Significance
Because individual cancer patients differ considerably in their clinical benefits from immunotherapies, early indicators of response could help physicians personalize treatments. Unfortunately, conventional clinical response criteria can be misleading for cancer vaccines. Herein, we show that early humoral responses to xenogenic Forssman disaccharide displayed on PROSTVAC-VF’s viral vectors correlate with long-term survival of vaccinated prostate cancer patients. The survival correlation for anti-Forssman responses was observed consistently when PROSTVAC-VF was used either as monotherapy or combined with the radiopharmaceutical Quadramet. Monitoring postvaccination anti-Forssman humoral responses could offer a simple indicator of response many months before conventional clinical response criteria become reliable. Finally, this study suggests that modifying glycans may improve poxvirus-based vaccines even when not specifically designed to target glycans.
Keywords: glycan array, Forssman antigen
Abstract
Therapeutic cancer vaccines can be effective for treating patients, but clinical responses vary considerably from patient to patient. Early indicators of a favorable response are crucial for making individualized treatment decisions and advancing vaccine design, but no validated biomarkers are currently available. In this study, we used glycan microarrays to profile antiglycan antibody responses induced by PROSTVAC-VF, a poxvirus-based cancer vaccine currently in phase III clinical trials. Although the vaccine is designed to induce T-cell responses to prostate-specific antigen, we demonstrate that this vaccine also induces humoral responses to a carbohydrate on the poxvirus, the Forssman disaccharide (GalNAcα1–3GalNAcβ). These responses had a statistically significant correlation with overall survival in two independent sample sets (P = 0.015 and 0.008) comprising more than 100 patients. Additionally, anti-Forssman humoral responses correlated with clinical outcome in a separate study of PROSTVAC-VF combined with a radiopharmaceutical (Quadramet). Studies on control subjects demonstrated that the survival correlation was specific to the vaccine. The results provide evidence that antiglycan antibody responses may serve as early biomarkers of a favorable response to PROSTVAC-VF and offer unique insights for improving vaccine design.
Therapeutic cancer vaccines are designed to reprogram a patient’s immune system to attack and lyse malignant cells. This approach for cancer treatment can lengthen survival significantly, as exemplified by the cancer vaccine PROVENGE recently approved by the Food and Drug Administration (FDA) (1), but clinical responses vary considerably from one patient to another. Unfortunately, it is not clear why some vaccinated patients live years longer than expected but seemingly similar patients receive little or no apparent benefit from vaccination. Moreover, it is difficult to determine whether a vaccinated patient is responding favorably, especially during the first few months of treatment (2, 3). Traditional Response Evaluation Criteria in Solid Tumors, which were developed to evaluate chemotherapies, can be unreliable for tracking response to cancer vaccines (4). For example, apparent tumor burden may increase during the first few months of cancer vaccine treatment because of inflammation even in patients who go on to have durable responses. Alternatively, tumors may progress during the buildup of antitumor immunity. Validated methods for evaluating vaccine efficacy thus are crucial for determining which vaccines in clinical trials show sufficient promise to continue development, for distinguishing patients who should continue receiving vaccine treatment from those who should consider alternative treatment options, and for optimizing vaccine design and administration. For these reasons, reliable indicators of a beneficial clinical response could have a major impact on cancer care.
Cancer vaccines are designed to stimulate antitumor immunity, and many features of the immune response potentially could serve as early indicators of vaccine efficacy (5, 6). The ideal biomarker would track responses early within the first months of treatment and be easily measured, independent of other clinical factors, and vaccine specific rather than generally prognostic. Many clinical trials have incorporated biomarker discovery, but this approach presents several challenges. False positives can arise because of small sample sizes, imbalances across groups, or insufficient consideration of multiple testing [i.e., statistical analyses must take into account the number of post hoc tests performed to identify the reported lead (7, 8)]. Moreover, studies aimed at identifying new biomarkers often lack controls necessary to assess whether a particular candidate biomarker is vaccine specific or is an epiphenomenon arising from nonspecific effects of disease progression (9). Although several promising studies have linked postvaccination immune responses with favorable clinical responses (1, 5, 6, 10, 11), none to date has led to a validated companion diagnostic. Therefore new clinically relevant biomarkers of vaccine efficacy are urgently needed.
Although there have been numerous advances in our understanding of immunology, many aspects of the immune response remain poorly understood. In particular, the great majority of prior studies have focused on immune responses to proteins, but carbohydrates also are an abundant and essential class of antigens. Abnormal glycosylation is a hallmark of malignancy (12, 13), and changes in carbohydrate expression can serve as focal points of immune responses. For example, a number of cancer vaccines in clinical trials have been designed specifically to target tumor-associated carbohydrate antigens (14–16). Other cancer vaccines in clinical development, such as glycoprotein-targeted vaccines and whole-cell vaccines, display a diverse set of glycans to the immune system that potentially induce antiglycan responses. Although immune responses to carbohydrates could contribute to clinical efficacy in several ways (inducing antibody-dependent cell-mediated cytotoxicity, blocking metastasis, or directly killing tumors), technical challenges have limited studies into this class of responses. It is particularly difficult to predict relevant responses, and traditional methods for profiling antiglycan responses lack the necessary throughput for comprehensive evaluations. Although relatively few studies of humoral responses to glycans have been published, several associations between clinical outcome and antiglycan antibody responses have been reported (17–19). These studies analyzed only a few glycans in a small number of patients; more in-depth studies could uncover other important antiglycan responses. Studies on immune responses to glycans also could provide new insights for improving vaccine design.
Glycan microarrays (20, 21), which contain hundreds of carbohydrates immobilized on microscope slides, provide a multiplex assay for analyzing serum antiglycan antibodies that uses only minute amounts of valuable glycans and clinical samples (e.g., only 2–4 µL of serum). This approach, referred to as “glycoimmunomics,” generates a comprehensive profile of antiglycan immune responses. In this study, we applied glycan microarray technology to PROSTVAC-VF, a prostate cancer vaccine currently in a phase III clinical trial (22). PROSTVAC-VF induces immunity for prostate-specific antigen (PSA) using genetically modified vaccinia and fowlpox encoding PSA and three costimulatory molecules (LFA-3, B7.1, and ICAM-1, designated “TRICOM”) coadministered with GM-CSF. In two phase II clinical trials (23, 24), PROSTVAC-VF lengthened median survival by 8–9 mo, surpassing the survival benefit of the only FDA-approved cancer vaccine, PROVENGE (4.1 mo) (1). Although PROSTVAC-VF was not designed to simulate immunity to glycans, cancer vaccines can induce responses beyond the target antigen(s) through antigen spreading (25). This fact, coupled with our previous finding that preexisting IgM to blood group A correlates with survival (26), prompted us to evaluate whether PROSTVAC-VF induces immune responses to glycans. Here we demonstrate that this vaccine does induce antibody responses to glycans and that responses to the Forssman glycan correlate positively with survival. In addition to providing a potential biomarker which may be useful for evaluating the efficacy of PROSTVAC-VF, these studies highlight the importance of antiglycan immune responses for cancer vaccines, including vaccines that are not specifically designed to induce responses to glycans.
Results
Antibody Responses Are Induced by PROSTVAC-VF.
PROSTVAC-VF is designed to stimulate T-cell responses to PSA, but several features of the vaccine could result in antiglycan responses. First, glycans associated with the vaccine could induce immune responses. In particular, PSA is a glycoprotein containing an N-linked glycan at Asn45 that accounts for ∼8% of the protein’s weight, and PROSTVAC-VF’s enveloped viral vectors are glycosylated. Second, prostate tumors abnormally express tumor-associated carbohydrate antigens, and immune responses to these glycans could occur through antigen spreading (25). Because many glycans could trigger responses, and nothing was known about anticarbohydrate responses induced by PROSTVAC-VF, a high-throughput approach was needed to study antiglycan antibody responses.
