Significance
HIV-1 viral protein U (Vpu) facilitates virus release from infected cells by down-regulating and degrading tetherin, a transmembrane protein upregulated by IFN that impedes the detachment of enveloped viruses from infected cells. Here we show that this activity of Vpu protects HIV-infected cells from antibodies that are capable of directing their elimination by antibody-dependent cell-mediated cytotoxicity. A direct implication of these observations is that tetherin serves as a link between innate and adaptive immunity to enhance the susceptibility of virus-infected cells to antibodies. Thus, the antiviral activity of tetherin may be much greater than previously appreciated based on its ability to inhibit virus release in cell culture assays.
Keywords: BST-2, CD317, AIDS, lentivirus
Abstract
Tetherin is an IFN-inducible transmembrane protein that inhibits the detachment of enveloped viruses from infected cells. HIV-1 overcomes this restriction factor by expressing HIV-1 viral protein U (Vpu), which down-regulates and degrades tetherin. We report that mutations in Vpu that impair tetherin antagonism increase the susceptibility of HIV-infected cells to antibody-dependent cell-mediated cytotoxicity (ADCC), and conversely that RNAi knockdown of tetherin, but not other cellular proteins down-modulated by Vpu, decreases the susceptibility of HIV-infected cells to ADCC. These results reveal that Vpu protects HIV-infected cells from ADCC as a function of its ability to counteract tetherin. By serving as link between innate and adaptive immunity, the antiviral activity of tetherin may be augmented by virus-specific antibodies, and hence much greater than previously appreciated.
Under conditions of IFN induction, tetherin is rapidly up-regulated on the surface of infected cells and prevents virus release by physically bridging nascent virions to the cell membrane (1–3). This activity can be explained by the unusual topology of tetherin, which includes an N-terminal transmembrane domain and a C-terminal glycosyl-phosphatidylinositol tail that allow both ends of the molecule to be anchored in lipid membranes (4). Although tetherin was initially identified as the cellular gene product that accounts for a late-stage defect in the release of vpu-deleted HIV-1 from restrictive cells (5, 6), it is now recognized to have antiviral activity against diverse families of enveloped viruses (7–11).
The primate lentiviruses have evolved to use at least three different viral proteins to counteract restriction by tetherin. Whereas most simian immunodeficiency viruses (SIVs) use Nef to counteract the tetherin proteins of their nonhuman primate hosts (12–14), HIV-1 and HIV-2 use their Vpu and Env proteins, respectively, to counteract human tetherin because of the absence of sequences in the cytoplasmic domain of the protein required for susceptibility to Nef (5, 6, 15). Tetherin has therefore had a significant impact on shaping the course of lentiviral evolution in primates.
Tetherin can inhibit retroviral replication in vivo as revealed by an IFN-dependent effect on the suppression of murine leukemia virus in WT, but not tetherin-deficient, mice (16). Instances of lentiviral adaptation to tetherin, including the acquisition of compensatory changes in gp41 of a nef-deleted strain of SIV passaged in rhesus macaques (17), and changes that restore the anti-tetherin activity of Nef in HIV-1–infected chimpanzees (18), further underscore the importance of tetherin antagonism for efficient virus replication in vivo. However, under certain circumstances, tetherin can also facilitate virus replication by enhancing cell-to-cell transmission (19). Indeed, a role for tetherin in promoting cell-to-cell transmission probably accounts for the selection of vpu-deficient HIV-1 under cell culture conditions designed to mimic rapid T-cell turnover (20). Thus, although the net effect of tetherin on virus replication in infected hosts is antiviral, the immunological mechanisms underlying this antiviral activity are not fully understood.
By using an assay designed to measure the ability of antibodies to direct the killing of virus-infected cells by antibody-dependent cell-mediated cytotoxicity (ADCC) (21), we show that Vpu protects HIV-infected cells from elimination by ADCC. We further demonstrate that this protection reflects the role of Vpu in counteracting restriction by tetherin. These results imply that tetherin enhances the susceptibility of HIV-infected cells to antibodies, thereby revealing an unappreciated link between innate and adaptive immunity. These results also suggest that the antiviral activity of tetherin may be augmented by virus-specific antibodies, and hence may be much greater than previously appreciated based solely on its ability to suppress virus replication in cell culture.
Results
Deletion of Vpu Increases the Susceptibility of HIV-1–Infected Cells to ADCC.
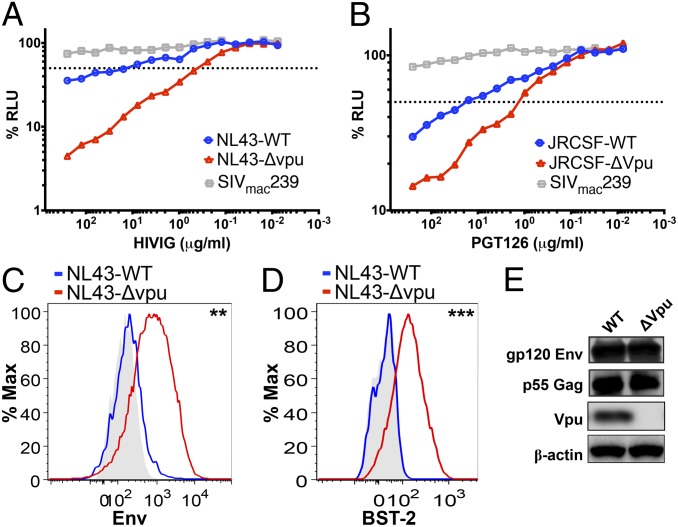
In the absence of Vpu, virions accumulate at the plasma membrane of HIV-1–infected cells as a consequence of the inhibitory effects of tetherin on virus release, resulting in visually striking electron micrographs in which infected cells are often coated with captured virus particles (6). We hypothesized that the accumulation of virus particles on the cell surface might increase the susceptibility of HIV-infected cells to elimination by ADCC. Cells infected with WT vs. vpu-deleted HIV-1 were therefore tested for susceptibility to ADCC using an assay designed to measure the ability of antibodies to direct the killing of HIV-infected cells expressing native conformations of the viral envelope glycoprotein (21). In the presence of Ig purified from the plasma of HIV-positive donors (HIVIG), cells infected with vpu-deleted HIV-1NL4-3 were ∼60-fold more susceptible to ADCC than cells infected with WT HIV-1NL4-3 (Fig. 1A and Fig. S1A). A similar difference in susceptibility to ADCC was observed for cells infected with WT vs. vpu-deleted HIV-1JR-CSF, a primary isolate that expresses an envelope glycoprotein that is more resistant to neutralizing antibodies, by using the gp120-specific monoclonal antibody PGT126 (Fig. 1B and Fig. S1D). These observations are further supported by statistical analyses of three independent experiments revealing significant differences in mean antibody concentrations resulting in half-maximal killing of virus-infected cells (i.e., 50% ADCC) and mean area under the curve (AUC) values for WT vs. vpu-deleted HIV-1NL4-3 (P < 0.0001 and P = 0.006, unpaired t test; Fig. S1 B and C) and for WT vs. vpu-deleted HIV-1JR-CSF (P = 0.017 and P = 0.007, unpaired t test; Fig. S1E and F).
