Significance
Serotonin is an important modulator of anxious states. Serotonergic neurotransmission in the dorsal raphe nuclei is known to be regulated by different presynaptic and postsynaptic feedback mechanisms involving G protein-coupled receptor signals, but the influence of such feedback mechanisms on anxiety-related behavior has not been investigated until now. We found that optogenetic activation of the Gq-coupled receptor signals in 5-HT2c receptor locations in GABAergic neurons in dorsal raphe nuclei decreases the activity of 5-HT neurons and relieves anxiety in mice. 5-HT2c receptors are drug targets for mental disorders. Our results demonstrate a previously unidentified negative feedback mechanism by 5-HT2c/Gq signals for the fine-tuning of anxiety behavior, and point to a promising therapeutic strategy for treating anxiety-related disorders.
Abstract
Serotonin 2c receptors (5-HT2c-Rs) are drug targets for certain mental disorders, including schizophrenia, depression, and anxiety. 5-HT2c-Rs are expressed throughout the brain, making it difficult to link behavioral changes to circuit specific receptor expression. Various 5-HT-Rs, including 5-HT2c-Rs, are found in the dorsal raphe nucleus (DRN); however, the function of 5-HT2c-Rs and their influence on the serotonergic signals mediating mood disorders remain unclear. To investigate the role of 5-HT2c-Rs in the DRN in mice, we developed a melanopsin-based optogenetic probe for activation of Gq signals in cellular domains, where 5-HT2c-Rs are localized. Our results demonstrate that precise temporal control of Gq signals in 5-HT2c-R domains in GABAergic neurons upstream of 5-HT neurons provides negative feedback regulation of serotonergic firing to modulate anxiety-like behavior in mice.
Serotonin (5-hydroxytryptamine, or 5-HT) is an important modulator of anxiety circuits (1). The diverse effects of serotonin are mediated through various 5-HT receptors (5-HT-Rs), including 5-HT1–7-Rs (2). Recent pharmacologic and genetic studies have highlighted an important role of 5-HT2c-Rs in anxiety disorders; however, the interpretation of physiological and behavioral data remains difficult owing to a lack of selective pharmacologic ligands (3).
5-HT2c-Rs are expressed in various cell types and brain regions of the anxiety circuit, including the amygdala and the dorsal raphe nucleus (DRN), a midbrain region containing high concentrations of 5-HT neurons. It has been suggested that 5-HT2c-Rs are expressed in GABAergic neurons, and that 5-HT2c-R activation may contribute to an inhibitory feedback control of 5-HT cell firing (4). The functional and behavioral consequences of such a possible inhibitory feedback mechanism for 5-HT firing have not yet been investigated, however.
Unfortunately, current techniques for identifying the functions of 5-HT2c-Rs in vertebrate brains are of limited value. For example, agonists and antagonists of 5-HT2c-Rs are often unspecific, and their action is not restricted to a specific cell type. Complete and conditional knockouts of the receptor gene have limited control of developmental and compensation effects by other G-protein–coupled receptors (GPCRs), and none of the current techniques allows for the physiological control of the 5-HT2c-R activation on a millisecond to second time scale.
To overcome the limitations of pharmacologic and genetic approaches, we have developed a new light-activated GPCR based on vertebrate melanopsin (vMo). Both 5-HT2c-Rs and vMo couple to the Gq signaling pathway (5, 6). To investigate the functional consequence of Gq signal activation in the cell types and cellular structures where 5-HT2c-Rs are located, we virally expressed vMo carrying the C terminus (CT) of the 5-HT2c-R in GABAergic neurons in the DRN. We found that light activation of vMo-CT5-HT2c decreases the firing of 5-HT neurons and modulates anxiety behaviors in mice. Our results demonstrate a previously unidentified, autoregulatory negative feedback mechanism for the firing of serotonergic neurons to control anxiety in mice.
Results
C-Terminally Tagged Vertebrate Melanopsin as a New Optogenetic Tool to Control Gq Signals in 5-HT2c Receptor Domains.
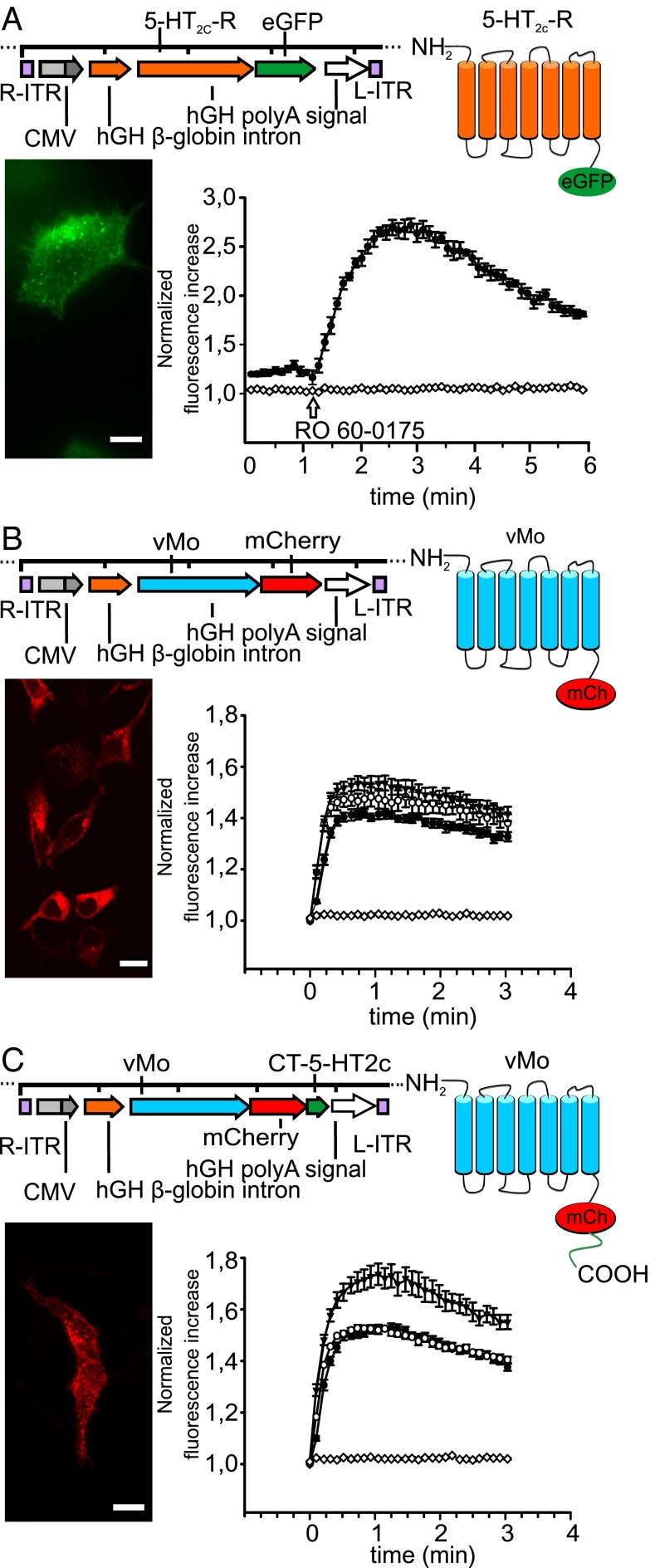
