Abstract
For the last 150 y scholars have focused upon the roles of intentional breeding and genetic isolation as fundamental to understanding the process of animal domestication. This analysis of ethnoarchaeological, archaeological, and genetic data suggests that long-term gene flow between wild and domestic stocks was much more common than previously assumed, and that selective breeding of females was largely absent during the early phases of animal domestication. These findings challenge assumptions about severe genetic bottlenecks during domestication, expectations regarding monophyletic origins, and interpretations of multiple domestications. The findings also raise new questions regarding ways in which behavioral and phenotypic domestication traits were developed and maintained.
Keywords: reproductive isolation, selected breeding, zooarchaeology, donkey, pig
Domestication resulted in diverse phenotypic and behavioral changes to wild animals, including decreased flight responses, increased sociality, earlier reproduction, and modification of endocrine and metabolic systems (1–4). Darwin’s (5) seminal research, heavily influenced by European animal breeding practices during the 19th century, led subsequent scholars studying animal domestication to prioritize the central roles of human intentionality, directed or controlled breeding of individuals, and genetic isolation of captive herds from wild relatives (6). This anthropocentric legacy is evident in various widely used definitions of domestication that emphasize isolation of captive animals from wild species and total human control over breeding and animal care (6–8). However, a growing body of archaeological, genetic, and ethnohistorical evidence discussed here shows that neither reproductive isolation nor intentional breeding of individuals was as significant as traditionally thought. Our findings indicate long-term gene flow between managed and wild animal populations, and little control of breeding of domestic females. These findings challenge assumptions about severe genetic bottlenecks during domestication and interpretations of genetic variability in terms of multiple instances of domestication. The findings also raise questions about ways in which behavioral and phenotypic domestication traits were maintained.
Research into dog and pig domestication over the last several decades has drawn attention to the roles of nonhuman drivers in the domestication process (9, 10) with early domestication routes for these taxa now widely viewed as commensal (3). Prey pathways provided other trajectories to domestication for goats, sheep, and cattle (11), whereas more directed routes to domestication have been proposed for animals such as donkeys (3). Despite these new emphases on varied human–animal relations, most models still rely on human-directed breeding over generations (3, 12, 13) and reproductive isolation to delineate all but the very earliest phases of domestication (14). The creation of separate breeding populations of animals, wholly isolated from their wild progenitors, persists as a fundamental assumption of classic speciation-based models (14, 15).
To date, there has been little discussion of how variabilities in the biology and behavior of captive animals, human environments, management regimes, and migration and dispersal of domestic animals affected directed breeding and gene flow between domestic and wild populations. These processes are explored here through archaeological, biological, ethnographic, and genetic evidence, focusing on large ungulates (Table 1).
Table 1.
Domestic animals, key archaeological sites, and domestication time-ranges
| Animal | Domestication | Sites | Sources |
| Donkey, Equus asinus | 6000–3500 B.P. | Maadi, Abydos, Uan Muhuggiag | 17, 19, 23 |
| Horse, Equus caballus | 5500 B.P. | Botai | 25, 26, 28 |
| Bactrian camel, Camelus bactrianus | 6000–4000 B.P. | Anau | 29–31 |
| Dromedary, Camelus dromedarius | 4000–3000 B.P. | Shahr-i-Sokhta | 35–37 |
| Llama, Lama glama | 6000–4000 B.P. | Pikimachay, Tulan, Inca Cueva | 39–42, 44 |
| Alpaca, Vicugna pacos | 5000–3000 B.P. | Telarmachay | 39–42, 44 |
| Pig, Sus scrofa | 12000–8300 B.P. | Çayönü Tepesi, Jiahu | 10, 48–52, 54 |
| Goat, Capra hircus | 11000–9000 B.P. | Asiab, Ganj Dareh, Ali Kosh | 58, 63 |
| Sheep, Ovis aries | 12000–10500 B.P. | Cafer Hüyük, Zawi Chemi Shanidar | 56–58 |
| Taurine cattle, Bos taurus | 10500–10000 B.P. | Dja’de, Çayönü | 66, 67 |
| Zebu cattle, Bos indicus | 8000–7500 B.P. | Mehrgarh | 68, 69 |
| Yak, Bos grunniens | ? | Tibetan Plateau | 45, 46 |
Wild-domestic gene-flow occurred among all taxa. Large transport animals were subject to low culling and high out-crossing potentials.
Management and Gene Flow
Equids, Camelids, and Yaks.
Humans have relied heavily on horses, donkeys, camelids, and yaks for transport, food, fiber, and ritual practices over the millennia. Physiologically well adapted to extreme environments, these animals enable mobile herders to survive in cold steppe, desert, and mountainous regions. With the exception of horses and yaks, transport animals are territorial and challenging to manage; they are also large-bodied with correspondingly slow gestation and herd growth rates that do not permit high levels of culling. These biological influences on human management mean herders value the adaptations of wild relatives of their domestic animals, manage animals lightly, cull at low levels, and grow herds through capture of more wild animals. Consequently, transport animals reflect low levels of directed selection resulting from intentional human management, including breeding, culling, or castration of selected animals, and high levels of gene flow.
Donkey’s desert adaptations, lack of sociality, long gestation rates, and use by mobile herders for long-distance movement have resulted in particularly low levels of management, little directed breeding, and constant gene flow with their wild and feral relatives, at least within their wild range. Much like cats, donkeys have often been treated as an exception to the accepted rules for domestication and, by definitions that focus on reproductive isolation (6, 8), they could, perhaps, not even be considered a domestic animal.
