Significance
Agriculture was a transformative development in the history of human societies and natural environments and drove the evolution of new domesticated species. Crop plants are the predominant domesticated species in most agricultural systems and are an essential component in all the food production systems that underpinned the development of urban societies. Archaeological plant remains provide a range of insights into the processes by which plants were domesticated in different parts of the world. The present paper provides a unique synthesis of evidence, including quantitative evidence on the trajectory and rate of domestication in seed crops and patterns in the development of tropical vegetatively propagated crops.
Keywords: archaeobotany, Neolithic, agriculture, archaeology, vegeculture
Abstract
Recent increases in archaeobotanical evidence offer insights into the processes of plant domestication and agricultural origins, which evolved in parallel in several world regions. Many different crop species underwent convergent evolution and acquired domestication syndrome traits. For a growing number of seed crop species, these traits can be quantified by proxy from archaeological evidence, providing measures of the rates of change during domestication. Among domestication traits, nonshattering cereal ears evolved more quickly in general than seed size. Nevertheless, most domestication traits show similarly slow rates of phenotypic change over several centuries to millennia, and these rates were similar across different regions of origin. Crops reproduced vegetatively, including tubers and many fruit trees, are less easily documented in terms of morphological domestication, but multiple lines of evidence outline some patterns in the development of vegecultural systems across the New World and Old World tropics. Pathways to plant domestication can also be compared in terms of the cultural and economic factors occurring at the start of the process. Whereas agricultural societies have tended to converge on higher population densities and sedentism, in some instances cultivation began among sedentary hunter–gatherers whereas more often it was initiated by mobile societies of hunter–gatherers or herder–gatherers.
Domestication offers an ideal laboratory for understanding evolution because it is a recent phenomenon in terms of geological time scales and because the selection pressures that affect harvestability by humans are often known (1). Domestication is a product of human behaviors that regulate or increase food supply, but may also inadvertently lock humans into an increased reliance on managed taxa (2). Archaeological research provides a fossil record of past organisms undergoing domestication, often accompanied by cultural artifacts associated with habitat management or niche construction (3, 4). The effects of agriculture in terms of intensifying land productivity to support larger populations has been fundamental to the development of civilizations and the ongoing impact on and management of ecosystems (5, 6).
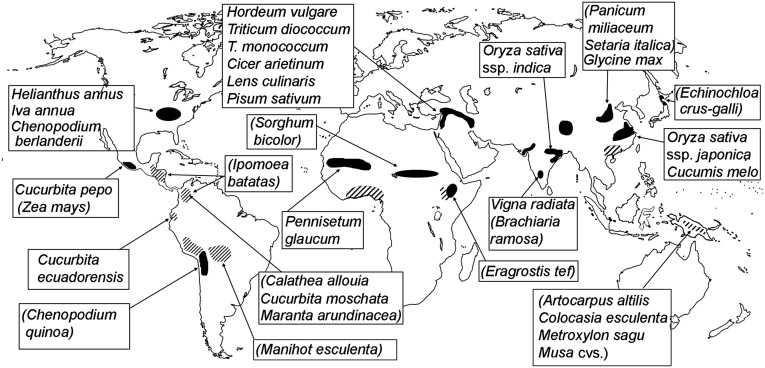
Domestications have occurred separately on different continents and in different cultural traditions, and thus represent a set of parallel experiments from which to infer recurrent processes (Fig. 1). In some cases this represents parallelism of phylogenetically related organisms that have been subjected to similar selection pressures and developed identical or similar adaptations in different places. In others, we can consider domestication as convergent evolution, in as much as similar adaptations have evolved across crops in different plant families. These parallel adaptations have been defined as the “domestication syndrome” (7, 8). A distinction can be made between true convergence, in which analogous states have been reached from very different and unrelated starting points, versus parallelism, in which similar pathways of change follow on from similar starting points, for example, as with taxa that share the same underlying developmental ontogeny and orthologous genetic loci (9). Some domestication traits, such as seasonality controls, have evolved in parallel across many species on the basis of the same genetic and developmental mechanisms; other traits, such as loss of wild-type seed dispersal or changes to seed and fruit size, have been attained through homoplasy based on different genetic and developmental changes. Similarly, in terms of the trajectories of domestication and their cultural causes, we can consider whether these were truly parallel, as is the case for wheat, barley, and Chinese rice, or have converged on similarly domesticated forms through different pathways, as seen for domesticated pulses (Fabaceae) and probably African pearl millet (9, 10).
Fig. 1.
Map of centers of domestication. Black areas indicate key areas of early seed crop domestication and hatched regions have an early focus on vegeculture. Species with quantified domestication rates are indicated, whereas others (species in parentheses) are discussed in the text.
This paper develops the perspective of domestication as a laboratory to understand evolution in relation to different cultural contexts of domestication. It examines whether instances of early agriculture worldwide converged through very different processes and from different starting points, or were parallel in terms of working from the same behavioral and botanical materials. We offer an updated review on the archaeobotany of plant domestication, including key processes for both plant and cultural evolution. For the purposes of this paper we will use the term “cultivation” to refer to a group of behaviors aimed at modifying soil environments and the management of the plants that grow in them. “Domestication” will be restricted to phenotypic changes in cultivars that make them different from unmanaged wild populations. Such phenotypic changes necessarily increase gradually at a population level, and therefore form a process, or an episode (which may take centuries or millennia) (2, 11). While selection on crops continues to occur and is behind varietal improvement and crop diversification, we interpret the “domestication episode” as the period in which key domestication syndrome traits underwent directional change and approached fixation within cultivated populations: these traits are now normally shared among all populations of a specific crop. “Agriculture” represents systems of land use in which cultivation behaviors became dominant, with domesticated species often the major cultivated taxa, and which took place at a large enough scale to become the primary economic activity of past populations. In this sense domestication emerges following a period of predomestication cultivation (12), with agriculture an outcome of both cultivation and domestication.