vMo, previously reported to enable in vivo neural control in mice (7), was coupled to the fluorescent marker protein mCherry (vMo-mCherry), tagged with the C-terminal domain of the 5-HT2c-R (vMo-CT5-HT2c) (Fig. 1 B and C). We recently demonstrated that a C-terminal domain of a 5-HT-R fused to vertebrate rhodopsin was sufficient for functional targeting into cellular domains where the corresponding endogenous 5-HT-R is localized (8). On confocal images of transfected human embryonic kidney cells (HEK tsA-201 cells, a subclone of the human embryonic kidney cell line HEK-293 that expresses the temperature-sensitive simian virus 40 T antigen), vMo-CT5-HT2c showed a similar distribution and membrane trafficking as the eGFP tagged 5-HT2c WT receptor (Fig. 1 A and C), whereas vMo-mCherry alone exhibited agglomeration and inclusion in cellular compartments (Fig. 1B). Both GPCR chimeras triggered intracellular Ca2+ release in HEK tsA cells when activated by a 2-s blue light pulse (Fig. 1 B and C). vMo-CT5-HT2c–induced Ca2+ responses were greater than vMo-mCherry–induced Ca2+ release. The Ca2+ response was dependent on the retinal compound supplied. In the presence of all-trans retinal, the Ca2+ signal induced by the different vMos was increased compared with that in cells incubated either with or without 9-cis retinal. Compared with the 5-HT2c-R, the Ca2+ response kinetics were faster, but lower by 30%. These findings support the use of vMo-CT5-HT2c as a unique optogenetic tool for controlling Gq-mediated signals with high temporal precision.
Fig. 1.
Light-induced changes in intracellular Ca2+ levels by Gq-coupled, C-terminally tagged vertebrate melanopsin. (A–C) (Upper) Schematic representation of the GPCRs in AAV expression vectors used in the experiments (Left) and corresponding predicted structure of the GPCRs (Right). Calcium responses were measured with a microplate reader using the fluorescent calcium indicator dye Fluo-4. Fluorescent counts were normalized to minimum fluorescence signal. Data from a representative experiment are shown as mean ± SEM from six wells. (Lower) Representative photomicrographs (Left) and quantification of Ca2+ responses (Right) of (A) heterologous expression of the WT 5-HT2c-R C-terminally tagged with eGFP in HEK cells and Ca2+ responses induced with 10 µM RO 60-0175 (arrowhead) in untransfected HEK cells (white diamonds) and those expressing 5-HT2c-eGFP (black circles) after 30-s baseline measurements (n = 6); (B) heterologous expression of vMo C-terminally tagged with mCherry in HEK cells and Ca2+ responses in HEK cells transiently expressing vMo, supplemented with 10 µM 9-cis retinal (white circles; n = 6), all-trans retinal (black triangles; n = 6) and without supplementation (black circles; n = 6) after repeated light stimulation (2-s light pulse, 1-s delay, 60 repeats), with HEK cells transfected with mCherry as control (white diamonds; n = 6); and (C) heterologous expression of vMo-CT5-HT2c C-terminally tagged with mCherry fused to the C-terminal domain of the human 5-HT2c-R in HEK cells and Ca2+ responses in HEK cells transiently expressing vMo-CT5-HT2c, supplemented with 10 µM 9-cis retinal (white circles; n = 6), all-trans retinal (black triangles; n = 6) and without any supplements (black circles; n = 6) after repeated light stimulation (2-s light pulse, 1-s delay, 60 repeats; 485 nm) with HEK cells transfected with mCherry (white diamonds; n = 6) as a control. (Scale bars: 15 µm.)
5-HT2c-Rs Are Located on GABAergic Neurons in the DRN and Modulate Firing of 5-HT Neurons.
It has been suggested that 5-HT2c-R activation in the DRN may inhibit 5-HT cell firing via GABAergic mechanisms. To demonstrate this inhibitory feedback loop for 5-HT firing, we first showed that 5-HT2c-Rs are expressed on GABAergic neurons, but not on serotonergic neurons, in the DRN (Fig. 2 A–C). We used double-immunohistochemical analysis to simultaneously visualize 5-HT2c-R immunoreactivity with either tryptophan hydroxylase (TPH), the rate-limiting enzyme of serotonin synthesis, or glutamate decarboxylase 65 (GAD65), an enzyme that catalyzes the decarboxylation of glutamate to GABA. The results indicate that 5-HT2c-immunoreactive (IR) cells are widely distributed throughout the DRN, mainly in the ventrolateral part (DRVL), the ventrolateral part of the periaqueductal gray (VLPAG), and the DR lateral wings (lwDRN) in the midrostrocaudal region (Fig. 2A). 5-HT2c positive cell bodies partly intermingle with TPH-positive cells in the DRV and the dorsal part of the dorsal raphe (DRD); however, higher-magnification pictures detected no 5-HT2c-IR on serotonergic cells.