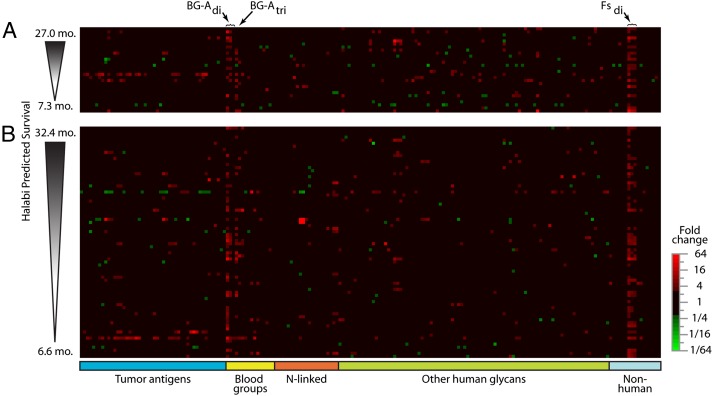
For our initial assessment, we profiled free antiglycan antibody levels in sera of 28 patients collected before and after priming and at least one booster from a phase II study of PROSTVAC-VF (23). We used glycan microarrays containing 171 structurally distinct glycans and glycopeptides to compare serum antiglycan IgG, IgM, and total Ig profiles in patients before and 2–4 mo after initiating treatment with PROSTVAC-VF. All clinical and survival data were blinded during the profiling of serum samples during this initial discovery phase. In a previous study of healthy humans (27), spontaneous changes of ≥2.6-fold occurred only rarely over a 3-mo period; therefore, this threshold was applied to this study. Interestingly, numerous antibody responses to glycans and glycopeptides were observed 2–4 mo after the initial vaccination (Fig. 1A). Increases significantly outnumbered decreases by a ratio of 4:1, supporting a response to vaccination rather than normal physiological variation. The magnitude of the responses ranged from a 52-fold increase in antibodies to the terminal disaccharide of the Forssman antigen (Fsdi) in one patient to an 8.3-fold decrease in antibody levels for Galβ1–3GalNAcβ (GA1di) in another patient. Although many responses were observed in the group, each patient had responses to only a small percentage of glycans, so that their overall antiglycan antibody profiles were largely unchanged after receiving PROSTVAC-VF. These results are consistent with our previous studies showing that serum antiglycan antibody profiles were stable over weeks to months in normal controls (27). Neither immunomodulation with low-dose GM-CSF given at the vaccine site nor disease progression systematically altered antibody levels.
Fig. 1.
Postvaccination changes in levels of serum antiglycan antibodies. Heat maps show postvaccination fold changes in antiglycan antibody levels (IgM + IgG + IgA) for (A) discovery and (B) validation sets measured at a serum dilution of 1:200. Columns correspond to 204 array components (ordered alphabetically within glycan families), and rows are individual patients (arranged by expected survival calculated using the Halabi nomogram). Increases are colored red, and decreases are green. Black indicates no significant change (increase <160% or decrease <61%).
Responses to a wide variety of glycans were observed, but there was considerable variability from one patient to another. In most cases, responses to individual glycans occurred in only one or in a small subset of patients. For example, antibody responses to tumor-associated carbohydrate antigens occurred only infrequently. For three glycans, however, responses were observed in many patients. The most common IgM, IgG, and total Ig (IgG + IgM + IgA) responses were directed at the terminal disaccharide of the Forssman xenoantigen (Fsdi = GalNAcα1–3GalNAcβ) (Fig. 2A), the blood group A trisaccharide [BG-Atri = GalNAcα1–3(Fucα1–2)Gal] (Fig. 2A), and a nonfucosylated substructure of blood group A (BG-Adi = GalNAcα1–3Galβ) (Fig. 2A). Beyond these three glycans, we detected humoral responses in at least 25% of patients for only one other case: Eight patients showed statistically significant changes for total Ig against the glycoprotein asialo-fetuin when serum was diluted 1:50.
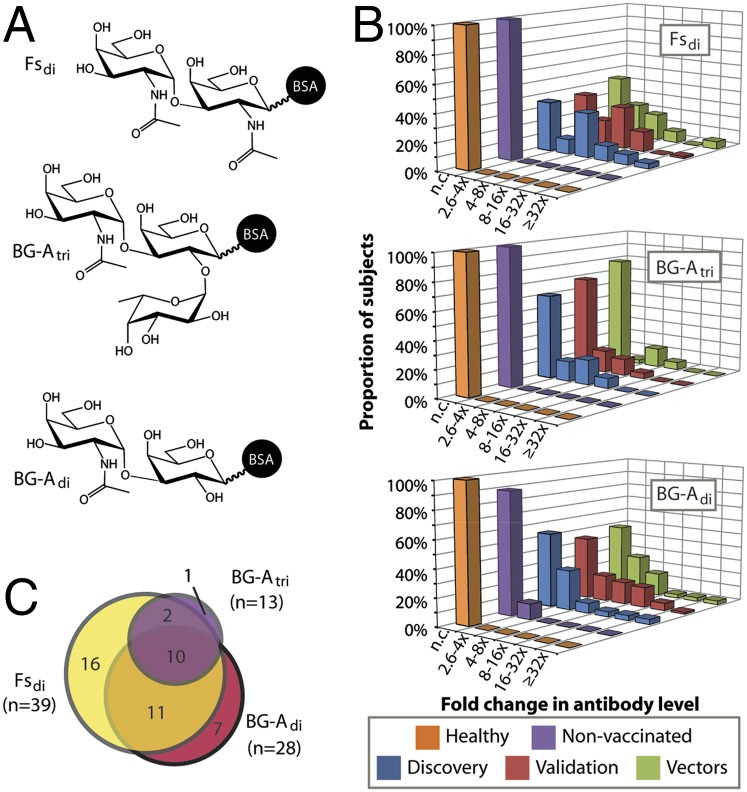
Fig. 2.
Humoral responses to terminal saccharides of blood group A and Forssman glycans. (A) Structures of glycans with largest changes in postvaccination antibody levels. Glycan density on the array was controlled by varying the number of glycans conjugated to the albumin carrier of the neoglycoprotein. Presented data were obtained with median conjugation ratios (glycans/albumin molecule) of 4, 4, and 19 for Fsdi, BG-Adi, and BG-Atri, respectively. (B) Levels of total Ig for BG-Adi, BG-Atri, and Fsdi were stable in healthy subjects (orange) and in nonvaccinated prostate cancer patients treated with radiation (purple). However, humoral responses to these glycans occurred in patients inoculated with PROSTVAC-VF (blue and red) or its viral vectors (green). Subjects with no significant change in antibody levels (<2.6-fold change) are labeled as n.c. (C) Venn diagram showing that fourfold or greater humoral responses to these three glycans typically co-occurred in the same patients.
Over the entire array, total Ig responses to low-density Fsdi (four copies per BSA molecule) typically were the largest in magnitude and occurred in the most patients (discovery set = 64%; maximum fold change = 52×) (Fig. 2B). A number of patients also showed changes for BG-Adi (discovery set = 46%; maximum fold change = 33.7×) and BG-Atri (discovery set = 39%; maximum fold change = 9.2×). However, spontaneous changes in antibody levels to these glycans were not found in healthy subjects or nonvaccinated prostate cancer patients treated with radiation in another study (28) (Fig. 2B). Thus we concluded that these responses are not general changes that occur physiologically or during disease progression.
To evaluate the consistency of these changes, we analyzed sera from several patients at multiple time points after initiating treatment with PROSTVAC-VF. Changes at 1, 2, and 4 mo after the first injection of PROSTVAC-VF displayed similar levels of change relative to prevaccination sera (Fig. S1). Moreover, these glycan-specific antibody changes are not the result of cross-reactivity for linkers used to conjugate glycans to carrier proteins because humoral responses did not occur for other glycans containing the same linker.
Antibody Responses to Fsdi Correlate with Survival.
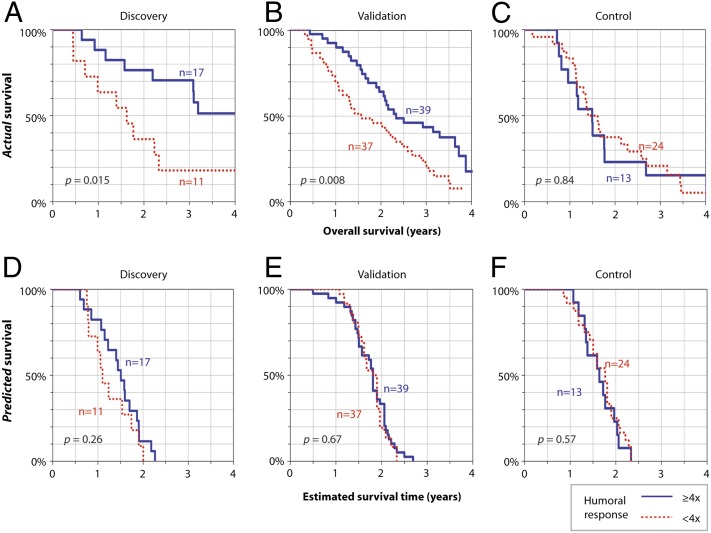
Once we had determined that antiglycan immune responses were induced by PROSTVAC-VF, we next evaluated whether any of the responses correlated with survival. Only responses that occurred in at least 25% of patients were considered, because disproportionately small strata (e.g., four vs. 24 subjects) show chance survival differences more frequently than predicted by log-rank P values. In addition, responses that occur infrequently would be more difficult to develop as biomarkers. The only glycans satisfying this criterion were BG-Adi, BG-Atri, Fsdi, and asialo-fetuin, and antibody response to only one of these correlated with survival. In particular, overall survival correlated positively with change in anti-Fsdi total Ig measured at a dilution of 1:200 (P = 0.015) (Fig. 3A).
Fig. 3.
Kaplan–Meier survival curves for patients stratified by postvaccination response to Fsdi. Kaplan–Meier curves were generated using actual overall survival data (A–C) or HPS data (D–F) for patients stratified according to anti-Fsdi responses (IgG+IgM+IgA). (A and B) In both discovery (A) and validation (B) sets, humoral responses to Fsdi were associated with improved overall survival. (C) Increased anti-Fsdi antibody levels also were seen after inoculation with wild-type viral vectors; however, humoral responses did not correlate with improved survival for control patients. (D–F)To verify that there were no systematic differences between the strata at baseline, Kaplan–Meier survival curves were generated using the HPS based on prevaccination prognostic data.
