Abstract
It is difficult to overstate the cultural and biological impacts that the domestication of plants and animals has had on our species. Fundamental questions regarding where, when, and how many times domestication took place have been of primary interest within a wide range of academic disciplines. Within the last two decades, the advent of new archaeological and genetic techniques has revolutionized our understanding of the pattern and process of domestication and agricultural origins that led to our modern way of life. In the spring of 2011, 25 scholars with a central interest in domestication representing the fields of genetics, archaeobotany, zooarchaeology, geoarchaeology, and archaeology met at the National Evolutionary Synthesis Center to discuss recent domestication research progress and identify challenges for the future. In this introduction to the resulting Special Feature, we present the state of the art in the field by discussing what is known about the spatial and temporal patterns of domestication, and controversies surrounding the speed, intentionality, and evolutionary aspects of the domestication process. We then highlight three key challenges for future research. We conclude by arguing that although recent progress has been impressive, the next decade will yield even more substantial insights not only into how domestication took place, but also when and where it did, and where and why it did not.
Keywords: evolution, selection, agriculture, human ecology, human history
The domestication of plants and animals was one of the most significant cultural and evolutionary transitions in the ∼200,000-y history of our species. Investigating when, where, and how domestication took place is therefore crucial for understanding the roots of complex societies. Domestication research is equally important to scholars from a wide range of disciplines, from evolutionary biology to sustainability science (1, 2). Research into both the process and spatiotemporal origins of domestication has accelerated significantly over the past decade through archaeological research, advances in DNA/RNA sequencing technology, and methods used to recover and formally identify changes in interactions among plants and animals leading to domestication (2–4). In the spring of 2011, 25 scholars with a central interest in domestication and representing the fields of genetics, archaeobotany, zooarchaeology, geoarchaeology, and archaeology met at the National Evolutionary Synthesis Center to discuss recent progress in domestication research and identify challenges for the future. Our goal was to begin reconsidering plant and animal domestication within an integrated evolutionary and cultural framework that takes into account not just new genetic and archaeological data, but also ideas related to epigenetics, plasticity, gene-by-environment interactions, gene-culture coevolution, and niche construction. Each of these concepts is relevant to understanding phenotypic change, heritability, and selection, and they are all fundamental components of the New Biology (5) and Expanded Modern Evolutionary Synthesis (6).
This PNAS Special Feature presents a collection of papers emanating from that meeting. Some evaluate past evidence and views on fundamental aspects of plant and animal domestication and offer a consensus perspective through the lens of more recent empirical findings and ideas. Others explore how best to investigate challenging research questions. All of the papers provide examples of how domestication research has illuminated, and will continue to enrich, our understanding of evolutionary and cultural change. In this introduction to the Special Feature, we present an outline of what is currently known about the pattern and process of domestication and we discuss foundational issues in domestication research, both in general and in light of the collected contributions. We conclude with a summary of outstanding questions and challenges.
Spatial and Temporal Patterns of Domestication
The beginnings of plant and animal domestication related to food production began globally 12,000–11,000 y ago at the end of the most recent ice age and during the transition to the Present Interglacial Period (7) (Figs. 1 and 2). Although often characterized as rapid and the result of explicit human intention (8, 9), domestication is a complex process along a continuum of human, plant, and animal relationships that often took place over a long time period and was driven by a mix of ecological, biological, and human cultural factors (2, 3). The process encompassed a wide range of relationships, from commensalism/mutualism to low-level management, and directed control over reproduction (10, 11), although these stages did not necessarily progress in a ratchet-like fashion from wild to domestic.
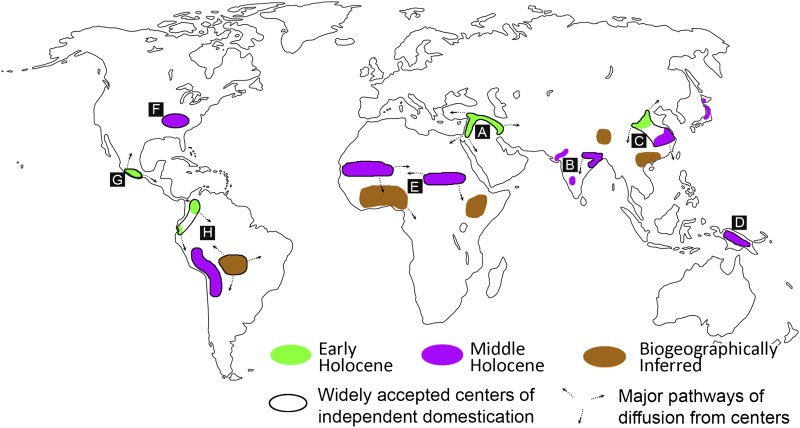
Fig. 1.
A map depicting likely centers where the domestication of at least one plant or animal took place. Black outlines surround the most widely accepted independent centers of domestication, and sources of major diffusions of domesticates are indicated by arrows. Green and purple regions, respectively, are those where the domestication process took place during the late Pleistocene to early Holocene transition (12,000–8,200 B.P.), and in the middle Holocene (8,200–4,200 B.P.). Brown regions represent areas where, at present, the evidence for domestication is interpreted based upon the presence of domestic forms indigenous to these regions found outside of their native distributions. Letters A–H correspond to those listed in Fig. 2. Additional detail and references associated with each region are found in the SI Text.
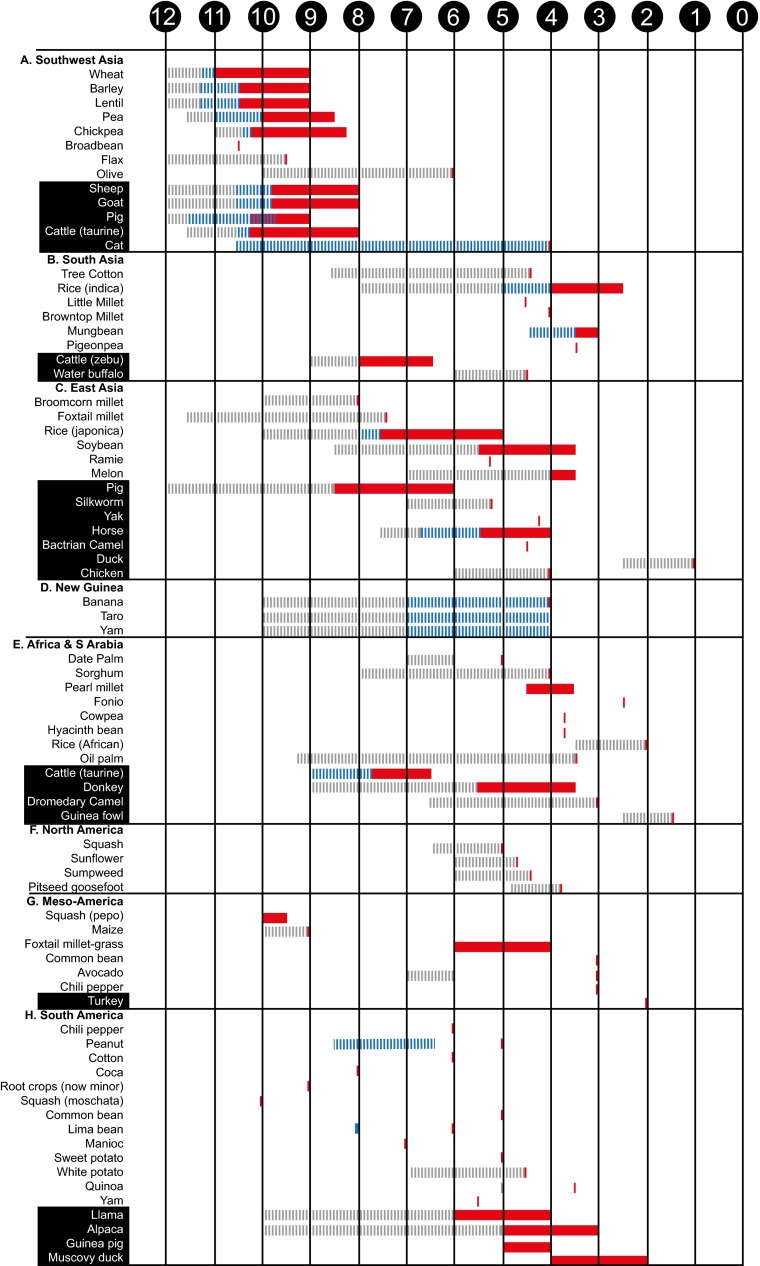
Fig. 2.
