Abstract
Objectives. We compared pneumonia and influenza death rates among American Indian/Alaska Native (AI/AN) people with rates among Whites and examined geographic differences in pneumonia and influenza death rates for AI/AN persons.
Methods. We adjusted National Vital Statistics Surveillance mortality data for racial misclassification of AI/AN people through linkages with Indian Health Service (IHS) registration records. Pneumonia and influenza deaths were defined as those who died from 1990 through 1998 and 1999 through 2009 according to codes for pneumonia and influenza from the International Classification of Diseases, 9th and 10th Revision, respectively. We limited the analysis to IHS Contract Health Service Delivery Area counties, and compared pneumonia and influenza death rates between AI/ANs and Whites by calculating rate ratios for the 2 periods.
Results. Compared with Whites, the pneumonia and influenza death rate for AI/AN persons in both periods was significantly higher. AI/AN populations in the Alaska, Northern Plains, and Southwest regions had rates more than 2 times higher than those of Whites. The pneumonia and influenza death rate for AI/AN populations decreased from 39.6 in 1999 to 2003 to 33.9 in 2004 to 2009.
Conclusions. Although progress has been made in reducing pneumonia and influenza mortality, disparities between AI/AN persons and Whites persist. Strategies to improve vaccination coverage and address risk factors that contribute to pneumonia and influenza mortality are needed.
Although infectious disease rates have fallen dramatically in the United States,1 infectious disease continues to be a significant health problem, and substantial racial and ethnic disparities for some such diseases remain.2,3 American Indian and Alaska Native (AI/AN) people are at higher risk for many chronic and infectious diseases, have a higher prevalence of certain health risk behaviors, and experience higher infectious disease hospitalization rates compared with the general US population.4–13 Lower respiratory tract infections are a leading cause of infectious disease hospitalizations for AI/AN people,10 and previous studies have found significant disparities in lower respiratory tract infection hospitalization rates for AI/AN people compared with the US population in some regions, especially for infants (children aged < 1 year).12 Hospitalization and death rates for overall lower respiratory tract infections are significantly higher for AI/AN infants compared with other US infants.14–16
Pneumonia is a common cause of lower respiratory tract infection,14 and influenza is one of the leading causes of pneumonia. Pneumonia and influenza together are one of the top 10 leading causes of death in the United States. In 2010, pneumonia and influenza ranked ninth as the leading cause of death for all races in the United States.17 Pneumonia and influenza also ranks among the top 10 causes of death for AI/AN populations; elevated rates of pneumonia and influenza mortality compared with other races have been documented since the early 1990s.18–21 In 2010, pneumonia and influenza ranked 10th as a leading cause of death for the AI/AN population.17
Among the AI/AN population, hospitalization and disease rates for the leading causes of pneumonia (e.g., respiratory syncytial virus, influenza, and Streptococcus pneumoniae) are higher compared with other US population groups. For example, respiratory syncytial virus hospitalization rates are significantly higher for AI/AN infants, particularly in the Southwest and Alaska, compared with other US infants22; respiratory syncytial virus hospitalizations for Alaska Native infants in the Alaskan Yukon-Kuskokwim Delta are 5 times higher than rates for the general US infant population.23 Before the introduction of pneumococcal vaccines, rates of invasive pneumococcal disease in AI/AN adults and children in Alaska and some tribes in the Southwest were higher than rates for the general US population.3,24–26 Although rates of invasive pneumococcal disease declined significantly in US children and adults and among AI/AN children3,26–33 after the introduction of pneumococcal vaccines, invasive pneumococcal disease rates among certain AI/AN populations remain elevated compared with the general US population.3,26,29,32 Influenza, a leading cause of pneumonia, has also been found to disproportionately affect AI/AN populations. In the last 100 years, numerous reports have described the high rates of influenza-associated morbidity and mortality in AI/AN communities.34,35 More recently, during the 2009 H1N1 influenza A pandemic, several studies found higher influenza mortality and hospitalization rates among AI/AN people compared with the general population,36–38 as well as a higher prevalence of self-reported influenza-like illness and influenza-associated hospitalizations compared with non-Hispanic Whites.39
Racial misclassification has been found to contribute to an underestimation of death rates for AI/AN people, who are often misclassified as non-AI/AN persons in death certificate data.40,41 In 1 evaluation of the validity of racial classification in death certificates, only 55% of self-identified AI/AN decedents were correctly classified on their death certificates from 1990 to 1998.40 In another study, included in this supplement, misclassification of AI/AN race in mortality data was 17.7%.41 Although it is clear that pneumonia and influenza are significant health burdens for AI/AN people, racial misclassification in previous studies that relied on death certificate data likely underestimated the death rate for AI/AN persons.40
To provide more accurate estimates of the true burden of pneumonia and influenza mortality among AI/AN people, we used data from the National Death Index linked to Indian Health Service (IHS) patient registration data to improve AI/AN racial classification. Using these data, we compared pneumonia and influenza death rates for AI/AN populations to those for Whites. We described pneumonia and influenza related deaths among AI/AN people from 1990 to 2009, examined geographic differences in pneumonia and influenza death rates for AI/ANs, compared overall and age-specific pneumonia and influenza death rates among AI/AN people with rates among Whites, and described progress in addressing disparities in pneumonia and influenza mortality.
METHODS
A detailed description of the data sources and the methods used to create the database are provided in another article in this supplement.41
Bridged single-race population estimates developed by the Centers for Disease Control and Prevention National Center for Health Statistics (NCHS) and adjusted for the population shifts because of Hurricanes Katrina and Rita in 2005 were included as denominators in the calculations of death rates.41 Bridged single-race data allowed for comparability between the pre- and post-2000 race/ethnic population estimates during this study period. During preliminary analyses, it was discovered that the updated bridged intercensal population estimates significantly overestimated AI/AN persons of Hispanic origin.42 To avoid underestimating mortality in AI/AN persons, we limited analyses to non-Hispanic AI/AN individuals. Non-Hispanic White was chosen as the most homogeneous referent group. Therefore, all analyses were limited to non-Hispanic persons. Henceforth, the term “non-Hispanic” was omitted when discussing both groups.
Death Records
Death certificate data are compiled by each state and sent to the NCHS. The NCHS makes this information available to the research community as part of the National Vital Statistics System and includes underlying and multiple cause of death fields, state of residence, age, gender, race, and ethnicity.41 NCHS applies a bridging algorithm nearly identical to the one used by the Census Bureau to assign a single race to decedents with multiple races reported on the death certificate.41
The IHS patient registration database was linked to death certificate data in the National Death Index to identify AI/AN deaths misclassified as non-AI/AN deaths.41 Following this linkage, a flag indicating a positive link to IHS was added as an additional indicator of AI/AN ancestry to the National Vital Statistics System mortality file. This file, which includes all deaths for all races reported to NCHS from 1990 to 2009 (AI/AN-US Mortality Database), was combined with the population estimates to create an analytic file in SEER*Stat (version 8.0.4; available at: http://seer.cancer.gov/seerstat).
Race for AI/AN deaths in this report was assigned as reported elsewhere in this supplement.41 In short, it combined race classification by NCHS based on the death certificate and information derived from data linkages between the IHS patient registration database and the National Death Index.41
We examined all reported pneumonia and influenza deaths that occurred from 1990 to 2009. Pneumonia and influenza deaths were defined as those with an underlying cause of death listed as either pneumonia or influenza. Pneumonia has multiple etiologies, and although it may be listed as 1 of multiple causes of death, it may not necessarily be assigned as the underlying cause of death. Differences between AI/AN persons and Whites with regard to coding pneumonia as the underlying cause of death could result in bias. Therefore, to assess for potential bias, we calculated the ratio of pneumonia and influenza deaths using underlying versus multiple causes for AI/AN persons and compared that to the underlying versus multiple cause ratio for Whites. The ratios were similar for AI/AN populations and Whites, suggesting that there were no significant differences between the 2 groups in the coding of pneumonia and influenza as an underlying cause of death. Pneumonia and influenza as the underlying cause of death was therefore used for all subsequent analyses.