Advances in the Archaeobotany of Plant Domestication
Archaeobotany consists of the recovery and study of plant remains from archaeological sites. Although methods of recovery and analysis have improved, there has also been an increase in the number of sites and species studied and a broadening range of geographical regions subject to analysis (7, 9, 13–16). Along with botanical field studies of wild relatives of crops, this has led to recognition of a larger number of centers of agricultural origins, perhaps more than 20 (1, 8). For a number of crops, it is now becoming possible to compare domestication processes across species and geographical centers, thus allowing us to characterize similarities and differences. The present paper makes explicit comparisons of seed crop domestication and vegecultural domestication processes from several regions.
Cereal crops are at the core of many agricultural systems and the seeds are highly visible in the archaeological record and, thus, lend themselves to quantitative studies on domestication and agricultural origins. Further, they may be directly dated by radiocarbon (15). The past decades have seen considerable methodological advances in identification criteria for crop subspecies and cereal varieties (e.g., refs. 16 and 17). Whereas the morphological distinction between wild and domesticated cereals has long been recognized (18), it is only in the last decade that substantial quantities of preserved remains have become available from Southwest Asia (7, 19, 20) and from Asian rice (21). In the New World, important pseudocereals, such as Chenopodium spp., have seen increased attention in terms of seed coat traits that relate to germination inhibition, an important target of selection during domestication (9, 15). In a wide range of taxa, metrical traits of seed size or phytolith size can be compared over time.
Where vegeculture was the focus of food production, poor preservation has made domestication traits harder to document. Advances have been made, however, in the study of phytoliths and starches, most notably of tuber crops, as well as evidence of landscape modification (22, 23). In the Neotropics, the field has been revolutionized by microfossil evidence of domesticated crops that precede, in some cases by many millennia, empirical evidence for established agricultural practices (22, 24). Additional inferences have relied on cross-examining geoarchaeological, genetic, and botanical evidence, suggesting, for example, the influence of cultivation practices on the development of varietal differences of manioc (Manihot esculenta) (25). In New Guinea, early human-managed habitats have been inferred. For instance, early agriculture based on the vegetative propagation of plants, including bananas, taro, and some yams, has been dated to 7000–6400 B.P. based on archaeological remains of former cultivation plots on old land surfaces, dramatic degradation of montane rainforest to grasslands, and microbotanical evidence for high frequencies of crop plants (26, 27). Plant microremains have also recently allowed recognition of palm (sago) starch consumption in tropical South China (28) and Borneo (23).
Where evidence for the presence of morphological domesticates is available alongside regional environmental modification, it appears that agriculture succeeds the establishment of domesticated crops. In early Neolithic Europe, archaeobotanical evidence for the presence of domesticated cereals in archaeological sites precedes palynological indicators of forest reduction and increases in arable pollen indicators (29). Early European and Anatolian weed floras and N15 isotope data from cereal grains indicate that these first crops were manured, suggesting intensively managed, small-scale fields, or grain “gardens” (30). The earliest preserved field systems for rice cultivation in China, ca. 6000 B.P., indicate small individual fields less than 2 m in diameter which allowed the careful management of soil and water conditions (31). As in Europe, regional deforestation in China proceeds gradually after this period (32). In the millet-dominated area of northern China, forest reduction occurred from 5000 B.P., millennia after domesticated millet production was widespread (33), whereas deforestation of the South Indian hills is evident around 3500 B.P., centuries after the first village-farming cultures (34).
Similar delays in agricultural systems are evident in the New World. In the Eastern Woodlands of North America, morphological changes document the domestication of several seed crops by 4500–4000 B.P. (35, 36), although a shift toward these crops over wild nuts only happened after 2,000 y ago (37). In the Balsas region of Mexico, starch and phytoliths indicate the exclusive processing of domesticated maize, without comparable finds of wild teosinte, at 8700 B.P., whereas lake core data in the region indicate landscape modification for slash-and- burn agriculture starting from 7600 B.P. (38, 39). In the Ñanchoc Valley (northwestern Peru), starch from human teeth indicates the consumption of a range of crops, including introduced domesticates by 8000 B.P.; this is some 2,000 y before evidence for irrigation ditches indicate agricultural landscape modification in the region (40). In the tropical lowlands of eastern South America, anthropogenic soils and raised fields occur millennia after the appearance of fossil remains of domesticated crops in the region (22, 25, 41). Thus agriculture itself as a system of major landscape modification was a convergent development that evolved after the establishment of cultivation and morphological domestication of crops.
Comparing Timing and Tempo of Domestication in Seed Crops
The domestication syndrome is likely to differ for various crop plants, according primarily to how they are reproduced (by seed or by cuttings) and according to which plant organ is the target of selection. The best-defined and -studied domestication syndrome is for grain crops, including cereals, pulses, and oilseeds (7, 9). Foremost are the traits selected by harvesting and a crop’s growing reliance on humans for seed dispersal, including the loss of natural seed dispersal mechanisms. Second, there are traits related to the more friable soil conditions within tilled fields, leading to the deeper burial of seeds. The increase in seed size seen in most crops is hypothesized to aid seedling establishment, including from deeper burial (1, 7, 9), and is the most widely documented change in archaeobotanical evidence. Another key change is the loss of germination inhibition, in which germination occurs shortly after planting; this is regarded as the key domestication trait of pseudocereals like Chenopodium (7, 15, 35) and many pulses (9). For some taxa this is visible in preserved seed coat structure.
