contraindicated or not tolerated. In OSA, patients submitted to tracheostomy showed a severe reduction in blood pressure and hypoglycaemia 65. Tracheostomy is preferably carried out with the skin-lined technique 66 to guarantee greater stability, less risk of granulation tissue and wider opening than tracheotomy.
Considerations
Among the phenomena that modulate the activity of the brain, sleep plays an important role. Normal breathing and sleep patterns are essential to survival, and thus it is not unexpected that the importance of SDB and OSAS and their effect on glucose metabolism and diabetes have been evalauted recently in several important publications; in the last Meeting of the European Association for the Study of Diabetes (EASD) an entire session was devoted to SDB and its impact on the diabetes 1-3.
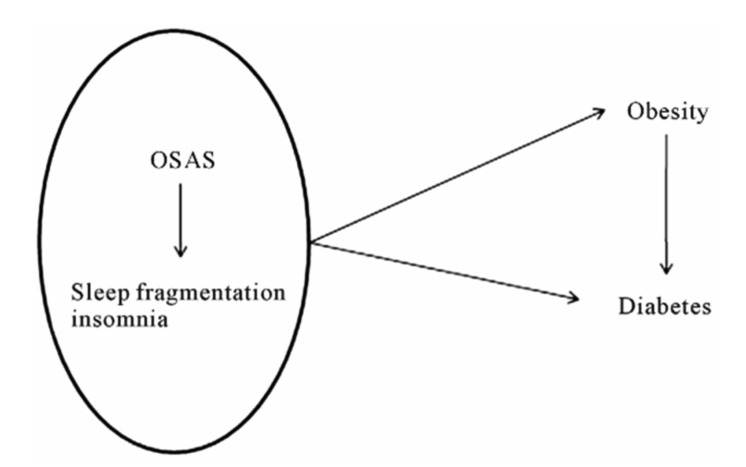
At present there is sufficient data to accept the existence of an interaction among OSAS and some recently described metabolic disorders (18), but understanding the mechanisms behind this is much more complicated. We have recently proposed a model of this interaction (Fig. 2).
Fig. 2.
A simplified scheme of the interactions between respiratory distress and metabolic disorders.
References
- 1.Tatti P, Passali D, Bellussi LM. The undisclosed role of anoxia/ hypoxia and disturbed sleep on glucose metabolism. J Diabetes Mellitus. 2012;2:186–190. [Google Scholar]
- 2.Spiegel K. Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Internal Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 3.Passàli D, Tatti P, Passàli FM, et al. The undisclosed role of disturbed sleep and hypoxia on metabolism: the importance of upper airways pathology. Sleep Breath. 2013;17:5–6. doi: 10.1007/s11325-012-0679-1. [DOI] [PubMed] [Google Scholar]
- 4.Tiihonen M, Partinen M, Närvänen S. The severity of obstructive sleep apnoea is associated with insulin resistance. J Sleep Res. 1993;2:56–61. doi: 10.1111/j.1365-2869.1993.tb00062.x. [DOI] [PubMed] [Google Scholar]
- 5.Punjabi NM, Beamer BA. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med. 2009;179:235–240. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman RN. Toward physiological understanding of glucose tolerance: Minimal model approach. Diabetes. 1989;38:1512–1527. doi: 10.2337/diab.38.12.1512. [DOI] [PubMed] [Google Scholar]
- 7.Tatti P, Passali D. Sleep disturbances and glucose variability. In: Lopez Garcia CM, Perez Gonzalez PA, editors. Metabolic Syndrome: Classification, Risk Factors and Health Impact. Nova Publisher; in Press. [Google Scholar]
- 8.Tatti P, Lehmann ED, Barber AE, Passali D, et al. Application of technology in endocrine disease. Int J Endocrinol. 2014;2014:715029–715029. doi: 10.1155/2014/715029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popko K, Gorska E, Potapinska O, et al. Frequency of distribution of inflammatory cytokines il-1, il-6 and tnf-α gene polymorphism in patients with obstructive sleep apnea. J Physiol Pharmacol. 2008;59(Suppl. 6):607–614. [PubMed] [Google Scholar]
- 10.Ciftci TU, Kokturk O, Bukan N, et al. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28:87–91. doi: 10.1016/j.cyto.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Williams CJ, Hu FB, Patel SR, et al. Sleep duration and snoring in relation to cardiovascular disease risk in women with type 2 diabetes. Diabetes Care. 2007;30:1233–1240. doi: 10.2337/dc06-2107. [DOI] [PubMed] [Google Scholar]
- 12.Mary L. Obesity and OSA: More than BMI. Sleep Review. 2004;4(D):36–39. [Google Scholar]
- 13.Tatti P, Barber A. Human obesity: an overview of the mechanisms and the basis for treatment. Advances in Medicine and Biology. 2011;15:341–352. [Google Scholar]
- 14.Phillips BG, Kato M, Narkiewicz K, et al. Higher leptin levels in OSA, independent of body fat content, suggest that OSA is associated with resistance to the weight reducing effects of Leptin. Am Physiol Heart Circ Physiol. 2000;279:234–237. [Google Scholar]