Because of the transition from International Classifications of Diseases, 9th Revision (ICD-9) to ICD-10 in 1999 and changes in the rules (especially Rule 3) for selecting the underlying cause of death, fewer pneumonia cases were identified as the underlying cause of death from 1999 onward.43 (Rule 3 addresses how direct sequelae should be handled. In ICD-10, any conditions that could be considered the consequence of another reported condition should not be reported as the underlying condition. In ICD-10, the applicability of Rule 3 to pneumonia is broader than in ICD-9; as a result, pneumonia is much less likely to be selected as the underlying cause of death under ICD-10 than under ICD-9.43) As a result, we were unable to directly compare pneumonia and influenza death rates across the entire time period. We therefore divided the 1990 to 2009 period into 2 time periods: 1990 to 1998 (when ICD-9 was used) and 1999 to 2009 (when ICD-10 was used), and examined pneumonia and influenza death rates separately in those 2 periods. From 1990 to 1998, we identified pneumonia and influenza deaths as any death with an underlying cause listed as ICD-9 codes 480–488. From 1999 to 2009, we identified pneumonia and influenza deaths as any death with an underlying cause of death listed as ICD-10 codes J09–J18. A table with a list of the codes and their corresponding definitions is included as a supplement to the online version of this article at http://www.ajph.org.
Geographic Coverage
Analyses were restricted to Contract Health Service Delivery Area (CHSDA) counties which, in general, contain federally recognized tribal lands or are adjacent to tribal lands.41 CHSDA residence is used by the IHS to determine eligibility for services not directly available within the IHS. Linkages studies indicate less misclassification of race for AI/AN persons in these counties.44 The CHSDA counties also have higher proportions of AI/AN persons in relation to the total population than do non-CHSDA counties, with 64% of the US AI/AN population residing in the 637 counties designated as CHSDA (these counties represent 20% of the 3141 counties in the United States).41 Although the result was less geographically representative, we restricted analyses to CHSDA counties for death rates for the purpose of offering improved accuracy in interpreting mortality statistics for AI/AN populations.
The analyses were completed for all regions combined and by individual IHS regions: Northern Plains, Alaska, Southern Plains, Southwest, Pacific Coast, and East.41 A map of the CHSDA counties and the IHS regions is included as data available as a supplement to the online version of this article at http://www.ajph.org and described in Table 1. These regions were used for other health-related publications focusing on AI/AN populations.41 Additional details about CHSDA counties and IHS regions are provided elsewhere.41
TABLE 1—
Pneumonia and Influenza Death Rates by Age Group and Indian Health Service Region for American Indians/Alaska Natives Compared with Whites: Contract Health Service Delivery Areas, United States, 1990–1998 and 1999–2009
| 1990–1998 |
1999–2009 |
|||||||||
| IHS Region/Age Range | AI/AN Count | AI/AN Rate | White Count | White Rate | AI/AN:White RR (95% CI) | AI/AN Count | AI/AN Rate | White Count | White Rate | AI/AN:White RR (95% CI) |
| Northern Plains | ||||||||||
| < 1 y | 32 | 69.9 | 108 | 12.6 | 5.55* (3.62, 8.30) | 15 | 22.0 | 48 | 4.9 | 4.49* (2.34, 8.16) |
| 1–4 y | … | 2.1 | 46 | 1.3 | 1.63 (0.40, 4.40) | … | 2.4 | 37 | 0.9 | 2.64 (0.90, 6.29) |
| 5–19 y | … | 0.6 | 47 | 0.3 | 1.86 (0.48, 5.12) | … | 0.8 | 53 | 0.3 | 2.50 (0.96, 5.52) |
| 20–49 y | 73 | 10.6 | 454 | 1.6 | 6.68* (5.13, 8.57) | 81 | 7.4 | 512 | 1.4 | 5.17* (4.03, 6.54) |
| 50–64 y | 81 | 46.1 | 965 | 9.9 | 4.67* (3.68, 5.87) | 94 | 27.9 | 1041 | 6.5 | 4.26* (3.41, 5.26) |
| 65–74 y | 85 | 145.1 | 2523 | 49.2 | 2.94* (2.30, 3.60) | 75 | 78.1 | 1734 | 27.6 | 2.83* (2.21, 3.57) |
| ≥ 75 y | 238 | 785.3 | 19 978 | 450.5 | 1.74* (1.50, 1.90) | 206 | 419.9 | 16 509 | 249.7 | 1.68* (1.45, 1.93) |
| All ages | 517 | 69.7 | 24 121 | 32.9 | 2.12* (1.92, 2.33) | 484 | 38.5 | 19 934 | 18.7 | 2.06* (1.85, 2.27) |
| Alaska | ||||||||||
| < 1 y | 15 | 75.1 | … | 11.8 | 6.38* (2.45, 18.49) | 15 | 64.1 | … | 7.8 | 8.22* (2.84, 28.92) |
| 1–4 y | … | 3.6 | … | 0.4 | 9.00 (0.72, 472.45) | … | 2.2 | … | 0.8 | 2.84 (0.20, 39.13) |
| 5–19 y | … | 0.7 | … | 0.1 | 6.25 (0.32, 385.74) | … | 1.6 | … | 0.2 | 8.37* (1.50, 86.13) |
| 20–49 y | 22 | 6.5 | 14 | 0.7 | 9.77* (4.70, 20.79) | 17 | 3.7 | 17 | 0.7 | 5.31* (2.54, 11.08) |
| 50–64 y | 19 | 23.9 | 32 | 7.5 | 3.19* (1.71, 5.82) | 26 | 18.3 | 37 | 4.1 | 4.52* (2.62, 7.68) |
| 65–74 y | 19 | 70.6 | 43 | 35.9 | 1.96* (1.07, 3.45) | 23 | 50.8 | 33 | 15.4 | 3.30* (1.84, 5.82) |
| ≥ 75 y | 80 | 558.5 | 139 | 267.4 | 2.09* (1.56, 2.77) | 104 | 431.3 | 209 | 170.5 | 2.53* (1.97, 3.22) |
| All ages | 160 | 46.2 | 237 | 20.1 | 2.29* (1.82, 2.87) | 193 | 35.1 | 305 | 12.5 | 2.83* (2.31, 3.44) |
| Southern Plains | ||||||||||
| < 1 y | … | 24.9 | … | 6.4 | 3.90 (0.63, 18.28) | … | 12.2 | 24 | 6.4 | 1.90 (0.74, 4.38) |
| 1–4 y | … | 0 | … | 0 | NA | … | 1.6 | 21 | 1.4 | 1.10 (0.27, 3.25) |
| 5–19 y | … | … | … | 0.2 | NA | … | 0.7 | 29 | 0.5 | 1.56 (0.61, 3.50) |