Antibody Responses and Survival Correlation Are Consistent in an Independent Patient Set.
Next, we focused on validating our initial findings in an independent group of patients treated with PROSTVAC-VF. We performed an analysis similar to that described above on sera from a second, separate phase II study of PROSTVAC-VF that included 76 vaccinated patients and 37 controls randomly assigned to receive wild-type viral vectors (24). As before, all clinical and survival data were blinded during the profiling of serum samples in this validation phase.
As seen in the discovery phase, numerous antiglycan IgM, IgG, and total Ig responses were observed 2–4 mo after the initial vaccination (Fig. 1B). Again, responses were directed most frequently at three structurally related glycans: Fsdi, BG-Atri, and BG-Adi. The frequency and magnitude of responses were similar in the discovery and validation sets (Fig. 2B). Once again total Ig responses to Fsdi were the largest in magnitude and showed statistically significant changes (≥2.6-fold) in the most patients (validation set = 64%; maximum change = 219×). Fewer patients showed changes for BG-Adi (validation set = 55%; maximum fold change = 74×) and BG-Atri (validation set = 32%; maximum fold change = 14×). Changes to these glycans tended to occur in many of the same individuals (Fig. 2C). Humoral responses to all other array components occurred in fewer than 25% of patients.
Humoral responses to Fsdi were the only responses that showed a statistically significant correlation with survival in our initial study; therefore, this was the only response that was examined in the validation set for a survival correlation. Importantly, we used the initial study to specify the threshold for stratifying patients according to anti-Fsdi responses in the validation phase. This conservative step of using the same threshold in the discovery and validation phases was necessary to prevent multiple hidden comparisons. Additionally, this step avoided the need to adjust for multiple comparisons because only one hypothesis (anti-Fsdi humoral responses at a specified threshold) was carried over to the validation set.
Postvaccination overall survival also correlated with humoral responses to Fsdi in the validation sample set. At the threshold identified by the discovery set, fourfold and larger increases in total serum Ig to Fsdi were associated with statistically significant improvements in survival (P = 0.008; Fig. 3B). Patients with fourfold and larger responses had a median survival that was ∼9 mo longer than that in patients with little or no response (Fig. 3 A and B). The odds ratio for living at least 3 y was 3.2 (95% confidence interval, 1.2–8.8; P = 0.02). Patients with fourfold and larger responses also lived significantly longer than control patients, with an improvement in median survival of about 11 mo (29.0 vs. 17.9 mo; P = 0.004). In contrast, patients in the vaccine arm who had little or no Fsdi response did not have a statistically significant difference in survival relative to the control patients (19.4 vs. 17.9 mo; P = 0.84). For comparison, the median survival for the 76 PROSTVAC-VF patients in this study was 25.5 mo. (Note that we did not receive serum samples from all the patients from this clinical trial; therefore, the median survival for our subset is somewhat different from the value reported for the entire group.) Taken together, these analyses demonstrate a significant improvement in survival for patients treated with PROSTVAC-VF who had Fsdi responses.
In addition to overall survival, we also evaluated survival relative to predicted survival based on the Halabi model. The Halabi model uses seven independent prognostic markers to predict survival of men with metastatic castration-resistant prostate cancer (mCRPC) treated with chemotherapy (23). It is based on multivariable analysis of more than 1,100 patients and provides a powerful tool for determining balanced prognostic criteria among groups of men. For each individual, the survival relative to expectations was calculated by subtracting the Halabi predicted survival (HPS) from the actual overall survival (OS). When a threshold of fourfold for Fsdi responses is used, the odds ratio for living longer than expected (i.e., OS − HPS > 0) was 3.67 (95% confidence interval, 1.38–9.75; P = 0.009). For patients above the threshold, the median improvement in actual survival relative to expected survival was 8.7 mo. For patients below the threshold, there was no increase in median survival relative to expectations (median OS − HPS = −1.0 mo). These data indicate that patients with an Fsdi response have both longer survival and longer survival relative to expected survival.
With the larger number of samples in the validation study, we could analyze survival correlations further over a broader range of anti-Fsdi humoral responses to assess consistency and robustness. Odds of long-term survival increased steadily with magnitude of response to Fsdi (Fig. S2). For example, patients with 10-fold or higher response had a median survival >3.5 y—more than twice as long as patients with little or no response and also twice as long as control patients vaccinated with wild-type poxviruses (Fig. S3). In addition, we also used the Kaplan–Meier survival estimator to evaluate all thresholds in which both strata had at least seven patients. Of the 69 thresholds studied for patients receiving PROSTVAC-VF, 20 had a statistically significant improvement in survival (P < 0.05), and another 12 showed a trend toward increased survival (P < 0.10) for patients with larger Forssman responses. These results demonstrate that the survival correlation is consistent across a broad range of anti-Fsdi humoral response thresholds. Interestingly, patients vaccinated with control vectors mounted similar immune responses to Fsdi, BG-Atri, and BG-Adi, although patients vaccinated with PROSTVAC-VF tended to have more robust responses to Fsdi. As mentioned previously, spontaneous changes in antibody levels to these glycans were not found in healthy subjects or in nonvaccinated prostate cancer patients treated with radiation in another study (Fig. 2B) (28). This pattern of antibody changes occurring after inoculation with PROSTVAC-VF or its control viral vectors, but not in healthy subjects or nonvaccinated patients, is consistent with a response to glycans on the viral vectors, as discussed in more detail below.
Anti-Fsdi Response Is Prognostic Specifically for Vaccinated Patients.
Once a consistent survival association for anti-Fsdi responses was observed in patients from the two phase II trials, we next assessed the prognostic specificity of this potential biomarker. In particular, patients who are able to mount an immune response to Fsdi may have less advanced disease or higher-functioning immune systems that could lead to longer overall survival regardless of treatment. To evaluate this possibility, we used a multipronged approach. First, we examined potential relationships between immune response to Fsdi and various measures of general health and disease severity. We evaluated relationships between anti-Fsdi responses and HPS. Before vaccination, patients with and without responses to Fsdi had similar HPS (Fig. 3 D–F), indicating equally severe disease and comparable expected outcomes. In addition, anti-Fsdi responses did not correlate with age, PSA level, or Gleason score (Fig. 4), indicating that patients with robust responses to the Fsdi are not simply the healthiest patients or the patients with the least aggressive disease.
Fig. 4.
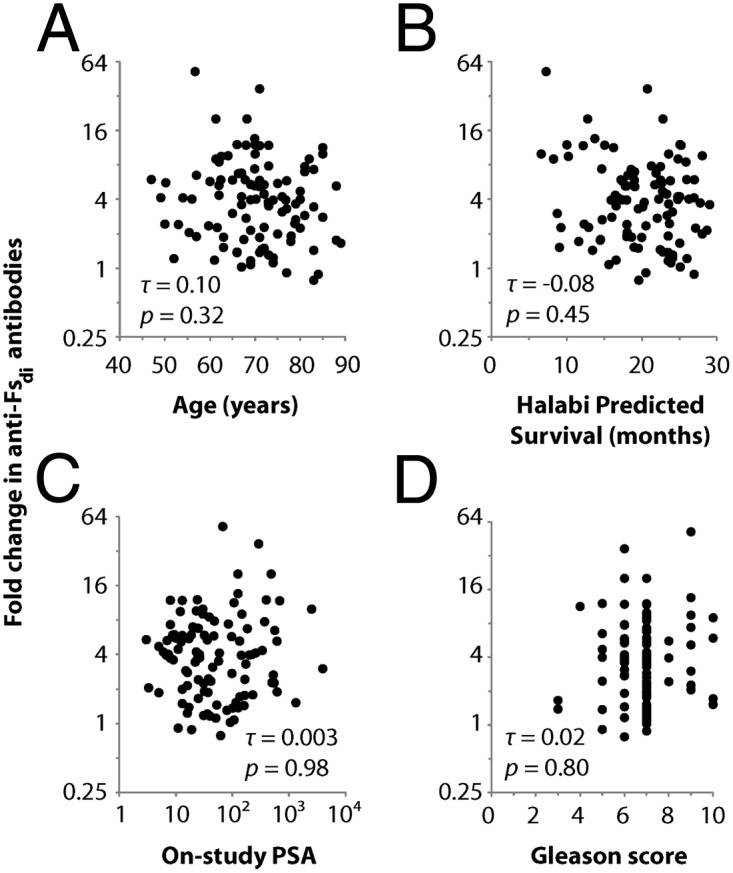
Correlation between humoral response to Fsdi and other prognostic factors. There was no association between anti-Fsdi humoral responses and (A) age, (B) HPS, (C) on-study PSA levels, or (D) Gleason score of the primary prostate tumor. The HPS calculation was based on seven prognostic factors: PSA, Gleason score, serum lactate dehydrogenase, AP, hematocrit, presence of visceral disease, and Eastern Cooperative Oncology Group functional status. Correlations were assessed with Kendall correlation coefficients (τ) and their associated P values.