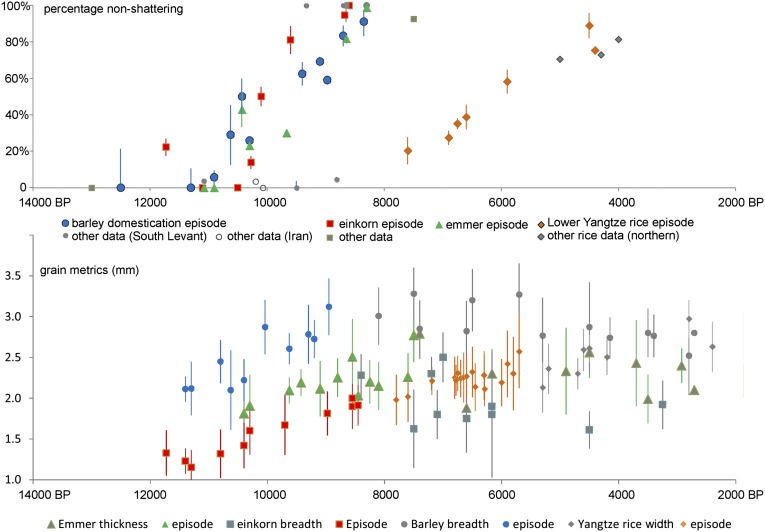
Nonshattering is often taken as a sine qua non of domesticated seed crops, making these species dependent on humans for reproduction by planting (1, 18, 19). In cereals this difference can be documented by the preserved abscission scar on the base of spikelets or rachis segments. Domestication in terms of this trait took at least 2,000–2,500 y (Fig. 2). Grain size change is more gradual over the same period as the shift from shattering (wild-type) to nonshattering seed dispersal (Fig. 2). After the episode of domestication, grain size becomes variable, fluctuating both up and down, suggesting processes of varietal differentiation and local adaptation; however, by the time of diversification, directional selection of the domestication episode is complete. Nonshattering becomes fixed at ∼100% in wheat and barley, whereas percentages as low as 70% are returned from archaeological rice populations, due to the persistence of weedy rices as a major contaminant of fields. Fig. 3 summarizes the variation in domestication rates and inferred coefficients of selection across 15 taxa and 18 traits (Tables S1–S4).
Fig. 2.
Evidence for protracted domestication episodes in Old World cereals, including proportion of nonshattering spikelet scars (Upper) and grain size indices (Lower).
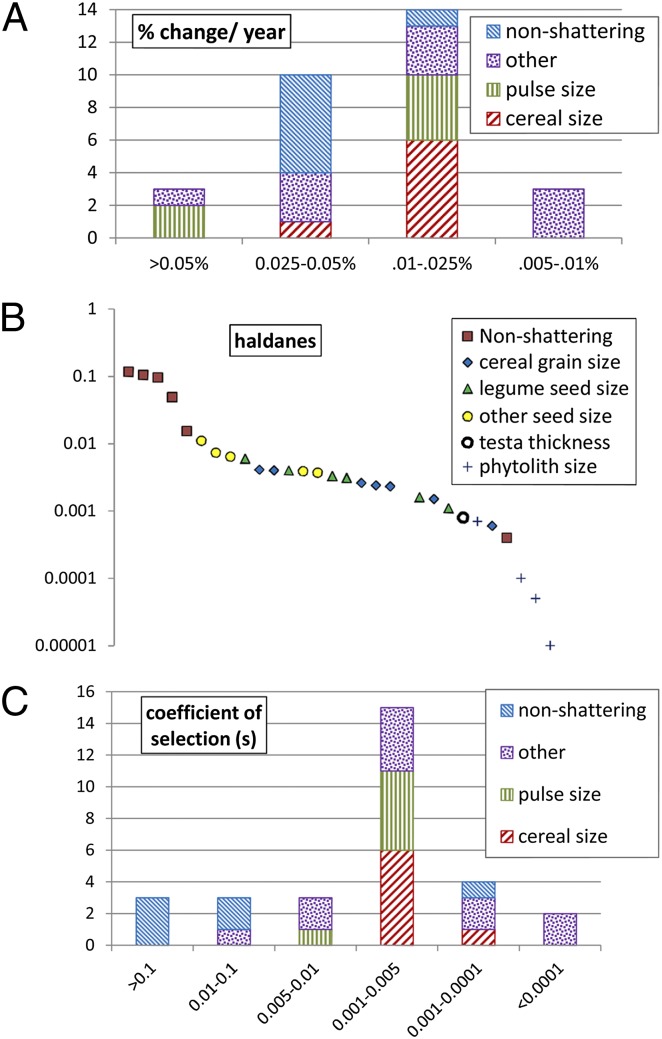
Fig. 3.
Rates of change in domestication traits across the collected dataset. (A) Graph comparing frequency of rates in terms of percentage change in trait per year. (B) Scatter of all haldane rate estimates, indicating trait/crop type. (C) Frequency of estimated selection coefficients in the dataset.
Evolutionary change in nonshattering is generally faster than grain size change, especially when measured in terms of haldanes (H). A haldane represents a change of one SD of a trait value per generation (11). For emmer wheat (Triticum dicoccum), the low estimate of H (Fig. 3) may be due to the relatively limited sample size available, as expanded datasets for Triticum monococcum and Hordeum vulgare have increased the estimates of rate somewhat over those made previously from a smaller dataset (11). Compared with reported phenotypic evolution rates in wild plant and animal studies that average around 0.03 H, our range of phenotypic evolution rates in domestication traits are generally similar. These rates of phenotypic change can be used to estimate the coefficient of selection, namely, the average increase per generation in gene(s) for a trait, which ranges from 0.0007 for nonshattering in T. diccocum to ∼0.1 in T. monococcum and H. vulgare.
