Abstract
Background
Kojic acid (5-Hydroxy-2-(hydroxymethyl)-4-pyrone) is one of the major secondary metabolites in Aspergillus oryzae. It is widely used in food, pharmaceuticals, and cosmetics. The production cost, however, is too high for its use in many applications. Thus, an efficient and cost-effective kojic acid production process would be valuable. However, little is known about the complete set of genes for kojic acid production. Currently, kojic acid is produced from glucose. The efficient production of kojic acid using cellulose as an inexpensive substrate would help establish cost-effective kojic acid production.
Results
A kojic acid transcription factor gene over-expressing the A. oryzae strain was constructed. Three genes related to kojic acid production in this strain were transcribed in higher amounts than those found in the wild-type strain. This strain produced 26.4 g/L kojic acid from 80 g/L glucose. Furthermore, this strain was transformed with plasmid harboring 3 cellulase genes. The resultant A. oryzae strain successfully produced 0.18 g/L of kojic acid in 6 days of fermentation from the phosphoric acid swollen cellulose.
Conclusions
Kojic acid was produced directly from cellulose material using genetically engineered A. oryzae. Because A. oryzae has efficient protein secretion ability and secondary metabolite productivity, an A. oryzae-based cell factory could be a platform for the production of various kinds of bio-based chemicals.
Keywords: Aspergillus oryzae, Kojic acid, Cellulose, Cellulase, Starch
Background
Kojic acid (5-Hydroxy-2-(hydroxymethyl)-4-pyrone) is widely used in the food industry, pharmaceutical industry, and in cosmetics [1,2]. Furthermore, kojic acid is also used as a building block for biodegradable plastics [3,4]. Plastics have phenolic hydroxyl groups that are derived from kojic acid. Thus, it has some unique properties. Futamura et al. [5] reported that the production cost of kojic acid is about $10/kg. It must be decreased to less than $2/kg before it could be used in many applications. Thus, an efficient and cost-effective kojic acid production process would be desirable.
The utilization of biomass, particularly cellulosic materials, is desirable because it is abundant, inexpensive, renewable, and has favorable environmental properties. However, an efficient and cost-effective method for the degradation of cellulosic materials into glucose would be required before it could be used for the production of bio-based chemicals.
Cellulose has a structure that is very rigid with a high degree of crystallinity. Thus, the degradation of cellulose requires large amounts of various types of cellulase. The degradation of cellulose requires a synergistic reaction of at least three types of cellulase: endoglucanase (EG), cellobiohydrolase (CBH), and β-glucosidase (BGL). Although many studies have reduced the cost of cellulosic material degradation using recombinant bacteria, fungi, and yeast [6], cellulase degradation efficiency has not been sufficiently improved. The filamentous fungus Trichoderma reesei degrades cellulose effectively and is known to produce more than several dozen g/L of cellulase in the medium [7], therefore, it is known for its high cellulose degradation ability. Aspergillus oryzae is known as a high protein-secreting microorganism and its genetic recombination procedures are well developed. There are many reports concerning the production of a large amount of protein by A. oryzae[8]. Thus, A. oryzae shows promise as a host strain that could produce a large amount of cellulase and bio-based chemicals from cellulose. There have been reports concerning a single cellulase gene expression by A. oryzae, but no studies have focused on the cultivation of the 3 types of cellulase expressing A. oryzae on cellulose as a sole carbon source.
Kojic acid is one of the major secondary metabolites in A. oryzae. In the 100 years since it was discovered, there has been no elucidation of the entire pathway of kojic acid biosynthesis in A. oryzae. Recently, some genes regarding the production of kojic acid in A. oryzae were reported [9]. According to the report, 14 genes from AO090113000132 to AO090113000145 including a transcription factor gene (kojR; AO090113000137), an enzyme gene (kojA, AO090113000136), and a transporter gene (kojT AO090113000138) form a cluster. These genes are engaged in kojic acid production. The kojR gene encodes a fungal-specific Zn(II)2Cy6 transcription factor that is located between kojA (upstream 743 bp) and kojT (downstream 383 bp). The kojR regulates the transcription of kojA and kojT. A strain with a disrupted transcription factor gene could not produce kojic acid at all [10]. Thus, kojR seems to be a key factor in kojic acid production.
The goal of the present study was to construct an A. oryzae-based cell factory for direct kojic acid production from cellulose. First, A. oryzae over-expressing the transcription factor gene kojR was constructed, and kojic acid production from glucose and starch by the resultant strain was carried out. Then, A. oryzae co-expressing the transcription factor and 3-types of cellulase genes was constructed. Finally, direct kojic acid production from cellulose by the resultant strain was carried out.
Methods
Strains and media
Table 1 summarizes the genetic properties of all strains used. The A. oryzae strain RIB40 [11] was used as the wild-type strain. The strain NSPlD1 [12] derived from A. oryzae RIB40 was used as a transformation host strain. Potato dextrose agar (PDA; Nissui Pharmaceutical, Tokyo, Japan) media and GPY medium (3% glucose, 0.2% KCl, 0.1% KH2PO4, 0.05% MgSO4 · 7H2O, 1% peptone, and 0.5% yeast extract, pH 6.0) were used for the cultivation of A. oryzae. Czapek-Dox (CD) medium (2% glucose, 0.3% NaNO3(CD-NO3), 0.2% KCl, 0.1% KH2PO4, 0.05% MgSO4 · 7H2O, and 0.8 M NaCl, 1.5% agar, pH 6.0) with a required supplement (0.0015% methionine, 20 mM uridine, 0.2% uracil) was used for the niaD-based plasmid transformation. CD medium was used for sC-based plasmid transformation.