Fig. 2.
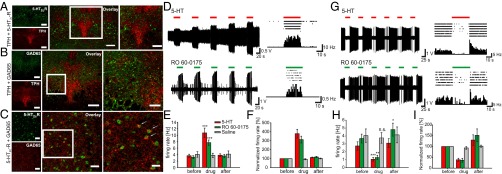
5-HT2c receptors are localized in GABAergic neurons in the DRN and modulate GABAergic and serotonergic neuronal firing. (A–C) Distribution of 5-HT2cRs in the DRN. (A) (Left) Representative photomicrographs displaying immunoreactivity (IR) for the 5-HT2cR (green) and TPH (red) in the DRN. (Center) Overlay of 5-HT2cR-IR and TPH-IR demonstrating no colocalization of 5-HT2cR on serotonergic cells. (Right) Higher-magnification view of the boxed region in the center panel. (B) (Left) Distribution of GAD65 (green) and TPH-IR (red) in the DRN. (Center) Overlay of GAD65 and TPH-IR. (Right) Higher-magnification view of the boxed region in the center panel. (C) (Left) Photomicrographs of the DRN displaying colocalization for the 5-HT2cR–positive and GAD65-positive cells. (Center) Overlay image of cells containing IR for both 5-HT2cR (green) and GAD65 (red), which appear yellow. (Right) Higher-magnification image of the boxed areas. Arrows indicate cells double-labeled for 5-HT2cR and GAD65. (Scale bars: 200 µm in A and B, Left and Center; 100 µm in B, Right ; 50 µm in C, Left and Center; 25 µm in C, Right.) (D–I) Activation of 5-HT2cRs in the DRN leads to either activation or inhibition of DRN neurons representing GABAergic and serotonergic neurons, respectively. (D) (Left) Example trace of repetitive activation of in vivo neuronal firing of GABAergic neurons in the DRN by application of 5-HT (Upper) or the 5-HT2c-R agonist RO 60-0175 (Lower) in anesthetized mice. (Right) Raster plot (Upper) and PSTH (Lower) during drug application to DRN neurons. The raster plot exemplifies a single cell response during five drug applications, whereas the PSTH shows the averaged single cell response during these five repetitions (1-s bin width). (E) Average firing rate of DRN neurons before, during, and after application of 5-HT (red; n = 25), RO 60-0175 (green; n = 25) or saline (gray; n = 6). (F) Normalized firing rates of DRN neurons for the data shown in E. (G) (Left) Example trace of repetitive inhibition of in vivo neuronal firing of serotonergic neurons in the DRN by the application of 5-HT (Upper) or RO 60-0175 (Lower) in anesthetized mice. (Right) Raster plot (Upper) and PSTH (Lower) during drug application to DRN neurons. The raster plot exemplifies a single cell response during six drug applications, whereas the PSTH shows the averaged single cell response during these six repetitions (1-s bin width). (H) Average firing rate of DRN neurons before, during, and after application of 5-HT (red; n = 13), RO 60-0175 (green; n = 10), or saline (gray; n = 6). (I) Normalized firing rates of DRN neurons for the data shown in E.
Based on the common belief that the 5-HT2c-R is expressed on GABAergic interneurons in the DRN, we performed colabeling for GAD65 and TPH to analyze the distribution pattern of GAD65-positive cells in the DRN (Fig. 2B). The anatomic distribution of GABAergic cells in the DRN indicates a correlation between GAD65-positive cells and the 5-HT2c-R expression pattern. A double-labeling experiment with GAD65 and 5-HT2C revealed 5-HT2c-R expression by GABAergic cells of the DRN containing the GAD65 isoform (Fig. 2C). We next demonstrated that 5-HT2c-Rs modulate firing of DRN neurons in vivo. Application of the 5-HT2c-R–selective agonist RO 60-0175 leads to either an increase or decrease in neuronal firing in two populations of neurons in the DRN in anesthetized mice (Fig. 2 D–I and Table S1). These two neuronal types, which most likely represent GABAergic and 5-HT neurons, were identified by their cell type-specific firing pattern and firing frequencies (9, 10). GABAergic neurons are activated by 5-HT and RO 60-0175 (Fig. 2 D–F), whereas 5-HT neurons are inhibited by 5-HT and RO 60-0175 (Fig. 2 G–I). Thus, activation of GABAergic 5-HT2c-Rs reduces the firing of 5-HT neurons in the DRN in vivo.
Light Activation of vMo-CT5-HT2c Expressed in Dorsal Raphe GABAergic Neurons Decrease 5-HT Cell Firing in Vivo.
