Significance
Persistent hepatitis C virus (HCV) infection is associated with mitochondrial liver injury. Mitochondrial quality control is established as a physiological adaptation to mitochondrial injury. This study provides a new insight into how HCV disrupts mitochondrial dynamics and evades apoptosis and innate immunity to sustain persistent viral infection. HCV promoted dynamin-related protein 1-mediated mitochondrial fission, followed by mitophagy. Interference of HCV-induced mitochondrial fission and mitophagy led to the suppression of virus secretion, a decrease in glycolysis and ATP generation, an increase in interferon synthesis, and an increase in apoptotic death of infected cells via enhanced apoptotic signaling. These observations implicate the functional relevance of altered mitochondrial dynamics in the pathogenesis of chronic liver disease associated with HCV infection.
Keywords: HCV persistence, innate immunity, autophagy
Abstract
Mitochondrial dynamics is crucial for the regulation of cell homeostasis. Our recent findings suggest that hepatitis C virus (HCV) promotes Parkin-mediated elimination of damaged mitochondria (mitophagy). Here we show that HCV perturbs mitochondrial dynamics by promoting mitochondrial fission followed by mitophagy, which attenuates HCV-induced apoptosis. HCV infection stimulated expression of dynamin-related protein 1 (Drp1) and its mitochondrial receptor, mitochondrial fission factor. HCV further induced the phosphorylation of Drp1 (Ser616) and caused its subsequent translocation to the mitochondria, followed by mitophagy. Interference of HCV-induced mitochondrial fission and mitophagy by Drp1 silencing suppressed HCV secretion, with a concomitant decrease in cellular glycolysis and ATP levels, as well as enhanced innate immune signaling. More importantly, silencing Drp1 or Parkin caused significant increase in apoptotic signaling, evidenced by increased cytochrome C release from mitochondria, caspase 3 activity, and cleavage of poly(ADP-ribose) polymerase. These results suggest that HCV-induced mitochondrial fission and mitophagy serve to attenuate apoptosis and may contribute to persistent HCV infection.
Hepatitis C virus (HCV) infection often leads to chronic hepatitis that can progress to fibrosis, cirrhosis, and hepatocellular carcinoma (1). HCV is a hepatotropic, noncytopathic (2, 3), single-stranded, positive-sense RNA virus that replicates its RNA genome on the endoplasmic reticulum (ER)-derived membranous structures (4, 5). HCV stimulates lipogenesis, leading to the accumulation of lipid droplets that facilitate virion assembly and maturation (5–8). HCV infection also induces mitochondrial dysfunction via ER and oxidative stress that results in mitochondrial Ca2+ overload, collapse of mitochondrial transmembrane potential (ΔΨm), elevated levels of reactive oxygen species, and disruption of mitochondrial respiration (9–15). Liver tissues of patients with chronic hepatitis C frequently exhibit traits of mitochondrial injury such as swollen, ruptured, and empty mitochondria (16).
Mitochondria are dynamic organelles that constantly undergo fission, fusion, and mitophagy to facilitate mitochondrial quality control, which is crucial for maintaining cell viability and bioenergetics (17). Aberrant mitochondrial dynamics are associated with the pathogenesis of several genetic and neurological disorders, cardiac dysfunctions, cancer, and metabolic diseases such as diabetes and obesity (18). Depending on their physiological and cellular context, the balance between mitochondrial fission and fusion processes modulates the mitochondrial morphology (17). Mitochondrial fission/fragmentation is mediated by recruitment of cytosolic Drp1 to the mitochondria, forming spirals that constrict both the inner and outer mitochondrial membranes (19). The mitochondrial fission is modulated by mitochondrial outer membrane proteins, which include mitochondrial fission 1 (Fis1), mitochondria fission factor (Mff), and mitochondrial dynamics proteins of 49 and 51 kDa. These proteins coordinate to recruit Drp1 to mitochondria (20, 21). Mitochondrial fusion involves mitofusin 1 and 2 proteins and the inner mitochondrial membrane protein optic atrophy 1 (19, 21). More specifically, Drp1 recruitment to mitochondria is regulated by phosphorylation and dephosphorylation of respective serine residues by putative kinases and phosphatases (19). Mitochondrial dynamics is tightly regulated in response to alterations in cellular physiology such as stress, infections, and nutrient supply, and is also shown to play a critical role in apoptosis (18, 22).
In this study, we investigated the HCV-induced modulation of mitochondrial dynamics, which plays a crucial role in attenuating apoptosis of infected cells resulting from mitochondrial injury associated with infection. We show that HCV stimulates the gene expression of Drp1 and Mff and promotes Drp1 recruitment to mitochondria by stimulating the phosphorylation of Drp1 (Ser616), leading to mitochondrial fission analyzed by confocal and electron microscopy. By using a dual-fluorescence mito-monomeric red fluorescent protein (mRFP)-EGFP reporter for monitoring complete mitophagy, we demonstrate that HCV-induced Drp1-mediated mitochondrial fission was followed by mitophagy. Interference of HCV-induced mitochondrial fission by silencing either Drp1 or Mff led to the accumulation of swollen mitochondria that resisted mitophagic degradation. Interestingly, interference of mitochondrial fission also suppressed viral secretion and glycolysis paralleled by a concomitant decline in cellular ATP levels and increased IFN synthesis in the HCV-infected cells. More importantly, inhibition of HCV-induced aberrant mitochondrial fission and mitophagy triggered robust apoptosis evidenced by a marked increase in cytochrome C release, caspase 3 activity, and cleavage of poly(ADP-ribose) polymerase. These observations unambiguously implicate the functional relevance of mitochondrial dynamics in the maintenance of persistent HCV infection.
Results
HCV Promotes Mitochondrial Fission.