Table 1.
Strains and plasmid used in this study
| Strains and plasmids | Relevant features | Reference |
|---|---|---|
|
Escherichia coli strain |
|
|
| NovaBlue |
endA1 hsdR17(r K12-m K12+) supE44 thi-I gyrA96 relA1 lac recA1/F’[proAB + lacIq ZΔM15::Tn10(Tetr)] |
Novagen |
|
Aspergillus oryzae strains |
|
|
| RIB40 |
Wild type |
Machida et al.
[11] |
| NSPlD1 |
niaD
-
sC
-
adeA
-
ΔargB::adeA
-
ΔligD::argB ΔpyrG::adeA |
Maruyama and Kitamoto
[12] |
| NSPlD1/pIS1-kojR |
niaD
-
::pIS1-kojR [P-sodM::kojR::T-glaB] sC
-
adeA- ΔargB::adeA- ΔligD::argB ΔpyrG::adeA |
This study |
| NSPlD1/pIS1-kojR/pIS1sC-CBHI-EGI-BGLI |
niaD
-
::pIS1-kojR [P-sodM::kojR::T-glaB] sC- ::pIS1sC-CBHI-EGI-BGLI [P-svaA::CBHI::T-svaA, P-hlyA::EGI::T-hlyA, P-sodM::CutL-BGLI::T-glaB] adeA- ΔargB::adeA- ΔligD::argB ΔpyrG::adeA |
This study |
| Plasmids |
|
|
| pISI-CBHI |
CBHI gene from T. reesei containing plasmid |
This study |
| pIS1-svaA-CBHI |
Vector for expression of CBHI from T. reesei [P-svaA::CBHI::T-glaB]; niaD marker |
This study |
| pISI-EG |
EGI (T. reesei) gene containing plasmid |
This study |
| pIS1-hlyA-EGI |
Vector for expression of EGI from T. reesei [P-hlyA::EG::T-hlyA]; niaD marker |
This study |
| pIS1-BGLI |
Vector for expression BGLI from A. aculeatus [P-sodM::CutL-BGL::T-glaB]; niaD marker |
This study |
| pIS1-CBHI-EGI-BGLI |
Vector for expression of CBHI, EGI, and BGLI [P-svaA::CBHI::T-svaA, P-hlyA::EGI::T-hlyA, P-sodM::CutL-BGLI::T-glaB]; niaD marker |
This study |
| pIS1sC-CBHI-EGI-BGLI |
Vector for expression CBHI, EGI, and BGLI [P-svaA::CBHI::T-svaA, P-hlyA::EGI::T-hlyA, P-sodM::CutL-BGLI::T-glaB]; sC marker |
This study |
| pIS1-kojR | Vector for expression of transcription factor (AO090113000137) [P-sodM::kojR::T-glaB]; niaD marker | This study |
GY medium (8% glucose, 0.25% yeast extract, 0.1% K2HPO4, 0.05% MgSO4 · 7H2O, pH 6.0), SY medium (8% soluble starch, 0.25% yeast extract, 0.1% K2HPO4, 0.05% MgSO4 · 7H2O, pH 6.0), and CY medium (1% phosphoric acid swollen cellulose (PASC), 0.25% yeast extract, 0.1% K2HPO4, 0.05% MgSO4 · 7H2O, pH 6.0) was used for kojic acid production. PASC was prepared from Avicel PH-101 (Fluka Chemie GmbH, Buchs, Switzerland) as amorphous cellulose [13]. The transformant and wild-type strains used for kojic acid production with GY medium and SY medium were cultivated in 500 mL Erlenmeyer flasks containing 100 mL medium at 30°C that was shaken at 150 rpm using an orbital shaker with inocula of 1 × 105 spores/mL. The transformant and wild-type strain used for kojic acid fermentation in the CY medium was precultivated in a 200 mL Erlenmeyer flask with baffles containing 100 mL GPY medium at 30°C that was shaken at 150 rpm using an orbital shaker with inocula of 1 × 105 spores/mL. The mycelium in culture medium was separated from the culture medium by filtration using Miracloth (Calbiochem, Darmstadt, Germany) and was washed by distilled water. The washed mycelium was recultivated in 500 mL Erlenmeyer flasks with baffles containing 100 mL CY medium at 30°C that were shaken at 150 rpm using an orbital shaker.
E. coli NovaBlue (Novagen, Inc., Madison, WI, USA) was used as the cloning host for recombinant DNA manipulations. The bacterium was grown in Luria–Bertani medium (1% tryptone, 0.5% yeast extract, and 0.5% NaCl) containing 0.1 mg/mL of ampicillin.
Construction of plasmids
The primers used in the present study are summarized in Table 2. The PCR amplification of DNA fragments was performed using KOD plus neo DNA polymerase (Toyobo, Osaka, Japan).
Table 2.