African wild asses (Equus africanus) were the ancestors of domestic donkeys (16, 17) (Fig. 1 and Table 1). Today, African pastoralists rely on donkeys for transport and they are rarely slaughtered for food. As a result, drought and disease are the principal causes of donkey mortality. Herders value individual animals for strength and hardiness (18) and castrate difficult males, but prefer uncastrated ones for transport-use. The presence of multiple breeding males reduces directed selection (18). Moreover, because they are challenging to herd, donkeys range widely in search of mates and donkey-owners do little to manage reproduction (18). Slow herd growth and the value placed on the size, strength, and hardiness of transport donkeys led historic pastoralists (and Romans in North Africa) to capture feral donkeys and African wild asses, and to encourage interbreeding with wild males (19–21) (Fig. 1 and Table S1).
Fig. 1.
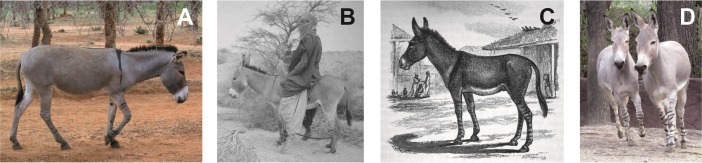
Intentional capture and out-crossing of donkeys, wild asses, and hybrids. (A) African donkey with shoulder cross (Image courtesy of Lior Weissbrod). (B) Tuareg taming captured Saharan wild ass or feral donkey, 1951 (21) (Image courtesy of Ida Nicolaisen and the Carlsberg Foundation). (C) Donkey-Somali wild ass hybrid with cross and striped legs, Berbera 1900s. Donkeys were tied outside the village to breed with Somali wild asses (20). (D) Somali wild asses with striped legs.
Modern pastoral use of donkeys presents a picture of weak directed selection principally resulting from castration and strong environmental selection. Environmental selection (15) primarily relates to unconscious or natural selection, resulting from mortality because of the effects of events, such as drought, disease, and predation on managed animals (Table S2). In regions where wild asses existed historically, continued gene flow resulted from managed or inadvertent breeding of domestic donkeys with wild asses (19–21). These aspects of the recent past are relevant to understanding ancient processes (22) because they reflect consistent mechanisms, biology, and transport use.
Archaeological and genetic data support conclusions that donkeys were domesticated in arid environments, bred with a variety of wild populations, and were used for transport and trade over long distances (Fig. S1). Archaeological evidence for specialized hunting of territorial desert asses goes back ca. 16,000 y in northeast Africa (18). However, desert assemblages are rare and evidence is lacking for the likely period of earliest management 9000–6000 B.P. (all dates are reported in calibrated years before present). The presence of two divergent mitochondrial lineages in donkeys has been interpreted as evidence for more than one domestication, but may be equally consistent with recurrent recruitment of females into domestic herds from genetically divergent Nubian wild ass populations (16, 17). A reduction in the size of some asses, often accepted as indicative of domestication, is first documented at Maadi in Egypt ca. 6000 B.P. (23). A thousand years later, despite expectations for significantly smaller animals, metacarpals from equids ritually buried at Abydos still fall within the size range of wild asses (19). Nevertheless, pathologies indicative of loading demonstrate that these morphologically wild animals were used for transport (19). Size decrease appears slow and inconsistent through time, with variability within and between archaeological sites indicating a nonlinear process of phenotypic change.
Herder reliance on donkeys for transport, the behavior of donkeys, and the long-term presence of wild asses near the Nile suggest that weak directed selection, continued recruitment of animals from the wild, and gene flow with wild asses contributed significantly to phenotypic variability among Predynastic and Early Dynastic donkeys in Egypt over at least a 2,500-y period. The value that donkey herders placed on strength is demonstrated by donkey-onager and subsequent donkey-horse hybrids (mules) bred in the ancient Near East (7, 24). Uncontrolled breeding among village donkeys and along trade routes also contributed to gene flow between founder populations and mitigated genetic drift (17, 18).
Zooarchaeological evidence, ethnographic observations, and genetic data suggest herd management has always been laissez faire and characterized by intentional and unintentional interbreeding with wild asses and feral donkeys, as well as by environmental selection for animals that survived in pastoral settlements. Together, these processes resulted in a prolonged and complicated process of domestication for donkeys.
Ethnographic and archaeological data for horses, Bactrian camels, dromedaries, llamas, alpacas, and yaks provide further insights into biological and human social factors affecting selective breeding and gene flow during the domestication of transport animals. Extinct Equus ferus from central Asia was the wild ancestor of domestic horses (Table 1 and Fig. S1). Evidence for bitting, milking, corralling, and size decrease documents domestication by horse-hunters at Botai in Kazakhstan ca. 5500 B.P. (25, 26). As with other species, mitochondrial DNA lineages were often initially interpreted in terms of multiple origins (25, 27), whereas genetic modeling now suggests domestication in a restricted region with subsequent incorporation of many different wild lineages into domestic stocks (28). Horse herds grow slowly and are subject to die-offs in severe storms, so the hardiness of wild horses is advantageous to herders. Accordingly, it has been argued that difficulties in maintaining domestic horse herd sizes during pastoral migrations led directly to restocking through the capture of wild females (25, 28).
Another transport animal subject to long-term gene flow is the Bactrian camel. Evidence is sparse, but ancient populations related to Camelus ferus are thought to have been domesticated in cold desert regions of Central Asia (Table 1 and Fig. S1). The presence of Bactrian camels found outside their likely wild range suggests domestication ca. 6000–4000 B.P. (29), with a geographically restricted domestication indicated by genetic data (30, 31). Extinction of their closest wild relatives (30) is thought to have resulted from both hunting and introgression with domestic camels (32). Historically herders have relied heavily on the strength of domestic Bactrian-dromedary crosses (33). Possibilities for increased strength and resilience may also have led nomads to encourage breeding of early domestic and wild camels, with chance admixture more likely occurring within their natural range (34). The domestication of a related camelid—the dromedary—also indicates both intentional and chance breeding of domestic and wild camels. Dromedaries are adapted to hot deserts and were domesticated in Arabia (35). Their wild ancestor (Camelus sp.) is now extinct (36) but increased frequencies of dromedaries at archaeological sites suggest domestication ca. 4000 B.P. (36, 37). Ethnographic data show that herders select bulls based on factors including size, color, family milk yields, and environmental adaptations (38), but all females are bred. Culling takes place at low levels and principally affects males, therefore directed selection is low. In contrast, high environmental selection on domestic camel herds is indicated by camelid genetics (30, 35). As shown by Bactrian-dromedary crosses, strength and hardiness were important to ancient herders and admixture is thought to have played a role in wild camelid extinctions.