A chronological chart listing the regions where, and the time frames over which, key plants and animals were domesticated. The numbers in the black circles represent thousands of years before present. Gray dashed lines represent documented exploitation before domestication or posited as necessary lead-time to domestication. Blue dashed lines represent either the management of plants or animals (including translocation) or predomestication cultivation of plants, neither of which were associated with morphological indications of domestication. Red bars frame the period over which morphological changes associated with domestication are first documented and a short, solid red bar represents the latest time by which domestication occurred. Although early Holocene plant domestication took place independently in both the Old and New Worlds, early Holocene animal domestication was restricted to the Near East. In addition, the majority of plants and animals on this list were domesticated in the middle Holocene. Additional details and references associated with each taxon are found in Table S1. Letters A–H correspond to those found in Fig. 1.
The addition of a human selective component on top of a natural selection regime has enhanced the power of domestication to reveal insights into long-standing evolutionary issues, including those highlighted below. Although we eschew one-size-fits-all definitions for either plants or animals, domestication can be generally considered a selection process for adaptation to human agro-ecological niches and, at some point in the process, human preferences. Importantly, the wild progenitor species of domesticated taxa must have possessed the potential to live in the context of human ecologies, and to express traits that were favorable for human use, harvesting, and edibility. Finally, the presence of gene flow between populations of domestic and wild plants and animals [and members of the same or closely related but geographically and genetically differentiated domesticated species (12)] often results in modern populations that appear as if they arose outside the regions where the initial domestication process took place (13). As a result, it is crucial that researchers carefully evaluate whether multiple domestications of a single species occurred (13, 14), making sure to reserve the term “domestication” solely for the initial independent process, and to avoid using the term to refer to subsequent admixture that often incorporated genetic and morphological characteristics of wild populations that were never independently domesticated (12, 13).
An increasingly rich and diverse corpus of data from molecular and archaeological research generated over the past 15 y now makes it clear that agriculture began independently over a much larger area of the globe than was once thought, and included a diverse range of plant and animal taxa (Figs. 1 and 2). At least 11 regions of the Old and New World were involved as independent centers of origin, encompassing geographically isolated regions on most continents, but several more have been suggested (Fig. 1) (3, 7, 11, 15, 16). Some of these regions were the sources of major domesticates that spread to adjacent regions, whereas others involved more regionally important species often regarded as “minor” crops today (7, 17). The combined data also clearly show that two major chronological periods are of greatest interest: the transition to the Holocene from about 12,000–9,000 B.P. (all ages are calendar years before CE 1950), and the middle Holocene between 7,000 and 4,000 B.P. (Fig. 1). Dogs were a significant exception and were certainly domesticated in the late Pleistocene before the establishment of agriculture, although both the geographical origins of dog domestication and claims for domestic dogs in ~30,000-y-old contexts remain contentious (18). In the New World, crop domestication occurred thousands of years before animal domestication, whereas the opposite was true in areas such as Africa, Arabia, and India. Some of the asynchronous patterns in individual plant and animal species from different regions may be the result of patchy evidence, as well as the lack of a clear distinction between “primary” (truly independent) vs. “secondary” (e.g., inspired by diffusionary processes) domestication.
Hunting and gathering was the primary subsistence strategy for more than 95% of the time since the origin of Homo sapiens 200,000 y ago (19). Theories and explanations for why human cultures abandoned this long-term and apparently successful subsistence strategy and turned to food production continue to attract discussion and intense debate. Traditionally, the transition to agriculture was viewed as the result of a few single agents or “prime movers” that operated at the onset of the Holocene. Climate change, human population pressure, and culturally driven alternatives, such as “competitive feasting,” are among numerous additional agents proposed by generations of archaeologists (20–24). Simple unidirectional explanations, however, have proved unsatisfying for a number of researchers, and significant tensions remain between camps advocating different explanatory blueprints. The issue poses an important remaining challenge in domestication research (25) (see below).
Early Domestication Stages
The initial stages of the multispecies networks involved in domestication were critical because humans acted as: (i) dispersal agents (managing the reproduction of cultivated plants and controlling the mobility, range and density of domestic livestock); (ii) agents of (conscious or unconscious) selection, favoring the reproductive success of particular behavioral and phenotypic variants); and (iii) ecosystem modifiers, who (along with natural environmental changes) alter the developmental conditions and hence the characteristics of associated organisms.
So what is a domesticated plant or animal and how does it differ from its wild ancestor? From a present-day perspective, it is possible to recognize suites of common traits that make up the so-called “domestication syndrome” (26–28), and presumably many of these were key to early selection along the wild-to-domesticated trajectory. In plants, the syndrome is defined by a wide variety of traits that, depending on the species, may include: a reduced ability to disperse seeds without human intervention, reduction in physical and chemical defenses, reduction in unproductive side-shoots, reduction in seed dormancy, bigger seeds, more predictable and synchronous germination, and in some seed-propagated species, bigger and more inflorescences. In animals, these traits include: endocrine changes, increased docility, altered reproduction pattern and output, altered coat color, floppy ears, facial neotony, usually a reduction in size, and other changes in body proportions (26). Recent genetic and archaeological research, however, has demonstrated that not all of these traits arose at the same time in either plants or animals. In addition, it has been helpful to separate genes that controlled the traits that were under early selection (domestication genes) from those that were selected later to produce diversified and improved crops and animals (improvement genes) (4).
The strength of selection for “domestication syndrome” gene variants and their speed of fixation remains controversial. Although strong selection with rapid evolution of domestication traits within as little as 100–200 y has been suggested (8, 9, 29), recent archaeological studies have questioned these conclusions, at least for cereal domestication. In wheat, barley, and rice, it took ∼2,000–4,000 y to fix the nonshattering spikelet phenotype, a key indicator of cereal domestication (7). There are other indications in the Near East of long periods of cultivation without morphological evidence of domestication, including specific field weed flora associated with morphologically wild cereals and legumes, and large stores, suggesting reliance on cultivated production of morphologically wild species (30, 31). Doust et al. (32) show that factors previously underappreciated, such as GxE (gene-by-environment) and epistasis (gene-by-gene) interactions may have been important in slowing domestication rates. A comparison of rates of phenotypic evolution between wild and domesticated species also indicates that, contrary to expectations, evolutionary rates in domesticated species are not generally faster than those observed in wild species (7). Indeed, selection strengths for some traits are at the same level as the strength of natural selection acting on wild species, or even slightly lower (33).