Polymerase chain reaction primers used in this study
| Primers | Sequence (5′-3′) | Template DNA |
|---|---|---|
| ascI-kojR-F |
AAAAAGGCGCGCCATGTCGTTGAATACCGACGATTCC |
A. oryzae genome DNA |
| notI-FLAG-kojR-R |
AAAGCGGCCGCTTACTTGTCATCATCGTCCTTATAGTCTCTATATCTCTGACCACCTGC |
|
| CBHI-F |
CCAAACCACCCAAAGGGCGCGCCATGTATCGGAAGTTGGCCGTCATC |
synthesized gene |
| CBHI-R |
GAAAGTACATGTCGAGCGGCCGCTTACAGGCACTGAGAGTAGTAAG |
|
| psvaA-ascI-CBHI-F |
GGCGCGCCAAAAAATGTATCGGAAGTTGGCCGTCA |
pISI-CBHI |
| tsvaA-notI-CBHI-R |
AAATGATGCGGCCGCTTACAGGCACTGAGAGTAGT |
|
| pIS1-psvaA-F |
CACAACACTCTCGACCTGCAGCGGTTTACACCGAAGACCGG |
A. oryzae genome DNA |
| CBHI-ascI-psvaA-R |
ATTTTTTGGCGCGCCCTTGCGAGCAGGGGGATAAT |
|
| CBHI-notI-tsvaA-F |
GCGGCCGCATCATTTTCCCGCTTTGATCTGGTCGGTTCCC |
A. oryzae genome DNA |
| pIS1-tsvaA-R |
GGTACCCGGGGATCCTCTAGATCAAGCATAACTACAACAGGGCAAGGAATACGG |
|
| EGI-F |
CCAAACCACCCAAAGGGCGCGCCATGGCGCCCTCAGTTACACTGC |
T. reesei cDNA |
| EGI-R |
GAAAGTACATGTCGAGCGGCCGCCTAAAGGCATTGCGAGTAGTCTG |
|
| phlyA-ascI-EGI-F |
GGCGCGCCAAAAAATGGCGCCCTCAGTTACACTGC |
pISI-EG |
| thlyA-notI-EGI-R |
AGAGAGAGCGGCCGCCTAAAGGCATTGCGAGTAGT |
|
| pIS1-phlyA-F |
CACAACACTCTCGACCTGCAGTACAGCATGGTCTGGATTCCAATCCACGCAGC |
A. oryzae genome DNA |
| EGI-ascI-phlyA-R |
ATTTTTTGGCGCGCCGGTGTTGTGGTGTGAAGGGTGATTGATGTGAGACC |
|
| EGI-notI-thlyA-F |
GCGGCCGCTCTCTCTCCCCTATACGTGATACCGTA |
A. oryzae genome DNA |
| pIS1-thlyA-R |
GGTACCCGGGGATCCTCTAGAGATGCAAATTGGAGTTAAAT |
|
| sCutL-F |
CCAAACCACCCAAAGGGCGCGCCATGCATCTTGCTATCAAGTCTC |
A. oryzae genome DNA |
| sCutL-R |
TCACTAGTTCTCTCAACCAGAGCATTGCTGGG |
|
| N28-F |
TTGAGAGAACTAGTGATGACAACTTGGTTGGTGGCATG |
pISI-GFP (Adachi et al.
[14]) |
| N28-R |
AGTTCATCCTCGACACGCTTGGCGCTGTTGG |
|
| BGL-F |
GTGTCGAGGATGAACTGGCGTTCTCTCCTC |
pdU-PGAGBGL (Yamada et al.
[15]) |
| BGL-R |
GAAAGTACATGTCGAGCGGCCGCTTACTTGTCATCGTCATCCTTG |
|
| pIS1-svaA-CBHI-F |
GGCTTTCCCCGTCAAGCTCT |
pISI-svaA-CBHI |
| pIS1-svaA-CBHI-R |
GGCGAACGTGGCGAGAAAGG |
|
| phlyA-into-pIS1-svaA-CBHI-F |
CTCGCCACGTTCGCCTACAGCATGGTCTGGATTCCAATCCACGCA |
pISI-hlyA-EGI |
| connect-thlyA-and-psodM-R |
TATTTAAATATCGATGATGCAAATTGGAGTTAAATATTAACTAAC |
|
| connect-psodM-and-thlyA-F |
ATCGATATTTAAATATTATGTACTCCGTACTCGGTTGATTATTAA |
pIS1-BGL1 |
| tglaB-into-pIS1-svaA-CBHI-R |
TTGACGGGGAAAGCCGGATGTAGTATGTATACTTAGTTTGATTGC |
|
| hlyA-EGI-svaA-CBHI-sodM-BGLI-F |
TTTATATCCAAGATCACCTGCAGCGGTTTACACCGAAGACCGGTA |
pISI-CBHI-EGI-BGL1 |
| hlyA-EGI-svaA-CBHI-sodM-BGLI-R |
TGCCAAGAGAAGCTTGGCGTAATCATGGTCATAGCTGTTT |
|
| sC-F |
AAGCTTCTCTTGGCAATAGCTGCCC |
pUSC (Yamada et al.
[16]) |
| sC-R |
GATCTTGGATATAAAAATCCAAATATGGCTCCTCG |
|
| kojR-RT-PCR-F |
TGGTGCAATCAGCGAAGGA |
wild type and engineered A. oryzae cDNA |
| kojR-RT-PCR-R |
AGACTACTCTCCTGCATCATGCC |
|
| kojA-RT-PCR-F |
ATCCGAAGGCGAATGGTTT |
wild type and engineered A. oryzae cDNA |
| kojA-RT-PCR-R |
ATGAACCCAGCGTCGCTATT |
|
| kojT-RT-PCR-F |
CATGGTGCCGCATATTTACTTC |
wild type and engineered A. oryzae cDNA |
| kojT-RT-PCR-R |
AATGGACACAATGGGTTGCC |
|
| ß-Tubulin-RT-PCR-F |
GCCAGTGTGGTAACCAAATAGGT |
wild type and engineered A. oryzae cDNA |
| ß-Tubulin-RT-PCR-R | TAAACACCGGAGCCGTCAA |
The transcription factor gene (kojR, AO090113000137) expression plasmid was constructed as follows. The kojR was amplified by PCR using primers ascI-kojR-F and notI-FLAG-kojR-R from A. oryzae genome DNA as a template. The amplified fragment was inserted into the AscI/NotI sites of the digested pISI, which contained the sodM promoter and glaB terminator from A. oryzae[17]. The resultant plasmid was named pISI-kojR.