There is also strong evidence for wild–domestic admixture and weak directed selection among domestic South American llama and alpaca and their wild relatives, guanaco (Llama guanicoe) and vicuña (Vicuna vicuna). These camelids are adapted to Andean high-altitude environments (Table 1 and Fig. S1). Zooarchaeological research suggests multiple processes of domestication by hunters and possibly early cultivators in the central and south central Andes ca. 6000–4000 B.P. (39, 40). Archaeological and ethnographic data indicate that, although initially used for meat, herders have increasingly relied on larger llamas for transport and managed alpacas for fiber production. In the Lake Titicaca basin, the zooarchaeological record documents increasingly intensified and controlled herding, continued hunting, and gene flow among camelids 3500–900 B.P. Evidence for continuous morphological variation implies long-term cross-breeding within and between South American camelids (41).
An extremely complex history of interbreeding, even blurring the taxonomy of these species, is indicated by the occurrence of maternal mitochondrial DNA (mtDNA) haplotypes from vicuñas and guanacos in both domesticated llamas and alpacas. Recent mtDNA-based research documents early divergences within the guanaco clade, interpreted as evidence for multiple centers of llama domestication (42). However, the nature of connections among early herders is not well known and these genetic and morphological patterns could, once again, simply reflect recurrent recruitment of individuals from diverse wild populations. Adaptations of wild ancestors to extreme environmental conditions may have contributed to intentional breeding of wild and domestic camelids. Because of the unpredictability of animals surviving extreme weather events and disease, contemporary herders prefer diverse herds, retaining rather than culling individuals with a wide variety of characters (43). In the southern Andes there are records of wild guanacos being tamed and hybridized with llamas (44). Chance breeding of wild and domestic animals also occurs when llamas and alpacas graze unsupervised in the same pastures and most hybrid offspring are fertile (44). Given prolonged interspecific and intraspecific gene flow among Andean camelids, an ancient chimera species is likely.
Low levels of selection and high levels of gene flow among transport animals are also indicated by ethnographic data for yak management on the Tibetan plateau, where limited archaeological data suggest domestication by sheep-herders some 5000–4000 B.P. (45) (Table 1 and Fig. S1). Because wild yaks (Bos mutus) are adapted to high-altitude environments (32), human reliance on them for transport and food allowed herders to survive year-round on the high plateau. Genetics show two mtDNA lineages in domestic yaks (45), which are now interpreted in terms of recurrent recruitment of diverse wild yak lineages into domestic herds (46). Ethnographic data show that breeding of wild and domestic animals is encouraged because domestic yaks are subject to frequent mortality during winter storms. These crosses have strong flight responses but are desired by herders because of their adaptation to the harsh plateau environment, size, and superior ability to protect herds from wolves (45, 47). Wild bulls move to lower elevations to mate with domestic females, where both encouraged and accidental breeding occurs (45, 47). Castration and limited culling are the only forms of directed breeding (47), but environmental selection on herded animals in pastoral camps and landscapes is strong (47).
These cases involving animals from extreme environments, primarily used for transport, all show relatively low levels of directed selection resulting from limited culling and castration, but strong environmental selection within the human niche. The examples also demonstrate practical difficulties for mobile herders of breeding selected animals and maintaining genetic isolation from wild relatives, and the advantages of wild adaptations. Given the demands placed on transport animals and their domestication history, it could be argued that this scenario is unlikely to hold more broadly. However, current evidence suggests that gene flow between domestic and wild populations is not unique to animals used for transport, but may well be true for most other domestic taxa, including animals kept for meat and secondary products, such as milk and wool.
Pigs.
Research into the domestication of wild boar provide some of the most comprehensive evidence for out-crossing and gene flow during and after initial domestication, as well as significant variability in these processes across Eurasia (Table 1 and Fig. S1). Wild boar (Sus sp.) are social animals, adapted to temperate or subtropical climates. Pigs are multiparous, with rapid gestation and herd growth rates leading to culling at much higher levels than equids, camelids, or bovines, and consequently to higher levels of selection. Unlike animals principally used for transport, intentional interbreeding of pigs with wild relatives confers no productive advantage. Gene flow is most likely to result from wild-capture as a herd-building strategy, or from chance breeding of domestic pigs with wild relatives (Table S1).
Zooarchaeological research indicates a long and complex process, possibly involving two different but related stages: initial commensalism followed by direct human involvement/control and resultant selection (10). Morphometric studies at early Neolithic sites dating to 9500–8600 B.P. in eastern Anatolia (10) and central China (48) indicate at least two separate domestications of Sus scrofa.
Genetic research over the last decade on both ancient and modern Sus reveals at least six phylogeographically distinct wild boar lineages have contributed mtDNA to domestic pig populations across the Old World, as well as clear evidence for out-crossing of domestic pigs and wild boar. Evidence also exists for the introduction and dispersal throughout Europe of several Near Eastern mtDNA S. scrofa haplotypes with early Neolithic farmers (49). Subsequent recruitment of European wild boar mtDNA lineages into these introduced domesticated swineherds led to the rapid replacement of Near Eastern lineages, first in Europe and then, during the late Bronze Age/Early Iron Age, eastwards across Anatolia (49, 50).