The evidence for a slow pace of domestication implies a cultural period in agricultural origins called “predomestication cultivation” (PDC) (34). These periods lasted for many centuries before fully domesticated cereals appeared, as has been inferred from evidence in the Near East and China (7, 31, 35). Instances of PDC have also recently been documented in northwestern South America (36). Increasing evidence for PDC goes hand-in-hand with increasing indications of a nonsimultaneous development of the suite of traits that make up the domestication syndrome, in turn raising questions about when exactly to call archaeological remains “domesticated” and how and in what order the domestication syndrome was assembled.
These factors also make it more likely that crops were independently brought under cultivation more than once, even within a given “nuclear region,” then hybridized with cultivated or domesticated plants from other regions to become the domesticated versions we study today (37, 38). Neither genetic nor archaeobotanical studies can easily sort out these different activities, which has led to increased skepticism of the traditional models that purport rapid events taking place in a single location to explain transitions from wild to domesticated species (39, 40). In addition, the recent reevaluation of the speed of cereal domestication has led to a renewed discussion of unconscious vs. conscious selection. Charles Darwin was the first to explicitly articulate the difference between conscious selection during domestication, in which humans directly select for desirable traits (called by Darwin “methodical” selection) (1), versus unconscious selection, where traits evolve as a by-product of growth and natural selection in field environments, or from selection on other traits. In rice, for example, glutinous grains most likely arose from conscious selection by certain Asian cultures for this cuisine-prized trait (41). In contrast, seed nonshattering in cereals is thought to have arisen as a by-product of stalk-harvesting by sickles or harvest knives, which select for seeds that do not readily fall off the stalk, rather than a result of a conscious strategy associated with beating seed heads into baskets (29). Other domestication traits in grasses are generally thought to result from unconscious selection, including seed size, seed dormancy, synchronous seed ripening, and apical dominance (27).
Most domesticated plants are not cereals, and other crops with different domestication syndromes may have had faster rates of domestication once humans targeted them for cultivation, and been more prone to have traits selected by conscious selection. The great cultural geographer Carl Sauer (42) insightfully noted that squashes, beans, and various root crops (along with maize, the premier cereal crop of the Americas) were not mass-harvested and mass-planted, nor likely mass-selected, as the Old World cereals were. Individual harvesting and selection by early farmers, who would be expected to choose and deliberately propagate the crop attributes most useful to them when they could distinguish the useful phenotypes, could foster conscious selection and result in faster fixation of crucial and preferred domestication traits, such as the loss of toxicity and increased size of starch storage organs in tubers and roots. Fruit nonbitterness in squashes and melons, major early domesticates in all regions of the Americas and parts of Asia and Africa may also have been rapidly and consciously selected. For example, botanical remains from human teeth indicate that the loss of fruit bitterness in the squash species Cucurbita moschata took place by at least 9200 B.P., only 800 y later than the first evidence for its domestication. In fact, the loss may have taken place even earlier because the seed traits used to document domestication do not inform fruit-flesh characteristics (36, 43). Arguments for relatively fast, conscious selection have also been made for the important seed dormancy trait in Old World lentils and peas (44).
What about conscious vs. unconscious selection in animals? Marshall et al. (12) make a compelling case that intentional breeding of females was largely absent during the early stages of domestication for a wide range of species. This theory, along with what probably was considerable gene flow between wild and early managed animals (13), poses challenges to a number of commonly held assumptions about early domestication in some species relating to interpretations of genetic bottlenecks and molecular sequences more generally, the number of times a species was domesticated, and how various domestication traits emerged and were maintained in the long term. Clearly, many questions persist about the roles of directed vs. undirected selection across the spectrum of domesticated plants and animals.
Research over the past few decades has made it clear that prehistoric humans around the world significantly modified their environments, sometimes before and during the process of plant and animal domestication, and the role of humans in the enduring modification of environments is no longer underestimated (45–49). A uniquely important aspect of human environmental modification is the additional role cultural transmission plays in maintaining patterns of enduring local ecologies, resulting in a strongly enculturated ecological inheritance. Because they can often be traced archaeologically, cultural transmission processes have received increasing interest and mathematical modeling in the social sciences (50–52) and are embedded both in practice and in material settings (e.g., terraces, canals, mounding, soil management, lassos, penning, somatic modifications such as castration, food-processing tools). Although the process of cultural inheritance differs from that of genetics, it plays a crucial role in maintaining both cultural practices over generations and environments in which domestication and husbandry occurred and were maintained. Human intentionality and knowledge systems must have been key components among the interacting mechanisms within these bio/eco-cultural environments, and cultural transmission provided a basis for the maintenance of cumulative innovation. Traditional ecological knowledge over the longer term has maintained crop landrace diversity, and remains important for biodiversity distribution and ecosystem services more generally (53).
Genetic and Evolutionary Insights from Domestication
The study of domesticated species has led to increased interest in several important issues in genetics and evolutionary biology, including the underlying genetic architecture of adaptations and parallel evolution. Genetic research is increasingly identifying domestication genes, especially in plants (4). By contrast, many fewer domestication genes have been identified in animals (13). With the exception of coat-color genes, genetic variants that can be unambiguously assigned to early stages in domestication in animals have not yet been revealed. There are several possible reasons for this. First, discovering the molecular basis of domestication traits is relatively easy and inexpensive in plants compared with animal populations because early animal selection likely focused on behavioral and other characteristics (such as tameness and altered reproduction), with complex genetic foundations that are more difficult to study than classic morphological traits (54). Second, there may simply be few domestication loci with major effects in animals. Early animal domestication may have happened by shifting the allele frequencies at many loci, each with small individual effects, thereby altering the phenotype. This scenario would be consistent with the observation that many domestic animals (e.g., pigs) can readily establish feral populations that in many aspects mimic the phenotype of their wild ancestors (55).
Thus, an important question for both plants and animals is whether the striking phenotypic changes seen during domestication are under the control of single or multiple genes. Thus far, separate studies have identified both single (or few) genes and combinations of numerous genes of small effect, depending on the approach and species in question (4). To some extent, different inferences concerning the genetic architecture of domestication can be because of different methodological approaches. Forward genetic approaches, such as quantitative trait loci (QTL) mapping and genome-wide association studies have the capability of finding multiple loci controlling phenotypic traits, and thus to interpret a domestication trait as under the control of multiple genes (4). Reverse genetic approaches concentrate on particular genes and cannot, by themselves, discover multiple loci for a particular phenotype. Genes in reverse genetic approaches are often chosen because their mutant phenotypes in model systems, such as chicken, mouse, Arabidopsis, maize, and rice, are analogous to phenotypic differences between wild and domesticated species. It is then possible to ask whether sequence changes in the locus explain phenotypic differences. An example of this approach involves a mutation of the transcription factor ramosa1 (ra1) locus in maize that results in loss of floral branches (56). Differences in the ra1 locus were later found to be correlated with differences in floral branching in maize and other grasses (57). However, it is not the only gene involved, as shown by QTL studies that indicated up to five significant QTL regions controlling these traits (58). Finally, a recent study (59) demonstrated that the action of sh4 in rice is not always sufficient to produce nonshattering phenotypes.