Second, we examined potential relationships between immune response to Fsdi and measures of overall immune function. In particular, we calculated nonparametric Kendall correlations (τ) to evaluate associations between anti-Fsdi responses and other aspects of immune function (Fig. 5). An absence of statistically significant associations would indicate that anti-Fsdi responses provide unique information about immune function. First, we considered the possibility that immunosuppression blunts anti-Fsdi responses. However, response to Fsdi did not correlate with the number of circulating T-regulatory cells (Fig. 5A), suggesting that these immunosuppressive cells are not the cause of heterogeneity in anti-Fsdi responses. As another measure of immune function, we assessed associations between responses to Fsdi and the vaccine’s viral vectors, which provide general indicators of immune function. There was no significant association between humoral responses to Fsdi and overall antivector titers (Fig. 5 B and C). Additionally, there was a weak correlation between responses to Fsdi and fold increase in PSA-specific T cells (Fig. 5D), which suggests that monitoring anti-Fsdi responses provides additional information about immune responses not captured by assaying T-cell function in peripheral blood. These results indicate that patients with responses to the Fsdi are not simply the ones with the highest immune capacity.
Fig. 5.
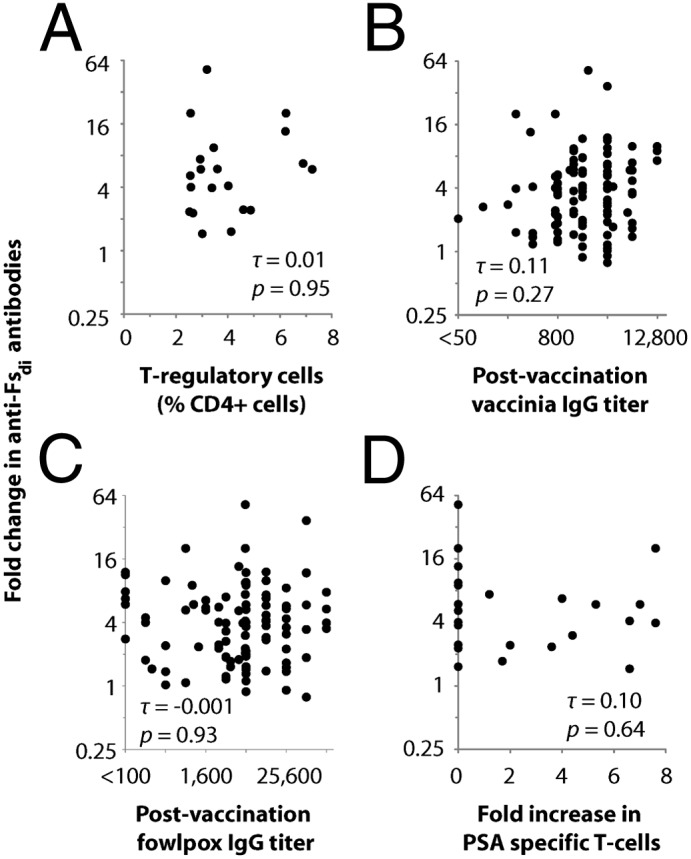
Correlation between humoral response to Fsdi and other measures of immune function. Sera collected before and after vaccination with PROSTVAC-VF were analyzed to determine changes in total Ig (IgG+IgM+IgA) reactive with Fsdi. Fold-changes in anti-Fsdi Ig were plotted against other immunological data. Correlations with (A) peripheral levels of T-regulatory cells, overall postvaccination IgG titers for (B) vaccinia and (C) fowlpox, and (D) fold increase in PSA-specific T cells by ELISPOT assay. τ, Kendall correlation coefficients.
Finally, we evaluated relationships between immune response to Fsdi and survival in control patients, who also mounted anti-Fsdi responses after inoculation with wild-type vectors lacking the key transgenes for PSA and the three costimulatory molecules. Unlike in the PROSTVAC-VF arm, however, humoral response to Fsdi in control patients did not have a statistically significant correlation with improved survival (Fig. 3C), even though these patients showed IgG and IgM changes similar to those in vaccinated patients (Fig. S4). More specifically, we used the Kaplan–Meier survival estimator to evaluate all potential thresholds in the control arm in which both strata had at least seven patients. In the control arm, none of the thresholds yielded a statistically significant difference in survival or a trend toward a difference in survival between strata (i.e., none had a P < 0.10). The lack of a survival correlation in controls suggests that anti-Fsdi responses are prognostic specifically for patients vaccinated with PROSTVAC-VF. Moreover, we used the Kaplan–Meier survival estimator to compare the survival of PROSTVAC-VF patients who mounted a fourfold or greater Fsdi response with the survival of the subset of control patients who had a fourfold or greater Fsdi response. Patients treated with PROSTVAC-VF were found to have significantly longer survival (P = 0.03), indicating that patients with robust responses to the Fsdi are not simply the healthiest patients or the patients with the least aggressive disease.
Anti-Fsdi Antibodies Correlate with Survival for Combined Therapy.
Having seen a consistent survival correlation in our discovery set and in an independent validation set, we next assessed the relationship between Fsdi antibody responses and survival for PROSTVAC-VF when used as part of a combination treatment, because combining PROSTVAC-VF with additional treatments may produce synergistic effects (29). Specifically, we analyzed anti-Fsdi responses induced during a phase 2.5 trial of PROSTVAC-VF combined with the radiopharmaceutical Quadramet (Samarium-153 lexidronam pentasodium) in patients with two or more bone lesions related to their prostate cancer despite prior hormonal ablation and treatment with docetaxel (30). Interestingly, despite being associated with leukopenia (31), Quadramet did not blunt increases in total anti-Fsdi Ig induced by PROSTVAC-VF. Of 13 patients who received Quadramet and PROSTVAC-VF, seven (54%) showed fourfold or larger anti-Fsdi responses, similar to the frequencies of response in the discovery (61%) and validation (51%) sets discussed above. Stratifying patients according to anti-Fsdi responses according to the threshold applied in Fig. 3 showed a statistically significant difference in overall survival (P = 0.028; difference in median survival, 1.9 y) (Fig. 6). This survival correlation further validates anti-Fsdi responses as a potential biomarker for PROSTVAC-VF and extends its clinical significance from mCRPC patients receiving PROSTVAC-VF as a single agent to include patients with radiographically evident metastases receiving combination therapy.
Fig. 6.
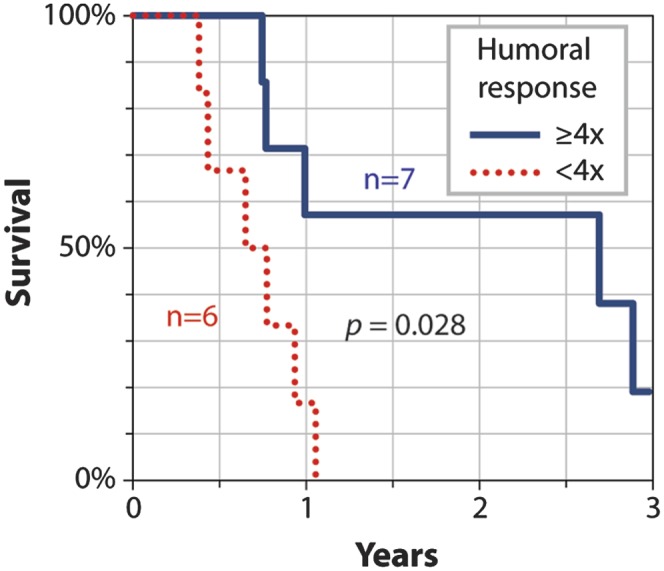
Kaplan–Meier survival curves for combination therapy stratified by postvaccination response to Fsdi. Patients (n = 13) treated with PROSTVAC-VF and Quadramet were stratified according to their postvaccination humoral anti-Fsdi responses. Probability of survival is plotted for patients with significant (solid blue) or little to no (dashed red) fold change in total Ig in response to Fsdi. The Kaplan–Meier curves were generated using the measurement technique and threshold used for stratifying Forssman responses in Fig. 3.
Anti-Fsdi Antibodies Are Directed at Viral Vectors.
To understand better how humoral responses to Fsdi are linked with improved survival, we next investigated the source of the antigen that produced these humoral responses. Several factors led us to hypothesize that the anti-Fsdi, BG-Adi, and BG-Atri responses are triggered by glycans on the viral vectors. First, these antiglycan humoral responses were seen following inoculation with either PROSTVAC-VF or wild-type viral vectors but not in nonvaccinated patients, indicating that the response is caused by a vaccine component other than the transgenes or GM-CSF. Second, a nonhuman source of the Forssman antigen is more likely than a human source because humans cannot biosynthesize the Forssman antigen, except in very rare instances (32, 33). Third, the chicken embryo dermal cells used as host cells for production of the poxviruses express blood group A (34) and the Forssman antigen (35, 36). Carryover of host-cell glycans during production of viruses is well known (37–40) and could cause PROSTVAC-VF’s viral vectors and control viral vectors to display both blood group A and the Forssman antigen.