The story of pig domestication in East and Southeast Asia is quite different from that of southwestern Asia and Europe. Here, mtDNA from both ancient and modern S. scrofa show that most contemporary Chinese lineages were never incorporated into domestic herds, nor exterminated as a result of hunting or introgression with feral pigs (51), suggesting control (even penning) of pigs from an early stage in the domestication process. Early agriculturalists moving into southeastern Asia deliberately or accidentally recruited local wild boar lineages into their domestic stock, with the result that ancient mainland and island southeastern Asian, New Guinea, and remote Oceanic domestic pigs share their maternal ancestry with lineages recruited from southeastern Asian wild boar populations (49, 52–54), and not with the earliest central Chinese domestic pigs. However, neutral markers, such as mtDNA, can themselves be rapidly replaced during the hybridization process between incoming domestic and local wild stock (53). The nuclear genome retains introgression signatures over longer evolutionary timescales and is now the principal focus for ancient DNA research (53).
These new Eurasian datasets for S. scrofa reveal significant introgression and gene flow between wild boar and domestic pig populations after domestication, indicating a rather different domestication process than traditionally purported: one involving initial domestication of a limited number of wild boar from discrete local populations, leading to a degree of genetic isolation. Extensive and mobile swineherding practices, along with subsequent migration/dispersal of early stock-keepers, led to introgression with new local wild boar lineages, which rapidly replaced “founding” lineages.
Historical and modern-day ethnographic observations of traditional pig keeping in, for example, the Mediterranean and Europe, point to the common practice of rather loose and extensive management of domestic pigs, along with long-distance mobility patterns linked with the search for summer and winter feeding (55). Such traditional pig husbandry was likely to have been the norm across Europe millennia earlier than the historical period, and in such circumstances it is likely that out-crossing of domestic pigs with wild boar was common.
Sheep, Goats, and Cattle.
Unlike pigs, domestic bovids were widely used for meat, milk, and fiber. Ancient populations of Capra aegagrus and Ovis aries are the southwestern Asian ancestors of domestic goats and sheep (Table 1 and Fig. S1). Zooarchaeological data document early culling or managed herds of both species by settled hunter-gatherers and early cultivators in eastern Anatolia and the Zagros mountains ca. 11,000–10,000 y ago (56, 57), with goats already displaying morphological changes by ca. 9400–8900 B.P. (11, 58). Compared with pigs, sheep and goat produce only one or two offspring at a time, altering the dynamics of herd management and culling. Traditional pastoralists today manage sheep and goats principally for growth, maximizing females in herds with male-offtake sustained up to 8–16% a year (59). Herders’ decisions regarding males spared for breeding or new stock acquisition (male or female) are informed by family histories of growth potential, color, milk production, and resilience (60–62). Nevertheless, acting primarily on males, directed selection remains weak.
Six wild bezoar lineages found in domestic goats suggest long-term recruitment of wild females to domestic herds (63). Long-distance pastoral movements of flocks through the Zagros provided continual opportunities for unintentional admixture within the natural range of sheep and goats. Morphological change, traditionally associated with domestication, may not have occurred in ancient goats until gene flow was reduced by the dispersal of managed herds outside the range of their wild relatives (58). Any decline in domestic herd size would have provided incentives for wild-capture with periodic weather events, drought, and disease strongly influencing pastoral herd dynamics and viability (59). Similar instability is implied in the case of pigs and goats introduced to Cyprus during the mid-11th millennium B.P. (13, 64). Once secondary products—such as milk or wool—became important, domestic traits, such as productivity and docility, would have become highly desirable, increasing the influence and intensity of directed selection.
Because of their large size, diverse use, and broad environmental adaptations, relations between humans and cattle differ greatly from those of sheep and goats. Cattle, native to temperate or semiarid subtropical environments, were principally used for meat, and at times depended on heavily for milk, traction, and ceremonial use. Bos primigenius, ancestral to taurine cattle, was domesticated in Anatolia 10,500–10,000 B.P. (65–67), whereas Bos namadicus, ancestral to zebu cattle, was domesticated in South Asia by ca. 8000–7500 B.P. (68, 69) (Table 1 and Fig. S1). The size of cattle, low growth, and culling rates, as well as early use for milk (70) or traction, implies lower levels of directed selection than even those experienced by pigs or sheep and goat. When selecting herd bulls today, African pastoralists consider similar factors to those discussed for camels, sheep, and goats (59, 71), although cattle are seldom culled at higher than 4–8%. Productive females are not culled, multiple bulls are kept, and natural mortality is often higher than that resulting from culling (72), which results in weak directed selection and strong environmental selection. Slow herd growth promotes gene flow, as does lightly supervised grazing.
The zooarchaeological record indicates a protracted process of domestication of taurine cattle (66) but genetic data suggest small numbers of wild cattle contributed to initial domestication in Anatolia (73), and that diverse wild populations were not incorporated into domestic herds. In contrast to pigs, there is no genetic support for interbreeding of domestic taurine cattle with wild cattle as herders moved across Europe (74), the one exception being data from Italy, where ancient mtDNA suggests female aurochs may have been recruited into domestic herds. The picture is different for South Asia, where high autosomal diversity indicates repeated crossing of domestic zebu cattle with wild males and females (75). Multiple mitochondrial lineages represent either two separate domestications or, again, recruitment of wild animals into domestic zebu herds (68). This variability highlights the roles of regional differences in management practices or herd viability in promoting gene flow. The debate over the question of local domestication of cattle in northeast Africa (76) versus interbreeding of Near Eastern cattle with African wild cattle indicates the extent to which scholars are grappling with the significant role of gene flow in patterning genetic data.
Despite differences in environments, biology, and husbandry practices between taxa, there is strong evidence for gene flow between pigs, sheep, goat, and cattle and their wild relatives in areas of common distribution. Set against the whole history of domestication, complete separation between wild and domestic populations was relatively late and region-specific. Regional variability in gene flow is demonstrated for pigs and cattle, which took several domestication “pathways” with different degrees of admixture in western, southern, and eastern Eurasia. These patterns of gene flow suggest regionally different approaches to management, with animals closely herded or provisioned in some settings and extensively ranging in others. Variability in herd size and viability was a contributory factor leading to admixture in some—but not all—regions.