An additional question is whether the same genes underlie similar phenotypic shifts in numerous domesticated plants and animals. In other words, is there parallelism from the same underlying genetic and developmental pathways or convergent evolution of unrelated taxa using unrelated gene networks (60)? In grasses, such forms are particularly striking, and similar awned and awnless spikelets, hulled and free-threshing grain, black-, red-, and straw-colored seed coats are found in multiple domesticated cereals. The geneticist Vavilov termed this phenomenon the Law of Homologous Series (61), and the first phase of comparative mapping in the grasses, using restriction fragment-length polymorphism markers, inferred QTL for shattering in rice, sorghum, and maize to be at the same location (62). Further work has proved equivocal, since most genes for shattering in grasses are unique to each domesticated lineage (63), though a recent study has shown that the major locus for shattering in sorghum corresponds to minor loci in rice and maize (64). Nevertheless, some mutations in domesticates are in fact parallel mutations. For example, variants of the MC1R locus are responsible for independently derived pig coloration patterns (65). Moreover, mutations at this gene appear to be associated with difference in color patterns in numerous domestic animals (66). A similar example of parallel evolution is associated with the rise of sticky cereals in northeast Asia, where glutinous rice, millets, and barley, among others (41, 67), are the result of alternative mutations at the Waxy gene (68–70).
Whether mutations selected during domestication were novel or were present as standing genetic variation in ancestral wild populations is a question of increasing interest. It has traditionally been assumed that phenotypic change and new adaptations arise from new mutations, but recent research increasingly shows that standing genetic variation plays important roles in a variety of species (71). For example, traits present as variants in wild progenitors today include the gene for tomato fruit size (fw2.2) (72), maize plant architecture (e.g., teosinte branched1) (73), seasonality controls (74, 75), and seed size [usually polygenic (76)]. Fast morphological evolution in cultivated plant populations may have ensued as favorable phenotypes, including those initially exposed by genetic or external environmental perturbations in response to the new field conditions, may have been preferentially selected by farmers who were not constrained by mutation rates (77, 78). Having said that, several traits in domesticated plants, including those associated with the reduction of seed-shattering in legumes and grasses, are deleterious in the wild, and if present, are rarely expressed phenotypically. In animals, analyses of modern dog genomes have revealed a handful of mutations (not found in extant wolves) with large effects on morphological variation, although given the predominance of selection for novel and unusual characteristics in dogs, this pattern is likely the exception (79). In many other domestic animals, humans likely selected for trait variants that were already present in ancestral populations, thereby altering the frequencies of the standing genetic variation.
As success in isolating domestication-related genes proceeds, it should become easier to distinguish between standing and new genetic variation, as well as to recognize parallelism in de novo mutations among domesticated species. Additionally, as the availability of genome-wide sequence data for domesticated species increases, it is becoming increasingly feasible to use selective sweep mapping to identify genomic regions that have been targets of selection during domestication without a priori information on candidate domestication genes (e.g., ref. 80). Challenges associated with this approach include the fact that the trait or traits affected by the selected genes may not be known, that selection that favors a de novo mutation during the domestication process will generate a more conspicuous signature of a selective sweep than selection for mutations that were already segregating in populations of the wild progenitor, and that some demographic processes can mimic the effects of selection on patterns of genetic variation. Understanding the different genetic architecture of domestication across crop types and in animals remains a major challenge for genetic research.
One new promising direction is the study of ancient DNA. Our increasing ability to identify selected mutations for domestication-associated traits in archaeological plant and animal remains is providing a unique temporal trajectory of the evolution of domesticated species, and the selection strengths that acted upon selected genes. One such example tested claims that two different genes (TSHR and BCDO2) were involved in early chicken domestication by typing the mutations in ancient European chickens. Because the wild-type alleles of both genes were segregating at a high frequency as recently as 500 y ago, the ancient DNA evidence demonstrated that the modern ubiquity of a mutation, even one that differentiates domestic and wild populations, cannot automatically be conflated with an ancient origin linked to early domestication (81).
Key Challenges for the Future
The enormous amount of empirical data compiled on domestication and associated human- and naturally driven circumstances during the past decades has naturally led to the generation of a number of questions, some of which pose key future challenges.
Filling in Gaps on Maps
One of the fundamental challenges of domestication research is filling the gaps that remain in both geographical and genomic maps. Genetic research provides a growing toolkit for elucidating the relationships between domesticates and their wild ancestors, and between the traits that make domesticates suited to anthropogenic environments and their underlying genetic architecture. The successes of genetics, touched upon above, at identifying domestication genes have been numerous and mostly recent. Expanding this repertoire remains a priority, but it is increasingly evident that we also need more evidence from ancient DNA, so that patterns found in modern populations can be compared with those of the past, and geographies and phylogeographic and adaptive hypotheses can be tested over the evolutionary time period of domestication.
In addition, archaeological research has many gaps on the chronology and regional sequences of domestication of plants and animals, and the contexts of agricultural origins. Recent research has shown that increased sampling and methodological developments have made it possible to clearly document cereal domestication [e.g., rice (82)], push back the earliest evidence for both the domestication of maize in southern Mexico (83) and the arrival of crops in northern Peru (36), and to recognize the likely independent processes of agricultural origins and domestication in New Guinea (45), parts of India (84), and Africa (85). These research successes within the past decade imply that more new information on more species from more regions and earlier periods can be expected and should be actively sought.
Related to this are important continuing challenges in determining why so few of the animal and plant species that were hunted and gathered by ancestral human populations were ever domesticated (86), and whether most species were domesticated once or multiple times. We recognize that distinguishing these options is complicated, and it is increasingly clear that incomplete archaeological evidence and genetic data are open to conflicting interpretation. This aspect highlights the importance of explicit modeling and simulation of a range of hypotheses concerning the starting conditions and processes of domestication (14, 87). Factors potentially leading to confusion include the fact that multiple domestication episodes may be hidden from genetic view today as a result of both bottlenecks (in some cases leading to extinction) and introgression. Archaeobotany, for example, has increasingly recognized extinct morphotypes of domesticated wheat (88, 89), and ancient DNA can help to identify lost genetic lineages of crops. Introduced domesticates may introgress with local wild populations, thus capturing genetic and phenotypic variation that can later be misinterpreted as the independent domestication of distinct wild animal (13) and plant (e.g., rice) (90–92) populations. Resolving these issues requires more targeted ancient DNA research and more realistic and sophisticated modeling.
Environmental and Ecological Contexts of Agricultural Origins
Although climate change remains the prime landscape and ecological modifier at the origins of agriculture, human behavior and the activities of diverse cultural traditions must be better understood. Beyond simply collecting more archaeological and paleoecological evidence, there is a need to broaden the study of past landscapes and their related ecosystems for both naturally derived features and the legacies of past human action. For example, more research should systematically map local and regional distributions of enriched soils, created through human activities, which are well known from Amazonia and Europe but much less well documented elsewhere (e.g., refs. 93, 94). Vegetation formations studied by plant ecologists and environmental historians may also be anthropogenic legacies, as has been suggested for a number of regions including South Asia and throughout the Americas (45, 53, 95, 94).