| 20–49 y | 12 | 5.0 | 87 | 2.7 | 1.87 (0.93, 3.43) | 72 | 5.1 | 352 | 2.7 | 1.91* (1.46, 2.47) |
| 50–64 y | 15 | 20.9 | 155 | 12.6 | 1.66 (0.91, 2.82) | 85 | 17.6 | 760 | 12.3 | 1.43* (1.13, 1.79) |
| 65–74 y | 29 | 107.1 | 385 | 61.1 | 1.75* (1.15, 2.55) | 85 | 54.0 | 1030 | 39.5 | 1.37* (1.08, 1.70) |
| ≥ 75 y | 95 | 544.9 | 2437 | 448.5 | 1.21 (0.97, 1.49) | 366 | 379.4 | 7123 | 294.5 | 1.29* (1.15, 1.43) |
| All ages | 154 | 45.6 | 3073 | 34.3 | 1.33* (1.11, 1.56) | 628 | 31.8 | 9339 | 23.7 | 1.34* (1.23, 1.45) |
| Southwest | ||||||||||
| < 1 y | 44 | 54.0 | 115 | 17.8 | 3.03* (2.09, 4.32) | 28 | 27.8 | 77 | 9.1 | 3.04* (1.90, 4.74) |
| 1–4 y | … | 2.7 | 39 | 1.6 | 1.70 (0.72, 3.57) | … | 2.1 | 26 | 0.8 | 2.69* (1.05, 6.10) |
| 5–19 y | 11 | 1.0 | 47 | 0.5 | 2.00 (0.93, 3.90) | 17 | 1.1 | 49 | 0.4 | 2.95* (1.59, 5.22) |
| 20–49 y | 135 | 10.3 | 590 | 2.9 | 3.54* (2.90, 4.29) | 208 | 10.3 | 747 | 2.6 | 4.02* (3.43, 4.70) |
| 50–64 y | 102 | 32.0 | 876 | 11.9 | 2.69* (2.17, 3.31) | 176 | 28.8 | 1473 | 10.4 | 2.77* (2.35, 3.24) |
| 65–74 y | 158 | 140.9 | 2099 | 50.2 | 2.80* (2.36, 3.30) | 159 | 79.3 | 2275 | 37.2 | 2.13* (1.80, 2.50) |
| ≥ 75 y | 576 | 743.8 | 10 936 | 406 | 1.83* (1.68, 1.99) | 699 | 561.0 | 12 327 | 243.6 | 2.30* (2.13, 2.48) |
| All ages | 1035 | 64.6 | 14 702 | 31.3 | 2.06* (1.92, 2.20) | 1295 | 48.7 | 16 974 | 20.1 | 2.42* (2.28, 2.56) |
| Pacific Coast | ||||||||||
| < 1 y | … | 22.1 | 158 | 10.2 | 2.16 (0.85, 4.55) | … | 17.6 | 50 | 3.1 | 5.64* (2.31, 12.00) |
| 1–4 y | … | 2.1 | 48 | 0.8 | 2.80 (0.55, 8.69) | … | 0 | 27 | 0.4 | 0.00 (0.00, 5.34) |
| 5–19 y | … | 1.0 | 82 | 0.4 | 2.97* (1.06, 6.73) | … | 0.5 | 73 | 0.3 | 2.09 (0.55, 5.55) |
| 20–49 y | 44 | 4.9 | 1224 | 2.3 | 2.11* (1.52, 2.85) | 54 | 4.2 | 1071 | 1.6 | 2.55* (1.90, 3.36) |
| 50–64 y | 52 | 22.4 | 2141 | 12.6 | 1.78* (1.32, 2.34) | 66 | 14.3 | 2236 | 7.5 | 1.91* (1.47, 2.44) |
| 65–74 y | 63 | 83.1 | 5271 | 56.6 | 1.47* (1.12, 1.88) | 53 | 41.6 | 3042 | 27.4 | 1.52* (1.13, 1.99) |
| ≥ 75 y | 199 | 519.4 | 37 227 | 503.5 | 1.03 (0.89, 1.18) | 200 | 299.5 | 26 894 | 227.8 | 1.31* (1.13, 1.51) |
| All ages | 374 | 43.0 | 46 151 | 37.3 | 1.15* (1.02, 1.28) | 385 | 25.2 | 33 393 | 17.5 | 1.44* (1.28, 1.59) |
| East | ||||||||||
| < 1 y | … | 29.1 | 119 | 7.8 | 3.73 (0.76, 11.16) | … | 10.0 | 61 | 3.8 | 2.65 (0.31, 9.99) |
| 1–4 y | … | 2.1 | 49 | 0.8 | 2.66 (0.6, 15.50) | … | 4.3 | 32 | 0.5 | 8.86* (1.73, 28.33) |
| 5–19 y | … | … | 79 | 0.3 | NA | … | … | 72 | 0.3 | NA |
| 20–49 y | … | 2.9 | 1120 | 2.1 | 1.38 (0.63, 2.62) | 10 | 2.1 | 1048 | 1.6 | 1.32 (0.63, 2.42) |
| 50–64 y | … | 11.2 | 2193 | 11.5 | 0.97 (0.45, 1.85) | 21 | 12.5 | 2279 | 7.7 | 1.61 (1.00, 2.47) |
| 65–74 y | 18 | 66.9 | 5341 | 50.4 | 1.33 (0.78, 2.09) | 20 | 44.5 | 3852 | 31.9 | 1.40 (0.84, 2.15) |
| ≥ 75 y | 67 | 454.6 | 35 684 | 409.7 | 1.11 (0.85, 1.40) | 67 | 242.6 | 33 058 | 255.3 | 0.95 (0.73, 1.20) |
| All ages | 107 | 35.3 | 44 585 | 30.9 | 1.14 (0.92, 1.38) | 123 | 20.8 | 40 402 | 19.5 | 1.06 (0.87, 1.27) |
| All regions | ||||||||||
| < 1 y | 104 | 51.7 | 513 | 10.9 | 4.76* (3.82, 5.89) | 76 | 23.5 | 265 | 4.8 | 4.86* (3.71, 6.29) |
| 1–4 y | 20 | 2.4 | 183 | 0.9 | 2.49* (1.48, 3.95) | 23 | 1.9 | 145 | 0.7 | 2.89* (1.77, 4.50) |
| 5–19 y | 23 | 0.8 | 259 | 0.4 | 2.15* (1.34, 3.31) | 42 | 0.8 | 278 | 0.3 | 2.89* (2.04, 4.01) |
| 20–49 y | 295 | 7.8 | 3489 | 2.2 | 3.57* (3.15, 4.03) | 442 | 6.6 | 3747 | 1.8 | 3.69* (3.33, 4.07) |
| 50–64 y | 278 | 29.0 | 6362 | 11.6 | 2.50* (2.21, 2.82) | 468 | 21.3 | 7826 | 8.1 | 2.62* (2.38, 2.88) |
| 65–74 y | 372 | 113.6 | 15 662 | 52.3 | 2.17* (1.95, 2.41) | 415 | 61.7 | 11 966 | 31.2 | 1.98* (1.79, 2.18) |
| ≥ 75 y | 1255 | 653.8 | 106 401 | 446.6 | 1.46* (1.38, 1.54) | 1642 | 423.5 | 96 120 | 246.9 | 1.72* (1.63, 1.80) |
| All ages | 2347 | 55.7 | 132 869 | 33.4 | 1.67* (1.59, 1.74) | 3108 | 36.3 | 120 347 | 19.1 | 1.90* (1.82, 1.97) |
Note. AI/AN = American Indian/Alaska Native; CI = confidence interval; CHSDA = Contract Health Service Delivery Area; IHS = Indian Health Service; NA = not applicable/able to be calculated; RR = rate ratio. Ellipses indicate that counts less than 10 were suppressed; if no cases reported, then rates and RRs could not be calculated. Analyses are limited to persons of non-Hispanic origin. AI/AN race is reported from death certificates or through linkage with the IHS patient registration database. Rates are per 100 000 persons and are age-adjusted to the 2000 US standard population (11 age groups; Census P25-113045). Rate ratios are calculated in SEER*Stat before rounding of rates and may not equal rate ratios calculated from rates presented in the table. IHS regions are defined as follows: Alaskaa; Northern Plains (IL, IN,a IA,a MI,a MN,a MT,a NE,a ND,a SD,a WI,a WYa); Southern Plains (OK,a KS,a TX,a); Southwest (AZ,a CO,a NV,a NM,a UTa); Pacific Coast (CA,a ID,a OR,a WA,a HI); East (AL,a AR, CT,a DE, FL,a GA, KY, LA,a ME,a MD, MA,a MS,a MO, NH, NJ, NY,a NC,a OH, PA,a RI,a SC,a TN, VT, VA, WV, DC). Percentage of AI/AN persons in CHSDA counties: Northern Plains = 64.8%; Alaska = 100%; Southern Plains = 76.3%; Southwest = 91.3%; Pacific Coast = 71.3%; East = 18.2%; total US = 64.2%. States and years of data excluded because Hispanic origin was not collected on the death certificate: LA: 1990; NH: 1990–1992; OK: 1990–1996.