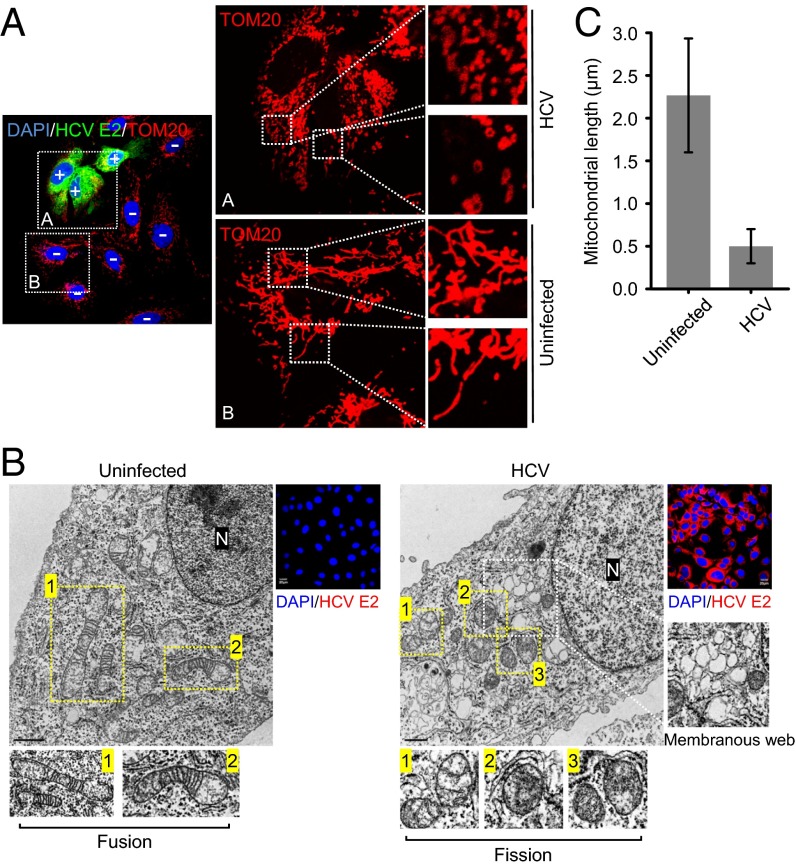
HCV infection induces ER and oxidative stress and alters calcium homeostasis, which causes mitochondrial dysfunction and damage (23–25). To investigate the HCV-induced alterations of mitochondrial dynamics, metabolism, and physiology, human hepatoma (Huh7) cells were infected with cell culture-derived HCV (HCVcc) of JC1 strain [genotype2a chimera of J6 and Japanese fulminant hepatitis (JFH) 1 strains]. As shown in Fig. 1A, HCV-infected cells displayed distinct fragmented mitochondria (mitochondrial fission), in contrast to uninfected cells, which displayed a typical tubular mitochondrial network indicative of normal healthy cells. We also observed similar mitochondrial fragmentation in cells infected with the HCV JFH1 strain, which replicates with lower efficiency compared with the HCV JC1 strain (SI Appendix, Fig. S1). This confirms that mitochondrial fission is a consequence of HCV infection and is not selectively induced in JC1-infected cells because of its higher replication rates. Ultrastructural analysis of HCV-infected cells by transmission electron microscopy further substantiated the occurrence of fragmented mitochondria in HCV-infected cells, in contrast to uninfected cells, which displayed elongated and tubular mitochondria (Fig. 1B). Also seen in Fig. 1B are the membranous web-like structure and features of mitochondrial injury such as swollen mitochondria devoid of mitochondrial cristae. A quantitative analysis of relative mitochondrial length in uninfected versus HCV-infected Huh7 cells is presented in Fig. 1C. These analyses demonstrate that HCV infection induces mitochondrial fission.
Fig. 1.
HCV infection induces mitochondrial fission. (A) Immunofluorescence analysis showing mitochondrial fission in HCV-infected cells. At 3 d postinfection, Huh7 cells infected with HCVcc (MOI, 1) were immunostained with antibodies specific to translocase of the outer mitochondrial membrane 20 (TOM20) (red) and HCV E2 protein (green). Nuclei, DAPI (blue). Infected cells, +; uninfected cells, −. The zoomed images display the typical tubular mitochondrial network in uninfected cells and fragmented mitochondria in infected cells, respectively. (B) Electron microscopy of HCV-infected Huh7 cells showing the fragmented mitochondria. The ultrastructure of uninfected (naive Huh7) and infected (HCV) cells was examined by electron microscope, as described in Materials and Methods. In the zoomed images, normal elongated tubular mitochondria in uninfected cells and fragmented mitochondria with loss of cristae in infected cells are shown. Membranous, web-like structure in infected cells is also shown. (Scale bar, uninfected, 1 μm; infected, 0.5 μm.) Organelle marker: N, nucleus. Confocal images: HCV E2, red; nuclei, blue (DAPI). (C) Quantification of mitochondrial length shown in B.
HCV Induces Drp1 Ser616 Phosphorylation and Mitochondrial Translocation.
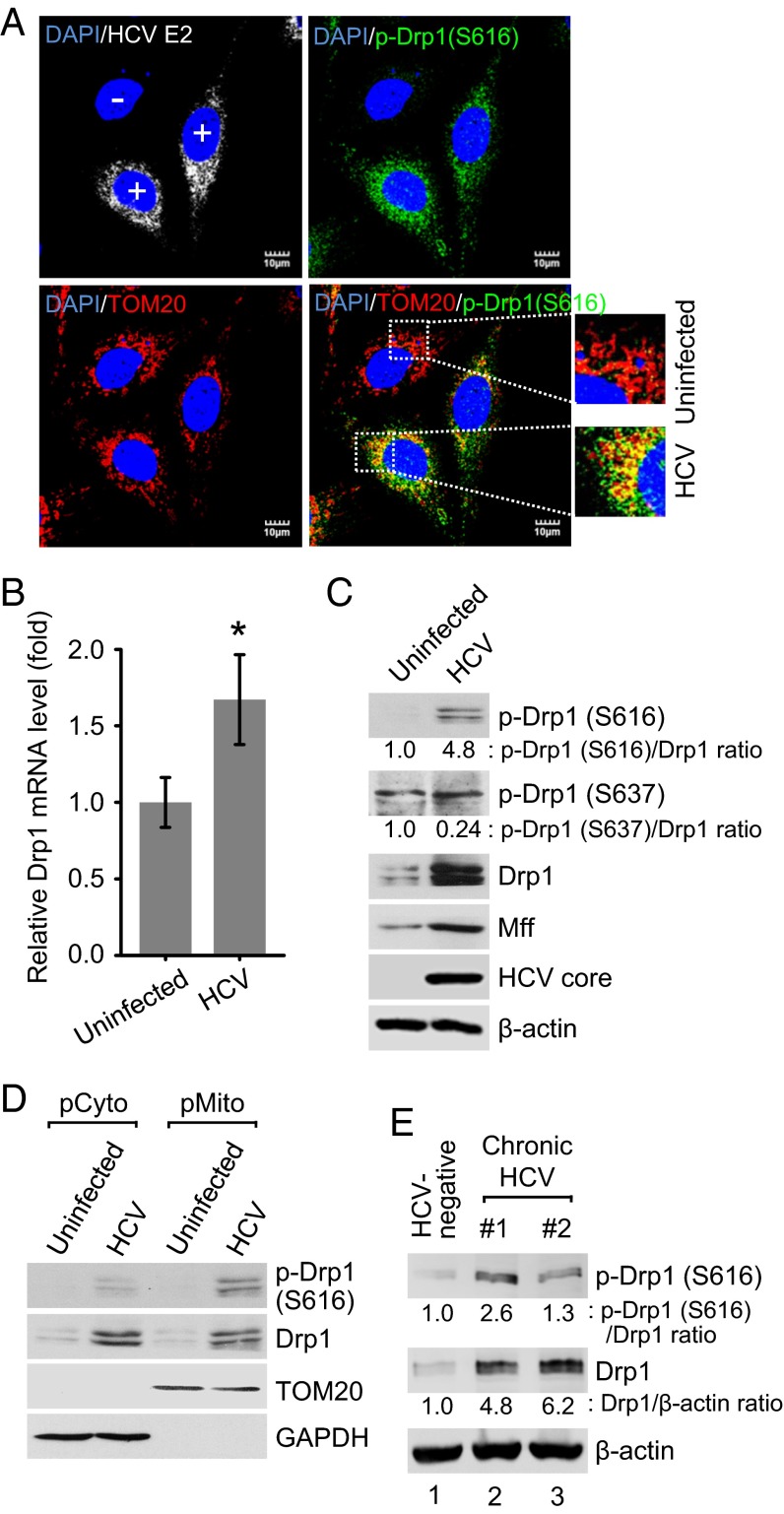
Drp1 recruitment to mitochondria can be triggered by Drp1 phosphorylation at serine 616 by cyclin-dependent kinase 1 (CDK1)/cyclin B (25). HCV core protein has been previously shown to promote CDK1/cyclin B complex activity (26). To demonstrate whether HCV induces mitochondrial fission by promoting Drp1 Ser616 phosphorylation and its subsequent translocation to mitochondria, HCV-infected cells were analyzed by confocal microscopy using a specific anti-Drp1 antibody that recognizes the Ser616-phosphorylated Drp1. We observed a dramatic stimulation of Drp1 Ser616 phosphorylation in HCV-infected cells compared with uninfected cells (Fig. 2A). Confocal