- 15.Spiegel K, Tasali E, Penev P, et al. Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Internal Med. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 16.Tatti P, Masselli L, Buonanno A, et al. Leptin levels in diabetic and nondiabetic subjects. Endocrine. 2001;15:305–308. doi: 10.1385/ENDO:15:3:305. [DOI] [PubMed] [Google Scholar]
- 17.Budweiser S, Enderlein S, Jörres RA, et al. Sleep apnea is an independent correlate of erectile and sexual dysfunction. J Sex Med. 2009;6:3147–3157. doi: 10.1111/j.1743-6109.2009.01372.x. [DOI] [PubMed] [Google Scholar]
- 18.Fisher WJ, et al. Current clinical issues: is sleep the new vital sign? Ann Int Med. 2005;10:877–877. [Google Scholar]
- 19.Toraldo DM, Peverini F, Benedetto M, et al. Obstructive sleep apnea syndrome: blood viscosity, blood coagulation abnormalities, and early atherosclerosis. Lung. 2013;191:1–7. doi: 10.1007/s00408-012-9427-3. [DOI] [PubMed] [Google Scholar]
- 20.Lugaresi E, Coccagna G, Mantovani M, et al. Effects of tracheastomy in two case of hyperinsomnia with periodic breathing. J Neuro Neurosurg Psychiatry. 1973;36:15–26. doi: 10.1136/jnnp.36.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passali D, Caruso G, Arigliano LC, et al. Database application for patients with obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital. 2012;32:252–255. [PMC free article] [PubMed] [Google Scholar]
- 22.Passali FM, Bellussi L, Mazzone S, et al. Predictive role of nasal functionality tests in the evaluation of patients before nocturnal polysomnographic recording. Acta Otorhinolaryngol Ital. 2011;31:103–108. [PMC free article] [PubMed] [Google Scholar]
- 23.Blakley BW, Mahowald MW. Nasal resistance and sleep apnea. Laryngoscope. 1987;97:752–754. doi: 10.1288/00005537-198706000-00023. [DOI] [PubMed] [Google Scholar]
- 24.Cole P, Haight JSJ. Mechanism of nasal obstruction in sleep. Laryngoscope. 1984;94:1557–1559. [PubMed] [Google Scholar]
- 25.Duchna HW. Anamnestic and polygraphic parameters in obstructive sleep apnea syndrome patients with reduced nasal respiration during the day in comparison with obstructive sleep apnea patients with normal nasal respiration. Wien Med Wochenschr. 1996;146:348–349. [PubMed] [Google Scholar]
- 26.Sugiura T, Noda A, Nakata S, et al. Influence of nasal resistance on initial acceptance of continuous positive airway pressure in treatment for obstructive sleep apnea syndrome. Respiration. 2007;74:56–60. doi: 10.1159/000089836. [DOI] [PubMed] [Google Scholar]
- 27.Croft CB, Pringle M. Sleep nasendoscopy: a technique of assessment in snoring and obstructive sleep apnoea. Clin Otolaryngol Allied Sci. 1991;16:504–509. doi: 10.1111/j.1365-2273.1991.tb01050.x. [DOI] [PubMed] [Google Scholar]
- 28.Kezirian KJ, Hohenhorst W, Vries N. Drug-induced sleep endoscopy: the VOTE classification. Eur Arch Otorhinolaryngol. 2011;268:1233–1236. doi: 10.1007/s00405-011-1633-8. [DOI] [PubMed] [Google Scholar]
- 29.Vicini C, Vito A, Benazzo M, et al. The nose oropharynx hypopharynx and larynx (NOHL) classification: a new system of diagnostic standardized examination for OSAHS patients. Eur Arch Otorhinolaryngol. 2012;269:1297–1300. doi: 10.1007/s00405-012-1965-z. [DOI] [PubMed] [Google Scholar]
- 30.Faber CE, Grymer L. Available techniques for objective assessment of upper airway narrowing in snoring and sleep apnea. Sleep Breath. 2003;7:77–86. doi: 10.1007/s11325-003-0077-9. [DOI] [PubMed] [Google Scholar]
- 31.Abdullah VJ, Wing YK, Hasselt CA. Video sleep nosendoscopy: the Hong Kong experience. Otolaryngol Clin North Am. 2003;36:461–471. doi: 10.1016/s0030-6665(02)00176-7. [DOI] [PubMed] [Google Scholar]