Source. AI/AN Mortality Supplement Database (AMD 1990–2009).
aIdentifies states with at least 1 county designated as CHSDA.
*P < .05.
Statistical Methods
We calculated rates per 100 000 population for all ages combined, and for the following age groups: infants (< 1 year), children (1–4 and 5–19 years), and adults (20–49, 50–64, 65–74, and ≥ 75 years). Comparisons of overall and age-specific rates between AI/AN and White people were generated by calculating the rate ratio (RR), AI/AN:White, for each period. Ninety-five percent confidence intervals (CIs) for age-adjusted rates and RRs were calculated using SEER*Stat 8.0.4.46 To describe recent progress in addressing disparities in pneumonia and influenza mortality, we compared death rates for AI/AN persons and Whites from 1999 to 2003 to rates from 2004 to 2009, and calculated the difference between death rates in AI/AN persons and Whites from 1999 to 2003 versus 2004 to 2009.
RESULTS
From 1990 to 1998, based on ICD-9 codes, there were 2347 pneumonia and influenza deaths in the AI/AN population and 132 869 pneumonia and influenza deaths in the White population. The average annual age-adjusted pneumonia and influenza death rate for AI/AN persons was 55.7, and ranged from 35.3 in the East region to 69.7 in the Northern Plains region. For Whites, death rates ranged from 20.1 in the Alaska region to 37.3 in the Pacific Coast region (Table 1). From 1999 to 2009, using ICD-10 codes, there were 3108 pneumonia and influenza deaths among AI/AN persons and 120 347 deaths among Whites. The average annual age-adjusted pneumonia and influenza death rate for AI/AN persons was 36.3 and ranged from a low of 20.8 in the East region to 48.7 in the Southwest region (Table 1). For Whites, pneumonia and influenza death rates ranged from 12.5 in Alaska to 23.7 in the Southern Plains region. The majority of pneumonia and influenza deaths in both periods were caused by pneumonia (Figure 1). Rates were higher for males than females in both periods and in all regions (data not shown). From 1990 to 1998, the rate among AI/AN males was 73.4 compared with 43.8 for females, and among Whites, 42.2 for males versus 28.2 for females. From 1999 to 2009, the AI/AN male pneumonia and influenza death rate for all ages was 43.2 versus 31.8 for females; among Whites, male versus female pneumonia and influenza death rates were 22.4 versus 17.0, respectively.
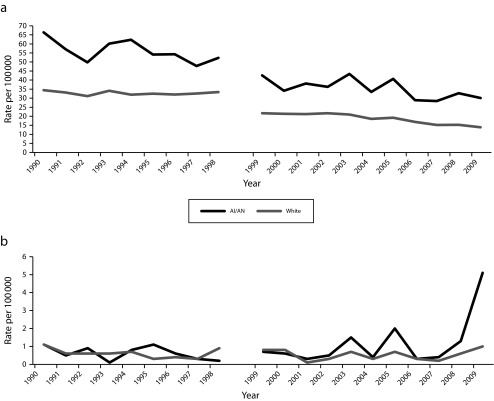
FIGURE 1—
Annual death rates for American Indians/Alaska Natives (AI/ANs) and Whites from (a) pneumonia and (b) influenza: Contract Health Service Delivery Areas, United States, 1990–2009.
Note. Rates are per 100 000 persons and are age-adjusted to the 2000 US standard population (11 age groups; Census P25-113045). Analyses are limited to persons of non-Hispanic origin. AI/AN race is reported from death certificates or through linkage with the Indian Health Service patient registration database. The following states and years of data are excluded because Hispanic origin was not collected on the death certificate: LA: 1990; NH: 1990–1992; OK: 1990–1996.
Source. AI/AN Mortality Database (AMD 1990–2009).
Compared with Whites, the all-ages pneumonia and influenza death rate for AI/AN persons in both periods was significantly higher from 1990 to 1998 (RR = 1.67; 95% CI = 1.59, 1.74) and 1999 to 2009 (RR = 1.90; 95% CI = 1.82, 1.97). In addition, in both periods, the pneumonia and influenza death rate was significantly higher for AI/AN persons compared with Whites in all regions except the East. AI/AN populations in the Alaska, Northern Plains, and Southwest regions had rates that were more than 2 times higher compared with rates in Whites (Table 1).
For all regions combined, the highest pneumonia and influenza death rate among AI/AN persons and Whites was in the 75 years and older age group in both periods (Table 1). Among AI/AN people, from 1990 to 1998, the next highest rates in all regions were among those aged 65 to 74 years followed by infants; among Whites, the second highest rates were in those aged 65 to 74 years followed by those aged 50 to 64 years, with the exception of the Northern Plains region, in which infants had the third highest rate for both AI/AN persons and Whites (Table 1). From 1999 to 2009, rates in AI/AN infants were higher than rates for AI/ANs aged 50 to 64 years in Alaska. In all other regions, for both AI/ANs and Whites, rates for those aged 50 to 64 years were higher than infant rates.
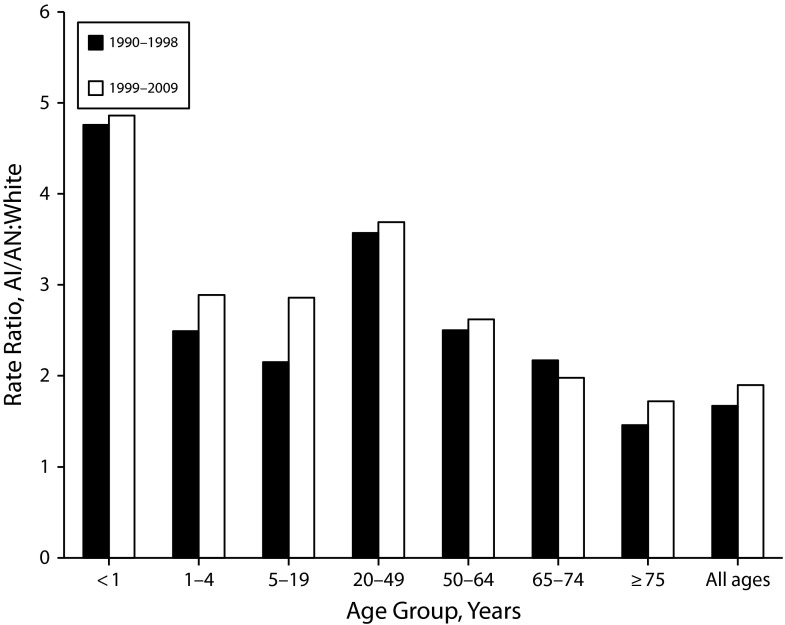
Although those 75 years and older had the highest overall pneumonia and influenza death rates, AI/AN infants and those aged 20 to 49 years had the highest RRs compared with Whites (Table 1 and Figure 2). The overall RR for AI/AN versus White infants was more than 4-fold higher in both periods. Among those aged 20 to 49 years, the RR for AI/AN persons versus Whites was more than 3-fold higher for both periods.
FIGURE 2—
Pneumonia and influenza death rate ratios by age group for American Indians/Alaska Natives (AI/ANs) and Whites: Contract Health Service Delivery Areas, United States, 1990–2009.
Note. Rate ratios are calculated in SEER*Stat before rounding of rates and may not equal RRs calculated from rates presented in Table 1. Analyses are limited to persons of non-Hispanic origin. AI/AN race is reported from death certificates or through linkage with the Indian Health Service patient registration database. The following states and years of data are excluded because Hispanic origin was not collected on the death certificate: LA 1990; NH: 1990–1992; OK: 1990–1996.