Fig. 1.
Deletion of vpu increases the susceptibility of HIV-infected cells to ADCC. CEM.NKR-CCR5-sLTR-Luc target cells containing a Tat-inducible luciferase reporter gene were infected with WT vs. vpu-deleted HIV-1NL4-3 (A) or HIV-1JR-CSF (B) and incubated at a 10:1 effector:target ratio with an NK cell line that constitutively expresses CD16 in the presence of serial dilutions of antibody, either purified Ig from HIV-1+ donors (i.e., HIVIG) or the gp120-specific monoclonal antibody PGT126. SIVmac239-infected target cells were included as a control for nonspecific killing. ADCC activity was measured as the dose-dependent loss of luciferase activity in RLU over an 8-h incubation as previously described (21). The dotted line indicates 50% killing of HIV-infected cells. Surface expression of HIV-1 Env (C) and tetherin (D) was compared on cells infected with WT vs. vpu-deleted HIV-1. CEM.NKR-CCR5-sLTR-Luc cells infected with WT HIV-1 (blue lines) and vpu-deleted HIV-1 (red lines) were stained for Env and tetherin (BST-2), followed by permeabilization and intracellular staining for Gag as described in Materials and Methods. Histograms represent the fluorescence intensity of surface staining for Env (C) and tetherin (D) after gating on viable, virus-infected (Gag+) CD45+ cells relative to control samples (shaded) stained with normal human IgG (C) or an isotype control for the BST-2–specific antibody (D). Differences in the geometric mean fluorescence intensity were significant for Env (**P = 0.01, unpaired t test) (C) and tetherin (D; ***P = 0.00009). (E) Immunoblot analysis of total levels of Env, Gag, and Vpu expression in whole-cell lysates of 293T cells (tetherin-negative) transfected with WT or vpu-deleted HIV-1NL4-3 proviral DNA. The membrane was subsequently reprobed with a β-actin–specific antibody to control for sample loading. The data in A–E are representative of three different experiments. A comparison of the data from three separate ADCC assays is provided in Fig. S1.
In accordance with greater susceptibility to ADCC mediated by Env-specific antibodies, surface expression of the viral envelope glycoprotein was fivefold higher on cells infected with HIV-1 Δvpu compared with cells infected with WT HIV-1 (Fig. 1C). Moreover, this increase in the availability of Env corresponded to a 10-fold increase in surface expression of tetherin (Fig. 1D). Importantly, deletion of the vpu gene did not change total Env expression levels in tetherin-negative 293T cells (Fig. 1E), verifying that the accumulation of Env on the surface of HIV-1 Δvpu-infected cells was not a result of an overall increase in env transcription or translation.
These observations are consistent with the possibility that Vpu protects HIV-infected cells from ADCC as a result of its anti-tetherin activity. However, Vpu also has other functional activities that could contribute to the resistance of HIV-infected cells to ADCC. In addition to down-regulating tetherin, Vpu down-regulates CD4, the primary receptor for virus entry (22), and NK-, T- and B-cell antigen (NTB-A), a costimulatory molecule required for natural killer (NK) cell activation (23). We therefore sought to determine which of these activities of Vpu account for the resistance of HIV-infected cells to ADCC.
Treatment with IFNα Enhances the Susceptibility of HIV-Infected Cells to ADCC.
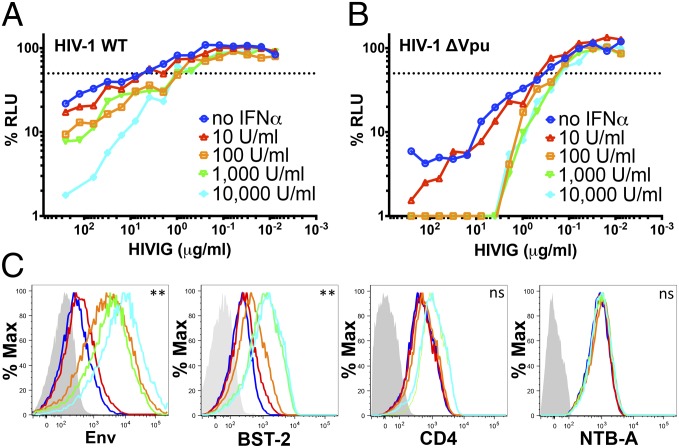
One feature that distinguishes tetherin from other cellular gene products down-modulated by Vpu is that it is strongly up-regulated in response to type I interferons. We therefore asked if IFN-α treatment could increase the susceptibility of HIV-infected cells to ADCC. Cells infected with WT and vpu-deleted HIV-1NL4-3 were incubated with increasing concentrations of IFNα ranging from 10 to 10,000 U/mL for 24 h before using them as target cells in an ADCC assay with HIVIG. Treatment with IFN-α resulted in a dose-dependent increase in the susceptibility of HIV-1 and HIV-1 Δvpu-infected cells to ADCC. Whereas cells infected with WT HIV-1 exhibited progressive increases in their sensitivity to ADCC with increasing concentrations of IFN-α (Fig. 2A), the sensitivity of HIV-1 Δvpu-infected cells did not continue to increase at IFN-α concentrations of more than 100 U/mL (Fig. 2B), suggesting that maximum sensitivity to ADCC may be achieved at a lower concentration of IFN-α in the absence of Vpu. This was reflected by comparisons of 50% ADCC concentrations and AUC values for three independent experiments. Whereas significant dose-dependent increases in the susceptibility of WT HIV-1–infected cells to ADCC were observed in mean 50% ADCC concentrations and mean AUC values (Fig. S2 B and C), significant increases in the susceptibility of HIV-1 Δvpu-infected cells to ADCC were only observed for comparisons of mean AUC values (Fig. S2 E and F).