microscopy images also revealed that most of the Ser616 phosphorylated Drp1 localized to the mitochondria (Fig. 2A), suggesting that HCV promoted Drp1 recruitment to mitochondria by stimulating Ser616 phosphorylation. Furthermore, the siRNA-mediated interference of CDK1 also abrogated HCV-induced Drp1 S616 phosphorylation (SI Appendix, Fig. S2). Drp1 dephosphorylation at Ser637 acts in parallel with Drp1 Ser616 phosphorylation to promote mitochondrial fission (27). We examined the Drp1 Ser637 phosphorylation levels and noted a modest decrease in HCV-infected cells (Fig. 2C). In addition, we investigated whether any HCV proteins can individually induce Drp1 activation via Drp1 Ser616 phosphorylation. The results show that HCV envelope glycoprotein 2 (E2), core, and nonstructural protein 3/4A (NS3/4A) proteins only modestly induced Drp1 Ser616 phosphorylation (SI Appendix, Fig. S3). However, Drp1 Ser616 phosphorylation induced by HCV infection was significantly higher compared with the induction by each HCV protein (SI Appendix, Fig. S3). HCV stimulated Drp1 expression at both the transcriptional and translational levels (Fig. 2 B and C). HCV also stimulated the expression of Mff protein, which serves as a receptor for Drp1 recruitment to the mitochondria (Fig. 2C) (20, 28). We have previously shown that HCV down-regulated mitofusin 2, which is one of the mediators of mitochondrial fusion (11). To further substantiate the observation of HCV-induced Drp1 translocation to the mitochondria, we purified mitochondrial fractions from HCV-infected and uninfected Huh7 cells. Western blot analysis of subcellular fractions demonstrated that phosphorylated Drp1 is substantially enriched in the mitochondrial fraction in HCV-infected cells (Fig. 2D). To establish the clinical relevance of these observations, we also investigated the activation of Drp1 in the liver biopsies of patients with chronic hepatitis C and observed an increase in the expression levels of total Drp1 and enhanced Drp1 Ser616 phosphorylation (Fig. 2E). Together, these results demonstrate that HCV causes Drp1 translocation to mitochondria to initiate fission activities.
Fig. 2.
HCV stimulates the expression of Drp1 and Mff and promotes Drp1 Ser616 phosphorylation and subsequent translocation to mitochondria. (A) Immunofluorescence analysis showing the induction of Drp1 Ser616 phosphorylation and its localization on the mitochondria in HCV-infected cells. At 2 d postinfection, HCV-infected Huh7 cells were immunostained with antibodies specific to TOM20 (red), p-Drp1 (Ser616) (green), and HCV E2 protein (white). In the zoomed images, yellow spots indicate the merge of p-Drp1 (Ser616) with mitochondria. Infected cells, +; uninfected cells, −. Nuclei, DAPI (blue). (B–D) Analyses of Drp1 mRNA level (B) and protein expression (C and D). Huh7 cells infected with HCVcc (MOI, 5) were used for RNA analysis by qRT-PCR at 1 d postinfection (mean ± SD; n = 3; *P ≤ 0.01, by unpaired Student t test) (B) and protein expression at 5 d postinfection (C). (D) Western blot analysis of purified subcellular fractions from uninfected (mock) and HCV-infected cells. Fractions: purified cytosolic, pCyto; purified mitochondria, pMito. Organelle markers: TOM20, mitochondria; GAPDH, cytoplasm. (E) Western blot analysis of p-Drp1 (S616) and total Drp1 expression levels in liver biopsies of chronic hepatitis C patients. Lane 1, HCV-negative; lanes 2–3, HCV-positive. β-actin, protein-loading control. (C and E) The band intensities were analyzed by ImageJ software.
HCV Induces Autophagy of Mitochondria that Undergo Drp1-Mediated Fission.