New or underdeveloped fields, such as ecological developmental biology (eco-devo) (96) and epigenetics (97)—together with mechanisms, such as developmental plasticity (98, 99)—are assuming increasing importance in the study of diversification, the origin of novelties, and evolutionary change. These fields should be extended to the realm of domestication research, in part because phenotypic and genetic responses to natural- and human-created environmental variability are among the most neglected issues in domestication studies. As recent work with teosinte has shown, field- and laboratory controlled experiments are needed to better understand them (99). Another example is that although it has been inferred that large seed size was selected by soil disturbance and depth of burial (e.g., ref. 100), as presumably seeds with the largest mass were better able to emerge from deeper burial depths associated with cultivation practices, others have suggested that seed size increase may be a plastic phenotypic response to enriched soils of early cultivation (101). New experimental research (102) on different legume crops indicate seed mass was important for emergence in some species, including those predicted to conform to the burial hypothesis (60), but not in others, suggesting a common single mechanism for seed size increase was not at work. In another vein, Blumler’s analysis (103), suggesting that the Near East was unusually well endowed with large seeded grasses preadapted to domestication, might explain the early and diverse domestication of plants in that region. In addition, Marshall et al. (12) make the point that epigenetic mechanisms should also be investigated in animal genetic responses during the domestication process.
A few scholars have discussed the potential role of climate shifts and atmospheric gas concentrations on biota at the transition between the late Pleistocene and early Holocene. More specifically, the authors have suggested that agriculture was a more favorable strategy in the Holocene as a result of these environmental shifts (e.g., refs. 99, and 104–107). Lower CO2 and temperature reduced plant productivity, in part by reducing photosynthetic efficiency, thus exacerbating drought stress: effects that were more marked on C3 plants but also present to a surprising degree in C4 plants. Did the rapid increase of CO2 and temperature at the onset of the Holocene make plants more attractive as a readily intensifiable resource and make cultivation more efficient? As plant productivity increased, why would some cultural traditions delay the shift in cultivation until the middle Holocene, and how can we connect the adoption of animal herding to changes in plant productivity? Although important global processes have doubtless impacted foragers and early cultivators, a great deal more research is necessary to unravel the causes, constraints, and exceptions to the early or middle Holocene transitions to farming.
Further experimental data on the impact of late-glacial and early Holocene temperatures and CO2 levels on the biological characteristics of wild progenitors of crops are needed to also understand how they may have influenced other phenotypic attributes of crop and animal progenitors on the eve of and during agricultural beginnings (99). Just as genetic studies of domestication have shown that conclusions drawn only from modern populations provide an incomplete and sometimes biased picture of the past (81, 108, 109), we need to better understand the interplay between past ecology, climate, plant phenotypic responses, and human activities.
Why Hunters and Gatherers Turned to Cultivation and Herding
Explaining the origins of agriculture is still one of the most contentious issues for social scientists. Few dispute that the interplay of climate, human demography, and social systems through time and space played a significant role (110). Although some consider the primary driving factors to be patterns of climatic and ecological change, others argue for the primacy of social imperatives and changes within social systems (23, 24, 111). More generally, some scholars have claimed that no explanations are likely to be universally applicable (112), whereas others have adopted an explicitly comparative approach, identifying parallel processes and exploring common underlying patterns (7, 15, 25). Further progress on this issue should focus not only on the acquisition of more data, but also on marshaling and discussing existing evidence, which may suggest which factors driving agricultural origins were of greater importance. In a number of nuclear centers there are now fewer disagreements about the cultural history of early agriculture (including the chronology and the organisms involved), which should make explanatory endeavors less complicated. As known instances of agricultural origins are further clarified, we will have more parallel histories of domestication from which to derive commonalities or process and patterns of causation.
Conclusions
The collection of papers presented in this Special Feature attempts to rise to the challenges outlined above. The articles illustrate a range of approaches to the study of domestication, including genetics, archaeological science, and anthropology, and raise new questions and hypotheses that are ripe for further testing. Even so, the new evidence and ideas presented here highlight a minority of the many species that were domesticated and subsequently improved by prehistoric cultures. Domestication remains a vibrant research area in biology and archaeology 145 y after Darwin’s seminal work (1), and the coming decade will no doubt generate satisfying and perhaps definitive answers to a wide range of outstanding questions.
Supplementary Material
Acknowledgments
We thank Joris Peters for assistance regarding the archaeological record of animal domestication. This manuscript resulted from a catalysis meeting entitled “Domestication as an Evolutionary Phenomenon: Expanding the Synthesis,” held April 7–11, 2011, that was awarded and hosted by the National Evolutionary Synthesis Centre (National Science Foundation EF-0905606) in 2011.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323964111/-/DCSupplemental.
References
- 1.Darwin C. The Variation of Animals and Plants under Domestication. London: John Murray; 1868. [Google Scholar]
- 2.Gepts P, et al., editors. Biodiversity in Agriculture: Domestication, Evolution, and Sustainability. Cambridge: Cambridge Univ Press; 2012. [Google Scholar]
- 3. Price D, Bar-Yosef O eds (2011) The Beginnings of Agriculture: New Data, New Ideas. Curr Anthropol 52 (Supp 4. Wenner-Gren Symposium Series; Univ of Chicago Press, Chicago)
- 4.Olsen KM, Wendel JF. A bountiful harvest: Genomic insights into crop domestication phenotypes. Annu Rev Plant Biol. 2013;64:47–70. doi: 10.1146/annurev-arplant-050312-120048. [DOI] [PubMed] [Google Scholar]
- 5.Council MotNR . A New Biology for the 21st century. Committee on a New Biology for the 21st Century: Ensuring the United States Leads the Coming Biology Revolution. Washington, DC: National Academies; 2009. [PubMed] [Google Scholar]