Fig. 2.
Treatment with IFN-α enhances the susceptibility of HIV-infected cells to ADCC. HIV-1NL4-3 (A) and HIV-1NL4-3 Δvpu (B)–infected CEM.NKR-CCR5-sLTR-Luc cells were treated with the indicated concentrations of IFN-α 24 h before they were used as target cells in an ADCC assay with serial dilutions of HIVIG. The dotted line indicates 50% killing of HIV-infected cells. A comparison of the data from three separate ADCC assays is provided in Fig. S2. (C) Changes in the surface expression of Env, tetherin (BST-2), CD4, and NTB-A on HIV-1 Δvpu-infected cells in response to IFN-α were measured by flow cytometry as described in Materials and Methods. The colored lines correspond to the indicated concentrations of IFN-α in A, and the shaded histograms indicate background staining with normal human IgG or an isotype control antibody. Differences in the geometric mean fluorescence intensity of staining were significant for cells treated with 0 vs. 10,000 U/mL IFN-α for Env (**P = 0.004, unpaired t test) and for tetherin (**P = 0.002), but not for CD4 or NTB-A (not significant). ADCC responses and surface expression of tetherin, but not CD4 or NTB-A, correlated with surface levels of Env (Fig. S2 G–J).
Surface staining also revealed a corresponding dose-dependent increase in the expression of Env and tetherin, but only marginal increases in CD4 and no increases in NTB-A, with increasing concentrations of IFN-α (Fig. 2C). Moreover, Env levels on HIV-infected cells correlated with susceptibility to ADCC (r2 = 0.81, P = 0.037, Pearson correlation test) and surface expression of tetherin (r2 = 0.77, P = 0.049), but not with the surface expression of CD4 (r2 = 0.54, P = 0.16) or NTB-A (r2 = 0.46, P = 0.21; Fig. S2 G–J). These results are consistent with greater susceptibility to ADCC as a result of IFN-α–dependent accumulation of tethered virions on the surface of HIV-infected cells.
Vpu Substitutions That Impair Tetherin Antagonism Increase the Susceptibility of HIV-Infected Cells to ADCC.
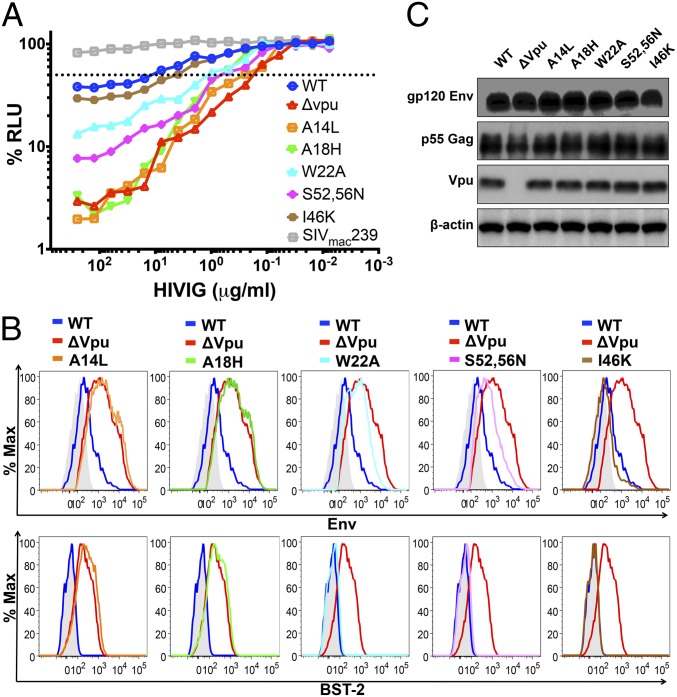
HIV-1 mutants with substitutions that differentially affect the down-modulation of tetherin, CD4 and NTB-A were tested to determine which of these Vpu activities contribute to the resistance of virus-infected cells to ADCC. Tetherin antagonism by Vpu is dependent on a physical interaction between the membrane-spanning domains of Vpu and tetherin (24–27). Thus, substitutions in the transmembrane domain of Vpu that impair binding to tetherin result in partial (W22A) to nearly complete (A14L and A18H) loss of anti-tetherin activity (28, 29). Whereas W22A and A14L directly impair Vpu binding to tetherin (28), changes in the subcellular distribution of the A18H mutant may also account for its inability to interact with tetherin (29). Notably, the A14L substitution does not affect CD4 down-regulation (28). Tetherin antagonism by Vpu also depends in part on the recruitment of βTrCP-2, an adaptor protein for an E3 ubiquitin ligase involved in targeting tetherin for degradation (24, 25, 30, 31). Hence, substitutions in a conserved DSGxxS motif in the cytoplasmic tail of Vpu (S52,56N) that prevent the recruitment of βTrCP-2 partially impair tetherin antagonism by preventing its degradation, but not its endosomal sequestration (25, 30–33). These substitutions are also known to abrogate CD4 down-regulation, but do not affect Vpu-mediated down-modulation of NTB-A (23).
The effects of each these Vpu mutations on the susceptibility of HIV-infected cells to ADCC correspond to their effects on tetherin antagonism. Whereas the A14L and A18H substitutions dramatically increased the sensitivity of HIV-infected cells to ADCC to a similar extent as wholesale deletion of the vpu gene, the W22A and S52,56N substitutions resulted in intermediate increases in sensitivity to ADCC commensurate with their partial effects on tetherin antagonism (Fig. 3A). In contrast, a substitution in the cytoplasmic tail of Vpu previously shown to interfere with CD4 binding (I46K) (34) resulted in a much smaller change in susceptibility to ADCC (Fig. 3A). These observations are supported by analyses of three independent experiments revealing highly significant differences in mean 50% ADCC concentrations and mean AUC values for the A14L, A18H, W22A, and S52,56N mutants with a more modest but reproducible difference in 50% ADCC concentrations for the I46K mutant (Fig. S3 B and C).