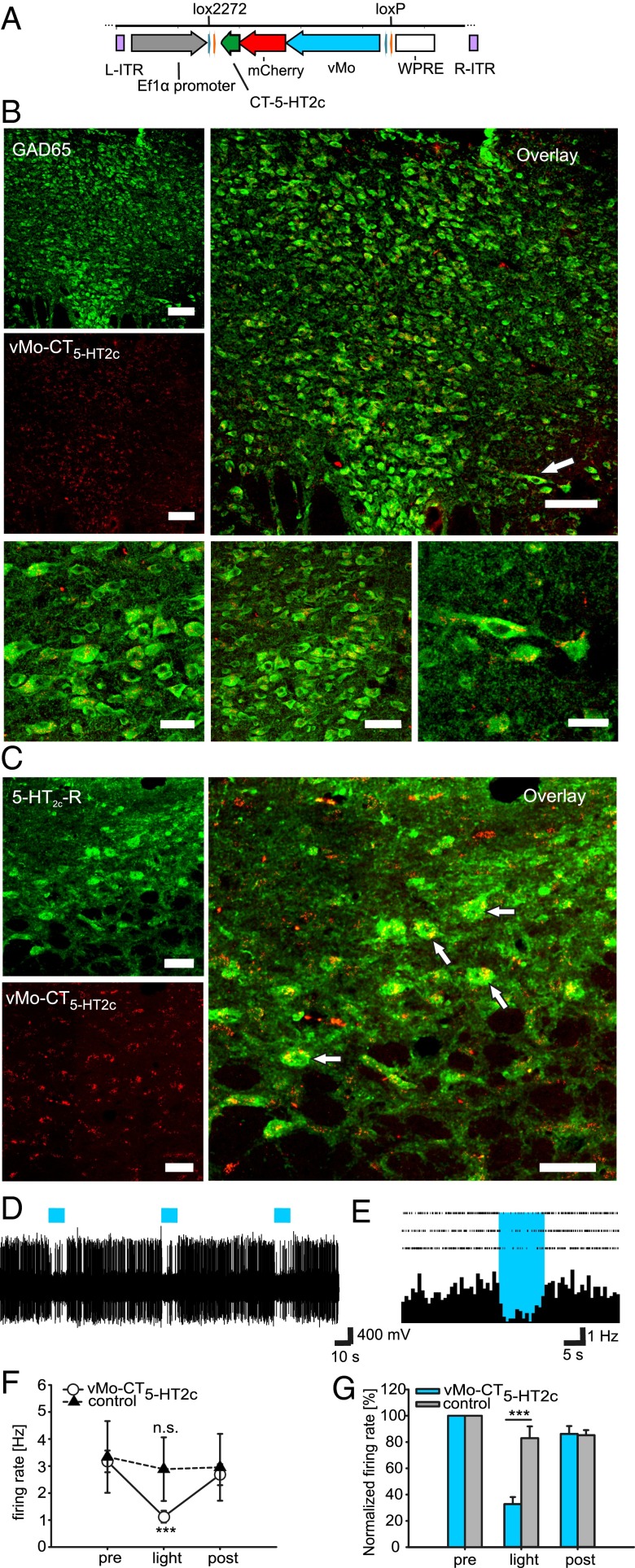
To investigate the functional consequence of 5-HT2c-R activation in GABAergic neurons in the DRN, we engineered an adeno-associated viral vector (AAV) with double-floxed vMo-CT5-HT2c under the control of the EF1α promoter to produce infectious virus particles (Fig. 3A). To selectively target GABAergic neurons, AAVs were stereotactically injected into the DRN of GAD65-Ires-Cre mice (expressing cre-recombinase in 65-kDa isoform-positive GABAergic cells). We first confirmed that vMo-CT5-HT2c is restricted to GAD65-positive GABAergic neurons (Fig. 3B) and is colocalized with the endogenous 5-HT2c-R. The colocalization of native and optogenetic GPCRs in vesicular structures indicates that Gq signals are activated in cellular domains in which 5-HT2c-Rs are localized (Fig. 3C and Fig. S1).
Fig. 3.
vMo-CT5-HT2c colocalizes with 5-HT2c-Rs in dorsal raphe GABAergic neurons and repetitively modulates neuronal firing after light-activation. (A) AAV expression vector carrying inverted vMo-CT5-HT2c (AAV-DIO-vMo-CT5-HT2c) under the control of EF-1α promoter for cre-dependent expression in dorsal raphe GABAergic cells. ITR, inverted terminal repeat; WPRE, woodchuck hepatitis B virus posttranscriptional element. (B and C) vMo-CT5-HT2c is expressed in GABAergic neurons and colocalizes with endogenous 5-HT2c-Rs. (B) (Upper Left) GAD65 immunostaining (green) indicating tissue-specific expression of vMo-CT5-HT2c (red) on dorsal raphe GABAergic cells. (Upper Right) Overlay of GAD65 and vMo-CT5-HT2c. (Lower) Higher-magnification images of individual cells and example cell marked with arrowheads in the overlay. (C) Endogenous 5-HT2c-Rs (green) colocalizing with vMo-CT5-HT2c (red) in dorsal raphe GABAergic cells. White arrows indicate colocalization between native HT2c-Rs and vMo-CT5-HT2c in exemplary cells. (Scale bars: 100 µm in B, Upper and C; 50 µm in B, Lower Center; 30 µm in B, Lower Left and Right.) (D) Single trace from in vivo optrode recordings from dorsal raphe serotonergic cells with light stimulation (blue bar; 10 s) of vMo-CT5-HT2c. (E) PSTH (1-s bins) and raster plot of three sweeps. (F) Changes in DR 5-HT firing rate before (pre), during (light), and after (post) light stimulation for control (AAV-eGFP: n = 5 cells from one animal; black triangles) and vMo-CT5-HT2c (n = 12 cells from three animals; white circles). Numerical data are provided in the text. (G) Normalized firing rate for recorded 5-HT cells. Light activation of dorsal raphe GABAergic cells significantly decreased 5-HT cell firing compared with control recordings. Numerical data are provided in the text.
We next obtained extracellular multiunit recordings from 5-HT cells using an optrode (Materials and Methods) to demonstrate that activation of vMo-CT5-HT2c modulates 5-HT firing consistent with the pharmacologic manipulations (Fig. 2) (11, 12). Fig. 3D shows a typical result recorded from exemplary 5-HT neurons over three stimulation periods with the appropriate peristimulus time histogram (PSTH) (Fig. 3E). Population data from all recorded 5-HT neurons indicate that optogenetic activation of DRN GABAergic cells (Fig. S2) significantly decreased 5-HT cell firing during a 10-s blue light pulse (baseline firing rate, 3.17 ± 0.4 Hz; light firing rate, 1.12 ± 0.22 Hz; P < 0.001) (Fig. 3F, white circles). The decreased firing rates persisted over the entire stimulation period before returning to baseline activity (post, 2.68 ± 0.39 Hz). Repetitive light stimulation induced comparable inhibition over time. No significant differences in the firing rate were detected for the control virus expressing eGFP during light stimulation (baseline firing rate, 3.34 ± 1.33 Hz; light firing rate, 2.88 ± 1.18 Hz; P = 0.184) (Fig. 3F, black triangles). Thus, optogenetic activation of the Gq pathway in DRN GABAergic neurons was sufficient to transiently decrease 5-HT cell firing to 33 ± 5%, as evidenced by normalized population data (P < 0.001) (Fig. 3G).