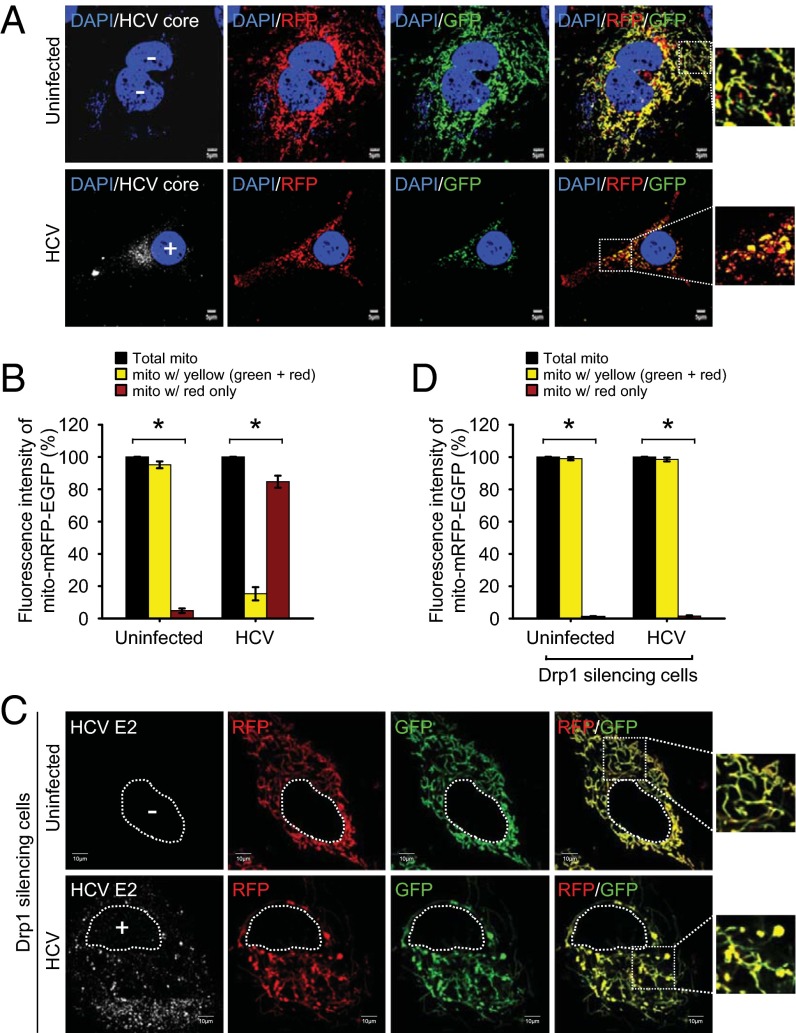
We have recently reported that HCV infection induces Parkin-dependent mitophagy (11). To examine whether Drp1-mediated mitochondrial fission facilitates mitophagy in HCV-infected cells, we used a tandem-tagged mRFP-EGFP chimeric fluorescence reporter (plasmid pAT016) encoding a mitochondrial targeting signal sequence fused in-frame with mRFP and EGFP genes (SI Appendix, Fig. S4) (29). The use of this expression scheme exploits the differential stabilities of RFP and GFP in the lysosomes (SI Appendix, Fig. S4) (29). The mito-mRFP-EGFP tagged mitochondria in the cytosol normally display a yellow color because of the merge of red and green fluorescence from mRFP and EGFP. However, on completion of mitophagy by fusion of autophagic vesicles containing mitochondria with lysosomes, only red fluorescence from mRFP is observed because of the higher stability of RFP over GFP in the acidic environment of lysosome. As shown in Fig. 3A, consistent with the typical tubular mitochondrial network in uninfected cells expressing mito-mRFP-EGFP, we observed predominantly a yellow color, indicating the merge of both green (EGFP) and red (mRFP) fluorescence from mitochondria in the cytosol, whereas in HCV-infected cells, most of the fragmented mitochondria predominantly displayed red fluorescence, which is indicative of the delivery of mitochondria (mitophagosomes) to lysosomes. The relative quantification of the number of mitochondria in the cytosol and the lysosomes is shown in Fig. 3B. We also confirmed the fusion of mitophagosomes with lysosomes by staining the mito-mRFP-EGFP-transfected HCV-infected cells with lysosomal-associated membrane protein 1 antibody (SI Appendix, Fig. S5). Delivery of mitophagosome (red) to lysosomes (blue) is shown as pink puncta (SI Appendix, Fig. S5).
Fig. 3.
HCV induces mitophagy. (A) Confocal images showing HCV-induced mitophagy. HCV-infected cells transiently expressing mito-mRFP-EGFP were immunostained with anti-HCV core antibody (white). Nuclei, DAPI (blue). Infected cells, +; uninfected cells, −. In the zoomed images, fluorescence signals indicate the expression of mito-mRFP-EGFP targeting mitochondria: yellow color, no mitophagy; red color, mitophagy. (C) Confocal images showing the inhibition of HCV-induced mitophagy by silencing Drp1. Drp1-silenced HCV-infected cells transiently expressing mito-mRFP-EGFP were immunostained with anti-HCV E2 antibody (white). Nuclei, white dots circle. Infected cells, +; uninfected cells, −. In the zoomed images, tubular mitochondria in uninfected cells and tubular/swollen mitochondria in infected cells are shown. (B and D) Quantitative analyses of the fluorescence signal targeted to mitochondria in A and C, respectively (mean ± SEM; n ≥ 10 cells; *P ≤ 0.01, by unpaired Student t test).
Mitochondrial fission leads to mitophagy by facilitating the segregation of dysfunctional mitochondria (30). Hence, we examined whether interference of mitochondrial fission affects the HCV-induced mitophagic process. HCV-infected Huh7 cells cotransfected with mito-mRFP-EGFP and Drp1-specific siRNA did not display delivery of mitochondria to lysosomes, as evidenced by the lack of distinct red puncta (Fig. 3C; see Fig. 3D for quantification), whereas cells transfected with nontargeting siRNA show red puncta, indicating mitophagy (SI Appendix, Fig. S6). In Drp1-silenced cells, both the uninfected and HCV-infected cells presented tubular mitochondria because of the absence of Drp1-dependent fission activity (Fig. 3C). In contrast, HCV-infected cells also displayed swollen mitochondria (Fig. 3C). We then examined the formation of mitophagosome in Drp1-silenced HCV-infected cells expressing GFP-tagged microtubule-associated protein 1 light chain 3 (GFP-LC3). Although we observed the punctate distribution pattern of the GFP-LC3 protein, indicative of the induction of general autophagy, we did not observe colocalization of GFP-LC3 puncta (green) with mitochondria (red), barring a few regions showing overlap (SI Appendix, Fig. S7). These results suggest that inhibition of mitochondrial fission hinders HCV-induced mitophagy and support the notion that Drp1-mediated mitochondrial fission precedes the subsequent events of mitophagy (19, 21, 30).