The CBHI gene from Trichoderma sp. expressing plasmid pIS1-svaA-CBHI was constructed as follows. The DNA fragment encoding the Trichoderma sp. CBHI gene (GenBank accession no. X69976) was amplified by PCR using primers CBHI-F and CBHI-R from a synthesized gene (GENEWIZ, South Plainfield, NJ, USA). The fragment was inserted into the AscI/NotI site of the plasmid pISI using an In-Fusion HD Cloning Kit (Takara Bio Inc., Shiga, Japan). The resultant plasmid was named pISI-CBHI. Then, the CBHI gene was amplified by PCR using primers psvaA-ascI-CBHI-F and tsvaA-notI-CBHI-R with pISI-CBHI as a template. The svaA promoter [17] and svaA terminator were amplified by PCR using primers pIS1-psvaA-F and CBHI-ascI-psvaA-R, and CBHI-notI-tsvaA-F and pIS1-tsvaA-R, respectively, with A. oryzae genome DNA as a template. These PCR-amplified DNA fragments were simultaneously inserted into the XbaI and PstI sites of pIS1 using an In-Fusion PCR Cloning kit. The resultant plasmid was named pISI-svaA-CBHI.
An EGI gene from the T. reesei-expressing plasmid pIS1-hlyA-EGI was constructed. The EGI-expressing plasmid was constructed as follows. The DNA fragment encoding the T. reesei EGI gene was amplified by PCR using the primers EGI-F and EGI-R with T. reesei cDNA as a template. The fragment was inserted into the AscI/NotI site of the plasmid pISI using an In-Fusion HD Cloning Kit. The resultant plasmid was named pISI-EG. The T. reesei EGI gene was then amplified by PCR using the primers phlyA-ascI-EGI-F and thlyA-notI-EGI-R with pISI-EG as a template. The hlyA promoter [18] and hlyA terminator were amplified by PCR using primers pIS1-phlyA-F and EGI-ascI-phlyA-R, and EGI-notI-thlyA-F and pIS1-thlyA-R, respectively, using the A. oryzae genome DNA as a template. These PCR-amplified DNA fragments were simultaneously inserted into the XbaI and PstI sites of pIS1 using an In-Fusion PCR Cloning kit. The resultant plasmid was named pISI-hlyA-EGI.
The BGL1 gene from A. aculeatus expressing plasmid pIS1-BGL1 was constructed as follows. The DNA fragment encoding the secretion signal from A. oryzae cutinase, the 28 amino acids from the N-terminal region of Rhizopus oryzae lipase, and the A. aculeatus BGL gene were amplified by PCR using the primers sCutL-F and sCutL-R, N28-F and N28-R, and BGL-F and BGL-R, respectively, from the genome DNA of A. oryzae, pISI-GFP [14], and pδU-PGAGBGL [15], respectively. These fragments were simultaneously inserted into the AscI/NotI site of the plasmid pISI using an In-Fusion HD Cloning Kit. The resultant plasmid was named pISI-BGL.
The pIS1-CBHI-EGI-BGL1 plasmid was constructed as follows. The pISI-svaA-CBHI was linearized by PCR using the primers pIS1-svaA-CBHI-F and pIS1-svaA-CBHI-R. The EGI- and BGL1-expressing cassettes were amplified by PCR using primers phlyA-into-pIS1-svaA-CBHI-F and connect-thlyA-and-psodM-R, and connect-psodM-and-thlyA-F and tglaB-into-pIS1-svaA-CBHI-R, respectively, from pISI-hlyA-EGI and pIS1-BGL1, respectively. These fragments were fused using an In-Fusion PCR Cloning kit. The resultant plasmid was named pISI-CBHI-EGI-BGL1.
The pIS1sC-CBHI-EGI-BGL1 plasmid was constructed as follows. The CBHI-, EGI-, and BGL1-expressing cassettes were amplified by PCR using primer (hlyA-EGI-svaA-CBHI-sodM-BGL1-F and hlyA-EGI-svaA-CBHI-sodM-BGL1-R). The sC gene was amplified by PCR using primer (sC-F and sC-R) from a pUSC plasmid [16]. These amplified fragments were fused using an In-Fusion PCR Cloning System. The resultant plasmid was named pISIsC-CBHI-EGI-BGL1.
Transformation of A. oryzae
The transformation of A. oryzae was carried out according to the previously described method with minor modifications [19].
The niaD-based pIS1-kojR plasmids were introduced into the NSPlD1 strain. The resultant strain was named NSPlD1/pIS1-kojR. The sC-based plasmid pIS1sC-CBHI-EGI-BGL1 was introduced into the NSPlD1/pIS1-kojR strain. The resultant strain was named NSPlD1/pIS1-kojR/pIS1sC-CBHI-EGI-BGL1.
RNA extraction and reverse transcription
The RNA was isolated from fungus bodies cultivated in GY medium for 6 days at 30°C using NucleoSpin RNA II (Takara Bio Inc.) according to the manufacturer’s protocol. The cDNA synthesis was carried out using a ReverTra Ace qPCR RT Kit (Toyobo) according to the manufacturer’s protocol.
Real-time PCR analysis
The transcription level of Kojic-acid-producing genes (kojR, kojA, kojT) was quantified by real-time PCR. Quantitative real-time PCR was performed using an Mx3005P Real-Time QPCR System (Agilent Technologies, Santa Clara, CA, USA) with a Thunderbird SYBR qPCR Mix (Toyobo). The normalized transcription level was calculated using the standard curve method with β-tubulin as the house-keeping gene [20]. All primers used for real-time PCR analysis are summarized in Table 2.