Light Activation of vMo-CT5-HT2c Expressed in Dorsal Raphe GABAergic Neurons Relieves Anxiety in Mice via Down-Regulation of 5-HT Cell Firing.
The findings of our pharmacologic and optogenetic experiments confirm that activation of postsynaptic 5-HT2c-Rs contributes to the inhibition of 5-HT cell firing and are consistent with earlier studies emphasizing extensive synaptic interactions between GABAergic and 5-HT neurons in the DRN (13). Based on this knowledge, we predicted that 5-HT2c-R–mediated activation of DRN GABAergic neurons might influence anxiety-like behavior in freely moving mice. Given that 5-HT2c-R is a main therapeutic target for anxiety disorders (3), we chose two well-established anxiety tests, the novelty-suppressed feeding (NSF) test and the open-field test (OFT), to specifically investigate the influence of Gq signals in 5-HT2c-R domains in DRN GABAergic neurons on anxious behavior (Fig. 4).
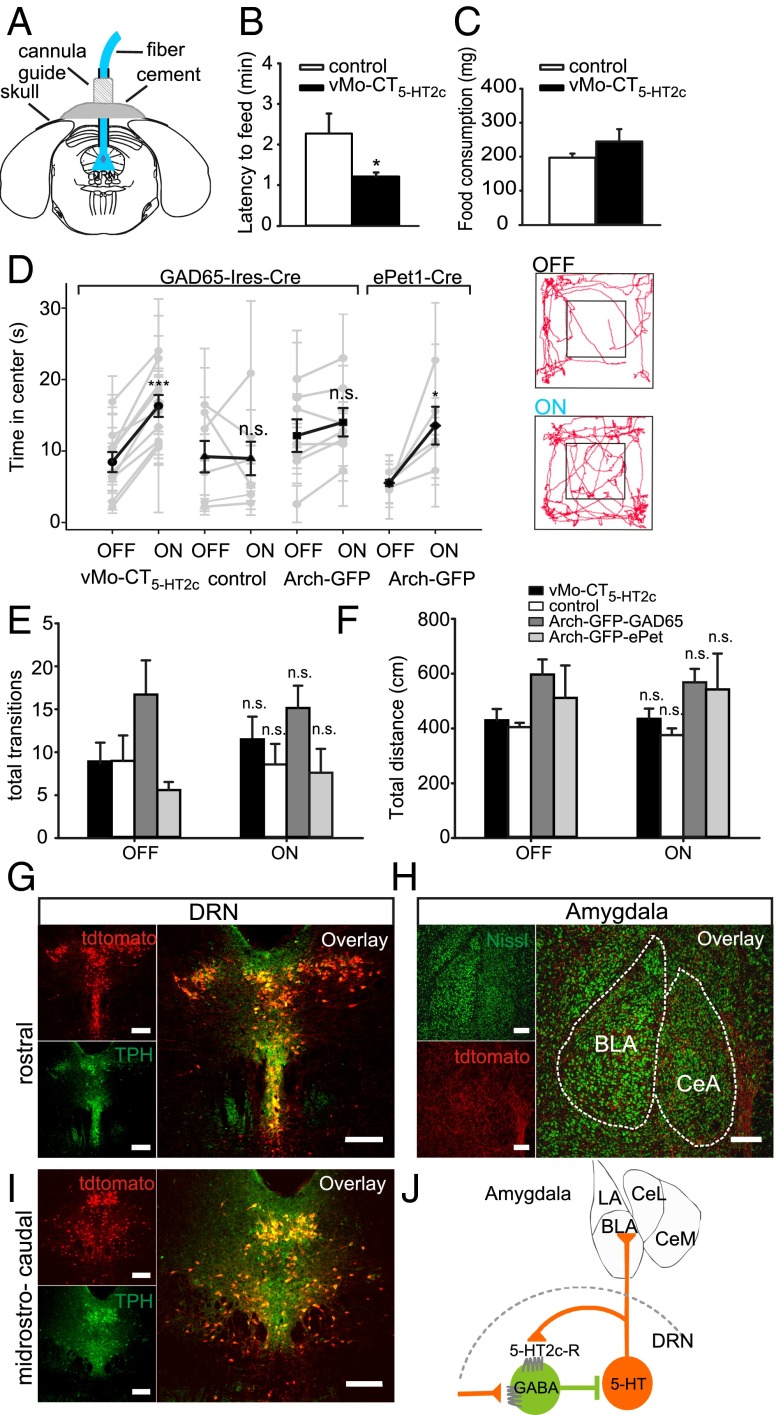
Fig. 4.