Fig. 3.
Substitutions in Vpu that impair tetherin antagonism increase the sensitivity of HIV-infected cells to ADCC. (A) CEM.NKR-CCR5-sLTR-Luc cells infected with HIV-1NL4-3 mutants with Vpu substitutions A14L, A18H, W22A, S52,56N, and I46K were tested for susceptibility to ADCC in the presence of the indicated concentrations of HIVIG. Target cells infected with WT HIV-1NL4-3, HIV-1NL4-3 Δvpu, and SIVmac239 were also included as controls. The dotted line indicates 50% ADCC activity. A comparison of data from three independent experiments is provided in Fig. S3 A–C. (B) Surface expression of Env and tetherin (BST-2) for CEM.NKR-CCR5-sLTR-Luc cells infected with each Vpu mutant was compared with cells infected with WT (blue lines) and vpu-deleted HIV-1NL4-3 (red lines). The color scheme for each of the mutants is the same as in A. The histogram plots represent the fluorescence intensity of surface staining for Env (Upper) and tetherin (Lower) in viable virus-infected (Gag+) CD45+ cells in relation to control samples (shaded) stained with normal human IgG (Upper) or with an isotype control for the BST-2-specific antibody (Lower). ADCC responses and surface expression of tetherin, but not CD4 or NTB-A, correlated with surface levels of Env (Fig. S3 D–G). (C) Immunoblot analysis of total levels of Env, Gag, and Vpu expression in whole-cell lysates of 293T cells (tetherin-negative) transfected with proviral DNA for WT HIV-1NL4-3 or each of the indicated vpu mutants. Reprobing with a β-actin–specific antibody was used to control for sample loading.
The effects of these mutations on susceptibility to ADCC are also reflected by increases in the surface expression of Env and tetherin. The levels of Env and tetherin on cells infected with the A14L and A18H mutants were nearly identical to cells infected with vpu-deleted HIV-1 (Fig. 3B). As expected, Env expression on cells infected with the W22A and S52,56N mutants was intermediate to Env levels on cells infected with WT and vpu-deleted virus (Fig. 3B). In this case, only a slight increase in tetherin expression was detected for these mutants vs. WT HIV-infected cells (Fig. 3B). Although the effects of these mutants on tetherin down-modulation are less than previously observed by other assays (28), their intermediate effects on Env expression and sensitivity to ADCC are consistent with partial anti-tetherin activity. For the I46K mutant, no increase in the surface expression of Env or tetherin was observed in accordance with the minor shift in the susceptibility of this mutant to ADCC (Fig. 3B). Similar to the vpu deletion mutant, none of the substitutions in Vpu affected total levels of Env expression in tetherin-negative 293T cells (Fig. 3C), confirming that these mutations do not affect transcription or translation from the partially overlapping env reading frame. Thus, the effects of each of these Vpu substitutions on the surface expression of Env and susceptibility to ADCC mirror their effects on tetherin antagonism. Indeed, surface levels of Env strongly correlated with susceptibility to ADCC (r2 = 0.93, P = 0.0004) and with surface expression of tetherin (r2 = 0.95, P = 0.0002), but not with the surface expression of CD4 (r2 = 0.0005, P = 0.96) or NTB-A (r2 = 0.17, P = 0.36; Fig. S3 D–G). Moreover, because the A14L substitution does not affect CD4 down-regulation (28), and the S52,56N substitutions do not affect NTB-A down-modulation (23), these results indicate that the resistance of HIV-infected cells to ADCC is a function of the role of Vpu in counteracting restriction by tetherin, rather than its role in CD4 or NTB-A down-modulation.
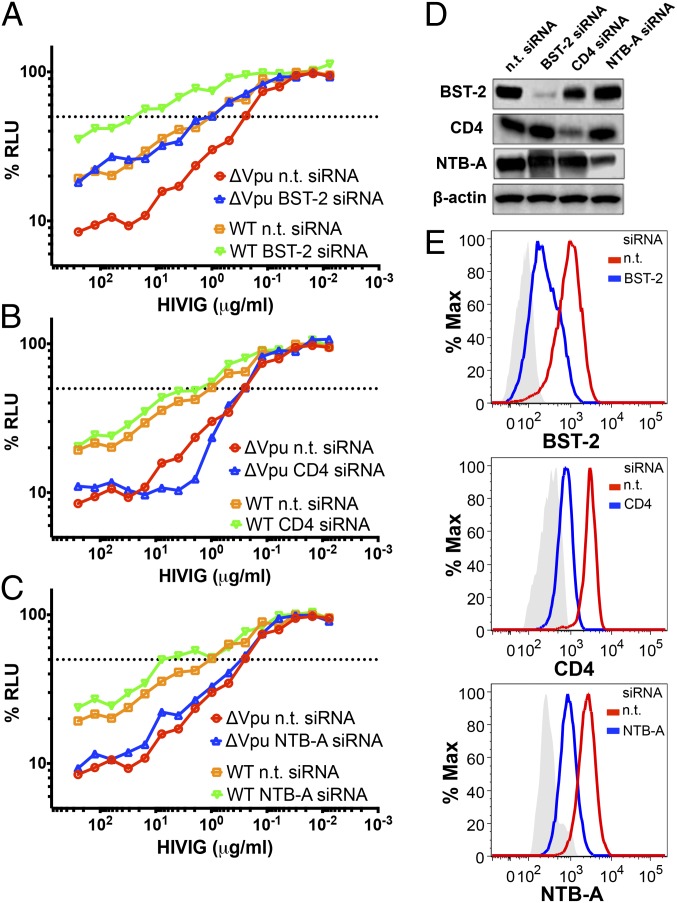
RNAi-Knockdown of Tetherin, but Not CD4 or NTB-A Increases the Resistance of HIV-Infected Cells to ADCC.