Enzyme assay
A wild-type strain and 3-types of cellulase gene-expressing strains were cultivated in GY medium for 6 days at 30°C, and the culture supernatant was used for the determination of PASC degradation activity. PASC degradation activity was measured in 25 mM sodium acetate buffer (pH 5.0) at 30°C with 5 g/L of PASC. After hydrolysis, the supernatant was separated by centrifugation for 5 min at 10,000 × g and 4°C, and the produced glucose concentration was measured. One unit of PASC degradation activity was defined as the amount of enzyme producing 1 μmol/min glucose at 30°C, pH 5.0.
Analytical methods
The kojic acid concentration was determined using a colorimetric method described previously [21]. The total sugar concentration was determined by a colorimetric phenol-sulfuric acid method described previously [22]. The glucose concentration was determined using a Wako Glucose CII-Test kit (Wako Pure Chemical Industries, Osaka, Japan).
Results
Kojic acid production from glucose using the engineered A. oryzae
To confirm the effect of the over-expression of the transcription factor for kojic acid production, the kojic acid productivity from the transcription factor over-expressing strain NSPlD1/pIS1-kojR from glucose was evaluated. As shown in Figure 1 the wild-type strain produced 16.4 g/L of kojic acid after 14 days of fermentation. By contrast, the transcription factor over-expressing strain NSPlD1/pIS1-kojR produced 26.4 g/L of kojic acid after 14 days of fermentation and it was 1.6-fold higher than that of the wild-type strain.
Figure 1.
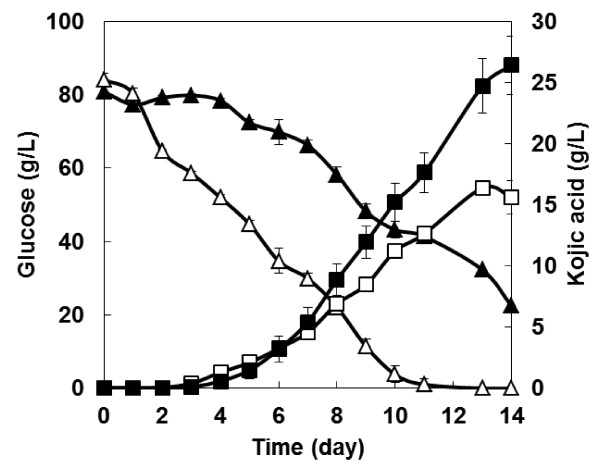
Time course of kojic acid production from glucose. Triangles, glucose; squares, kojic acid; open symbols, wild-type strain; and, closed symbols, transcription factor over-expressing strain NSPlD1/pIS1-kojR. The data represent the averages from three independent experiments (error bars represent SE).
Quantification of the transcription level of kojic-acid-producing genes by real-time PCR
To confirm the effect of the over-expression of transcription factor at the level of the kojic-acid-producing genes, the transcription level of the transcription factor, the kojic-acid-producing enzyme, and the transporter gene were evaluated by real-time PCR. As shown in Figure 2, the transcription factor of the over-expressing strain NSPlD1/pIS1-kojR showed a 1.8, 1.8, and 5.4-fold increases in the transcription level of transcription factor, enzyme, and transporter gene, respectively, compared with the wild-type strain.
Figure 2.
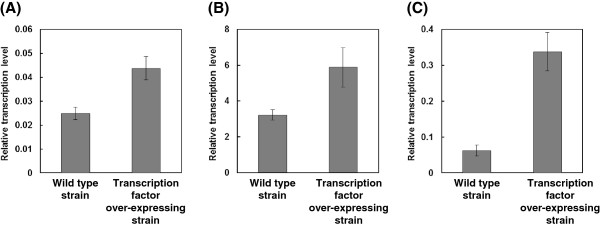
Transcription levels of kojic acid producing genes. (A) Transcription factor gene, (B) enzyme gene, (C) transporter gene. The data represent the averages from three independent experiments (error bars represent SE).
Kojic acid production from soluble starch by recombinant A. oryzae
Since A. oryzae can assimilate starch as a sole carbon source intrinsically, direct kojic acid production from starch using the transcription factor over-expressing strain NSPlD1/pIS1-kojR was carried out. As shown in Figure 3, the wild-type strain produced 6.2 g/L of kojic acid after 18 days of fermentation. In contrast, the transcription factor over-expressing strain produced 19.4 g/L of kojic acid after 18 days of fermentation, i.e. 3.1-fold gain.
Figure 3.
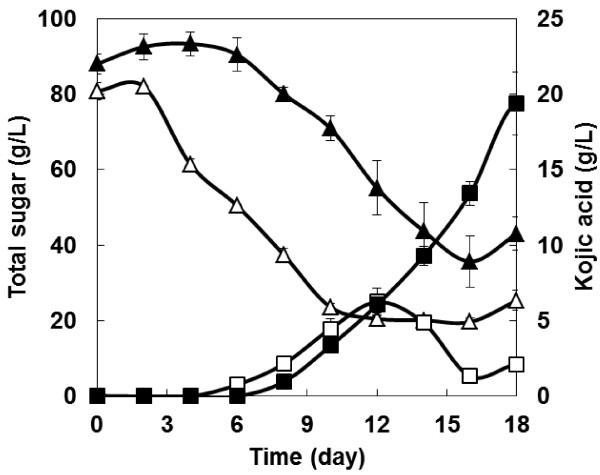
Time course of kojic acid production from starch. Triangles, total sugar; squares, kojic acid; open symbols, wild-type strain; and, closed symbols, transcription factor over-expressing strain NSPlD1/pIS1-kojR. The data represent the averages from three independent experiments (error bars represent SE).