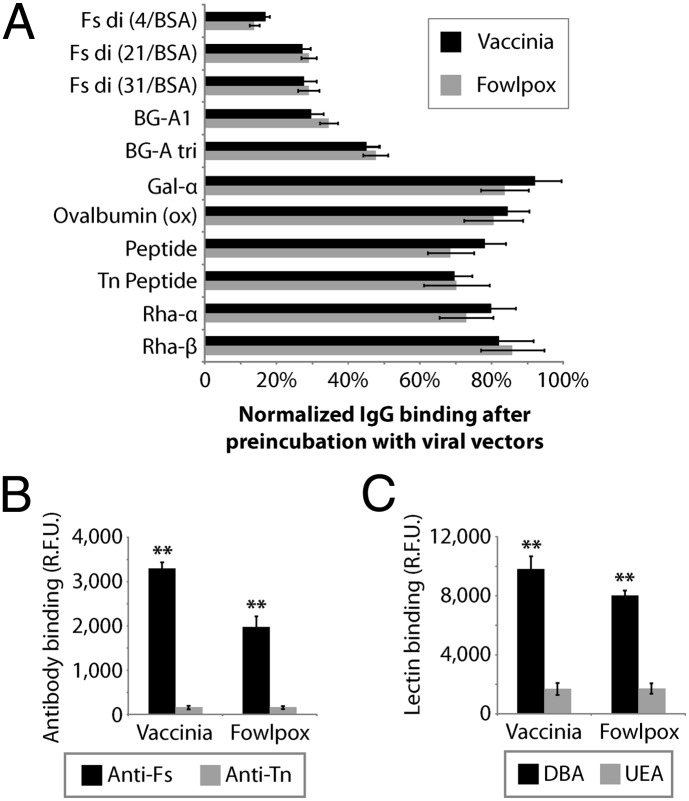
Two approaches were used to assay the viral vectors for Forssman antigen. First, we used a competitive binding assay to detect binding of serum antibodies to the viral vectors (Fig. 7A). Sera from patients with the largest responses to Forssman antigen and blood group A were pooled and preincubated with the viral vectors. Binding of serum antibodies to the viral vectors was detected as a reduction in signal intensity on the array. Preincubation of pooled serum with vaccinia reduced binding of IgG antibodies to Fsdi by 80%, which was the most substantial reduction for any component on the array. Substantial reductions also were observed for BG-A–related glycans. ELISA experiments with monoclonal antibodies and lectins provided additional evidence that the viral vectors contain Fsdi (Fig. 7 B and C) and BG-A (26).
Fig. 7.
Glycan profiling of PROSTVAC-VF’s viral vectors. (A) IgG antibody binding to glycans after preincubating serum with PROSTVAC-VF’s viral vectors was normalized to IgG binding under standard conditions in the absence of the viral vectors. The peptide sequence was Ac-S-S-S-G, and the clustered Tn peptide was Ac-Tn(Thr)-Tn(Thr)-Tn(Thr)-G (20/BSA). Error bars ± SD (n = 4); P = 2e-7 by two-way ANOVA.] (B) Additionally, the vectors differentially bound monoclonal antibodies specific for Forssman antigen (Fs) or control (Tn antigen). (C) Comparison of vaccinia and fowlpox binding to lectins reactive for Forssman antigen (DBA) or blood group H (UEA). Error bars ± SD (n = 3). **P < 0.001 for Forssman-specific binding relative to control.
Discussion
Therapeutic cancer vaccines are promising treatments, but clinical responses vary considerably from one patient to another. Strategies to distinguish responders from nonresponders could improve patient care substantially by allowing more personalized treatment decisions. For example, the ability to identify nonresponders within the first 3 mo of treatment could enable clinicians to adjust treatments rationally many months to years before clinical assessments (e.g., tumor burden, PSA levels) become reliable indicators of an effective response. Although previous investigations of immune responses to vaccination have found potential associations with clinical responses (1, 5, 6, 10, 11), no clinically validated biomarker of efficacy is available for any cancer vaccine. Detailed investigations of T-cell responses, humoral responses to protein antigens, and immunosuppressive T-regulatory cells have not accounted consistently for variation in survival benefit among patients (2, 3, 5, 6).
Major challenges in identifying promising biomarkers for cancer vaccines include difficulty in obtaining enough samples for independent discovery and validation stages as well as a lack of control groups for assessing a biomarker’s vaccine specificity. In this study, we were fortunate to have access to serum samples from multiple clinical trials of PROSTVAC-VF, a poxvirus-based cancer vaccine currently in phase III clinical trials for prostate cancer, comprising more than 100 vaccinated patients. In addition, we obtained sera from control subjects vaccinated with wild-type viral vectors as well as nonvaccinated control subjects treated with radiation. This unique collection of samples provided an opportunity to identify responses that correlated with survival and to assess the specificity and independence of these responses from other clinical factors.
In this study, we profiled antiglycan antibody levels during the first few months on treatment with PROSTVAC-VF. Although this vaccine is not specifically designed to generate antiglycan immunity, we demonstrate that antiglycan antibody responses are induced by this vaccine. In addition, we demonstrate that antibody responses to the Forssman antigen have a statistically significant correlation with survival for patients treated with PROSTVAC-VF as a single agent or in combination with Quadramet in mCRPC. The correlation was consistent in patients from three separate phase II clinical trials. Responses to Fsdi induced 2–3 mo after the first vaccination were associated with an eight times better likelihood of surviving 3.5 y and an increase in median survival of ∼9 mo. The correlation was specific to patients receiving PROSTVAC-VF, because antibody responses to the Forssman antigen did not show a significant correlation with general prognostic indicators, features of disease severity, or measures of general immune function. Moreover, no significant correlation was observed in controls vaccinated with wild-type poxviruses lacking the key tumor-associated antigen (i.e., PSA).
These results on antibody responses provide unique insights beyond our earlier studies on the relationships between preexisting antiglycan antibodies before vaccination and survival (26). In the previous study, we found that preexisting IgM to BG-Atri correlated positively with survival. Although this result was consistent with our central hypothesis that antiglycan antibodies are relevant to clinical outcomes for cancer vaccines, the correlations of preexisting antibodies with survival are not, in general, good indicators that an antibody response will be produced by a vaccine, nor are they good indicators that an antibody response will correlate with survival. This caveat is especially true for a vaccine designed to stimulate T-cell responses, such as PROSTVAC-VF. Given that (i) antibody responses to the overall viral vectors do not correlate with survival, (ii) preexisting IgM, IgG, and total Ig levels for Fsdi do not correlate with survival (26), and (iii) the vaccine does not induce antibody responses to PSA (41), the induction of a response to a viral vector glycan and its correlation with survival was unexpected and represents an aspect of the immune response to PROSTVAC-VF, and to poxviruses in general, not previously appreciated.
Anti-Forssman humoral responses were traced to the viral vectors based on several lines of evidence. First, the Forssman antigen, a glycolipid composed of a pentasaccharide attached to a ceramide, is not expressed in humans, who lack a functional Forssman synthase to catalyze the addition of the penultimate GalNAc residue to a tetrasaccharide glycolipid precursor, Gb4 (also referred to as the “P antigen”). Although polymorphisms that reactivate Forssman synthase do occur in humans (33), they are extremely rare and are unlikely to account for the high frequency of anti-Fsdi responses observed in this study. Therefore, the Forssman antigen likely is of nonhuman origin. Second, anti-Forssman responses were detected in subjects inoculated with either PROSTVAC-VF or wild-type vectors but not in nonvaccinated patients. This result indicates that vaccination with a poxvirus is necessary (i.e., changes in anti-Forssman antibody levels do not occur spontaneously in patients with prostate cancer), but the presence of a tumor-associated antigen and costimulatory proteins on the vaccine is not required for this antibody response. Third, the Forssman antigen was detected on viral vectors, and these vectors could competitively inhibit binding of serum antibodies to surface-bound Forssman glycans. Fourth, carryover of cell-surface glycans onto enveloped viruses is well known, and the chicken embryo dermal cells used to produce PROSTVAC-VF can biosynthesize the Forssman antigen.
Interestingly, antibody responses to the Forssman antigen were not simply a surrogate for immune capacity or overall response to the viral vectors. Forssman responses did not correlate well with overall titers to the poxviruses. In addition, previous studies found no link between survival and overall antivector titers (23, 24). Therefore, responses to Fsdi on the viral vectors provided unique information not captured by overall titers to the viral vectors, which may overlook heterogeneity in how individuals recognize specific epitopes and do not distinguish between neutralizing and nonneutralizing antibodies.