To further differentiate the activities of Vpu that contribute to resistance to ADCC, the sensitivity of HIV-infected cells to ADCC was compared following RNAi knockdown of tetherin, CD4, and NTB-A. CEM.NKR-CCR5-sLTR-Luc cells were infected with WT and vpu-deleted HIV-1, and then transfected with nontargeting siRNA or siRNAs specific for tetherin, CD4, or NTB-A 2 d before measuring their susceptibility to ADCC (Fig. 4 A–C). RNAi knockdown of protein expression was confirmed by Western blot analysis (Fig. 4D) and by flow cytometry (Fig. 4E). Whereas RNAi knockdown of tetherin significantly increased the resistance of cells infected with WT and vpu-deleted HIV-1 to ADCC (Fig. S4 A–C), RNAi knockdown of CD4 or NTB-A did not have a detectable effect (Fig. S4 D–I). These results are in accordance with IFN-dependent increases in susceptibility to ADCC and the mutational analysis of Vpu, indicating that tetherin enhances the sensitivity of HIV-infected cells to ADCC and that this sensitivity is at least partially counteracted by Vpu.
Fig. 4.
RNAi knockdown of tetherin increases the resistance of HIV-infected cells to ADCC. CEM.NKR-CCR5-sLTR-Luc cells infected with WT and vpu-deleted HIV-1NL4-3 were treated with nontargeting (n.t.) siRNA and siRNAs specific for tetherin (A), CD4 (B), and NTB-A (C) 48 h before they were used as target cells in an ADCC assay with HIVIG. The dotted line indicates 50% killing of HIV-infected cells. RNAi knockdown of tetherin (BST-2), CD4, and NTB-A was confirmed by immunoblot analysis of cell lysates (D) and by flow cytometry (E). (D) Immunoblot analysis of tetherin, CD4, and NTB-A expression in whole-cell lysates of HIV-1 Δvpu-infected CEM.NKR-CCR5-sLTR-Luc cells treated with a nontargeting (n.t.) siRNA or specific siRNAs targeting tetherin, CD4, or NTB-A. Reprobing with a β-actin–specific antibody was used to control for sample loading. Histogram plots in E represent the change in surface expression of tetherin, CD4, and NTB-A on HIV-1 Δvpu-infected target cells treated with specific siRNAs (blue lines) targeting tetherin (Top), CD4 (Middle), or NTB-A (Bottom) in relation to samples treated with a nontargeting (n.t.) siRNA (red lines). The shaded histograms indicate background staining with an isotype control antibody. A comparison of data from three independent experiments is provided in Fig. S4.
Discussion
Our results reveal a role for Vpu in protecting HIV-infected cells from ADCC, and demonstrate that this protection is a function of the ability of Vpu to counteract restriction by tetherin. These findings are supported by the following observations, which differentiate tetherin antagonism from other functional activities of Vpu: (i) increasing concentrations of IFN-α result in a dose-dependent increase in the susceptibility of HIV-infected cells to ADCC, which corresponds to a dose-dependent increase in the cell-surface expression of tetherin, but not of CD4, or NTB-A; (ii) mutations in Vpu that specifically impair tetherin antagonism without affecting CD4 or NTB-A down-regulation increase the susceptibility HIV-infected cells to ADCC; and (iii) RNAi knockdown of tetherin, but not NTB-A or CD4, increases the resistance of HIV-infected cells to ADCC.
A direct implication of these observations is that tetherin serves as a link between innate and adaptive immunity to enhance the susceptibility of virus-infected cells to antibodies. Although we measured ADCC, the same antibodies may have other antiviral activities that could potentially be magnified by tetherin, such as complement fixation and antibody-dependent phagocytosis. Tetherin may also enhance virus-specific T-cell responses via lysosomal degradation of captured virus particles for exogenous antigen presentation of viral peptides to CD4+ T cells or cross-presentation of viral peptides to CD8+ T cells. Therefore, although our data show that tetherin can increase the sensitivity of HIV-infected cells to ADCC, it is possible that tetherin also enhances other immune responses that contribute to the containment of virus replication in vivo.
A role for tetherin in enhancing the antiviral activity of antibodies may help to reconcile the strong selective pressure for HIV-1 and SIV to counteract tetherin with the comparatively modest—and, in some cases, seemingly contradictory—effects of tetherin on the replication of these viruses in vitro. Tetherin has had a significant impact on shaping the course of lentiviral evolution in primates, having selected for at least three different viral antagonists, SIV Nef, HIV-1 Vpu, and HIV-2 Env (35). Instances of HIV-1 and SIV adaptation to tetherin in nonhuman primates further underscore the selective pressure for these viruses to overcome restriction by tetherin (17, 18). However, in contrast to other restriction factors, such as the TRIM5 and APOBEC3 proteins, which often impose a complete block to virus replication, vpu-deleted HIV-1 and nef-deleted SIV still replicate in primary CD4+ T cells under conditions of IFN-induced up-regulation of tetherin (17, 36). Moreover, under certain conditions, tetherin may actually enhance virus replication by facilitating cell-to-cell virus transmission (19). A role for tetherin in increasing the susceptibility of HIV-infected cells to antibodies offers a resolution to this paradox. By enhancing the susceptibility of virus-infected cells to antibodies, the antiviral activity of tetherin in vivo may be much greater than previously appreciated based on its ability to inhibit virus replication in cell culture.
Recent reports suggest that tetherin also serves as an innate sensor of viral infection. Overexpression or virion-induced cross-linking of tetherin triggers NF-κB activation, which can lead to the induction of proinflammatory cytokines (37–39). As a consequence of these proinflammatory signals, cellular mediators of antibody-dependent responses, such as NK cells and macrophages, may be attracted to sites of virus infection. Signal transduction by tetherin may therefore serve to amplify antibody-dependent cellular immunity by attracting mediators of ADCC and phagocytosis to sites of virus replication.
A number of studies suggest that nonneutralizing, Fc-receptor (FcR)–mediated functions of antibodies such as ADCC may be important for protection against HIV-1. Substitutions that disrupted FcR binding of a broadly neutralizing monoclonal antibody diminished protection against a simian-human immunodeficiency virus challenge after passive transfer to macaques (40). ADCC activity was also associated with the maturation of protection against pathogenic SIV challenge in macaques immunized with nef-deleted, live-attenuated SIV (41). Higher ADCC responses were also observed among vaccinated subjects of the RV144 trial who did not become infected with HIV-1 than among those who did, although this difference was not statistically significant (42).