Evaluation of cellulase activity
To confirm the cellulase expression of engineered A. oryzae, the PASC degradation activity of 3-types of cellulase were evaluated. As shown in Figure 4, the 3-types of cellulase gene-expressing strains NSPlD1/pIS1-kojR/pIS1sC-CBHI-EGI-BGLI showed 29.9 U/L after 6 days of cultivation. In contrast, the wild-type strain showed significantly lower PASCase activity (1.4 U/L).
Figure 4.
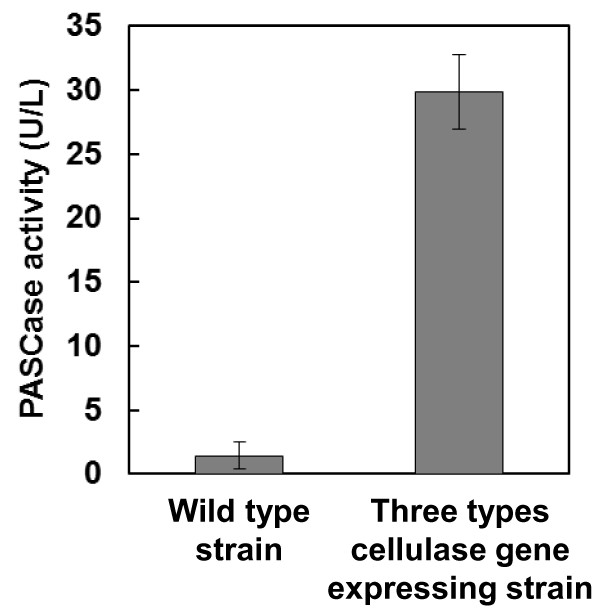
PASCase activity of 3-types of cellulase gene-expressing strains. The data represent the averages from three independent experiments (error bars represent SE).
Direct kojic acid production from PASC
To confirm direct kojic acid productivity from cellulose, kojic acid production from PASC was carried out. As shown in Figure 5, there was 0.18 g/L of kojic acid produced after 6 days of fermentation when using a transcription factor gene and the 3-types of cellulase genes co-expressing strain NSPlD1/pIS1-kojR/pIS1sC-CBHI-EGI-BGLI. In contrast, the wild-type strain did not produce a detectable amount of kojic acid.
Figure 5.
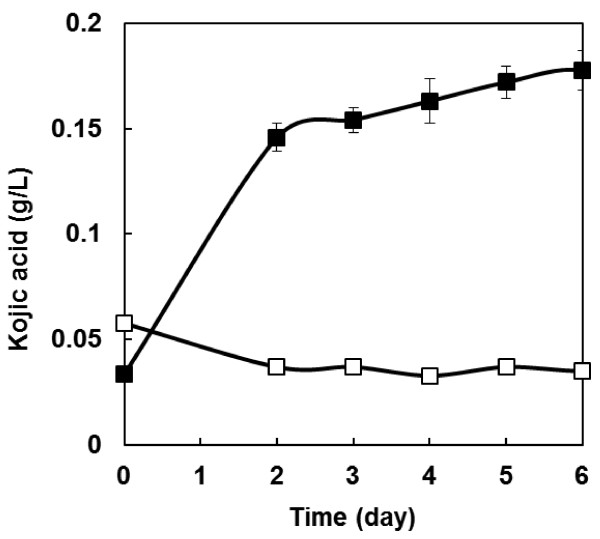
Time course of kojic acid production from PASC. Open symbols, wild-type strain; closed symbols, transcription factor and 3-types of cellulase gene over-expressing strains NSPlD1/pIS1-kojR/pIS1sC-CBHI-EGI-BGLI. The data represent the averages from three independent experiments (error bars represent SE).
Discussion
In the present study, a transcription factor gene over-expressing A. oryzae NSPlD1/pIS1-kojR was developed by gene recombination strategy. Furthermore, the transcription factor gene and 3-types of cellulase genes co-expressing A. oryzae NSPlD1/pIS1-kojR/pIS1sC-CBHI-EGI-BGLI was constructed and the strain successfully produced kojic acid directly from PASC.
The transcription factor gene kojR, which is responsible for kojic acid production, was over-expressed in this study, and as a result, kojic acid productivity was improved. As shown in Figure 2, the transcription levels of both the kojic acid-producing enzyme kojA and the exporting transporter kojT were enhanced. This was because the kojic acid productivity was improved. Terabayashi et al. [9] reported that the kojR, kojA, and kojT genes would encode the transcription factor, the kojic acid-producing enzyme, and the transporter, respectively. In the present study, the transcription level of the enzyme and the transporter were enhanced by an over-expression of the transcription factor, which correlates well with that report. Although there are reports of improved kojic acid productivity through fermentation engineering strategies [1,5,23], to our knowledge, this study is the first to report an improvement in kojic acid productivity via genetic engineering. Kojic acid productivity could be improved even more by combining fermentation engineering and genetic engineering in future work.
Direct kojic acid production from cellulose was successfully demonstrated by A. oryzae expressing 3-types of cellulase genes. However, the yield of kojic acid remained low. This was due to the low levels of cellulase production. In previous reports, some microorganisms such as Saccharomyces cerevisiae, Kluyveromyces marxianus, Bacillus subtilis, and E. coli were used for bio-based chemical production from cellulose by conferring cellulolytic activity [6]. Although most reports did not indicate the amount of cellulase production, Zhang et al. [24] reported that about 14 mg/L of endoglucanase was produced by recombinant Bacillus subtilis. In any case, because T. reesei produces several dozen g/L of cellulase [7], the amount of cellulase expression from cellulolytic activity conferred microorganisms has not yet reached the level of T. reesei. Although A. oryzae has an extremely high protein secreting ability, cellulase expression of T. reesei is still higher than that of A. oryzae. Because the maximum protein production is approximately 20 g/L in Aspergillus organisms [25], the cellulase productivity could be improved with genetic engineering.