Implications of Widespread Gene Flow
Because the role of gene flow in the domestication of large herbivores has, until now, largely been considered minor or peripheral to more dominant processes, drivers of gene flow have not been systematically investigated. Ethnographic and ethnoarchaeological data clearly demonstrate that admixture is not simply an occasional or accidental process. Recent and historic herders intentionally captured wild relatives of their domestic animals and encouraged directed breeding between them. Both herders’ goals and unintended circumstances influenced the extent of gene flow between wild and domestic animals (Table S1). At the same time as discounting gene flow as a significant component of early domestication history, the primacy of strong directional selection in the process has often been assumed (15). It appears that under most historic and prehistoric management regimes, weak directed selection was driven primarily by culling or castration of male surplus to the growth needs of herds. Environmental selection was also a key factor for domestication histories in human-influenced environments.
These findings have significant implications for our interpretation of the archaeological record, determinations of the timing and location of initial domestication, and interpretations of genetic data on domestication. Trends in the extent of directed selection and in gene-flow potentials reinforce many of the distinctions proposed among commensal, prey, and directed pathways to domestication (11, 13), and point to additional selective mechanisms that differentiate them. Culling rates were lower and out-crossing potentials higher for larger transport animals, horses, donkeys, camelids, and yaks. Correspondingly, higher rates of culling, and therefore of directed selection characterized sheep, goats, and pigs, or more rapidly maturing animals domesticated and managed in less extreme environments.
Interbreeding among domestic, feral, and wild animals, augmented by the opportunities afforded by migrations and trade, has created long and complex evolutionary and domestication histories that challenge assumptions regarding genetic isolation and long-held definitions of domestication. Given differences of degree between domestic and wild animals, some might question whether domestication remains a useful concept. We consider it is essential to treat changing human–animal relations as a continuum, specifying domestication traits that vary with taxon and context—animal–human relationship, place, and time—rather than focusing on general expectations or arbitrary boundaries. This is the direction in which recent archaeological research has been moving (11, 13, 77).
Current assumptions regarding severe domestication bottlenecks and monophyletic origins have complicated attempts by zooarchaeologists and geneticists alike to study the domestication histories of animals such as South American camelids (41), or to interpret coalescence data and estimate domestication time-frames for cats (15). Recurrent gene flow makes wild and domestic animals more similar and the perceived time of divergence more recent. The same assumptions have resulted in widespread (mis)-interpretation of mitochondrial variability in terms of multiple instances of domestication. Recognition of the extent of long-term gene flow within and between wild and domestic animals better reconciles archaeological and genetic data for many species and suggests longer and more complex domestication processes (53). Long-term gene flow also undermines the ability of modern genetic data derived from highly developed modern-day breeds to shed light on the earliest phases of domestication (78).
If gene flow resulting from breeding between wild and domestic animals was common during domestication and has not ceased until recent historic times, it raises many fascinating questions regarding ways in which behavioral and phenotypic domestication traits were maintained, and just what a domestic population was. To address these issues, we need better characterization of animal–human relationships through time, including better integration of multiple scales of analysis: from the molecular level, to whole animals, to the social contexts and landscapes within which domestication occurs. Diverse zooarchaeological, biochemical, and geoarchaeological approaches to documenting changes in herd sizes, penning, milking and feeding strategies, as well as culling and castration across ancient sites, offer promise for eliciting temporal and site-specific data on selection processes and gene flow. We need to know, for example, exactly where and when out-crossing was common or directed selection high before we can begin to evaluate the respective importance of these processes in the domestication of particular species or to understand regional variability. Other questions, such as the amount of gene flow required to counter directed selection at different levels of culling or natural mortality in human environments, are amenable to modeling (79).
We identify environmental selection under human management as an important force in animal domestication, an area that genomic studies are currently exploring (4) (Table S2). Understanding epigenetic mechanisms, such as patterns of DNA methylation that cause genes to express themselves differently in human compared with wild settings or under varying management regimes (e.g., under stress), promise to provide new insights into ways in which selection was maintained (80, 81). Finally, landscape genetic studies of how small-scale social and biological processes, such as household mobility and exchange or captive animal breeding rates affect movement, interbreeding, and gene flow at large scales, have the potential to integrate anthropological, behavioral, and genetic data (82).
Instead of assuming strong intentional and directional selection during the early stage of animal domestication, the challenge is to investigate sources of selection more critically, bearing in mind the complex interplay of human and environmental selection and the likelihood of long-term gene flow from the wild. These insights on gene flow and unintentional breeding provide new perspectives on early animal domestication, alter current sets of assumptions and questions, and enhance our understanding of domestication as a complex biocultural process.
Supplementary Material
Acknowledgments
We thank Ida Nicolaisen and Lior Weissbrod for providing images, and the reviewers for their exceptionally thoughtful comments. This manuscript resulted from a catalysis meeting entitled “Domestication as an Evolutionary Phenomenon: Expanding the Synthesis” that was awarded and hosted by the National Evolutionary Synthesis Centre (National Science Foundation EF-090560) in 2011.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.L. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312984110/-/DCSupplemental.
References
- 1.Trut L, Oskina I, Kharlamova A. Animal evolution during domestication: The domesticated fox as a model. Bioessays. 2009;31(3):349–360. doi: 10.1002/bies.200800070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hemmer H. Domestication. Cambridge, UK: Cambridge Univ Press; 1990. [Google Scholar]
- 3. Zeder MA (2011) Pathways to animal domestication. Harlan II: Biodiversity in Agriculture: Domestication, Evolution and Sustainability, eds Damania A, Gepts P (Univ California Press, Davis, CA), pp 227–259.