Rates of phenotypic change for nonshattering were often one or two orders of magnitude greater than for grain size (Fig. 3), although this was still a protracted process. In the case of pearl millet (Pennisetum glaucum), domesticated in West Africa, chaff impressions in early Malian pottery indicate nonshattering types predominated by 4000 B.P., suggesting a preceding domestication episode of at least 1,000–2,000 y (10). In the case of maize (Zea mays mays), phytoliths indicative of nonshattering and absence of wild teosinte (Zea mays parviglumis) in the Rio Balsas region of Mexico at 8700 B.P. suggest that the domestication process and evolution of nonshattering occurred earlier (39). However, a sequence of evidence tracking the transition from nonshattering to shattering, or grain size changes, during this period is not yet available. For other cereals (various millets), archaeological evidence for nonshattering is similarly elusive. Remains of sorghum (Sorghum bicolor) from sites in the eastern Sahara indicate the consumption of wild (shattering) forms from 10000 to 5500 B.P. (42, 43), whereas the first domesticated forms are only documented following the crop’s introduction to India after 4000 B.P. (44). This suggests that domestication took place in Africa between 6000 and 4000 B.P., but hard evidence for the process is lacking. Widespread use of cultivars suggests they had evolved by 8000–7500 B.P. for north Chinese millets (45, 46) and before 4500 B.P. for some Indian millets (44).
A more widely documented domestication trait in seed crops is increased seed size (2, 47). Most seeds increase by 20–60% in one or two dimensions, mainly thickness or breadth (Figs. S1 and S2 and Table S1). A 20% increase is reported for barnyard millet (Echinochloa crus-galli) domestication in northern Japan over 2,000–3,000 y (48), whereas the smallest change in the current dataset is the 19% increase in peas. At the upper end are reported thickness changes in some millets, including a 72% difference in grain thickness between modern wild and domesticated Eragrostis tef (49), and 76% in archaeological P. glaucum. A few taxa show much more dramatic increases on the order 100% or more, i.e., doubling seed dimensions, such as in Indian mungbean (Vigna radiata), Chinese soybean (Glycine max) (50), and North American Iva annua (51). The rate of change ranges between 0.0006 and 0.11 H. Evidence for einkorn wheat at early occupations on Cyprus suggests the rate of change in domestication traits was accelerated in an island context, perhaps due to genetic isolation from the wild population or bottleneck effects (52). Here einkorn grain size increased at 0.06 H as opposed to 0.006 H on the Eurasian mainland (52).
Cucurbit seeds appear to be closer to the lower end of size increases, with about 15% increase found with the domestication of Cucurbita pepo and recorded through the squash seeds of Guila Naquitz in southern Mexico 10000–8000 B.P. (15). In the case of Chinese melons, mean seed sizes increase by about 22% over 500 y, with a moderately fast 0.0073 H. Phytolith size in squash rinds also correlates with increasing fruit size (Fig. S3) showing a significant size increase in Ecuadorean assemblages (>40%) over the Early Holocene (53), but with a low H (1 × 10−5). A more rapid increase (1 × 10−4 H) is associated with an initial domestication that took place by 11000 B.P.
Seed size increase may not always be an indicator of domestication. In some starchy fruits, reduction of seeds correlates with increase in starch content, as was clearly the case in the development of domesticated bananas (Musa cvs.) (54). In some cases, regional selection trajectories have been divergent. For example, breadfruit (Artocarpus altilis) has been selected for seedless cultivars with larger fruits (consumed for edible pulp), as well as seeded cultivars in which seeds are eaten. The degree of seediness decreases eastwards and away from the New Guinea region (55).
Comparing Timing of Domestication in Vegecultural Crops
It has generally been thought that vegetative reproduction made the domestication of tuberous plants possible through piecemeal replication of the characteristics of parent clones followed by selection and multiplication of useful phenotypic variations arising in planted stock (e.g., larger and smooth-skinned tubers, or less toxic/bitter forms). Unlike domesticated seed crops, some vegetative crops are not dependent on human efforts to reproduce and spread (23). However, sexual reproduction cycles and cooption of volunteer wild seedlings provide an important source of genetic diversity and local adaptations in at least some tubers (56). Indeed, it is clear that domesticated forms have been genetically altered from their wild progenitors on the basis of differing functional traits, such as those relating to toxicity, as well as on evidence for genetic bottlenecks between cultivars and wild relatives (57). Although increase in tuber size may be expected to correlate with an increase in the size of individual parenchyma cells, archaeologically recovered tuber fragments tend to preserve few morphological attributes relevant to phenotypic change. However, some research suggests that microremains such as starch grains have increased in size with tuber domestication (14, 58) and banana phytoliths have increased in size between diploid and triploid cultivars (59). Notwithstanding, compared with seed crops, it is harder to document archaeologically phenotypic changes in crops that are vegetatively propagated, such as tubers and some fruits, e.g., banana, grape, and olive (60, 61).
In some cases, cultivation practices may induce phenotypic alteration without genotypic change. Larger tubers develop in yams replanted in loosened, prepared soil as opposed to harder unprepared soils (62, 63). Thus some tuber crops could be cultivated for long periods without undergoing morphological domestication. Recent work on SSR molecular markers of live germplasm of Ipomoea batatas (64) and Manihot esculenta (65) permit disentangling complex histories of domestication. The latter study provides support for the argument (25) that one of the two manioc macro varieties (sweet manioc, the lower starch yielder that is less resistant to pests and poor soils) was domesticated earlier than bitter manioc (which grows well on poor soils, is resistant to pests, and yields more starch). The sequence most likely reflects an initial selection of plants in dump heaps with subsequent cultivation and range expansion. Subsequently in Amazonia, detoxification and cultivation techniques were innovated and led to the relaxation of selective pressures against cyanogenic glucosides to the point where a more toxic, but high starch-yielder evolved (66). Recent research has identified traits that facilitate vegetative propagation of cropped manioc, such as pronounced parenchymatous swellings at the nodes leading to brittle stems that can be readily broken for replanting, are absent in wild relatives (67).