Three types of cellulase genes and specific transcription factors were successfully over-expressed simultaneously in recombinant A. oryzae. To this point, only a few reports have focused on the co-expression of more than 3-kinds of genes in A. oryzae. This is because the selection marker for gene recombination is limited in A. oryzae compared with other microorganisms such as yeast S. cerevisiae[26]. In the present study, a 3-cassette plasmid with different promoters was constructed and the simultaneous overexpression of 4 genes was successful. Thus, the simultaneous multi gene expression technique, which is an important technique for the construction of a cell factory in A. oryzae, has been well established. By using this technique, an A. oryzae-based cell factory could be a promising strategy for producing various bio-based chemicals.
Conclusions
The productivity of kojic acid from A. oryzae was made more efficient by the over-expression of specific transcription factors. To accomplish this, recombinant A. oryzae expressing 3 types of cellulase genes was constructed. Direct kojic acid production from PASC by genetically engineered A. oryzae was successfully demonstrated. Although this research was conducted as the proof of a concept, the results showed that an A. oryzae-based cell factory could be established. Because, A. oryzae has efficient protein secretion ability and secondary metabolite productivity, an A. oryzae-based cell factory could be a promising platform for producing various kinds of bio-based chemicals.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TY designed and performed the experiments. NAN performed the experiments. TY and RY wrote the paper. SW, FO, CO, HH, HT, YH and AK commented and supervised on the manuscript. All the authors approved the final manuscript.
Contributor Information
Ryosuke Yamada, Email: yamada@chemeng.osakafu-u.ac.jp.
Toshihide Yoshie, Email: 113t473t@stu.kobe-u.ac.jp.
Satoshi Wakai, Email: wakaists@pegasus.kobe-u.ac.jp.
Nanami Asai-Nakashima, Email: nnakashima@kitty.kobe-u.ac.jp.
Fumiyoshi Okazaki, Email: okazaki@port.kobe-u.ac.jp.
Chiaki Ogino, Email: ochiaki@port.kobe-u.ac.jp.
Hiromoto Hisada, Email: hisada@gekkeikan.co.jp.
Hiroko Tsutsumi, Email: h_tsutsumi@gekkeikan.co.jp.
Yoji Hata, Email: y_hata@gekkeikan.co.jp.
Akihiko Kondo, Email: akondo@kobe-u.ac.jp.
Acknowledgements
The authors gratefully acknowledge Dr. Katsuhiko Kitamoto (The University of Tokyo, Tokyo, Japan.) for providing the A. oryzae strain NSPlD1.
This work was supported by Special Coordination Funds for Promoting Science and Technology, Creation of Innovation Centers for Advanced Interdisciplinary Research Areas (Innovative Bioproduction Kobe), MEXT, Japan.
References
- Rosfarizan M, Ariff AB, Hassan MA, Karim MI. Kojic acid production by Aspergillus flavus using gelatinized and hydrolyzed sago starch as carbon sources. Folia Microbiol (Praha) 1998;43:459–464. doi: 10.1007/BF02820791. [DOI] [PubMed] [Google Scholar]
- Wan HM, Chen CC, Chang TS, Giridhar RN, Wu WT. Combining induced mutation and protoplasting for strain improvement of Aspergillus oryzae for kojic acid production. Biotechnol Lett. 2004;26:1163–1166. doi: 10.1023/B:BILE.0000035490.49252.38. [DOI] [PubMed] [Google Scholar]
- Tomita I, Mitsuhashi K, Endo T. Synthesis and radical polymerization of styrene derivative bearing kojic acid moieties. J Polym Sci A Polym Chem. 1996;34:271–276. doi: 10.1002/(SICI)1099-0518(19960130)34:2<271::AID-POLA13>3.0.CO;2-N. [DOI] [Google Scholar]
- Ochiai B, Kamiya M, Endo T. Synthesis and Fe(III)-complexation ability of polyurethane bearing kojic acid skeleton in the main chain prepared by polyaddition of aliphatic hydroxyl groups without protection of phenolic hydroxyl groups. J Polym Sci A Polym Chem. 2012;50:3493–3498. doi: 10.1002/pola.26161. [DOI] [Google Scholar]
- Futamura T, Ishihara H, Tamura T, Yasutake T, Huang G, Kojima M, Okabe M. Kojic acid production in an airlift bioreactor using partially hydrolyzed raw corn starch. J Biosci Bioeng. 2001;92:360–365. doi: 10.1263/jbb.92.360. [DOI] [PubMed] [Google Scholar]
- Yamada R, Hasunuma T, Kondo A. Endowing non-cellulolytic microorganisms with cellulolytic activity aiming for consolidated bioprocessing. Biotechnol Adv. 2013;31:754–763. doi: 10.1016/j.biotechadv.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Peterson R, Nevalainen H. Trichoderma reesei RUT-C30–thirty years of strain improvement. Microbiology. 2012;158:58–68. doi: 10.1099/mic.0.054031-0. [DOI] [PubMed] [Google Scholar]
- Fleissner A, Dersch P. Expression and export: recombinant protein production systems for Aspergillus. Appl Microbiol Biotechnol. 2010;87:1255–1270. doi: 10.1007/s00253-010-2672-6. [DOI] [PubMed] [Google Scholar]