Light activation of vMo-CT5-HT2c specifically in GABAergic neurons relieves anxiety behavior through inhibition of serotonergic neurons. (A–C) NSF. (A) Stereotactic targeting of the DRN through a cannula guide in vivo. (B) Constant illumination with blue light significantly decreased latency to feed in a novel environment. Control group (white), n = 4 animals; vMo-CT5-HT2c group (black), n = 5 animals. (C) Food consumption during NSF was not altered in both groups. (D–F) OFT. (D) Comparison of center time duration in the OFT without light stimulation (OFF) and with light stimulation (ON). Individual traces (light gray) and mean center time duration (black) for GAD65-Ires-Cre mice injected with AAV-DIO-vMo-CT5-HT2c (n = 10 animals), AAV-eGFP (n = 7 animals), or AAV-flex-Arch-GFP (n = 7 animals) and ePet1-Cre mice injected with AAV-flex-Arch-GFP (n = 5 animals). Numerical data are provided in the text. (Right) Exemplary paths demonstrating increased center time in the OFT during light-on epochs for a vMo-CT5-HT2c–injected animal. (E and F) Border-to-center transitions and total distance traveled in the OFT for control (white; n = 7 animals), vMo-CT5-HT2c in GAD65-Ires-Cre–expressing mice (black; n = 10 animals), Arch-GFP in GAD65-Cre–expressing mice (dark gray; n = 7 animals), and Arch-GFP in ePet1-Cre–expressing mice (light gray; n = 5 animals) without (OFF) and with (ON) constant blue light stimulation. (G–I) DRN serotonergic projections into the amygdala. Serotonergic projection sites in ePet1-Cre transgenic mice were analyzed using AAV2/1.CAG.FLEX.tdTomato.WPRE. bGH tracer virus. (G and I) tdTomato expression (red) at two distinct dorsal raphe sites (rostral and midrostrocaudal). Colabeling for TPH (green) shows restricted expression in 5-HT containing cells (yellow). (H) tdTomato expression of 5-HT containing nerve terminals in the amygdala (red) of an ePet1-Cre transgenic mouse colabeled with fluorescent Nissl (green). The overlay shows 5-HT innervation sites in the basolateral amygdala (BLA) and central amygdala (CeA); boundaries are outlined by dashed lines. (J) Schematic representation of a purposed dorsal raphe–amygdala circuit comprising 5-HT2c-Rs to control anxious behavior. LA, basolateral amygdaloid nucleus, CeL, central amygdaloid nucleus, lateral devision, CeM, central amygdaloid nucleus, medial devision. (Scale bars: 200 µm in G–I.)
At 2–6 wk after virus injection and implantation, GAD65-Ires-Cre mice were coupled to a fiber patch cord to specifically target the DRN (Fig. 4A). 5-HT2c-R–mediated activation of dorsal raphe GABAergic neurons significantly reduced the latency to feed in a novel environment for the vMo-CT5-HT2c group relative to the control group (vMo-CT5-HT2c group; 1.21 ± 0.1 min; control group: 2.27 ± 0.49 min; P = 0.049) (Fig. 4B), with no significant changes in food consumption (vMo-CT5-HT2c group: 244.6 ± 36.49 mg; control group: 197.1 ± 12.16 mg; P = 0.556) (Fig. 4C).
To provide independent support for the 5-HT2c-R circuit in anxiety-related behavior, we next investigated behavior in the OFT. In Fig. 4 D–F, a representative path from the vMo-CT5-HT2c group is shown along with population data indicating that mice expressing vMo-CT5-HT2c spent significantly more time in the center of the open field during the light stimulation period (OFF: 8.45 ± 1.41 s; ON: 16.30 ± 1.53 s; P ≤ 0.001) (Fig. 4D), whereas the control group expressing eGFP and inhibition of GABAergic neurons by light activation of archaerhodopsin from Halorubrum sp. (Arch-GFP) exhibited no significant changes in time in the center of the opne field owing to light stimulation (control: OFF, 9.2 ± 2.2 s; ON, 9.0 ± 2.34 s; P = 0.908; Arch-GFP: OFF, 12.15 ± 2.28 s; ON, 14.02 ± 1.99 s; P = 0.08) (Fig. 4D). Thus, optical activation of the Gq pathway in DRN GABAergic cells has anxiolytic effects on OFT results, indicated by increased time in the center of the field without impaired locomotion (vMo-CT5-HT2c: OFF, 429 ± 41.5 cm; ON, 435 ± 37.4 cm; P = 0.772) (Fig. 4F and Table S2), but has no significant effect on total border-to-center transitions (vMo-5-HT2c OFF, 8.7 ± 2.42; ON, 11.5 ± 2.64; P = 0.284) (Fig. 4E and Table S2).
To verify that the observed anxiolytic effect is caused by down-regulation of serotonergic cell firing, we used the transgenic ePet1-Cre mouse line, which allows for specific expression in 5-HT neurons by cre-mediated expression of double-floxed constructs (14). We used AAV-mediated expression of Arch-GFP for silencing of 5-HT activity. Under the same light-stimulation protocols as before, mice spent significantly more time in the center of the field during light stimulation (OFF, 5.53 ± 0.43 s; ON, 13.55 ± 2.64; P = 0.048) (Fig. 4D). Arch-GFP expression in ePet1-Cre mice was localized in the DRD and the lateral wings of the DRN (Fig. S3). Thus, the results indicate that the induced anxiolytic effect results from down-regulation of serotonergic cell firing by upstream modulation of 5-HT2c-Rs in GABAergic neurons.
Consequently, we analyzed dorsal raphe 5-HT forebrain innervation to the amygdala, known to be a key modulator of anxious states. To selectively target 5-HT neurons, a double-floxed tdTomato tracer virus was injected into the DRN of ePet1-Cre mice (Fig. 4 G–I). Analysis of TPH-IR cells showed that expression of the tracer was restricted to 5-HT–positive neurons throughout the rostral (Fig. 4G) and midrostrocaudal (Fig. 4I) parts of the DRN. Projection sites have been identified in the basolateral amygdala (BLA) and central nucleus of the amygdala (CeA) (Fig. 4H). Our tracing results are consistent with those of previous studies, emphasizing the amygdala as important target of serotonergic forebrain innervation. In Fig. 4J, electrophysiological, behavioral, and immunohistochemical data are summarized in a schematic representation of a presumed dorsal raphe–amygdala circuit including 5-HT2c-Rs to control anxious behavior.
Discussion
Previous investigations of 5-HT2c-R function in the brain have relied mainly on pharmacologic approaches together with systematic drug administration; however, chemical compounds have only limited specificity for distinct receptor subtypes and distinct cell types. Moreover, the 5-HT2c-R activation kinetics after drug application are slow and almost irreversible, hindering interpretation of behavioral and physiological responses. Thus, activation of 5-HT2c-Rs with defined stimulation patterns and in specific cell types in the mammalian CNS to dissect 5-HT2cR function had not been addressed until now.