Although it is not possible yet to provide quantitative data on domestication rates for vegetatively reproduced crops, we can provide comparisons of some general trajectories. In both New Guinea and Central/South America, evidence for consumption of starchy plants has been found back to the start of the Holocene or into the Pleistocene, whereas pollen evidence indicates human-disturbed forests, often including intentional burning (22, 24, 27, 68). This indicates human management of the landscape, within which some plants could be encouraged and planted. Artificial cultivation mounds in New Guinea (26, 27) suggest cultivation of tubers by the Mid-Holocene (7000–6400 B.P.), whereas microfossils beyond the range of the wild species, as well as dedicated lithic tools for cultivation and processing of roots, suggest tuber cultivation in Central and South America by 7000 B.P. (22, 24). In New Guinea, agricultural dependence and intensification of production can be suggested from 5000 to 3500 B.P., with ditched fields, wooden spades, and probable sedentism (68). A similar timing is suggested for the introduction of rice and possible spread of banana in Southeast Asia (28, 69). In northern South America, sedentism associated with tuber crops and other cultivars (squashes, fruit trees, and maize) dates to the Mid-Holocene in different regions (70). These data suggest a shift from initial management of forest gaps and edges, where gap-colonizing species were exploited for food, to the creation of patches of food plants through planting. Vicariance induced by deliberate diffusion beyond the range of the wild species before the adoption of dedicated agricultural practices, along with the formalization of systematic plots where selection pressures and genetic isolation from wild populations increased, both played a role in the trajectories of domestication of vegecultural crops.
Trajectories to Agriculture: Parallelism and Convergence in Cultural Evolution
The morphological changes of domestication are only one aspect of documenting the origins of agriculture. A domestication episode can be regarded as providing a species-specific time scale against which evidence for cultivation and management practices can be charted to reveal the interplay of human action and domestication. Although the evolution of domestication traits tends to increase the efficiency of harvests and yields, it also requires adjustments in human activities. Some of these may be characterized as labor traps, such as the additional requirement of threshing and winnowing as nonshattering rose to dominance, or the need to add nutrients to soils as erect crop growth habits packed more plants into the same units of soil, or the relocation of plots following nutrient depletion of soils (2). Thus, over the course of domestication there would have been fluctuations toward efficiency alternating with increased labor demands, but with an overall direction toward increasing yields and intensification of cultivation activities.
Taken at a comparative global level, the long-term impact of agricultural origins has been to support denser human populations through intensification of land use (5), including sedentism and fostering a greater reliance on a limited range of domesticated food stuffs. Although this represents convergence at a global level, the different domestication processes that can be documented across crops suggest that we should also look for multiple cultural patterns of agricultural origins, for example, in terms of the mobility of past societies and nature of crop reproduction.
Pristine domestications of crops have often occurred within mobile societies, which might include either hunter–gatherers or noncultivating pastoralists. In the case of vegecultural origins, both in New Guinea and Central/America, sedentism dates back only 4,000–5,000 y, long after the earliest inferred cultivation in these regions. Similarly seed crop cultivation of maize in Mesoamerica precedes settled villages by ∼5,000 y. In northern China, settled village farmers of millets date from at least 6500 B.P., whereas millet exploitation occurred by 9500 B.P., with clear cultivation by 8000 B.P. (45, 71). Both maize and Chinese millet cultivation correlate with periods of climatic amelioration in the Early Holocene (39, 46). In the case of the Old World savannah millet domestications, mobile gatherer–pastoralists with domesticated ungulates entered the Sahel of western Africa and then cultivated millet, whereas in peninsular India seasonally mobile herder–hunters precede sedentism or crops (44). In these cases of Mid-Holocene grain domestication, there was climatic aridification shortly before evidence for cultivation, including desertification processes of the Sahara or savannah expansion in South India (10, 44). In eastern North America, cultivation began among seasonally mobile Late Archaic hunter–fisher–gatherers ca. 5000 B.P. (15, 35). Among the key factors suggested for domestication by mobile groups are risk avoidance and seasonal conflicts in resource availability, leading to cultivation to make such resources readily available when seasonally needed (44, 72) or to buffer risks in wild food availability (46, 73).
Although sedentism based on agricultural economies was to become universal, only in a few instances is there possible evidence for sedentary foragers involved in the initial cultivation of crops. In China’s Yangtze Valley, substantial settlements at ∼8000 B.P. indicate sedentism before morphological domestication of rice and alongside early cultivation (31, 32). In Southwest Asia, the Late Pleistocene Natufian culture is often regarded as sedentary or nearly so, by 13,500 y ago, and a precursor of the cultivating villages during the Pre-Pottery Neolithic from ca. 11500 B.P. (74). Although the extent of year-round sedentism at villages focused on farming can be queried (74), it is nevertheless the case that architecturally permanent sites became increasingly important for cultivation, storage, and consumption of early crops like wheats and barley. Early cultivation by the Jomon of Japan was in the context of sedentism (48). The adoption of crops in the Ñanchoc Valley of northwestern Peru after 9000 B.P. is associated with sedentism (76). In these cases, pressure on resources and the need to support growing, and sedentary, populations are often considered part of the explanation of the origins of cultivation (77).
Conclusion
Agriculture is increasingly recognized as the coalescence of human activities and genetically transformed species that extends the widespread proclivity of Homo sapiens for niche construction (4–6) into a more intensive coevolutionary relationship that enhances the fitness, population size, and density of both humans and their crop plants. The pathways to agriculture were prolonged episodes of coevolution, genetic adaptations on the part of the plants, and cultural shifts and innovations on the part of people. These processes demand long-term and interregional comparative study. For seed crops, domestication trajectories are increasingly documented by quantitative patterns in archaeological plant assemblages, whereas for vegeculture only some general outlines have begun to emerge. The development of cultivation among mobile forager societies focusing on tubers as well as seeds indicates some parallelism across the seed crop and vegetative crop divide, whereas sedentary collectors turned cultivators was a less common development. In terms of cultural history, the domestication of wild plant species has been a process of convergence from different regional, environmental, and economic starting points.