Although these observations suggest that ADCC may be important for preventing HIV-1 acquisition under certain circumstances, as with other immune responses, ADCC ultimately fails to contain HIV-1 replication in most individuals. A role for Vpu in reducing the sensitivity of virus-infected cells to antibodies may in part explain why ADCC is not more effective at controlling chronic HIV-1 infection. By reducing the accumulation of virions of the cell surface, Vpu may lower the sensitivity of HIV-infected cells to antibodies, facilitating ongoing virus replication and immune escape. Partial resistance to ADCC is consistent with evidence that antibodies capable of directing ADCC may select for immune escape variants (43). In this regard, Vpu-mediated resistance to ADCC is perhaps analogous to Nef-mediated resistance to cytotoxic T lymphocytes (CTLs), in which MHC class I down-regulation by Nef reduces, but does not eliminate, CD8+ T-cell recognition of HIV-infected cells, as reflected by the well-documented emergence of CTL escape variants (44).
The potential synergy between tetherin and virus-specific antibody responses has important therapeutic implications. Similar to the increase in sensitivity to ADCC observed for cells infected with HIV-1 carrying the A14L substitution in the transmembrane domain of Vpu, compounds designed to impair tetherin antagonism by Vpu could enhance the elimination of HIV-infected cells by antibodies. Indeed, greater stimulation of Env-specific antibody responses as a consequence of an inability to counteract tetherin may have contributed to the robust ADCC activity observed in rhesus macaques infected with nef-deleted SIV (41). Drugs designed to interfere with the anti-tetherin activity of Vpu may therefore achieve substantial reductions in HIV-1 replication in patients through a combination of inhibitory effects on virion detachment and greater potency of antibody-mediated responses to virus-infected cells.
In summary, we show that the anti-tetherin activity of Vpu protects HIV-infected cells from antibodies capable of directing the killing of infected cells by ADCC. This suggests that by trapping virions on the cell surface, tetherin increases the susceptibility of virus-infected cells to antibodies. By serving as a link between innate and adaptive immunity, the antiviral activity of tetherin may be magnified by antibodies, and therefore may be much greater than previously appreciated based solely on the capacity of tetherin to inhibit virus release in cell culture assays.
Materials and Methods
ADCC Assay.
ADCC was measured as previously described (21, 41). An NK cell line transduced with a retroviral vector to stably express human CD16a (FCGR3A) served as effector cells (21, 45). Target cells (CEM.NKR-CCR5-sLTR-Luc) were derived from CEM.NKR-CCR5 CD4+ T cells obtained through the AIDS Reagent Program [ARP; Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH)] from Alexandra Trkola (46), and modified to express firefly luciferase upon HIV-1 infection (21). Target cells were infected with HIV-1 by spinoculation. Four days after infection, NK cells and HIV-1–infected target cells were incubated for 8 h at an effector:target ratio of 10:1 in triplicate wells at each antibody concentration . Background and maximal luciferase activity were determined, respectively, from six wells containing uninfected target cells and six wells containing HIV-infected target cells incubated with NK cells, but without antibody. ADCC activity [as a percentage of relative light units (RLU)] was calculated as follows: (mean RLU at a given antibody concentration − mean background RLU)/(mean maximal RLU − mean background RLU) × 100.
RNAi Knockdown.
siRNA oligonucleotides targeting tetherin, NTB-A, and CD4 were synthesized by Dharmacon (Thermo Scientific) as 21-nt duplexes with 3′dTdT overhangs. The siGENOME Control RNAi no. 2 and siGLO were used as nontargeting and transfection efficiency controls, respectively. Cells were transfected with 100 nM siRNA duplexes using DharmaFECT 1 transfection reagent at a density of 5 × 105 cells per milliliter in six-well plates according to manufacturer instructions (Thermo Scientific). Two days after transfection, the efficiency of RNAi knockdown was evaluated by flow cytometry and by immunoblot analysis.
Plasmid DNA Constructs.
HIV-1NL4-3 (pNL4-3) and HIV-1JR-CSF were obtained through the ARP from Malcolm Martin and Irvin Chen (NIAID, NIH), respectively (47, 48). The vpu-deleted variant of HIV-1NL4-3 (pNL4-3.Δvpu) was generated by deleting nucleotide 6076, resulting in multiple stop codons after the fifth codon of vpu (49). HIV-1JR-CSF Δvpu was generated by the same approach. Vpu substitutions A14L, A18H, W22A, S52,56N, and I46K were introduced into HIV-1NL4-3 3′ (p83-10) by PCR-based mutagenesis as previously described (50). Of these Vpu changes, only the substitution at position 56 resulted in an amino acid change in Env (27). All plasmid DNA expression constructs were sequence-confirmed.
Immunoblotting.
Cell lysates were prepared in ice-cold RIPA buffer (Pierce Biotechnology) containing EDTA and protease inhibitor mixture (Pierce Biotechnology), cleared by centrifugation at 2,500 × g at 4 °C for 5 min, and suspended in 2× Laemmli buffer (Sigma-Aldrich). Proteins were subsequently separated on 12% (wt/vol) polyacrylamide gels or Mini-Protean TGX Any kD gradient gels (Bio-Rad), transferred to PVDF membranes (GE Healthcare), blocked with PBS solution plus 2% (vol/vol) BSA, and probed with commercially available monoclonal antibodies to tetherin (clone RS38E), NTB-A (clone NT-7), CD4 (clone mAb51312), β-actin (clone ACTN05), HIV-1 Env (clone 1994), HIV-1 Gag (clone 3A1), or a rabbit polyclonal antibody to Vpu obtained through the ARP from Klaus Strebel (NIAID, NIH) (51). Blots were then washed with PBS solution plus 0.05% Tween-20 and probed with an HRP-conjugated goat anti-mouse antibody (Pierce) or a HRP-conjugated goat anti-rabbit antibody (Bio-Rad). Immunoblots were developed with enhanced chemiluminescence (GE Healthcare) and imaged by using an Image Reader LAS-4000 (FujiFilm) (17).
Flow Cytometry.