HCV-Induced Mitochondrial Fission Facilitates Virus Secretion and Evasion of Innate Immunity.
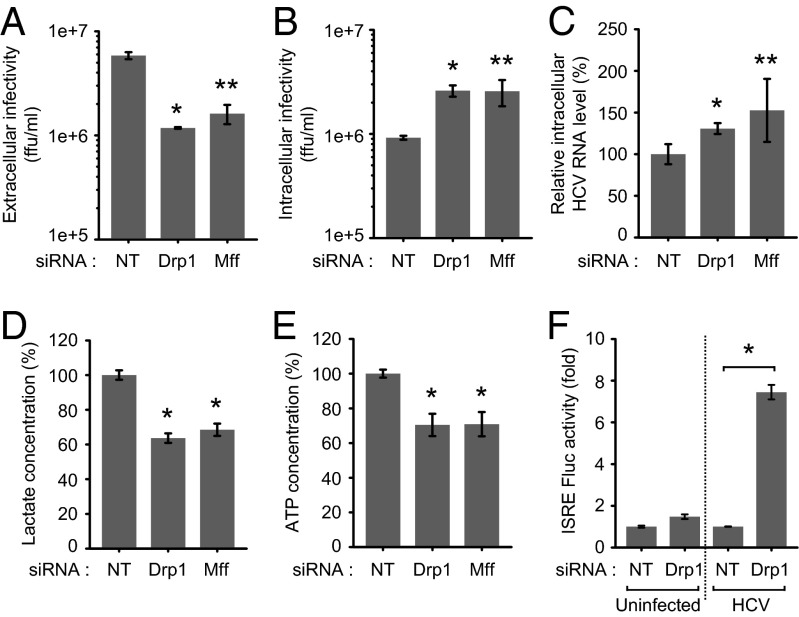
To characterize the functional significance of mitochondrial fission in the HCV infectious process, we determined the effect of inhibiting mitochondrial fission in HCV RNA replication and secretion. Huh7 cells transfected with nontargeting or gene-specific siRNA pools specific to Drp1 and Mff were infected with HCVcc at a multiplicity of infection (MOI) of 5 at 12 h posttransfection. HCV secretion was analyzed by determining the infectivity of culture medium by foci-forming unit assay, as described previously (31). HCV RNA replication was determined by quantitative RT-PCR (qRT-PCR). As shown in Fig. 4A, extracellular virus particle titer was decreased in Drp1- or Mff-silenced cells, indicating the functional role of mitochondrial fission in HCV secretion. Intracellular infectivity showed a notable increase, indicating the accumulation of mature HCV virions because of the inhibition of virus particle secretion/egress (Fig. 4B). Silencing Drp1 or Mff did not affect HCV replication (Fig. 4C). Drp1 silencing also did not affect HCV replication in subgenomic replicon-expressing cells, which are capable of autonomous replication (SI Appendix, Fig. S8). However, in HCV-infected cells, we observed an increase in the intracellular HCV RNA levels in the Drp1- or Mff-silenced cells, reflecting the accumulation of HCV RNA as a result of inhibition of secretion (Fig. 4C). Mitochondrial dynamics and quality control are tightly linked to cellular metabolic alterations and ATP levels (32). To investigate whether inhibition in HCV secretion is a result of reduction in cellular ATP levels, we determined the total ATP levels and rate of glycolysis, an alternative mode of ATP generation shown to be induced by HCV (33). As shown in Fig. 4 D and E, interference of mitochondrial fission negatively affected cellular glycolytic rates and total cellular ATP pool. We also investigated whether HCV-induced mitochondrial fission affects innate immunity, which is orchestrated at the mitochondrial level via retinoic acid-inducible gene 1–mitochondrial antiviral signaling protein (MAVS) interactions. HCV NS3/4A cleaves the MAVS protein localized to mitochondria, resulting in abrogation of the downstream signaling and IFN synthesis (34). Interestingly, interference of mitochondrial fission by Drp1 silencing significantly increased the luciferase activity under the transcriptional control of IFN-stimulated response element (ISRE), indicating that HCV-induced aberrant mitochondrial fission may also contribute in part to modulate the innate immune response (Fig. 4F). Taken together, these results suggest that HCV-induced mitochondrial fission both affects HCV secretion and contributes in part to the evasion of the innate immune system.
Fig. 4.
Inhibition of mitochondrial fission affects HCV secretion and innate immune response. (A–E) Huh7 cells transfected with nontargeting (NT) or gene-specific siRNA pools targeting Drp1 and Mff, respectively, were infected HCVcc (MOI, 5). At 3 d postinfection, culture medium and cells were used for analyses of extracellular infectivity (A) and intracellular infectivity (B) determined by Foci-forming unit assay, intracellular HCV RNA levels (C) analyzed by qRT-PCR, lactate concentration (D) determined by cell-based glycolysis assay kit, and intracellular ATP levels (E) determined by the ATP EnzyLight assay kit (mean ± SEM; n = 3; *P ≤ 0.05; **P ≤ 0.005, by unpaired Student t test). (F) Drp1 silencing increases ISRE promoter activity in HCV-infected cells. Huh7 cells cotransfected with plasmids encoding ISRE Firefly luciferase reporter and wild-type Renilla luciferase reporter were subsequently transfected with NT or Drp1-specific siRNA pools and, 12 h later, infected with HCVcc (MOI, 5). At 2 d postinfection, the relative ISRE-luciferase activity was determined as described in Materials and Methods (mean ± SD; n = 3; *P ≤ 0.01, by unpaired Student t test).