Supplementary Material
Acknowledgments
This paper emerged from a meeting in Durham, NC (April 2011), supported by the National Evolutionary Synthesis Center, National Science Foundation (NSF) EF-0905606. Current research on Comparative Pathways to Agriculture (ComPAg) by D.Q.F., C.J.S., and L.L. is supported by European Research Council Grant 323842; research on early rice cultivation by D.Q.F. and L.Q. was supported by UK Natural Environment Research Council (London) Grants NE/G005540/1 and NE/K003402/1; research on early agriculture in the highlands of New Guinea was supported by Monash and Australian Research Council postdoctoral fellowships (to T.D.); research by M.A.-K. is supported by a British Academy postdoctoral fellowship; and research on the evolution of crop species by M.D.P. is supported by the US NSF Plant Genome Research Program and the New York University Abu Dhabi Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1308937110/-/DCSupplemental.
References
- 1.Purugganan MD, Fuller DQ. The nature of selection during plant domestication. Nature. 2009;457(7231):843–848. doi: 10.1038/nature07895. [DOI] [PubMed] [Google Scholar]
- 2.Fuller DQ, Allaby RG, Stevens C. Domestication as innovation: The entanglement of techniques, technology and chance in the domestication of cereal crops. World Archaeol. 2010;42(1):13–28. [Google Scholar]
- 3.Laland KN, Odling-Smee J, Feldman MW. Niche construction, biological evolution, and cultural change. Behav Brain Sci. 2000;23(1):131–146. doi: 10.1017/s0140525x00002417. discussion 146–175. [DOI] [PubMed] [Google Scholar]
- 4.Smith BD. General patterns of niche construction and the management of ‘wild’ plant and animal resources by small-scale pre-industrial societies. Philos Trans R Soc Lond B Biol Sci. 2011;366(1566):836–848. doi: 10.1098/rstb.2010.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis EC, et al. Used planet: A global history. Proc Natl Acad Sci USA. 2013;110(20):7978–7985. doi: 10.1073/pnas.1217241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith BD, Zeder MA. 2013. The onset of the Anthropocene. Anthropocene, 10.1016/j.ancene.2013.05.001.
- 7.Fuller DQ. Contrasting patterns in crop domestication and domestication rates: Recent archaeobotanical insights from the Old World. Ann Bot (Lond) 2007;100(5):903–924. doi: 10.1093/aob/mcm048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer RS, DuVal AE, Jensen HR. Patterns and processes in crop domestication: An historical review and quantitative analysis of 203 global food crops. New Phytol. 2012;196(1):29–48. doi: 10.1111/j.1469-8137.2012.04253.x. [DOI] [PubMed] [Google Scholar]
- 9.Fuller DQ, Allaby R. 2009. Fruit Development and Seed Dispersal. Annual Plant Reviews, ed Østergaard L (Wiley-Blackwell, Oxford), Vol 38, pp 238–295.
- 10.Manning K, Pelling R, Higham T, Schwenniger J-L, Fuller DQ. 4500-year old domesticated pearl millet (Pennisetum glaucum) from the Tilemsi Valley, Mali: New insights into an alternative cereal domestication pathway. J Archaeol Sci. 2011;38(2):312–322. [Google Scholar]
- 11.Purugganan MD, Fuller DQ. Archaeological data reveal slow rates of evolution during plant domestication. Evolution. 2011;65(1):171–183. doi: 10.1111/j.1558-5646.2010.01093.x. [DOI] [PubMed] [Google Scholar]
- 12.Hillman G, Davies MS. Domestication rates in wild wheats and barley under primitive cultivation. Biol J Linn Soc Lond. 1990;39(1):39–78. [Google Scholar]
- 13.Denham TP, Haberle SG, Pierret A. In: New Directions in Archaeological Science (Terra Australis 28) Fairbairn A, O’Connor S, Marwick B, editors. Canberra, Australia: ANU E Press; 2009. pp. 139–154. [Google Scholar]
- 14.Piperno D. New archaeobotanical information on early cultivation and plant domestication. In: Gepts P, et al., editors. Biodiversity in Agriculture. Domestication, Evolution, and Sustainability. Cambridge, UK: Cambridge Univ Press; 2012. pp. 110–135. [Google Scholar]
- 15.Smith BD. Eastern North America as an independent center of plant domestication. Proc Natl Acad Sci USA. 2006;103(33):12223–12228. doi: 10.1073/pnas.0604335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones G, Valamoti S, Charles M. Early Crop diversity: A “new” glume wheat from northern Greece. Veg Hist Archaeobot. 2000;9(3):133–146. [Google Scholar]
- 17.Fuller DQ, Willcox G, Allaby RG. Early agricultural pathways: Moving outside the ‘core area’ hypothesis in Southwest Asia. J Exp Bot. 2012;63(2):617–633. doi: 10.1093/jxb/err307. [DOI] [PubMed] [Google Scholar]
- 18.Helbaek H. Commentary on the phylogenesis of Triticum and Hordeum. Econ Bot. 1966;20(4):350–360. [Google Scholar]
- 19.Tanno KI, Willcox G. Distinguishing wild and domestic wheat and barley spikelets from early Holocene sites in the Near East. Veg Hist Archaeobot. 2012;21(2):107–115. [Google Scholar]
- 20.Riehl S, Zeidi M, Conard NJ. Emergence of agriculture in the foothills of the Zagros Mountains of Iran. Science. 2013;341(6141):65–67. doi: 10.1126/science.1236743. [DOI] [PubMed] [Google Scholar]
- 21.Fuller DQ, et al. The domestication process and domestication rate in rice: Spikelet bases from the Lower Yangtze. Science. 2009;323(5921):1607–1610. doi: 10.1126/science.1166605. [DOI] [PubMed] [Google Scholar]
- 22.Piperno DR, Pearsall D. The Origins of Agriculture in the Lowland Neotropics. San Diego: Academic; 1998. [Google Scholar]