- 32.Ravesloot MJL, Vries N. One hundred consecutive patients undergoing drug-induced sleep endoscopy: results and evaluation. Laryngoscope. 2011;121:2710–2716. doi: 10.1002/lary.22369. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Bruno K, Goldberg A, McCulloch C, et al. Testretest reliability of drug-induced sleep endoscopy. Otolaryngol Head Neck Surg. 2009;140:646–651. doi: 10.1016/j.otohns.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salamanca F, Costantini F, Bianchi A, et al. Identification of obstructive sites and patterns in obstructive sleep apnea syndrome by sleep endoscopy in 614 patients. Acta Otorhinolaryngol Ital. 2013;33:261–266. [PMC free article] [PubMed] [Google Scholar]
- 35.Corso E, Fiorita A, Rizzotto MG, et al. The role of druginduced sleep endoscopy in the diagnosis and management of obstructive sleep apnoea syndrome: our personal experience. Acta Otorhinolaryngol Ital. 2013;33:405–413. [PMC free article] [PubMed] [Google Scholar]
- 36.Nock NL, Li L, Larkin EK, et al. Empirical evidence for "syndrome Z": a hierarchical 5-factor model of the metabolic syndrome incorporating sleep disturbance measures. Sleep. 2009;32:615–622. doi: 10.1093/sleep/32.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor F, Ward K, Moore TH, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2011 Jan 19;1:CD004816–CD004816. doi: 10.1002/14651858.CD004816.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baguet J P, Nadra M, Barone-Rochette G, et al. Early cardiovascular abnormalities in newly diagnosed obstructive sleep apnea. Vasc Health Risk Manag. 2009;5:1063–1073. doi: 10.2147/vhrm.s8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye L, Ma GH, Chen L, et al. Quantification of circulatingcell- free DNA in the serum of patients with obstructive sleepapnea- hypopnea syndrome. Lung. 2010;188:469–474. doi: 10.1007/s00408-010-9253-4. [DOI] [PubMed] [Google Scholar]
- 40.Kohler M, Bloch KE, Stradling JR. The role of the nose in the pathogenesis of obstructive sleep apnea. Curr Opin Otolaryngol Head Neck Surg. 2009;17:33–37. doi: 10.1097/MOO.0b013e32831b9e17. [DOI] [PubMed] [Google Scholar]
- 41.Randerath WJ, Verbraecken J, Andreas S, et al. Non- CPAP therapies in obstructive sleep apnoea. Eur Resp J. 2011;37:1000–1023. doi: 10.1183/09031936.00099710. [DOI] [PubMed] [Google Scholar]
- 42.Bäck LJ, Liukko T, Rantanen I, et al. Radiofrequency surgery of the soft palate in the treatment of mild obstructive sleep apnea is not effective as a single-stage procedure: A randomized single-blinded placebo-controlled trial. Laryngoscope. 2009;119:1621–1627. doi: 10.1002/lary.20562. [DOI] [PubMed] [Google Scholar]
- 43.Tolsdorff P, Honnef B. Gaumen-Knorpel-Stabilisierung bei Schnarchen und mildem bis mittelschwerem obstruktivem Schlaf-Apnoe-Syndrom. Laryngorhinootologie. 2009;88:696–698. doi: 10.1055/s-0029-1242893. [DOI] [PubMed] [Google Scholar]
- 44.Fujita S, Conway W, Zorick F, et al. Surgical correction of anatomic abnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg. 1981;89:923–934. doi: 10.1177/019459988108900609. [DOI] [PubMed] [Google Scholar]
- 45.Mantovani M, Minetti A, Torretta S, et al. The velo-uvulopharyngeal lift or "roman blinds" technique for treatment of snoring: a preliminary report. Acta Otorhinolaryngol Ital. 2012;32:48–53. [PMC free article] [PubMed] [Google Scholar]
- 46.Fujita S, Simmons FB. Pharyngeal surgery for obstructive sleep apnea and snoring. In: Fairbanks DNF, Fujita S, Ikematsu T, editors. Snoring and Obstructive Sleep Apnea. NY: Raven Press; 1987. pp. 101–128. [Google Scholar]
- 47.Sher AE, Schectman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156–177. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 48.Pang KP, Tan R, Puraviappan P, et al. Anterior palatoplasty for the treatment of OSA: three-year results. Otolaryngol Head Neck Surg. 2009;141:253–256. doi: 10.1016/j.otohns.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 49.Tucker Woodson B. Transpalatal advancement pharyngoplasty. Operative Techniques in Otolaryngology. 2007;18:11–16. [Google Scholar]