- 4.Andersson LS, et al. Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature. 2012;488(7413):642–646. doi: 10.1038/nature11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darwin C. The Variation of Animals and Plants Under Domestication. London: John Murray; 1868. [Google Scholar]
- 6.Clutton-Brock J. The process of domestication. Mammal Rev. 1992;22(2):79–85. [Google Scholar]
- 7.Clutton-Brock J. A Natural History of Domesticated Animals. Cambridge, UK: Cambridge Univ Press; 1999. [Google Scholar]
- 8. Bӧkӧnyi S (1989) Definitions of animal domestication. The Walking Larder: Patterns of Domestication, Pastoralism and Predation, ed Clutton-Brock J (Unwin Hyman, London), pp 22–27.
- 9.Morey D. The early evolution of the domestic dog. Am Sci. 1994;82(4):336–347. [Google Scholar]
- 10.Ervynck A, Dobney K, Hongo H, Meadow R. Born free? New evidence for the status of “Sus scrofa” at Neolithic Çayönü Tepesi (Southeastern Anatolia, Turkey) Paleorient. 2002;27(2):47–73. [Google Scholar]
- 11.Zeder M. The domestication of animals. J Anthropol Res. 2012;68(2):161–190. [Google Scholar]
- 12.Price E. Animal Domestication and Behavior. New York: CABI Publishing; 2002. [Google Scholar]
- 13.Vigne J-D. The origins of animal domestication and husbandry: A major change in the history of humanity and the biosphere. C R Biol. 2011;334(3):171–181. doi: 10.1016/j.crvi.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor T. Wild or domestic? Biometric variation in the cat Felis silvestris Schreber. Int J Osteoarchaeol. 2007;17:581–595. [Google Scholar]
- 15.Driscoll CA, Macdonald DW, O’Brien SJ. From wild animals to domestic pets, an evolutionary view of domestication. Proc Natl Acad Sci USA. 2009;106(Suppl 1):9971–9978. doi: 10.1073/pnas.0901586106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beja-Pereira A, et al. African origins of the domestic donkey. Science. 2004;304(5678):1781. doi: 10.1126/science.1096008. [DOI] [PubMed] [Google Scholar]
- 17.Kimura B, et al. Ancient DNA from Nubian and Somali wild ass provides insights into donkey ancestry and domestication. Proc Biol Sci. 2011;278(1702):50–57. doi: 10.1098/rspb.2010.0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall F, Weissbrod L. Domestication processes and morphological change through the lens of the donkey and African pastoralism. Curr Anthropol. 2011;52(S4):S397–S413. [Google Scholar]
- 19.Rossel S, et al. Domestication of the donkey: Timing, processes, and indicators. Proc Natl Acad Sci USA. 2008;105(10):3715–3720. doi: 10.1073/pnas.0709692105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bland-Sutton J (1911) Man and Beast in Eastern Ethiopia (McMillan, London), pp 189.
- 21.Nicolaisen J, Nicolaisen I. The Pastoral Tuareg. New York: The Carlsberg Foundations Nomad Research Project. Thames and Hudson; 1997. p. 163. [Google Scholar]
- 22.Wylie A. Thinking from Things. Berkeley: Univ California Press; 2002. [Google Scholar]
- 23.Boessneck J, von den Driesch A, Ziegler R. Die Tierreste von Maadi und Wadi Digla [The animal remains of Maadi and Wadi Digla] In: Rizkana I, Seeher J, editors. Maadi III. Mainz: Phillipp von Zabern; 1989. pp. 87–128. German. [Google Scholar]
- 24.Weber J. Elite equids: Redefining equid burials of the mid- to late 3rd millennium BC from Umm el-Marra. In: Vila E, Gourichon L, Choyke MA, Buitenhuis H, editors. Archaeozoology of the Near East VIII. Lyon: Travaux de la Maison de l’Orient et de la Méditerrannée; 2008. pp. 499–520. [Google Scholar]
- 25.Olsen S. Early horse domestication on the Eurasian steppe. In: Zeder MA, Bradley DG, Emschwiller E, Smith BD, editors. Documenting Domestication: New Genetic and Archaeological Paradigms. Berkeley, CA: Univ California Press; 2006. pp. 245–269. [Google Scholar]
- 26.Outram AK, et al. The earliest horse harnessing and milking. Science. 2009;323(5919):1332–1335. doi: 10.1126/science.1168594. [DOI] [PubMed] [Google Scholar]
- 27.Vilà C, et al. Widespread origins of domestic horse lineages. Science. 2001;291(5503):474–477. doi: 10.1126/science.291.5503.474. [DOI] [PubMed] [Google Scholar]
- 28.Warmuth V, et al. Reconstructing the origin and spread of horse domestication in the Eurasian steppe. Proc Natl Acad Sci USA. 2012;109(21):8202–8206. doi: 10.1073/pnas.1111122109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters J, von den Driesch A. The two-humped camel (Camelus bactrianus): New light on its distribution, management and medical treatment in the past. J Zool (Lond) 1997;242(4):651–679. [Google Scholar]
- 30.Ji R, et al. Monophyletic origin of domestic bactrian camel (Camelus bactrianus) and its evolutionary relationship with the extant wild camel (Camelus bactrianus ferus) Anim Genet. 2009;40(4):377–382. doi: 10.1111/j.1365-2052.2008.01848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trinks A, Burger P, Benecke N, Burger J. Ancient DNA reveals domestication process: The case of the two-humped camel. In: Knoll E-M, Burger P, editors. Camels in Asia and North Africa: Interdisciplinary Perspectives on Their Past and Present Significance. Vienna: Austrian Academy of Sciences Press; 2012. pp. 79–86. [Google Scholar]
- 32.Schaller GB. Wildlife of the Tibetan Steppe. Chicago: Univ Chicago Press; 1998. [Google Scholar]
- 33.Potts DT. Camel hybridization and the role of Camelus bactrianus in the ancient Near East. J Econ Soc Hist Orient. 2004;47(2):143–165. [Google Scholar]