Cells were stained at room temperature in PBS solution plus 2% (vol/vol) FBS and 1% NaN3 with fluorochrome-conjugated antibodies specific for tetherin (APC; clone RS38E), NTB-A (PE; clone NT-7), CD4 (Alexa Fluor 700; clone RPA-T4) and CD45 (PerCP; clone 2D1). For Env staining, an indirect method was used; cells were first incubated with HIVIG obtained through the ARP from Luba Vujcic (NIAID, NIH) (52), or purified human IgG from HIV-negative donors (Invitrogen), followed by a fluorochrome-conjugated isotype-specific mouse anti-human IgG antibody (PE-Cy7; clone G18-145). For intracellular staining of Gag, cells were fixed and permeabilized with the Cytofix/Cytoperm kit (BD Biosciences), followed by staining with monoclonal antibody KC57 (FITC; clone FH190-1-1) (53). Samples were then washed, fixed in 2% (wt/vol) paraformaldehyde, and analyzed by using an LSR-II flow cytometer (Becton Dickinson). Dead cells were excluded by using the LIVE/DEAD fixable dead cell aqua stain (Invitrogen). After gating on viable, virus-infected (Gag+) CD45+ cells, the surface expression of Env, tetherin, CD4, and NTB-A was analyzed by using FlowJo 9.6.4 software (TreeStar).
Statistical Methods.
Antibody concentrations for half-maximal killing of HIV-infected cells (i.e., 50% ADCC) and AUC values were calculated for three independent experiments as previously described (21, 41). Unpaired t tests or one-way ANOVA followed by the Holm–Sidak test were used to compare differences in mean 50% ADCC concentrations and mean AUC values. Correlations between Env staining on the surface of infected cells and susceptibility to ADCC, and Env staining vs. surface expression of tetherin, CD4, and NTB-A were analyzed by using the Pearson correlation test. SI Materials and Methods provides additional details on statistical methods.
Supplementary Material
Acknowledgments
We thank Dr. Reiko Nishihara (Department of Medical Oncology, Dana–Farber Cancer Institute) for her guidance with the statistical methods used for this study and Jackie Gillis and Michelle Connole (Division of Immunology, New England Primate Research Center) for flow cytometry services. This work was supported by Public Health Service Grants AI098485, AI095098, and P51RR000167/P51OD011106. D.T.E. is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1321507111/-/DCSupplemental.
References
- 1.Perez-Caballero D, et al. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell. 2009;139(3):499–511. doi: 10.1016/j.cell.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammonds J, Wang J-J, Yi H, Spearman P. Immunoelectron microscopic evidence for Tetherin/BST2 as the physical bridge between HIV-1 virions and the plasma membrane. PLoS Pathog. 2010;6(2):e1000749. doi: 10.1371/journal.ppat.1000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzpatrick K, et al. Direct restriction of virus release and incorporation of the interferon-induced protein BST-2 into HIV-1 particles. PLoS Pathog. 2010;6(3):e1000701. doi: 10.1371/journal.ppat.1000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kupzig S, et al. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic. 2003;4(10):694–709. doi: 10.1034/j.1600-0854.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 5.VanDamme N, et al. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral vpu protein. Cell Host Microbe. 2008;3(4):245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neil SJD, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451(7177):425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 7.Mangeat B, et al. Influenza virus partially counteracts restriction imposed by tetherin/BST-2. J Biol Chem. 2012;287(26):22015–22029. doi: 10.1074/jbc.M111.319996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weidner JM, et al. Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J Virol. 2010;84(24):12646–12657. doi: 10.1128/JVI.01328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakuma T, Noda T, Urata S, Kawaoka Y, Yasuda J. Inhibition of Lassa and Marburg virus production by tetherin. J Virol. 2009;83(5):2382–2385. doi: 10.1128/JVI.01607-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansouri M, et al. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi’s sarcoma-associated herpesvirus. J Virol. 2009;83(19):9672–9681. doi: 10.1128/JVI.00597-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jouvenet N, et al. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J Virol. 2009;83(4):1837–1844. doi: 10.1128/JVI.02211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sauter D, et al. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe. 2009;6(5):409–421. doi: 10.1016/j.chom.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang F, et al. Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe. 2009;6(1):54–67. doi: 10.1016/j.chom.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia B, et al. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 2009;5(5):e1000429. doi: 10.1371/journal.ppat.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeTortorec A, Neil SJD. Antagonism and intracellular sequestration of human tetherin by the HIV-2 envelope glycoprotein. J Virol. 2009;83(22):11966–11978. doi: 10.1128/JVI.01515-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberatore RA, Bieniasz PD. Tetherin is a key effector of the antiretroviral activity of type I interferon in vitro and in vivo. Proc Natl Acad Sci USA. 2011;108(44):18097–18101. doi: 10.1073/pnas.1113694108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serra-Moreno R, Jia B, Breed M, Alvarez X, Evans DT. Compensatory changes in the cytoplasmic tail of gp41 confer resistance to tetherin/BST-2 in a pathogenic nef-deleted SIV. Cell Host Microbe. 2011;9(1):46–57. doi: 10.1016/j.chom.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Götz N, et al. Reacquisition of Nef-mediated tetherin antagonism in a single in vivo passage of HIV-1 through its original chimpanzee host. Cell Host Microbe. 2012;12(3):373–380. doi: 10.1016/j.chom.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jolly C, Booth NJ, Neil SJ. Cell-cell spread of human immunodeficiency virus type 1 overcomes tetherin/BST-2-mediated restriction in T cells. J Virol. 2010;84(23):12185–12199. doi: 10.1128/JVI.01447-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gummuluru S, Kinsey CM, Emerman M. An in vitro rapid-turnover assay for human immunodeficiency virus type 1 replication selects for cell-to-cell spread of virus. J Virol. 2000;74(23):10882–10891. doi: 10.1128/jvi.74.23.10882-10891.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alpert MD, et al. A novel assay for antibody-dependent cell-mediated cytotoxicity against HIV-1- or SIV-infected cells reveals incomplete overlap with antibodies measured by neutralization and binding assays. J Virol. 2012;86(22):12039–12052. doi: 10.1128/JVI.01650-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schubert U, et al. CD4 glycoprotein degradation induced by human immunodeficiency virus type 1 Vpu protein requires the function of proteasomes and the ubiquitin-conjugating pathway. J Virol. 