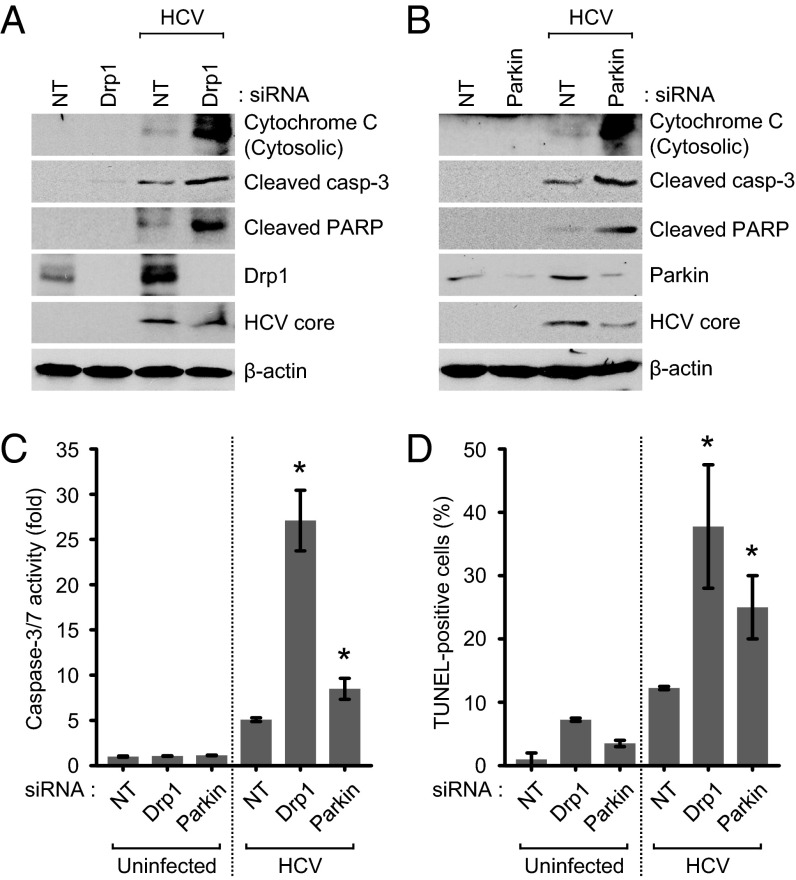
Disruption of Mitochondrial Fission and Mitophagy Lead to Induction of Apoptosis.
Mitochondrial dynamics is integrally linked to apoptosis (19, 32). Here we investigated apoptotic signaling in HCV-infected cells in which mitochondrial fission or mitophagy have been inhibited. We observed that silencing Drp1 or Mff in HCV-infected cells leads to the appearance of significant numbers of swollen/enlarged mitochondria, in contrast to uninfected cells, which mostly displayed tubular mitochondria (SI Appendix, Fig. S9). Previous reports have shown that cells depleted of mitochondrial fission machinery when subjected to oxidative stress accumulate swollen/enlarged mitochondria, which subsequently leads to the induction of apoptotic signaling initiated by cytochrome C leakage (35). Depletion of Drp1 and Parkin induced robust cytochrome C release from mitochondria and promoted activation of caspase 3/7 followed by subsequent cleavage of poly(ADP-ribose) polymerase, a caspase 3 substrate (Fig. 5 A–C). Induction of apoptosis was also substantiated by TUNEL assay (Fig. 5D), which shows accumulation of TUNEL-positive cells. HCV-infected cells not silenced for either gene did not exhibit any of the proapoptotic stimuli. Together, these results strongly suggest that HCV-mediated induction of mitochondrial fission and mitophagy, although serving as a quality control mechanism to eliminate damaged mitochondria, also protects virus-infected hepatocytes from apoptotic cell death, facilitating persistent viral infection.
Fig. 5.
HCV attenuates mitochondrial apoptosis. (A–D) Drp1 and Parkin silencing accelerates HCV-induced mitochondrial apoptotic signaling. Huh7 cells infected with HCVcc (MOI, 5) were transfected with nontargeting (NT) or gene-specific siRNA pools targeting Drp1 and Parkin, respectively, and analyzed at 3 d posttransfection by Western blot analysis using antibodies specific to the indicated proteins (A and B), caspase 3/7 activity assay (C), and TUNEL assay (D) (mean ± SD; n = 3; *P ≤ 0.05, by unpaired Student t test).
Discussion
Mitochondrial depolarization, membrane permeabilization, and swelling are common occurrences in HCV infection (36). Hence, survival of HCV-infected cells is probably dependent on their ability to clear dysfunctional mitochondria. Mitochondrial quality control maintains healthy mitochondria and cell viability. Malfunction of mitochondrial quality control results in the accumulation of defective mitochondria and leads to cell death (30). Removal of damaged mitochondria via mitophagy is recognized as the primary pathway in mitochondria quality control (19, 21, 30). Aberrations in cellular mitophagy pathway are linked to varied pathophysiological conditions such as neurodegeneration and cardiac dysfunctions (19, 21, 30).
It has been proposed that asymmetric fission/fragmentation of mitochondria helps in the segregation of depolarized mitochondria that are later targeted by the mitophagy machinery. How mitochondria achieve asymmetric fission is not known, but the notion that fission is a prerequisite for mitophagy is supported by the fact that genetic manipulation of fission proteins mitochondrial fission 1 and Drp1 by siRNA or overexpression of dominant negative Drp1 reduces mitophagy (30). In addition, tubular network formation has been shown to protect mitochondria from autophagosomal degradation during nutrient starvation (37). In correlation to our previous observations of HCV-induced mitophagy (11), here we present evidence for mitochondrial fragmentation/fission activities in HCV-infected cells (Fig. 1).
Mitochondrial fission is mediated by the mitochondrial recruitment of Drp1, which drives fission by oligomerization to form spirals that constrict mitochondria (19). We observed that HCV triggered mitochondrial recruitment of Drp1 by promoting its phosphorylation at serine 616 mediated by CDK1/cyclin B (Fig. 2). siRNA-mediated interference of CDK1 subverted HCV-induced Drp1 S616 phosphorylation (SI Appendix, Fig. S2), implicating its role in this process. In addition, we observed a modest decline in Drp1 S637 phosphorylation (Fig. 2), which is also known to promote mitochondrial recruitment of Drp1 (27). We also observed that HCV enhanced the expression levels of the fission proteins Drp1 and Mff (Fig. 2C). We have previously shown that mitofusin 2, one of the mediators of mitochondrial fusion, is ubiquitinated by Parkin and degraded in HCV-infected cells (11). Collectively, these findings suggest that HCV also selectively manipulates the expression levels of fission and fusion proteins to shift the balance of mitochondrial dynamics toward fission.
It has been proposed that asymmetric mitochondrial fission generates a segregated pool of depolarized mitochondria that are later subjected to mitophagy (30). We observed a higher number of mitochondria in lysosomes in HCV-infected cells (Fig. 3). Depletion of Drp1 during HCV infection compromises mitophagy. We did not observe colocalization between GFP-LC3 puncta and swollen mitochondria (SI Appendix, Fig. S7B), suggesting that efficient engulfment of these swollen mitochondria by phagophore is impaired, probably because of their larger size (38). The tubular mitochondrial network has also been shown to resist autophagic degradation during nutrient stress, probably because of nonproductive engulfment of continuous mitochondrial network (37).