- 34.Yadamsuren A, Dulamtseren E, Reading R. The conservation status and management of wild camels in Mongolia. In: Knoll E-M, Burger P, editors. Camels in Asia and North Africa: Interdisciplinary Perspectives on Their Past and Present Significance. Vienna: Austrian Academy of Sciences Press; 2012. pp. 45–54. [Google Scholar]
- 35.Al-Swailem AM, et al. Sequencing, analysis, and annotation of expressed sequence tags for Camelus dromedarius. PLoS ONE. 2010;5(5):e10720. doi: 10.1371/journal.pone.0010720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters J. [The dromedary: Ancestry, history of domestication and medical treatment in early historic times] Tierarztl Prax Ausg G Grosstiere Nutztiere. 1997;25(6):559–565. [PubMed] [Google Scholar]
- 37.Uerpmann H-P, Uerpmann M. The appearance of the domestic camel in south-east Arabia. J Oman Stud. 2002;12:235–260. [Google Scholar]
- 38.Köhler-Rollefson I, Rathore HS. Indigenous versus official knowledge, concepts and institutions: Raika pastoralists and the outside world. Nomad People. 2004;8(2):150–167. [Google Scholar]
- 39.Mengoni-Goñalons GL. Camelids in ancient Andean societies: A review of the zooarchaeological evidence. Quat Int. 2008;185(1):59–68. [Google Scholar]
- 40.Mengoni-Goñalons G, Yacobaccio HD. The domestication of South American camelids: A view from the south-central Andes. In: Zeder M, Bradley DG, Emschwiller E, Smith BD, editors. Documenting Domestication: New Genetic and Archaeological Paradigms. Berkeley: Univ California Press; 2006. pp. 228–244. [Google Scholar]
- 41.Moore KM. Grace under pressure: Responses to changing environments by herders and fishers in the Formative Lake Titicaca Basin, Bolivia. In: Miller NF, Moore KM, Ryan K, editors. Sustainable Lifeways: Cultural Persistence in an Ever-Changing Environment. Philadelphia: Univ Pennsylvania Museum of Archaeology and Anthropology; 2011. pp. 244–272. [Google Scholar]
- 42.Barreta J, et al. Analysis of mitochondrial DNA in Bolivian llama, alpaca and vicuna populations: A contribution to the phylogeny of the South American camelids. Anim Genet. 2013;44(2):158–168. doi: 10.1111/j.1365-2052.2012.02376.x. [DOI] [PubMed] [Google Scholar]
- 43.Bolton M. Genetic defects or generative prototypes? Competing models for livestock improvement in southern Bolivia. J R Anthropol Inst. 2006;12(3):531–549. [Google Scholar]
- 44.Bonavia D. The South American Camelids. Los Angeles: Cotsen Institute of Archaeology, Univ of California; 2008. [Google Scholar]
- 45.Rhode D, Madsen DB, Brantingham PJ, Dargye T. Yaks, yak dung, and prehistoric human habitation of the Tibetan Plateau. Developments in Quaternary Sciences. 2007;9:205–224. [Google Scholar]
- 46.Wang Z, et al. Phylogeographical analyses of domestic and wild yaks based on mitochondrial DNA: New data and reappraisal. J Biogeogr. 2010;37(12):2332–2344. [Google Scholar]
- 47. Wiener G, Han J, Long R (2003) The Yak (FAO, Bankok) 2nd. Ed.
- 48.Cucchi T, et al. Early Neolithic pig domestication at Jiahu, Henan Province, China: Clues from molar shape analyses using geometric morphometric approaches. J Archaeol Sci. 2011;38(1):11–22. [Google Scholar]
- 49.Larson G, et al. Ancient DNA, pig domestication, and the spread of the Neolithic into Europe. Proc Natl Acad Sci USA. 2007;104(39):15276–15281. doi: 10.1073/pnas.0703411104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ottoni C, et al. Pig domestication and human-mediated dispersal in western Eurasia revealed through ancient DNA and geometric morphometrics. Mol Biol Evol. 2013;30(4):824–832. doi: 10.1093/molbev/mss261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larson G, et al. Patterns of East Asian pig domestication, migration, and turnover revealed by modern and ancient DNA. Proc Natl Acad Sci USA. 2010;107(17):7686–7691. doi: 10.1073/pnas.0912264107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Larson G, Cucchi T, Dobney K. Genetic aspects of pig domestication. In: Rothschild MF, Ruvinsky A, editors. The Genetics of the Pig. Wallingford: CAB International; 2011. pp. 14–37. [Google Scholar]
- 53.Larson G, Burger J. A population genetics view of animal domestication. Trends Genet. 2013;29(4):197–205. doi: 10.1016/j.tig.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Dobney K, Cucchi T, Larson G. The pigs of Island Southeast Asia and the Pacific: New evidence for taxonomic status and human-mediated dispersal. Asian Perspect. 2008;47(1):59–74. [Google Scholar]
- 55.Albarella U, Manconi F, Trentacoste A. A week on the plateau: Pig husbandry, mobility and resource exploitation in Central Sardinia. In: Albarella U, Trentacoste A, editors. Ethnozooarchaeology. The Present and Past of Human-animal Relationships. Oxford: Oxbow Books; 2011. pp. 143–159. [Google Scholar]
- 56.Hiendleder S, Kaupe B, Wassmuth R, Janke A. Molecular analysis of wild and domestic sheep questions current nomenclature and provides evidence for domestication from two different subspecies. Proc Biol Sci. 2002;269(1494):893–904. doi: 10.1098/rspb.2002.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peters J, von den Driesch A, Helmer D. The upper Euphrates-Tigris Basin: Cradle of agro-pastoralism? In: Vigne JD, Peters J, Helmer D, editors. The First Steps of Animal Domestication. New Archaeological Approaches. Oxford: Oxbow Books; 2005. pp. 96–124. [Google Scholar]