- 6.Wake M. Development in the real world. Review of Ecological Developmental Biology: Integrating Epigenetics, Medicine, and Evolution by Gilbert, S.F., Epel, D. Am Sci. 2010;98(1):75–78. [Google Scholar]
- 7.Fuller DQ, et al. Convergent evolution and parallelism in plant domestication revealed by an expanding archaeological record. Proc Natl Acad Sci USA. 2014;111:6147–6152. doi: 10.1073/pnas.1308937110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbo S, Lev-Yadun S, Gopher A. Agricultural origins: Centers and noncenters; a Near Eastern reappraisal. Crit Rev Plant Sci. 2010;29(5):317–328. [Google Scholar]
- 9.Innan H, Kim Y. Pattern of polymorphism after strong artificial selection in a domestication event. Proc Natl Acad Sci USA. 2004;101(29):10667–10672. doi: 10.1073/pnas.0401720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeder MA. Pathways to animal domestication. In: Gepts P, et al., editors. Biodiversity in Agriculture: Domestication, Evolution and Sustainability. Cambridge: Cambridge Univ Press; 2012. [Google Scholar]
- 11.Piperno DR. The origins of plant cultivation and domestication in the New World tropics. Curr Anthropol. 2011;52(S4):S453–S470. [Google Scholar]
- 12.Marshall FB, Dobney K, Denham T, Capriles JM. Evaluating the roles of directed breeding and gene flow in animal domestication. Proc Natl Acad Sci USA. 2014;111:6153–6158. doi: 10.1073/pnas.1312984110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larson G, Burger J. A population genetics view of animal domestication. Trends Genet. 2013;29(4):197–205. doi: 10.1016/j.tig.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Gerbault P, et al. Storytelling and story testing in domestication. Proc Natl Acad Sci USA. 2014;111:6159–6164. doi: 10.1073/pnas.1400425111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barker G. The Agricultural Revolution in Prehistory: Why did Foragers Become Farmers? Oxford: Oxford Univ Press; 2006. [Google Scholar]
- 16.Fuller DQ. An emerging paradigm shift in the origins of agriculture. General Anthropology. 2010;17(2):1–12. [Google Scholar]
- 17.Kraft KH, et al. Multiple lines of evidence for the origin of domesticated chili pepper, Capsicum annuum, in Mexico. Proc Natl Acad Sci USA. 2014;111:6165–6170. doi: 10.1073/pnas.1308933111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson G, et al. Rethinking dog domestication by integrating genetics, archeology, and biogeography. Proc Natl Acad Sci USA. 2012;109(23):8878–8883. doi: 10.1073/pnas.1203005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDougall I, Brown FH, Fleagle JG. Stratigraphic placement and age of modern humans from Kibish, Ethiopia. Nature. 2005;433(7027):733–736. doi: 10.1038/nature03258. [DOI] [PubMed] [Google Scholar]
- 20.Binford LR. In: Post-Pleistocene Adaptations. Binford SR, Binford LR, editors. Chicago: Aldine; 1968. pp. 313–342. [Google Scholar]
- 21.Moore A, Hillman GC. The Pleistocene to Holocene transition and human economy in Southwest Asia: The impact of the Younger Dryas. Am Antiq. 1992;57:482–494. [Google Scholar]
- 22.Cohen MN. Introduction: Rethinking the origins of agriculture. Curr Anthropol. 2009;50(5):591–595. doi: 10.1086/603548. [DOI] [PubMed] [Google Scholar]
- 23.Hayden B. The proof is in the pudding: Feasting and the origins of domestication. Curr Anthropol. 2009;50(5):597–601. doi: 10.1086/605110. [DOI] [PubMed] [Google Scholar]
- 24.Bowles S, Choi J-K. Coevolution of farming and private property during the early Holocene. Proc Natl Acad Sci USA. 2013;110(22):8830–8835. doi: 10.1073/pnas.1212149110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gremillion KJ, Barton L, Piperno DR. Particularism and the retreat from theory in the archaeology of agricultural origins. Proc Natl Acad Sci USA. 2014;111:6171–6177. doi: 10.1073/pnas.1308938110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammer K. Das domestikationssyndrom. Kulturpflanze. 1984;32(1):11–34. [Google Scholar]
- 27.Harlan JR. Crops and Man. 2nd Ed. Madison, WI: American Society of Agronomy; 1992. [Google Scholar]
- 28.Darwin C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. London: John Murray; 1859. [PMC free article] [PubMed] [Google Scholar]
- 29.Hillman GC, Davies MS. Domestication rates in wild wheats and barley under primitive cultivation. In: Anderson PC, editor. Préhistoire de l'Agriculture: Nouvelles Approches Expérimentales et Ethnographiques, Monographie du CRA. Paris: Centre de Rercherches Archéologiques, CNRS; 1992. pp. 113–158. [Google Scholar]
- 30.Weiss E, Kislev ME, Hartmann A. Anthropology. Autonomous cultivation before domestication. Science. 2006;312(5780):1608–1610. doi: 10.1126/science.1127235. [DOI] [PubMed] [Google Scholar]
- 31.Willcox G, Stordeur D. Large-scale cereal processing before domestication during the tenth millennium cal BC in northern Syria. Antiquity. 2012;86(331):99–114. [Google Scholar]
- 32.Doust AN, et al. Beyond the single gene: How epistasis and gene-by-environment effects influence crop domestication. Proc Natl Acad Sci USA. 2014;111:6178–6183. doi: 10.1073/pnas.1308940110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purugganan MD, Fuller DQ. Archaeological data reveal slow rates of evolution during plant domestication. Evolution. 2011;65(1):171–183. doi: 10.1111/j.1558-5646.2010.01093.x. [DOI] [PubMed] [Google Scholar]
- 34.Harris DR, Hillman GC. Foraging and Farming: The Evolution of Plant Exploitation. London: Unwin Hyman; 1989. [Google Scholar]
- 35.Yang X, et al. Early millet use in northern China. Proc Natl Acad Sci USA. 2012;109(10):3726–3730. doi: 10.1073/pnas.1115430109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piperno DR, Dillehay TD. Starch grains on human teeth reveal early broad crop diet in northern Peru. Proc Natl Acad Sci USA. 2008;105(50):19622–19627. doi: 10.1073/pnas.0808752105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allaby RG, Fuller DQ, Brown TA. The genetic expectations of a protracted model for the origins of domesticated crops. Proc Natl Acad Sci USA. 2008;105(37):13982–13986. doi: 10.1073/pnas.0803780105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bettinger R, Barton L, Morgan C. The origins of food production in North China: A different kind of agricultural revolution. Evol Anthropol. 2010;19:9–21. [Google Scholar]
- 39.Brown TA, Jones MK, Powell W, Allaby RG. The complex origins of domesticated crops in the Fertile Crescent. Trends Ecol Evol. 2009;24(2):103–109. doi: 10.1016/j.tree.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 40.Fuller DQ, Willcox G, Allaby RG. Cultivation and domestication had multiple origins: Arguments against the core area hypothesis for the origins of agriculture in the Near East. World Archaeol. 2011;43(4):628–652. [Google Scholar]
- 41.Fuller D, Rowlands M. Ingestion and food technologies: Maintaining differences over the long-term in West, South and East Asia. In: Bennet J, Sherratt S, Wilkinson TC, editors. Interweaving Worlds: Systematic Interactions in Eurasia, 7th to 1st millennia BC. Essays from a Conference in Memory of Professor Andrew Sherratt. Oxford: Oxbow Books; 2011. [Google Scholar]