- 50.Cahali MB. Lateral pharyngoplasty: a new treatment for obstructive sleep apnea hypopnea syndrome. Laryngoscope. 2003;113:1961–1968. doi: 10.1097/00005537-200311000-00020. [DOI] [PubMed] [Google Scholar]
- 51.Babademez MA, Ciftci B, Acar B, et al. Low-temperature bipolar radiofrequency ablation (coblation) of the tongue base for supine-position-associated obstructive sleep apnea. ORL J Otorhinolaryngol Relat Spec. 2010;72:51–55. doi: 10.1159/000298945. [DOI] [PubMed] [Google Scholar]
- 52.Barakate M, Havas T. Lingual tonsillectomy: A review of 5 years' experience and evolution of surgical technique. Otolaryngol Head Neck Surg. 2008;139:222–227. doi: 10.1016/j.otohns.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 53.Fujita S, Woodson BT, Clark JL, et al. Laser midline glossectomy as a treatment for obstructive sleep apnea. Laryngoscope. 1991;101:805–809. doi: 10.1288/00005537-199108000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Maturo SC, Mair EA. Submucosal minimally invasive lingual excision (SMILE): Technique for tonguebase reduction. Operative Techniques in Otolaryngology. 2007;18:29–32. [Google Scholar]
- 55.Chabolle F, Wagner I, Blumen M, et al. Tongue base reduction with hyoepiglottoplasty: A treatment for severe obstructive sleep apnea. Laryngoscope. 1999;109:1273–1280. doi: 10.1097/00005537-199908000-00017. [DOI] [PubMed] [Google Scholar]
- 56.Fibbi A, Peirano M, Bertino G. Mini-invasive surgical approach for obstructive sleep apnea syndrome (OSAS). A case report. Acta Otorhinolaryngol Ital. 2000;20:129–133. [PubMed] [Google Scholar]
- 57.Friedman M, Hamilton C, Samuelson GC, et al. Transoral robotic glossectomy for the treatment of obstructive sleep apnea-hypopnea syndrome. Otolaryngol Head Neck Surg. 2012;146:854–862. doi: 10.1177/0194599811434262. [DOI] [PubMed] [Google Scholar]
- 58.Riley RW, Powell NB, Guilleminault C. Inferior sagittal osteotomy of the mandible with hyoid myotomy-suspension: A new procedure for obstructive sleep apnea. Otolaryngol Head Neck Surg. 1986;94:589–593. doi: 10.1177/019459988609400510. [DOI] [PubMed] [Google Scholar]
- 59.Hörmann K, Baisch A. How I do it: The hyoid suspension. Laryngoscope. 2004;114:1677–1679. doi: 10.1097/00005537-200409000-00033. [DOI] [PubMed] [Google Scholar]
- 60.Lewis R. A modified technique of hyoid advancement. Operative Techniques in Otolaryngology. 2007;18:17–19. [Google Scholar]
- 61.Lin H-C, Friedman M, Chang H-W, et al. The efficacy of multilevel surgery of the upper airway in adults with obstructive sleep apnea/hypopnea syndrome. Laryngoscope. 2008;118:902–908. doi: 10.1097/MLG.0b013e31816422ea. [DOI] [PubMed] [Google Scholar]
- 62.Milano F, Mondini S, Billi MC, et al. The impact of a multidisciplinary approach on response rate of mandibular advancing device therapy in patients with obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital. 2013;33:337–342. [PMC free article] [PubMed] [Google Scholar]
- 63.Giarda M, Brucoli M, Arcuri F, et al. Efficacy and safety of maxillomandibular advancement in treatment of obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital. 2013;33:43–46. [PMC free article] [PubMed] [Google Scholar]
- 64.Gazayerli M, Bleibel W, Elhorr A, et al. A correlation between the shape of the epiglottis and obstructive sleep apnea. Surg Endosc. 2006;20:836–837. doi: 10.1007/s00464-005-0641-4. [DOI] [PubMed] [Google Scholar]
- 65.Bhimaraj A, Havaligi N, Ramachandran S. Rapid reduction of antihypertensive medications and insulin requirements after tracheostomy in a patient with severe obstructive sleep apnea syndrome. J Clin Sleep Med. 2007;3:297–299. [PMC free article] [PubMed] [Google Scholar]
- 66.Campanini A, Vito A, Frassineti S, et al. Role of skinlined tracheotomy in obstructive sleep apnea syndrome: personal experience. Acta Otorhinolaryngol Ital. 2004;24:68–74. [PubMed] [Google Scholar]