- 58.Zeder MA. Domestication and early agriculture in the Mediterranean Basin: Origins, diffusion, and impact. Proc Natl Acad Sci USA. 2008;105(33):11597–11604. doi: 10.1073/pnas.0801317105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dahl G, Hjort A. Having Herds: Pastoral Herd Growth and Household Economy. Stockholm: Department of Social Anthropology, Univ Stockholm; 1976. [Google Scholar]
- 60.Kebede T, Haile A, Dadi H. Smallholder goat breeding and flock management practices in the central rift valley of Ethiopia. Trop Anim Health Prod. 2012;44(5):999–1006. doi: 10.1007/s11250-011-0033-9. [DOI] [PubMed] [Google Scholar]
- 61.Tabbaa M, Al-Atiyat R. Breeding objectives, selection criteria and factors influencing them for goat breeds in Jordan. Small Rumin Res. 2009;84(1):8–15. [Google Scholar]
- 62.Mbuku SM, Kosgey IS, Kahi AK. Identification systems and selection criteria for small ruminants among pastoralist communities in northern Kenya: Prospects for a breeding programme. Trop Anim Health Prod. 2010;42(7):1487–1492. doi: 10.1007/s11250-010-9584-4. [DOI] [PubMed] [Google Scholar]
- 63.Naderi S, et al. Large scale mitochondrial DNA analysis of the domestic goat reveals six maternal lineages with high haplotype diversity. PLoS ONE. 2007;2(10):e1012. doi: 10.1371/journal.pone.0001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vigne J-D. Domestication Process and Domestic Ungulates: New Observations from Cyprus. In: Colledge S, Conolly J, Dobney K, Manning K, Shennan S, editors. The Origins and Spread of Domestic Animals in Southwest Asia and Europe. Walnut Creek: Left Coast Press; 2013. pp. 115–128. [Google Scholar]
- 65.Hanotte O, et al. African pastoralism: Genetic imprints of origins and migrations. Science. 2002;296(5566):336–339. doi: 10.1126/science.1069878. [DOI] [PubMed] [Google Scholar]
- 66.Hongo H, et al. The process of unguate domestication at Çayönü, Southeastern Turkey: A multidisciplinary approach focusing on Bos sp. and Cervus elaphus. Anthropozoologica. 2009;44(1):63–73. [Google Scholar]
- 67.Loftus RT, MacHugh DE, Bradley DG, Sharp PM, Cunningham P. Evidence for two independent domestications of cattle. Proc Natl Acad Sci USA. 1994;91(7):2757–2761. doi: 10.1073/pnas.91.7.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen S, et al. Zebu cattle are an exclusive legacy of the South Asia neolithic. Mol Biol Evol. 2010;27(1):1–6. doi: 10.1093/molbev/msp213. [DOI] [PubMed] [Google Scholar]
- 69.Meadow RH. The origins and spread of agriculture and pastoralism in northwestern South Asia. In: Harris DR, editor. The Origins and Spread of Agriculture and Pastoralism in Eurasia. London: Univ College London Press; 1996. pp. 390–412. [Google Scholar]
- 70.Evershed RP, et al. Earliest date for milk use in the Near East and southeastern Europe linked to cattle herding. Nature. 2008;455(7212):528–531. doi: 10.1038/nature07180. [DOI] [PubMed] [Google Scholar]
- 71.Kugonza DR, Nabasirye M, Hanotte O, Mpairwe D, Okeyo AM. Pastoralists’ indigenous selection criteria and other breeding practices of the long-horned Ankole cattle in Uganda. Trop Anim Health Prod. 2012;44(3):557–565. doi: 10.1007/s11250-011-9935-9. [DOI] [PubMed] [Google Scholar]
- 72.Mutundu K. Domestic stock age profiles and herd management practices: Ethnoarchaeological implications from Maasai settlements in southern Kenya. Archaeofauna. 2005;14:83–92. [Google Scholar]
- 73.Bollongino R, et al. Modern taurine cattle descended from small number of near-eastern founders. Mol Biol Evol. 2012;29(9):2101–2104. doi: 10.1093/molbev/mss092. [DOI] [PubMed] [Google Scholar]
- 74.Edwards CJ, et al. Mitochondrial DNA analysis shows a Near Eastern Neolithic origin for domestic cattle and no indication of domestication of European aurochs. Proc Biol Sci. 2007;274(1616):1377–1385. doi: 10.1098/rspb.2007.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murray C, Huerta-Sanchez E, Casey F, Bradley DG. Cattle demographic history modelled from autosomal sequence variation. Philos Trans R Soc Lond B Biol Sci. 2010;365(1552):2531–2539. doi: 10.1098/rstb.2010.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stock F, Gifford-Gonzalez DP. Genetics and African cattle domestication. Af Arch Rev. 2013;30(1):51–72. [Google Scholar]
- 77.Denham T, Iriarte J. In: Rethinking Agriculture: Archaeological and Ethnoarchaeological Perspectives. Vrydachs L, editor. Walnut Creek: Left Coast Press; 2007. [Google Scholar]
- 78.Larson G, et al. Rethinking dog domestication by integrating genetics, archeology, and biogeography. Proc Natl Acad Sci USA. 2012;109(23):8878–8883. doi: 10.1073/pnas.1203005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gerbault P, et al. (2014) Storytelling and story testing in domestication. Proc Natl Acad Sci USA 111:6159–6164. [DOI] [PMC free article] [PubMed]
- 80.Jablonka E. Behavioral epigenetics in ecological context. Behav Ecol. 2013;24:325–326. [Google Scholar]
- 81.Nätt D, et al. Heritable genome-wide variation of gene expression and promoter methylation between wild and domesticated chickens. BMC Genomics. 2012;13:59. doi: 10.1186/1471-2164-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Segelbacher G, et al. Application of landscape genetics in conservation biology: Concepts and challenges. Conserv Genet. 2010;11(2):375–385. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