Responses to anti-Forssman antibody could be linked to clinical outcomes in a variety of ways. One possibility is that the antibodies act directly on prostate tumors; however, this seems unlikely, considering that human cells rarely produce the Forssman antigen and that no survival correlation was observed in control subjects who mounted an anti-Forssman response. Rather, the presence of the Forssman antigen on the virus suggests this xenoantigen may function as an adjuvant. Several reports have demonstrated that serum antibodies against xenogenic carbohydrate antigens on vaccines can enhance immune responses (42–44). For example, recognition of α-Gal on viruses by antibodies facilitates trafficking to dendritic cells, opsonization, and cross-presentation (45); these effects are notable, considering that immune complexes have been shown specifically to enhance the presentation of PSA on dendritic cells (46). Moreover, genetic modification of cancer cell vaccines to express α-Gal can enhance immune responses and break tolerance (42, 47). Because Forssman antigen and α-Gal are both T-cell–independent antigens (not presented on the MHC), and are both capable of inducing hyperacute rejection, anti-Forssman and anti–α-Gal antibodies may act through similar mechanisms. Alternatively, the activated B cells that produce the Forssman antibodies may play a key role in shaping the immune response. Additional studies will be necessary to evaluate the mechanistic link with survival more fully.
Our results have a number of important implications for PROSTVAC-VF and other vaccines produced in nonhuman cells. First, tracking changes in a patient’s anti-Forssman antibody levels during the initial 2–3 mo of treatment with PROSTVAC-VF could enable clinicians to adjust treatment rationally many months to years before clinical assessments (e.g., tumor burden, PSA levels) become reliable indicators of an effective response (48). With treatment options for prostate cancer expanding rapidly (with five recent drug approvals and one more expected shortly) (1, 49–53), there is a clear need to determine which particular therapy is best suited for each of the >240,000 men diagnosed with prostate cancer annually in the United States (54). Because therapies with the fewest side effects, such as therapeutic vaccines, often are considered first for patients with minimal or no symptoms, a test that could determine the likelihood of eventual clinical benefit within a few months of taking the vaccine would be highly valued in this rapidly changing clinical landscape. This type of test eventually may guide physicians in selecting patients for further vaccine alone, adding additional therapy, such as immune checkpoint inhibition, to the vaccine (55), or pursuing an altogether different course of therapy, such as chemotherapy. Prospective studies of PROSTVAC-VF could include monitoring anti-Forssman humoral responses to define further the potential clinical utility of this biomarker.
Second, the link between postvaccination survival and a xenogenic glycan on the vaccine’s viral vector suggests that immunogenic glycans on viral vectors may be an underappreciated feature of vaccine design and characterization, especially for vaccines that are not designed to induce immune responses to glycans. Analysis of a viral vector’s glycans may be an important aspect of quality control. The type of host cell and growth conditions, as well as other factors that influence glycans displayed on viral vectors, may influence clinical outcomes for poxvirus-based vaccines significantly. Moreover, engineering vaccines to display Forssman xenoantigen could be a generally useful strategy to enhance vaccine immunogenicity.
Third, Forssman antibodies may be relevant to a variety of other vaccines and biological agents. For example, the wild-type vaccinia vector used to immunize control subjects has been used as a smallpox vaccine for many years. There is considerable interest in defining the key antigens targeted by the immune system upon immunization with the small pox vaccine and in understanding the factors that influence the development of protective immunity. In this study we specifically demonstrate human antibody responses to glycans on vaccinia. Therefore, our results reveal a previously unrecognized aspect of the immune response to this vaccine/virus. Because only 50–60% of patients had a response to the Forssman antigen, our results also demonstrate that the variability in responses to poxvirus glycans can be a previously unrecognized contributor to the heterogeneity of immune responses. A number of other enveloped viruses also are produced in chicken-derived cells and are likely to display the Forssman xenoantigen. Some examples that currently are in clinical trials or clinical use include other poxvirus-based cancer vaccines [e.g., PANVAC (56) and rV-NY-ESO-1 (57)], poxvirus-based HIV vaccines [e.g., ALVAC-HIV (58)], oncolytic poxviruses (59, 60), and the influenza vaccine. Moreover, enveloped viruses produced in other Forssman-positive host cells (e.g., CHO, MDCK, NIH 3T3, BHK21-F) also may display the Forssman antigen. Therefore, anti-Forssman immunity could influence the clinical efficacy of a broad range of vaccines and biological agents, even when they are not designed to induce antiglycan immunity.
Although these results are promising, certain limitations should be noted. In particular, our study was retrospective and observational, like other biomarker studies done in conjunction with randomized clinical trials. To guard against bias inherent in observational studies, we analyzed two independent sample sets and verified that strata defined by an anti-Fsdi response were balanced according to a number of prognostic factors. Furthermore, we confirmed the findings within a third patient population receiving combination therapy. Nevertheless, it remains possible that stratifying according to anti-Fsdi response resulted in subtle differences between groups in disease severity or other clinical factors that were not accounted for by our control analyses. Although our study comprised more than 100 vaccinated patients, as well as controls to demonstrate the prognostic specificity of anti-Fsdi responses, additional prospective studies with larger numbers of patients and control subjects will be an important focus of future research. An ongoing phase III clinical trial evaluating overall survival is anticipated to enroll 1,200 patients and could provide additional studies on Forssman responses as early indicators of a favorable immune response. A second limitation of our study is that only a small subset of the human and chicken glycomes is represented on our glycan array. Therefore, the array may have missed antibody responses to other glycans, such as additional xenogenic glycans, on PROSTVAC-VF’s viral vectors. Third, our analyses focused on antibody responses that occurred frequently in patients; therefore, infrequent responses that correlate with survival may not have been detected. Moreover, combinations of infrequent responses were not evaluated. Fourth, the glycan array detects only free antibodies in serum; antibody responses that result in antibody–antigen complexes would not have been detected.
Our results underscore the importance of studying antiglycan immune responses for biomarker discovery and vaccine development. Immune responses to carbohydrates traditionally have been very difficult to evaluate, when a complex assortment of glycans is present on an immunogen. With advances in glycan microarray technology, high-throughput analyses of antiglycan antibody responses to hundreds of potential glycan antigens in hundreds of patient samples can be accomplished rapidly and efficiently. Our study demonstrates how more comprehensive profiling of antiglycan responses can uncover previously unrecognized aspects of the immune response that are relevant to our basic understanding of vaccine immunology and may lead to the development of new biomarkers and the design of improved vaccines. In addition, these findings highlight the potential of antiglycan immune responses to advance personalized medicine.
Materials and Methods
Serum Samples.
Serum samples were collected during two previously reported phase II clinical trials of PROSTVAC-VF (23, 24) and a completed phase 2.5 trial of Quadramet alone or combined with PROSTVAC-VF (30). For the discovery set, serum samples came from 28 patients with mCRPC who enrolled in a single-center phase II study of PROSTVAC-VF (23). The independent validation set (76 vaccinated patients and 37 controls randomly assigned to receive wild-type vaccinia and fowlpox) came from a placebo-controlled, multicenter study of PROSTVAC-VF (24) in which all patients received immunizations according to a single regimen. All patients received the same dose of vaccinia and fowlpox vectors, and the vaccination schedule was the same except that the validation subjects received an additional boost on day 14. Across all study centers, sera were obtained in serum-separator tubes, processed within 4 h, and stored at −80 °C until assayed. We included all study sites in our analysis after clustering showed no systematic differences in antiglycan antibody profiles that might have resulted from variations across different sites in sample collection or processing (Figs. S5 and S6). Sera were collected before vaccination and at least one postvaccination time point (Dataset S1). For the discovery set, we analyzed data from the time point closest to 3 mo after initial vaccination in cases when multiple postvaccination samples were available. For the validation set, all reported postvaccination changes were measured by comparing prevaccination antibody levels with postvaccination levels measured 2–4 mo after initial vaccination. (Consistency of postvaccination measurements was verified by comparing multiple time points from 2 to 4 mo.) (Fig. S1.) Samples for the Quadramet/PROSTVAC combination trial were analyzed from the available time point closest to 3 mo postvaccination (Dataset S1). Additionally, serum samples were collected over 2–3 mo from healthy subjects (n = 7) and from prostate cancer patients (n = 9) before and after treatment with local definitive radiotherapy instead of PROSTVAC-VF (28).
High-Throughput Profiling of Serum Anti-Glycan Antibodies.
Serum antiglycan antibodies were profiled on a glycan microarray (array components are listed in Dataset S1). Before array printing, glycans were conjugated to albumin via short, flexible linkers with the goal of mimicking the density and spacing of native glycans (61, 62). For example, the Forssman disaccharide was conjugated to BSA via mercaptoethylamine glutarate to measure the anti-Fsdi antibodies reported here. The array format and assay have been described previously (63), along with analysis of reproducibility (27) and validation with numerous antibodies and lectins (61–64). Arrays were printed as previously described (63) using SMP2 pins (TeleChem). Print buffer included DyLight 649 (0.7 μg/mL) (Thermo Scientific) to assess print quality before being washed away before serum assays. Sera were diluted at 1:50 or 1:200 to obtain the necessary dynamic range to measure changes in both high- and low-abundance antibodies. Unless otherwise noted, all changes reported were measured at a dilution of 1:200. Bound antiglycan antibodies were detected with fluorescent secondary antibodies specific for IgM, IgG, or total Ig (Jackson ImmunoResearch) (Table S1 and Dataset S1). Clinical data were blinded during data collection and processing.