- 42.Sauer CE. American agricultural origins: a consideration of nature and culture. In: Leighly J, editor. Land and Life. A Selection of the Writing of Carl Ortwin Sauer. Berkeley: Univ of California Press; 1965. [Google Scholar]
- 43.Dillehay TD, Rossen J, Andres TC, Williams DE. Preceramic adoption of peanut, squash, and cotton in northern Peru. Science. 2007;316(5833):1890–1893. doi: 10.1126/science.1141395. [DOI] [PubMed] [Google Scholar]
- 44.Abbo S, et al. Experimental growing of wild pea in Israel and its bearing on Near Eastern plant domestication. Ann Bot (Lond) 2011;107(8):1399–1404. doi: 10.1093/aob/mcr081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denham TP, et al. Origins of agriculture at Kuk Swamp in the highlands of New Guinea. Science. 2003;301(5630):189–193. doi: 10.1126/science.1085255. [DOI] [PubMed] [Google Scholar]
- 46.Piperno DR. Quaternary environmental history and agricultural impact on vegetation in Central America. Ann Mo Bot Gard. 2006;93(2):274–296. [Google Scholar]
- 47.Lentz DL, editor. Imperfect Balance: Landscape Transformations in the Precolumbian Americas. New York: Columbia Univ Press; 2000. [Google Scholar]
- 48.Ellis EC, et al. Used planet: A global history. Proc Natl Acad Sci USA. 2013;110(20):7978–7985. doi: 10.1073/pnas.1217241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arroyo-Kalin M. Slash-burn-and-churn: Landscape history and crop cultivation in pre-Columbian Amazonia. Quat Int. 2012;249:4–18. [Google Scholar]
- 50.Richerson PJ, Boyd R. Not By Genes Alone. Chicago: Univ of Chicago Press; 2005. [Google Scholar]
- 51.Shennan SJ. Genes, Memes and Human History: Darwinian Archaeology and Cultural Evolution. London: Thames and Hudson; 2002. [Google Scholar]
- 52.Powell A, Shennan S, Thomas MG. Late Pleistocene demography and the appearance of modern human behavior. Science. 2009;324(5932):1298–1301. doi: 10.1126/science.1170165. [DOI] [PubMed] [Google Scholar]
- 53.Ford A, Nigh R. Origins of the Maya forest gardens. J Ethnobiol. 2009;29:213–236. [Google Scholar]
- 54.Albert FW, et al. Genetic architecture of tameness in a rat model of animal domestication. Genetics. 2009;182(2):541–554. doi: 10.1534/genetics.109.102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Albarella U, Dobney K, Rowley-Conwy P. The domestication of the pig (Sus scrofa): New challenges and approaches. In: Zeder MA, Bradley DG, Emshwiller E, Smith BD, editors. Documenting Domestication: New Genetic and Archaeological Paradigms. Berkeley: Univ of California Press; 2006. pp. 209–227. [Google Scholar]
- 56.Vollbrecht E, Springer PS, Goh L, Buckler ES, 4th, Martienssen R. Architecture of floral branch systems in maize and related grasses. Nature. 2005;436(7054):1119–1126. doi: 10.1038/nature03892. [DOI] [PubMed] [Google Scholar]
- 57.Sigmon B, Vollbrecht E. Evidence of selection at the ramosa1 locus during maize domestication. Mol Ecol. 2010;19(7):1296–1311. doi: 10.1111/j.1365-294X.2010.04562.x. [DOI] [PubMed] [Google Scholar]
- 58.Upadyayula N, da Silva HS, Bohn MO, Rocheford TR. Genetic and QTL analysis of maize tassel and ear inflorescence architecture. Theor Appl Genet. 2006;112(4):592–606. doi: 10.1007/s00122-005-0133-x. [DOI] [PubMed] [Google Scholar]
- 59.Ishikawa R, et al. Allelic interaction at seed-shattering loci in the genetic backgrounds of wild and cultivated rice species. Genes Genet Syst. 2010;85(4):265–271. doi: 10.1266/ggs.85.265. [DOI] [PubMed] [Google Scholar]
- 60.Fuller DQ, Allaby RG. Seed dispersal and crop domestication: Shattering, germination and seasonality in evolution under cultivation. In: Ostergaard L, editor. Fruit Development and Seed Dispersal, Annual Plant Reviews. Vol 38. Oxford: Wiley-Blackwell; 2009. pp. 238–295. [Google Scholar]
- 61.Vavilov NI. Phytogeographic basis of plant breeding. The origin, variation, immunity and breeding of cultivated plants. Chron Bot. 1951;13:1–366. [Google Scholar]
- 62.Paterson AH, et al. Convergent domestication of cereal crops by independent mutations at corresponding genetic Loci. Science. 1995;269(5231):1714–1718. doi: 10.1126/science.269.5231.1714. [DOI] [PubMed] [Google Scholar]
- 63.Li W, Gill BS. Multiple genetic pathways for seed shattering in the grasses. Funct Integr Genomics. 2006;6(4):300–309. doi: 10.1007/s10142-005-0015-y. [DOI] [PubMed] [Google Scholar]
- 64.Lin ZW, et al. Parallel domestication of the Shattering1 genes in cereals. Nat Genet. 2012;44(6):720–U154. doi: 10.1038/ng.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fang M, Larson G, Ribeiro HS, Li N, Andersson L. Contrasting mode of evolution at a coat color locus in wild and domestic pigs. PLoS Genet. 2009;5(1):e1000341. doi: 10.1371/journal.pgen.1000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Linderholm A, Larson G. The role of humans in facilitating and sustaining coat colour variation in domestic animals. Semin Cell Dev Biol. 2013;24(6–7):587–593. doi: 10.1016/j.semcdb.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 67.Erikksson G. The waxy character. Hereditas. 1969;63(1–2):180–204. [Google Scholar]
- 68.Ma J, et al. Molecular characterization and comparative analysis of two waxy alleles in barley. Genes & Genomics. 2010;32(6):513–520. [Google Scholar]
- 69.Araki M, Numaoka A, Kawase M, Fukunaga K. Origin of waxy common millet, Panicum miliaceum L. in Japan. Genet Resour Crop Evol. 2012;59(7):1303–1308. [Google Scholar]
- 70.Olsen KM, Purugganan MD. Molecular evidence on the origin and evolution of glutinous rice. Genetics. 2002;162(2):941–950. doi: 10.1093/genetics/162.2.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barrett RD, Schluter D. Adaptation from standing genetic variation. Trends Ecol Evol. 2008;23(1):38–44. doi: 10.1016/j.tree.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 72.Nesbitt TC, Tanksley SD. Comparative sequencing in the genus Lycopersicon. Implications for the evolution of fruit size in the domestication of cultivated tomatoes. Genetics. 2002;162(1):365–379. doi: 10.1093/genetics/162.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Studer A, Zhao Q, Ross-Ibarra J, Doebley J. Identification of a functional transposon insertion in the maize domestication gene tb1. Nat Genet. 2011;43(11):1160–1163. doi: 10.1038/ng.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones H, et al. Population-based resequencing reveals that the flowering time adaptation of cultivated barley originated east of the Fertile Crescent. Mol Biol Evol. 2008;25(10):2211–2219. doi: 10.1093/molbev/msn167. [DOI] [PubMed] [Google Scholar]
- 75.Clotault J, et al. Evolutionary history of pearl millet (Pennisetum glaucum [L.] R. Br.) and selection on flowering genes since its domestication. Mol Biol Evol. 2012;29(4):1199–1212. doi: 10.1093/molbev/msr287. [DOI] [PubMed] [Google Scholar]