To overcome this problem, we have developed a chimeric construct based on the light-activated, Gq-coupled GPCR vMo tagged with the CT of 5-HT2c-R to reliably and repetitively activate Gq signals in cellular compartments where native 5-HT2c-Rs are localized. The advantage of vMo-CT5-HT2c compared with other optogenetic tools, such as ChR2, is that physiologically meaningful signaling cascades are controlled and activated to modulate neuronal circuits rather than switching neuronal circuits on or off. Using this construct, we were able to analyze the effect of 5-HT2c/Gq-mediated pathway activation in the DRN on anxiety-related behavior. Our behavioral data suggest that specific activation of Gq signals in 5-HT2c-R domains on GABAergic DRN neurons fine-tunes anxiety behavior in mice, most likely via down-regulation of 5-HT neuron activity.
Several lines of evidence indicate that reduction of serotonergic neurotransmission in the DRN can have anxiolytic effects, including infusion of drugs expected to decrease serotonergic activity, such as 5-HT1A-R agonists and GABA (15). GABA most likely inhibits serotonergic projection neurons located in the dorsal raphe as part of a negative-feedback loop, which can be activated by 5-HT via 5-HT2c-Rs. This hypothesis is supported by our data showing that 5-HT2c-R immunoreactivity is most abundant on GABAergic neurons in a subregion of the DRN, the lwDR (Fig. 2). The lwDR includes a large population of GABAergic interneurons that appear to be important in the regulation of serotonergic tone of the midrostrocaudal region of the DRD (16, 17). These GABAergic neurons most likely receive serotonergic input from recurrent collaterals and/or axon terminals from other 5-HT neurons within the dorsal raphe or from other raphe nuclei (4, 18, 19). The projection area of DRD neurons, the BLA (20), has been implicated as part of the anxiety circuit (Fig. 4 G–J). Thus, based on our results, we hypothesize that 5-HT neurons projecting to the amygdala are under control of neighboring GABAergic cells expressing 5-HT2c-Rs as part of a negative-feedback loop. Thus, depending on the cellular site of action and the receptor subtype involved (i.e., 5-HT2c or GABAA-R), a reduction in serotonin release via GABAergic neurons might attenuate anxiety-related behavior (15, 21–23).
Taken together, our findings suggest that Gq pathway activation in 5-HT2c-R domains in GABAergic neurons in the DRN provides a fine-tuning mechanism in anxiety-related circuits. With the aid of the light-activated GPCRs, which activate G protein signals in cellular compartments where 5-HT receptors are localized, we have created a powerful tool for investigating the function of 5-HT2c-R signaling and its contributions to various neuropsychiatric diseases in different brain regions.
Materials and Methods
Construction of vMo, vMo-CT5-HT2c, and 5-HT2c-eGFP and AAV2 Virus Production, Surgery, Virus Injection, and Implantation.
Human vMo (cDNA clone BC113558.1; Source BioScience) was cloned into pmCherry-N1 vector. The 5-HT2c-R cDNA (Missouri S&T cDNA Resource Center) was cloned into a peGFP-N1 vector. The C terminus of the 5-HT2c-R was fused to the mCherry-tagged vMo using fusion PCR to create vMo-CT5-HT2c. DIO viral vectors were created for cell type-specific expression of vMo-CT5-HT2c (AAV-DIO-vMo-CT5-HT2c) in Cre recombinase driver mouse lines. AAV expression vectors (Stratagene) were modified using a Gateway Vector Conversion System (Life Technologies). Recombinant AAV stocks, serotype 2, were produced according to the AAV Helper-Free System manual (Stratagene). Detailed information on primers, cloning, virus production, surgery, virus injection, and implantation is provided in SI Materials and Methods.
Fluo-4 Calcium Assay.
HEK tsA-201 cells (American Type Culture Collection) were transiently transfected with vMo, vMo-CT5-HT2c, and 5-HT2c-eGFP. At 3 d after transfection, the release of intracellular Ca2+ was measured using the Ca2+ indicator dye Fluo-4 (Fluo-4 Direct Calcium Assay Kit; Life Technologies) on a multilabel plate reader (Victor X3; PerkinElmer). The calcium assay is described in more detail in SI Materials and Methods.
In Vivo Extracellular Recordings and Optical Stimulation, Microiontophoresis, Spike Sorting, and Data Analysis.
For extracellular in vivo recordings, anesthetized mice were placed into a stereotactic frame, and 200- µm-diameter custom-made optrodes coupled to a blue diode-pumped laser were lowered into the brain. Single-unit and multiunit potentials were amplified, filtered, and stored. For the test, 10-s blue light pulses were applied. One trial lasted 70 s, including a 30-s baseline recording, a 10-s blue light pulse, and an additional 30-s baseline recording. Ten trials were recorded for each cell. After the experiments, mice were perfused with 4% paraformaldehyde for histological analysis.
Microiontophoresis experiments were performed using combined three-barrel recording/iontophoresis carbon fiber electrodes (Carbostar-3; Kation Europe). All drugs (Sigma-Aldrich) were prepared in 0.9% NaCl (pH 4) and delivered using a constant-current pump (Union-40 iontophoresis pump; Kation Scientific). Data were analyzed offline using Spike2 version 7 (Cambridge Electronic Design). More information on recordings, microiontophoresis, and analysis is provided in SI Materials and Methods.
Immunohistochemistry and Image Analysis.
Immunohistochemical localization of 5-HT2c receptors, tryptophan-hydroxylase, and GAD65 in the DRN and tracer studies with tdTomato were performed as described in SI Materials and Methods.
Behavioral Assays.
For the behavioral tests, male and female mice age 2–6 mo were injected stereotactically with virus solution and implanted as described in SI Materials and Methods. Mice were caged individually and allowed to recover for at least 2 wk before behavioral testing. The mice were moved from the holding room to the behavior room at least 1 h before testing to allow for acclimatization. The OFT and the NSF test were performed as described in SI Materials and Methods.
All experiments were approved by the Institutional Animal Research Facility.
Supplementary Material
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft Grants MA 4692/3-1 (to O.A.M.) and He2471/8-1, He2471/12-1 Priority Program (SPP1665), and SFB874 (to S.H.) and by National Institutes of Health Grants P50 MH096972 and R01 MH062723 (to E.S.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321576111/-/DCSupplemental.