- 23.Barton H, Denham T. In: Anthropological and Archaeological Approaches to Foraging-Farming Transitions in Southeast Asia, McDonald Institute Monographs. Barker G, Janowski M, editors. Cambridge, UK: McDonald Institute for Archaeological Research; 2011. pp. 17–25. [Google Scholar]
- 24.Piperno DR. The origins of plant cultivation and domestication in the New World tropics: Patterns, process, and new developments. Curr Anthropol. 2011;52(S4):53–470. [Google Scholar]
- 25.Arroyo-Kalin M. The Amazonian Formative: Crop domestication and anthropogenic soils. Diversity. 2010;2(4):473–504. [Google Scholar]
- 26.Denham TP, et al. Origins of agriculture at Kuk Swamp in the highlands of New Guinea. Science. 2003;301(5630):189–193. doi: 10.1126/science.1085255. [DOI] [PubMed] [Google Scholar]
- 27.Haberle S, Lentfer C, O'Donnell S, Denham T. The palaeoenvironments of Kuk Swamp from the beginnings of agriculture in the highlands of Papua New Guinea. Quat Int. 2012;249:129–139. [Google Scholar]
- 28.Yang X, et al. Sago-type palms were an important plant food prior to rice in southern subtropical China. PLoS ONE. 2013;8(5):e63148. doi: 10.1371/journal.pone.0063148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willis KJ, Bennett KD. The Neolithic transition-fact or fiction? Palaeoecological evidence from the Balkans. Holocene. 1994;4(3):326–330. [Google Scholar]
- 30.Bogaard A, et al. Crop manuring and intensive land management by Europe's first farmers. Proc Natl Acad Sci USA. 2013;110(31):12589–12594. doi: 10.1073/pnas.1305918110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuller DQ, Qin L. Water management and labour in the origins and dispersal of Asian rice. World Archaeol. 2009;41(1):88–111. [Google Scholar]
- 32.Fuller DQ, Qin L. Declining oaks, increasing artistry, and cultivating rice: The environmental and social context of the emergence of farming in the Lower Yangtze Region. Environmental Archaeology. 2010;15(2):139–159. [Google Scholar]
- 33.Ren G. Changes in forest cover in China during the Holocene. Veg Hist Archaeobot. 2007;16(2-3):119–126. [Google Scholar]
- 34.Kingwell-Banham E, Fuller DQ. Shifting cultivators in South Asia: Expansion, marginalisation and specialisation over the long-term. Quat Int. 2012;249:84–95. [Google Scholar]
- 35.Smith BD. Origins of agriculture in eastern North America. Science. 1989;246(4937):1566–1571. doi: 10.1126/science.246.4937.1566. [DOI] [PubMed] [Google Scholar]
- 36.Smith BD. 2006. Documenting Domestication, eds Zeder MA, Bradley D, Emshwiller E, Smith BD (Univ of California Press, Berkeley, CA), pp 25–31.
- 37.Milner GR. The Moundbuilders: Ancient Peoples of Eastern North America. London: Thames and Hudson; 2004. [Google Scholar]
- 38.Piperno DR, et al. Late Pleistocene and Holocene environmental history of the Iguala Valley, Central Balsas Watershed of Mexico. Proc Natl Acad Sci USA. 2007;104(29):11874–11881. doi: 10.1073/pnas.0703442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piperno DR, Ranere AJ, Holst I, Iriarte J, Dickau R. Starch grain and phytolith evidence for early ninth millennium B.P. maize from the Central Balsas River Valley, Mexico. Proc Natl Acad Sci USA. 2009;106(13):5019–5024. doi: 10.1073/pnas.0812525106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piperno DR, Dillehay TD. Starch grains on human teeth reveal early broad crop diet in northern Peru. Proc Natl Acad Sci USA. 2008;105(50):19622–19627. doi: 10.1073/pnas.0808752105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKey DS, et al. Pre-Columbian agricultural landscapes, ecosystem engineers, and self-organized patchiness in Amazonia. Proc Natl Acad Sci USA. 2010;107(17):7823–7828. doi: 10.1073/pnas.0908925107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stemler AB. A scanning electron microscopic analysis of plant impressions in pottery from the sites of Kadero, El Zakiab, Um Direiwa and El Kadada. Archéologie du Nil Moyen. 1990;4:87–105. [Google Scholar]
- 43.Wasylikowa K, et al. Archaeobotany of the early Neolithic site E-75-6 at Nabta Playa, Western Desert, South Egypt (preliminary results) Acta Palaeobotanica. 1995;35(1):133–155. [Google Scholar]
- 44.Fuller DQ. Finding plant domestication in the Indian subcontinent. Curr Anthropol. 2011;52(4):S347–S362. [Google Scholar]
- 45.Liu X, Hunt HV, Jones MK. River valleys and foothills: Changing archaeological perceptions of North China’s earliest farms. Antiquity. 2009;83(319):82–95. [Google Scholar]
- 46.Bettinger RL, Barton L, Morgan C. The origins of food production in north China: A different kind of agricultural revolution. Evol Anthropol. 2010;19(1):9–21. [Google Scholar]
- 47.Harlan JR, De Wet JMJ, Price EG. Comparative evolution of cereals. Evolution. 1973;27(2):311–325. doi: 10.1111/j.1558-5646.1973.tb00676.x. [DOI] [PubMed] [Google Scholar]
- 48.Crawford G. Advances in understanding early agriculture in Japan. Curr Anthropol. 2011;52(S4):S331–S345. [Google Scholar]
- 49.D’Andrea AC. T’ef (Eragrostis tef) in ancient agricultural systems of highland Ethiopia. Econ Bot. 2008;62(4):547–566. [Google Scholar]
- 50.Lee G-A, Crawford GW, Liu L, Sasaki Y, Chen X. Archaeological soybean (Glycine max) in East Asia: Does size matter? PLoS ONE. 2011;6(11):e26720. doi: 10.1371/journal.pone.0026720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asch DL, Asch NB. 1985. Prehistoric food production in North America. Anthropological Papers 75, ed Ford RI (Museum of Anthropology, Univ of Michigan, Ann Arbor, MI), pp 149–204.