- 76.Gupta PK, Rustgi S, Kumar N. Genetic and molecular basis of grain size and grain number and its relevance to grain productivity in higher plants. Genome. 2006;49(6):565–571. doi: 10.1139/g06-063. [DOI] [PubMed] [Google Scholar]
- 77.Pickersgill B. Domestication of plants in the Americas: Insights from Mendelian and molecular genetics. Ann Bot (Lond) 2007;100(5):925–940. doi: 10.1093/aob/mcm193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gremillion KJ, Piperno DR. Human behavioral ecology, phynotipic (developmental) plasticity, and agricultural origins. Curr Anthropol. 2009;50(5):615–619. doi: 10.1086/605360. [DOI] [PubMed] [Google Scholar]
- 79.Boyko AR, et al. A simple genetic architecture underlies morphological variation in dogs. PLoS Biol. 2010;8(8):e1000451. doi: 10.1371/journal.pbio.1000451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rubin C-J, et al. Whole-genome resequencing reveals loci under selection during chicken domestication. Nature. 2010;464(7288):587–591. doi: 10.1038/nature08832. [DOI] [PubMed] [Google Scholar]
- 81.Flink LG, et al. Establishing the validity of domestication genes using DNA from ancient chickens. Proc Natl Acad Sci USA. 2014;111:6184–6189. doi: 10.1073/pnas.1308939110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fuller DQ, et al. The domestication process and domestication rate in rice: Spikelet bases from the Lower Yangtze. Science. 2009;323(5921):1607–1610. doi: 10.1126/science.1166605. [DOI] [PubMed] [Google Scholar]
- 83.Piperno DR, Ranere AJ, Holst I, Iriarte J, Dickau R. Starch grain and phytolith evidence for early ninth millennium B.P. maize from the Central Balsas River Valley, Mexico. Proc Natl Acad Sci USA. 2009;106(13):5019–5024. doi: 10.1073/pnas.0812525106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fuller DQ. Finding plant domestication in the Indian subcontinent. Curr Anthropol. 2011;52(S4):S347–S362. [Google Scholar]
- 85.Fuller DQ, Hildebrand L. In: Domesticating Plants in Africa. The Oxford Handbook of African Archaeology. Mitchell P, Lane P, editors. Oxford: Oxford Univ Press; 2013. pp. 507–525. [Google Scholar]
- 86.Diamond J. Evolution, consequences and future of plant and animal domestication. Nature. 2002;418(6898):700–707. doi: 10.1038/nature01019. [DOI] [PubMed] [Google Scholar]
- 87.Allaby R. Integrating the processes in the evolutionary system of domestication. J Exp Bot. 2010;61(4):935–944. doi: 10.1093/jxb/erp382. [DOI] [PubMed] [Google Scholar]
- 88.Jones G, Valamoti S, Charles M. Early crop diversity: A “new” glume wheat from northern Greece. Vegetation History and Archaeobotany. 2000;9(3):133–146. [Google Scholar]
- 89.Fuller DQ, Willcox G, Allaby RG. Early agricultural pathways: Moving outside the ‘core area’ hypothesis in Southwest Asia. J Exp Bot. 2012;63(2):617–633. doi: 10.1093/jxb/err307. [DOI] [PubMed] [Google Scholar]
- 90.Fuller DQ. Pathways to Asian civilizations: Tracing the origins and spread of rice and rice cultures. Rice. 2011;4:78–92. [Google Scholar]
- 91.Molina J, et al. Molecular evidence for a single evolutionary origin of domesticated rice. Proc Natl Acad Sci USA. 2011;108(20):8351–8356. doi: 10.1073/pnas.1104686108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gross BL, Zhao Z. Archaeological and genetic insights into the origins of domesticated rice. Proc Natl Acad Sci USA. 2014;111:6190–6197. doi: 10.1073/pnas.1308942110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baade J. Irragric anthrosols—Artifacts of human adaptation to arid conditions: Examples from the high Himalaya and the coastal desert of Peru. Climates, Landscapes, and Civilizations Geophysical Monograph Series. 2012;198:203–208. [Google Scholar]
- 94.Fairhead J, Leach M. Amazonian Dark Earths in Africa? Amazonian Dark Earths: Wim Sombroek’s Vision. New York: Springer; 2009. pp. 265–278. [Google Scholar]
- 95.Woods WI, et al. Amazonian Dark Earths: Wim Sombroek’s Vision. New York: Springer; 2009. [Google Scholar]
- 96.Gilbert SF, Epel D. Ecological Developmental Biology: Integrating Epigenetics, Medicine and Evolution. Sunderland, MA: Sinauer Associates, Inc.; 2009. [Google Scholar]
- 97.Jablonka E, Raz G. Transgenerational epigenetic inheritance: Prevalence, mechanisms, and implications for the study of heredity and evolution. Q Rev Biol. 2009;84(2):131–176. doi: 10.1086/598822. [DOI] [PubMed] [Google Scholar]
- 98.West-Eberhard MJ. Developmental Plasticity and Evolution. New York: Oxford Univ Press; 2003. [Google Scholar]
- 99.Piperno DR, Holst I, Winter K, McMillan O. Teosinte before domestication: Experimental study of growth and phenotypic variability in late Pleistocene and early Holocene environments. Quat Int. 2014 doi: 10.1016/j.quaint.2013.12.049. [DOI] [Google Scholar]
- 100.Fuller DQ, Harvey E, Qin L. Presumed domestication? Evidence for wild rice cultivation and domestication in the fifth millennium BC of the Lower Yangtze region. Antiquity. 2007;81(312):316–331. [Google Scholar]
- 101.Willcox G. Measuring grain size and identifying Near Eastern cereal domestication: Evidence from the Euphrates valley. J Archaeol Sci. 2004;31(2):145–150. [Google Scholar]
- 102.Kluyver TA, Charles M, Jones G, Rees M, Osborne CP. Did greater burial depth increase the seed size of domesticated legumes? J Exp Bot. 2013;64(13):4101–4108. doi: 10.1093/jxb/ert304. [DOI] [PubMed] [Google Scholar]
- 103.Blumler MA. Evolution of Caryopsis Gigantism and Agricultural Origins. New York: Binghamton Univ; 1998. [Google Scholar]
- 104.Piperno DR, Pearsall DM. The Origins of Agriculture in the Lowland Neotropics. San Diego, CA: Academic; 1998. [Google Scholar]
- 105.Richerson PJ, Boyd R, Bettinger RL. Was agriculture impossible during the Pleistocene but mandatory during the Holocene? A climate change hypothesis. Am Antiq. 2001;66(3):387–411. [Google Scholar]
- 106.Cunniff J, Charles M, Jones G, Osborne CP. Was low atmospheric CO2 a limiting factor in the origin of agriculture? Environmental Archaeology. 2010;15(2):113–123. [Google Scholar]
- 107.Sage RF. Was low atmospheric CO2 during the Pleistocene a limiting factor for the origin of agriculture? Glob Change Biol. 1995;1(2):93–106. [Google Scholar]
- 108.Asplund L, Hagenblad J, Leino MW. Re-evaluating the history of the wheat domestication gene NAM-B1 using historical plant material. J Archaeol Sci. 2010;37(9):2303–2307. [Google Scholar]
- 109.Roullier C, Benoit L, McKey DB, Lebot V. Historical collections reveal patterns of diffusion of sweet potato in Oceania obscured by modern plant movements and recombination. Proc Natl Acad Sci USA. 2013;110(6):2205–2210. doi: 10.1073/pnas.1211049110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Belfer-Cohen A, Goring-Morris AN. Becoming farmers. Curr Anthropol. 2011;52(S4):S209–S220. [Google Scholar]
- 111.Hayden B, Canuel N, Shanse J. What was brewing in the Natufian? An archaeological assessment of brewing technology in the Epipaleolithic. J Archaeol Method Theory. 2013;20(1):102–150. [Google Scholar]
- 112.Zeder MA, Smith BD. A conversation on agricultural origins. Curr Anthropol. 2009;50(5):681–690. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.