Source. AI/AN Mortality Database (AMD 1990–2009).
Among the regions for the 1990 to 1998 period, infant pneumonia and influenza death rates for AI/AN populations were significantly higher compared with rates among Whites in 3 regions—Alaska, the Southwest, and the Northern Plains—ranging from 3.03 in the Southwest to 6.38 times higher in Alaska (Table 1). From 1999 to 2009, infant pneumonia and influenza death rates were again significantly higher in those 3 regions, as well as in the Pacific Coast region. RRs ranged from 3.04 in the Southwest to 8.22 in Alaska (Table 1).
From 1990 to 1998, pneumonia and influenza death rates among AI/AN persons aged 20 to 49 years were significantly higher than rates in Whites in Alaska (RR = 9.77; 95% CI = 4.70, 20.79), the Northern Plains (RR = 6.68; 95% CI 5.13, 8.57), the Southwest (RR = 3.54; 95% CI = 2.90, 4.29), and the Pacific Coast (RR = 2.11; 95% CI = 1.52, 2.85; Table 1). From 1999 to 2009, rates for AI/AN persons in this age group were significantly higher in all regions, except for the East, and RRs ranged from 1.91 in the Southern Plains to 5.31 in Alaska (Table 1).
Underlying Cause of Death
The majority of pneumonia and influenza deaths in both periods were caused by pneumonia, which was responsible for more than 95% of all pneumonia and influenza deaths for AI/ANs and Whites (data available as a supplement to the online version of this article at http://www.ajph.org). Annual pneumonia death rates for AI/AN persons from 1990 to 2009 were significantly higher than rates for Whites in all years, ranging from 1.4 to 2.1 times higher compared with rates among Whites (Figure 1). From 1990 to 1998, influenza death rates were similar for AI/AN persons and Whites. From 1999 to 2009, influenza death rates among AI/AN persons tended to be higher compared with rates in Whites (Figure 1). In 2003, 2005, and 2008, influenza death rates ranged from 2.1 to 2.8 times higher compared with Whites, and in 2009, the year of the H1N1 influenza A pandemic, the overall influenza death rate for AI/AN persons was 5.1 (RR = 4.9; 95% CI = 3.8, 6.4) compared with 1.0 among Whites. In 2009, rates were significantly higher for AI/AN persons compared with Whites in all age groups, except for infants and children aged 5 to 19 years; the highest RR occurred in children aged 1 to 4 years, with a RR of 8.2 (95% CI = 1.3, 38.6), followed by those aged 65 to 74 years (RR = 6.29; 95% CI = 2.17, 14.9), 20 to 49 years (RR = 6.10; RR = 4.09, 8.86), 75 years and older (RR = 3.94; 95% CI = 1.04, 10.4), and 50 to 64 years (RR = 3.83; 95% CI = 2.2, 6.15; data not shown).
Progress in Addressing Disparities
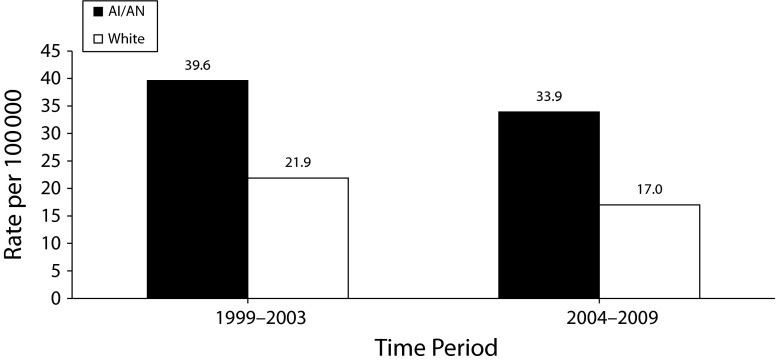
From 1999 to 2009, rates for pneumonia and influenza deaths declined for AI/AN and White people. Rates for AI/AN individuals decreased from 39.6 in 1999 to 2003 to 33.9 in 2004 to 2009. Rates for Whites decreased from 21.9 in 1999 to 2003 to 17.0 in 2004 to 2009. The difference in death rates between AI/AN individuals and Whites also decreased. From 1999 to 2003, the difference in death rates between AI/AN individuals and Whites was 17.7, and decreased slightly to 16.9 from 2004 to 2009 (Figure 3). The RR increased slightly, although the CIs overlapped (1999–2003: RR = 1.8; 95% CI = 1.7, 1.9; 2004–2009: RR = 1.9; 95% CI = 1.8, 2.0).
FIGURE 3—
Pneumonia and influenza death rates for American Indians/Alaska Natives (AI/ANs) and Whites: Contract Health Service Delivery Areas, United States, 1999–2003 and 2004–2009.
Note. Rates are per 100 000 persons and are age-adjusted to the 2000 US standard population (11 age groups; Census P25-113045). Analyses are limited to persons of non-Hispanic origin. AI/AN race is reported from death certificates or through linkage with the IHS patient registration database. The following states and years of data are excluded because Hispanic origin was not collected on the death certificate: LA: 1990; NH: 1990–1992; OK: 1990–1996.
Source. AI/AN Mortality Database (AMD 1990–2009).
DISCUSSION
Although the changes in ICD coding complicated the examination of trends in pneumonia and influenza mortality for the entire 1990 to 2009 period, from 1999 to 2009, pneumonia and influenza death rates for both AI/ANs and Whites declined. Although the difference in death rates between AI/AN persons and Whites also decreased, disparities between AI/AN and White populations persisted. AI/AN people continued to have almost a 2-fold increased risk of dying from pneumonia and influenza compared with Whites. This disparity persisted in all regions except for the East, and was the highest for the Alaska, Northern Plains, and Southwest regions.
The geographic differences in pneumonia and influenza mortality were not surprising, and likely reflected differences in population characteristics known to contribute to pneumonia and influenza mortality. The prevalence of underlying medical conditions, such as diabetes, cardiovascular disease, and obesity were higher among certain AI/AN tribes compared with others.5,6,8 Although, overall, AI/AN people were more likely to live in poverty compared with non-Hispanic Whites,6–8,21 some regions (Alaska, Northern Plains, Southwest, and Pacific Coast) had a higher proportion of AI/AN people with low incomes compared with other regions.8 Low socioeconomic status could contribute to household and environmental factors, such as household crowding, lack of running water in the home, and poor indoor air quality, all of which have been associated with higher rates of infectious diseases and lower respiratory tract infections.30,47–54 In the Alaska, Southwest, and Northern Plains regions, where the pneumonia and influenza mortality disparity appeared to be the greatest, AI/AN people were more likely to live in crowded housing55,56 and reside on reservations and tribal villages in rural, isolated, and remote areas where running water in the home might not be available, use of wood-burning stoves might be more common, and household ventilation might be suboptimal during cold weather months.50,51 Tobacco smoking, which can contribute to poor indoor air quality and is a known risk factor for respiratory infections among children,57 was more prevalent in Alaska and in the Northern Plains than in other regions8; this might contribute to the increased pneumonia and influenza mortality burden in these regions. Finally, previous studies conducted during the time represented by our data found that AI/AN people were generally less likely to access preventive care6,8 and were more likely to report not receiving needed health care because of cost.7 Barriers to accessing health care and delays in antiviral medication treatment, which were found to contribute to increased rates of influenza mortality,58 might be more likely to occur in the more remote and rural reservations that are common to the Alaska, Southwest,38 and Northern Plains regions. In rural and remote communities, there might be fewer health care options, distances to health care facilities might be greater, and transportation options limited.34
The differences in the age distribution of pneumonia and influenza mortality among AI/AN and White people are worth noting; AI/AN infants and those aged 20 to 49 years had the highest RRs compared with Whites, and the oldest age group had the lowest RR. From 1999 to 2009, for all regions combined, a disparity for AI/AN persons compared with Whites existed for all age groups. Not surprisingly, those aged 75 years and older had the highest pneumonia and influenza death rates for both groups, and although rates among AI/AN individuals were significantly higher compared with rates for Whites in this age group, the disparity was less than for other age groups. This finding was consistent with previous studies of elderly AI/AN hospitalizations.11 For all regions combined, the greatest disparity was found among infants, where the pneumonia and influenza death rate among AI/AN infants was 4.86 times higher compared with Whites. AI/AN infants in Alaska, the Northern Plains, and the Southwest regions, as well as the Pacific Coast, had significantly higher pneumonia and influenza death rates. The highest RR was found in the Alaska region, where AI/AN infants were 8.22 times more likely to die from pneumonia and influenza compared with their White counterparts. Previous studies showed that AI/AN infants had higher hospitalization rates for infectious diseases and other respiratory infections compared with non-Hispanic Whites14,15; therefore, it was not surprising that the pneumonia and influenza death rate would also be significantly higher. Although improvements in access to health care and treatment contributed to a reduction in infant pneumonia and influenza deaths, household and environmental factors, such as crowding, lack of access to running water in the home, poor indoor air quality, and exposure to tobacco and wood smoke, some of which are more common in Alaska, the Northern Plains and the Southwest, likely continued to contribute to the ongoing disparity.16,30,50–57 The emergence of a disparity among the infant age group in the Pacific Coast region in the 1999 to 2009 period might be related to the presence of some of these risk factors among AI/AN communities in the Pacific Coast region, although the relatively small number of cases in this region made it difficult to determine if this was a true disparity.