- 52.Lucas L, Colledge S, Simmons A, Fuller DQ. Crop introduction and accelerated island evolution: Archaeobotanical evidence from ‘Ais Yiorkis and Pre-Pottery Neolithic Cyprus. Veg Hist Archaeobot. 2012;21(2):117–129. [Google Scholar]
- 53.Piperno DR, Stothert KE. Phytolith evidence for early Holocene Cucurbita domestication in southwest Ecuador. Science. 2003;299(5609):1054–1057. doi: 10.1126/science.1080365. [DOI] [PubMed] [Google Scholar]
- 54.Perrier X, et al. Multidisciplinary perspectives on banana (Musa spp.) domestication. Proc Natl Acad Sci USA. 2011;108(28):11311–11318. doi: 10.1073/pnas.1102001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zerega NJC, Ragone D, Motley TJ. Complex origins of breadfruit (Artocarpus altilis, Moraceae): Implications for human migrations in Oceania. Am J Bot. 2004;91(5):760–766. doi: 10.3732/ajb.91.5.760. [DOI] [PubMed] [Google Scholar]
- 56.McKey D, Elias M, Pujol B, Duputié A. In: Biodiversity in Agriculture. Domestication, Evolution, and Sustainability. Gepts P, et al., editors. Cambridge, UK: Cambridge Univ Press; 2012. pp. 377–406. [Google Scholar]
- 57.Olsen KM, Schall BA. In: Documenting Domestication: New Genetic and Archaeological Paradigms. Zeder MA, et al., editors. Berkeley, CA: Univ of California Press; 2006. pp. 123–133. [Google Scholar]
- 58.Perry L. Starch granule size and the domestication of manioc (Manihot esculenta) and sweet potato (Ipomoea batatas) Econ Bot. 2002;56(4):335–349. [Google Scholar]
- 59.Vrydaghs L, et al. Differentiating the Volcaniform Phytoliths of Bananas: Musa acuminata. Ethnobotanical Research and Applications. 2009;7:239–246. [Google Scholar]
- 60.Hather JG. In: The Origins and Spread of Agriculture and Pastoralism in Eurasia. Harris DR, editor. London: UCL Press; 1996. pp. 538–551. [Google Scholar]
- 61.Zohary D, Hopf M, Weiss E. Domestication of Plants in the Old World: The Origin and Spread of Domesticated Plants in Southwest Asia, Europe, and the Mediterranean Basin. Oxford: Oxford Univ Press; 2012. [Google Scholar]
- 62.Chikwendu VE, Okezie CEA. In: Foraging and Farming: The Evolution of Plant Exploitation. Harris DR, Hillman GC, editors. London: Unwin Hyman; 1989. pp. 344–357. [Google Scholar]
- 63.Hildebrand EA. In: Rethinking Agriculture. Archaeological and Ethnoarchaeological Perspectives. Denham T, Vrydaghs L, Iriarte J, editors. Walnut Creek, CA: Left Coast Press; 2007. pp. 273–298. [Google Scholar]
- 64.Roullier C, et al. Disentangling the origins of cultivated sweet potato (Ipomoea batatas (L.) Lam.) PLoS ONE. 2013;8(5):e62707. doi: 10.1371/journal.pone.0062707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mühlen GS, Alves-Pereira AA, Clement CR, Valle TR. 2013. Genetic diversity and differentiation of Brazilian bitter and sweet manioc varieties (Manihot esculenta Crantz, Euphorbiaceae) based on SSR molecular markers. Tipití 11(2):66–73.
- 66.Arroyo-Kalin M. Slash-burn-and-churn: Landscape History and Crop cultivation in pre-Columbian Amazonia. Quat Int. 2012;249:4–18. [Google Scholar]
- 67.Ménard L, McKey D, Mühlen GS, Clair B, Rowe NP. The evolutionary fate of phenotypic plasticity and functional traits under domestication in manioc: Changes in stem biomechanics and the appearance of stem brittleness. PLoS ONE. 2013;8(9):e74727. doi: 10.1371/journal.pone.0074727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Denham TP, The Cambridge World Prehistory, eds Bahn P, Renfrew C (Cambridge Univ Press, Cambridge, UK)
- 69.Fuller DQ, Madella M. Banana cultivation in South Asia and East Asia: A review of the evidence from archaeology and linguistics. Ethnobot Res Applications. 2009;7:333–351. [Google Scholar]
- 70.Raymond JS. The process of sedentism in northwestern South America. In: Silverman H, Isbell W, editors. Handbook of South American Archaeology. New York: Springer; 2008. pp. 79–90. [Google Scholar]
- 71.Yang X, et al. Early millet use in northern China. Proc Natl Acad Sci USA. 2012;109(10):3726–3730. doi: 10.1073/pnas.1115430109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marshall F, Hildebrand EA. Cattle before crops: The beginnings of food production in Africa. J World Prehist. 2002;16(2):99–143. [Google Scholar]
- 73.Clement CR. In: Time and Complexity in the Neotropical Lowlands: Studies in Historical Ecology. Balée W, Erickson CL, editors. New York: Columbia Univ Press; 2006. pp. 165–185. [Google Scholar]
- 74.Asouti E, Fuller DQ. A contextual approach to the emergence of agriculture in Southwest Asia. Curr Anthropol. 2013;54(3):299–345. [Google Scholar]
- 75.Willcox G, Strodeur D. Large-scale cereal processing before domestication during the tenth millennium cal BC in northern Syria. Antiquity. 2012;86(331):99–114. [Google Scholar]
- 76.Dillehay TD, Rossen J, Andres TC, Williams DE. Preceramic adoption of peanut, squash, and cotton in northern Peru. Science. 2007;316(5833):1890–1893. doi: 10.1126/science.1141395. [DOI] [PubMed] [Google Scholar]
- 77.Cohen MN. Rethinking the origins of agriculture. Introduction. Curr Anthropol. 2009;50(5):591–595. doi: 10.1086/603548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.