Because >2,000 arrays were required, precautions were taken to minimize technical variations and to monitor print quality (65). Slides came from the fewest possible print batches, and consistency of print batches was checked using reference serum. Samples were analyzed in a random order to ensure intermixing of controls and vaccinated patients with varied responses to PROSTVAC-VF. Additionally, the same experimenter collected all array data, and samples were analyzed on replicate slides to identify technical faults.
Glycan Profiling of Viral Vectors.
Glycans on viral vectors were assayed by adapting the glycan microarray for a competitive binding assay. Postvaccination serum (diluted 1:500 in 1% BSA and 0.3% HSA) was preblocked for 1.5 h at 37 °C with vaccinia (9.1 × 109 pfu) and fowlpox (6.9 × 1010 pfu) that had been propagated in chicken embryo dermal cells, purified with a sucrose cushion, and UV-inactivated. The presence of particular glycans on viral vectors was detected as reduced binding of preblocked serum relative to binding of control serum.
Second, the expression of Forssman antigen on viral vectors was assayed via ELISA. Viral vectors (10 µg/mL in carbonate buffer, pH 9.6) were coated onto Maxisorp plates (Nunc) and incubated with monoclonal anti-Forssman antibody M1/87 (2 μg/mL) (Santa Cruz Biotechnology) or biotinylated Dolichos biflorus agglutinin (DBA) (10 μg/mL) (Vector Labs), which is highly reactive for the Forssman antigen. Controls included monoclonal antibody BRIC111 (10 μg/mL) (Accurate Chemical) specific for the Tn antigen (GalNAc-α linked to serine or threonine) and biotinylated Ulex europaeus agglutinin (10 μg/mL) (Vector Labs), which reacts with blood group H. All antibodies and lectins were diluted in PBS containing 3% (wt/vol) BSA. Next, bound monoclonal antibody or lectin was detected with either an alkaline phosphatase (AP)-conjugated secondary antibody [2.4 μg/mL in PBS + 3% (wt/vol) BSA] (Jackson ImmunoResearch) or AP-conjugated streptavidin (2 μg/mL in PBS + 3% BSA) (Vector Labs) that catalyzed the conversion of methylumbelliferyl phosphate (26 µg/mL in Tris, pH 9.0) (Sigma) into a fluorescent indicator.
Measurements of Peripheral T Cells and Overall Titers to Viral Vectors.
Assays of peripheral T cells along with antivaccinia and antifowlpox IgG titers (Dataset S1) were previously reported (23, 24).
Statistical Analyses.
To reduce unintentional overfitting common for high-throughput assays, leads were identified in a discovery set and were further tested with independent samples in a blinded manner. In the discovery set, antiglycan antibody profiles were screened for statistically significant survival differences in patients with high or low fold-changes in antibody levels after inoculation (nonparametric log-rank P value < 0.05 for Kaplan–Meier survival estimator). Based on a previous study of typical variations in serum antiglycan antibody occurring over 3 mo in healthy controls (27), we considered only increases >2.6-fold (i.e., increase >160%) or decreases <2.6 fold (i.e., decreases >62%). Additionally, analysis considered only strata containing seven or more patients because disproportionate strata (e.g., five vs. 23 patients) show chance survival differences more frequently than predicted by log-rank P values. Lead validation required consistent, statistically significant survival differences in the independent, blinded validation set. The survival correlation for anti-Fsdi responses was validated using samples from a multicenter phase II trial of PROSTVAC-VF and separately in a phase II trial of PROSTVAC-VF combined with Quadramet. Because only one change (anti-Fsdi total Ig) correlated with survival in the discovery set, adjusting for multiple comparisons was unnecessary.
Correlations between antiglycan antibodies and clinical or other immunologic data were assessed by nonparametric Kendall correlations (τ) and their associated P values (66). Analyses were performed in Microsoft Excel and Partek Genomics Suite (version 6.4).
Supplementary Material
Acknowledgments
We thank Kevin Camphausen for providing sera from nonvaccinated patients with prostate cancer and Professor Tom Tolbert (University of Kansas), Professor Lai-Xi Wang (University of Maryland), and Dr. Joseph Barchi (National Cancer Institute) for contributing glycans for the array. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute. C.T.C. received fellowship funding from the Pharmacology Research Associate Program of the National Institute of General Medical Sciences.
Footnotes
Conflict of interest statement: C.T.C., J.L.G., O.O., J.S., and J.C.G. are co-inventors on a patent application covering the new biomarker reported in this manuscript. PROSTVAC-VF is being developed under a Cooperative Research and Development Agreement between Bovarian Nordic and the Center for Cancer Research.
This article is a PNAS Direct Submission.
Data deposition: Glycan microarray data are supplied in Dataset S1.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314722111/-/DCSupplemental.
References
- 1.Kantoff PW, et al. IMPACT Study Investigators Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 2.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12(4):265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolchok JD, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 5.Cipriani B. Immunological markers of cancer vaccine efficacy and their clinical relevance. Biomarkers Med. 2009;3(3):253–264. doi: 10.2217/bmm.09.18. [DOI] [PubMed] [Google Scholar]
- 6.Disis ML. Immunologic biomarkers as correlates of clinical response to cancer immunotherapy. Cancer Immunol Immunother. 2011;60(3):433–442. doi: 10.1007/s00262-010-0960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine—reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189–2194. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 8.Andre F, et al. Biomarker studies: A call for a comprehensive biomarker study registry. Nat Rev Clin Oncol. 2011;8(3):171–176. doi: 10.1038/nrclinonc.2011.4. [DOI] [PubMed] [Google Scholar]
- 9.Ransohoff DF, Gourlay ML. Sources of bias in specimens for research about molecular markers for cancer. J Clin Oncol. 2010;28(4):698–704. doi: 10.1200/JCO.2009.25.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walter S, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18(8):1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 11.Sheikh NA, et al. Sipuleucel-T immune parameters correlate with survival: An analysis of the randomized phase 3 clinical trials in men with castration-resistant prostate cancer. Cancer Immunol Immunother. 2013;62(1):137–147. doi: 10.1007/s00262-012-1317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakomori S. Glycosylation defining cancer malignancy: New wine in an old bottle. Proc Natl Acad Sci USA. 2002;99(16):10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dube DH, Bertozzi CR. Glycans in cancer and inflammation—potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4(6):477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 14.Gilewski TA, et al. Immunization of high-risk breast cancer patients with clustered sTn-KLH conjugate plus the immunologic adjuvant QS-21. Clin Cancer Res. 2007;13(10):2977–2985. doi: 10.1158/1078-0432.CCR-06-2189. [DOI] [PubMed] [Google Scholar]
- 15.Ragupathi G, et al. Synthesis of sialyl Lewis(a) (sLe (a), CA19-9) and construction of an immunogenic sLe(a) vaccine. Cancer Immunol Immunother. 2009;58(9):1397–1405. doi: 10.1007/s00262-008-0654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Astronomo RD, Burton DR. Carbohydrate vaccines: Developing sweet solutions to sticky situations? Nat Rev Drug Discov. 2010;9(4):308–324. doi: 10.1038/nrd3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi T, Johnson TD, Nishinaka Y, Morton DL, Irie RF. IgM anti-ganglioside antibodies induced by melanoma cell vaccine correlate with survival of melanoma patients. J Invest Dermatol. 1999;112(2):205–209. doi: 10.1046/j.1523-1747.1999.00493.x. [DOI] [PubMed] [Google Scholar]
- 18.Ravindranath MH, et al. Ratio of IgG:IgM antibodies to sialyl Lewis(x) and GM3 correlates with tumor growth after immunization with melanoma-cell vaccine with different adjuvants in mice. Int J Cancer. 1998;75(1):117–124. doi: 10.1002/(sici)1097-0215(19980105)75:1<117::aid-ijc18>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 19.Reddish MA, MacLean GD, Poppema S, Berg A, Longenecker BM. Pre-immunotherapy serum CA27.29 (MUC-1) mucin level and CD69+ lymphocytes correlate with effects of Theratope sialyl-Tn-KLH cancer vaccine in active specific immunotherapy. Cancer Immunol Immunother. 1996;42(5):303–309. doi: 10.1007/s002620050287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rillahan CD, Paulson JC. Glycan microarrays for decoding the glycome. Annu Rev Biochem. 2011;80:797–823. doi: 10.1146/annurev-biochem-061809-152236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oyelaran O, Gildersleeve JC. Glycan arrays: Recent advances and future challenges. Curr Opin Chem Biol. 2009;13(4):406–413. doi: 10.1016/j.cbpa.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.BN ImmunoTherapeutics 2014. A randomized, double-blind, phase 3 efficacy trial of PROSTVAC +/− GM-CSF in men with asymptomatic or minimally symptomatic metastatic castrate-resistant prostate cancer (Prospect). Available at: http://clinicaltrials.gov/ct/show/NCT01322490. Accessed April 3, 2014.