Mitochondrial fission and mitophagy are the key determinants of mitochondrial quality control, and HCV modulates these key processes in the adaptation to cellular physiological perturbations associated with infection to promote viral persistence. Mitochondrial fission is not invariably associated with cell death but can also protect cells from death induced by oxidative stress and Ca2+-dependent apoptotic stimuli (18, 22, 38). In this study, we observed that despite high levels of mitochondrial fission in HCV-infected cells, there was no upregulation of apoptotic signaling. However, interference of mitochondrial fission and mitophagy led to a dramatic increase in apoptotic signaling (Fig. 5), suggesting that mitochondrial fission during HCV infection promotes cell survival (see model in SI Appendix, Fig. S10). Interestingly, we also observed that inhibition of mitochondrial fission resulted in the inhibition of HCV secretion with a concomitant accumulation of infective intracellular virus particles (Fig. 4). HCV infection has been previously shown to promote glycolysis (33). Hence, we investigated whether perturbing mitochondrial dynamics affects mitochondrial functions and metabolic reprogramming. Inhibition of mitochondrial fission led to a decline in glycolysis and total cellular ATP levels, which may directly affect HCV secretion (Fig. 4). Alternatively, reactive oxygen species emanating from accumulated dysfunctional mitochondria can damage lipids and proteins that could play a crucial role in HCV secretion. However, the precise molecular mechanisms underlying the role of mitochondrial dynamics in HCV secretion still remain to be fully characterized.
Viral persistence is a primary determinant of chronic viral infection and pathogenesis of associated diseases. The occurrence of immunologically distinct viral variants (quasispecies) and the intrinsic ability of HCV to abrogate cellular immunity helps to evade the immune attack and facilitate viral persistence (2). Mitochondrial dynamics have been shown to regulate innate immunity (39). Mitochondrial fusion may serve to increase the interaction of key molecules such as MAVS in downstream signaling of IFN synthesis, and mitochondrial fission may prevent these interactions (39, 40). Consistent with this idea, we observed an increase in IFN synthesis on depletion of Drp1 (Fig. 4F), suggesting that mitochondrial fission may also participate in some way in evading innate immune response, in addition to the cleavage of MAVS by HCV NS3/4A protease (34). In the case of HCV, we observed that Huh7.5 cells, which are devoid of retinoic acid-inducible gene 1 (34), maintained mitochondrial fission, suggesting that mitochondrial fission is independent of the retinoic acid-inducible gene 1 signaling pathway (SI Appendix, Fig. S11). The molecular details about how HCV-induced mitochondrial fission may affect innate immunity remains to be characterized. Apart from the immune escape, the viability of the infected cells is a primary requirement for viral persistence. Despite the infection-associated stresses and mitochondrial injury, HCV does not produce any notable cytopathic effects (2, 41). Perhaps the physiological and metabolic alterations triggered by HCV in the infected hepatocyte play a role in abating the stress associated with viral infection and prevent the manifestation of viral cytopathic effects to sustain persistent infection. The present study highlights the physiological adaptation involving up-regulation of mitochondria quality control in HCV-infected hepatocytes to sustain cellular viability and promote viral persistence. The primary cause of persistent HCV infection is a result of the failure to mount an efficient immune response to eliminate infected hepatocytes (2). However, HCV-induced aberrant mitochondrial dynamics may also contribute to the viral persistence by attenuating apoptosis of infected cells. A similar strategy of perturbation of mitochondrial dynamics and attenuation of apoptosis is also used by hepatitis B virus (29). Our findings implicate the mitochondrial quality control pathway as a potential therapeutic target against HCV infection and associated liver disease pathogenesis.
Materials and Methods
Additional information is provided in SI Appendix, Materials and Methods.
Cell Culture and Virus.
The human hepatoma cell line Huh7 was cultured as described previously (11). Cell culture-derived HCV (HCVcc) of JC1 and JFH1 strains were used in this study and prepared as described previously (11, 42).
DNA Constructs.
The pEGFP-LC3 plasmid DNA was a kind gift from Tamotsu Yoshimori (National Institute of Genetics, Japan). The p-mito-mRFP-EGFP plasmid DNA (pAT016) was described previously (29).
Immunofluorescence.
HCV-infected cells and those transfected with siRNA were grown on coverslips and used for immunofluorescence assay, as described previously (11). Images were visualized under a 60× or 100× oil objectives, using an Olympus FluoView 1000 confocal microscope.
Supplementary Material
Acknowledgments
We thank Dr. Charles Rice (Rockefeller University) for providing HCV JC1 clone and NS5A (9E10) antibody, Dr. Takaji Wakita (National Institute of Infectious Disease, Japan) for providing HCV pJFH1 plasmid, Dr. Mansun Law (The Scripps Research Institute) for providing HCV E2 (AR3A) antibody, Dr. Tamotsu Yoshimori (National Institute of Genetics, Japan) for providing pEGFP-LC3 DNA construct, Dr. Marilyn Farquhar for advice on analysis of electron microscopy, and Ying Jones and Timo Meerloo for technical assistance with electron microscopy. Electron microscopy was carried out at the University of California, San Diego (UCSD) electron microscopy facility. Confocal analysis was performed at the UCSD Neuroscience Microscopy Shared Facility (Grant P30 NS047101). This work was supported in whole or in part by National Institutes of Health Grants AI085087, DK077704, and DK08379 (to A.S.), and T32 DK07202 (to M.A.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.G. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321114111/-/DCSupplemental.