1998;72(3):2280–2288. doi: 10.1128/jvi.72.3.2280-2288.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah AH, et al. Degranulation of natural killer cells following interaction with HIV-1-infected cells is hindered by downmodulation of NTB-A by Vpu. Cell Host Microbe. 2010;8(5):397–409. doi: 10.1016/j.chom.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwabu Y, et al. HIV-1 accessory protein Vpu internalizes cell-surface BST-2/tetherin through transmembrane interactions leading to lysosomes. J Biol Chem. 2009;284(50):35060–35072. doi: 10.1074/jbc.M109.058305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mangeat B, et al. HIV-1 Vpu neutralizes the antiviral factor Tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation. PLoS Pathog. 2009;5(9):e1000574. doi: 10.1371/journal.ppat.1000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skasko M, et al. HIV-1 Vpu protein antagonizes innate restriction factor BST-2 via lipid-embedded helix-helix interactions. J Biol Chem. 2012;287(1):58–67. doi: 10.1074/jbc.M111.296772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNatt MW, Zang T, Bieniasz PD. Vpu binds directly to tetherin and displaces it from nascent virions. PLoS Pathog. 2013;9(4):e1003299. doi: 10.1371/journal.ppat.1003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vigan R, Neil SJ. Determinants of tetherin antagonism in the transmembrane domain of the human immunodeficiency virus type 1 Vpu protein. J Virol. 2010;84(24):12958–12970. doi: 10.1128/JVI.01699-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skasko M, et al. BST-2 is rapidly down-regulated from the cell surface by the HIV-1 protein Vpu: evidence for a post-ER mechanism of Vpu-action. Virology. 2011;411(1):65–77. doi: 10.1016/j.virol.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell RS, et al. Vpu antagonizes BST-2-mediated restriction of HIV-1 release via β-TrCP and endo-lysosomal trafficking. PLoS Pathog. 2009;5(5):e1000450. doi: 10.1371/journal.ppat.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Douglas JL, et al. Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a βTrCP-dependent mechanism. J Virol. 2009;83(16):7931–7947. doi: 10.1128/JVI.00242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tokarev AA, Munguia J, Guatelli JC. Serine-threonine ubiquitination mediates downregulation of BST-2/tetherin and relief of restricted virion release by HIV-1 Vpu. J Virol. 2011;85(1):51–63. doi: 10.1128/JVI.01795-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gustin JK, Douglas JL, Bai Y, Moses AV. Ubiquitination of BST-2 protein by HIV-1 Vpu protein does not require lysine, serine, or threonine residues within the BST-2 cytoplasmic domain. J Biol Chem. 2012;287(18):14837–14850. doi: 10.1074/jbc.M112.349928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margottin F, et al. Interaction between the cytoplasmic domains of HIV-1 Vpu and CD4: role of Vpu residues involved in CD4 interaction and in vitro CD4 degradation. Virology. 1996;223(2):381–386. doi: 10.1006/viro.1996.0491. [DOI] [PubMed] [Google Scholar]
- 35.Evans DT, Serra-Moreno R, Singh RK, Guatelli JC. BST-2/tetherin: a new component of the innate immune response to enveloped viruses. Trends Microbiol. 2010;18(9):388–396. doi: 10.1016/j.tim.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neil SJD, Sandrin V, Sundquist WI, Bieniasz PD. An interferon-α-induced tethering mechanism inhibits HIV-1 and Ebola virus particle release but is counteracted by the HIV-1 Vpu protein. Cell Host Microbe. 2007;2(3):193–203. doi: 10.1016/j.chom.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cocka LJ, Bates P. Identification of alternatively translated Tetherin isoforms with differing antiviral and signaling activities. PLoS Pathog. 2012;8(9):e1002931. doi: 10.1371/journal.ppat.1002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galão RP, Le Tortorec A, Pickering S, Kueck T, Neil SJ. Innate sensing of HIV-1 assembly by Tetherin induces NFκB-dependent proinflammatory responses. Cell Host Microbe. 2012;12(5):633–644. doi: 10.1016/j.chom.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tokarev A, et al. Stimulation of NF-κB activity by the HIV restriction factor BST2. J Virol. 2013;87(4):2046–2057. doi: 10.1128/JVI.02272-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hessell AJ, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449(7158):101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 41.Alpert MD, et al. ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIV(mac)251 challenge. PLoS Pathog. 2012;8(8):e1002890. doi: 10.1371/journal.ppat.1002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haynes BF, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366(14):1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung AW, et al. Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc Natl Acad Sci USA. 2011;108(18):7505–7510. doi: 10.1073/pnas.1016048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carlson JM, Brumme ZL. HIV evolution in response to HLA-restricted CTL selection pressures: A population-based perspective. Microbes Infect. 2008;10(5):455–461. doi: 10.1016/j.micinf.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 45.Yagita M, et al. A novel natural killer cell line (KHYG-1) from a patient with aggressive natural killer cell leukemia carrying a p53 point mutation. Leukemia. 2000;14(5):922–930. doi: 10.1038/sj.leu.2401769. [DOI] [PubMed] [Google Scholar]
- 46.Trkola A, Matthews J, Gordon C, Ketas T, Moore JP. A cell line-based neutralization assay for primary human immunodeficiency virus type 1 isolates that use either the CCR5 or the CXCR4 coreceptor. J Virol. 1999;73(11):8966–8974. doi: 10.1128/jvi.73.11.8966-8974.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adachi A, et al. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koyanagi Y, et al. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236(4803):819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 49.Wong SK, et al. A New World primate deficient in tetherin-mediated restriction of human immunodeficiency virus type 1. J Virol. 2009;83(17):8771–8780. doi: 10.1128/JVI.00112-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gibbs JS, Regier DA, Desrosiers RC. Construction and in vitro properties of HIV-1 mutants with deletions in “nonessential” genes. AIDS Res Hum Retroviruses. 1994;10(4):343–350. doi: 10.1089/aid.1994.10.343. [DOI] [PubMed] [Google Scholar]
- 51.Maldarelli F, Chen MY, Willey RL, Strebel K. Human immunodeficiency virus type 1 Vpu protein is an oligomeric type I integral membrane protein. J Virol. 1993;67(8):5056–5061. doi: 10.1128/jvi.67.8.5056-5061.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vujcic LK, Quinnan GV., Jr Preparation and characterization of human HIV type 1 neutralizing reference sera. AIDS Res Hum Retroviruses. 1995;11(7):783–787. doi: 10.1089/aid.1995.11.783. [DOI] [PubMed] [Google Scholar]
- 53.Carter CC, et al. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med. 2010;16(4):446–451. doi: 10.1038/nm.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.