References
- 1.Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8(4):233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- 2.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38(8):1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 3.Millan MJ. Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: Focus on novel therapeutic strategies. Therapie. 2005;60(5):441–460. doi: 10.2515/therapie:2005065. [DOI] [PubMed] [Google Scholar]
- 4.Sharp T, Boothman L, Raley J, Quérée P. Important messages in the “post”: Recent discoveries in 5-HT neurone feedback control. Trends Pharmacol Sci. 2007;28(12):629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Sanders-Bush E, Burris KD, Knoth K. Lysergic acid diethylamide and 2,5-dimethoxy-4-methylamphetamine are partial agonists at serotonin receptors linked to phosphoinositide hydrolysis. J Pharmacol Exp Ther. 1988;246(3):924–928. [PubMed] [Google Scholar]
- 6.Isoldi MC, Rollag MD, Castrucci AM, Provencio I. Rhabdomeric phototransduction initiated by the vertebrate photopigment melanopsin. Proc Natl Acad Sci USA. 2005;102(4):1217–1221. doi: 10.1073/pnas.0409252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsunematsu T, Tanaka KF, Yamanaka A, Koizumi A. Ectopic expression of melanopsin in orexin/hypocretin neurons enables control of wakefulness of mice in vivo by blue light. Neurosci Res. 2013;75(1):23–28. doi: 10.1016/j.neures.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Oh E, Maejima T, Liu C, Deneris E, Herlitze S. Substitution of 5-HT1A receptor signaling by a light-activated G protein-coupled receptor. J Biol Chem. 2010;285(40):30825–30836. doi: 10.1074/jbc.M110.147298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allers KA, Sharp T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience. 2003;122(1):193–204. doi: 10.1016/s0306-4522(03)00518-9. [DOI] [PubMed] [Google Scholar]
- 10.Challis C, et al. Raphe GABAergic neurons mediate the acquisition of avoidance after social defeat. J Neurosci. 2013;33(35):13978–13988, 13988a. doi: 10.1523/JNEUROSCI.2383-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boothman L, Raley J, Denk F, Hirani E, Sharp T. In vivo evidence that 5-HT(2C) receptors inhibit 5-HT neuronal activity via a GABAergic mechanism. Br J Pharmacol. 2006;149(7):861–869. doi: 10.1038/sj.bjp.0706935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quérée P, Peters S, Sharp T. Further pharmacological characterization of 5-HT(2C) receptor agonist-induced inhibition of 5-HT neuronal activity in the dorsal raphe nucleus in vivo. Br J Pharmacol. 2009;158(6):1477–1485. doi: 10.1111/j.1476-5381.2009.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang QP, Ochiai H, Nakai Y. GABAergic innervation of serotonergic neurons in the dorsal raphe nucleus of the rat studied by electron microscopy double immunostaining. Brain Res Bull. 1992;29(6):943–948. doi: 10.1016/0361-9230(92)90169-x. [DOI] [PubMed] [Google Scholar]
- 14.Scott MM, et al. A genetic approach to access serotonin neurons for in vivo and in vitro studies. Proc Natl Acad Sci USA. 2005;102(45):16472–16477. doi: 10.1073/pnas.0504510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins GA, Bradbury AJ, Jones BJ, Oakley NR. Behavioural and biochemical consequences following activation of 5HT1-like and GABA receptors in the dorsal raphé nucleus of the rat. Neuropharmacology. 1988;27(10):993–1001. doi: 10.1016/0028-3908(88)90058-5. [DOI] [PubMed] [Google Scholar]
- 16.Hale MW, Lowry CA. Functional topography of midbrain and pontine serotonergic systems: Implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl) 2011;213(2-3):243–264. doi: 10.1007/s00213-010-2089-z. [DOI] [PubMed] [Google Scholar]
- 17.Calizo LH, et al. Raphe serotonin neurons are not homogenous: Electrophysiological, morphological and neurochemical evidence. Neuropharmacology. 2011;61(3):524–543. doi: 10.1016/j.neuropharm.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lechin F, van der Dijs B, Hernández-Adrián G. Dorsal raphe vs. median raphe serotonergic antagonism. Anatomical, physiological, behavioral, neuroendocrinological, neuropharmacological and clinical evidence: Relevance for neuropharmacological therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(4):565–585. doi: 10.1016/j.pnpbp.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 19.Bagdy E, Kiraly I, Harsing LG., Jr Reciprocal innervation between serotonergic and GABAergic neurons in raphe nuclei of the rat. Neurochem Res. 2000;25(11):1465–1473. doi: 10.1023/a:1007672008297. [DOI] [PubMed] [Google Scholar]
- 20.Muller JF, Mascagni F, McDonald AJ. Serotonin-immunoreactive axon terminals innervate pyramidal cells and interneurons in the rat basolateral amygdala. J Comp Neurol. 2007;505(3):314–335. doi: 10.1002/cne.21486. [DOI] [PubMed] [Google Scholar]
- 21.Maier SF, Busch CR, Maswood S, Grahn RE, Watkins LR. The dorsal raphe nucleus is a site of action mediating the behavioral effects of the benzodiazepine receptor inverse agonist DMCM. Behav Neurosci. 1995;109(4):759–766. doi: 10.1037//0735-7044.109.4.759. [DOI] [PubMed] [Google Scholar]
- 22.Sena LM, et al. The dorsal raphe nucleus exerts opposed control on generalized anxiety and panic-related defensive responses in rats. Behav Brain Res. 2003;142(1-2):125–133. doi: 10.1016/s0166-4328(02)00399-6. [DOI] [PubMed] [Google Scholar]
- 23.Sidor MM, Amath A, MacQueen G, Foster JA. A developmental characterization of mesolimbocortical serotonergic gene expression changes following early immune challenge. Neuroscience. 2010;171(3):734–746. doi: 10.1016/j.neuroscience.2010.08.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.