AI/AN persons aged 20 to 49 years also appeared to bear a disproportionate burden of pneumonia and influenza mortality compared with Whites. Pneumonia and influenza death rates for AI/AN persons in this age group were 3.69 times higher compared with Whites from 1999 to 2009, with rates in the Northern Plains, Alaska, and the Southwest 4 to 5 times higher. Reasons for this increased disparity were likely related not only to the previously mentioned household and other environmental factors, but might also be related to the high prevalence of underlying chronic health conditions among adults that have been documented in the AI/AN population,5,7,13,32 and the higher rates of smoking, particularly in the Alaska and Northern Plains regions.6–8,32
Progress in reducing the overall pneumonia and influenza death rate was likely related to better access to health services and medical treatment, particularly for pneumonia, and improvements in living conditions. The introduction and expansion of vaccination programs for both influenza and pneumococcal vaccines also contributed to the decline in the pneumonia and influenza death rate since 1999. Among AI/AN people seen in the IHS health care system, overall coverage among AI/AN adults aged 65 years and older with the 23-valent pneumococcal polysaccharide vaccine increased from 58% in 2000 to 82% in 2009, and coverage with influenza vaccine increased from 48% in 2000 to 59% in 2009 (personal communication, Diane Leach, IHS Government Performance Results Act Team, May 2013). The introduction of pneumococcal conjugate vaccine for children in 2000 resulted in an overall decline in invasive pneumococcal disease in all age groups in the United States59,60 and among AI/AN children.3,29 Coverage with 4 doses of pneumococcal conjugate vaccine among AI/AN children served by IHS was 77% in 2009.61 Further progress in prevention of invasive pneumococcal disease and pneumococcal pneumonia is anticipated with the widespread use of the 13-valent pneumococcal conjugate vaccine, which replaced the 7-valent vaccine in 2010. Because of multiple causative agents and diagnostic challenges related to identifying the organisms causing pneumonia,62 it is difficult to say how much of the pneumonia and influenza mortality can be further reduced through vaccination. In both time periods and for both groups, 80% or more of pneumonia and influenza deaths were attributed to pneumonia caused by an unspecified organism, although some of these deaths might have been caused by S. pneumonia infection.62 Although vaccination can play a role in further reducing pneumonia and influenza mortality, other interventions are needed.
Of note, the influenza mortality RR for 2009 was considerably higher than the RR reported for other years. In 2009, the influenza death rate among AI/AN persons was almost 5 times higher compared with the rate for Whites. This finding was consistent with a 12-state study that found that AI/AN people were 4 times more likely to die from H1N1 influenza infections compared with other populations.36 A follow-up study to identify risk factors for increased influenza mortality found that advanced age, underlying medical conditions, smoking, delayed initiation of antiviral medications, and barriers to health care access were associated with an increased risk of influenza mortality (personal communication, Thomas Hennessy, Director, Arctic Investigations Program, Anchorage, AK, May 2013). In addition, a study examining H1N1 influenza-related hospitalizations in 4 rural AI/AN communities in the Southwest found substantially higher hospitalization rates compared with other US populations, which was attributed in part to underlying chronic medical conditions and low antiviral treatment rates.38
Limitations
Limitations to this study include the transition from ICD-9 to ICD-10 codes, possible racial misclassification, exclusion of Hispanic AI/AN persons, small numbers that limited our ability to examine geographic variation, and potential underreporting of pneumonia and influenza as a cause of death. The transition from ICD-9 to ICD-10 made it difficult to determine if pneumonia and influenza mortality really decreased over time, although the comparison of RRs for the 2 time periods allowed for a comparison of the magnitude of the disparity over time. Small numbers for certain regions, however, limited our ability to distinguish true differences. Although the methods used in this study improved the racial classification methods used in previous studies, they were limited to AI/AN people who receive care from IHS. There is substantial variation between federally recognized tribes in the proportion of Native ancestry required for tribal membership, and therefore, for eligibility for IHS services. Whether and how this discrepancy in tribal membership requirements might influence some of our findings was unclear, although our findings were consistent with previous reports. In addition, racial misclassification of AI/AN people who did not receive care from the IHS might contribute to an underestimate in the death rates for AI/ANs presented here. Limiting our analysis to CHSDA counties, however, should have minimized this issue, although as a result, our findings might not be generalizable to those residing outside CHSDA counties. Although the exclusion of Hispanic AI/AN individuals from the analyses for reasons described in another article in this supplement41 reduced the overall AI/AN deaths by less than 5%, it might disproportionately affect some states. Finally, previous studies found that influenza and pneumonia, although noted on the death certificate as contributing causes, were often not assigned as the cause of death.63 Influenza was often not reported as the cause of death because people die of complications from the disease, not directly from influenza.64 Pneumonia, which has multiple etiologies, might be the immediate cause of death, but not necessarily the underlying cause of death. Because of this, the pneumonia and influenza death rates reported here might be an underestimate.
Conclusions
Reasons for the continued pneumonia and influenza mortality disparity between AI/AN and White people were difficult to isolate. The disparities were most likely the result of a complex interaction of factors. These included lower socioeconomic status that was associated with household and environmental factors that increased transmission of infectious diseases, an increased burden of chronic health conditions and health risk behaviors that might increase infectious disease susceptibility and severity, and barriers to accessing health care that might result in less use of prevention and treatment services. Although reducing socioeconomic disparities is challenging, focused efforts to improve household living conditions could have a direct impact on reducing the infectious disease burden among AI/AN people30 and might help to further reduce pneumonia and influenza death rates. Although only a subset of the pneumonia deaths might be vaccine preventable, ensuring access to pneumococcal and influenza vaccines, as well as antiviral treatment, is critical for AI/AN communities, particularly in the event of another pandemic, because of the higher background rates of both invasive pneumococcal disease and influenza, and the documented increased risk for severe outcomes.36,38 Continuing efforts to improve pneumococcal conjugate vaccine coverage among AI/AN children, and increased awareness of the importance of early antiviral treatment of influenza among providers and AI/AN communities, is needed. Finally, continuing to strengthen on-going surveillance efforts to better identify the disease burden among AI/AN people at local and regional levels is essential to increase public health action to address disparities.