References
- 1.Pawlotsky JM. Pathophysiology of hepatitis C virus infection and related liver disease. Trends Microbiol. 2004;12(2):96–102. doi: 10.1016/j.tim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 3.Paula T, et al. New drug targets for hepatitis C and other Flaviviridae viruses. Infect Disord Drug Targets. 2009;9(2):133–147. doi: 10.2174/187152609787847749. [DOI] [PubMed] [Google Scholar]
- 4.Egger D, et al. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J Virol. 2002;76(12):5974–5984. doi: 10.1128/JVI.76.12.5974-5984.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5(6):453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- 6.Miyanari Y, et al. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007;9(9):1089–1097. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- 7.Waris G, Felmlee DJ, Negro F, Siddiqui A. Hepatitis C virus induces proteolytic cleavage of sterol regulatory element binding proteins and stimulates their phosphorylation via oxidative stress. J Virol. 2007;81(15):8122–8130. doi: 10.1128/JVI.00125-07. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Syed GH, Amako Y, Siddiqui A. Hepatitis C virus hijacks host lipid metabolism. Trends Endocrinol Metab. 2010;21(1):33–40. doi: 10.1016/j.tem.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tardif KD, Mori K, Siddiqui A. Hepatitis C virus subgenomic replicons induce endoplasmic reticulum stress activating an intracellular signaling pathway. J Virol. 2002;76(15):7453–7459. doi: 10.1128/JVI.76.15.7453-7459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong G, Waris G, Tanveer R, Siddiqui A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci USA. 2001;98(17):9599–9604. doi: 10.1073/pnas.171311298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SJ, Syed GH, Siddiqui A. Hepatitis C virus induces the mitochondrial translocation of Parkin and subsequent mitophagy. PLoS Pathog. 2013;9(3):e1003285. doi: 10.1371/journal.ppat.1003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korenaga M, et al. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J Biol Chem. 2005;280(45):37481–37488. doi: 10.1074/jbc.M506412200. [DOI] [PubMed] [Google Scholar]
- 13.Piccoli C, et al. Hepatitis C virus protein expression causes calcium-mediated mitochondrial bioenergetic dysfunction and nitro-oxidative stress. Hepatology. 2007;46(1):58–65. doi: 10.1002/hep.21679. [DOI] [PubMed] [Google Scholar]
- 14.Waris G, Siddiqui A. Hepatitis C virus stimulates the expression of cyclooxygenase-2 via oxidative stress: Role of prostaglandin E2 in RNA replication. J Virol. 2005;79(15):9725–9734. doi: 10.1128/JVI.79.15.9725-9734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Waris G, Turkson J, Hassanein T, Siddiqui A. Hepatitis C virus (HCV) constitutively activates STAT-3 via oxidative stress: Role of STAT-3 in HCV replication. J Virol. 2005;79(3):1569–1580. doi: 10.1128/JVI.79.3.1569-1580.2005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Barbaro G, et al. Hepatocellular mitochondrial alterations in patients with chronic hepatitis C: Ultrastructural and biochemical findings. Am J Gastroenterol. 1999;94(8):2198–2205. doi: 10.1111/j.1572-0241.1999.01294.x. [DOI] [PubMed] [Google Scholar]
- 17.Chan DC. Fusion and fission: Interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- 18.Liesa M, Palacín M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89(3):799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 19.Otera H, Ishihara N, Mihara K. New insights into the function and regulation of mitochondrial fission. Biochim Biophys Acta. 2013;1833(5):1256–1268. doi: 10.1016/j.bbamcr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Losón OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 2013;24(5):659–667. doi: 10.1091/mbc.E12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337(6098):1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22(12):1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng L, et al. Hepatitis C virus infection induces apoptosis through a Bax-triggered, mitochondrion-mediated, caspase 3-dependent pathway. J Virol. 2008;82(21):10375–10385. doi: 10.1128/JVI.00395-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Boehning DF, Qian T, Popov VL, Weinman SA. Hepatitis C virus core protein increases mitochondrial ROS production by stimulation of Ca2+ uniporter activity. FASEB J. 2007;21(10):2474–2485. doi: 10.1096/fj.06-7345com. [DOI] [PubMed] [Google Scholar]
- 25.Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9(7):505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spaziani A, Alisi A, Sanna D, Balsano C. Role of p38 MAPK and RNA-dependent protein kinase (PKR) in hepatitis C virus core-dependent nuclear delocalization of cyclin B1. J Biol Chem. 2006;281(16):10983–10989. doi: 10.1074/jbc.M512536200. [DOI] [PubMed] [Google Scholar]
- 27.Cereghetti GM, et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci USA. 2008;105(41):15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otera H, et al. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol. 2010;191(6):1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SJ, et al. Hepatitis B virus disrupts mitochondrial dynamics: Induces fission and mitophagy to attenuate apoptosis. PLoS Pathog. 2013;9(12):e1003722. doi: 10.1371/journal.ppat.1003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Twig G, Shirihai OS. The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal. 2011;14(10):1939–1951. doi: 10.1089/ars.2010.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Syed GH, Siddiqui A. Effects of hypolipidemic agent nordihydroguaiaretic acid on lipid droplets and hepatitis C virus. Hepatology. 2011;54(6):1936–1946. doi: 10.1002/hep.24619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen H, Chan DC. Mitochondrial dynamics—fusion, fission, movement, and mitophagy—in neurodegenerative diseases. Hum Mol Genet. 2009;18(R2):R169–R176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ripoli M, et al. Hepatitis C virus-linked mitochondrial dysfunction promotes hypoxia-inducible factor 1 alpha-mediated glycolytic adaptation. J Virol. 2010;84(1):647–660. doi: 10.1128/JVI.00769-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sumpter R, Jr, et al. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol. 2005;79(5):2689–2699. doi: 10.1128/JVI.79.5.2689-2699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: A tale of two deaths? Hepatology. 2006;43(2, Suppl 1):S31–S44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 36.Korenaga M, et al. Mitochondrial dysfunction in hepatitis C. J Clin Gastroenterol. 2005;39(4, Suppl 2):S162–S166. doi: 10.1097/01.mcg.0000155517.02468.46. [DOI] [PubMed] [Google Scholar]
- 37.Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci USA. 2011;108(25):10190–10195. doi: 10.1073/pnas.1107402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kageyama Y, et al. Mitochondrial division ensures the survival of postmitotic neurons by suppressing oxidative damage. J Cell Biol. 2012;197(4):535–551. doi: 10.1083/jcb.201110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castanier C, Garcin D, Vazquez A, Arnoult D. Mitochondrial dynamics regulate the RIG-I-like receptor antiviral pathway. EMBO Rep. 2010;11(2):133–138. doi: 10.1038/embor.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11(6):389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wieland SF, Chisari FV. Stealth and cunning: Hepatitis B and hepatitis C viruses. J Virol. 2005;79(15):9369–9380. doi: 10.1128/JVI.79.15.9369-9380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amako Y, Sarkeshik A, Hotta H, Yates J, 3rd, Siddiqui A. Role of oxysterol binding protein in hepatitis C virus infection. J Virol. 2009;83(18):9237–9246. doi: 10.1128/JVI.00958-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.