- Terabayashi Y, Sano M, Yamane N, Marui J, Tamano K, Sagara J, Dohmoto M, Oda K, Ohshima E, Tachibana K, Higa Y, Ohashi S, Koike H, Machida M. Identification and characterization of genes responsible for biosynthesis of kojic acid, an industrially important compound from Aspergillus oryzae. Fungal Genet Biol. 2010;47:953–961. doi: 10.1016/j.fgb.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Marui J, Yamane N, Ohashi-Kunihiro S, Ando T, Terabayashi Y, Sano M, Ohashi S, Ohshima E, Tachibana K, Higa Y, Nishimura M, Koike H, Machida M. Kojic acid biosynthesis in Aspergillus oryzae is regulated by a Zn(II)(2)Cys(6) transcriptional activator and induced by kojic acid at the transcriptional level. J Biosci Bioeng. 2011;112:40–43. doi: 10.1016/j.jbiosc.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Machida M, Asai K, Sano M, Tanaka T, Kumagai T, Terai G, Kusumoto K, Arima T, Akita O, Kashiwagi Y, Abe K, Gomi K, Horiuchi H, Kitamoto K, Kobayashi T, Takeuchi M, Denning DW, Galagan JE, Nierman WC, Yu J, Archer DB, Bennett JW, Bhatnagar D, Cleveland TE, Fedorova ND, Gotoh O, Horikawa H, Hosoyama A, Ichinomiya M, Igarashi R. et al. Genome sequencing and analysis of Aspergillus oryzae. Nature. 2005;438:1157–1161. doi: 10.1038/nature04300. [DOI] [PubMed] [Google Scholar]
- Maruyama J, Kitamoto K. Multiple gene disruptions by marker recycling with highly efficient gene-targeting background (ΔligD) in Aspergillus oryzae. Biotechnol Lett. 2008;30:1811–1817. doi: 10.1007/s10529-008-9763-9. [DOI] [PubMed] [Google Scholar]
- Den Haan R, Rose SH, Lynd LR, van Zyl WH. Hydrolysis and fermentation of amorphous cellulose by recombinant Saccharomyces cerevisiae. Metab Eng. 2007;9:87–94. doi: 10.1016/j.ymben.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Adachi T, Ito J, Kawata K, Kaya M, Ishida H, Sahara H, Hata Y, Ogino C, Fukuda H, Kondo A. Construction of an Aspergillus oryzae cell-surface display system using a putative GPI-anchored protein. Appl Microbiol Biotechnol. 2008;81:711–719. doi: 10.1007/s00253-008-1687-8. [DOI] [PubMed] [Google Scholar]
- Yamada R, Taniguchi N, Tanaka T, Ogino C, Fukuda H, Kondo A. Cocktail delta-integration: a novel method to construct cellulolytic enzyme expression ratio-optimized yeast strains. Microb Cell Fact. 2010;9:32. doi: 10.1186/1475-2859-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada O, Lee BR, Gomi K. Transformation system for Aspergillus oryzae with double auxotrophic mutations, niaD and sC. Biosci Biotechnol Biochem. 1997;61:1367–1369. doi: 10.1271/bbb.61.1367. [DOI] [Google Scholar]
- Ishida H, Hata Y, Kawato A, Abe Y, Kashiwagi Y. Isolation of a novel promoter for efficient protein production in Aspergillus oryzae. Biosci Biotechnol Biochem. 2004;68:1849–1857. doi: 10.1271/bbb.68.1849. [DOI] [PubMed] [Google Scholar]
- Bando H, Hisada H, Ishida H, Hata Y, Katakura Y, Kondo A. Isolation of a novel promoter for efficient protein expression by Aspergillus oryzae in solid-state culture. Appl Microbiol Biotechnol. 2011;92:561–569. doi: 10.1007/s00253-011-3446-5. [DOI] [PubMed] [Google Scholar]
- Kitamoto K. Molecular biology of the Koji molds. Adv Appl Microbiol. 2002;51:129–153. doi: 10.1016/s0065-2164(02)51004-2. [DOI] [PubMed] [Google Scholar]
- McKelvey SM, Murphy RA. Analysis of wide-domain transcriptional regulation in solid-state cultures of Aspergillus oryzae. J Ind Microbiol Biotechnol. 2010;37:455–469. doi: 10.1007/s10295-010-0691-z. [DOI] [PubMed] [Google Scholar]
- Bentley R. Preparation and analysis of kojic acid. Methods Enzymol. 1957;3:238–241. [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Wan HM, Chen CC, Giridhar R, Chang TS, Wu WT. Repeated-batch production of kojic acid in a cell-retention fermenter using Aspergillus oryzae M3B9. J Ind Microbiol Biotechnol. 2005;32:227–233. doi: 10.1007/s10295-005-0230-5. [DOI] [PubMed] [Google Scholar]
- Zhang XZ, Sathitsuksanoh N, Zhu Z, Percival Zhang YH. One-step production of lactate from cellulose as the sole carbon source without any other organic nutrient by recombinant cellulolytic Bacillus subtilis. Metab Eng. 2011;13:364–372. doi: 10.1016/j.ymben.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Su X, Schmitz G, Zhang M, Mackie RI, Cann IK. Heterologous gene expression in filamentous fungi. Adv Appl Microbiol. 2012;81:1–61. doi: 10.1016/B978-0-12-394382-8.00001-0. [DOI] [PubMed] [Google Scholar]
- Ruiz-Díez B. Strategies for the transformation of filamentous fungi. J Appl Microbiol. 2002;92:189–195. doi: 10.1046/j.1365-2672.2002.01516.x. [DOI] [PubMed] [Google Scholar]