Acknowledgments
We thank Melissa Jim and Diana Roberts for their assistance with the data analysis, and David Espey for his dedication, support, and guidance throughout this project.
Human Participant Protection
This project was determined to constitute public health practice and not research; therefore, no formal institutional review board approvals were required.
References
- 1.Armstrong GL, Conn LA, Pinner RW. Trends in infectious disease mortality in the United States during the 20th century. JAMA. 1999;281(1):61–66. doi: 10.1001/jama.281.1.61. [DOI] [PubMed] [Google Scholar]
- 2.Hutchins SS, Fiscella K, Levine RS et al. Protection of racial/ethnic minority populations during an influenza pandemic. Am J Public Health. 2009;99(suppl 2):S261–S270. doi: 10.2105/AJPH.2009.161505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singleton R, Holve S, Groom A et al. Impact of immunizations on the disease burden of American Indian and Alaska native children. Arch Pediatr Adolesc Med. 2009;163(5):446–453. doi: 10.1001/archpediatrics.2009.44. [DOI] [PubMed] [Google Scholar]
- 4.Oraka E, Iqbal S, Flanders W et al. Racial and ethnic disparities in current asthma and emergency department visits: findings from the National Health Interview Survey, 2001–2010. J Asthma. 2013;50(5):488–496. doi: 10.3109/02770903.2013.790417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denny CH, Holtzman D, Cobb N. Surveillance for health behaviors of American Indians and Alaska Natives. Findings from the Behavioral Risk Factor Surveillance System, 1997–2000. MMWR Surveill Summ. 2003;52(7):1–13. [PubMed] [Google Scholar]
- 6.Steele CB, Cardinez CJ, Richardson LC et al. Surveillance for health behaviors of American Indians and Alaska Natives—findings from the Behavioral Risk Factor Surveillance System, 2000–2006. Cancer. 2008;113(5 suppl):1131–1141. doi: 10.1002/cncr.23727. [DOI] [PubMed] [Google Scholar]
- 7.Barnes PM, Adams PF, Powell-Griner E. Health characteristics of the American Indian or Alaska Native adult population: United States, 2004–2008. Natl Health Stat Report. 2010;20:1–22. [PubMed] [Google Scholar]
- 8.Cobb N, Espey D, King J. Health behaviors and risk factors among American Indians and Alaska Natives, 2000–2010. Am J Public Health. 2014;104(6 suppl 3):S481–S489. doi: 10.2105/AJPH.2014.301879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holman RC, Curns AT, Cheek JE et al. Infectious disease hospitalizations among American Indian and Alaska native infants. Pediatrics. 2003;111(2):E176–E182. doi: 10.1542/peds.111.2.e176. [DOI] [PubMed] [Google Scholar]
- 10.Holman RC, Curns AT, Kaufman SF et al. Trends in infectious disease hospitalizations among American Indians and Alaska Natives. Am J Public Health. 2001;91(3):425–431. doi: 10.2105/ajph.91.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holman RC, Curns AT, Singleton RF et al. Infectious disease hospitalizations among older American Indian and Alaska Native adults. Public Health Rep. 2006;121(6):674–683. doi: 10.1177/003335490612100607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holman RC, Folkema AM, Singleton R et al. Disparities in infectious disease hospitalizations for American Indian/Alaska Native people. Public Health Rep. 2011;126(4):508–521. doi: 10.1177/003335491112600407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao Y, Bang D, Cosgrove S et al. Surveillance of health status in minority communities—Racial and Ethnic Approaches to Community Health Across the U.S. (REACH U.S.) Risk Factor Survey, United States, 2009. MMWR Surveill Summ. 2011;60(6):1–44. [PubMed] [Google Scholar]
- 14.Singleton RJ, Holman RC, Folkema AM et al. Trends in lower respiratory tract infection hospitalizations among American Indian/Alaska Native children and the general US child population. J Pediatr. 2012;161(2):296–302. doi: 10.1016/j.jpeds.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Peck AJ, Holman RC, Curns A et al. Lower respiratory tract infections among American Indian and Alaska Native children and the general population of U.S. children. Pediatr Infect Dis J. 2005;24(4):342–351. doi: 10.1097/01.inf.0000157250.95880.91. [DOI] [PubMed] [Google Scholar]
- 16.Singleton RJ, Wirsing EA, Haberling DL et al. Risk factors for lower respiratory tract infection death among infants in the United States, 1999–2004. Pediatrics. 2009;124(4):e768–e776. doi: 10.1542/peds.2009-0109. [DOI] [PubMed] [Google Scholar]
- 17.National Center for Health Statistics. Hyattsville, MD: US Government Printing Office; 2012. Health, United States, 2011: With Special Feature on Socioeconomic Status and Health. [PubMed] [Google Scholar]
- 18.Indian Health Service. Trends in Indian Health – 1995. Rockville, MD: Indian Health Service; 1996. [Google Scholar]
- 19.Indian Health Service. Trends in Indian Health 1998–1999. Rockville, MD: Indian Health Service; 2000. [Google Scholar]
- 20.Indian Health Service. Trends in Indian Health 2000–2001. Rockville, MD: Indian Health Service; 2002. [Google Scholar]
- 21.Indian Health Service. Trends in Indian Health 2002–2003. Rockville, MD: Indian Health Service; 2008. [Google Scholar]
- 22.Holman RC, Curns AT, Cheek JE et al. Respiratory syncytial virus hospitalizations among American Indian and Alaska Native infants and the general United States infant population. Pediatrics. 2004;114(4):e437–e444. doi: 10.1542/peds.2004-0049. [DOI] [PubMed] [Google Scholar]
- 23.Singleton RJ, Bruden D, Bulkow L. Respiratory syncytial virus season and hospitalizations in the Alaskan Yukon-Kuskokwim Delta. Pediatr Infect Dis J. 2007;26(11 suppl):S46–S50. doi: 10.1097/INF.0b013e318157da9b. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien KL, Shaw J, Weatherholtz R et al. Epidemiology of invasive Streptococcus pneumoniae among Navajo children in the era before use of conjugate pneumococcal vaccines, 1989–1996. Am J Epidemiol. 2004;160(3):270–278. doi: 10.1093/aje/kwh191. [DOI] [PubMed] [Google Scholar]
- 25.Watt JP, O’Brien KL, Benin AL et al. Invasive pneumococcal disease among Navajo adults, 1989–1998. Clin Infect Dis. 2004;38(4):496–501. doi: 10.1086/381198. [DOI] [PubMed] [Google Scholar]
- 26.Said MA, O’Brien KL, Nuorti JP et al. The epidemiologic evidence underlying recommendations for use of pneumococcal polysaccharide vaccine among American Indian and Alaska Native populations. Vaccine. 2011;29(33):5355–5362. doi: 10.1016/j.vaccine.2011.05.086. [DOI] [PubMed] [Google Scholar]
- 27.Whitney CG. Impact of conjugate pneumococcal vaccines. Pediatr Infect Dis J. 2005;24(8):729–730. doi: 10.1097/01.inf.0000174138.25465.ec. [DOI] [PubMed] [Google Scholar]
- 28.Pilishvili T, Lexau C, Farley M et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 29.Weatherholtz R, Millar E, Moulton L et al. Invasive pneumococcal disease a decade after pneumococcal conjugate vaccine use in an American Indian population at high risk for disease. Clin Infect Dis. 2010;50(9):1238–1246. doi: 10.1086/651680. [DOI] [PubMed] [Google Scholar]
- 30.Wenger JD, Zulz T, Bruden D et al. Invasive pneumococcal disease in Alaskan children: impact of the seven-valent pneumococcal conjugate vaccine and the role of water supply. Pediatr Infect Dis J. 2010;29(3):251–256. doi: 10.1097/INF.0b013e3181bdbed5. [DOI] [PubMed] [Google Scholar]