- 23.Gulley JL, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. 2010;59(5):663–674. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantoff PW, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(7):1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranieri E, et al. Dendritic cell/peptide cancer vaccines: Clinical responsiveness and epitope spreading. Immunol Invest. 2000;29(2):121–125. doi: 10.3109/08820130009062294. [DOI] [PubMed] [Google Scholar]
- 26.Campbell CT, et al. Serum antibodies to blood group A predict survival on PROSTVAC-VF. Clin Cancer Res. 2013;19(5):1290–1299. doi: 10.1158/1078-0432.CCR-12-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oyelaran O, McShane LM, Dodd L, Gildersleeve JC. Profiling human serum antibodies with a carbohydrate antigen microarray. J Proteome Res. 2009;8(9):4301–4310. doi: 10.1021/pr900515y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ménard C, et al. Discovering clinical biomarkers of ionizing radiation exposure with serum proteomic analysis. Cancer Res. 2006;66(3):1844–1850. doi: 10.1158/0008-5472.CAN-05-3466. [DOI] [PubMed] [Google Scholar]
- 29.Gulley JL, Madan RA, Arlen PM. Enhancing efficacy of therapeutic vaccinations by combination with other modalities. Vaccine. 2007;25(Suppl 2):B89–B96. doi: 10.1016/j.vaccine.2007.04.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Cancer Institute 2013. 153Sm-EDTMP with or without a PSA/TRICOM vaccine to treat men with androgen-insensitive prostate cancer. National Library of Medicine NLM Identifier: NCT00450619. Available at: http://clinicaltrials.gov/ct/show/NCT00450619. Accessed April 3, 2014.
- 31.Farhanghi M, Holmes RA, Volkert WA, Logan KW, Singh A. Samarium-153-EDTMP: Pharmacokinetic, toxicity and pain response using an escalating dose schedule in treatment of metastatic bone cancer. J Nucl Med. 1992;33(8):1451–1458. [PubMed] [Google Scholar]
- 32.Xu H, Storch T, Yu M, Elliott SP, Haslam DB. Characterization of the human Forssman synthetase gene. An evolving association between glycolipid synthesis and host-microbial interactions. J Biol Chem. 1999;274(41):29390–29398. doi: 10.1074/jbc.274.41.29390. [DOI] [PubMed] [Google Scholar]
- 33.Svensson L, et al. Forssman expression on human erythrocytes: Biochemical and genetic evidence of a new histo-blood group system. Blood. 2013;121(8):1459–1468. doi: 10.1182/blood-2012-10-455055. [DOI] [PubMed] [Google Scholar]
- 34.Briles WE. Early chicken blood group investigations. Immunogenetics. 1984;20(3):217–226. doi: 10.1007/BF00364204. [DOI] [PubMed] [Google Scholar]
- 35.Szepsenwol J, Witersky E. Research on the “Forssman” antigen in eggs and in various parts of chicken embryos. C R Seances Soc Biol Fil. 1934;115:1019–1020. [Google Scholar]
- 36.Leduc EH, Tanaka N. A study of the cellular distribution of Forssman antigen in various species. J Immunol. 1956;77(3):198–212. [PubMed] [Google Scholar]
- 37.Krishnamoorthy L, Bess JW, Jr, Preston AB, Nagashima K, Mahal LK. HIV-1 and microvesicles from T cells share a common glycome, arguing for a common origin. Nat Chem Biol. 2009;5(4):244–250. doi: 10.1038/nchembio.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Springer GF, Schuster R. [Blood group A-like Forssman antigens in myxoviruses cultured in a chicken egg: Their possible pathogenetic significance in vaccines] Klin Wochenschr. 1964;42:821–823. doi: 10.1007/BF01479140. German. [DOI] [PubMed] [Google Scholar]
- 39.Springer GF, Tritel H. Blood group A active substances in embryonated chicken eggs and their relation to egggrown virus. Science. 1962;138(3541):687–688. doi: 10.1126/science.138.3541.687. [DOI] [PubMed] [Google Scholar]
- 40.Rott R, Drzenick R, Saber MS, Reichert E. Blood group substances forssman and mononucleosis antigens in lipid-containing RNA viruses. Arch Gesamte Virusforsch. 1966;19(3):273–288. [Google Scholar]
- 41.Gulley JL, et al. Immune Impact Induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immun. Res. 2014;2:133–141. doi: 10.1158/2326-6066.CIR-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi GR, et al. Effective treatment of preexisting melanoma with whole cell vaccines expressing alpha(1,3)-galactosyl epitopes. Cancer Res. 2005;65(22):10555–10561. doi: 10.1158/0008-5472.CAN-05-0627. [DOI] [PubMed] [Google Scholar]
- 43.Mandell RB, et al. The αGal HyperAcute(®) technology: Enhancing immunogenicity of antiviral vaccines by exploiting the natural αGal-mediated zoonotic blockade. Zoonoses Public Health. 2009;56(6-7):391–406. doi: 10.1111/j.1863-2378.2008.01191.x. [DOI] [PubMed] [Google Scholar]
- 44.Deguchi T, et al. Increased immunogenicity of tumor-associated antigen, mucin 1, engineered to express alpha-gal epitopes: A novel approach to immunotherapy in pancreatic cancer. Cancer Res. 2010;70(13):5259–5269. doi: 10.1158/0008-5472.CAN-09-4313. [DOI] [PubMed] [Google Scholar]
- 45.Dürrbach A, Baple E, Preece AF, Charpentier B, Gustafsson K. Virus recognition by specific natural antibodies and complement results in MHC I cross-presentation. Eur J Immunol. 2007;37(5):1254–1265. doi: 10.1002/eji.200636129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berlyn KA, et al. Generation of CD4(+) and CD8(+) T lymphocyte responses by dendritic cells armed with PSA/anti-PSA (antigen/antibody) complexes. Clin Immunol. 2001;101(3):276–283. doi: 10.1006/clim.2001.5115. [DOI] [PubMed] [Google Scholar]
- 47.NewLink Genetics Corporation 2013. Immunotherapy study for surgically resected pancreatic cancer. National Library of Medicine Identifier: NCT0107298. Available at: www.clinicaltrials.gov/ct2/show/NCT01072981. Accessed April 3, 2014.
- 48.Gulley JL, Drake CG. Immunotherapy for prostate cancer: Recent advances, lessons learned, and areas for further research. Clin Cancer Res. 2011;17(12):3884–3891. doi: 10.1158/1078-0432.CCR-10-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petrylak DP, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 50.Tannock IF, et al. TAX 327 Investigators Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 51.de Bono JS, et al. TROPIC Investigators Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376(9747):1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 52.Scher HI, et al. AFFIRM Investigators Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 53.de Bono JS, et al. COU-AA-301 Investigators Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 55.Madan RA, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: A phase 1 dose-escalation trial. Lancet Oncol. 2012;13(5):501–508. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gulley JL, et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14(10):3060–3069. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jäger E, et al. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc Natl Acad Sci USA. 2006;103(39):14453–14458. doi: 10.1073/pnas.0606512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rerks-Ngarm S, et al. MOPH-TAVEG Investigators Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 59.Kirn DH, Thorne SH. Targeted and armed oncolytic poxviruses: A novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9(1):64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- 60.Breitbach CJ, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477(7362):99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- 61.Manimala JC, Roach TA, Li Z, Gildersleeve JC. High-throughput carbohydrate microarray profiling of 27 antibodies demonstrates widespread specificity problems. Glycobiology. 2007;17(8):17C–23C. doi: 10.1093/glycob/cwm047. [DOI] [PubMed] [Google Scholar]
- 62.Manimala JC, Roach TA, Li Z, Gildersleeve JC. High-throughput carbohydrate microarray analysis of 24 lectins. Angew Chem Int Ed Engl. 2006;45(22):3607–3610. doi: 10.1002/anie.200600591. [DOI] [PubMed] [Google Scholar]
- 63.Campbell CT, Zhang Y, Gildersleeve JC. Construction and use of glycan microarrays. Curr Protoc Chem Biol. 2010;2(1):37–53. doi: 10.1002/9780470559277.ch090228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manimala JC, Li Z, Jain A, VedBrat S, Gildersleeve JC. Carbohydrate array analysis of anti-Tn antibodies and lectins reveals unexpected specificities: Implications for diagnostic and vaccine development. ChemBioChem. 2005;6(12):2229–2241. doi: 10.1002/cbic.200500165. [DOI] [PubMed] [Google Scholar]
- 65.Allison DB, Cui X, Page GP, Sabripour M. Microarray data analysis: From disarray to consolidation and consensus. Nat Rev Genet. 2006;7(1):55–65. doi: 10.1038/nrg1749. [DOI] [PubMed] [Google Scholar]
- 66.Lin JT. Alternatives to hamakers approximations to the cumulative normal-distribution and its inverse. Statistician. 1988;37(4–5):413–414. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.