- 31.Singleton RJ, Hennessy TW, Bulkow L et al. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297(16):1784–1792. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 32.Singleton RJ, Butler JC, Bulkow L et al. Invasive pneumococcal disease epidemiology and effectiveness of 23-valent pneumococcal polysaccharide vaccine in Alaska native adults. Vaccine. 2007;25(12):2288–2295. doi: 10.1016/j.vaccine.2006.11.065. [DOI] [PubMed] [Google Scholar]
- 33.Benin AL, Watt JP, O’Brien KL et al. Delivering pneumococcal vaccine to a high risk population: the Navajo experience. Hum Vaccin. 2005;1(2):66–69. doi: 10.4161/hv.1.2.1562. [DOI] [PubMed] [Google Scholar]
- 34.Groom AV, Jim C, Laroque M et al. Pandemic influenza preparedness and vulnerable populations in tribal communities. Am J Public Health. 2009;99(suppl 2):S271–S278. doi: 10.2105/AJPH.2008.157453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reid AH, Fanning TG, Hultin JV et al. Origin and evolution of the 1918 “Spanish” influenza virus hemagglutinin gene. Proc Natl Acad Sci USA. 1999;96(4):1651–1656. doi: 10.1073/pnas.96.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Deaths related to 2009 pandemic influenza A (H1N1) among American Indian/Alaska Natives - 12 states, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(48):1341–1344. [PubMed] [Google Scholar]
- 37.Wenger JD, Castrodale L, Bruden D et al. 2009 Pandemic influenza A H1N1 in Alaska: temporal and geographic characteristics of spread and increased risk of hospitalization among Alaska Native and Asian/Pacific Islander people. Clin Infect Dis. 2011;52(suppl 1):S189–S197. doi: 10.1093/cid/ciq037. [DOI] [PubMed] [Google Scholar]
- 38.Suryaprasad A, Redd J, Hancock K et al. Severe acute respiratory infections caused by 2009 pandemic influenza A (H1N1) among American Indians — Southwestern United States, May 1–July 21, 2009. Influenza Other Respir Viruses. 2013;7(6):1361–1369. doi: 10.1111/irv.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dee DL, Bensyl DM, Gindler J et al. Racial and ethnic disparities in hospitalizations and deaths associated with 2009 pandemic influenza A (H1N1) virus infections in the United States. Ann Epidemiol. 2011;21(8):623–630. doi: 10.1016/j.annepidem.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Arias E, Schauman WS, Eschbach K, Sorlie PD. The validity of race and Hispanic origin reporting on death certificates in the United States. National Center for Health Statistics. Vital Health Stat 2. 2008;(148):1–23. [PubMed] [Google Scholar]
- 41.Espey D, Jim MA, Richards T, Begay C, Haverkamp D, Roberts D. Methods for improving the quality and completeness of mortality data for American Indians and Alaska Natives. Am J Public Health. 2014;104(6 suppl 3):S286–S294. doi: 10.2105/AJPH.2013.301716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards BK, Noone AM, Mariotto AB et al. Annual report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2013 doi: 10.1002/cncr.28509. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson RN, Minino AM, Hoyert D et al. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl Vital Stat Rep. 2001;49(2):1–32. [PubMed] [Google Scholar]
- 44.Jim MA, Arias E, Seneca DS et al. Racial misclassification of American Indians and Alaska Natives by Indian Health Service Contract Health Service Delivery Area. Am J Public Health. 2014;104(6 suppl 3):S295–S302. doi: 10.2105/AJPH.2014.301933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Day JC. Population Projections of the United States by Age, Sex, Race, and Hispanic Origin: 1995 to 2050, US Bureau of the Census, Current Population Reports, P25-1130. Washington, DC: US Government Printing Office; 1996. [Google Scholar]
- 46.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547–569. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 47.Bulkow LR, Singleton RJ, DeByle C et al. Risk factors for hospitalization with lower respiratory tract infections in children in rural Alaska. Pediatrics. 2012;129(5):e1220–e1227. doi: 10.1542/peds.2011-1943. [DOI] [PubMed] [Google Scholar]
- 48.Bulkow LR, Singleton RJ, Karron RA et al. Risk factors for severe respiratory syncytial virus infection among Alaska native children. Pediatrics. 2002;109(2):210–216. doi: 10.1542/peds.109.2.210. [DOI] [PubMed] [Google Scholar]
- 49.Sood A. Indoor fuel exposure and the lung in both developing and developed countries: an update. Clin Chest Med. 2012;33(4):649–665. doi: 10.1016/j.ccm.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robin LF, Less PS, Winget M et al. Wood-burning stoves and lower respiratory illnesses in Navajo children. Pediatr Infect Dis J. 1996;15(10):859–865. doi: 10.1097/00006454-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Morris K, Morgenlander M, Coulehan J et al. Wood-burning stoves and lower respiratory tract infection in American Indian children. Am J Dis Child. 1990;144(1):105–108. doi: 10.1001/archpedi.1990.02150250117047. erratum, 1990;144(4):490. [DOI] [PubMed] [Google Scholar]
- 52.Gessner BD. Lack of piped water and sewage services is associated with pediatric lower respiratory tract infection in Alaska. J Pediatr. 2008;152(5):666–670. doi: 10.1016/j.jpeds.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 53.Hennessy TW, Ritter T, Holman RC et al. The relationship between in-home water service and the risk of respiratory tract, skin, and gastrointestinal tract infections among rural Alaska natives. Am J Public Health. 2008;98(11):2072–2078. doi: 10.2105/AJPH.2007.115618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graham NM. The epidemiology of acute respiratory infections in children and adults: a global perspective. Epidemiol Rev. 1990;12:149–178. doi: 10.1093/oxfordjournals.epirev.a036050. [DOI] [PubMed] [Google Scholar]
- 55.Kingsley T, Spencer V, Simonson J. Assessment of American Indian Housing Needs and Programs: Final Report. Washington, DC: Urban Institute; 1995. [Google Scholar]
- 56.Myers D, Baer WC, Choi S-Y. The changing problem of overcrowded housing. J Am Plann Assoc. 1996;62(1):66–84. [Google Scholar]
- 57.Pedreira FA, Guandolo VL, Feroli EJ et al. Involuntary smoking and incidence of respiratory illness during the first year of life. Pediatrics. 1985;75(3):594–597. [PubMed] [Google Scholar]
- 58.Kumar A. Early versus late oseltamivir treatment in severely ill patients with 2009 pandemic influenza A (H1N1): speed is life. J Antimicrob Chemother. 2011;66(5):959–963. doi: 10.1093/jac/dkr090. [DOI] [PubMed] [Google Scholar]
- 59.Simonsen L, Taylor R, Young-Xu Y et al. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. MBio. 2011;2(1):e00309–e00310. doi: 10.1128/mBio.00309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klugman KP, Chien YW, Madhi SA. Pneumococcal pneumonia and influenza: a deadly combination. Vaccine. 2009;27(suppl 3):C9–C14. doi: 10.1016/j.vaccine.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 61. Indian Health Service. IHS quarterly immunization coverage reports, 2009. Available at: http://www.ihs.gov/epi/index.cfm?module=epi_vaccine_reports. Accessed May 31, 2013.
- 62.Guevara RE, Butler JC, Marston BJ et al. Accuracy of ICD-9-CM codes in detecting community-acquired pneumococcal pneumonia for incidence and vaccine efficacy studies. Am J Epidemiol. 1999;149(3):282–289. doi: 10.1093/oxfordjournals.aje.a009804. [DOI] [PubMed] [Google Scholar]
- 63.Barker WH, Mullooly JP. Underestimation of the role of pneumonia and influenza in causing excess mortality. Am J Public Health. 1981;71(6):643–645. doi: 10.2105/ajph.71.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thompson WW, Shay DK, Weintraub E et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]